Vitamins and Radioprotective Effect: A Review
Abstract
1. Introduction
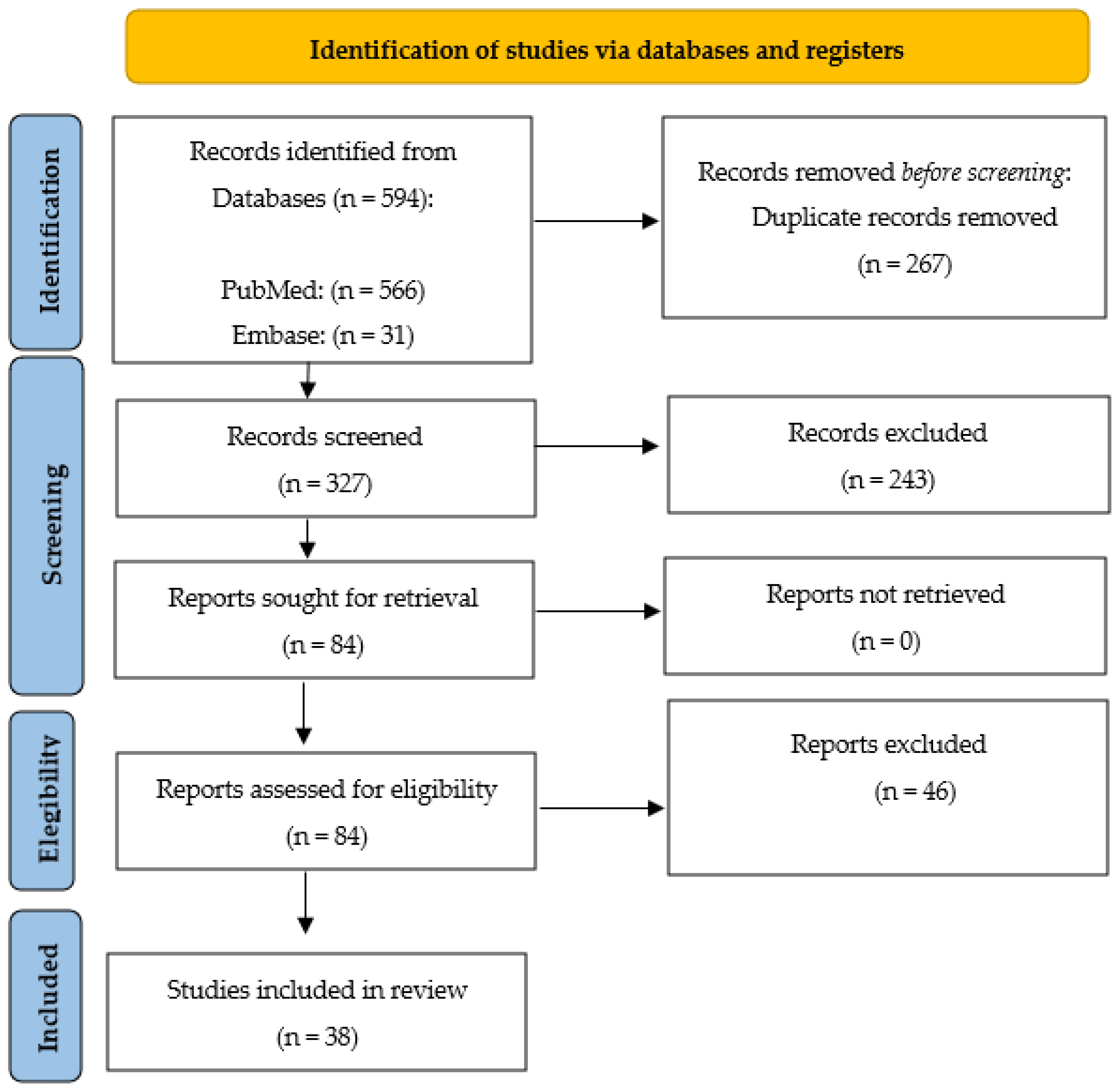
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gonçalves, D.; Pires, A.S.; Marques, I.A.; Gomes, I.; Sousa, G.; Botelho, M.F.; Abrantes, A.M. An overview on radiation sensitivity in hereditary breast and ovarian cancer syndrome. Cancers 2022, 14, 3254. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, F. More about radioactive pollution. Health Scope 2012, 1, 99–100. [Google Scholar] [CrossRef]
- Thomas, G.A.; Symonds, P. Radiation exposure and health effects—Is it time to reassess the real consequences? Clin. Oncol. R. Coll. Radiol. 2016, 28, 231–236. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Radiation Quantities and Units. Available online: http://www.fda.gov/Radiation-EmittingProducts/RadiationEmittingProductsandProcedures/MedicalImaging/MedicalX-rays/ucm115335.htm (accessed on 11 February 2023).
- Ahmadinejad, F.; Geir Møller, S.; Hashemzadeh-Chaleshtori, M.; Bidkhori, G.; Jami, M.-S. Molecular mechanisms behind free radical scavengers function against oxidative stress. Antioxidants 2017, 6, 51. [Google Scholar] [CrossRef]
- Obrador, E.; Salvador, R.; Villaescusa, J.I.; Soriano, J.M.; Estrela, J.M.; Montoro, A. Radioprotection and radiomitigation: From the bench to clinical practice. Biomedicines 2020, 8, 461. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Chandrasekharan, D.K.; Kagiya, T.V.; Nair, C.K.K. Radiation protection by 6-palmitoyl ascorbic acid-2-glucoside: Studies on DNA damage in vitro, ex vivo, in vivo and oxidative stress in vivo. J. Radiat. Res. 2009, 50, 203–212. [Google Scholar] [CrossRef]
- Garg, S.; Sadhukhan, R.; Banerjee, S.; Savenka, A.V.; Basnakian, A.G.; McHargue, V.; Wang, J.; Pawar, S.A.; Ghosh, S.P.; Ware, J.; et al. Gamma-tocotrienol protects the intestine from radiation potentially by accelerating mesenchymal immune cell recovery. Antioxidants 2019, 8, 57. [Google Scholar] [CrossRef]
- Marampon, F.; Gravina, G.L.; Festuccia, C.; Popov, V.M.; Colapietro, E.A.; Sanita, P.; Musio, D.; De Felice, F.; Lenzi, A.; Jannini, E.A.; et al. Vitamin D protects endothelial cells from irradiation-induced senescence and apoptosis by modulating MAPK/SirT1 axis. J. Endocrinol. Investig. 2016, 39, 411–422. [Google Scholar] [CrossRef]
- Xiao, L.; Tsutsui, T.; Miwa, N. The lipophilic vitamin C derivative, 6-o-palmitoylascorbate, protects human lymphocytes, preferentially over ascorbate, against X-ray-induced DNA damage, lipid peroxidation, and protein carbonylation. Mol. Cell. Biochem. 2014, 394, 247–259. [Google Scholar] [CrossRef]
- Darlina, D.; Teja, K.; Mukh, S. Capability of vitamin E as a radioprotector in suppressing DNA damage determined with comet assay. Biosaintifika 2017, 9, 201–208. [Google Scholar] [CrossRef]
- Zarei, H.; Bahreinipour, M.; Sefidbakht, Y.; Rezaei, S.; Gheisari, R.; Ardestani, S.K.; Uskoković, V.; Watabe, H. Radioprotective role of vitamins C and E against the gamma ray-induced damage to the chemical structure of bovine serum albumin. Antioxidants 2021, 10, 1875. [Google Scholar] [CrossRef] [PubMed]
- Safaei, M.; Jafarpour, S.M.; Mohseni, M.; Salimian, M.; Akbari, H.; Karami, F.; Aliasgharzadeh, A.; Farhood, B. Vitamins E and C prevent DNA double-strand breaks in peripheral lymphocytes exposed to radiations from Iodine-131. Indian J. Nucl. Med. 2018, 33, 20–24. [Google Scholar] [PubMed]
- Konopacka, M.; Rzeszowska-Wolny, J. Antioxidant vitamins C, E and β-carotene reduce DNA damage before as well as after γ-ray irradiation of human lymphocytes in vitro. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2001, 491, 1–7. [Google Scholar] [CrossRef]
- Rostami, A.; Moosavi, S.A.; Changizi, V.; Ardakani, A.A. Radioprotective effects of selenium and vitamin-E against 6MV X-rays in human blood lymphocytes by micronucleus assay. Med. J. Islam. Repub. Iran 2016, 30, 367. [Google Scholar]
- Cai, L.; Koropatnick, J.; Cherian, M.G. Roles of vitamin C in radiation-induced DNA damage in presence and absence of copper. Chem. Biol. Interact. 2001, 137, 75–88. [Google Scholar] [CrossRef]
- Popov, B.; Radev, R.; Georgieva, S. In vitro incidence of chromosome aberrations in gamma-irradiated rabbit lymphocytes, treated with Haberlea rhodopensis extract and vitamin C. Bulg. J. Vet. Med. 2010, 13, 148–153. [Google Scholar]
- Jafari, E.; Alavi, M.; Zal, F. The evaluation of protective and mitigating effects of vitamin C against side effects induced by radioiodine therapy. Radiat. Environ. Biophys. 2018, 57, 233–240. [Google Scholar] [CrossRef]
- Eksioglu, U.; Atilgan, H.I.; Yakin, M.; Yazihan, N.; Altiparmak, U.E.; Yumusak, N.; Korkmaz, M.; Demir, A.; Ornek, F.; Ayral, P.A.; et al. Antioxidant effects of vitamin D on lacrimal glands against high dose radioiodine-associated damage in an animal model. Cutan. Ocul. Toxicol. 2019, 38, 18–24. [Google Scholar] [CrossRef]
- de Moraes Ramos, F.M.; dos Anjos Pontual, M.L.; de Almeida, S.M.; Bóscolo, F.N.; Tabchoury, C.P.M.; Novaes, P.D. Evaluation of radioprotective effect of vitamin E in salivary dysfunction in irradiated rats. Arch. Oral Biol. 2006, 51, 96–101. [Google Scholar] [CrossRef]
- Sree, K.K.; Srinivasan, V.; Toles, R.; Jobe, L.; Seed, T.M. Nutritional approaches to radioprotection: Vitamin E. Mil. Med. 2002, 167, 57–59. [Google Scholar] [CrossRef]
- Upadhyaya, A.; Zhou, P.; Meng, Z.; Wang, P.; Zhang, G.; Jia, Q.; Tan, J.; Li, X.; Hu, T.; Liu, N.; et al. Radioprotective effect of vitamin E on salivary glands after radioiodine therapy for differentiated thyroid cancer: A randomized-controlled trial. Nucl. Med. Commun. 2017, 38, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.C.; Ramos-Perez, F.M.D.M.; Perez, D.E.D.C.; Novaes, P.D.; Boscolo, F.N.; Almeida, S.M.D. Radioprotective effect of vitamin E in parotid glands: A morphometric analysis in rats. Braz. Dent. J. 2013, 24, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Abedi, S.M.; Yarmand, F.; Motallebnejad, M.; Seyedmajidi, M.; Moslemi, D.; Ashrafpour, M.; Bijani, A.; Moghadamnia, A.; Mardanshahi, A.; Hosseinimehr, S.J. Vitamin E protects salivary glands dysfunction induced by ionizing radiation in rats. Arch. Oral Biol. 2015, 60, 1403–1409. [Google Scholar] [CrossRef]
- Acar, U.; Atilgan, H.I.; Acar, D.E.; Yalniz-Akkaya, Z.; Yumusak, N.; Korkmaz, M.; Koca, G. The effect of short-term vitamin E against radioiodine-induced early lacrimal gland damage. Ann. Nucl. Med. 2013, 27, 886–891. [Google Scholar] [CrossRef]
- Kulkarni, S.; Singh, P.K.; Ghosh, S.P.; Posarac, A.; Singh, V.K. Granulocyte colony-stimulating factor antibody abrogates radioprotective efficacy of gamma-tocotrienol, a promising radiation countermeasure. Cytokine 2013, 62, 278–285. [Google Scholar] [CrossRef]
- Garg, S.; Garg, T.K.; Wise, S.Y.; Fatanmi, O.O.; Miousse, I.R.; Savenka, A.V.; Basnakian, A.G.; Singh, V.K.; Hauer-Jensen, M. Effects of gamma-tocotrienol on intestinal injury in a GI-specific acute radiation syndrome model in nonhuman primate. Int. J. Mol. Sci. 2022, 23, 4643. [Google Scholar] [CrossRef]
- Li, X.H.; Fu, D.; Latif, N.H.; Mullaney, C.P.; Ney, P.H.; Mog, S.R.; Whitnall, M.H.; Srinivasan, V.; Xiao, M. δ-tocotrienol protects mouse and human hematopoietic progenitors from γ-irradiation through extracellular signal-regulated kinase/mammalian target of rapamycin signaling. Haematologica 2010, 95, 1996. [Google Scholar] [CrossRef]
- Satyamitra, M.M.; Kulkarni, S.; Ghosh, S.P.; Mullaney, C.P.; Condliffe, D.; Srinivasan, V. Hematopoietic recovery and amelioration of radiation-induced lethality by the vitamin E isoform δ-tocotrienol. Radiat. Res. 2011, 175, 736–745. [Google Scholar] [CrossRef]
- Ghosh, S.P.; Kulkarni, S.; Hieber, K.; Toles, R.; Romanyukha, L.; Kao, T.C.; Hauer-Jensen, M.; Kumar, K.S. Gamma-tocotrienol, a tocol antioxidant as a potent radioprotector. Int. J. Radiat. Biol. 2009, 85, 598–606. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Cary, L.H.; Gambles, K.; Hauer-Jensen, M.; Kumar, K.S.; Ghosh, S.P. Gamma-tocotrienol, a radiation prophylaxis agent, induces high levels of granulocyte colony-stimulating factor. Int. Immunopharmacol. 2012, 14, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Wise, S.Y.; Scott, J.R.; Romaine, P.L.; Newman, V.L.; Fatanmi, O.O. Radioprotective efficacy of delta-tocotrienol, a vitamin E isoform, is mediated through granulocyte colony-stimulating factor. Life Sci. 2014, 98, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Anzai, K.; Ueno, M.; Matsumoto, K.I.; Ikota, N.; Takata, J. Gamma-tocopherol-N, N-dimethylglycine ester as a potent post-irradiation mitigator against whole body X-irradiation-induced bone marrow death in mice. J. Radiat. Res. 2014, 55, 67–74. [Google Scholar] [CrossRef]
- Vasilyeva, I.; Bespalov, V.; Baranova, A. Radioprotective combination of α-tocopherol and ascorbic acid promotes apoptosis that is evident by release of low-molecular weight DNA fragments into circulation. Int. J. Radiat. Biol. 2015, 91, 872–877. [Google Scholar] [CrossRef]
- Anwar, M.; Nanda, N.; Bhatia, A.; Akhtar, R.; Mahmood, S. Effect of antioxidant supplementation on digestive enzymes in radiation induced intestinal damage in rats. Int. J. Radiat. Biol. 2013, 89, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, S.M.J.; Rahimi, S.; Mosleh-Shirazi, M.A.; Arjomandi, M.; Soleimani, A.; Hossein-Abadi, O.K.; Haghani, M.; Alavi, M. A comparative study on the life-saving radioprotective effects of vitamins a, E, C and over-the-counter multivitamins. J. Biomed. Phys. Eng. 2015, 5, 59. [Google Scholar]
- Mirdoraghi, M.; Changizi, V.; Haeri, S.A.; Rajabi, Z.; Amini, P.; Abassi, S. The radioprotective effect of magnesium sulfate and vitamin a on radiation-induced micronuclei and the expression of NOX4 in bone marrow cells of mice. J. Kerman Univ. Med. Sci. 2022, 29, 237–245. [Google Scholar]
- Tabeie, F.; Tabatabaei, S.M.; Mahmoud-Pashazadeh, A.; Assadi, M. Radioprotective effect of beta D-glucan and vitamin E on gamma irradiated mouse. J. Clin. Diagn. Res. 2017, 11, TC08–TC11. [Google Scholar] [CrossRef]
- Nair, C.K.K.; Menon, A. Consumption of antioxidant dietary agents, curcumin and vitamin C, protects cellular DNA from gamma-radiation. Int. J. Radiat. Res. 2013, 11, 11. [Google Scholar]
- Naeeji, A.; Mozdarani, H.; Monfared, A.S.; Faeghi, F.; Ahmadi, A.A.; Gholami, M.; Behzadi, R.; Momtaz, M.R. Oral administration of vitamin C, cimetidine and famotidine on micronuclei induced by low dose radiation in mouse bone marrow cells. J. Biomed. Phys. Eng. 2017, 7, 117. [Google Scholar]
- Zangeneh, M.; Mozdarani, H.; Mahmoudzadeh, A. Potent radioprotective effects of combined regimens of famotidine and vitamin C against radiation-induced micronuclei in mouse bone marrow erythrocytes. Radiat. Environ. Biophys. 2015, 54, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Aram, R.; Seyed Akbar, M.; Hassan Dianat, M.; Eftekhar Rajab, B. Micronuclei assessment of the radioprotective effects of melatonin and vitamin c in human lymphocytes. Cell J. 2016, 18, 46–51. [Google Scholar]
- Torun, N.; Muratli, A.; Serim, B.D.; Ergulen, A.; Altun, G.D. Radioprotective effects of amifostine, L-carnitine and vitamin E in preventing early salivary gland injury due to radioactive iodine treatment. Curr. Med. Imaging 2019, 15, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Nor-Eldin, E.K.; Elsayed, H.M. The possible radioprotective effects of vitamin E, Nigella sativa oil, and melatonin against X-ray induced early acute changes in cerebral and cerebellar cortices in Albino rats: Histological and Immunohistochemical. Egypt. J. Histol. 2019, 42, 767–782. [Google Scholar]
- Malick, M.A.; Roy, R.M.; Sternberg, J. Effect of vitamin E on post irradiation death in mice. Experientia 1978, 34, 1216–1217. [Google Scholar] [CrossRef] [PubMed]
- Konings, A.W.T.; Drijver, E.B. Radiation effects on membranes: I. Vitamin E deficiency and lipid peroxidation. Radiat. Res. 1979, 80, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Empey, L.R.; Papp, J.D.; Jewell, L.D.; Fedorak, R.N. Mucosal protective effects of vitamin E and misoprostol during acute radiation-induced enteritis in rats. Digest. Dis. Sci. 1992, 37, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Weiss, J.F. Radioprotection by vitamin E: Injectable vitamin E administered alone or with WR-3689 enhances survival of irradiated mice. Int. J. Radiat. Oncol. Biol. Phys. 1992, 23, 841–845. [Google Scholar] [CrossRef]
- Paranich, A.V.; Pocherniaeva, V.F.; Dubinskaia, G.M.; Mishchenko, V.P.; Mironova, N.G.; Gugalo, V.P.; Nazarets, V.V. Effect of supposed radioprotectors on oxidation-reduction of vitamin E in the tissues of irradiated rats. Radiats. Biol. Radioecol. 1993, 33, 653–657. [Google Scholar]
- Meclain, D.E.; Kalinich, J.F.; Ramakrishnan, N. Trolox inhibits apoptosis in irradiated MOLT-4 lymphocytes. FASEB J. 1995, 9, 1345–1354. [Google Scholar] [CrossRef]
- Jha, M.N.; Bedford, J.S.; Cole, W.C.; Edward-Prasad, J.; Prasad, K.N. Vitamin E (d-Alpha-tocopheryl succinate) decreases mitotic accumulation in gamma-irradiated human tumor, but not in normal, cells. Nutr. Cancer 1999, 35, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Lefaix, J.L.; Delanian, S.; Vozenin, M.C.; Leplat, J.J.; Tricaud, Y.; Martin, M. Striking regression of subcutaneous fibrosis induced by high doses of gamma rays using a combination of pentoxifylline and alpha-tocopherol: An experimental study. Int. J. Radiat. Oncol. Biol. Phys. 1999, 43, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Mutalip, S.S.M. Vitamin E: Nature’s gift to fight cancer. In Anticancer Plants: Properties and Application; Akhtar, M.S., Swamy, M.K., Eds.; Springer: Singapore, 2018; Volume 1, pp. 367–393. [Google Scholar]
- Nukala, U.; Thakkar, S.; Krager, K.J.; Breen, P.J.; Compadre, C.M.; Aykin-Burns, N. Antioxidant tocols as radiation countermeasures (challenges to be addressed to use tocols as radiation countermeasures in humans). Antioxidants 2018, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Hauer-Jensen, M. γ-Tocotrienol as a promising countermeasure for acute radiation syndrome: Current status. Int. J. Mol. Sci. 2016, 17, 663. [Google Scholar] [CrossRef]
- Suhardi, J.V.; Morgan, D.F.A.; Muratoglu, O.K.; Oral, E. Radioprotection and cross-linking of allograft bone in the presence of vitamin E. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2354–2367. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two faces of vitamin C—Antioxidative and pro-oxidative agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef] [PubMed]
- Okunieff, P. Interactions between ascorbic acid and the radiation of bone marrow, skin, and tumor. Am. J. Clin. Nutr. 1991, 54, 1281S–1283S. [Google Scholar] [CrossRef]
- Sarma, L.; Kesavan, P.C. Protective effects of vitamins C and E against gamma-ray-induced chromosomal damage in mouse. Int. J. Radiat. Biol. 1993, 63, 759–764. [Google Scholar] [CrossRef]
- Narra, V.R.; Howell, R.W.; Sastry, K.S.; Rao, D.V. Vitamin C as a radioprotector against Iodine-131 in vivo. J. Nucl. Med. 1993, 34, 637–640. [Google Scholar]
- Simpson, G.L.; Ortwerth, B.J. The non-oxidative degradation of ascorbic acid at physiological conditions. Biochim. Biophys. Acta 2000, 1501, 12–24. [Google Scholar] [CrossRef]
| Compound/s | Radiation Type and Dose | Studied Sample | Main Outcomes | Reference |
|---|---|---|---|---|
| 6-palmitoyl ascorbic acid-2-glucoside (PAsAG) | 60Co γ -irradiation (6 Gy) | Mouse spleen cells | Cellular DNA protected due to the presence of 1.6 mM PAsAG during radiation so that the comet parameters. | Chandrasekharan et al., 2009 [8] |
| γ -tocotrienol (GT3) | 137Cs (8 Gy to measure apoptosis of epithelial cells and recovery of the immune cells and 10 and 12 Gy for the intestinal permeability assay) | Male CD2F1 mice | Cell death and decrease in villus height were suppressed and decreased, respectively, together with other intestinal effects, such as are crypt depth, attenuated intestinal permeability, and upregulated occludin level in the intestine, due to the use of GT3 (200 mg/kg b.w.) administered 24 h before irradiation. | Garg et al., 2019 [9] |
| Compound/s | Radiation Type and Dose | Studied Sample | Main Outcomes | Reference |
|---|---|---|---|---|
| Vitamin D | X-rays (4 Gy) | Human umbilical vein endothelial cells (HUVECs) | It was shown that vit D reduced IR-induced reactive oxygen species production protecting proliferating and quiescent HUVECs from cellular apoptosis or senescence, respectively, by regulating positively the mitogen-activated protein kinase (MAPK) pathways. | Marampon et al., 2016 [10] |
| Vitamin C (6-o-palmitoylascorbate (PlmtVC)) | X-rays (1.5 Gy) | HEV0082 cells | PlmtVC markedly inhibited X-ray induced caspase 3 activation. So, PlmtVC could prevent X-ray-induced DNA damages and inhibited intracellular ROS production and lipid peroxidation. | Xiao et al., 2014 [11] |
| Vitamin E (as dl-α-tocopherol). | 60Co γ-irradiation (6 Gy) | Human lymphocytes | Reduction of DNA damage up to 94.2% using concentration of 0.8 mM of vitamin E. | Darlina et al., 2017 [12] |
| Vitamins C and E | 60Co γ-irradiation (0.1–3 Gy) | Bovine serum albumin (BSA) | Irradiated BSA, with the presence of both vitamins, was protected of structural changes caused by ROS. | Zarei et al., 2021 [13] |
| Vitamins E and C | 131I (20 µCi) | Human lymphocytes | Vitamins E and C can reduce the toxicity of 131I. Vitamin C provided the more protection for DNA followed of vitamin E. | Safaei et al., 2018 [14] |
| Vitamins C and E and β-carotene | 60Co γ-irradiation (2 Gy) | Human lymphocytes | The strongest effect is more effective when they were added no later than 1 h after irradiation. Furthermore, vitamin C at low concentration (1 μg/mL), vitamin E at the concentration above 2 μg/mL and β-carotene were effective at all tested concentrations between 1–5 μg/mL reduced the number of micronuclei in irradiated cells. | Konopacka et al., 2001 [15] |
| Vitamin E and selenium | X-rays (2 Gy) | Human lymphocytes | 1 hr after administration of both nutrients was observed the maximum protection and decrease in frequency of micronuclei (50%). | Rostami et al., 2016 [16] |
| Vitamin C and copper | 60Co γ-irradiation (3 Gy) | Calf thymus DNA | Vitamin C with copper (50 μM) significantly enhanced γ-radiation-induced DNA damage. | Cai et al., 2001 [17] |
| Vitamin C and Haberlea rhodopensis extract | 60Co γ-irradiation (2 Gy) | Rabbits peripheral blood lymphocytes | The frequency of dicentrics and double acentric fragments was similar with both compounds. | Popov et al., 2010 [18] |
| Vitamin/s | Radiation Type and Dose | Studied Sample | Main Outcomes | Reference |
|---|---|---|---|---|
| Vitamin C | 131I (5550 MBq) | Differentiated thyroid cancer patients ablated with radioiodine | This vitamin, used after radioiodine therapy, ameliorated serum oxidative stress. | Jafari et al., 2018 [19] |
| Vitamin D | 131I (3 mCi) | Wistar albino rats | Anti-inflammatory, antioxidant and radio-protective effects on lacrimal glands were observed. | Eksioglu et al., 2019 [20] |
| Vitamin E | 60Co γ-irradiation (15 Gy) | Male Wistar rats between 8 and 10 weeks old divided into six groups. | Vitamin E protected the salivary function 30 days after irradiation. | De Moraes ramos et al., 2006 [21] |
| Vitamin E | 60Co γ-irradiation (0.6 Gy/min) | Male CD2F1 mice | Vitamin E at a dose of 400 IU/kg acts as radioprotectant against lethal doses of radiation being more efficacious when is given subcutaneously than when given orally. | Sree et al., 2002 [22] |
| Vitamin E | 131I (100 mCi) | Eighty-two patients with differentiated thyroid cancer | Vitamin E exerts significant protective effects on the parotid and submandibular glands after 131I therapy. | Upadhyaya et al., 2017 [23] |
| Vitamin E | 60Co γ-irradiation (15 Gy) | Sixty male Wistar rats | Vitamin E was not effective as a radioprotective agent on acinar cells in rats’ parotid glands. | Gomes et al., 2013 [24] |
| Vitamin E | 60Co γ-irradiation (15 Gy) | Male Wistar rats | Vitamin E at dose 400 IU/Kg significantly protected and improved salivary gland function against toxicity induced by ionizing radiation. | Abedi et al., 2015 [25] |
| Vitamin E | 131I (3 mCi) | Wistar albino rats | Histopathological examinations revealed that vitamin E protects rat lacrimal glands against radioiodine-related early damage. | Acar et al., 2013 [26] |
| γ-tocotrienol | 60Co γ-irradiation (9.2 Gy) | Male CD2F1 mice | Induction of high levels of granulocyte colony-stimulating factor by γ-tocotrienol administration is responsible for its protective efficacy against radiation injury. | Kulkarni et al., 2013 [27] |
| γ-tocotrienol | 60Co γ-irradiation (12 Gy) | Rhesus macaques were treated with 37.5 mg/kg γ-tocotrienol subcutaneously 24 h prior to radiation exposure | γ-tocotrienol has radioprotective function in intestinal epithelial and crypt cells. | Garg et al., 2022 [28] |
| δ-tocotrienol | 60Co γ-irradiation (0, 5 or 8.75 Gy) | CD2F1 male mice | δ-tocotrienol protects mouse bone marrow from radiation-induced damage through extracellular signal-related kinase activation-associated mammalian target of rapamycin survival pathways. | Li et al., 2010 [29] |
| δ-tocotrienol | 60Co γ-irradiation (7 Gy) | Mouse liver microsomes | 24 h prior to 7 Gy reduced pancytopenia significantly with 300 mg/kg δ-tocotrienol. | Satyamitra et al., 2011 [30] |
| δ-tocotrienol | 60Co γ-irradiation (0.5, 11 or 11.5 Gy) | Male CD2F1 mice | 200 mg/kg subcutaneously of this vitamin 24 h before irradiation in mice obtained a dose reduction factor of 1.29 and accelerated the recovery of several parameters, such as total white blood cells, neutrophils, monocytes, platelet, and reticulocytes. | Ghosh et al., 2009 [31] |
| δ-tocotrienol | 60Co γ-irradiation (0 or 7 Gy) | Male CD2F1 mice | This vitamin increased serum levels of G-CSF, IL-6, KC and several other cytokines within 12–24 h post-administration. | Kulkarni et al., 2012 [32] |
| δ-tocotrienol | 60Co γ-irradiation (9.2 Gy) | Male CD2F1 mice | Granulocyte colony-stimulating factor induced by δ-tocotrienol administration a protective efficacy against radiation injury. | Singh et al., 2014 [33] |
| γ-tocopherol- N, N-dimethyl glycine ester (GTDMG) | X-rays (7.5 Gy) | Male C3H mice | GTDMG enhanced the recovery of red and white blood cells, platelet counts and significantly increased the number of endogenous spleen colonies. | Anzai et al., 2014 [34] |
| Vitamin E and vitamin C | 137Cs γ-irradiation (2 or 8 Gy) | Wistar male rats | The pretreatment with both vitamins provided radioprotection partially by aiding non-inflammatory, apoptotic elimination of several damaged cells. | Vasilyeva et al., 2015 [35] |
| Vitamin A, C, E and lycopene | 60Co γ-irradiation (8 Gy) | Swiss Albino rats were divided into six groups. | Vitamin E supplementation, compared to other vitamins, was most potent in ameliorating the intestinal aberrations. | Anwar et al., 2013 [36] |
| Vitamins A, E, C and over-the-counter multivitamins | 60Co γ-irradiation (8.8 Gy) | Male Balb/c mice | The radioprotective effect of vitamin C is more efficient than the effect of other vitamins. Even high doses of vitamin C can show lifesaving radioprotective effects. | Mortazavi et al., 2015 [37] |
| Vitamin A and magnesium sulphate | X-rays (2 Gy) | Mice were treated intraperitoneally with 9 different combined doses of vitamin A (100, 200 and 400 mg/kg) and MgSO4 (75, 150 and 300 mg/kg) | Combination of 200 mg/kg vitamin A + 150 mg/kg MgSO4 produced high protection against 2Gy X-ray protecting the bone marrow cells of mice. | Mirdoraghi et al., 2022 [38] |
| Vitamin E and β-D-glucan | 60Co γ-irradiation (6, 7 or 8 Gy) | 240 female mice | Β-D-glucan in the body of mice, during exposure to ionizing radiation, leads to dose reduction factor higher than one. Furthermore, both increased resistance of mice against ionizing radiation. | Tabeie et al., 2017 [39] |
| Vitamin C and curcumin | γ-radiation (0, 3 or 6 6 Gy) | Human non-smoking male volunteers were treated with orally given 100 µg/mL of vitamin or curcumin before the radiation | The extent of DNA damage was significantly decreased either in the presence or following the intake of curcumin and ascorbic acid. The intake of dietary antioxidants such as ascorbic acid could offer protection against ionizing radiation reflected, in peripheral blood leukocytes, cellular DNA damage. | Nair and Menon, 2013 [40] |
| Vitamin C, famotidine and cimetidine | 60Co γ-irradiation (2 Gy) | Male NMRI mice | Oral administration of Famotidine, vitamin C and Cimetidine, in single use or combination form, demonstrates reliable and similar radioprotective effects. | Naeeji et al., 2017 [41] |
| Vitamin C and famotidine | 60Co γ-irradiation (2 or 4 Gy) | Male NMRI mice | Combination of both compounds was more effective in reducing the frequency of micronucleated polychromatic erythrocytes leading to a protection factor of 4.3 after irradiation. | Zangeneh et al., 2015 [42] |
| Vitamin C and melatonin | X-rays (7.5 Gy) | Volunteers | Before irradiation, in human peripheral blood lymphocytes, the use of both compounds caused a reduction in DNA damage. | Aram et al., 2016 [43] |
| Vitamin E, amifostine and L-Carnitine | 131I (555–660 MBq) | Adult guinea pigs | The individual use of these compounds for radioprotection yields different levels of, but not absolute, protection against radioactive iodine treatment injury in salivary glands. | Torun et al., 2019 [44] |
| Vitamin E, Nigella sativa oil and melatonin | X-ray (8 Gy) | Albino rats | The use of these compounds could limit radiation induced injury in brain and cerebellum. | Nor-Eldin and Elsayed, 2019 [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lledó, I.; Ibáñez, B.; Melero, A.; Montoro, A.; Merino-Torres, J.F.; San Onofre, N.; Soriano, J.M. Vitamins and Radioprotective Effect: A Review. Antioxidants 2023, 12, 611. https://doi.org/10.3390/antiox12030611
Lledó I, Ibáñez B, Melero A, Montoro A, Merino-Torres JF, San Onofre N, Soriano JM. Vitamins and Radioprotective Effect: A Review. Antioxidants. 2023; 12(3):611. https://doi.org/10.3390/antiox12030611
Chicago/Turabian StyleLledó, Inés, Blanca Ibáñez, Ana Melero, Alegría Montoro, Juan F. Merino-Torres, Nadia San Onofre, and Jose M. Soriano. 2023. "Vitamins and Radioprotective Effect: A Review" Antioxidants 12, no. 3: 611. https://doi.org/10.3390/antiox12030611
APA StyleLledó, I., Ibáñez, B., Melero, A., Montoro, A., Merino-Torres, J. F., San Onofre, N., & Soriano, J. M. (2023). Vitamins and Radioprotective Effect: A Review. Antioxidants, 12(3), 611. https://doi.org/10.3390/antiox12030611









