Effects of Dietary Glutamine Supplementation on Heat-Induced Oxidative Stress in Broiler Chickens: A Systematic Review and Meta-Analysis
Abstract
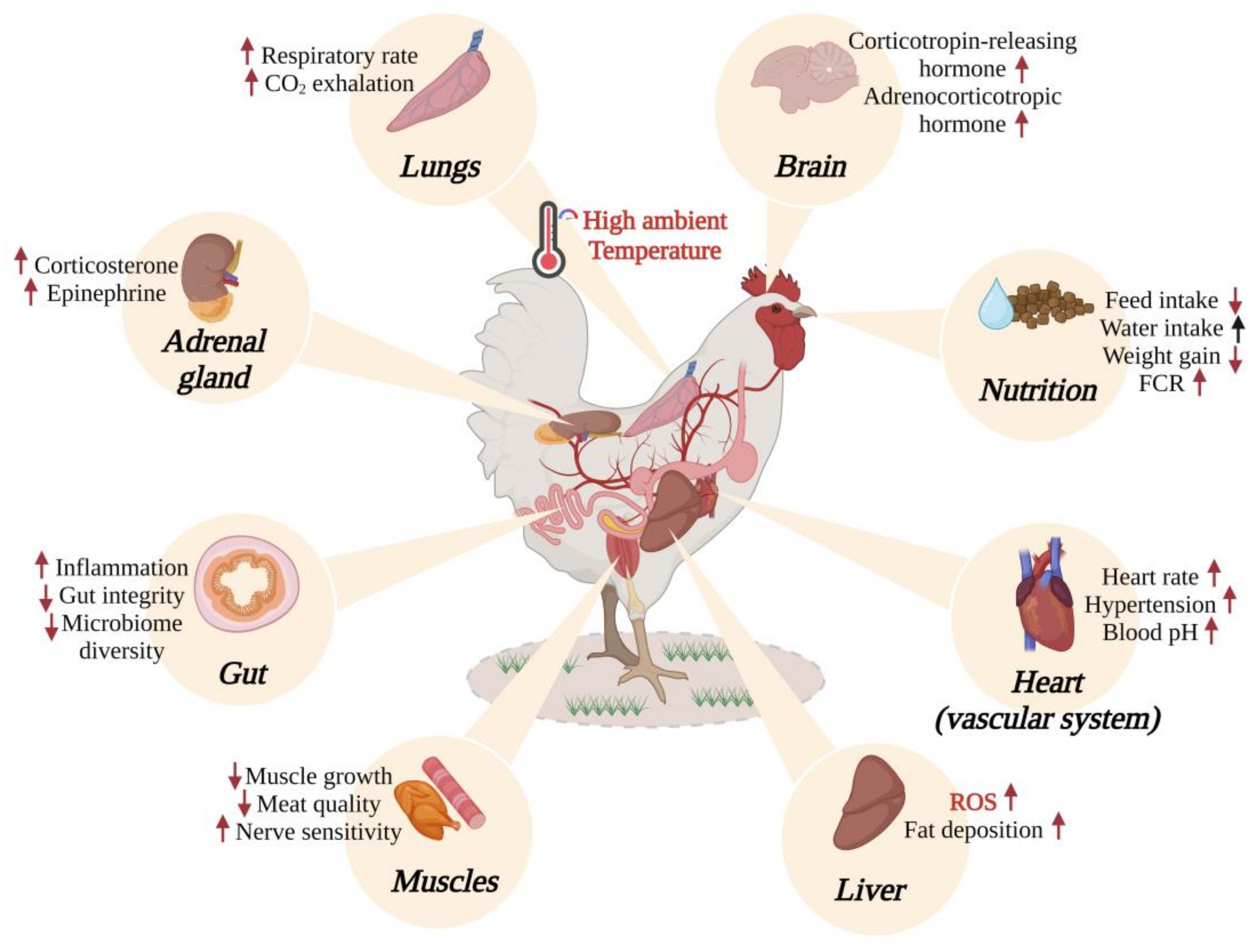
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Study Quality Assessment
2.4. Data Extraction and Processing
2.5. Statistical Analysis
3. Results
3.1. Study Selection Workflow
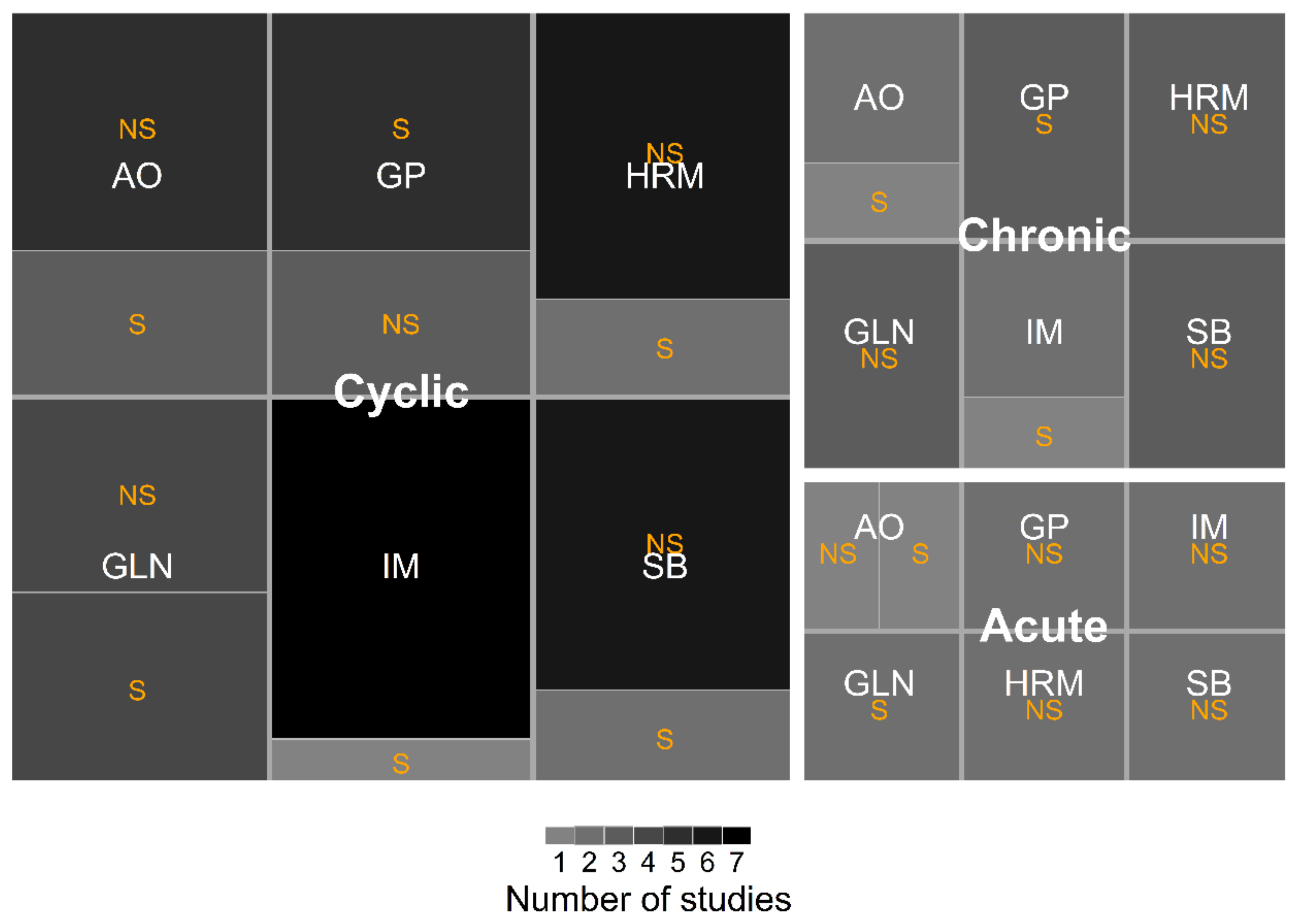
3.2. Study Characteristics
3.3. Study Quality
3.4. Effect of Dietary Glutamine on the Growth Performances of Broilers Reared under HS
3.5. Effect of Dietary Glutamine on the Glutamine Concentration in Different Tissues of Broilers Reared under HS
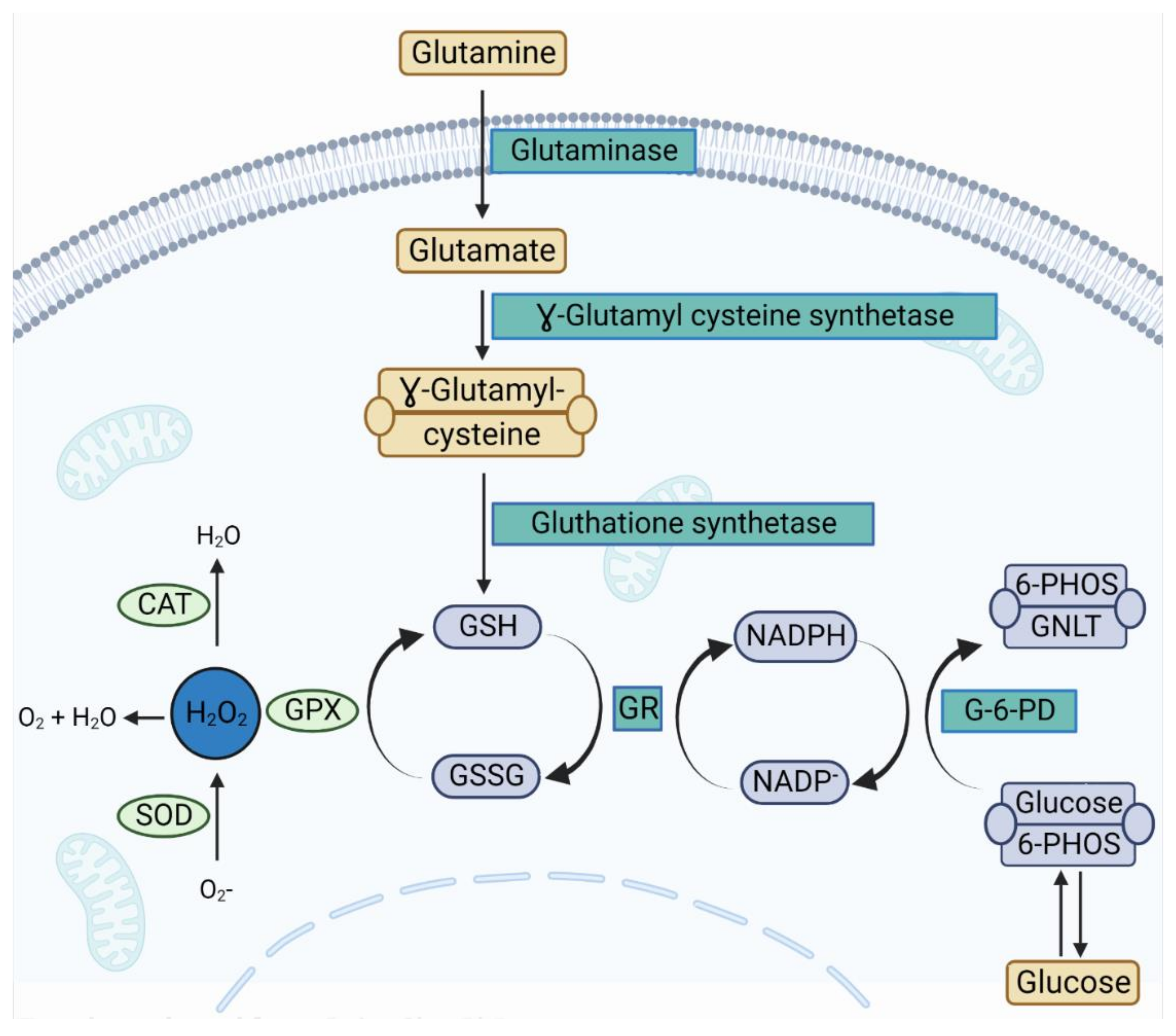
3.6. Effect of Dietary Glutamine on the Antioxidant Status of Broilers Reared under HS
3.7. Effects of the Glutamine Concentration and HS Duration on Growth and Antioxidant Parameters
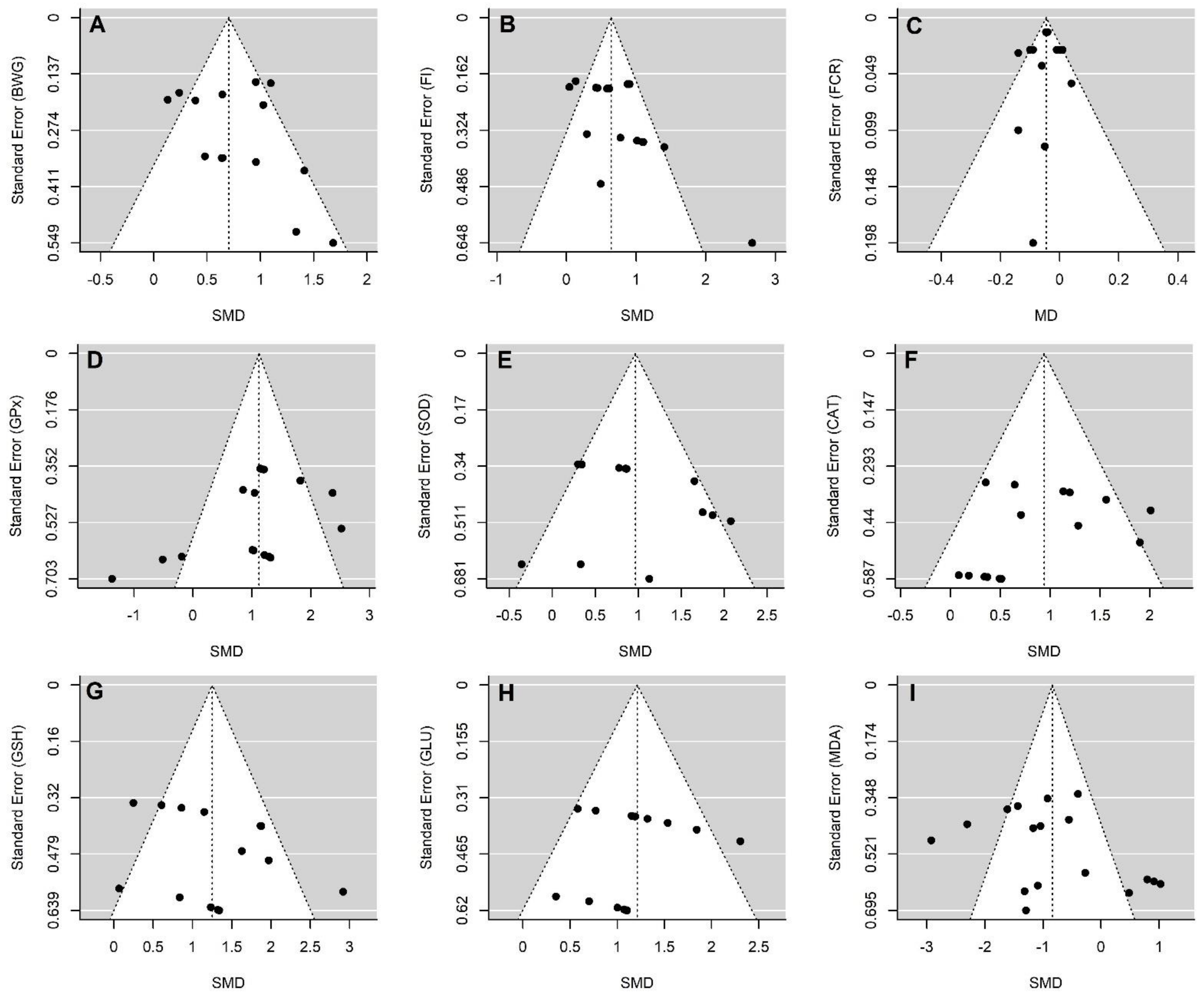
3.8. Analysis of Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torrey, S.; Mohammadigheisar, M.; Nascimento dos Santos, M.; Rothschild, D.; Dawson, L.C.; Liu, Z.; Kiarie, E.G.; Edwards, A.M.; Mandell, I.; Karrow, N.; et al. In pursuit of a better broiler: Growth, efficiency, and mortality of 16 strains of broiler chickens. Poult. Sci. 2021, 100, 100955. [Google Scholar] [CrossRef] [PubMed]
- Zou, A.; Nadeau, K.; Wang, P.W.; Lee, J.Y.; Guttman, D.S.; Sharif, S.; Korver, D.R.; Brumell, J.H.; Parkinson, J. Accumulation of genetic variants associated with immunity in the selective breeding of broilers. BMC Genet. 2020, 21, 5. [Google Scholar] [CrossRef] [PubMed]
- Agyare, C.; Boamah, V.E.; Zumbi, C.N.; Osei, F.B. Antibiotic Use in Poultry Production and Its Effects on Bacterial Resistance. In Antimicrob Resist-A Global Threat; IntechOpen: London, UK, 2018; pp. 33–50. [Google Scholar]
- Biswal, J.; Vijayalakshmy, K.; Bhattacharya, T.K.; Rahman, H. Impact of heat stress on poultry production. World Poult. Sci. J. 2022, 78, 179–196. [Google Scholar] [CrossRef]
- Nawab, A.; Ibtisham, F.; Li, G.; Kieser, B.; Wu, J.; Liu, W.; Zhao, Y.; Nawab, Y.; Li, K.; Xiao, M.; et al. Heat stress in poultry production: Mitigation strategies to overcome the future challenges facing the global poultry industry. J. Biol 2018, 78, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2022, 8, 383–395. [Google Scholar] [CrossRef]
- Siegel, H. Physiological stress in birds. Bioscience 1980, 30, 529–534. [Google Scholar] [CrossRef]
- Borges, S.A.; Fischer da Silva, A.V.; Majorka, A.; Hooge, D.M.; Cummings, K.R. Physiological responses of broiler chickens to heat stress and dietary electrolyte balance (sodium plus potassium minus chloride, milliequivalents per kilogram). Poult. Sci. 2004, 83, 1551–1558. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, N.; Wu, X.; Wang, G.; Lin, L. Glutamine alleviates heat stress-induced impairment of intestinal morphology, intestinal inflammatory response, and barrier integrity in broilers. Poult. Sci. 2018, 97, 2675–2683. [Google Scholar] [CrossRef]
- Ncho, C.M.; Goel, A.; Gupta, V.; Jeong, C.-M.; Choi, Y.-H. Embryonic manipulations modulate differential expressions of heat shock protein, fatty acid metabolism, and antioxidant-related genes in the liver of heat-stressed broilers. PLoS ONE 2022, 17, e0269748. [Google Scholar] [CrossRef]
- Ncho, C.M.; Goel, A.; Gupta, V.; Jeong, C.-M.; Choi, Y.-H. Impact of embryonic manipulations on core body temperature dynamics and survival in broilers exposed to cyclic heat stress. Sci. Rep. 2022, 12, 15110. [Google Scholar] [CrossRef]
- Gupta, V.; Ncho, C.M.; Goel, A.; Jeong, C.-M.; Choi, Y.-H. Effects of In Ovo Injection of α-Ketoglutaric Acid on Hatchability, Growth, Plasma Metabolites, and Antioxidant Status of Broilers. Antioxidants 2022, 11, 2102. [Google Scholar] [PubMed]
- Chowdhury, V.S.; Tomonaga, S.; Nishimura, S.; Tabata, S.; Furuse, M. Physiological and behavioral responses of young chicks to high ambient temperature. J. Poult. Sci. 2012, 49, 1203260160. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, J.; Wang, B.; Tang, J. Effect of γ-aminobutyric acid on digestive enzymes, absorption function, and immune function of intestinal mucosa in heat-stressed chicken1 1This work is supported by research grants from National Natural Science Foundation of China (NSFC31060312; 31260555, Beijing). Poult. Sci. 2014, 93, 2490–2500. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Zhou, Y.; Feng, J.; Zhang, M. Effects of Heat Stress on Gut-Microbial Metabolites, Gastrointestinal Peptides, Glycolipid Metabolism, and Performance of Broilers. Animals 2021, 11, 1286. [Google Scholar] [CrossRef]
- Sifa, D.; Bai, X.; Zhang, D.; Hu, H.; Wu, X.; Wen, A.; He, S.; Zhao, L. Dietary glutamine improves meat quality, skeletal muscle antioxidant capacity and glutamine metabolism in broilers under acute heat stress. J. Appl. Anim. Res. 2018, 46, 1412–1417. [Google Scholar] [CrossRef]
- Zhang, J.F.; Hu, Z.P.; Lu, C.H.; Yang, M.X.; Zhang, L.L.; Wang, T. Dietary curcumin supplementation protects against heat-stress-impaired growth performance of broilers possibly through a mitochondrial pathway1. J. Anim. Sci. 2015, 93, 1656–1665. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Esquerra, R.; Leeson, S. Physiological and metabolic responses of broilers to heat stress-implications for protein and amino acid nutrition. World Poult. Sci. J. 2006, 62, 282–295. [Google Scholar] [CrossRef]
- Goel, A.; Ncho, C.M.; Choi, Y.H. Regulation of gene expression in chickens by heat stress. J. Anim. Sci. Biotechnol. 2021, 12, 11. [Google Scholar] [CrossRef]
- Goel, A.; Ncho, C.M.; Gupta, V.; Choi, Y.-H. Embryonic modulation through thermal manipulation and in ovo feeding to develop heat tolerance in chickens. Anim. Nutr. 2023, in press. [Google Scholar] [CrossRef]
- Hu, H.; Bai, X.; Shah, A.A.; Dai, S.; Wang, L.; Hua, J.; Che, C.; He, S.; Wen, A.; Jiang, J. Interactive effects of glutamine and gamma-aminobutyric acid on growth performance and skeletal muscle amino acid metabolism of 22-42-day-old broilers exposed to hot environment. Int. J. Biometeorol. 2016, 60, 907–915. [Google Scholar] [CrossRef]
- Hu, H.; Bai, X.; Shah, A.A.; Wen, A.Y.; Hua, J.L.; Che, C.Y.; He, S.J.; Jiang, J.P.; Cai, Z.H.; Dai, S.F. Dietary supplementation with glutamine and gamma-aminobutyric acid improves growth performance and serum parameters in 22- to 35-day-old broilers exposed to hot environment. J. Anim. Physiol. Anim. Nutr. 2016, 100, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Chen, L.; Dai, S.; Li, J.; Bai, X. Effect of glutamine on antioxidant capacity and lipid peroxidation in the breast muscle of heat-stressed broilers via antioxidant genes and Hsp70 pathway. Animals 2020, 10, 404. [Google Scholar] [CrossRef] [PubMed]
- Vesterinen, H.; Sena, E.; Egan, K.; Hirst, T.; Churolov, L.; Currie, G.; Antonic, A.; Howells, D.; Macleod, M. Meta-analysis of data from animal studies: A practical guide. J. Neurosci. Methods 2014, 221, 92–102. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Bakhsh, A.; Lee, E.-Y.; Ncho, C.M.; Kim, C.-J.; Son, Y.-M.; Hwang, Y.-H.; Joo, S.-T. Quality Characteristics of Meat Analogs through the Incorporation of Textured Vegetable Protein: A Systematic Review. Foods 2022, 11, 1242. [Google Scholar] [CrossRef]
- Ncho, C.M.; Jeong, C.; Gupta, V.; Goel, A. The effect of gamma-aminobutyric acid supplementation on growth performances, immune responses, and blood parameters of chickens reared under stressful environment: A meta-analysis. Environ. Sci. Pollut. Res. 2021, 28, 45019–45028. [Google Scholar] [CrossRef]
- Ncho, C.M.; Gupta, V.; Goel, A. Effect of thermal conditioning on growth performance and thermotolerance in broilers: A systematic review and meta-analysis. J. Therm. Biol. 2021, 98, 102916. [Google Scholar] [CrossRef]
- Harrer, M.; Cuijpers, P.; Furukawa, T.A.; Ebert, D.D. Doing Meta-Analysis with R: A Hands-On Guide; Chapman and Hall/CRC: Boca Raton, FL, USA, 2021. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. Bmj 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Cui, T.; Xi, J.; Tang, C.; Song, J.; He, J.; Brytek-Matera, A. The Relationship between Music and Food Intake: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 2571. [Google Scholar] [CrossRef]
- Ayazi, M. The effect of dietary glutamine supplementation on performance and blood antioxidant status of broiler chickens under continuous heat stress condition. Int. J. Farming Allied Sci. 2014, 3, 1213–1219. [Google Scholar]
- Bai, X.; Dai, S.; Li, J.; Xiao, S.; Wen, A.; Hu, H. Glutamine improves the growth performance, serum biochemical profile and antioxidant status in broilers under medium-term chronic heat stress. J. Appl. Poult. Res. 2019, 28, 1248–1254. [Google Scholar] [CrossRef]
- Dai, S.F.; Gao, F.; Zhang, W.H.; Song, S.X.; Xu, X.L.; Zhou, G.H. Effects of dietary glutamine and gamma-aminobutyric acid on performance, carcass characteristics and serum parameters in broilers under circular heat stress. Anim. Feed Sci. Technol. 2011, 168, 51–60. [Google Scholar] [CrossRef]
- Jazideh, F.; Farhoomand, P.; Daneshyar, M.; Najafi, G. The effects of dietary glutamine supplementation on growth performance and intestinal morphology of broiler chickens reared under hot conditions. Turk. J. Vet. Anim. Sci. 2014, 38, 264–270. [Google Scholar] [CrossRef]
- Wu, Q.J.; Jiao, C.; Liu, Z.H.; Cheng, B.Y.; Liao, J.H.; Zhu, D.D.; Ma, Y.; Li, Y.X.; Li, W. Effect of glutamine on the growth performance, digestive enzyme activity, absorption function, and mRNA expression of intestinal transporters in heat-stressed chickens. Res. Vet. Sci. 2021, 134, 51–57. [Google Scholar] [CrossRef]
- Dai, S.; Wang, L.; Wen, A.; Wang, L.; Jin, G. Dietary glutamine supplementation improves growth performance, meat quality and colour stability of broilers under heat stress. Br. Poult. Sci. 2009, 50, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Dai, S.; Li, J.; Wen, A.; Bai, X. Glutamine improves heat stress–induced oxidative damage in the broiler thigh muscle by activating the nuclear factor erythroid 2–related 2/Kelch-like ECH-associated protein 1 signaling pathway. Poult. Sci. 2020, 99, 1454–1461. [Google Scholar] [CrossRef]
- Hu, H.; Bai, X.; Wen, A.; Shah, A.; Dai, S.; Ren, Q.; Wang, S.; He, S.; Wang, L. Assessment of interactions between glutamine and glucose on meat quality, AMPK, and glutamine concentrations in pectoralis major meat of broilers under acute heat stress. J. Appl. Poult. Res. 2016, 25, 370–378. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; 2nd, ed, Ed.; Erbaum Press: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Xi, P.; Jiang, Z.; Zheng, C.; Lin, Y.; Wu, G. Regulation of protein metabolism by glutamine: Implications for nutrition and health. Front Biosci 2011, 16, 578–597. [Google Scholar] [CrossRef]
- Amores-Sánchez, M.a.I.; Medina, M.Á. Glutamine, as a precursor of glutathione, and oxidative stress. Mol. Genet. Metab. 1999, 67, 100–105. [Google Scholar] [CrossRef]
- Matés, J.M.; Pérez-Gómez, C.; de Castro, I.N.; Asenjo, M.; Márquez, J. Glutamine and its relationship with intracellular redox status, oxidative stress and cell proliferation/death. Int. J. Biochem. Cell Biol. 2002, 34, 439–458. [Google Scholar] [CrossRef]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Kidd, M.T. Antioxidant Defence Systems and Oxidative Stress in Poultry Biology: An Update. Antioxidants 2019, 8, 235. [Google Scholar] [CrossRef] [PubMed]
- Sadaf, A.; Quinn, C.T. L-glutamine for sickle cell disease: Knight or pawn? Exp. Biol. Med. 2020, 245, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, J.; Djordjevic, A.; Adzic, M.; Niciforovic, A.; Radojcic, M.B. Chronic stress differentially affects antioxidant enzymes and modifies the acute stress response in liver of Wistar rats. Physiol. Res. 2010, 59, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Emami, N.K.; Jung, U.; Voy, B.; Dridi, S. Radical Response: Effects of Heat Stress-Induced Oxidative Stress on Lipid Metabolism in the Avian Liver. Antioxidants 2021, 10, 35. [Google Scholar] [CrossRef]
- Surai, P.; Kochish, I.; Fisinin, V. Antioxidant systems in poultry biology: Nutritional modulation of vitagenes. Eur. J. Poult. Sci. 2017, 81, 1612–9199. [Google Scholar]
- Hafeman, D.; Sunde, R.; Hoekstra, W. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J. Nutr. 1974, 104, 580–587. [Google Scholar] [CrossRef]
- Lennarz, W.J.; Lane, M.D. Encyclopedia of Biological Chemistry; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Surai, P.F. Antioxidant systems in poultry biology: Superoxide dismutase. J. Anim. Res. Nutr. 2016, 1, 8. [Google Scholar] [CrossRef]
- Wang, R.; Pan, X.; Peng, Z. Effects of heat exposure on muscle oxidation and protein functionalities of pectoralis majors in broilers. Poult. Sci. 2009, 88, 1078–1084. [Google Scholar] [CrossRef]
- Xiong, Y.L.; Cantor, A.H.; Pescatore, A.J.; Blanchard, S.P.; Straw, M.L. Variations in Muscle Chemical Composition, pH, and Protein Extractability Among Eight Different Broiler Crosses1. Poult. Sci. 1993, 72, 583–588. [Google Scholar] [CrossRef]
- Küçükyilmaz, K.; Bozkurt, M.; Çatli, A.; Herken, E.N.; Çinar, M.; Bintaş, E. Chemical composition, fatty acid profile and colour of broiler meat as affected by organic and conventional rearing systems. S. Afr. J. Anim. Sci. 2012, 42, 361–368. [Google Scholar] [CrossRef]
- Esterbauer, H.; Wäg, G.; Puhl, H. Lipid peroxidation and its role in atherosclerosis. Br. Med. Bull. 1993, 49, 566–576. [Google Scholar] [CrossRef]
- Gutteridge, J.M. Aspects to consider when detecting and measuring lipid peroxidation. Free Radic. Res. Commun. 1986, 1, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zou, X.; Hu, J.; Qiao, Y.; Sun, K. Effects of dietary supplementation of glutamine on meat quality and antioxidant indexes of broilers. Chin. J. Anim. Nutr. 2009, 21, 245–250. [Google Scholar]
- Wu, Q.; Liu, Z.; Li, S.; Jiao, C.; Wang, Y. Effects of glutamine on digestive function and redox regulation in the intestines of broiler chickens challenged with Salmonella Enteritidis. Braz. J. Poult. Sci. 2019, 21. [Google Scholar] [CrossRef]
- Takeshima, N.; Sozu, T.; Tajika, A.; Ogawa, Y.; Hayasaka, Y.; Furukawa, T.A. Which is more generalizable, powerful and interpretable in meta-analyses, mean difference or standardized mean difference? Bmc Med. Res. Methodol. 2014, 14, 30. [Google Scholar] [CrossRef]
- Ryder, A.; Feddes, J.; Zuidhof, M. Field study to relate heat stress index to broiler performance. J. Appl. Poult. Res. 2004, 13, 493–499. [Google Scholar] [CrossRef]
- Alverdy, J.; Aoys, E.; Weiss-Carrington, P.; Burke, D. The effect of glutamine-enriched TPN on gut immune cellularity. J. Surg. Res. 1992, 52, 34–38. [Google Scholar] [CrossRef]
- Wang, J.; Xue, X.; Liu, Q.; Zhang, S.; Peng, M.; Zhou, J.; Chen, L.; Fang, F. Effects of duration of thermal stress on growth performance, serum oxidative stress indices, the expression and localization of ABCG2 and mitochondria ROS production of skeletal muscle, small intestine and immune organs in broilers. J. Therm. Biol. 2019, 85, 102420. [Google Scholar] [CrossRef]
- Piestun, Y.; Halevy, O.; Shinder, D.; Ruzal, M.; Druyan, S.; Yahav, S. Thermal manipulations during broiler embryogenesis improves post-hatch performance under hot conditions. J. Therm. Biol. 2011, 36, 469–474. [Google Scholar] [CrossRef]
- Drubin, D.G.; Kellogg, D.R. English as the universal language of science: Opportunities and challenges. Mol. Biol. Cell 2012, 23, 1399. [Google Scholar] [CrossRef]
- He, J.; Sun, S.; Lin, Z.; Fan, X. The association between body appreciation and body mass index among males and females: A meta-analysis. Body Image 2020, 34, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Grégoire, G.; Derderian, F.; Le Lorier, J. Selecting the language of the publications included in a meta-analysis: Is there a Tower of Babel bias? J. Clin. Epidemiol. 1995, 48, 159–163. [Google Scholar] [CrossRef] [PubMed]

| Study (Authors and Year) | Breed | Sex | Country | HS Category | HS Starting Age (Days) | HS Ending Age (Days) | HS Duration in a Day (Hours) | Peak Temperature |
|---|---|---|---|---|---|---|---|---|
| Ayazi, 2014 [32] | Ross 308 | - | Iran | Chronic | 1 | 42 | 24 | 32 ± 1 °C |
| Bai et al., 2019 [33] | Arbor Acres | 50% each | China | Cyclic | 22 | 35 | 8 | 34 °C |
| Dai et al., 2011 [34] | Arbor Acres | Male | China | Cyclic | 22 | 42 | 9 | 34 °C |
| Hu et al., 2015a [21] | Arbor Acres | Male | China | Cyclic | 22 | 42 | 9 | 34 °C |
| Hu et al., 2015b [22] | Arbor Acres | Male | China | Cyclic | 22 | 35 | 9 | 35 °C |
| Jazideh et al., 2014 [35] | Ross 308 | Male | Iran | Chronic | 1 | 42 | 24 | 32 ± 1 °C |
| Sifa et al., 2018 [16] | Arbor Acres | - | China | Acute | 42 | 42 | 24 | 34 ± 1 °C |
| Wu et al., 2018 [9] | Arbor Acres | 50% each | China | Cyclic | 22 | 41 | 10 | 33 ± 1 °C |
| Wu et al., 2020 [36] | Arbor Acres | - | China | Cyclic | 21 | 42 | 10 | 36 ± 1 °C |
| Dai et al., 2009 [37] | Arbor Acres | Male | China | Chronic | 35 | 42 | 24 | 28 °C |
| Hu et al., 2020a [23] | Arbor Acres | - | China | Cyclic | 22 | 42 | 8 | 34 °C |
| Hu et al., 2020b [38] | Arbor Acres | 50% each | China | Cyclic | 22 | 42 | 8 | 35 °C |
| Hu et al., 2016 [39] | Arbor Acres | Male | China | Acute | 35 | 35 | 12 | 34 ± 1 °C |
| Studies | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Ayazi, 2014 [32] | - | - | ? | ? | ? | - | ? | ? | - | - |
| Bai et al., 2019 [33] | - | - | ? | ? | ? | ? | ? | ? | - | - |
| Dai et al., 2011 [34] | - | - | ? | ? | ? | - | ? | ? | - | - |
| Hu et al., 2015a [21] | - | - | ? | ? | ? | + | ? | ? | - | - |
| Hu et al., 2015b [22] | - | - | ? | ? | ? | ? | ? | ? | - | - |
| Jazideh et al., 2014 [35] | - | - | ? | ? | ? | - | ? | ? | - | - |
| Sifa et al., 2018 [16] | - | - | ? | ? | ? | - | ? | ? | - | - |
| Wu et al., 2018 [9] | - | - | ? | ? | ? | - | ? | ? | - | - |
| Wu et al., 2020 [36] | - | - | ? | ? | ? | - | ? | ? | - | - |
| Dai et al., 2009 [37] | - | - | ? | ? | ? | - | ? | ? | - | - |
| Hu et al., 2020a [23] | - | - | ? | ? | ? | ? | ? | ? | - | - |
| Hu et al., 2020b [38] | - | - | ? | ? | ? | ? | ? | ? | - | - |
| Hu et al., 2016 [39] | - | - | ? | ? | ? | - | ? | ? | - | - |
| BWG | FI | FCR | GLN | GPX | GSH | SOD | CAT | MDA | |
|---|---|---|---|---|---|---|---|---|---|
| Intercept | |||||||||
| Estimate | 1.039 | 1.077 | −0.075 | 0.946 | 1.697 | 1.182 | 1.988 | 0.375 | −0.397 |
| SE | 0.214 | 0.193 | 0.032 | 0.212 | 0.303 | 0.354 | 0.479 | 0.278 | 0.385 |
| p value | <0.001 | <0.001 | 0.021 | <0.001 | <0.001 | 0.001 | <0.001 | 0.177 | 0.302 |
| HS duration | |||||||||
| Estimate | −0.012 | −0.017 | 0.001 | 0.024 | −0.033 | 0.005 | −0.045 | 0.004 | −0.027 |
| SE | 0.007 | 0.006 | 0.001 | 0.015 | 0.014 | 0.022 | 0.019 | 0.017 | 0.018 |
| p value | 0.074 | 0.007 | 0.322 | 0.114 | 0.021 | 0.805 | 0.021 | 0.021 | 0.141 |
| Model | |||||||||
| R2 (%) | 9.48 | 40.4 | 4.47 | 14.5 | 24.71 | 0.09 | 43.09 | 33.83 | 9.88 |
| τ2 | 0.098 | 0.074 | 0.001 | 0.065 | 0.33 | 0.334 | 0.157 | 0.11 | 0.76 |
| I2 (%) | 64.75 | 54.69 | 61.49 | 25.8 | 58.9 | 61.79 | 48.18 | 36.52 | 77.23 |
| BWG | FI | FCR | GLN | GPX | GSH | SOD | CAT | MDA | |
|---|---|---|---|---|---|---|---|---|---|
| Intercept | |||||||||
| Estimate | 0.497 | 0.315 | −0.524 | 1.424 | 0.41 | 0.662 | 0.194 | 0.428 | −0.607 |
| SE | 0.261 | 0.221 | 0.034 | 0.271 | 0.402 | 0.421 | 0.422 | 0.333 | 0.557 |
| p value | 0.056 | 0.153 | 0.127 | <0.001 | 0.308 | 0.115 | 0.645 | 0.198 | 0.275 |
| GLNd | |||||||||
| Estimate | 0.331 | 0.481 | 0.011 | −0.244 | 0.703 | 0.54 | 0.828 | 0.462 | −0.221 |
| SE | 0.384 | 0.296 | 0.049 | 0.269 | 0.348 | 0.352 | 0.414 | 0.282 | 0.478 |
| p value | 0.388 | 0.104 | 0.831 | 0.365 | 0.043 | 0.124 | 0.045 | 0.101 | 0.643 |
| Model | |||||||||
| R2 (%) | 0.05 | 18.33 | 0.07 | 0.57 | 24.72 | 16.08 | 36.88 | 40.96 | 0.08 |
| τ2 | 0.112 | 0.102 | 0.001 | 0.076 | 0.33 | 0.247 | 0.174 | 0.098 | 0.89 |
| I2 (%) | 67.4 | 61.95 | 64.26 | 29.21 | 58.28 | 54.08 | 49.08 | 33.63 | 79.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ncho, C.M.; Gupta, V.; Choi, Y.-H. Effects of Dietary Glutamine Supplementation on Heat-Induced Oxidative Stress in Broiler Chickens: A Systematic Review and Meta-Analysis. Antioxidants 2023, 12, 570. https://doi.org/10.3390/antiox12030570
Ncho CM, Gupta V, Choi Y-H. Effects of Dietary Glutamine Supplementation on Heat-Induced Oxidative Stress in Broiler Chickens: A Systematic Review and Meta-Analysis. Antioxidants. 2023; 12(3):570. https://doi.org/10.3390/antiox12030570
Chicago/Turabian StyleNcho, Chris Major, Vaishali Gupta, and Yang-Ho Choi. 2023. "Effects of Dietary Glutamine Supplementation on Heat-Induced Oxidative Stress in Broiler Chickens: A Systematic Review and Meta-Analysis" Antioxidants 12, no. 3: 570. https://doi.org/10.3390/antiox12030570
APA StyleNcho, C. M., Gupta, V., & Choi, Y.-H. (2023). Effects of Dietary Glutamine Supplementation on Heat-Induced Oxidative Stress in Broiler Chickens: A Systematic Review and Meta-Analysis. Antioxidants, 12(3), 570. https://doi.org/10.3390/antiox12030570







