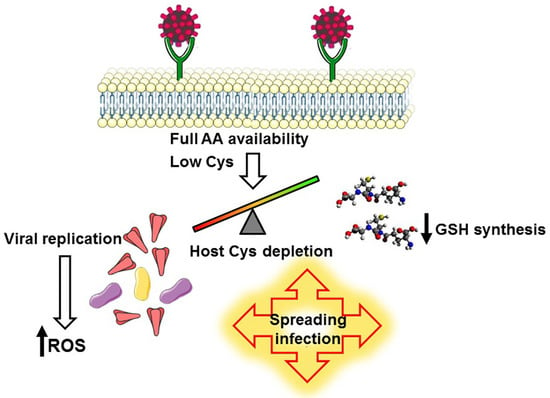
How the Competition for Cysteine May Promote Infection of SARS-CoV-2 by Triggering Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Protein Dataset Construction
2.2. Viral Protein Dataset Construction
2.3. Construction of Protein Datasets with Amino Acid Relative Content
2.4. Evaluation of Protein Similarities by Hierarchical Cluster Protein Analysis
- We used the method with a higher cophenetic correlation coefficient (“average”) to measure the similarities between human proteins and viral variants in terms of cophenetic distance. The heights of dendrograms obtained from the hierarchical clustering analysis were normalized to the maximum height to make comparison among the different analyses possible.
- We chose the four best methods (“average”, “single”, “median”, and “centroid”) with a cophenetic correlation coefficient greater than 0.7, and for each method, we selected a subset of protein clusters such that it belongs to the 5% of proteins closest (depending on the cophenetic distance) to spike from Omicron_BA.1. The resulting four subsets were merged, obtaining a list of proteins, with the proteins most similar to spike as calculated by at least 3 out of the 4 best methods. This approach was used to validate the similarity of proteins of interest.
3. Results
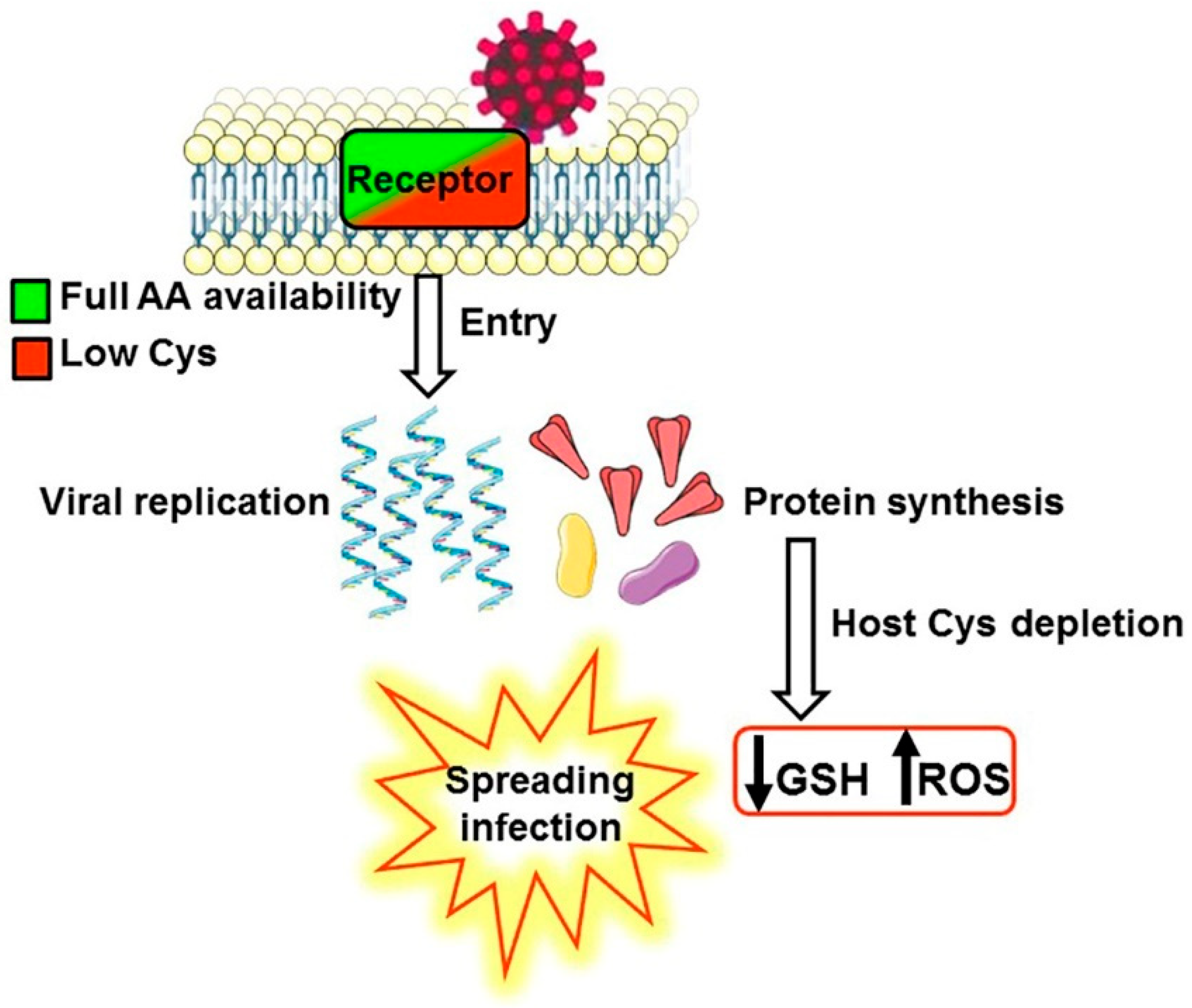
3.1. A Biochemical Model Envisaging the Competition for Cysteine as a Key Mechanism Promoting the Infection and the Selection of Host Receptors
3.2. The Selection of ACE2 as the Main SARS-CoV-2 Receptor Based on Its Amino Acid Composition
3.2.1. Cysteine Content of Viral Proteins and ACE2
3.2.2. Composition of Viral Proteins and ACE2
3.3. The Search for Novel Putative Receptors or Co-Receptors for SARS-CoV-2
- We calculated the relative content of each amino acid except Cys in all human proteins, and we searched the proteins most resembling the spike of the Omicron variant. We decided to exclude Cys content because the potential receptors should be almost identical to spike except for Cys. The similarity was obtained from the hierarchical cluster protein analysis with the “average” method as described in Methods. We found 1648 human proteins with a similarity to spike higher than or equal to the value found for ACE2. Among the listed proteins, 14 potential receptors have caught our attention and their similarity with spike is shown in Figure 2A.
- We searched the scientific literature and identified 18 surface proteins related to viral infection (SARS-CoV or other viruses); we calculated their similarity and discovered that they were very close to spike, as shown in Figure 2B. The total 32 proteins of interest (14 from step 1 and 18 from step 2) are listed in Supplementary Table S1.
- We validated the similarities using four methods. In this procedure, as described in Methods, a subset of proteins shared among the four methods with good cophenetic correlation coefficient was obtained, containing the proteins most similar to the spike of Omicron_BA.1. We discovered that 10 proteins out of 32 were consistently very similar to spike, as indicated in Figure 2A,B by red-outlined bars.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chiara, M.; D’Erchia, A.M.; Gissi, C.; Manzari, C.; Parisi, A.; Resta, N.; Zambelli, F.; Picardi, E.; Pavesi, G.; Horner, D.S.; et al. Next Generation Sequencing of SARS-CoV-2 Genomes: Challenges, Applications and Opportunities. Brief. Bioinform. 2021, 22, 616–630. [Google Scholar] [CrossRef]
- Romano, M.; Ruggiero, A.; Squeglia, F.; Maga, G.; Berisio, R. A Structural View of SARS-CoV-2 RNA Replication Machinery: RNA Synthesis, Proofreading and Final Capping. Cells 2020, 9, 1267. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Wu, L.-A.; Wang, Q.; Qi, J.; Gao, G.F. Cell Entry by SARS-CoV-2. Trends Biochem. Sci. 2021, 46, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Pöhlmann, S. A Multibasic Cleavage Site in the Spike Protein of SARS-CoV-2 Is Essential for Infection of Human Lung Cells. Mol. Cell 2020, 78, 779–784.e5. [Google Scholar] [CrossRef]
- Bestle, D.; Heindl, M.R.; Limburg, H.; Van Lam van, T.; Pilgram, O.; Moulton, H.; Stein, D.A.; Hardes, K.; Eickmann, M.; Dolnik, O.; et al. TMPRSS2 and Furin Are Both Essential for Proteolytic Activation of SARS-CoV-2 in Human Airway Cells. Life Sci. Alliance 2020, 3, e202000786. [Google Scholar] [CrossRef]
- Niehues, R.V.; Wozniak, J.; Wiersch, F.; Lilienthal, E.; Tacken, N.; Schumertl, T.; Garbers, C.; Ludwig, A.; Düsterhöft, S. The Collectrin-like Part of the SARS-CoV-1 and -2 Receptor ACE2 Is Shed by the Metalloproteinases ADAM10 and ADAM17. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2022, 36, e22234. [Google Scholar] [CrossRef]
- Heurich, A.; Hofmann-Winkler, H.; Gierer, S.; Liepold, T.; Jahn, O.; Pöhlmann, S. TMPRSS2 and ADAM17 Cleave ACE2 Differentially and Only Proteolysis by TMPRSS2 Augments Entry Driven by the Severe Acute Respiratory Syndrome Coronavirus Spike Protein. J. Virol. 2014, 88, 1293–1307. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; An, Y. ACE2 Shedding and the Role in COVID-19. Front. Cell. Infect. Microbiol. 2022, 11, 789180. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Liu, Q.; Yao, Q.; Wang, X.; Zhang, H.; Chen, R.; Ren, L.; Min, J.; Deng, F.; et al. SARS-CoV-2 Cell Tropism and Multiorgan Infection. Cell Discov. 2021, 7, 1–4. [Google Scholar] [CrossRef]
- Liu, L.; Chopra, P.; Li, X.; Bouwman, K.M.; Tompkins, S.M.; Wolfert, M.A.; de Vries, R.P.; Boons, G.-J. Heparan Sulfate Proteoglycans as Attachment Factor for SARS-CoV-2. ACS Cent. Sci. 2021, 7, 1009–1018. [Google Scholar] [CrossRef]
- Eslami, N.; Aghbash, P.S.; Shamekh, A.; Entezari-Maleki, T.; Nahand, J.S.; Sales, A.J.; Baghi, H.B. SARS-CoV-2: Receptor and Co-Receptor Tropism Probability. Curr. Microbiol. 2022, 79, 133. [Google Scholar] [CrossRef]
- Alipoor, S.D.; Mirsaeidi, M. SARS-CoV-2 Cell Entry beyond the ACE2 Receptor. Mol. Biol. Rep. 2022, 49, 10715–10727. [Google Scholar] [CrossRef]
- Chu, H.; Chan, C.-M.; Zhang, X.; Wang, Y.; Yuan, S.; Zhou, J.; Au-Yeung, R.K.-H.; Sze, K.-H.; Yang, D.; Shuai, H.; et al. Middle East Respiratory Syndrome Coronavirus and Bat Coronavirus HKU9 Both Can Utilize GRP78 for Attachment onto Host Cells. J. Biol. Chem. 2018, 293, 11709–11726. [Google Scholar] [CrossRef]
- Posadas-Sánchez, R.; Sánchez-Muñoz, F.; Guzmán-Martín, C.A.; Hernández-Díaz Couder, A.; Rojas-Velasco, G.; Fragoso, J.M.; Vargas-Alarcón, G. Dipeptidylpeptidase-4 Levels and DPP4 Gene Polymorphisms in Patients with COVID-19. Association with Disease and with Severity. Life Sci. 2021, 276, 119410. [Google Scholar] [CrossRef]
- Rodrigues-Diez, R.R.; Tejera-Muñoz, A.; Marquez-Exposito, L.; Rayego-Mateos, S.; Santos Sanchez, L.; Marchant, V.; Tejedor Santamaria, L.; Ramos, A.M.; Ortiz, A.; Egido, J.; et al. Statins: Could an Old Friend Help in the Fight against COVID-19? Br. J. Pharmacol. 2020, 177, 4873–4886. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M.; et al. Neuropilin-1 Facilitates SARS-CoV-2 Cell Entry and Infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef]
- Amraei, R.; Yin, W.; Napoleon, M.A.; Suder, E.L.; Berrigan, J.; Zhao, Q.; Olejnik, J.; Chandler, K.B.; Xia, C.; Feldman, J.; et al. CD209L/L-SIGN and CD209/DC-SIGN Act as Receptors for SARS-CoV-2. ACS Cent. Sci. 2021, 7, 1156–1165. [Google Scholar] [CrossRef]
- Qi, F.; Qian, S.; Zhang, S.; Zhang, Z. Single Cell RNA Sequencing of 13 Human Tissues Identify Cell Types and Receptors of Human Coronaviruses. Biochem. Biophys. Res. Commun. 2020, 526, 135–140. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, Q.; Fox, D.M.; Gao, C.; Stanley, S.A.; Luo, K. SARS-CoV-2 Downregulates ACE2 through Lysosomal Degradation. Mol. Biol. Cell 2022, 33, ar147. [Google Scholar] [CrossRef]
- Glowacka, I.; Bertram, S.; Müller, M.A.; Allen, P.; Soilleux, E.; Pfefferle, S.; Steffen, I.; Tsegaye, T.S.; He, Y.; Gnirss, K.; et al. Evidence That TMPRSS2 Activates the Severe Acute Respiratory Syndrome Coronavirus Spike Protein for Membrane Fusion and Reduces Viral Control by the Humoral Immune Response. J. Virol. 2011, 85, 4122–4134. [Google Scholar] [CrossRef] [PubMed]
- Silvagno, F.; Vernone, A.; Pescarmona, G.P. The Role of Glutathione in Protecting against the Severe Inflammatory Response Triggered by COVID-19. Antioxid. Basel Switz. 2020, 9, E624. [Google Scholar] [CrossRef] [PubMed]
- Polonikov, A. Endogenous Deficiency of Glutathione as the Most Likely Cause of Serious Manifestations and Death in COVID-19 Patients. ACS Infect. Dis. 2020, 6, 1558–1562. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-L.; Wu, C.-H.; Chien, K.-Y.; Lai, C.-H.; Li, G.-J.; Liu, Y.-Y.; Lin, G.; Ho, H.-Y. Enteroviral 2B Interacts with VDAC3 to Regulate Reactive Oxygen Species Generation That Is Essential to Viral Replication. Viruses 2022, 14, 1717. [Google Scholar] [CrossRef]
- Kumar, P.; Osahon, O.; Vides, D.B.; Hanania, N.; Minard, C.G.; Sekhar, R.V. Severe Glutathione Deficiency, Oxidative Stress and Oxidant Damage in Adults Hospitalized with COVID-19: Implications for GlyNAC (Glycine and N-Acetylcysteine) Supplementation. Antioxidants 2021, 11, 50. [Google Scholar] [CrossRef]
- Fernandes, I.G.; de Brito, C.A.; Dos Reis, V.M.S.; Sato, M.N.; Pereira, N.Z. SARS-CoV-2 and Other Respiratory Viruses: What Does Oxidative Stress Have to Do with It? Oxid. Med. Cell. Longev. 2020, 2020, 8844280. [Google Scholar] [CrossRef]
- Ciriolo, M.R.; Palamara, A.T.; Incerpi, S.; Lafavia, E.; Buè, M.C.; De Vito, P.; Garaci, E.; Rotilio, G. Loss of GSH, Oxidative Stress, and Decrease of Intracellular PH as Sequential Steps in Viral Infection. J. Biol. Chem. 1997, 272, 2700–2708. [Google Scholar] [CrossRef]
- Suhail, S.; Zajac, J.; Fossum, C.; Lowater, H.; McCracken, C.; Severson, N.; Laatsch, B.; Narkiewicz-Jodko, A.; Johnson, B.; Liebau, J.; et al. Role of Oxidative Stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) Infection: A Review. Protein J. 2020, 39, 644–656. [Google Scholar] [CrossRef]
- Nencioni, L.; Iuvara, A.; Aquilano, K.; Ciriolo, M.R.; Cozzolino, F.; Rotilio, G.; Garaci, E.; Palamara, A.T. Influenza A Virus Replication Is Dependent on an Antioxidant Pathway That Involves GSH and Bcl-2. FASEB J. 2003, 17, 758–760. [Google Scholar] [CrossRef]
- Garaci, E.; Palamara, A.T.; Di Francesco, P.; Favalli, C.; Ciriolo, M.R.; Rotilio, G. Glutathione Inhibits Replication and Expression of Viral Proteins in Cultured Cells Infected with Sendai Virus. Biochem. Biophys. Res. Commun. 1992, 188, 1090–1096. [Google Scholar] [CrossRef]
- Nucci, C.; Palamara, A.T.; Ciriolo, M.R.; Nencioni, L.; Savini, P.; D’Agostini, C.; Rotilio, G.; Cerulli, L.; Garaci, E. Imbalance in Corneal Redox State during Herpes Simplex Virus 1-Induced Keratitis in Rabbits. Effectiveness of Exogenous Glutathione Supply. Exp. Eye Res. 2000, 70, 215–220. [Google Scholar] [CrossRef]
- De Rosa, S.C.; Zaretsky, M.D.; Dubs, J.G.; Roederer, M.; Anderson, M.; Green, A.; Mitra, D.; Watanabe, N.; Nakamura, H.; Tjioe, I.; et al. N-Acetylcysteine Replenishes Glutathione in HIV Infection. Eur. J. Clin. Investig. 2000, 30, 915–929. [Google Scholar] [CrossRef]
- Schwarz, K.B. Oxidative Stress during Viral Infection: A Review. Free Radic. Biol. Med. 1996, 21, 641–649. [Google Scholar] [CrossRef]
- Thyrsted, J.; Holm, C.K. Virus-Induced Metabolic Reprogramming and Innate Sensing Hereof by the Infected Host. Curr. Opin. Biotechnol. 2021, 68, 44–50. [Google Scholar] [CrossRef]
- Rahbar Saadat, Y.; Hosseiniyan Khatibi, S.M.; Zununi Vahed, S.; Ardalan, M. Host Serine Proteases: A Potential Targeted Therapy for COVID-19 and Influenza. Front. Mol. Biosci. 2021, 8, 725528. [Google Scholar] [CrossRef]
- Meyer, M.; Jaspers, I. Respiratory Protease/Antiprotease Balance Determines Susceptibility to Viral Infection and Can Be Modified by Nutritional Antioxidants. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L1189–L1201. [Google Scholar] [CrossRef]
- Da Silva, M.C.; dos Santos, V.M.; da Silva, M.V.B.; Prazeres, T.C.M.M.; Maria do Socorro, S.C.; Calzerra, N.T.M.; de Queiroz, T.M. Involvement of Shedding Induced by ADAM17 on the Nitric Oxide Pathway in Hypertension. Front. Mol. Biosci. 2022, 9, 1032177. [Google Scholar] [CrossRef]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.-C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef]
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of Angiotensin-Converting Enzyme 2 (ACE2) in COVID-19. Crit. Care Lond. Engl. 2020, 24, 422. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hasan, M.; Ahmed, A. Potential Detrimental Role of Soluble ACE2 in Severe COVID-19 Comorbid Patients. Rev. Med. Virol. 2021, 31, e2213. [Google Scholar] [CrossRef]
- Esumi, M.; Ishibashi, M.; Yamaguchi, H.; Nakajima, S.; Tai, Y.; Kikuta, S.; Sugitani, M.; Takayama, T.; Tahara, M.; Takeda, M.; et al. Transmembrane Serine Protease TMPRSS2 Activates Hepatitis C Virus Infection. Hepatology 2015, 61, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.W.; Mao, H.J.; Wu, Y.L.; Tanaka, Y.; Zhang, W. TMPRSS2: A Potential Target for Treatment of Influenza Virus and Coronavirus Infections. Biochimie 2017, 142, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Luo, M. Influenza Virus Entry. Adv. Exp. Med. Biol. 2012, 726, 201–221. [Google Scholar] [CrossRef] [PubMed]
- Bertram, S.; Heurich, A.; Lavender, H.; Gierer, S.; Danisch, S.; Perin, P.; Lucas, J.M.; Nelson, P.S.; Pöhlmann, S.; Soilleux, E.J. Influenza and SARS-Coronavirus Activating Proteases TMPRSS2 and HAT Are Expressed at Multiple Sites in Human Respiratory and Gastrointestinal Tracts. PLoS ONE 2012, 7, e35876. [Google Scholar] [CrossRef]
- Paszti-Gere, E.; Barna, R.F.; Kovago, C.; Szauder, I.; Ujhelyi, G.; Jakab, C.; Meggyesházi, N.; Szekacs, A. Changes in the Distribution of Type II Transmembrane Serine Protease, TMPRSS2 and in Paracellular Permeability in IPEC-J2 Cells Exposed to Oxidative Stress. Inflammation 2015, 38, 775–783. [Google Scholar] [CrossRef]
- Cao, W.; Feng, Q.; Wang, X. Computational Analysis of TMPRSS2 Expression in Normal and SARS-CoV-2-Infected Human Tissues. Chem. Biol. Interact. 2021, 346, 109583. [Google Scholar] [CrossRef]
- Shen, L.-W.; Qian, M.-Q.; Yu, K.; Narva, S.; Yu, F.; Wu, Y.-L.; Zhang, W. Inhibition of Influenza A Virus Propagation by Benzoselenoxanthenes Stabilizing TMPRSS2 Gene G-Quadruplex and Hence down-Regulating TMPRSS2 Expression. Sci. Rep. 2020, 10, 7635. [Google Scholar] [CrossRef]
- Lavillette, D.; Barbouche, R.; Yao, Y.; Boson, B.; Cosset, F.-L.; Jones, I.M.; Fenouillet, E. Significant Redox Insensitivity of the Functions of the SARS-CoV Spike Glycoprotein: Comparison with HIV Envelope. J. Biol. Chem. 2006, 281, 9200–9204. [Google Scholar] [CrossRef]
- Hati, S.; Bhattacharyya, S. Impact of Thiol-Disulfide Balance on the Binding of Covid-19 Spike Protein with Angiotensin-Converting Enzyme 2 Receptor. ACS Omega 2020, 5, 16292–16298. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 Spike Receptor-Binding Domain Bound to the ACE2 Receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef]
- Sgarbanti, R.; Nencioni, L.; Amatore, D.; Coluccio, P.; Fraternale, A.; Sale, P.; Mammola, C.L.; Carpino, G.; Gaudio, E.; Magnani, M.; et al. Redox Regulation of the Influenza Hemagglutinin Maturation Process: A New Cell-Mediated Strategy for Anti-Influenza Therapy. Antioxid. Redox Signal. 2011, 15, 593–606. [Google Scholar] [CrossRef]
- Amatore, D.; Sgarbanti, R.; Aquilano, K.; Baldelli, S.; Limongi, D.; Civitelli, L.; Nencioni, L.; Garaci, E.; Ciriolo, M.R.; Palamara, A.T. Influenza Virus Replication in Lung Epithelial Cells Depends on Redox-Sensitive Pathways Activated by NOX4-Derived ROS. Cell. Microbiol. 2015, 17, 131–145. [Google Scholar] [CrossRef]
- To, E.E.; Broughton, B.R.S.; Hendricks, K.S.; Vlahos, R.; Selemidis, S. Influenza A Virus and TLR7 Activation Potentiate NOX2 Oxidase-Dependent ROS Production in Macrophages. Free Radic. Res. 2014, 48, 940–947. [Google Scholar] [CrossRef]
- Grishin, A.M.; Dolgova, N.V.; Landreth, S.; Fisette, O.; Pickering, I.J.; George, G.N.; Falzarano, D.; Cygler, M. Disulfide Bonds Play a Critical Role in the Structure and Function of the Receptor-Binding Domain of the SARS-CoV-2 Spike Antigen. J. Mol. Biol. 2022, 434, 167357. [Google Scholar] [CrossRef]
- Manček-Keber, M.; Hafner-Bratkovič, I.; Lainšček, D.; Benčina, M.; Govednik, T.; Orehek, S.; Plaper, T.; Jazbec, V.; Bergant, V.; Grass, V.; et al. Disruption of Disulfides within RBD of SARS-CoV-2 Spike Protein Prevents Fusion and Represents a Target for Viral Entry Inhibition by Registered Drugs. FASEB J. 2021, 35, e21651. [Google Scholar] [CrossRef]
- Murae, M.; Shimizu, Y.; Yamamoto, Y.; Kobayashi, A.; Houri, M.; Inoue, T.; Irie, T.; Gemba, R.; Kondo, Y.; Nakano, Y.; et al. The Function of SARS-CoV-2 Spike Protein Is Impaired by Disulfide-Bond Disruption with Mutation at Cysteine-488 and by Thiol-Reactive N-Acetyl-Cysteine and Glutathione. Biochem. Biophys. Res. Commun. 2022, 597, 30–36. [Google Scholar] [CrossRef]
- Lin, X.; Wang, R.; Zou, W.; Sun, X.; Liu, X.; Zhao, L.; Wang, S.; Jin, M. The Influenza Virus H5N1 Infection Can Induce ROS Production for Viral Replication and Host Cell Death in A549 Cells Modulated by Human Cu/Zn Superoxide Dismutase (SOD1) Overexpression. Viruses 2016, 8, 13. [Google Scholar] [CrossRef]
- Staal, F.J.; Roederer, M.; Herzenberg, L.A.; Herzenberg, L.A. Intracellular Thiols Regulate Activation of Nuclear Factor Kappa B and Transcription of Human Immunodeficiency Virus. Proc. Natl. Acad. Sci. USA 1990, 87, 9943–9947. [Google Scholar] [CrossRef]
- Wang, W.; Ye, L.; Ye, L.; Li, B.; Gao, B.; Zeng, Y.; Kong, L.; Fang, X.; Zheng, H.; Wu, Z.; et al. Up-Regulation of IL-6 and TNF-Alpha Induced by SARS-Coronavirus Spike Protein in Murine Macrophages via NF-KappaB Pathway. Virus Res. 2007, 128, 1–8. [Google Scholar] [CrossRef]
- Liao, Y.; Li, X.; Mou, T.; Zhou, X.; Li, D.; Wang, L.; Zhang, Y.; Dong, X.; Zheng, H.; Guo, L.; et al. Distinct Infection Process of SARS-CoV-2 in Human Bronchial Epithelial Cell Lines. J. Med. Virol. 2020, 92, 2830–2838. [Google Scholar] [CrossRef]
- Müllner, D. Fastcluster: Fast Hierarchical, Agglomerative Clustering Routines for R and Python. J. Stat. Softw. 2013, 53, 1–18. [Google Scholar] [CrossRef]
- Saraçli, S.; Doğan, N.; Doğan, İ. Comparison of Hierarchical Cluster Analysis Methods by Cophenetic Correlation. J. Inequalities Appl. 2013, 2013, 203. [Google Scholar] [CrossRef]
- Sharova, L.V.; Sharov, A.A.; Nedorezov, T.; Piao, Y.; Shaik, N.; Ko, M.S.H. Database for MRNA Half-Life of 19 977 Genes Obtained by DNA Microarray Analysis of Pluripotent and Differentiating Mouse Embryonic Stem Cells. DNA Res. 2009, 16, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Forrest, M.E.; Pinkard, O.; Martin, S.; Sweet, T.J.; Hanson, G.; Coller, J. Codon and Amino Acid Content Are Associated with MRNA Stability in Mammalian Cells. PLoS ONE 2020, 15, e0228730. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.J.; Gao, J.; Yamaguchi, N.; Pinzaru, A.; Wu, Q.; Mandayam, N.; Liberti, M.; Heissel, S.; Alwaseem, H.; Tavazoie, S.; et al. Arginine Limitation Drives a Directed Codon-Dependent DNA Sequence Evolution Response in Colorectal Cancer Cells. Sci. Adv. 2023, 9, eade9120. [Google Scholar] [CrossRef]
- Finkel, Y.; Gluck, A.; Nachshon, A.; Winkler, R.; Fisher, T.; Rozman, B.; Mizrahi, O.; Lubelsky, Y.; Zuckerman, B.; Slobodin, B.; et al. SARS-CoV-2 Uses a Multipronged Strategy to Impede Host Protein Synthesis. Nature 2021, 594, 240–245. [Google Scholar] [CrossRef]
- Vernone, A.; Ricca, C.; Merlo, D.; Pescarmona, G.; Silvagno, F. The Analysis of Glutamate and Glutamine Frequencies in Human Proteins as Marker of Tissue Oxygenation. R. Soc. Open Sci. 2019, 6, 181891. [Google Scholar] [CrossRef]
- Vernone, A.; Ricca, C.; Pescarmona, G.; Silvagno, F. Chromosome Walking: A Novel Approach to Analyse Amino Acid Content of Human Proteins Ordered by Gene Position. Appl. Sci. Switz. 2021, 11, 3511. [Google Scholar] [CrossRef]
- Violi, F.; Oliva, A.; Cangemi, R.; Ceccarelli, G.; Pignatelli, P.; Carnevale, R.; Cammisotto, V.; Lichtner, M.; Alessandri, F.; De Angelis, M.; et al. Nox2 Activation in Covid-19. Redox Biol. 2020, 36, 101655. [Google Scholar] [CrossRef]
- Al-kuraishy, H.M.; Al-Gareeb, A.I.; Al-Niemi, M.S.; Aljowaie, R.M.; Almutairi, S.M.; Alexiou, A.; Batiha, G.E.-S. The Prospective Effect of Allopurinol on the Oxidative Stress Index and Endothelial Dysfunction in Covid-19. Inflammation 2022, 45, 1651–1667. [Google Scholar] [CrossRef]
- Shang, C.; Liu, Z.; Zhu, Y.; Lu, J.; Ge, C.; Zhang, C.; Li, N.; Jin, N.; Li, Y.; Tian, M.; et al. SARS-CoV-2 Causes Mitochondrial Dysfunction and Mitophagy Impairment. Front. Microbiol. 2021, 12, 780768. [Google Scholar] [CrossRef]
- Li, Q.; Wang, L.; Dong, C.; Che, Y.; Jiang, L.; Liu, L.; Zhao, H.; Liao, Y.; Sheng, Y.; Dong, S.; et al. The Interaction of the SARS Coronavirus Non-Structural Protein 10 with the Cellular Oxido-Reductase System Causes an Extensive Cytopathic Effect. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2005, 34, 133–139. [Google Scholar] [CrossRef]
- Sanders, D.W.; Jumper, C.C.; Ackerman, P.J.; Bracha, D.; Donlic, A.; Kim, H.; Kenney, D.; Castello-Serrano, I.; Suzuki, S.; Tamura, T.; et al. SARS-CoV-2 Requires Cholesterol for Viral Entry and Pathological Syncytia Formation. eLife 2021, 10, e65962. [Google Scholar] [CrossRef]
- Flower, T.G.; Buffalo, C.Z.; Hooy, R.M.; Allaire, M.; Ren, X.; Hurley, J.H. Structure of SARS-CoV-2 ORF8, a Rapidly Evolving Immune Evasion Protein. Proc. Natl. Acad. Sci. USA 2021, 118, e2021785118. [Google Scholar] [CrossRef]
- Kneller, D.W.; Phillips, G.; Weiss, K.L.; Pant, S.; Zhang, Q.; O’Neill, H.M.; Coates, L.; Kovalevsky, A. Unusual Zwitterionic Catalytic Site of SARS–CoV-2 Main Protease Revealed by Neutron Crystallography. J. Biol. Chem. 2020, 295, 17365–17373. [Google Scholar] [CrossRef]
- Ravanfar, R.; Sheng, Y.; Shahgholi, M.; Lomenick, B.; Jones, J.; Chou, T.-F.; Gray, H.B.; Winkler, J.R. Surface Cysteines Could Protect the SARS-CoV-2 Main Protease from Oxidative Damage. J. Inorg. Biochem. 2022, 234, 111886. [Google Scholar] [CrossRef]
- Domovitz, T.; Ayoub, S.; Werbner, M.; Alter, J.; Izhaki Tavor, L.; Yahalom-Ronen, Y.; Tikhonov, E.; Meirson, T.; Maman, Y.; Paran, N.; et al. HCV Infection Increases the Expression of ACE2 Receptor, Leading to Enhanced Entry of Both HCV and SARS-CoV-2 into Hepatocytes and a Coinfection State. Microbiol. Spectr. 2022, 10, e0115022. [Google Scholar] [CrossRef]
- Schweitzer, K.S.; Crue, T.; Nall, J.M.; Foster, D.; Sajuthi, S.; Correll, K.A.; Nakamura, M.; Everman, J.L.; Downey, G.P.; Seibold, M.A.; et al. Influenza Virus Infection Increases ACE2 Expression and Shedding in Human Small Airway Epithelial Cells. Eur. Respir. J. 2021, 58, 2003988. [Google Scholar] [CrossRef]
- Torices, S.; Cabrera, R.; Stangis, M.; Naranjo, O.; Fattakhov, N.; Teglas, T.; Adesse, D.; Toborek, M. Expression of SARS-CoV-2-Related Receptors in Cells of the Neurovascular Unit: Implications for HIV-1 Infection. J. Neuroinflamm. 2021, 18, 167. [Google Scholar] [CrossRef]
- Vernone, A.; Berchialla, P.; Pescarmona, G. Human Protein Cluster Analysis Using Amino Acid Frequencies. PLoS ONE 2013, 8, e60220. [Google Scholar] [CrossRef]
- Collins, D.P.; Steer, C.J. Binding of the SARS-CoV-2 Spike Protein to the Asialoglycoprotein Receptor on Human Primary Hepatocytes and Immortalized Hepatocyte-Like Cells by Confocal Analysis. Hepatic Med. Evid. Res. 2021, 13, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, J.A.; Tremblay, B.J.-M.; Mansfield, M.J.; Woody, O.; Lobb, B.; Banerjee, A.; Chandiramohan, A.; Tiessen, N.; Cao, Q.; Dvorkin-Gheva, A.; et al. Gene Expression and in Situ Protein Profiling of Candidate SARS-CoV-2 Receptors in Human Airway Epithelial Cells and Lung Tissue. Eur. Respir. J. 2020, 56, 2001123. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.M.; Abdelmalek, D.H.; Elshahat, M.E.; Elfiky, A.A. COVID-19 Spike-Host Cell Receptor GRP78 Binding Site Prediction. J. Infect. 2020, 80, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wei, C.; He, J.; Zhang, L.; Zhou, J.; Balaji, K.S.; Shen, S.; Peng, J.; Sharma, A.; Fu, J. Evaluation and Characterization of HSPA5 (GRP78) Expression Profiles in Normal Individuals and Cancer Patients with COVID-19. Int. J. Biol. Sci. 2021, 17, 897–910. [Google Scholar] [CrossRef]
- Shin, W.-J.; Ha, D.P.; Machida, K.; Lee, A.S. The Stress-Inducible ER Chaperone GRP78/BiP Is Upregulated during SARS-CoV-2 Infection and Acts as a pro-Viral Protein. Nat. Commun. 2022, 13, 6551. [Google Scholar] [CrossRef]
- Shaban, M.S.; Müller, C.; Mayr-Buro, C.; Weiser, H.; Meier-Soelch, J.; Albert, B.V.; Weber, A.; Linne, U.; Hain, T.; Babayev, I.; et al. Multi-Level Inhibition of Coronavirus Replication by Chemical ER Stress. Nat. Commun. 2021, 12, 5536. [Google Scholar] [CrossRef]
- Jocher, G.; Grass, V.; Tschirner, S.K.; Riepler, L.; Breimann, S.; Kaya, T.; Oelsner, M.; Hamad, M.S.; Hofmann, L.I.; Blobel, C.P.; et al. ADAM10 and ADAM17 Promote SARS-CoV-2 Cell Entry and Spike Protein-Mediated Lung Cell Fusion. EMBO Rep. 2022, 23, e54305. [Google Scholar] [CrossRef]
- Carapito, R.; Li, R.; Helms, J.; Carapito, C.; Gujja, S.; Rolli, V.; Guimaraes, R.; Malagon-Lopez, J.; Spinnhirny, P.; Lederle, A.; et al. Identification of Driver Genes for Critical Forms of COVID-19 in a Deeply Phenotyped Young Patient Cohort. Sci. Transl. Med. 2022, 14, eabj7521. [Google Scholar] [CrossRef]
- Rosen, H.R.; O’Connell, C.; Nadim, M.K.; DeClerck, B.; Sheibani, S.; DePasquale, E.; Sanossian, N.; Blodget, E.; Angell, T. Extrapulmonary Manifestations of Severe Acute Respiratory Syndrome Coronavirus-2 Infection. J. Med. Virol. 2021, 93, 2645–2653. [Google Scholar] [CrossRef]
- Wang, W.; McKinnie, S.M.K.; Farhan, M.; Paul, M.; McDonald, T.; McLean, B.; Llorens-Cortes, C.; Hazra, S.; Murray, A.G.; Vederas, J.C.; et al. Angiotensin-Converting Enzyme 2 Metabolizes and Partially Inactivates Pyr-Apelin-13 and Apelin-17: Physiological Effects in the Cardiovascular System. Hypertension 2016, 68, 365–377. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Blume, C.; Jackson, C.L.; Spalluto, C.M.; Legebeke, J.; Nazlamova, L.; Conforti, F.; Perotin, J.-M.; Frank, M.; Butler, J.; Crispin, M.; et al. A Novel ACE2 Isoform Is Expressed in Human Respiratory Epithelia and Is Upregulated in Response to Interferons and RNA Respiratory Virus Infection. Nat. Genet. 2021, 53, 205–214. [Google Scholar] [CrossRef]
- Mustapha, M.; Weil, D.; Chardenoux, S.; Elias, S.; El-Zir, E.; Beckmann, J.S.; Loiselet, J.; Petit, C. An Alpha-Tectorin Gene Defect Causes a Newly Identified Autosomal Recessive Form of Sensorineural Pre-Lingual Non-Syndromic Deafness, DFNB21. Hum. Mol. Genet. 1999, 8, 409–412. [Google Scholar] [CrossRef]
- Ghaffari, R.; Aranyosi, A.J.; Richardson, G.P.; Freeman, D.M. Tectorial Membrane Travelling Waves Underlie Abnormal Hearing in Tectb Mutant Mice. Nat. Commun. 2010, 1, 96. [Google Scholar] [CrossRef]
- Zhou, D.; Zhao, Y.; Kotecha, A.; Fry, E.E.; Kelly, J.T.; Wang, X.; Rao, Z.; Rowlands, D.J.; Ren, J.; Stuart, D.I. Unexpected Mode of Engagement between Enterovirus 71 and Its Receptor SCARB2. Nat. Microbiol. 2019, 4, 414–419. [Google Scholar] [CrossRef]
- Wells, A.I.; Coyne, C.B. Enteroviruses: A Gut-Wrenching Game of Entry, Detection, and Evasion. Viruses 2019, 11, 460. [Google Scholar] [CrossRef]
- Xing, J.; Wang, K.; Wang, G.; Li, N.; Zhang, Y. Recent Advances in Enterovirus A71 Pathogenesis: A Focus on Fatal Human Enterovirus A71 Infection. Arch. Virol. 2022. [Google Scholar] [CrossRef]
- Bochkov, Y.A.; Watters, K.; Ashraf, S.; Griggs, T.F.; Devries, M.K.; Jackson, D.J.; Palmenberg, A.C.; Gern, J.E. Cadherin-Related Family Member 3, a Childhood Asthma Susceptibility Gene Product, Mediates Rhinovirus C Binding and Replication. Proc. Natl. Acad. Sci. USA 2015, 112, 5485–5490. [Google Scholar] [CrossRef]
- Bønnelykke, K.; Sleiman, P.; Nielsen, K.; Kreiner-Møller, E.; Mercader, J.M.; Belgrave, D.; den Dekker, H.T.; Husby, A.; Sevelsted, A.; Faura-Tellez, G.; et al. A Genome-Wide Association Study Identifies CDHR3 as a Susceptibility Locus for Early Childhood Asthma with Severe Exacerbations. Nat. Genet. 2014, 46, 51–55. [Google Scholar] [CrossRef]
- Everman, J.L.; Sajuthi, S.; Saef, B.; Rios, C.; Stoner, A.M.; Numata, M.; Hu, D.; Eng, C.; Oh, S.; Rodriguez-Santana, J.; et al. Functional Genomics of CDHR3 Confirms Its Role in HRV-C Infection and Childhood Asthma Exacerbations. J. Allergy Clin. Immunol. 2019, 144, 962–971. [Google Scholar] [CrossRef]
- Mansoor, S.E.; Lü, W.; Oosterheert, W.; Shekhar, M.; Tajkhorshid, E.; Gouaux, E. X-Ray Structures Define Human P2X(3) Receptor Gating Cycle and Antagonist Action. Nature 2016, 538, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Finger, T.; Kinnamon, S. Purinergic Neurotransmission in the Gustatory System. Auton. Neurosci. Basic Clin. 2021, 236, 102874. [Google Scholar] [CrossRef] [PubMed]
- Roelvink, P.W.; Lizonova, A.; Lee, J.G.; Li, Y.; Bergelson, J.M.; Finberg, R.W.; Brough, D.E.; Kovesdi, I.; Wickham, T.J. The Coxsackievirus-Adenovirus Receptor Protein Can Function as a Cellular Attachment Protein for Adenovirus Serotypes from Subgroups A, C, D, E, and F. J. Virol. 1998, 72, 7909–7915. [Google Scholar] [CrossRef] [PubMed]
- Martino, T.A.; Petric, M.; Weingartl, H.; Bergelson, J.M.; Opavsky, M.A.; Richardson, C.D.; Modlin, J.F.; Finberg, R.W.; Kain, K.C.; Willis, N.; et al. The Coxsackie-Adenovirus Receptor (CAR) Is Used by Reference Strains and Clinical Isolates Representing All Six Serotypes of Coxsackievirus Group B and by Swine Vesicular Disease Virus. Virology 2000, 271, 99–108. [Google Scholar] [CrossRef]
- Noutsias, M.; Fechner, H.; de Jonge, H.; Wang, X.; Dekkers, D.; Houtsmuller, A.B.; Pauschinger, M.; Bergelson, J.; Warraich, R.; Yacoub, M.; et al. Human Coxsackie-Adenovirus Receptor Is Colocalized with Integrins Alpha(v)Beta(3) and Alpha(v)Beta(5) on the Cardiomyocyte Sarcolemma and Upregulated in Dilated Cardiomyopathy: Implications for Cardiotropic Viral Infections. Circulation 2001, 104, 275–280. [Google Scholar] [CrossRef]
- Clark, L.E.; Clark, S.A.; Lin, C.; Liu, J.; Coscia, A.; Nabel, K.G.; Yang, P.; Neel, D.V.; Lee, H.; Brusic, V.; et al. VLDLR and ApoER2 Are Receptors for Multiple Alphaviruses. Nature 2022, 602, 475–480. [Google Scholar] [CrossRef]
- Clatworthy, A.E.; Stockinger, W.; Christie, R.H.; Schneider, W.J.; Nimpf, J.; Hyman, B.T.; Rebeck, G.W. Expression and Alternate Splicing of Apolipoprotein E Receptor 2 in Brain. Neuroscience 1999, 90, 903–911. [Google Scholar] [CrossRef]
- Riddell, D.R.; Vinogradov, D.V.; Stannard, A.K.; Chadwick, N.; Owen, J.S. Identification and Characterization of LRP8 (ApoER2) in Human Blood Platelets. J. Lipid Res. 1999, 40, 1925–1930. [Google Scholar] [CrossRef]
- Ganaie, S.S.; Schwarz, M.M.; McMillen, C.M.; Price, D.A.; Feng, A.X.; Albe, J.R.; Wang, W.; Miersch, S.; Orvedahl, A.; Cole, A.R.; et al. Lrp1 Is a Host Entry Factor for Rift Valley Fever Virus. Cell 2021, 184, 5163–5178.e24. [Google Scholar] [CrossRef]
- Pieper-Fürst, U.; Lammert, F. Low-Density Lipoprotein Receptors in Liver: Old Acquaintances and a Newcomer. Biochim. Biophys. Acta BBA—Mol. Cell Biol. Lipids 2013, 1831, 1191–1198. [Google Scholar] [CrossRef]
- García-Fernández, P.; Üçeyler, N.; Sommer, C. From the Low-Density Lipoprotein Receptor-Related Protein 1 to Neuropathic Pain: A Potentially Novel Target. Pain Rep. 2021, 6, e898. [Google Scholar] [CrossRef]
- Wujak, L.; Markart, P.; Wygrecka, M. The Low Density Lipoprotein Receptor-Related Protein (LRP) 1 and Its Function in Lung Diseases. Histol. Histopathol. 2016, 31, 733–745. [Google Scholar] [CrossRef]
- Neumann, E.; Moser, R.; Snyers, L.; Blaas, D.; Hewat, E.A. A Cellular Receptor of Human Rhinovirus Type 2, the Very-Low-Density Lipoprotein Receptor, Binds to Two Neighboring Proteins of the Viral Capsid. J. Virol. 2003, 77, 8504–8511. [Google Scholar] [CrossRef]
- Ujino, S.; Nishitsuji, H.; Hishiki, T.; Sugiyama, K.; Takaku, H.; Shimotohno, K. Hepatitis C Virus Utilizes VLDLR as a Novel Entry Pathway. Proc. Natl. Acad. Sci. USA 2016, 113, 188–193. [Google Scholar] [CrossRef]
- Chung, E.Y.M.; Wang, Y.M.; Keung, K.; Hu, M.; McCarthy, H.; Wong, G.; Kairaitis, L.; Bose, B.; Harris, D.C.H.; Alexander, S.I. Membranous Nephropathy: Clearer Pathology and Mechanisms Identify Potential Strategies for Treatment. Front. Immunol. 2022, 13, 1036249. [Google Scholar] [CrossRef]
- Zang, R.; Gomez Castro, M.F.; McCune, B.T.; Zeng, Q.; Rothlauf, P.W.; Sonnek, N.M.; Liu, Z.; Brulois, K.F.; Wang, X.; Greenberg, H.B.; et al. TMPRSS2 and TMPRSS4 Promote SARS-CoV-2 Infection of Human Small Intestinal Enterocytes. Sci. Immunol. 2020, 5, eabc3582. [Google Scholar] [CrossRef]
- Bertram, S.; Dijkman, R.; Habjan, M.; Heurich, A.; Gierer, S.; Glowacka, I.; Welsch, K.; Winkler, M.; Schneider, H.; Hofmann-Winkler, H.; et al. TMPRSS2 Activates the Human Coronavirus 229E for Cathepsin-Independent Host Cell Entry and Is Expressed in Viral Target Cells in the Respiratory Epithelium. J. Virol. 2013, 87, 6150–6160. [Google Scholar] [CrossRef]
- Abe, M.; Tahara, M.; Sakai, K.; Yamaguchi, H.; Kanou, K.; Shirato, K.; Kawase, M.; Noda, M.; Kimura, H.; Matsuyama, S.; et al. TMPRSS2 Is an Activating Protease for Respiratory Parainfluenza Viruses. J. Virol. 2013, 87, 11930–11935. [Google Scholar] [CrossRef]
- Sato, K.; Hayashi, H.; Shimotai, Y.; Yamaya, M.; Hongo, S.; Kawakami, K.; Matsuzaki, Y.; Nishimura, H. TMPRSS2 Activates Hemagglutinin-Esterase Glycoprotein of Influenza C Virus. J. Virol. 2021, 95, e0129621. [Google Scholar] [CrossRef]
- Vaarala, M.H.; Porvari, K.S.; Kellokumpu, S.; Kyllönen, A.P.; Vihko, P.T. Expression of Transmembrane Serine Protease TMPRSS2 in Mouse and Human Tissues. J. Pathol. 2001, 193, 134–140. [Google Scholar] [CrossRef]
- Ma, R.; Gan, L.; Jiang, S.; Ding, P.; Chen, D.; Wu, J.; Qian, J. High Expression of SARS-CoV-2 Entry Factors in Human Conjunctival Goblet Cells. Exp. Eye Res. 2021, 205, 108501. [Google Scholar] [CrossRef] [PubMed]
- Osan, J.; Talukdar, S.N.; Feldmann, F.; DeMontigny, B.A.; Jerome, K.; Bailey, K.L.; Feldmann, H.; Mehedi, M. Goblet Cell Hyperplasia Increases SARS-CoV-2 Infection in Chronic Obstructive Pulmonary Disease. Microbiol. Spectr. 2022, 10, e0045922. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, M.; Uemura, K.; Sanaki, T.; Sato, A.; Hall, W.W.; Kariwa, H.; Orba, Y.; Sawa, H.; Sasaki, M. TMPRSS11D and TMPRSS13 Activate the SARS-CoV-2 Spike Protein. Viruses 2021, 13, 384. [Google Scholar] [CrossRef] [PubMed]
- Drozdzik, A.; Drozdzik, M. Oral Pathology in COVID-19 and SARS-CoV-2 Infection-Molecular Aspects. Int. J. Mol. Sci. 2022, 23, 1431. [Google Scholar] [CrossRef] [PubMed]
- Graham, K.L.; Halasz, P.; Tan, Y.; Hewish, M.J.; Takada, Y.; Mackow, E.R.; Robinson, M.K.; Coulson, B.S. Integrin-Using Rotaviruses Bind Alpha2beta1 Integrin Alpha2 I Domain via VP4 DGE Sequence and Recognize AlphaXbeta2 and AlphaVbeta3 by Using VP7 during Cell Entry. J. Virol. 2003, 77, 9969–9978. [Google Scholar] [CrossRef]
- Bergelson, J.M.; St John, N.; Kawaguchi, S.; Chan, M.; Stubdal, H.; Modlin, J.; Finberg, R.W. Infection by Echoviruses 1 and 8 Depends on the Alpha 2 Subunit of Human VLA-2. J. Virol. 1993, 67, 6847–6852. [Google Scholar] [CrossRef]
- Zutter, M.M.; Santoro, S.A. Widespread Histologic Distribution of the Alpha 2 Beta 1 Integrin Cell-Surface Collagen Receptor. Am. J. Pathol. 1990, 137, 113–120. [Google Scholar]
- Nykjaer, A.; Fyfe, J.C.; Kozyraki, R.; Leheste, J.R.; Jacobsen, C.; Nielsen, M.S.; Verroust, P.J.; Aminoff, M.; de la Chapelle, A.; Moestrup, S.K.; et al. Cubilin Dysfunction Causes Abnormal Metabolism of the Steroid Hormone 25(OH) Vitamin D(3). Proc. Natl. Acad. Sci. USA 2001, 98, 13895–13900. [Google Scholar] [CrossRef]
- Elsakka, E.G.E.; Mokhtar, M.M.; Hegazy, M.; Ismail, A.; Doghish, A.S. Megalin, a Multi-Ligand Endocytic Receptor, and Its Participation in Renal Function and Diseases: A Review. Life Sci. 2022, 308, 120923. [Google Scholar] [CrossRef]
- Shafren, D.R.; Dorahy, D.J.; Ingham, R.A.; Burns, G.F.; Barry, R.D. Coxsackievirus A21 Binds to Decay-Accelerating Factor but Requires Intercellular Adhesion Molecule 1 for Cell Entry. J. Virol. 1997, 71, 4736–4743. [Google Scholar] [CrossRef]
- Nishimura, Y.; Shimojima, M.; Tano, Y.; Miyamura, T.; Wakita, T.; Shimizu, H. Human P-Selectin Glycoprotein Ligand-1 Is a Functional Receptor for Enterovirus 71. Nat. Med. 2009, 15, 794–797. [Google Scholar] [CrossRef]
- Mendelsohn, C.L.; Wimmer, E.; Racaniello, V.R. Cellular Receptor for Poliovirus: Molecular Cloning, Nucleotide Sequence, and Expression of a New Member of the Immunoglobulin Superfamily. Cell 1989, 56, 855–865. [Google Scholar] [CrossRef]
- Jindadamrongwech, S.; Thepparit, C.; Smith, D.R. Identification of GRP 78 (BiP) as a Liver Cell Expressed Receptor Element for Dengue Virus Serotype 2. Arch. Virol. 2004, 149, 915–927. [Google Scholar] [CrossRef]
- Nain, M.; Mukherjee, S.; Karmakar, S.P.; Paton, A.W.; Paton, J.C.; Abdin, M.Z.; Basu, A.; Kalia, M.; Vrati, S. GRP78 Is an Important Host Factor for Japanese Encephalitis Virus Entry and Replication in Mammalian Cells. J. Virol. 2017, 91, e02274-16. [Google Scholar] [CrossRef]
- Wang, N.; Shi, X.; Jiang, L.; Zhang, S.; Wang, D.; Tong, P.; Guo, D.; Fu, L.; Cui, Y.; Liu, X.; et al. Structure of MERS-CoV Spike Receptor-Binding Domain Complexed with Human Receptor DPP4. Cell Res. 2013, 23, 986–993. [Google Scholar] [CrossRef]
- Ohnuma, K.; Uchiyama, M.; Yamochi, T.; Nishibashi, K.; Hosono, O.; Takahashi, N.; Kina, S.; Tanaka, H.; Lin, X.; Dang, N.H.; et al. Caveolin-1 Triggers T-Cell Activation via CD26 in Association with CARMA1. J. Biol. Chem. 2007, 282, 10117–10131. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiang, R.; Huo, S.; Zhou, Y.; Jiang, S.; Wang, Q.; Yu, F. Molecular Mechanism of Interaction between SARS-CoV-2 and Host Cells and Interventional Therapy. Signal Transduct. Target. Ther. 2021, 6, 233. [Google Scholar] [CrossRef]
- Chen, Z.; Mi, L.; Xu, J.; Yu, J.; Wang, X.; Jiang, J.; Xing, J.; Shang, P.; Qian, A.; Li, Y.; et al. Function of HAb18G/CD147 in Invasion of Host Cells by Severe Acute Respiratory Syndrome Coronavirus. J. Infect. Dis. 2005, 191, 755–760. [Google Scholar] [CrossRef]
- Zhong, F.-Y.; Zhao, Y.-C.; Zhao, C.-X.; Gu, Z.-C.; Lu, X.-Y.; Jiang, W.-L.; Gao, L.-C.; Li, W.-L.; Qin, Z.-H.; Ge, H.; et al. The Role of CD147 in Pathological Cardiac Hypertrophy Is Regulated by Glycosylation. Oxid. Med. Cell. Longev. 2022, 2022, 6603296. [Google Scholar] [CrossRef]
- Feigelstock, D.; Thompson, P.; Mattoo, P.; Zhang, Y.; Kaplan, G.G. The Human Homolog of HAVcr-1 Codes for a Hepatitis A Virus Cellular Receptor. J. Virol. 1998, 72, 6621–6628. [Google Scholar] [CrossRef]
- Van Timmeren, M.M.; van den Heuvel, M.C.; Bailly, V.; Bakker, S.J.L.; van Goor, H.; Stegeman, C.A. Tubular Kidney Injury Molecule-1 (KIM-1) in Human Renal Disease. J. Pathol. 2007, 212, 209–217. [Google Scholar] [CrossRef] [PubMed]
- White, J.M. ADAMs: Modulators of Cell-Cell and Cell-Matrix Interactions. Curr. Opin. Cell Biol. 2003, 15, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Poon, M.-W.; Tsang, W.-H.; Chan, S.-O.; Li, H.-M.; Ng, H.-K.; Waye, M.M.-Y. Dyslexia-Associated Kiaa0319-like Protein Interacts with Axon Guidance Receptor Nogo Receptor 1. Cell. Mol. Neurobiol. 2011, 31, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Pillay, S.; Meyer, N.L.; Puschnik, A.S.; Davulcu, O.; Diep, J.; Ishikawa, Y.; Jae, L.T.; Wosen, J.E.; Nagamine, C.M.; Chapman, M.S.; et al. An Essential Receptor for Adeno-Associated Virus Infection. Nature 2016, 530, 108–112. [Google Scholar] [CrossRef]

| Spike: | Alpha | Beta | Delta | Gamma | BA.1 | BA.2 | BA.2.12.1 | BA.2.75 | BA.4 | BA.5 |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.031 | 0.031 | 0.031 | 0.031 | 0.031 | 0.031 | 0.031 | 0.031 | 0.032 | 0.032 | |
| Polyproteins: | R1A | R1AB | ||||||||
| 0.031 | 0.032 | |||||||||
| SP: | E | M | N | |||||||
| 0.040 | 0.018 | 0 | ||||||||
| Human proteins: | ACE2 | Median of all proteins (Q1–Q3) | ||||||||
| 0.010 | 0.021 (0.01–0.03) |
| Alpha | Beta | Delta | Gamma | BA.1 a | GLYC | POLG | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| RIAB | R1A | HRSVA | HCV | |||||||
| ACE2 | 0.074 | 0.074 | 0.074 | 0.074 | 0.074 | 0.071 | 0.070 | 0.219 | 0.098 | without Cys |
| ACE2 | 0.082 | 0.082 | 0.082 | 0.082 | 0.065 | 0.072 | 0.070 | 0.235 | 0.092 | with Cys |
| Main Target Tissue | SARS-CoV-2 Receptor (Putative or Verified) | Relative Cys Content | Signal for Oxidative Stress Relative to Spike (Cys Less than 0.031) |
|---|---|---|---|
| Ubiquitous | ACE2 | 0.010 | YES |
| LRP1 | 0.073 | NO | |
| TMPRSS2 | 0.045 | NO | |
| TMPRSS11D | 0.022 | YES | |
| ADAM17 | 0.042 | NO | |
| ADAM10 | 0.048 | NO | |
| ADAM9 | 0.054 | NO | |
| DPP4 | 0.016 | YES | |
| UFO | 0.025 | YES | |
| CD209/DC-SIGN | 0.022 | YES | |
| Ear | TECTA | 0.068 | NO |
| TECTB | 0.040 | NO | |
| Taste | P2RX3 | 0.030 | YES |
| Nasal airway epithelium | CDHR3 | 0.008 | YES |
| NRP1 | 0.024 | YES | |
| Lungs | BiP/GRP78 | 0.003 | YES |
| CDHR3 | 0.008 | YES | |
| Heart | CAR | 0.027 | YES |
| Basigin | 0.018 | YES | |
| Intestine | SCARB2 | 0.017 | YES |
| CAR | 0.027 | YES | |
| ITA-2 | 0.019 | YES | |
| DAF | 0.047 | NO | |
| PSGL-1 | 0.007 | YES | |
| Kremen-1 | 0.036 | NO | |
| PVR | 0.022 | YES | |
| FcRn | 0.016 | YES | |
| Kidney | LRP2/megalin | 0.071 | NO |
| Cubilin | 0.043 | NO | |
| HAVR1 | 0.019 | YES | |
| Nervous system | LRP-8 | 0.065 | NO |
| VLDLR | 0.077 | NO | |
| K319L | 0.018 | YES | |
| PVR | 0.022 | YES | |
| SCARB2 | 0.017 | YES | |
| Liver | ASGR1 | 0.034 | NO |
| VLDLR | 0.077 | NO | |
| Endothelium | CD209L/L-SIGN | 0.023 | YES |
| Platelets | LRP-8 | 0.065 | NO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vernone, A.; Bergandi, L.; Pernice, S.; Pescarmona, G.; Silvagno, F. How the Competition for Cysteine May Promote Infection of SARS-CoV-2 by Triggering Oxidative Stress. Antioxidants 2023, 12, 483. https://doi.org/10.3390/antiox12020483
Vernone A, Bergandi L, Pernice S, Pescarmona G, Silvagno F. How the Competition for Cysteine May Promote Infection of SARS-CoV-2 by Triggering Oxidative Stress. Antioxidants. 2023; 12(2):483. https://doi.org/10.3390/antiox12020483
Chicago/Turabian StyleVernone, Annamaria, Loredana Bergandi, Simone Pernice, Gianpiero Pescarmona, and Francesca Silvagno. 2023. "How the Competition for Cysteine May Promote Infection of SARS-CoV-2 by Triggering Oxidative Stress" Antioxidants 12, no. 2: 483. https://doi.org/10.3390/antiox12020483
APA StyleVernone, A., Bergandi, L., Pernice, S., Pescarmona, G., & Silvagno, F. (2023). How the Competition for Cysteine May Promote Infection of SARS-CoV-2 by Triggering Oxidative Stress. Antioxidants, 12(2), 483. https://doi.org/10.3390/antiox12020483