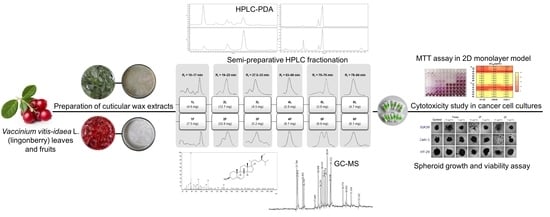
Fractionation and Characterization of Triterpenoids from Vaccinium vitis-idaea L. Cuticular Waxes and Their Potential as Anticancer Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Cultures
2.3. Plant Material
2.4. Preparation of the Crude Extracts
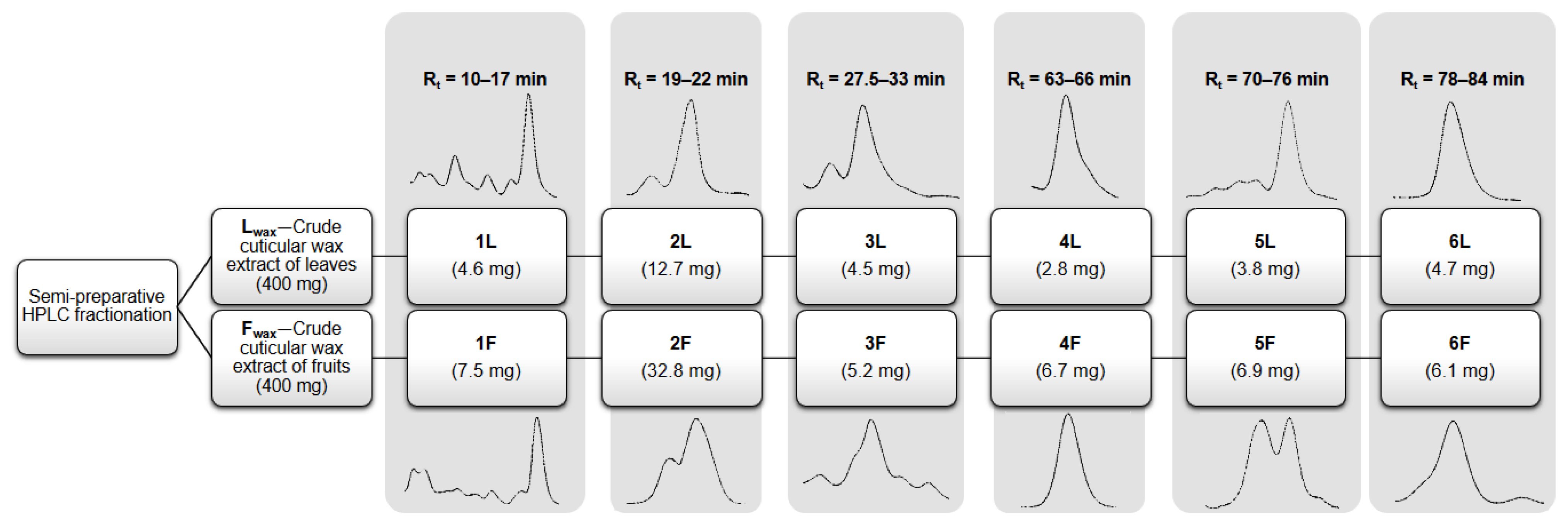
2.5. Fractionation Using Semi-Preparative HPLC
2.6. Phytochemical Characterization Techniques
2.6.1. HPLC-PDA for the Quantification of Triterpenoids and Other Compounds
2.6.2. GC-MS for the Qualitative Analysis of Triterpenoids
2.7. Screening of Anticancer Agents
2.7.1. MTT Assay
2.7.2. Spheroid Growth and Viability Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Phytochemical Characterization
3.1.1. Qualitative and Quantitative Analysis of Cuticular Wax Extracts
3.1.2. Qualitative and Quantitative Analysis of Obtained Fractions
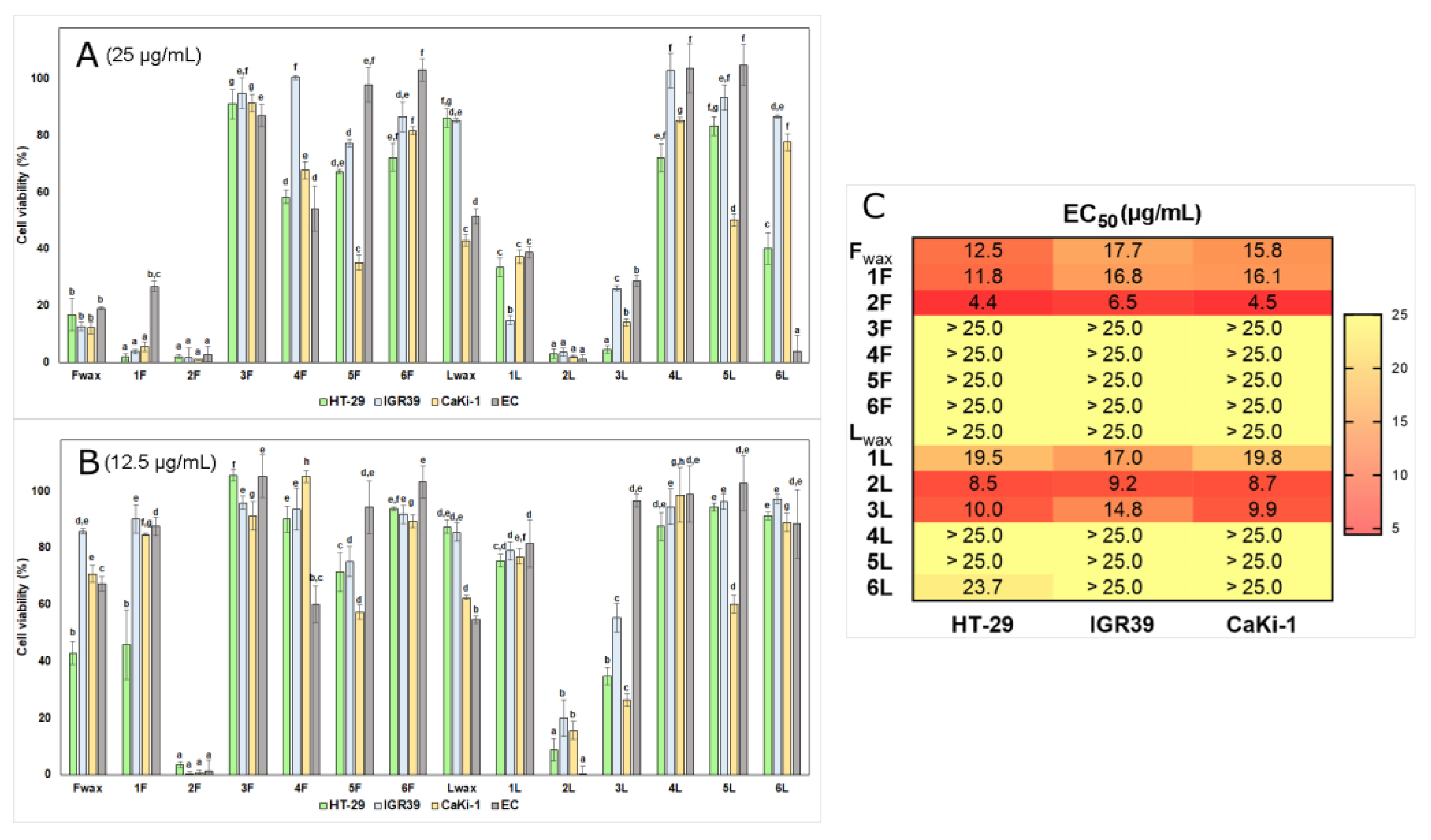
3.2. Anticancer Activity of Cuticular Wax Extracts and Obtained Fractions
3.2.1. Cytotoxicity Study in 2D Cultures
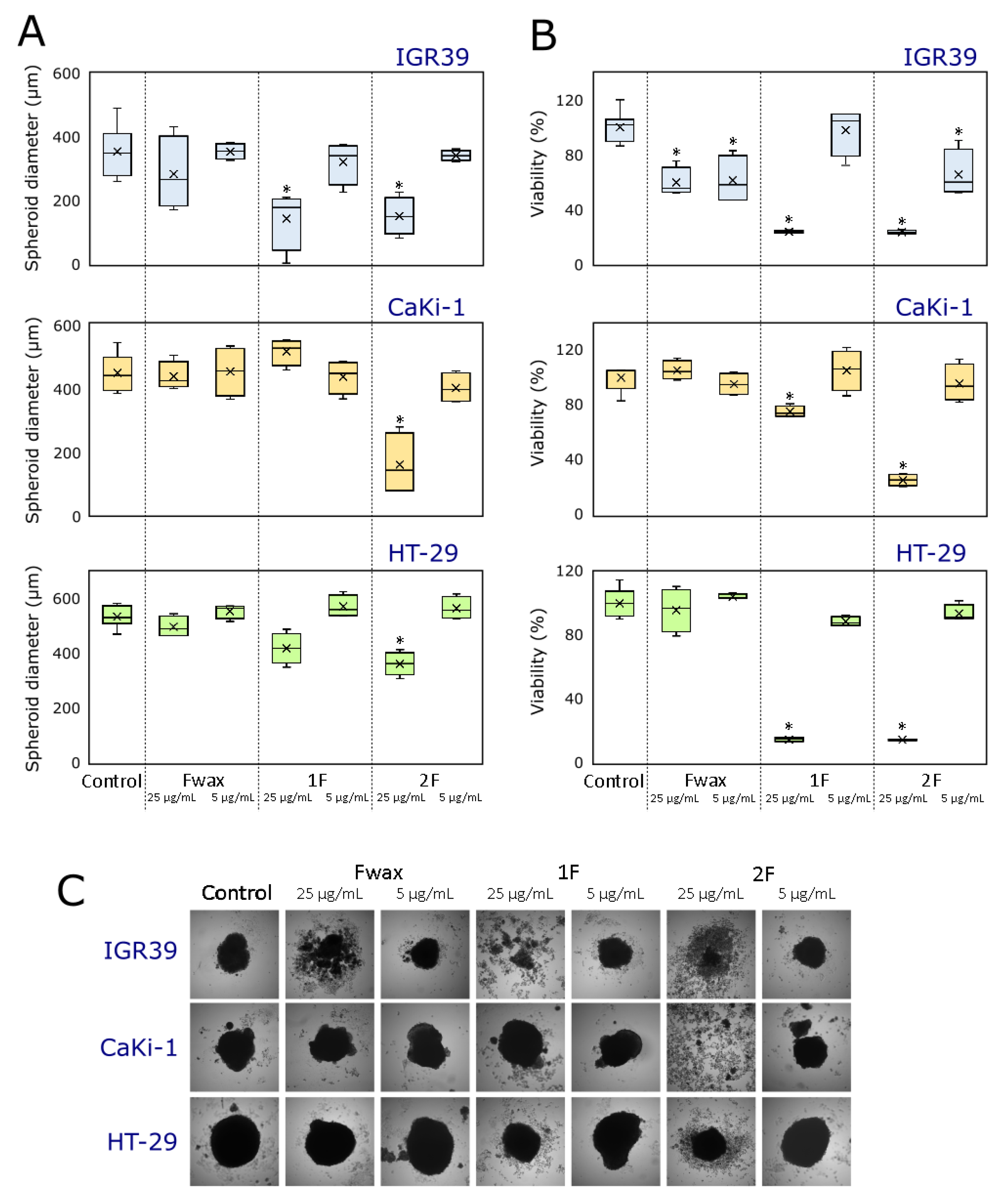
3.2.2. Effect on 3D Cultures (Spheroids) Growth and Viability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kubczak, M.; Szustka, A.; Rogalińska, M. Molecular targets of natural compounds with anti-cancer properties. Int. J. Mol. Sci. 2021, 22, 13659. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, Z.; Wimmer, Z. Selected plant triterpenoids and their amide derivatives in cancer treatment: A review. Phytochemistry 2022, 203, 113340. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.W.K.; Mocan, A.; Atanasov, A.G. Let food be thy medicine and medicine be thy food: A bibliometric analysis of the most cited papers focusing on nutraceuticals and functional foods. Food Chem. 2018, 269, 455–465. [Google Scholar] [CrossRef]
- Shukla, D.; Rawal, R.; Jain, N. A brief review on plant-derived natural compounds as an anti-cancer agents. Int. J. Herb. Med. 2018, 6, 28–36. [Google Scholar]
- Siddiqui, A.J.; Jahan, S.; Singh, R.; Saxena, J.; Ashraf, S.A.; Khan, A.; Choudhary, R.K.; Balakrishnan, S.; Badraoui, R.; Bardakci, F.; et al. Plants in anticancer drug discovery: From molecular mechanism to chemoprevention. BioMed Res. Int. 2022, 2022, 5425485. [Google Scholar] [CrossRef]
- Noushahi, H.A.; Khan, A.H.; Noushahi, U.F.; Hussain, M.; Javed, T.; Zafar, M.; Batool, M.; Ahmed, U.; Liu, K.; Harrison, M.T.; et al. Biosynthetic pathways of triterpenoids and strategies to improve their biosynthetic efficiency. Plant Growth Regul. 2022, 97, 439–454. [Google Scholar] [CrossRef]
- Becker, R.; Dashbaldan, S.; Pączkowski, C.; Golis, T.; Szakiel, A. Comparison of steroids and triterpenoids in leaf cuticular waxes of selected Polish and Russian cultivars and genotypes of edible honeysuckle. Phytochem. Lett. 2019, 30, 238–244. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Gegechkori, V.; Mohammed, E.U.R.; Ku, H.; Morton, D.W. Isolation of bioactive pentacyclic triterpenoid acids from olive tree leaves with flash chromatography. Appl. Sci. 2022, 12, 996. [Google Scholar] [CrossRef]
- Chen, H.; Gao, Y.; Wang, A.; Zhou, X.; Zheng, Y.; Zhou, J. Evolution in medicinal chemistry of ursolic acid derivatives as anticancer agents. Eur. J. Med. Chem. 2015, 92, 648–655. [Google Scholar] [CrossRef]
- Ghante, M.H.; Jamkhande, P.G. Role of pentacyclic triterpenoids in chemoprevention and anticancer treatment: An overview on targets and underling mechanisms. J. Pharmacopunct. 2019, 22, 55–67. [Google Scholar] [CrossRef]
- Kang, H.R.; Eom, H.J.; Lee, S.R.; Choi, S.U.; Kang, K.S.; Lee, K.R.; Kim, K.H. Bioassay-guided isolation of antiproliferative triterpenoids from Euonymus alatus twigs. Nat. Prod. Commun. 2015, 10, 1929–1932. [Google Scholar] [CrossRef]
- Szakiel, A.; Pączkowski, C.; Pensec, F.; Bertsch, C. Fruit cuticular waxes as a source of biologically active triterpenoids. Phytochem. Rev. 2012, 11, 263–284. [Google Scholar] [CrossRef]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Latha, L.Y. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef]
- Boyanova, P.; Gradinarska, D.; Dobreva, V.; Ivanov, I.; Petkova, N. Effects of lingonberry extract (Vaccinium vitis-idaea L.) on the antioxidant, physicochemical and sensory characteristics of ice cream. BIO Web Conf. 2022, 45, 01008. [Google Scholar] [CrossRef]
- Zhang, D.; Adaelina, N.M.; Fan, Z.; Liu, J. Phytochemical profile and biological activities from different parts of Vaccinium vitis-idaea. J. Berry Res. 2022, 12, 445–462. [Google Scholar] [CrossRef]
- Kostka, T.; Ostberg-Potthoff, J.J.; Stärke, J.; Guigas, C.; Matsugo, S.; Mirčeski, V.; Stojanov, L.; Veličkovska, S.K.; Winterhalter, P.; Esatbeyoglu, T. Bioactive phenolic compounds from lingonberry (Vaccinium vitis-idaea L.): Extraction, chemical characterization, fractionation and cellular antioxidant activity. Antioxidants 2022, 11, 467. [Google Scholar] [CrossRef]
- Shamilov, A.A.; Bubenchikova, V.N.; Chernikov, M.V.; Pozdnyakov, D.I.; Garsiya, E.R. Vaccinium vitis-idaea L.: Chemical contents, pharmacological activities. Pharm. Sci. 2020, 26, 344–362. [Google Scholar] [CrossRef]
- Kowalska, K. Lingonberry (Vaccinium vitis-idaea L.) fruit as a source of bioactive compounds with health-promoting effects—A review. Int. J. Mol. Sci. 2021, 22, 5126. [Google Scholar] [CrossRef]
- Vilkickyte, G.; Petrikaite, V.; Pukalskas, A.; Sipailiene, A.; Raudone, L. Exploring Vaccinium vitis-idaea L. as a potential source of therapeutic agents: Antimicrobial, antioxidant, and anti-inflammatory activities of extracts and fractions. J. Ethnopharmacol. 2022, 292, 115207. [Google Scholar] [CrossRef]
- Vilkickyte, G.; Raudone, L.; Petrikaite, V. Phenolic fractions from Vaccinium vitis-idaea L. and their antioxidant and anticancer activities assessment. Antioxidants 2020, 9, 1261. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chemposov, V.V.; Chirikova, N.K. Polymeric compounds of lingonberry waste: Characterization of antioxidant and hypolipidemic polysaccharides and polyphenol-polysaccharide conjugates from Vaccinium vitis-idaea press cake. Foods 2022, 11, 2801. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Karppinen, K.; Klavins, L.; Kviesis, J.; Sundqvist, P.; Nguyen, N.; Heinonen, E.; Klavins, M.; Jaakola, L.; Väänänen, J.; et al. Compositional and morphological analyses of wax in northern wild berry species. Food Chem. 2019, 295, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, M.; Durazzo, A.; Bernini, R.; Campo, M.; Vita, C.; Souto, E.B.; Lombardi-Boccia, G.; Ramadan, M.F.; Santini, A.; Romani, A. Fruit wastes as a valuable source of value-added compounds: A collaborative perspective. Molecules 2021, 26, 6338. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.M.; Ansari, M.-R.; Ali, Q.; Awan, S. Plant cuticular waxes: A review on functions, composition, biosyntheses mechanism and transportation. Life Sci. J. 2015, 12, 60–67. [Google Scholar] [CrossRef]
- Wang, X.; Kong, L.; Zhi, P.; Chang, C. Update on cuticular wax biosynthesis and its roles in plant disease resistance. Int. J. Mol. Sci. 2020, 21, 5514. [Google Scholar] [CrossRef]
- Klavins, L.; Klavins, M. Cuticular wax composition of wild and cultivated northern berries. Foods 2020, 9, 587. [Google Scholar] [CrossRef]
- Ferlemi, A.-V.; Lamari, F.N. Berry leaves: An alternative source of bioactive natural products of nutritional and medicinal value. Antioxidants 2016, 5, 17. [Google Scholar] [CrossRef]
- Pensec, F.; Pączkowski, C.; Grabarczyk, M.; Woźniak, A.; Bénard-Gellon, M.; Bertsch, C.; Chong, J.; Szakiel, A. Changes in the triterpenoid content of cuticular waxes during fruit ripening of eight grape (Vitis vinifera) cultivars grown in the Upper Rhine Valley. J. Agric. Food Chem. 2014, 62, 7998–8007. [Google Scholar] [CrossRef]
- Fernandez-Moreno, J.-P.; Malitsky, S.; Lashbrooke, J.; Biswal, A.K.; Racovita, R.C.; Mellerowicz, E.J.; Jetter, R.; Orzaez, D.; Aharoni, A.; Granell, A. An efficient method for medium throughput screening of cuticular wax composition in different plant species. Metabolomics 2016, 12, 73. [Google Scholar] [CrossRef]
- Razeq, F.M.; Kosma, D.K.; Rowland, O.; Molina, I. Extracellular lipids of Camelina sativa: Characterization of chloroform-extractable waxes from aerial and subterranean surfaces. Phytochemistry 2014, 106, 188–196. [Google Scholar] [CrossRef]
- Vilkickyte, G.; Raudone, L. Optimization, validation and application of HPLC-PDA methods for quantification of triterpenoids in Vaccinium vitis-idaea L. Molecules 2021, 26, 1645. [Google Scholar] [CrossRef]
- Caligiani, A.; Malavasi, G.; Palla, G.; Marseglia, A.; Tognolini, M.; Bruni, R. A simple GC-MS method for the screening of betulinic, corosolic, maslinic, oleanolic and ursolic acid contents in commercial botanicals used as food supplement ingredients. Food Chem. 2013, 136, 735–741. [Google Scholar] [CrossRef]
- Sánchez Avila, N.; Priego Capote, F.; Luque de Castro, M.D. Ultrasound-assisted extraction and silylation prior to gas chromatography-mass spectrometry for the characterization of the triterpenic fraction in olive leaves. J. Chromatogr. A 2007, 1165, 158–165. [Google Scholar] [CrossRef]
- Grigalius, I.; Petrikaite, V. Relationship between antioxidant and anticancer activity of trihydroxyflavones. Mol. J. Synth. Chem. Nat. Prod. Chem. 2017, 22, 2169. [Google Scholar] [CrossRef]
- Fallahi-Sichani, M.; Honarnejad, S.; Heiser, L.M.; Gray, J.W.; Sorger, P.K. Metrics other than potency reveal systematic variation in responses to cancer drugs. Nat. Chem. Biol. 2013, 9, 708–714. [Google Scholar] [CrossRef]
- Šermukšnytė, A.; Kantminienė, K.; Jonuškienė, I.; Tumosienė, I.; Petrikaitė, V. The effect of 1,2,4-triazole-3-thiol derivatives bearing hydrazone moiety on cancer cell migration and growth of melanoma, breast, and pancreatic cancer spheroids. Pharmaceuticals 2022, 15, 1026. [Google Scholar] [CrossRef]
- Dashbaldan, S.; Becker, R.; Pączkowski, C.; Szakiel, A. Various patterns of composition and accumulation of steroids and triterpenoids in cuticular waxes from screened Ericaceae and Caprifoliaceae berries during fruit development. Molecules 2019, 24, 3826. [Google Scholar] [CrossRef]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic triterpene distribution in various plants—Rich sources for a new group of multi-potent plant extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef]
- Szakiel, A.; Pączkowski, C.; Huttunen, S. Triterpenoid content of berries and leaves of bilberry Vaccinium myrtillus from Finland and Poland. J. Agric. Food Chem. 2012, 60, 11839–11849. [Google Scholar] [CrossRef]
- Sedbare, R.; Raudone, L.; Zvikas, V.; Viskelis, J.; Liaudanskas, M.; Janulis, V. Development and validation of the UPLC-DAD methodology for the detection of triterpenoids and phytosterols in fruit samples of Vaccinium Macrocarpon Aiton and Vaccinium Oxycoccos L. Molecules 2022, 27, 4403. [Google Scholar] [CrossRef]
- Szakiel, A.; Pączkowski, C.; Koivuniemi, H.; Huttunen, S. Comparison of the triterpenoid content of berries and leaves of lingonberry Vaccinium vitis-idaea from Finland and Poland. J. Agric. Food Chem. 2012, 60, 4994–5002. [Google Scholar] [CrossRef]
- Vrancheva, R.; Ivanov, I.; Dincheva, I.; Badjakov, I.; Pavlov, A. Triterpenoids and other non-polar compounds in leaves of wild and cultivated Vaccinium species. Plants 2021, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Schlag, S.; Huang, Y.; Vetter, W. GC/EI-MS method for the determination of phytosterols in vegetable oils. Anal. Bioanal. Chem. 2022, 414, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; MacKinnon, S.L.; Craft, C.C.; Matchett, M.D.; Hurta, R.A.R.; Neto, C.C. Ursolic acid and its esters: Occurrence in cranberries and other Vaccinium fruit and effects on matrix metalloproteinase activity in DU145 prostate tumor cells. J. Sci. Food Agric. 2011, 91, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.F.P.; Gomes, L.N.L.F.; Mazzei, J.L.; Fontão, A.P.A.; Sampaio, A.L.F.; Siani, A.C.; Valente, L.M.M. Polyphenol and triterpenoid constituents of Eugenia Florida DC. (Myrtaceae) leaves and their antioxidant and cytotoxic potential. Quím. Nova 2018, 41, 1140–1149. [Google Scholar] [CrossRef]
- Lourenço, A.; Marques, A.V.; Gominho, J. The identification of new triterpenoids in Eucalyptus globulus wood. Molecules 2021, 26, 3495. [Google Scholar] [CrossRef]
- Klavins, L.; Mezulis, M.; Nikolajeva, V.; Klavins, M. Composition, sun protective and antimicrobial activity of lipophilic bilberry (Vaccinium myrtillus L.) and lingonberry (Vaccinium vitis-idaea L.) extract fractions. LWT 2021, 138, 110784. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S.; Vongkamjan, K. Antioxidant and antibacterial properties of guava leaf extracts as affected by solvents used for prior dechlorophyllization. J. Food Biochem. 2018, 42, e12600. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, K.; Xu, Y.; Meng, Q.; Liu, Z.; Sun, P.; Ye, X. Paleovegetational reconstruction and implications on formation of oil shale and coal in the lower Cretaceous Laoheishan Basin (NE China): Evidence from palynology and terpenoid biomarkers. Energies 2021, 14, 4704. [Google Scholar] [CrossRef]
- Alvarado, H.L.; Abrego, G.; Garduño-Ramirez, M.L.; Clares, B.; Calpena, A.C.; García, M.L. Design and optimization of oleanolic/ursolic acid-loaded nanoplatforms for ocular anti-inflammatory applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 521–530. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Zhao, S.; Sun, W.; Tong, S. Preparative separation of structural isomeric pentacyclic triterpene oleanolic acid and ursolic acid from natural products by pH-zone-refining countercurrent chromatography. RSC Adv. 2019, 9, 38860–38866. [Google Scholar] [CrossRef] [PubMed]
- Vilkickyte, G.; Raudone, L. Phenological and geographical effects on phenolic and triterpenoid content in Vaccinium vitis-idaea L. leaves. Plants 2021, 10, 1986. [Google Scholar] [CrossRef] [PubMed]
- Pardo, J.E.; Tello, J.; Suárez, M.; Rabadán, A.; De Miguel, C.; Álvarez-Orti, M. Variety characterization and influence of olive maturity in virgin olive oils from the area assigned to the protected designation of origin “Aceite de La Alcarria” (Spain). Agronomy 2020, 10, 38. [Google Scholar] [CrossRef]
- Klavins, L.; Kviesis, J.; Kļaviņš, M. Surface Wax Composition of Wild and Cultivated Northern Berries. Agron. Res. 2019, 17, 1337–1345. [Google Scholar]
- Osafo, T.; Philips, T.J.; Adomako, A.K.; Borquaye, L.S.; Ekuadzi, E.; Appiah-Opong, R.; Dickson, R.A. In vitro antileishmanial activity and molecular docking studies of lupeol and monostearin, isolated from Parkia biglobosa. Sci. Afr. 2023, 19, e01464. [Google Scholar] [CrossRef]
- Dos Santos, B.M.; Ferreira, G.M.; Tavares, M.T.; De Bona, J.C.; Hirata, M.H.; De Paula, V.F.; Saturnino, K.C.; Soares, A.M.; Mendes, M.M. Antiophidic activity of the secondary metabolite lupeol isolated from Zanthoxylum monogynum. Toxicon 2021, 193, 38–47. [Google Scholar] [CrossRef]
- Mongalo, N.I.; McGaw, L.J.; Finnie, J.F.; Van Staden, J. Isolation and characterization of antimicrobial and anti-inflammatory triterpenoids from the acetone extract of Grewia flava DC. (Malvaceae) Roots. S. Afr. J. Bot. 2022, 149, 87–95. [Google Scholar] [CrossRef]
- Nakamura, H.; Fang, J.; Maeda, H. Development of next-generation macromolecular drugs based on the EPR effect: Challenges and pitfalls. Expert Opin. Drug Deliv. 2015, 12, 53–64. [Google Scholar] [CrossRef]
- Danhier, F.; Feron, O.; Préat, V. To Exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef]
- Juan, M.E.; Planas, J.M.; Ruiz-Gutierrez, V.; Daniel, H.; Wenzel, U. Antiproliferative and apoptosis-inducing effects of maslinic and oleanolic acids, two pentacyclic triterpenes from olives, on HT-29 colon cancer cells. Br. J. Nutr. 2008, 100, 36–43. [Google Scholar] [CrossRef]
- Su, D.; Gao, Y.-Q.; Dai, W.-B.; Hu, Y.; Wu, Y.-F.; Mei, Q.-X. Helicteric acid, oleanic acid, and betulinic acid, three triterpenes from Helicteres angustifolia L., inhibit proliferation and induce apoptosis in HT-29 colorectal cancer cells via suppressing NF-ΚB and STAT3 signaling. Evid. Based Complement. Altern. Med. ECAM 2017, 2017, 5180707. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, S.-S.; Zhang, X.-K.; He, F.; Duan, C.-Q. An effective method for the semi-preparative isolation of high-purity anthocyanin monomers from grape pomace. Food Chem. 2020, 310, 125830. [Google Scholar] [CrossRef]
- Radić, M.; Vlašić, I.; Jazvinšćak Jembrek, M.; Horvat, A.; Tadijan, A.; Sabol, M.; Dužević, M.; Herak Bosnar, M.; Slade, N. Characterization of vemurafenib-resistant melanoma cell lines reveals novel hallmarks of targeted therapy resistance. Int. J. Mol. Sci. 2022, 23, 9910. [Google Scholar] [CrossRef]
- Yenjit, P.; Issarakraisila, M.; Intana, W.; Chantrapromma, K. Fungicidal activity of compounds extracted from the pericarp of Areca catechu against Colletotrichum gloeosporioides in vitro and in mango fruit. Postharvest Biol. Technol. 2010, 55, 129–132. [Google Scholar] [CrossRef]
- Li, J.; Zhang, B.; Liu, H.; Zhang, X.; Shang, X.; Zhao, C. Triterpenoids from Ainsliaea yunnanensis Franch. and their biological activities. Molecules 2016, 21, 1481. [Google Scholar] [CrossRef]
- Ma, C.-M.; Cai, S.-Q.; Cui, J.-R.; Wang, R.-Q.; Tu, P.-F.; Hattori, M.; Daneshtalab, M. The cytotoxic activity of ursolic acid derivatives. Eur. J. Med. Chem. 2005, 40, 582–589. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Soon, C.Y.; Tan, J.B.L.; Wong, S.K.; Hui, Y.W. Ursolic acid: An overview on its cytotoxic activities against breast and colorectal cancer cells. J. Integr. Med. 2019, 17, 155–160. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Ahmad, R.; Mahmood, T.; Kanwal, S.; Ali, B.; Khalil, A.T.; Shah, S.A.; Alam, M.M.; Badshah, H. Ursolic acid a promising candidate in the therapeutics of breast cancer: Current status and future implications. Biomed. Pharmacother. 2018, 108, 752–756. [Google Scholar] [CrossRef]
- Hashem, F.A.; Sengab, A.E.; Shabana, M.H.; Khaled, S. Antioxidant activity of Mayodendron Igneum Kurz and the cytotoxicity of the isolated terpenoids. J. Med. Act. Plants 2012, 1, 88–97. [Google Scholar] [CrossRef]
- Yin, M.-C.; Chan, K.-C. Nonenzymatic antioxidative and antiglycative effects of oleanolic acid and ursolic acid. J. Agric. Food Chem. 2007, 55, 7177–7181. [Google Scholar] [CrossRef]
- Jiménez-Arellanes, A.; Luna-Herrera, J.; Cornejo-Garrido, J.; López-García, S.; Castro-Mussot, M.E.; Meckes-Fischer, M.; Mata-Espinosa, D.; Marquina, B.; Torres, J.; Hernández-Pando, R. Ursolic and oleanolic acids as antimicrobial and immunomodulatory compounds for tuberculosis treatment. BMC Complement. Altern. Med. 2013, 13, 258. [Google Scholar] [CrossRef] [PubMed]
- Soica, C.; Oprean, C.; Borcan, F.; Danciu, C.; Trandafirescu, C.; Coricovac, D.; Crăiniceanu, Z.; Dehelean, C.A.; Munteanu, M. The synergistic biologic activity of oleanolic and ursolic acids in complex with hydroxypropyl-γ-cyclodextrin. Molecules 2014, 19, 4924–4940. [Google Scholar] [CrossRef] [PubMed]
- Bonel-Pérez, G.C.; Pérez-Jiménez, A.; Gris-Cárdenas, I.; Parra-Pérez, A.M.; Lupiáñez, J.A.; Reyes-Zurita, F.J.; Siles, E.; Csuk, R.; Peragón, J.; Rufino-Palomares, E.E. Antiproliferative and pro-apoptotic effect of uvaol in human hepatocarcinoma HepG2 cells by affecting G0/G1 cell cycle arrest, ROS Production and AKT/PI3K signaling pathway. Molecules 2020, 25, 4254. [Google Scholar] [CrossRef] [PubMed]
- Peñas-Fuentes, J.L.; Siles, E.; Rufino-Palomares, E.E.; Pérez-Jiménez, A.; Reyes-Zurita, F.J.; Lupiáñez, J.A.; Fuentes-Almagro, C.; Peragón-Sánchez, J. Effects of erythrodiol on the antioxidant response and proteome of HepG2 cells. Antioxidants 2021, 11, 73. [Google Scholar] [CrossRef]
- Martín, R.; Ibeas, E.; Carvalho-Tavares, J.; Hernández, M.; Ruiz-Gutierrez, V.; Nieto, M.L. Natural triterpenic diols promote apoptosis in astrocytoma cells through ros-mediated mitochondrial depolarization and JNK activation. PLoS ONE 2009, 4, e5975. [Google Scholar] [CrossRef]
- Allouche, Y.; Beltrán, G.; Gaforio, J.J.; Uceda, M.; Mesa, M.D. Antioxidant and Antiatherogenic Activities of Pentacyclic Triterpenic Diols and Acids. Food Chem. Toxicol. 2010, 48, 2885–2890. [Google Scholar] [CrossRef]
- Ombui, H.; Moraa, M.; Kerubo Omosa, L.; Mbaveng, A.; Nchiozem-Ngnitedem, V.-A.; Masila, V.; Okemwa, E.; Heydenreich, M.; Efferth, T.; Kuete, V. Cytotoxicity of lupeol from the stem bark of Zanthoxylum gilletii against multi-factorial drug resistant cancer cell lines. Investig. Med. Chem. Pharmacol. 2018, 1, 10. [Google Scholar]
- Cmoch, P.; Pakulski, Z.; Swaczynová, J.; Strnad, M. Synthesis of lupane-type saponins bearing mannosyl and 3,6-branched trimannosyl residues and their evaluation as anticancer agents. Carbohydr. Res. 2008, 343, 995–1003. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, X.; Xie, L.; Deng, M.; Chen, H.; Song, J.; Long, J.; Li, X.; Luo, J. Lupeol and its derivatives as anticancer and anti-inflammatory agents: Molecular mechanisms and therapeutic efficacy. Pharmacol. Res. 2021, 164, 105373. [Google Scholar] [CrossRef]
- Khan, Z.; Nath, N.; Rauf, A.; Emran, T.B.; Mitra, S.; Islam, F.; Chandran, D.; Barua, J.; Khandaker, M.U.; Idris, A.M.; et al. Multifunctional roles and pharmacological potential of β-sitosterol: Emerging evidence toward clinical applications. Chem. Biol. Interact. 2022, 365, 110117. [Google Scholar] [CrossRef]
- Sharmila, R.; Sindhu, G. Evaluate the Antigenotoxicity and Anticancer Role of β-Sitosterol by Determining Oxidative DNA Damage and the Expression of Phosphorylated Mitogen-Activated Protein Kinases’, C-Fos, C-Jun, and Endothelial Growth Factor Receptor. Pharmacogn. Mag. 2017, 13, 95–101. [Google Scholar] [CrossRef]
- Blanco-Vaca, F.; Cedó, L.; Julve, J. Phytosterols in Cancer: From Molecular Mechanisms to Preventive and Therapeutic Potentials. Curr. Med. Chem. 2019, 26, 6735–6749. [Google Scholar] [CrossRef]
- Gonçalves Pereira, R.C.; Gontijo Evangelista, F.C.; Dos Santos Júnior, V.S.; de Paula Sabino, A.; Gonçalves Maltarollo, V.; de Freitas, R.P.; Pains Duarte, L. Cytotoxic activity of triterpenoids from Cheiloclinium cognatum branches against chronic and acute leukemia cell Lines. Chem. Biodivers. 2020, 17, e2000773. [Google Scholar] [CrossRef]
- Asif, M.; Shafaei, A.; Abdul Majid, A.S.; Ezzat, M.O.; Dahham, S.S.; Ahamed, M.B.K.; Oon, C.E.; Abdul Majid, A.M.S. Mesua ferrea stem bark extract induces apoptosis and inhibits metastasis in human colorectal carcinoma HCT 116 cells, through modulation of multiple cell signalling pathways. Chin. J. Nat. Med. 2017, 15, 505–514. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Souza, A.G.; Silva, I.B.B.; Campos-Fernandez, E.; Barcelos, L.S.; Souza, J.B.; Marangoni, K.; Goulart, L.R.; Alonso-Goulart, V. Comparative assay of 2D and 3D cell culture models: Proliferation, gene expression and anticancer drug response. Curr. Pharm. Des. 2018, 24, 1689–1694. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, N.; Sun, X.; Li, Q.; Zeng, Y.; Chen, F.; Sun, S.; Xu, J.; Zhang, J.; Ye, H.; et al. Automated evaluation of tumor spheroid behavior in 3D culture using deep learning-based recognition. Biomaterials 2021, 272, 120770. [Google Scholar] [CrossRef]
- Butkeviciute, A.; Petrikaite, V.; Jurgaityte, V.; Liaudanskas, M.; Janulis, V. Antioxidant, anti-inflammatory, and cytotoxic activity of extracts from some commercial apple cultivars in two colorectal and glioblastoma human cell lines. Antioxidants 2021, 10, 1098. [Google Scholar] [CrossRef]
- Piet, M.; Paduch, R. Ursolic and oleanolic acids in combination therapy inhibit migration of colon cancer cells through down-regulation of the UPA/UPAR-dependent MMPs pathway. Chem. Biol. Interact. 2022, 368, 110202. [Google Scholar] [CrossRef]
- Braciuliene, A.; Janulis, V.; Petrikaite, V. The chemo-sensitizing effect of doxorubicin of apple extract-enriched triterpenic complex on human colon adenocarcinoma and human glioblastoma cell lines. Pharmaceutics 2022, 14, 2593. [Google Scholar] [CrossRef]
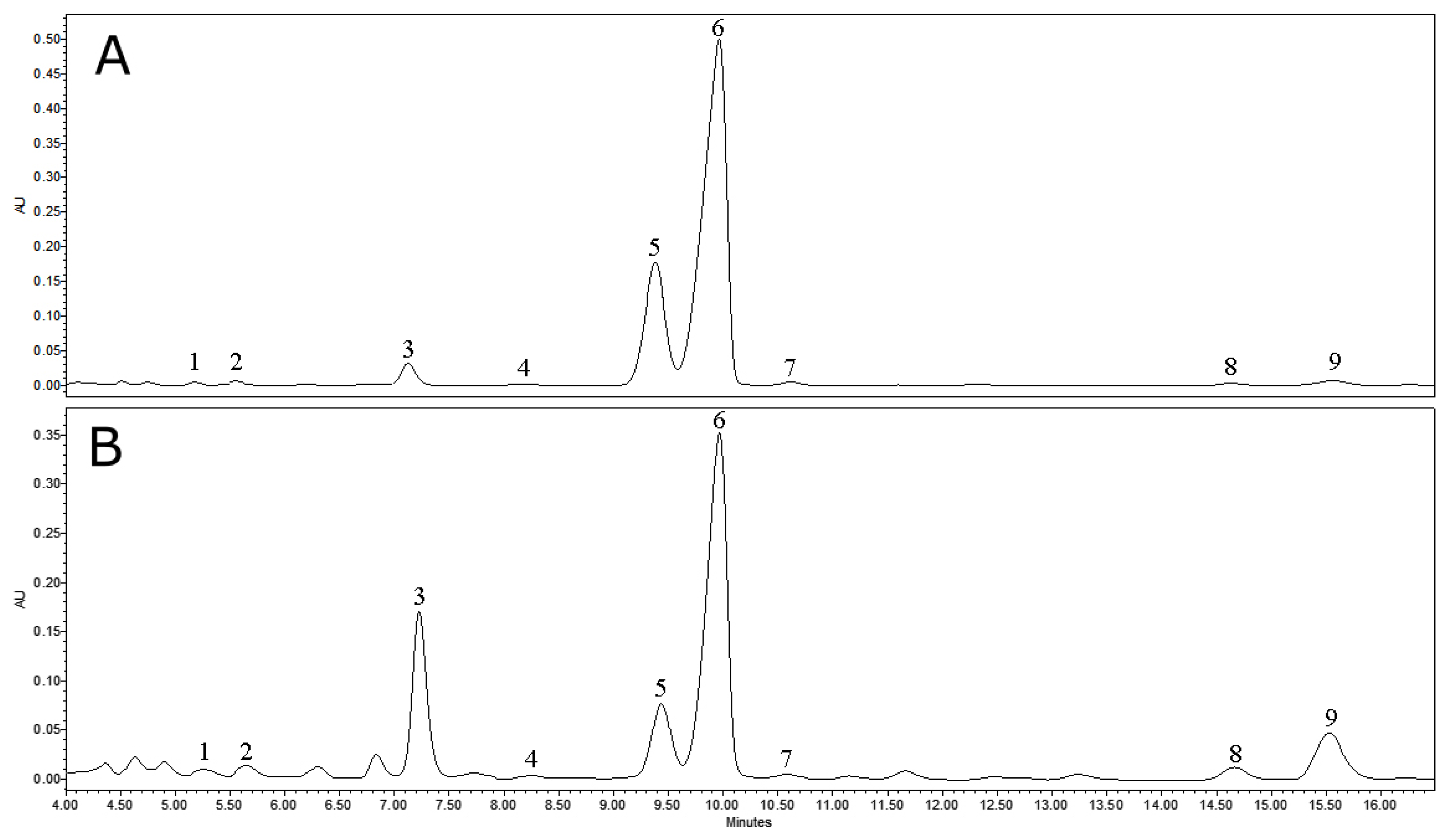
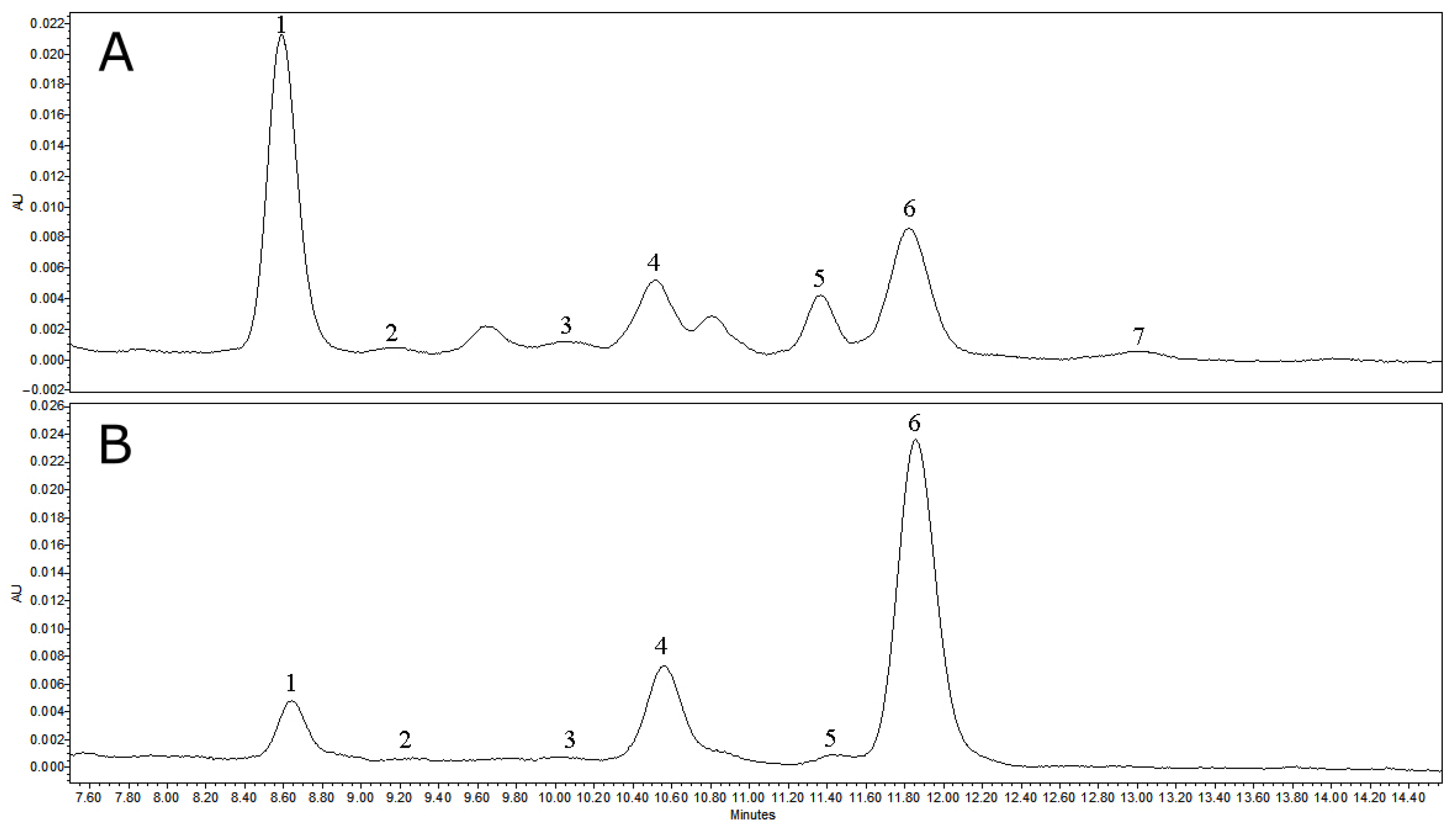
| Triterpenoid | Lwax | 1L | 2L | 3L | 4L | 5L | 6L |
|---|---|---|---|---|---|---|---|
| Maslinic acid | 0.59 ± 0.04 h,i | 39.94 ± 1.86 c | ND | ND | ND | ND | ND |
| Corosolic acid | 2.74 ± 0.12 g,i | 88.53 ± 3.90 b | ND | ND | ND | ND | ND |
| Betulinic acid | 0.14 ± 0.01 h | 4.17 ± 0.12 d | ND | ND | ND | ND | ND |
| Oleanolic acid | 18.12 ± 0.60 d | ND | 195.77 ± 4.74 b | ND | ND | ND | ND |
| Ursolic acid | 113.79 ± 2.00 a | ND | 718.99 ± 27.51 a | ND | ND | ND | ND |
| Sum of triterpenoid acids | 135.38 | 132.64 | 914.76 | - | - | - | - |
| Fernenol | 29.40 ± 1.08 c | 549.24 ± 19.26 a | ND | ND | ND | ND | ND |
| Betulin | 2.56 ± 0.08 g,i | ND | 19.38 ± 0.69 c | ND | ND | ND | ND |
| Erythrodiol | 3.46 ± 0.09 g | ND | ND | 130.99 ± 6.32 b | ND | ND | ND |
| Uvaol | 23.31 ± 0.83 c | ND | ND | 874.47 ± 39.46 a | ND | ND | ND |
| Lupeol | 7.42 ± 0.21 f | ND | ND | ND | 848.47 ± 35.14 a | ND | ND |
| β-Amyrin | 15.11 ± 0.55 e | ND | ND | ND | ND | 762.69 ± 22.31 a | ND |
| α-Amyrin | 73.11 ± 1.38 b | ND | ND | ND | ND | ND | 994.24 ± 18.96 a |
| Friedelin | ND | ND | ND | ND | ND | ND | ND |
| Sum of neutral triterpenoids | 154.37 | 549.24 | 19.38 | 1005.47 | 848.47 | 762.69 | 994.24 |
| Lanosterol | NQ | ND | ND | ND | 12.53 ± 0.37 b | 5.15 ± 0.18 c | ND |
| Taraxasterol | NQ | ND | ND | ND | ND | 23.45 ± 0.77 c | ND |
| β-Sitosterol | 1.29 ± 0.04 g,h | ND | ND | ND | ND | 82.62 ± 2.44 b | ND |
| Sum of phytosterols | 1.29 | - | - | - | 12.53 | 111.22 | - |
| Total identified | 291.01 | 681.88 | 934.14 | 1005.47 | 861.00 | 873.91 | 994.24 |
| Triterpenoid | Fwax | 1F | 2F | 3F | 4F | 5F | 6F |
|---|---|---|---|---|---|---|---|
| Maslinic acid | 1.09 ± 0.04 f | 66.09 ± 2.46 c | ND | ND | ND | ND | ND |
| Corosolic acid | 3.22 ± 0.13 e,f | 105.20 ± 4.08 b | ND | ND | ND | ND | ND |
| Betulinic acid | 0.31 ± 0.01 f | 15.74 ± 0.48 d | ND | ND | ND | ND | ND |
| Oleanolic acid | 127.57 ± 4.86 b | ND | 335.65 ± 13.63 b | ND | ND | ND | ND |
| Ursolic acid | 584.96 ± 21.42 a | ND | 723.73 ± 33.64 a | ND | ND | ND | ND |
| Sum of triterpenoid acids | 717.15 | 187.03 | 1059.38 | - | - | - | - |
| Fernenol | 17.05 ± 0.68 d | 395.41 ± 7.78 a | ND | ND | ND | ND | ND |
| Betulin | 3.54 ± 0.14 e,f | ND | 15.82 ± 0.68 c | ND | ND | ND | ND |
| Erythrodiol | 2.07 ± 0.08 e,f | ND | ND | 133.88 ± 2.38 b | ND | ND | ND |
| Uvaol | 10.78 ± 0.34 d,e | ND | ND | 539.29 ± 14.73 a | ND | ND | ND |
| Lupeol | 31.71 ± 1.46 c | ND | ND | ND | 1004.90 ± 46.23 a | ND | ND |
| β-Amyrin | 10.86 ± 0.22 d,e | ND | ND | ND | ND | 348.80 ± 13.36 b | ND |
| α-Amyrin | 16.38 ± 0.49 d | ND | ND | ND | ND | ND | 663.80 ± 14.28 a |
| Friedelin | 4.70 ± 0.18 e,f | ND | ND | ND | ND | ND | 119.65 ± 3.65 b |
| Sum of neutral triterpenoids | 97.09 | 395.41 | 15.82 | 673.17 | 1004.90 | 348.80 | 783.45 |
| Lanosterol | 0.10 ± 0.00 f | ND | ND | ND | ND | 13.82 ± 0.42 c | ND |
| Taraxasterol | 0.57 ± 0.03 f | ND | ND | ND | ND | 30.65 ± 0.85 c | ND |
| β-Sitosterol | 9.14 ± 0.29 d,e | ND | ND | ND | ND | 427.79 ± 15.51 a | 79.36 ± 2.75 c |
| Sum of phytosterols | 9.81 | - | - | - | - | 472.26 | 79.36 |
| Total identified | 824.05 | 582.44 | 1075.20 | 673.17 | 1004.90 | 821.06 | 862.81 |
| Triterpenoid | Retention Time, min | Main Fragment Ions, m/z (Relative Intensity %) | Where Identified |
|---|---|---|---|
| Unknown phytosterol I | 23.82 | 386 (3), 329 (15), 129 (30), 105 (28), 95 (42), 73 (95), 69 (47), 57 (35), 43 (35), 41 (45) | Lwax, 4L |
| Lanosterol | 24.94 | 426 (3), 393 (20), 129 (25), 109 (65), 95 (72), 73 (70), 69 (100), 55 (41), 41 (92) | Lwax, Fwax, 4L, 5L, 5F |
| β-Sitosterol | 25.01 | 414 (8), 396 (15), 357 (14), 145 (15), 129 (68), 95 (23), 73 (62), 55 (35), 57 (56), 43 (100), 41 (29) | Lwax, Fwax, 5L, 5F, 6F |
| β-Amyrin | 25.23 | 426 (4), 218 (100), 203 (33), 189 (24), 107 (32), 95 (60), 73 (90), 69 (54), 55 (29) | Lwax, Fwax, 5L, 5F |
| Unknown phytosterol II | 25.56 | 410 (4), 374 (16), 269 (17), 174 (20), 129 (33), 121 (45), 107 (26), 73 (100), 69 (61), 43 (2), 41 (32) | Lwax, Fwax, 5L, 5F |
| α-Amyrin | 25.75 | 426 (7), 218 (100), 203 (20), 189 (31), 107 (32), 95 (43), 73 (64), 55 (47) | Lwax, Fwax, 6L, 6F |
| Unknown neutral triterpenoid I | 25.79 | 426 (3), 218 (49), 201 (51), 189 (25), 121 (34), 109 (62), 107 (57), 95 (47), 73 (100), 69 (90), 55 (50) | Lwax, Fwax, 1L, 1F |
| Lupeol | 25.89 | 426 (3), 218 (13), 189 (24), 129 (38), 109 (37), 95 (42), 73 (100), 68 (62), 55 (30) | Lwax, Fwax, 4L, 4F |
| Fernenol | 26.32 | 426 (3), 393 (20), 331 (12), 255 (14), 241 (35), 229 (10), 189 (10), 159 (11), 119 (25), 95 (52), 73 (100), 43 (64) | Lwax, Fwax, 1L, 1F |
| Unknown phytosterol III | 26.78 | 440 (2), 422 (8), 129 (22), 121 (24), 119 (26), 107 (34), 95 (54), 73 (86), 69 (100), 55 (80) | Lwax, Fwax, 5L, 5F |
| Taraxasterol | 27.15 | 426 (3), 408 (7), 189 (53), 175 (12), 161 (14), 147 (22), 129 (49), 121 (58), 109 (100), 95 (86), 73 (97), 55 (34) | Lwax, Fwax, 5L, 5F |
| Erythrodiol | 27.33 | 442 (4), 216 (65), 203 (29), 119 (15), 95 (32), 75 (42), 73 (100), 55 (16) | Lwax, Fwax, 3L, 3F |
| Friedelin | 27.51 | 426 (4), 163 (12), 149 (8), 123 (30), 109 (40), 95 (64), 81 (65), 69 (100), 55 (92) | Fwax, 6F |
| Uvaol | 27.76 | 442 (3), 216 (44), 203 (44), 188 (27), 133 (31), 73 (100), 55 (16) | Lwax, Fwax, 3L, 3F |
| Betulin | 27.98 | 442 (4), 189 (13), 147 (10), 95 (26), 75 (39), 73 (100), 55 (14) | Lwax, Fwax, 2L, 2F |
| Oleanolic acid | 28.19 | 482 (12), 456 (3), 320 (15), 203 (64), 189 (25), 129 (30), 119 (18), 73 (100), 55 (9) | Lwax, Fwax, 2L, 2F |
| Betulinic acid | 28.34 | 456 (3), 320 (5), 203 (9), 189 (16), 129 (30), 73 (100), 55 (11) | Lwax, Fwax, 1L, 1F |
| Ursolic acid | 28.77 | 482 (12), 456 (3), 320 (47), 203 (76), 189 (29), 133 (57), 73 (100), 55 (10) | Lwax, Fwax, 2L, 2F |
| Unknown derivative of triterpenoid acid I | 29.21 | 452 (4), 320 (13), 203 (100), 189 (16), 148 (35), 133 (45), 119 (5), 75 (70), 73 (44), 69 (7) | Lwax, Fwax, 1L, 1F |
| Unknown neutral triterpenoid II | 29.55 | 424 (3), 322 (9), 219 (8), 189 (20), 122 (16), 103 (35), 95 (28), 75 (37), 73 (100), 69 (15), 55 (12) | Fwax, 3F |
| Unknown derivative of triterpenoid acid II | 29.62 | 468 (3), 320 (15), 203 (50), 189 (16), 148 (40), 133 (45), 119 (17), 75 (25), 73 (100), 55 (8) | Lwax, Fwax, 1L, 1F |
| Unknown derivative of triterpenoid acid III | 29.78 | 452 (6), 426 (3), 411 (3), 320 (12), 203 (75), 133 (35), 119 (18), 105 (13), 95 (15), 73 (100), 55 (9), 43 (58) | Fwax, 3F |
| Maslinic acid | 29.91 | 472 (3), 320 (9), 203 (22), 189 (9), 133 (12), 147 (39), 73 (100), 55 (6) | Lwax, Fwax, 1L, 1F |
| Corosolic acid | 30.27 | 472 (3), 320 (12), 203 (22), 189 (6), 147 (42), 133 (23), 73 (100), 55 (8) | Lwax, Fwax, 1L, 1F |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilkickyte, G.; Petrikaite, V.; Marksa, M.; Ivanauskas, L.; Jakstas, V.; Raudone, L. Fractionation and Characterization of Triterpenoids from Vaccinium vitis-idaea L. Cuticular Waxes and Their Potential as Anticancer Agents. Antioxidants 2023, 12, 465. https://doi.org/10.3390/antiox12020465
Vilkickyte G, Petrikaite V, Marksa M, Ivanauskas L, Jakstas V, Raudone L. Fractionation and Characterization of Triterpenoids from Vaccinium vitis-idaea L. Cuticular Waxes and Their Potential as Anticancer Agents. Antioxidants. 2023; 12(2):465. https://doi.org/10.3390/antiox12020465
Chicago/Turabian StyleVilkickyte, Gabriele, Vilma Petrikaite, Mindaugas Marksa, Liudas Ivanauskas, Valdas Jakstas, and Lina Raudone. 2023. "Fractionation and Characterization of Triterpenoids from Vaccinium vitis-idaea L. Cuticular Waxes and Their Potential as Anticancer Agents" Antioxidants 12, no. 2: 465. https://doi.org/10.3390/antiox12020465
APA StyleVilkickyte, G., Petrikaite, V., Marksa, M., Ivanauskas, L., Jakstas, V., & Raudone, L. (2023). Fractionation and Characterization of Triterpenoids from Vaccinium vitis-idaea L. Cuticular Waxes and Their Potential as Anticancer Agents. Antioxidants, 12(2), 465. https://doi.org/10.3390/antiox12020465