Se-Enriched Cardamine violifolia Improves Laying Performance and Regulates Ovarian Antioxidative Function in Aging Laying Hens
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal and Experimental Design
2.2. Laying Performance
2.3. Egg Quality
2.4. Sample Collection
2.5. Histology
2.6. Lipid Peroxidation and Antioxidant Enzyme Activity
2.7. Real-Time Quantitative PCR
2.8. Immunofluorescence Staining
2.9. TUNEL Assay
2.10. Statistical Analysis
3. Results
3.1. Laying Performance
3.2. Egg Quality
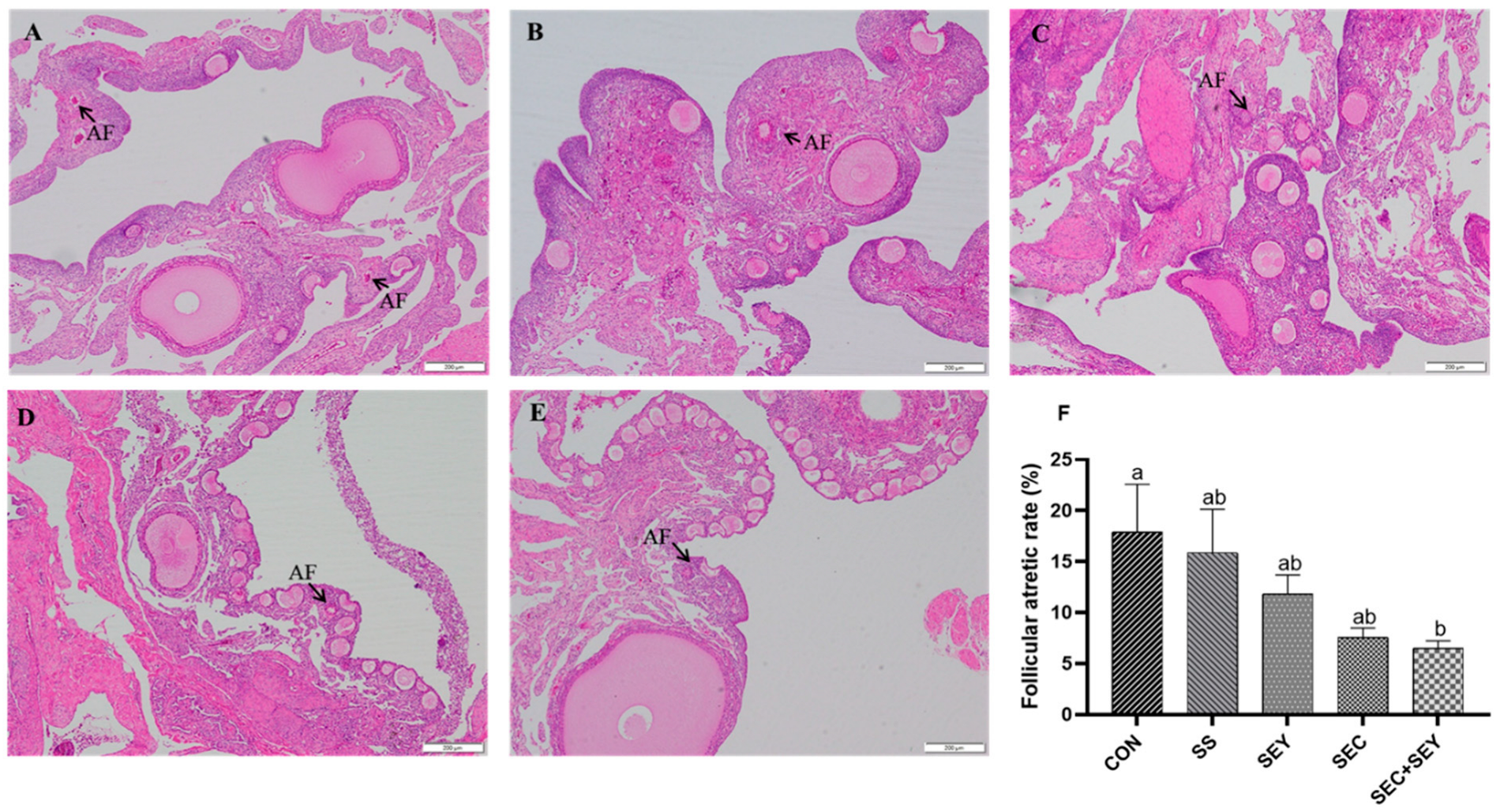
3.3. Ovarian Follicle Development
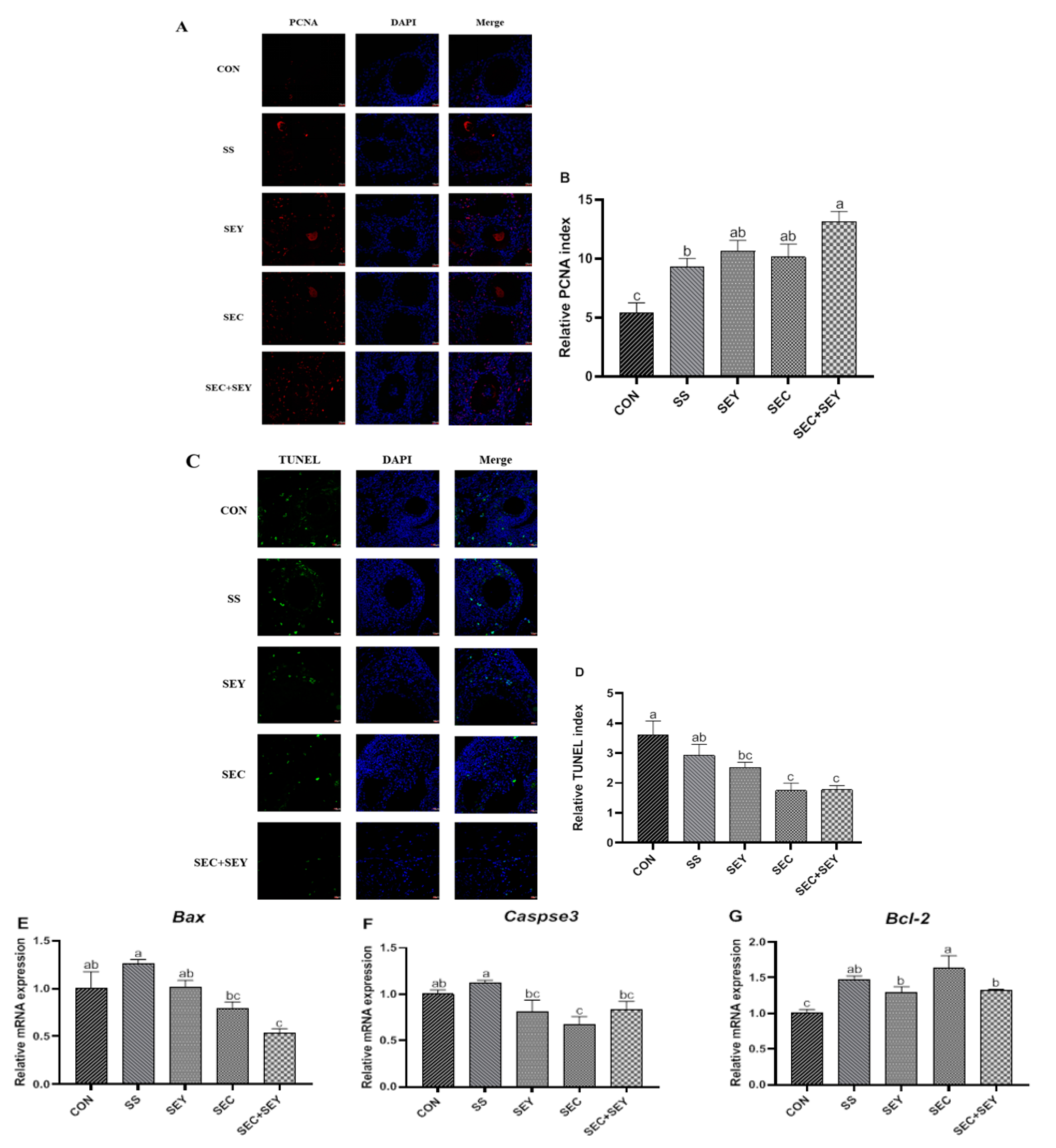
3.4. Proliferation and Apoptosis in Ovary
3.5. Antioxidant Status
3.6. Relative mRNA Expression of Selenoproteins and Nrf2/Keap1 Pathway-Related Genes in the Ovaries of Hens
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, G.M. The biology of aging: 1985–2010 and beyond. FASEB J. 2011, 25, 3756–3762. [Google Scholar] [CrossRef]
- Liu, X.T.; Lin, X.; Zhang, S.Y.; Guo, C.Q.; Li, J.; Mi, Y.L.; Zhao, C.Q. Lycopene ameliorates oxidative stress in the aging chicken ovary via activation of Nrf2/HO-1 pathway. Aging 2018, 10, 2016–2036. [Google Scholar] [CrossRef]
- Yao, H.H.; Volentine, K.K.; Bahr, J.M. Destruction of the germinal disc region of an immature preovulatory chicken follicle induces atresia and apoptosis. Biol. Reprod. 1998, 59, 516–521. [Google Scholar] [PubMed]
- Hao, E.Y.; Wang, D.; Chang, L.Y.; Huang, C.X.; Chen, H.; Yue, Q.X.; Zhou, R.Y.; Huang, R.L. Melatonin regulates chicken granulosa cell proliferation and apoptosis by activating the mTOR signaling pathway via its receptors. Poult. Sci. 2020, 96, 6147–6162. [Google Scholar] [CrossRef] [PubMed]
- Devine, P.J.; Perreault, S.D.; Luderer, U. Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol. Reprod. 2012, 86, 27. [Google Scholar] [CrossRef] [PubMed]
- Luderer, U. Ovarian toxicity from reactive oxygen species. Vitam. Horm. 2014, 94, 99–127. [Google Scholar]
- Silva-Islas, C.A.; Maldonado, P.D. Canonical and non-canonical mechanisms of Nrf2 activation. Pharmacol. Res. 2018, 134, 92–99. [Google Scholar] [CrossRef]
- Amevor, F.K.; Cui, Z.F.; Ning, Z.F.; Du, X.X.; Jin, N.N.; Shu, G.; Deng, X.; Zhu, Q.; Tian, Y.F.; Li, D.; et al. Synergistic effects of quercetin and vitamin E on egg production, egg quality, and immunity in aging breeder hens. Poult. Sci. 2021, 100, 101481. [Google Scholar] [CrossRef]
- Zhou, S.; Zhao, A.; Wu, Y.; Mi, Y.; Zhang, C. Protective effect of grape seed proanthocyanidins on oxidative damage of chicken follicular granulosa cells by inhibiting FoxO1-mediated autophagy. Front. Cell. Dev. Biol. 2022, 10, 762228. [Google Scholar] [CrossRef]
- Danielle, R.E.; David, E.S. Plants, selenium and human health. Curr. Opin. Plant Biol. 2003, 6, 273–279. [Google Scholar]
- Kieliszek, M.; Błażejak, S. Current knowledge on the importance of selenium in food for living organisms: A review. Molecules 2016, 21, 609. [Google Scholar] [CrossRef] [PubMed]
- Han, X.J.; Qin, P.; Li, W.X.; Ma, G.; Ji, C.; Zhang, J.Y.; Zhao, L.H. Effect of sodium selenite and selenium yeast on performance, egg quality, antioxidant capacity, and selenium deposition of laying hens. Poult Sci. 2017, 96, 3973–3980. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.X.; Qazi, I.H.; Pan, B.; Angel, C.; Guo, S.C.; Yang, J.Y.; Zhang, Y.; Ming, Z.; Zeng, C.J.; Meng, Q.Y.; et al. Dietary selenium supplementation ameliorates female reproductive efficiency in aging mice. Antioxidants 2019, 8, 634. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Guo, J.; Zhu, S.; Li, M.; Zhu, Z.; Cheng, S.; Wang, S.; Sun, Y.; Cong, X. Protective effects of selenium-enriched peptides from Cardamine violifolia against high-fat diet induced obesity and its associated metabolic disorders in mice. RSC Adv. 2020, 10, 31411–31424. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, Y.; Chen, Q.; Kuang, Y.; Fan, J.; Xu, X.; Zhu, H.; Gao, Q.; Cheng, S.; Cong, X.; et al. Selenium-enriched Cardamine violifolia improves growth performance with potential regulation of intestinal health and antioxidant function in weaned pigs. Front. Vet. Sci. 2022, 9, 964766. [Google Scholar] [CrossRef]
- Xu, X.; Wei, Y.; Zhang, Y.; Jing, X.; Cong, X.; Gao, Q.; Cheng, S.; Zhu, Z.; Zhu, H.; Zhao, J.; et al. A new selenium source from Se-enriched Cardamine violifolia improves growth performance, anti-oxidative capacity and meat quality in broilers. Front. Nutr. 2022, 9, 996932. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Poultry; National Academic Press: Washington, DC, USA, 1994. [Google Scholar]
- Gupta, S.K.; Gilbert, A.B.; Walker, M.A. Histological study of follicular atresia in the ovary of the domestic hen (Gallus domesticus). J. Reprod Fertil. 1988, 82, 219–225. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and 2-(Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lin, X.; Lin, X.; Guo, C.; Liu, M.; Mi, Y.; Zhang, C. Promotion of the prehierarchical follicle growth by postovulatory follicles involving PGE2-EP2 signaling in chickens. J. Cell Physiol. 2018, 233, 8984–8995. [Google Scholar] [CrossRef]
- Perheentupa, A.; Huhtaniemi, I. Aging of the human ovary and testis. Mol. Cell Endocrinol. 2009, 299, 2–13. [Google Scholar] [CrossRef]
- Wang, L.; Tang, J.; Wang, L.; Tan, F.; Song, H.; Zhou, J.; Li, F. Oxidative stress in oocyte aging and female reproduction. J. Cell Physiol. 2021, 236, 7966–7983. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2005, 3, 28. [Google Scholar] [PubMed]
- Lim, J.; Luderer, U. Oxidative damage increases and antioxidant gene expression decreases with aging in the mouse ovary. Biol. Reprod. 2011, 84, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.J.; Wu, C.X.; Gong, L.M.; Song, T.; Wu, H.; Zhang, L.Y. Effects of nano-selenium on performance, meat quality, immune function, oxidation resistance, and tissue selenium content in broilers. Poult. Sci. 2012, 91, 2532–2539. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F.; Fisinin, V.I. Selenium in poultry breeder nutrition: An update. Anim. Feed. Sci. Technol. 2014, 191, 1–15. [Google Scholar]
- Thiry, C.A.; Ruttens, A.; Temmerman, L.D.; Schneider, Y.; Pussemier, L. Current knowledge in species-related bioavailability of selenium in food. Food Chem. 2012, 130, 767–784. [Google Scholar]
- Lu, J.; Qu, L.; Shen, M.M.; Wang, X.G.; Guo, J.; Hu, Y.P.; Dou, T.C.; Wang, K.H. Effects of high-dose selenium-enriched yeast on laying performance, egg quality, clinical blood parameters, organ development, and selenium deposition in laying hens. Poult. Sci. 2019, 98, 2522–2530. [Google Scholar] [CrossRef]
- Pan, C.; Zhao, Y.; Liao, S.F.; Chen, F.; Qin, S.; Wu, X.; Zhou, H.; Huang, K. Effect of selenium-enriched probiotics on laying performance, egg quality, egg selenium content, and egg glutathione peroxidase activity. J. Agric. Food Chem. 2011, 59, 11424–11431. [Google Scholar] [CrossRef]
- Zhao, M.; Sun, Q.; Khogali, M.K.; Liu, L.; Geng, T.; Yu, L.; Gong, D. Dietary selenized glucose increases selenium concentration and antioxidant capacity of the liver, oviduct, and spleen in laying hens. Biol. Trace Elem. Res. 2021, 199, 4746–4752. [Google Scholar] [CrossRef]
- Bao, T.; Yao, J.; Zhou, S.; Ma, Y.; Dong, J.; Zhang, C.; Mi, Y. Naringin prevents follicular atresia by inhibiting oxidative stress in the aging chicken. Poult. Sci. 2022, 101, 101891. [Google Scholar] [CrossRef]
- Liu, X.; Lin, X.; Mi, Y.; Li, J.; Zhang, C. Grape seed proanthocyanidin extract prevents ovarian aging by inhibiting oxidative stress in the hens. Oxid. Med. Cell Longev. 2018, 2018, 9390810. [Google Scholar] [CrossRef] [PubMed]
- Sai, T.; Goto, Y.; Yoshioka, R.; Maeda, A.; Matsuda, F.; Sugimoto, M.; Wongpanit, K.; Jin, H.Z.; Li, J.Y.; Manabe, N. Bid and Bax are involved in granulosa cell apoptosis during follicular atresia in porcine ovaries. J. Reprod. Dev. 2011, 57, 421–427. [Google Scholar] [CrossRef]
- Qiu, X.G.; Chen, Y.D.; Yuan, J.; Zhang, N.; Lei, T.; Liu, J.; Yang, M. Functional BCL-2 rs2279115 promoter noncoding variant contributes to glioma predisposition, especially in males. DNA. Cell Biol. 2019, 38, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Wan, N.; Xu, Z.; Liu, T.; Min, Y.; Li, S. Ameliorative effects of selenium on cadmium-induced injury in the chicken ovary: Mechanisms of oxidative stress and endoplasmic reticulum stress in cadmium-induced apoptosis. Biol. Trace Elem. Res. 2018, 184, 463–473. [Google Scholar] [CrossRef]
- Zhang, H.; Davies, K.J.A.; Forman, H.J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015, 88, 314–336. [Google Scholar] [CrossRef]
- Lim, J.; Nakamura, B.N.; Mohar, I.; Kavanagh, T.J.; Luderer, U. Glutamate cysteine ligase modifier subunit (Gclm) null mice have increased ovarian oxidative stress and accelerated age-related ovarian failure. Endocrinology 2015, 156, 3329–3343. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.W.; Lu, L.; Li, W.X.; Zhang, L.Y.; Ji, C.; Lin, X.; Liu, H.C.; Odle, J.; Luo, X.G. Effect of dietary manganese on antioxidant status and expressions of heat shock proteins and factors in tissues of laying broiler breeder under normal and high environmental temperatures. Br. J. Nutr. 2016, 116, 1851–1860. [Google Scholar] [CrossRef]
- Behne, D.; Kyiakopoulos, A. Mammalian selenium-containing proteins. Annu. Rev. Nutr. 2001, 21, 453–473. [Google Scholar] [CrossRef]
- Kim, Y.B.; Lee, S.H.; Kim, D.H.; Lee, K.W. Effects of dietary methyl sulfonyl methane and selenium on laying performance, egg quality, gut health indicators, and antioxidant capacity of laying hens. Anim. Biosci. 2022, 35, 1566–1574. [Google Scholar] [CrossRef]
- Jing, C.L.; Dong, X.F.; Wang, Z.M.; Liu, S.; Tong, J.M. Comparative study of DL-selenomethionine vs sodium selenite and seleno-yeast on antioxidant activity and selenium status in laying hens. Poult. Sci. 2015, 94, 965–975. [Google Scholar] [CrossRef]
- Qazi, I.H.; Angel, C.; Yang, H.; Pan, B.; Zoidis, E.; Zeng, C.J.; Han, H.; Zhou, G.B. Selenium, selenoproteins, and female reproduction: A review. Molecules 2018, 23, 3053. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Dai, S.; Li, J.; Wen, A.; Bai, X. Glutamine improves heat stress-induced oxidative damage in the broiler thigh muscle by activating the nuclear factor erythroid 2-related 2/Kelch-like ECH-associated protein 1 signaling pathway. Poult. Sci. 2020, 99, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell Mol. Life. Sci. 2016, 73, 3221–3247. [Google Scholar]
- Baird, L.; Dinkova-Kostova, A.T. The cytoprotective role of the Keap1-Nfr2 pathway. Arch. Toxicol. 2011, 85, 241–272. [Google Scholar] [CrossRef]
- Reszka, E.; Wieczorek, E.; Jablonska, E.; Janasik, B.; Fendler, W.; Wasowicz, W. Association between plasma selenium level and NRF2 target genes expression in humans. J. Trace Elem. Med. Biol. 2015, 30, 102–106. [Google Scholar] [CrossRef] [PubMed]

| Ingredients | Nutrient Levels 2 | |||
|---|---|---|---|---|
| Corn | 63.25 | Metabolizable energy (MJ/kg) | 11.62 | |
| Soybean meal | 25.45 | Crude protein | 16.31 | |
| Limestone | 8.00 | Calcium | 3.66 | |
| Calcium hydrogen phosphate | 1.50 | Available phosphorus | 0.41 | |
| Sodium chloride | 0.30 | Lysine | 0.35 | |
| Premix 1 | 0.50 | Selenium 3 (mg/kg) | 0.156 | |
| DL-methionine | 0.10 |
| Gene | Forward (5′-3′) | Reverse (5′-3′) | Size (bp) | Accession Number |
|---|---|---|---|---|
| β-actin | GAGAAATTGTGCGTGACATCA | CCTGAACCTCTCATTGCCA | 152 | NM_205518.2 |
| Nrf2 | CTGCTAGTGGATGGCGAGAC | CTCCGAGTTCTCCCCGAAAG | 132 | NM_001030756.1 |
| Keap1 | GGTTACGATGGGACGGATCA | CACGTAGATCTTGCCCTGGT | 135 | XM_025145847.1 |
| HO-1 | AGCTTCGCACAAGGAGTGTT | GGAGAGGTGGTCAGCATGTC | 106 | NM_205344.1 |
| NQO1 | TCGCCGAGCAGAAGAAGATTGAAG | GGTGGTGAGTGACAGCATGGC | 191 | NM_001277619.1 |
| GPX1 | AGTACATCATCTGGTCGCCG | CTCGATGTCGTCCTGCAGTT | 137 | NM_001277853.1 |
| Selenof | GAGAACCTTGACTGCTAAC | CACACCTGACATCTGACTA | 208 | NM_001012926.3 |
| GPX4 | ATCCTTACGTGATCGAGAAG | GTGGACAGCAGATACTACAT | 249 | NM_204220.3 |
| Bax | ATCAATGCAGAGGACCAGGTG | CGTACCGCTTGTTGATGTCG | 208 | NM_001030920.1 |
| Bcl-2 | GATGACCGAGTACCTGAACC | CAGGAGAAATCGAACAAAGGC | 114 | NM_205339.2 |
| Caspase 3 | GAGTTGGAGATGTCCGTTAT | AGTAGGTCTTGTAAGTGATGG | 159 | XM_046915476.1 |
| Item | Treatments (T) 2 | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CON | SS | SEY | SEC | SEC + SEY | T | Time | T × Time 2 | ||
| Laying rate (%) | |||||||||
| 1–28d | 88.60 | 90.88 | 90.94 | 93.14 | 89.74 | 0.604 | 0.074 | 0.474 | 0.105 |
| 28–56d | 89.60 | 90.10 | 87.18 | 93.85 | 90.16 | 0.734 | |||
| 1–56d | 89.09 | 90.46 | 89.03 | 93.68 | 90.03 | 0.568 | |||
| Average egg weight (g) | |||||||||
| 1–28d | 64.93 | 64.56 | 65.33 | 65.33 | 65.53 | 0.220 | 0.709 | 0.004 | 0.538 |
| 28–56d | 65.53 | 65.07 | 65.53 | 65.97 | 65.89 | 0.227 | |||
| 1–56d | 65.22 | 64.79 | 65.32 | 65.62 | 65.70 | 0.214 | |||
| Average daily egg mass (g/bird per day) | |||||||||
| 1–28d | 59.53 | 58.74 | 59.74 | 61.34 | 59.30 | 0.418 | 0.303 | 0.023 | 0.149 |
| 28–56d | 59.43 | 58.60 | 57.01 | 61.16 | 57.79 | 0.560 | |||
| 1–56d | 59.47 | 58.69 | 58.41 | 61.28 | 58.49 | 0.465 | |||
| ADFI (g/day/bird) | |||||||||
| 1–28d | 132.27 | 129.71 | 137.82 | 130.82 | 126.51 | 2.641 | 0.402 | 0.020 | 0.777 |
| 28–56d | 123.29 | 117.58 | 129.21 | 127.73 | 122.02 | 2.120 | |||
| 1–56d | 127.36 | 122.62 | 133.76 | 128.91 | 124.39 | 1.700 | |||
| FCR (g of feed/g of egg) | |||||||||
| 1–28d | 2.32 a | 2.18 ab | 2.19 ab | 2.02 b | 2.03 ab | 0.036 | 0.021 | 0.482 | 0.746 |
| 28–56d | 2.28 | 2.03 | 2.19 | 2.04 | 2.03 | 0.040 | |||
| 1–56d | 2.26 a | 2.07 b | 2.19 ab | 2.03 b | 2.03 b | 0.028 | |||
| Item | Treatment (T) 2 | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CON | SS | SEY | SEC | SEC + SEY | T | Time | T × Time 2 | ||
| Egg shape index | |||||||||
| 28d | 1.32 | 1.34 | 1.33 | 1.33 | 1.32 | 0.004 | 0.336 | 0.128 | 0.640 |
| 56d | 1.35 | 1.35 | 1.33 | 1.34 | 1.34 | 0.005 | |||
| Eggshell strength, N | |||||||||
| 28d | 38.13 | 41.13 | 40.63 | 43.29 | 40.51 | 0.722 | 0.045 | 0.151 | 0.169 |
| 56d | 40.39 ab | 44.66 a | 39.67 b | 43.01 a | 41.02 ab | 0.640 | |||
| Albumen height, mm | |||||||||
| 28d | 6.11 | 6.18 | 6.31 | 6.94 | 6.58 | 0.110 | 0.321 | 0.439 | 0.723 |
| 56d | 6.11 | 6.26 | 6.19 | 6.21 | 6.20 | 0.076 | |||
| Egg yolk color | |||||||||
| 28d | 6.57 | 5.96 | 6.59 | 6.30 | 6.61 | 0.098 | 0.152 | 0.332 | 0.286 |
| 56d | 6.00 | 6.48 | 6.29 | 5.74 | 6.54 | 0.120 | |||
| Egg yolk index | |||||||||
| 28d | 0.35 | 0.35 | 0.35 | 0.35 | 0.38 | 0.003 | 0.801 | 0.001 | 0.266 |
| 56d | 0.38 | 0.39 | 0.38 | 0.39 | 0.38 | 0.004 | |||
| Yolk percentage | |||||||||
| 28d | 26.15 | 26.09 | 26.14 | 27.23 | 26.25 | 0.171 | 0.660 | 0.001 | 0.127 |
| 56d | 28.58 | 28.17 | 28.10 | 27.70 | 28.38 | 0.190 | |||
| Haugh unit | |||||||||
| 28d | 70.27 b | 77.92 a | 75.05 ab | 79.78 a | 77.92 a | 1.050 | 0.017 | 0.819 | 0.161 |
| 56d | 75.07 | 76.23 | 74.39 | 76.56 | 76.55 | 0.567 | |||
| Item | CON | SS | SEY | SEC | SEC + SEY | SEM | p-Value |
|---|---|---|---|---|---|---|---|
| POFs (n) | 5.75 | 6.25 | 6.00 | 6.29 | 6.13 | 0.132 | 0.724 |
| SYFs (n) | 10.13 | 10.25 | 11.71 | 12.14 | 11.25 | 0.691 | 0.874 |
| LWFs (n) | 35.50 b | 41.73 ab | 38.00 ab | 49.20 a | 43.00 ab | 1.545 | 0.048 |
| Ovary index (%) | 2.18 | 2.19 | 2.24 | 2.29 | 2.05 | 0.041 | 0.443 |
| Item | CON | SS | SEY | SEC | SEC + SEY | SEM | p-Value |
|---|---|---|---|---|---|---|---|
| T-AOC (mmol/g prot) | 0.12 b | 0.22 a | 0.22 a | 0.22 a | 0.24 a | 0.010 | <0.001 |
| GSH-PX (U/mg prot) | 675.46 | 812.03 | 742.60 | 719.05 | 884.68 | 26.726 | 0.094 |
| MDA (nmol/mg prot) | 0.71 a | 0.66 ab | 0.52 b | 0.50 b | 0.60 ab | 0.026 | 0.046 |
| T-SOD (U/mg prot) | 764.46 | 800.35 | 836.41 | 798.87 | 891.86 | 23.070 | 0.469 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Cong, X.; Qin, K.; Yan, M.; Xu, X.; Liu, M.; Xu, X.; Zhang, Y.; Gao, Q.; Cheng, S.; et al. Se-Enriched Cardamine violifolia Improves Laying Performance and Regulates Ovarian Antioxidative Function in Aging Laying Hens. Antioxidants 2023, 12, 450. https://doi.org/10.3390/antiox12020450
Wang H, Cong X, Qin K, Yan M, Xu X, Liu M, Xu X, Zhang Y, Gao Q, Cheng S, et al. Se-Enriched Cardamine violifolia Improves Laying Performance and Regulates Ovarian Antioxidative Function in Aging Laying Hens. Antioxidants. 2023; 12(2):450. https://doi.org/10.3390/antiox12020450
Chicago/Turabian StyleWang, Hui, Xin Cong, Kun Qin, Mengke Yan, Xianfeng Xu, Mingkang Liu, Xiao Xu, Yue Zhang, Qingyu Gao, Shuiyuan Cheng, and et al. 2023. "Se-Enriched Cardamine violifolia Improves Laying Performance and Regulates Ovarian Antioxidative Function in Aging Laying Hens" Antioxidants 12, no. 2: 450. https://doi.org/10.3390/antiox12020450
APA StyleWang, H., Cong, X., Qin, K., Yan, M., Xu, X., Liu, M., Xu, X., Zhang, Y., Gao, Q., Cheng, S., Zhao, J., Zhu, H., & Liu, Y. (2023). Se-Enriched Cardamine violifolia Improves Laying Performance and Regulates Ovarian Antioxidative Function in Aging Laying Hens. Antioxidants, 12(2), 450. https://doi.org/10.3390/antiox12020450








