Electronegative LDL Is Associated with Plaque Vulnerability in Patients with Ischemic Stroke and Carotid Atherosclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Carotid Plaque Imaging
2.3.1. Carotid Ultrasound Protocol
2.3.2. Carotid 18F-FDG PET/CT Protocol
2.4. Collection of Blood Samples
2.5. Serum Determinations
2.6. LDL(−) Quantification
2.7. Effect of Lipoproteins on Cells
2.7.1. ChE Capacity of HDL
2.7.2. Inflammatory Effect of LDL(−) on Cells
2.8. Outcomes
2.9. Statistical Analysis
3. Results
3.1. Study Population
3.2. Lipid Profile and Lipoprotein-Related Molecules
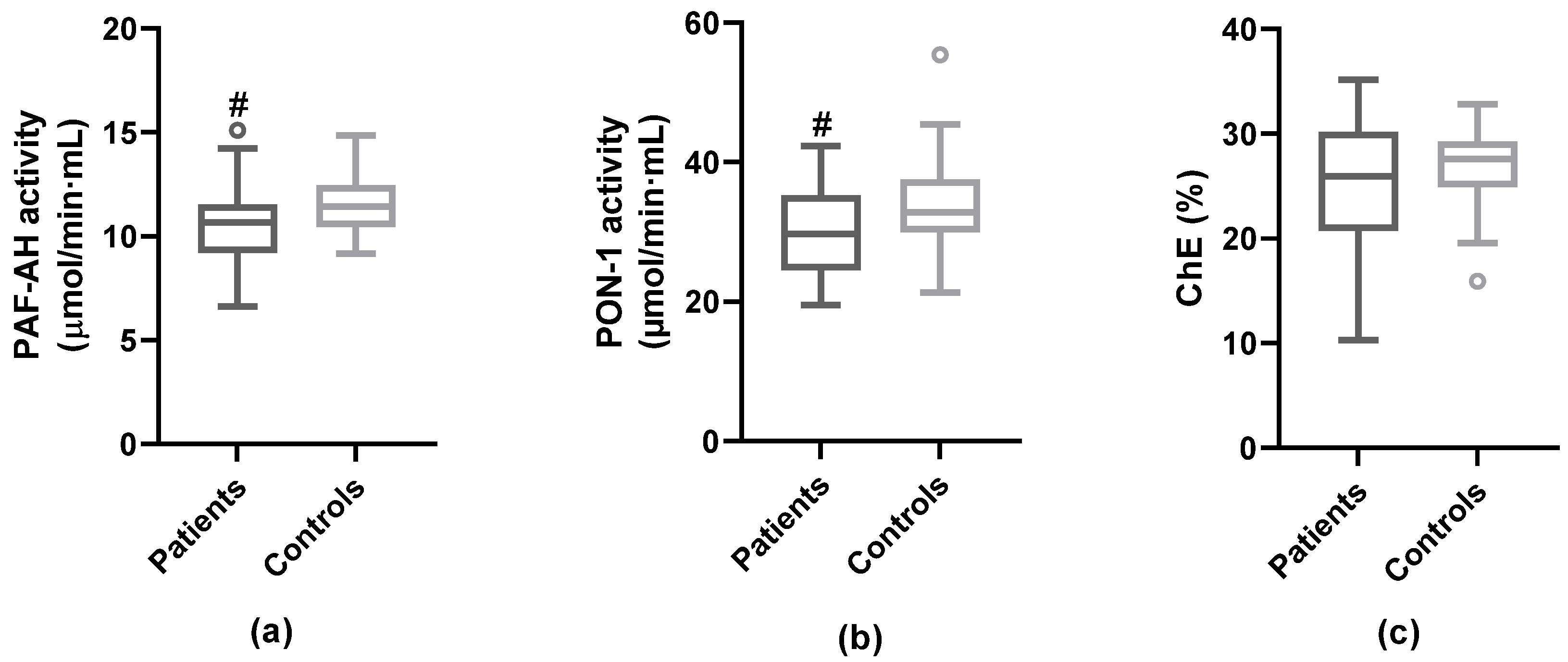
3.3. Anti-Atherogenic Properties of HDL
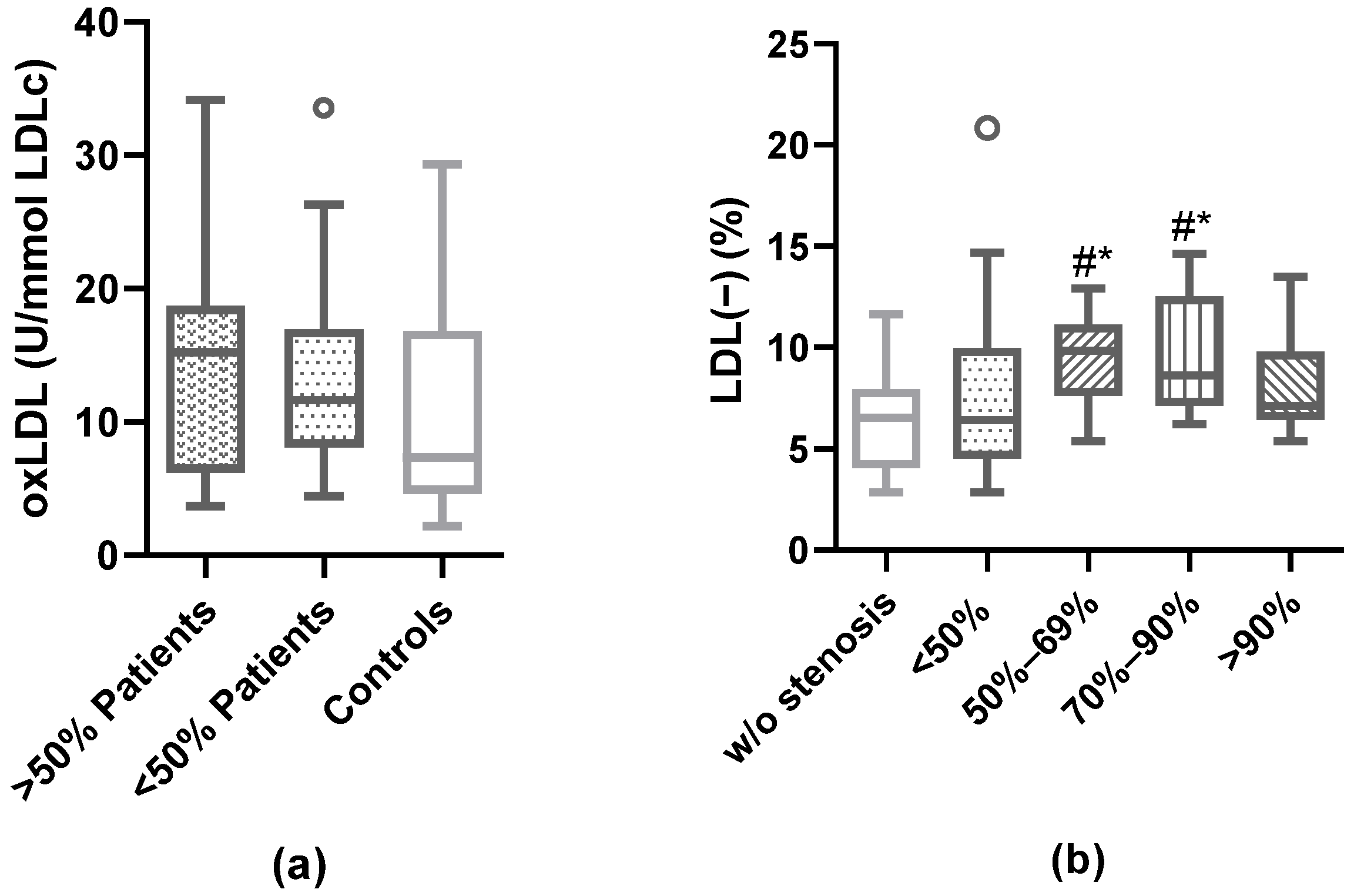
3.4. Modified LDL: oxLDL and LDL(−)
3.5. Association of Plaque Characteristics with Modified LDL
3.6. Prediction of Carotid Plaque Vulnerability According to the Plasma Proportion of LDL(−)
3.7. LDL(−) as an Inductor of Inflammation in Cultured Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonati, L.H.; Kakkos, S.; Berkefeld, J.; de Borst, G.J.; Bulbulia, R.; Halliday, A.; van Herzeele, I.; Koncar, I.; McCabe, D.J.; Lal, A.; et al. European Stroke Organisation guideline on endarterectomy and stenting for carotid artery stenosis. Eur. Stroke J. 2021, 6, I–XLVII. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.M. ACST: Which subgroups will benefit most from carotid endarterectomy? Lancet 2004, 364, 1122–1123, author reply 1125–1126. [Google Scholar] [CrossRef] [PubMed]
- Camps-Renom, P.; Prats-Sánchez, L.; Casoni, F.; González-de-Echávarri, J.M.; Marrero-González, P.; Castrillón, I.; Marín, R.; Jiménez-Xarrié, E.; Delgado-Mederos, R.; Martínez-Domeño, A.; et al. Plaque neovascularization detected with contrast-enhanced ultrasound predicts ischaemic stroke recurrence in patients with carotid atherosclerosis. Eur. J. Neurol. 2020, 27, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.J.; Camps-Renom, P.; Giannotti, N.; Martí-Fàbregas, J.; Murphy, S.; McNulty, J.; Barry, M.; Barry, P.; Calvet, D.; Coutts, S.B.; et al. Carotid Plaque Inflammation Imaged by 18F-Fluorodeoxyglucose Positron Emission Tomography and Risk of Early Recurrent Stroke. Stroke 2019, 50, 1766–1773. [Google Scholar] [CrossRef] [PubMed]
- Puig, N.; Camps-Renom, P.; Camacho, M.; Aguilera-Simón, A.; Jiménez-Altayó, F.; Fernández-León, A.; Marín, R.; Martí-Fàbregas, J.; Sánchez-Quesada, J.L.; Jiménez-Xarrié, E.; et al. Plasma sICAM-1 as a Biomarker of Carotid Plaque Inflammation in Patients with a Recent Ischemic Stroke. Transl. Stroke Res. 2022, 13, 745–756. [Google Scholar] [CrossRef]
- Kamtchum-Tatuene, J.; Saba, L.; Heldner, M.R.; Poorthuis, M.H.F.; de Borst, G.J.; Rundek, T.; Kakkos, S.K.; Chaturvedi, S.; Topakian, R.; Polak, J.F.; et al. Interleukin-6 Predicts Carotid Plaque Severity, Vulnerability, and Progression. Circ. Res. 2022, 131, e22–e33. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.J.; Tabas, I. The response-to-retention hypothesis of early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Hindy, G.; Engström, G.; Larsson, S.C.; Traylor, M.; Markus, H.S.; Melander, O.; Orho-Melander, M. Stroke Genetics Network (SiGN) Role of Blood Lipids in the Development of Ischemic Stroke and its Subtypes: A Mendelian Randomization Study. Stroke 2018, 49, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Amarenco, P.; Labreuche, J.; Elbaz, A.; Touboul, P.-J.; Driss, F.; Jaillard, A.; Bruckert, E. GENIC Investigators Blood Lipids in Brain Infarction Subtypes. Cerebrovasc. Dis. 2006, 22, 101–108. [Google Scholar] [CrossRef]
- Rader, D.J.; Hovingh, G.K. HDL and cardiovascular disease. Lancet 2014, 384, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Pan, Y.; Wang, M.; Meng, X.; Jiang, Y.; Li, Z.; Li, H.; Wang, Y.; Wang, Y. Inverse Association between High-Density Lipoprotein Cholesterol and Adverse Outcomes among Acute Ischemic Stroke Patients with Diabetes Mellitus. Biomedicines 2021, 9, 1947. [Google Scholar] [CrossRef] [PubMed]
- Nishi, K.; Itabe, H.; Uno, M.; Kitazato, K.T.; Horiguchi, H.; Shinno, K.; Nagahiro, S. Oxidized LDL in carotid plaques and plasma associates with plaque instability. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Lehti, S.; Nguyen, S.D.; Belevich, I.; Vihinen, H.; Heikkilä, H.M.; Soliymani, R.; Käkelä, R.; Saksi, J.; Jauhiainen, M.; Grabowski, G.A.; et al. Extracellular Lipids Accumulate in Human Carotid Arteries as Distinct Three-Dimensional Structures and Have Proinflammatory Properties. Am. J. Pathol. 2018, 188, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Kubo, T.; Okumoto, Y.; Ishibashi, K.; Komukai, K.; Tanimoto, T.; Ino, Y.; Kitabata, H.; Hirata, K.; Imanishi, T.; et al. Circulating malondialdehyde-modified low-density lipoprotein levels are associated with the presence of thin-cap fibroatheromas determined by optical coherence tomography in coronary artery disease. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Yang, Y.; Su, Z.; Yue, W.; Hao, H.; Ren, L.; Wang, Y.; Cao, Y.; Wang, Y. Association of Oxidized Low-Density Lipoprotein With Prognosis of Stroke and Stroke Subtypes. Stroke 2017, 48, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Dai, L.; Zhang, N.; Lin, J.; Chen, G.; Zuo, Y.; Li, H.; Wang, Y.; Meng, X.; Wang, Y. Oxidized low-density lipoprotein (LDL) and LDL cholesterol are associated with outcomes of minor stroke and TIA. Atherosclerosis 2020, 297, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-C.; Chang, P.-Y.; Lu, S.-C. L5-LDL from ST-elevation myocardial infarction patients induces IL-1β production via LOX-1 and NLRP3 inflammasome activation in macrophages. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H265–H274. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.-Y.; Chen, F.-Y.; Hsu, J.-F.; Fu, R.-H.; Chang, C.-M.; Chang, C.-T.; Liu, C.-H.; Wu, J.-R.; Lee, A.-S.; Chan, H.-C.; et al. Plasma L5 levels are elevated in ischemic stroke patients and enhance platelet aggregation. Blood 2016, 127, 1336–1345. [Google Scholar] [CrossRef]
- Estruch, M.; Sánchez-Quesada, J.L.; Ordóñez Llanos, J.; Benítez, S. Electronegative LDL: A circulating modified LDL with a role in inflammation. Mediators Inflamm. 2013, 2013, 181324. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Chen, C.-H.; Chen, Y.-M.; Hsieh, T.-Y.; Li, J.-P.; Shen, M.-Y.; Lan, J.-L.; Chen, D.-Y. Association between Negatively Charged Low-Density Lipoprotein L5 and Subclinical Atherosclerosis in Rheumatoid Arthritis Patients. J. Clin. Med. 2019, 8, E177. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, A.; Khera, A.; Berry, J.D.; Givens, E.G.; Ayers, C.R.; Wedin, K.E.; Neeland, I.J.; Yuhanna, I.S.; Rader, D.R.; de Lemos, J.A.; et al. HDL cholesterol efflux capacity and incident cardiovascular events. N. Engl. J. Med. 2014, 371, 2383–2393. [Google Scholar] [CrossRef] [PubMed]
- Rader, D.J.; Alexander, E.T.; Weibel, G.L.; Billheimer, J.; Rothblat, G.H. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J. Lipid Res. 2009, 50, S189–S194. [Google Scholar] [CrossRef] [PubMed]
- Navab, M.; Berliner, J.A.; Subbanagounder, G.; Hama, S.; Lusis, A.J.; Castellani, L.W.; Reddy, S.; Shih, D.; Shi, W.; Watson, A.D.; et al. HDL and the inflammatory response induced by LDL-derived oxidized phospholipids. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Mackness, M.I.; Durrington, P.N.; Mackness, B. The role of paraoxonase 1 activity in cardiovascular disease: Potential for therapeutic intervention. Am. J. Cardiovasc. Drugs Drugs Devices Interv. 2004, 4, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Kontush, A.; Chapman, M.J. Antiatherogenic small, dense HDL—Guardian angel of the arterial wall? Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, 144–153. [Google Scholar] [CrossRef]
- Bartlett, E.S.; Walters, T.D.; Symons, S.P.; Fox, A.J. Quantification of carotid stenosis on CT angiography. AJNR Am. J. Neuroradiol. 2006, 27, 13–19. [Google Scholar]
- von Reutern, G.-M.; Goertler, M.-W.; Bornstein, N.M.; Del Sette, M.; Evans, D.H.; Hetzel, A.; Kaps, M.; Perren, F.; Razumovky, A.; von Reutern, M.; et al. Grading carotid stenosis using ultrasonic methods. Stroke 2012, 43, 916–921. [Google Scholar] [CrossRef]
- Adams, H.P.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef]
- Norris, S.L.; Grothaus, L.C.; Buchner, D.M.; Pratt, M. Effectiveness of physician-based assessment and counseling for exercise in a staff model HMO. Prev. Med. 2000, 30, 513–523. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; García-Arellano, A.; Toledo, E.; Salas-Salvadó, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schröder, H.; Arós, F.; Gómez-Gracia, E.; et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: The PREDIMED trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef]
- Kernan, W.N.; Ovbiagele, B.; Black, H.R.; Bravata, D.M.; Chimowitz, M.I.; Ezekowitz, M.D.; Fang, M.C.; Fisher, M.; Furie, K.L.; Heck, D.V.; et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014, 45, 2160–2236. [Google Scholar] [CrossRef] [PubMed]
- Geroulakos, G.; Ramaswami, G.; Nicolaides, A.; James, K.; Labropoulos, N.; Belcaro, G.; Holloway, M. Characterization of symptomatic and asymptomatic carotid plaques using high-resolution real-time ultrasonography. Br. J. Surg. 1993, 80, 1274–1277. [Google Scholar] [CrossRef] [PubMed]
- Deyama, J.; Nakamura, T.; Takishima, I.; Fujioka, D.; Kawabata, K.; Obata, J.; Watanabe, K.; Watanabe, Y.; Saito, Y.; Mishina, H.; et al. Contrast-enhanced ultrasound imaging of carotid plaque neovascularization is useful for identifying high-risk patients with coronary artery disease. Circ. J. Off. J. Jpn. Circ. Soc. 2013, 77, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Benítez, S.; Sánchez-Quesada, J.L.; Ribas, V.; Jorba, O.; Blanco-Vaca, F.; González-Sastre, F.; Ordóñez-Llanos, J. Platelet-activating factor acetylhydrolase is mainly associated with electronegative low-density lipoprotein subfraction. Circulation 2003, 108, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Ribas, V.; Sánchez-Quesada, J.L.; Antón, R.; Camacho, M.; Julve, J.; Escolà-Gil, J.C.; Vila, L.; Ordóñez-Llanos, J.; Blanco-Vaca, F. Human apolipoprotein A-II enrichment displaces paraoxonase from HDL and impairs its antioxidant properties: A new mechanism linking HDL protein composition and antiatherogenic potential. Circ. Res. 2004, 95, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Quesada, J.L.; Vinagre, I.; De Juan-Franco, E.; Sánchez-Hernández, J.; Bonet-Marques, R.; Blanco-Vaca, F.; Ordóñez-Llanos, J.; Pérez, A. Impact of the LDL subfraction phenotype on Lp-PLA2 distribution, LDL modification and HDL composition in type 2 diabetes. Cardiovasc. Diabetol. 2013, 12, 112. [Google Scholar] [CrossRef]
- Benítez, S.; Sánchez-Quesada, J.L.; Lucero, L.; Arcelus, R.; Ribas, V.; Jorba, O.; Castellví, A.; Alonso, E.; Blanco-Vaca, F.; Ordóñez-Llanos, J. Changes in low-density lipoprotein electronegativity and oxidizability after aerobic exercise are related to the increase in associated non-esterified fatty acids. Atherosclerosis 2002, 160, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Cedó, L.; Fernández-Castillejo, S.; Rubió, L.; Metso, J.; Santos, D.; Muñoz-Aguayo, D.; Rivas-Urbina, A.; Tondo, M.; Méndez-Lara, K.A.; Farràs, M.; et al. Phenol-Enriched Virgin Olive Oil Promotes Macrophage-Specific Reverse Cholesterol Transport In Vivo. Biomedicines 2020, 8, E266. [Google Scholar] [CrossRef] [PubMed]
- Navab, M.; Hama-Levy, S.; Van Lenten, B.J.; Fonarow, G.C.; Cardinez, C.J.; Castellani, L.W.; Brennan, M.-L.; Lusis, A.J.; Fogelman, A.M. Mildly oxidized LDL induces an increased apolipoprotein J/paraoxonase ratio. J. Clin. Investig. 1997, 99, 2005–2019. [Google Scholar] [CrossRef]
- Cubedo, J.; Padró, T.; Vilahur, G.; Crea, F.; Storey, R.F.; Lopez Sendon, J.L.; Kaski, J.C.; Sionis, A.; Sans-Rosello, J.; Fernández-Peregrina, E.; et al. Glycosylated apolipoprotein J in cardiac ischaemia: Molecular processing and circulating levels in patients with acute ischaemic events. Eur. Heart J. 2022, 43, 153–163. [Google Scholar] [CrossRef]
- Zhang, B.; Matsunaga, A.; Rainwater, D.L.; Miura, S.-I.; Noda, K.; Nishikawa, H.; Uehara, Y.; Shirai, K.; Ogawa, M.; Saku, K. Effects of rosuvastatin on electronegative LDL as characterized by capillary isotachophoresis: The ROSARY Study. J. Lipid Res. 2009, 50, 1832–1841. [Google Scholar] [CrossRef] [PubMed]
- Benítez, S.; Ordóñez-Llanos, J.; Franco, M.; Marín, C.; Paz, E.; López-Miranda, J.; Otal, C.; Pérez-Jiménez, F.; Sánchez-Quesada, J.L. Effect of simvastatin in familial hypercholesterolemia on the affinity of electronegative low-density lipoprotein subfractions to the low-density lipoprotein receptor. Am. J. Cardiol. 2004, 93, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-S.; Ke, L.-Y.; Chan, H.-C.; Chan, H.-C.; Chen, C.-C.; Cheng, K.-H.; Lee, H.-C.; Kuo, H.-F.; Chang, C.-T.; Chang, K.-C.; et al. Four Statin Benefit Groups Defined by The 2013 ACC/AHA New Cholesterol Guideline are Characterized by Increased Plasma Level of Electronegative Low-Density Lipoprotein. Acta Cardiol. Sin. 2016, 32, 667–675. [Google Scholar] [CrossRef]
- Puig, N.; Montolio, L.; Camps-Renom, P.; Navarra, L.; Jiménez-Altayó, F.; Jiménez-Xarrié, E.; Sánchez-Quesada, J.L.; Benitez, S. Electronegative LDL Promotes Inflammation and Triglyceride Accumulation in Macrophages. Cells 2020, 9, 583. [Google Scholar] [CrossRef] [PubMed]
- De Castellarnau, C.; Sánchez-Quesada, J.L.; Benítez, S.; Rosa, R.; Caveda, L.; Vila, L.; Ordóñez-Llanos, J. Electronegative LDL from normolipemic subjects induces IL-8 and monocyte chemotactic protein secretion by human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2281–2287. [Google Scholar] [CrossRef]
- Bancells, C.; Benítez, S.; Jauhiainen, M.; Ordóñez-Llanos, J.; Kovanen, P.T.; Villegas, S.; Sánchez-Quesada, J.L.; Öörni, K. High binding affinity of electronegative LDL to human aortic proteoglycans depends on its aggregation level. J. Lipid Res. 2009, 50, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Ligi, D.; Benitez, S.; Croce, L.; Rivas-Urbina, A.; Puig, N.; Ordóñez-Llanos, J.; Mannello, F.; Sanchez-Quesada, J.L. Electronegative LDL induces MMP-9 and TIMP-1 release in monocytes through CD14 activation: Inhibitory effect of glycosaminoglycan sulodexide. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3559–3567. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Quesada, J.L.; Camacho, M.; Antón, R.; Benítez, S.; Vila, L.; Ordóñez-Llanos, J. Electronegative LDL of FH subjects: Chemical characterization and induction of chemokine release from human endothelial cells. Atherosclerosis 2003, 166, 261–270. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Lee, A.-S.; Lu, L.-S.; Ke, L.-Y.; Chen, W.-Y.; Dong, J.-W.; Lu, J.; Chen, Z.; Chu, C.-S.; Chan, H.-C.; et al. Human electronegative LDL induces mitochondrial dysfunction and premature senescence of vascular cells in vivo. Aging Cell 2018, 17, e12792. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Cui, R.; Chen, C.-H.; Du, J. Oxidized low-density lipoprotein stimulates p53-dependent activation of proapoptotic Bax leading to apoptosis of differentiated endothelial progenitor cells. Endocrinology 2007, 148, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yang, J.-H.; Burns, A.R.; Chen, H.-H.; Tang, D.; Walterscheid, J.P.; Suzuki, S.; Yang, C.-Y.; Sawamura, T.; Chen, C.-H. Mediation of electronegative low-density lipoprotein signaling by LOX-1: A possible mechanism of endothelial apoptosis. Circ. Res. 2009, 104, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-T.; Wang, G.-J.; Kuo, C.-C.; Hsieh, J.-Y.; Lee, A.-S.; Chang, C.-M.; Wang, C.-C.; Shen, M.-Y.; Huang, C.-C.; Sawamura, T.; et al. Electronegative Low-density Lipoprotein Increases Coronary Artery Disease Risk in Uremia Patients on Maintenance Hemodialysis. Medicine 2016, 95, e2265. [Google Scholar] [CrossRef] [PubMed]
- Puertas-Umbert, L.; Puig, N.; Camacho, M.; Dantas, A.P.; Marín, R.; Martí-Fàbregas, J.; Jiménez-Xarrié, E.; Benitez, S.; Camps-Renom, P.; Jiménez-Altayó, F. Serum from Stroke Patients with High-Grade Carotid Stenosis Promotes Cyclooxygenase-Dependent Endothelial Dysfunction in Non-ischemic Mice Carotid Arteries. Transl. Stroke Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Gho, Y.S.; Kleinman, H.K.; Sosne, G. Angiogenic activity of human soluble intercellular adhesion molecule-1. Cancer Res. 1999, 59, 5128–5132. [Google Scholar] [PubMed]
- Koch, A.E.; Halloran, M.M.; Haskell, C.J.; Shah, M.R.; Polverini, P.J. Angiogenesis mediated by soluble forms of E-selectin and vascular cell adhesion molecule-1. Nature 1995, 376, 517–519. [Google Scholar] [CrossRef]
- Lee, S.-J.; Namkoong, S.; Kim, Y.-M.; Kim, C.-K.; Lee, H.; Ha, K.-S.; Chung, H.-T.; Kwon, Y.-G.; Kim, Y.-M. Fractalkine stimulates angiogenesis by activating the Raf-1/MEK/ERK- and PI3K/Akt/eNOS-dependent signal pathways. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H2836–H2846. [Google Scholar] [CrossRef]




| <50% Group (n = 27) | ≥50% Group (n = 37) | p | All Stroke Patients (n = 64) | Control Group (n = 27) | p | |
|---|---|---|---|---|---|---|
| Triglycerides (mM), md (IQR) | 1.11 (0.88–1.53) | 1.20 (0.94–1.63) | 0.601 | 1.17 (0.92–1.61) | 1.18 (0.90–1.45) | 0.212 |
| Total cholesterol (mM), m ± sd/md (IQR) | 3.80 ± 1.03 | 3.95 ± 1.186 | 0.588 | 3.75 (2.91–4.65) | 4.64 (4.00–5.88) | <0.001 |
| VLDLc (mM), md (IQR) | 0.22 (0.18–0.31) | 0.24 (0.19–0.33) | 0.601 | 0.23 (0.18–0.32) | 0.24 (0.18–0.29) | 0.524 |
| LDLc (mM), md (IQR) | 2.48 (1.76–3.48) | 2.31 (1.88–3.20) | 0.949 | 2.41 (1.82–3.29) | 3.09 (2.58–3.91) | <0.001 |
| HDLc (mM), m ± sd/md (IQR) | 1.07 ± 0.37 | 1.02 ± 0.28 | 0.541 | 1.03 (0.80–1.32) | 1.34 (1.15–1.59) | <0.001 |
| LDLc/HDLc ratio, md (IQR) | 2.42 (1.85–2.93) | 2.57 (1.98–3.11) | 0.510 | 2.48 (1.92–3.03) | 2.25 (1.98–2.80) | 0.507 |
| NEFA (mM), md (IQR) | 0.47 (0.30–0.69) | 0.38 (0.26–0.57) | 0.404 | 0.42 (0.27–0.61) | 0.43 (0.24–0.48) | 0.621 |
| apoB (g/L), md (IQR) | 0.68 (0.56–0.90) | 0.66 (0.60–0.80) | 0.826 | 0.68 (0.58–0.83) | 0.91 (0.78–1.02) | <0.001 |
| apoA-I (g/L), md (IQR) | 1.22 (1.14–1.50) | 1.22 (1.10–1.47) | 0.703 | 1.22 (1.10–1.47) | 1.62 (1.44–1.87) | <0.001 |
| apoA-II (g/L), m ± sd | 0.30 ± 0.07 | 0.28 ± 0.08 | 0.468 | 0.30 ± 0.08 | 0.37 ± 0.08 | <0.001 |
| apoE (g/L), m ± sd | 0.04 ± 0.02 | 0.04 ± 0.02 | 0.978 | 0.04 ± 0.02 | 0.05 ± 0.02 | 0.006 |
| apoC-III (g/L), md (IQR) | 0.05 (0.03–0.09) | 0.05 (0.01–0.11) | 0.713 | 0.05 (0.02–0.10) | 0.07 (0.03–0.11) | 0.144 |
| apoJ (mg/L), md (IQR) | 170 (153–219) | 180 (147–218) | 0.952 | 179 (148–218) | 145 (114–170) | <0.001 |
| Total PAF-AH activity (µmol/min*mL) m ± sd | 19.10 ± 3.33 | 19.07 ± 3.58 | 0.974 | 19.08 ± 3.41 | 21.49 ± 2.66 | 0.003 |
| Echolucency | Predominantly Hypoechoic (n = 19) | Predominantly Hyperechoic (n = 45) | p |
|---|---|---|---|
| LDL(−) (%), md (IQR) | 9.5 (7.1–11.7) | 7.4 (5.6–9.9) | 0.010 |
| oxLDL (U/mmol LDLc), md (IQR) | 15.7 (5.4–21.6) | 11.4 (8.2–17.1) | 0.734 |
| LDLc (mM), md (IQR) | 3.02 (1.85–3.32) | 2.33 (1.76–2.90) | 0.228 |
| Intraplaque neovascularization | Present (n = 28) | Absent (n = 9) | p |
| LDL(−) (%), md (IQR) | 7.9 (6.5–10.5) | 6.5 (3.8–7.6) | 0.047 |
| oxLDL (U/mmol LDLc), md (IQR) | 13.2 (7.0–17.1) | 12.3 (11.6–13.1) | 0.976 |
| LDLc (mM), md (IQR) | 2.35 (1.89–3.20) | 2.71 (2.01–3.70) | 0.481 |
| Diffuse intraplaque neovascularization | Present (n = 14) | Absent (n = 23) | p |
| LDL(−) (%), md (IQR) | 9.0 (7.1–11.7) | 7.0 (4.6–9.2) | 0.033 |
| oxLDL (U/mmol LDLc), md (IQR) | 15.5 (6.2–17.1) | 11.9 (8.8–14.5) | 0.569 |
| LDLc (mM), md (IQR) | 3.03 (1.90–3.44) | 2.40 (1.82–3.1) | 0.468 |
| Intraplaque inflammation | SUVmax ≥ 2.85 g/mL (n = 26) | SUVmax < 2.85 g/mL (n = 38) | p |
| LDL(−) (%), md (IQR) | 7.2 (5.4–10.9) | 8.0 (6.2–10.2) | 0.642 |
| oxLDL (U/mmol LDLc), md (IQR) | 15.9 (10.1–18.7) | 9.9 (7.0–16.9) | 0.127 |
| LDLc (mM), md (IQR) | 2.26 (1.89–3.18) | 2.52 (1.75–3.37) | 0.603 |
| OR | 95% CI | p | |
|---|---|---|---|
| (a) Plaque stenosis ≥50% | |||
| Proportion of LDL(−) (×1 increase in the logarithm) | 5.40 | 1.15–25.44 | 0.033 |
| Hypertension | 0.12 | 0.01–1.02 | 0.052 |
| (b) Predominantly hypoechoic plaque | |||
| Proportion of LDL(−) (×1 increase in the logarithm) | 7.52 | 1.26–44.83 | 0.027 |
| Prior statin therapy | 0.23 | 0.06–0.79 | 0.020 |
| (c) Diffuse intraplaque neovascularization | |||
| Proportion of LDL(−) (×1 increase in the logarithm) | 10.77 | 1.21–95.93 | 0.033 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puig, N.; Camps-Renom, P.; Solé, A.; Aguilera-Simón, A.; Jiménez-Xarrié, E.; Fernández-León, A.; Camacho, M.; Guasch-Jiménez, M.; Marin, R.; Martí-Fàbregas, J.; et al. Electronegative LDL Is Associated with Plaque Vulnerability in Patients with Ischemic Stroke and Carotid Atherosclerosis. Antioxidants 2023, 12, 438. https://doi.org/10.3390/antiox12020438
Puig N, Camps-Renom P, Solé A, Aguilera-Simón A, Jiménez-Xarrié E, Fernández-León A, Camacho M, Guasch-Jiménez M, Marin R, Martí-Fàbregas J, et al. Electronegative LDL Is Associated with Plaque Vulnerability in Patients with Ischemic Stroke and Carotid Atherosclerosis. Antioxidants. 2023; 12(2):438. https://doi.org/10.3390/antiox12020438
Chicago/Turabian StylePuig, Núria, Pol Camps-Renom, Arnau Solé, Ana Aguilera-Simón, Elena Jiménez-Xarrié, Alejandro Fernández-León, Mercedes Camacho, Marina Guasch-Jiménez, Rebeca Marin, Joan Martí-Fàbregas, and et al. 2023. "Electronegative LDL Is Associated with Plaque Vulnerability in Patients with Ischemic Stroke and Carotid Atherosclerosis" Antioxidants 12, no. 2: 438. https://doi.org/10.3390/antiox12020438
APA StylePuig, N., Camps-Renom, P., Solé, A., Aguilera-Simón, A., Jiménez-Xarrié, E., Fernández-León, A., Camacho, M., Guasch-Jiménez, M., Marin, R., Martí-Fàbregas, J., Martínez-Domeño, A., Prats-Sánchez, L., Casoni, F., Pérez, B., Jiménez-Altayó, F., Sánchez-Quesada, J. L., & Benitez, S. (2023). Electronegative LDL Is Associated with Plaque Vulnerability in Patients with Ischemic Stroke and Carotid Atherosclerosis. Antioxidants, 12(2), 438. https://doi.org/10.3390/antiox12020438









