Physicochemical Properties and In Vivo Hepatoprotective Effect of Polysaccharides from Grape Pomace
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Extraction and Purification of GPPs
2.3. Experimental Design for Optimization
2.4. Characterization of GPPs
2.4.1. Molecular Weight Determination
2.4.2. UV and Fourier Transform Infrared Spectrometry (FT-IR) Analyses
2.4.3. Determination of Monosaccharide Composition
2.4.4. SEM Analysis
2.5. Antioxidant Properties
2.5.1. DPPH Radical Scavenging Assay
2.5.2. ABTS Radical Scavenging Activity
2.5.3. Hydroxyl Radical Scavenging Activity
2.6. Evaluation of In Vivo Hepatoprotective Effect of GPPs
2.6.1. Animals and Treatment
2.6.2. Determination of Liver Index and Liver Appearance
2.6.3. Biochemical Analysis
2.6.4. Histopathological Observation
2.7. Statistical Analysis
3. Results and Discussion
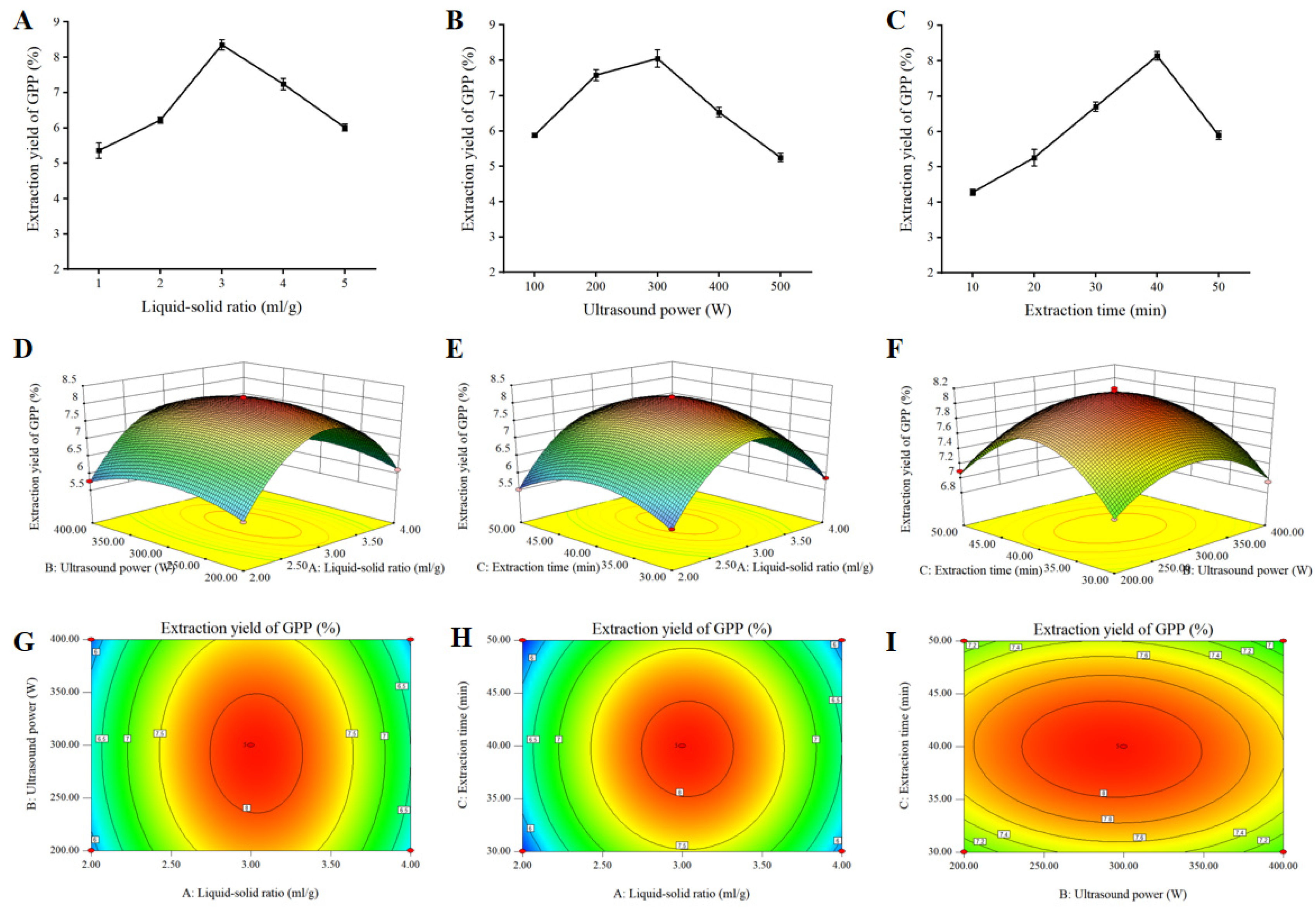
3.1. Single-Factor Tests
3.2. Response Surface Analysis
3.3. Structural Characterization of GPPs
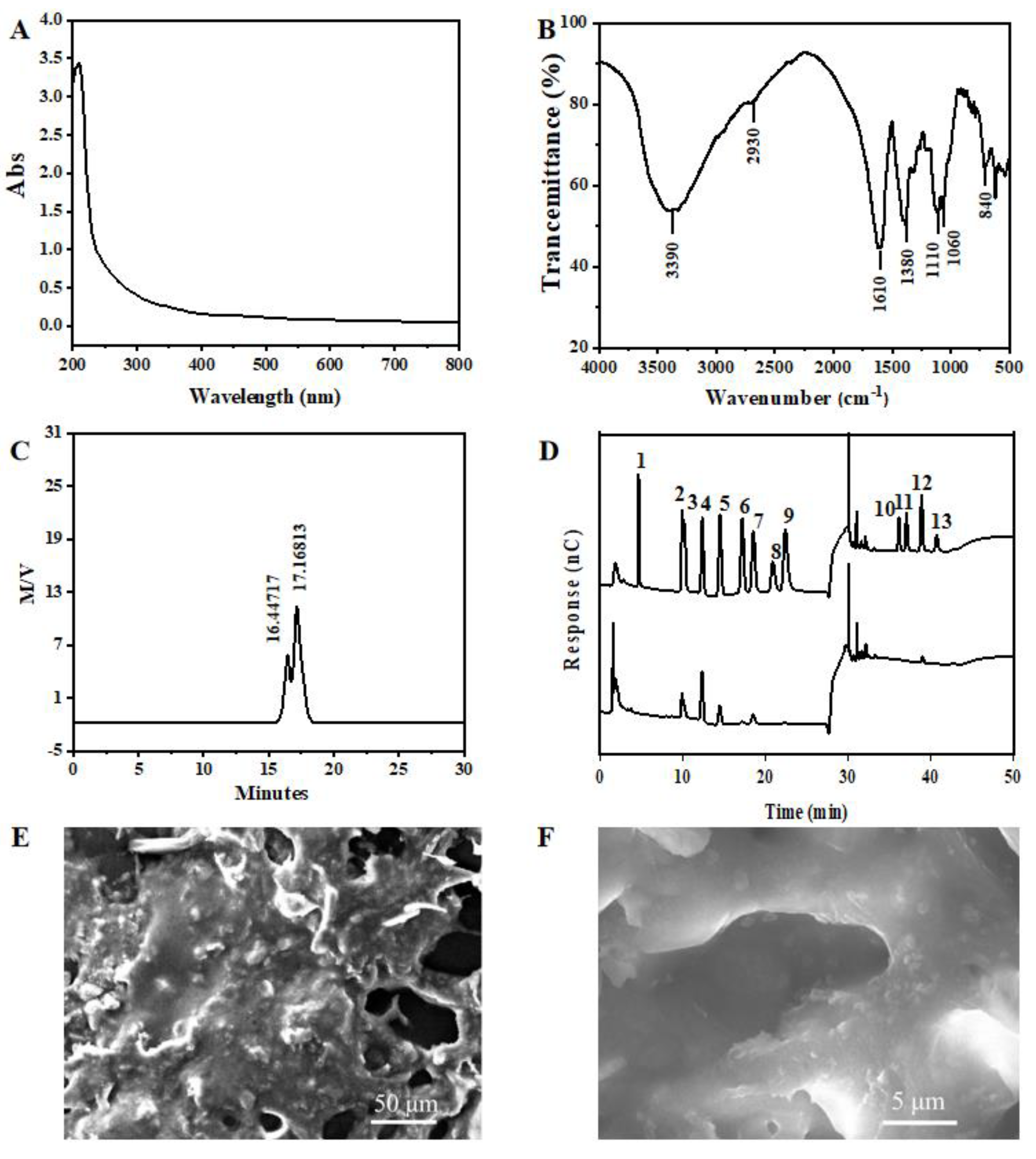
3.3.1. UV and FT-IR of GPPs
3.3.2. Molecular Weight and Monosaccharide Composition Analysis
3.3.3. SEM Analysis of GPPs
3.4. Antioxidant Activity Analysis
3.4.1. DPPH Radical Scavenging Activity
3.4.2. ABTS Radical Scavenging Activity
3.4.3. Hydroxyl Radical Scavenging Activity
3.5. Evaluation of In Vivo Hepatoprotective Activity of GPPs
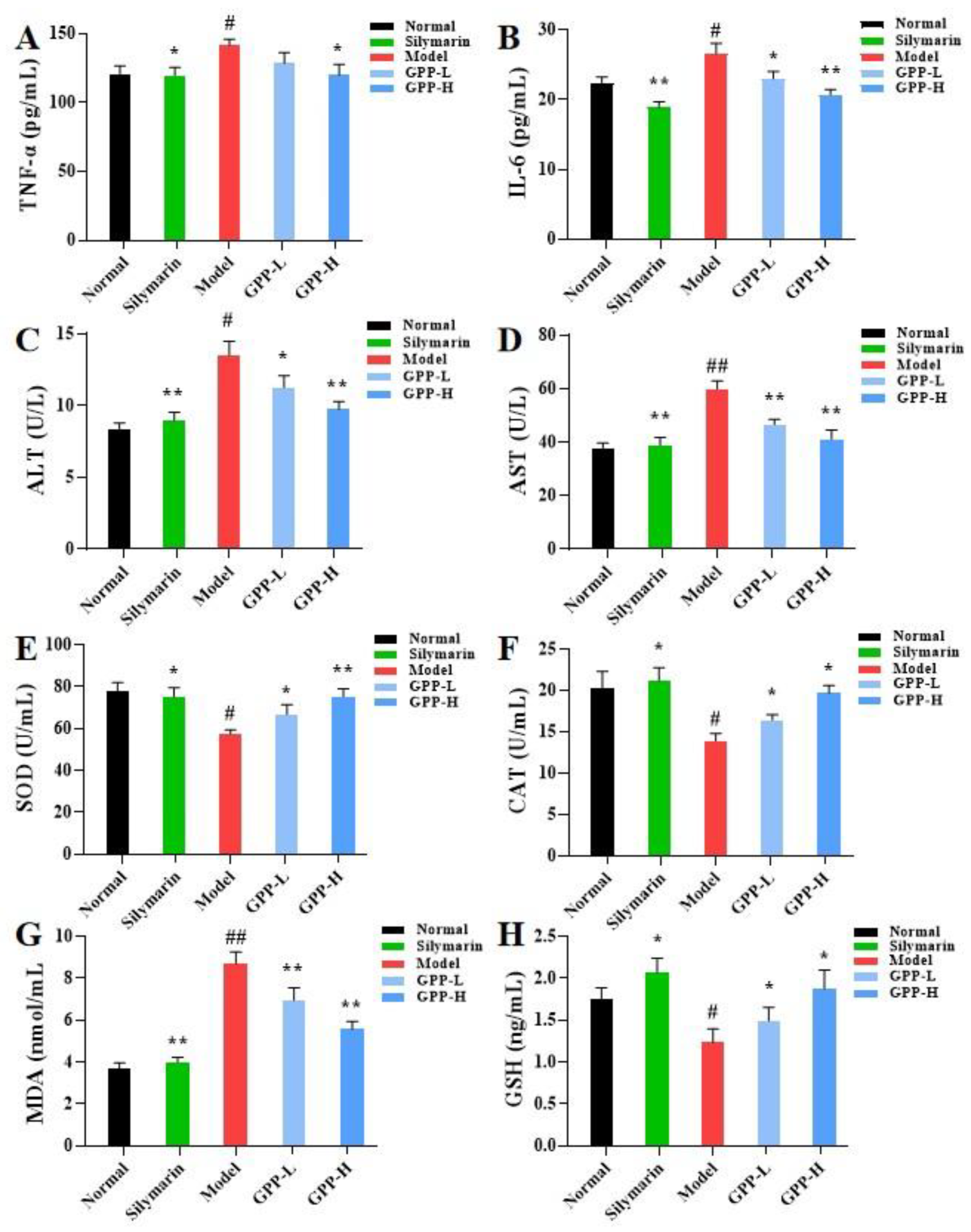
3.5.1. Effects of Inflammatory Cytokines in Serum Induced by GPPs
3.5.2. Effects of GPPs on Serum ALT and AST Activities
3.5.3. Effects of GPPs on Oxidative Stress in Liver
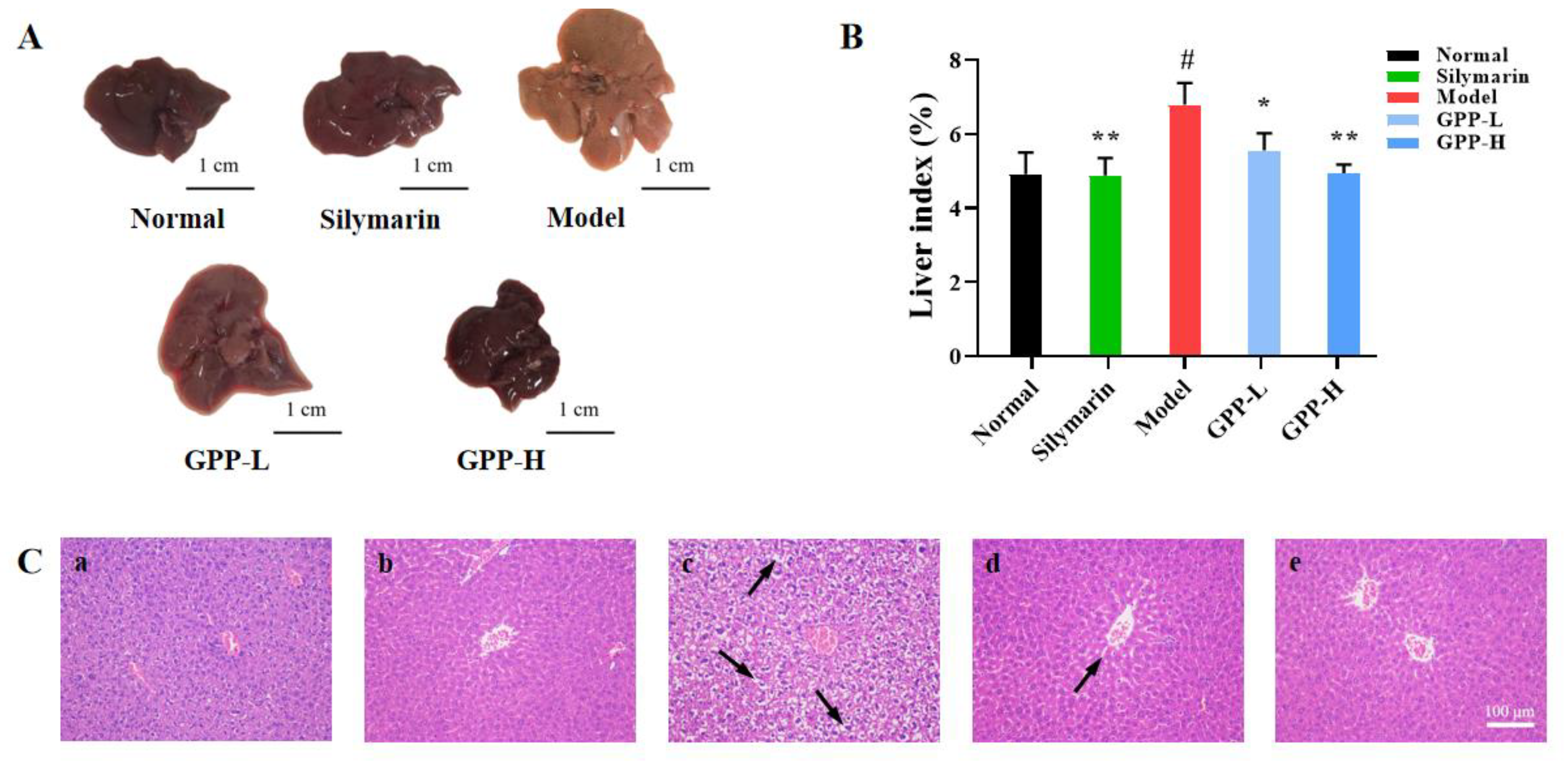
3.5.4. Effects of GPPs on Morphology in Mice and Liver Index
3.5.5. Histopathology of Liver
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Casadei, E.; Albert, J. Food and Agriculture Organization of the United Nations. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 749–753. [Google Scholar] [CrossRef]
- Martínez-Meza, Y.; Pérez-Jiménez, J.; Rocha-Guzmán, N.E.; Rodríguez-García, M.E.; Alonzo-Macías, M.; Reynoso-Camacho, R. Modification on the polyphenols and dietary fiber content of grape pomace by instant controlled pressure drop. Food Chem. 2021, 360, 130035. [Google Scholar] [CrossRef]
- Ky, I.; Teissedre, P.-L. Characterisation of Mediterranean Grape Pomace Seed and Skin Extracts: Polyphenolic Content and Antioxidant Activity. Molecules 2015, 20, 2190–2207. [Google Scholar] [CrossRef]
- Ferri, M.; Bin, S.; Vallini, V.; Fava, F.; Michelini, E.; Roda, A.; Minnucci, G.; Bucchi, G.; Tassoni, A. Recovery of polyphenols from red grape pomace and assessment of their antioxidant and anti-cholesterol activities. New Biotechnol. 2016, 33, 338–344. [Google Scholar] [CrossRef]
- Fiori, L.; Lavelli, V.; Duba, K.S.; Harsha, P.S.; Mohamed, H.B.; Guella, G. Supercritical CO2 extraction of oil from seeds of six grape cultivars: Modeling of mass transfer kinetics and evaluation of lipid profiles and tocol contents. J. Supercrit. Fluids 2014, 94, 71–80. [Google Scholar] [CrossRef]
- Canalejo, D.; Guadalupe, Z.; Martínez-Lapuente, L.; Ayestarán, B.; Pérez-Magariño, S. Optimization of a method to extract polysaccharides from white grape pomace by-products. Food Chem. 2021, 365, 130445. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M. Functional components of grape pomace: Their composition, biological properties and potential applications. Int. J. Food Sci. Technol. 2013, 48, 221–237. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qiu, Z.; Dong, H.; Ma, C.; Qiao, Y.; Zheng, Z. Structural characterization and antioxidant activities of one neutral polysaccharide and three acid polysaccharides from the roots of Arctium lappa L.: A comparison. Int. J. Biol. Macromol. 2021, 182, 187–196. [Google Scholar] [CrossRef]
- Huang, X.; Hou, R.; Pan, W.; Wu, D.; Zhao, W.; Li, Q. A functional polysaccharide from Eriobotrya japonica relieves myocardial ischemia injury via anti-oxidative and anti-inflammatory effects. Food Funct. 2022, 13, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, F.; Hou, R.; Huang, T.; Hao, Z. Post-screening characterization of an acidic polysaccharide from Echinacea purpurea with potent anti-inflammatory properties in vivo. Food Funct. 2020, 11, 7576–7583. [Google Scholar] [CrossRef]
- Qiu, Z.; Qiao, Y.; Zhang, B.; Sun-Waterhouse, D.; Zheng, Z. Bioactive polysaccharides and oligosaccharides from garlic (Allium sativum L.): Production, physicochemical and biological properties, and structure–function relationships. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3033–3095. [Google Scholar] [CrossRef] [PubMed]
- Dtwa, B.; Yuan, H.B.; Qin, Y.C.; Sw, C.; Ryga, D.; Ych, A.; Liang, Z.A. Effects of molecular weight and degree of branching on microbial fermentation characteristics of okra pectic-polysaccharide and its selective impact on gut microbial composition. Food Hydrocoll. 2022, 132, 107897. [Google Scholar]
- Björnsson, E.S. Hepatotoxicity by Drugs: The Most Common Implicated Agents. Int. J. Mol. Sci. 2016, 17, 224. [Google Scholar] [CrossRef]
- Li, J.; Jia, X.; So, K.F. Research progress on hepatic encephalopathy: Animal models and disease mechanisms. Abdomen 2015, 2. [Google Scholar]
- Nada, S.A.; Omara, E.A.; Abdel-Salam, O.M.E.; Zahran, H.G. Mushroom insoluble polysaccharides prevent carbon tetrachloride-induced hepatotoxicity in rat. Food Chem. Toxicol. 2010, 48, 3184–3188. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-C.; Hu, J.-L.; Li, J.; Wang, J.; Zhang, X.-Y.; Wu, X.-Y.; Li, X.; Guo, Z.-B.; Zou, L.; Wu, D.-T. Physicochemical characteristics and biological activities of soluble dietary fibers isolated from the leaves of different quinoa cultivars. Food Res. Int. 2023, 163, 112166. [Google Scholar] [CrossRef]
- Yang, B.; Wu, Q.; Luo, Y.; Yang, Q.; Chen, G.; Wei, X.; Kan, J. Japanese grape (Hovenia dulcis) polysaccharides: New insight into extraction, characterization, rheological properties, and bioactivities. Int. J. Biol. Macromol. 2019, 134, 631–644. [Google Scholar] [CrossRef]
- Zhu, S.; Qiu, Z.; Qiao, X.; Waterhouse, G.I.N.; Zhu, W.; Zhao, W.; He, Q.; Zheng, Z. Creating burdock polysaccharide-oleanolic acid-ursolic acid nanoparticles to deliver enhanced anti-inflammatory effects: Fabrication, structural characterization and property evaluation. Food Sci. Hum. Wellness 2023, 12, 454–466. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, B.; Xiao, J.; Huang, Q.; Li, C.; Fu, X. Physicochemical, functional, and biological properties of water-soluble polysaccharides from Rosa roxburghii Tratt fruit. Food Chem. 2018, 249, 127. [Google Scholar] [CrossRef]
- Zhu, Z.; Song, X.; Jiang, Y.; Yao, J.; Jiang, Y.; Li, Z.; Dai, F. Chemical structure and antioxidant activity of a neutral polysaccharide from Asteris Radix et Rhizoma. Carbohydr. Polym. 2022, 286, 119309. [Google Scholar] [CrossRef]
- Wu, D.T.; An, L.Y.; Liu, W.; Hu, Y.C.; Wang, S.P.; Zou, L. In vitro fecal fermentation properties of polysaccharides from Tremella fuciformis and related modulation effects on gut microbiota. Food Res. Int. 2022, 156, 111185. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.M.; Liu, X.Y.; Yan, Y.Y.; Gao, J.H.; Qin, Z.; Wang, X.D. Structural properties and antioxidant activities of polysaccharides isolated from sunflower meal after oil extraction. Arab. J. Chem. 2021, 14, 103420. [Google Scholar] [CrossRef]
- Liu, W.; Ren, Y.L.; Feng-Ju, R.; Xiao-Yan, H.E.; Ouyang, Y. Antioxidant and Alpha-glucosidase Inhibitory Activity of Crataegus songorica Leaves. Food Res. Dev. 2017, 38, 20–24. [Google Scholar]
- Li, Q.; Yang, S.; Li, Y.; Xue, X.; Huang, Y.; Luo, H.; Zhang, Y.; Lu, Z. Comparative Evaluation of Soluble and Insoluble-Bound Phenolics and Antioxidant Activity of Two Chinese Mistletoes. Molecules 2018, 23, 359. [Google Scholar] [CrossRef]
- Xie, L.; Shen, M.; Wen, P.; Hong, Y.; Xie, J. Preparation, characterization, antioxidant activity and protective effect against cellular oxidative stress of phosphorylated polysaccharide from Cyclocarya paliurus. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2020, 145, 111754. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.; Su, M.; Chen, Q.; Chang, Q.; Wang, W.; Li, H. Protective effect of a polysaccharide from Anoectochilus roxburghii against carbon tetrachloride-induced acute liver injury in mice. J. Ethnopharmacol. 2017, 200, 124–135. [Google Scholar] [CrossRef]
- Zhang, X.; Kuang, G.; Wan, J.; Jiang, R.; Ma, L.; Gong, X.; Liu, X. Salidroside protects mice against CCl4-induced acute liver injury via down-regulating CYP2E1 expression and inhibiting NLRP3 inflammasome activation. Int. Immunopharmacol. 2020, 85, 106662. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.; Wen, S.; Li, Q.; Chen, R.; Lai, X.; Zhang, Z.; Zhou, Z.; Xie, Y.; Zheng, X.; et al. Extract of Jasminum grandiflorum L. alleviates CCl4-induced liver injury by decreasing inflammation, oxidative stress and hepatic CYP2E1 expression in mice. Biomed. Pharmacother. 2022, 152, 113255. [Google Scholar] [CrossRef]
- Jia, S.; Chen, Q.; Wu, J.; Yao, X.; Shao, J.; Cheng, X.; Zhang, C.; Cen, D.; Wang, Y.; Shen, Z.; et al. Danshensu derivative ADTM ameliorates CCl4-induced acute liver injury in mice through inhibiting oxidative stress and apoptosis. Pathol.-Res. Pract. 2021, 228, 153656. [Google Scholar] [CrossRef]
- El-haskoury, R.; Al-Waili, N.; Kamoun, Z.; Makni, M.; Al-Waili, H.; Lyoussi, B. Antioxidant Activity and Protective Effect of Carob Honey in CCl4-induced Kidney and Liver Injury. Arch. Med. Res. 2018, 49, 306–313. [Google Scholar] [CrossRef]
- Bargougui, K.; Athmouni, K.; Chaieb, M. Optimization, characterization and hepatoprotective effect of polysaccharides isolated from Stipa parviflora Desf. against CCl4 induced liver injury in rats using surface response methodology (RSM). Int. J. Biol. Macromol. 2019, 132, 524–533. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, M.; Jiang, X.; Qiao, Q.; Liu, T.; Zhao, C.; Wang, M. Comprehensive Analysis of Fecal Microbiome and Metabolomics in Hepatic Fibrosis Rats Reveal Hepatoprotective Effects of Yinchen Wuling Powder from the Host-Microbial Metabolic Axis. Front. Pharmacol. 2021, 12, 713197. [Google Scholar] [CrossRef]
- Yin, D.; Sun, X.; Li, N.; Guo, Y.; Tian, Y.; Wang, L. Structural properties and antioxidant activity of polysaccharides extracted from Laminaria japonica using various methods. Process Biochem. 2021, 111, 201–209. [Google Scholar] [CrossRef]
- Chen, S.; Shang, H.; Yang, J.; Li, R.; Wu, H. Effects of different extraction techniques on physicochemical properties and activities of polysaccharides from comfrey (Symphytum officinale L.) root. Ind. Crops Prod. 2018, 121, 18–25. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, J.; Chen, Y.; Ma, Y.; Yang, Q.; Fan, Y.; Fu, C.; Limsila, B.; Li, R.; Liao, W. Extraction, structural characterization and antioxidant activity of turmeric polysaccharides. LWT 2022, 154, 112805. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, J.; Wang, B.; Cheng, Z.; Xu, J.; Gao, W.; Chen, K. Structural characterization and antioxidant activities of Bletilla striata polysaccharide extracted by different methods. Carbohydr. Polym. 2021, 266, 118149. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Fan, S.H.; Zheng, Y.L.; Lu, J.; Wu, D.M.; Shan, Q.; Hu, B. Troxerutin protects the mouse liver against oxidative stress-mediated injury induced by D-galactose. J. Agric. Food Chem. 2009, 57, 7731. [Google Scholar] [CrossRef]
- Unanma, H.C.; Anaduaka, E.G.; Uchendu, N.O.; Ononiwu, C.P.; Ogugua, V.N. Ananas comosus and Citrus sinensis peels ameliorate CCl4-induced liver injury in Wistar rats. Sci. Afr. 2021, 14, e01026. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, M.; Liu, Y.; Liu, T.; Zhao, C.; Wang, M. Investigation of the therapeutic effect of Yinchen Wuling Powder on CCl4-induced hepatic fibrosis in rats by 1H NMR and MS-based metabolomics analysis. J. Pharm. Biomed. Anal. 2021, 200, 114073. [Google Scholar] [CrossRef] [PubMed]
- Baradaran, A.; Samadi, F.; Ramezanpour, S.S.; Yousefdoust, S. Hepatoprotective effects of silymarin on CCl4-induced hepatic damage in broiler chickens model. Toxicol. Rep. 2019, 6, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Madrigal-Santillán, E.; Madrigal-Bujaidar, E.; Álvarez-González, I.; Sumaya-Martínez, M.T.; Gutiérrez-Salinas, J.; Bautista, M. Review of natural products with hepatoprotective effects. World J. Gastroenterol. 2014, 20, 14787. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Li, C.; Wang, S.; Mei, X.; Zhang, H.; Kan, J. Characterization of physicochemical properties and antioxidant activity of polysaccharides from shoot residues of bamboo (Chimonobambusa quadrangularis): Effect of drying procedures. Food Chem. 2019, 292, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, H.-M.; Qin, G.-Y. Structure characterization and antioxidant activity of polysaccharides from Chinese quince seed meal. Food Chem. 2017, 234, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, S.; Liu, J.; Cai, E.; Zhu, H.; He, Z.; Gao, Y.; Li, P.; Zhao, Y. Sesquiterpenoids from the root of Panax Ginseng protect CCl4–induced acute liver injury by anti-inflammatory and anti-oxidative capabilities in mice. Biomed. Pharmacother. 2018, 102, 412–419. [Google Scholar] [CrossRef]
- Meng, X.; Wang, Z.; Liang, S.; Tang, Z.; Liu, J.; Xin, Y.; Kuang, H.; Wang, Q. Hepatoprotective effect of a polysaccharide from Radix Cyathulae officinalis Kuan against CCl4-induced acute liver injury in rat. Int. J. Biol. Macromol. 2019, 132, 1057–1067. [Google Scholar] [CrossRef]
- Hamid, M.; Liu, D.; Abdulrahim, Y.; Liu, Y.; Qian, G.; Khan, A.; Gan, F.; Huang, K. Amelioration of CCl4-induced liver injury in rats by selenizing Astragalus polysaccharides: Role of proinflammatory cytokines, oxidative stress and hepatic stellate cells. Res. Vet. Sci. 2017, 114, 202–211. [Google Scholar] [CrossRef]

| Variable | Coded Factor Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Liquid–solid ratio (mL/g) (A) | 2 | 3 | 4 |
| Ultrasound power (W) (B) | 200 | 300 | 400 |
| Extraction time (min) (C) | 30 | 40 | 50 |
| Run | Liquid–Solid Ratio (A) | Ultrasonic Power (B) | Extraction Time (C) | Yield (%) |
|---|---|---|---|---|
| 1 | 0 | −1 | −1 | 7.02 |
| 2 | −1 | 1 | 0 | 5.77 |
| 3 | 0 | −1 | 1 | 7.1 |
| 4 | 0 | 0 | 0 | 8.14 |
| 5 | 0 | 0 | 0 | 8.17 |
| 6 | −1 | 0 | 1 | 5.5 |
| 7 | 0 | 1 | 1 | 6.81 |
| 8 | 1 | 0 | 1 | 5.72 |
| 9 | 1 | −1 | 0 | 6.11 |
| 10 | −1 | −1 | 0 | 5.83 |
| 11 | 0 | 0 | 0 | 8.13 |
| 12 | −1 | 0 | −1 | 5.62 |
| 13 | 1 | 0 | −1 | 5.85 |
| 14 | 0 | 0 | 0 | 8.08 |
| 15 | 0 | 1 | −1 | 6.95 |
| 16 | 1 | 1 | 0 | 5.96 |
| 17 | 0 | 0 | 0 | 8.2 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 17.5 | 9 | 1.94 | 878.22 | <0.0001 | *** |
| A | 0.11 | 1 | 0.11 | 47.8 | 0.0002 | |
| B | 0.041 | 1 | 0.041 | 18.35 | 0.0036 | |
| C | 0.012 | 1 | 0.012 | 5.43 | 0.0526 | |
| AB | 2.025E-003 | 1 | 2.025E-003 | 0.91 | 0.3707 | |
| AC | 2.500E-005 | 1 | 2.500E-005 | 0.011 | 0.9183 | |
| BC | 0.012 | 1 | 0.012 | 5.47 | 0.0520 | |
| A2 | 13.07 | 1 | 13.07 | 5905.47 | <0.0001 | *** |
| B2 | 0.91 | 1 | 0.91 | 410.41 | <0.0001 | *** |
| C2 | 2.12 | 1 | 2.12 | 957.52 | <0.0001 | *** |
| Residual | 0.015 | 7 | 2.561E-003 | |||
| Lack of fit | 7.375E-003 | 3 | 3.483E-003 | 1.86 | 0.2766 | Not significant |
| Pure error | 8.120E-003 | 4 | 1.870E-003 | |||
| Cor total | 17.51 | 16 | ||||
| R-squared | 0.9991 | |||||
| Adj R-squared | 0.9980 | |||||
| CV% | 0.70% | |||||
| Pred R-squared | 0.9825 | |||||
| Adeq precision | 72.683 |
| Sample | Percentage of Each Monosaccharide Group (%) | |||||
|---|---|---|---|---|---|---|
| Ara | Rha | Gal | Glc | Man | Glc-UA | |
| GPPs | 20.79 | 9.63 | 18.67 | 18.76 | 16.09 | 7.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, W.; Huang, R.; Huang, X.; Gao, F.; Leng, X.; Li, Q. Physicochemical Properties and In Vivo Hepatoprotective Effect of Polysaccharides from Grape Pomace. Antioxidants 2023, 12, 394. https://doi.org/10.3390/antiox12020394
Miao W, Huang R, Huang X, Gao F, Leng X, Li Q. Physicochemical Properties and In Vivo Hepatoprotective Effect of Polysaccharides from Grape Pomace. Antioxidants. 2023; 12(2):394. https://doi.org/10.3390/antiox12020394
Chicago/Turabian StyleMiao, Wenjun, Rong Huang, Xiaoli Huang, Fei Gao, Xiangpeng Leng, and Qiu Li. 2023. "Physicochemical Properties and In Vivo Hepatoprotective Effect of Polysaccharides from Grape Pomace" Antioxidants 12, no. 2: 394. https://doi.org/10.3390/antiox12020394
APA StyleMiao, W., Huang, R., Huang, X., Gao, F., Leng, X., & Li, Q. (2023). Physicochemical Properties and In Vivo Hepatoprotective Effect of Polysaccharides from Grape Pomace. Antioxidants, 12(2), 394. https://doi.org/10.3390/antiox12020394










