Abstract
The energy and metabolic state of sows will alter considerably over different phases of gestation. Maternal metabolism increases dramatically, particularly in late pregnancy. This is accompanied by the development of an increase in oxidative stress, which has a considerable negative effect on the maternal and the placenta. As the only link between the maternal and the fetus, the placenta is critical for the maternal to deliver nutrients to the fetus and for the fetus’ survival and development. This review aimed to clarify the changes in energy and metabolism in sows during different pregnancy periods, as well as the impact of maternal oxidative stress on the placenta, which affects the fetus’ survival and development.
1. Introduction
Maintaining sow performance has become critical in the modern pig industry for meeting output targets. The number of piglets weaned per sow per year (PSY) is an important metric of how efficiently pig farms run and how well sows reproduce [1].
Genetic selection and early weaning have made it possible for hyper-prolific sows to have PSYs greater than 31 [2], and this may cause a greater metabolic burden for sows, and possibly a shorter productive life. Simultaneously, the larger the litter size, the lower the uterine blood flow per fetus and piglet birth weight, and the more variation in piglet birth weight within the litter, which raises management expenses in modern all-in-all-out swine production systems [3,4,5]. The increased metabolic demands on sows have a number of detrimental consequences, including oxidative stress and decreased reproductive efficiency. Therefore, the key to increasing the efficiency of pig production is to reduce the metabolic burden of sows while maintaining the reproductive performance of high-yield sows. Pregnancy is a dynamic and finely coordinated process in which maternal metabolism plays a critical role in fetal survival and growth [6]. Maternal metabolic status varies throughout pregnancy, with major alterations in lipid and glucose metabolisms that facilitate nutrition supply to the developing fetus and have a significant impact on reproductive function [7,8]. The maternal endocrine state during gestation also undergoes drastic changes, which has an important impact on the maintenance of pregnancy and fetal development. Maternal metabolism during early pregnancy is predominantly anabolic, with catabolic circumstances common in late pregnancy [9]. Metabolic diseases can impede fetal and neonatal growth and increase morbidity and death at any stage of pregnancy [10]. The placenta serves as the vital link between the fetal and the maternal circulations, sustaining pregnancy and embryonic development and growth by facilitating the transfer of nutrients and waste, contributing to immunological response, and acting as an endocrine organ [11,12]. There is evidence that maternal factors influence the morphology and function of the placenta [13]. This paper discusses changes in energy supply and metabolism in sows during pregnancy, as well as the consequences of oxidative stress-induced placental malfunction on fetal survival and development.
2. Maternal Energy and Metabolism Changes during Pregnancy
2.1. Early Pregnancy
The first 30 days of pregnancy in sows is the phase of early pregnancy, which is the critical stage of embryo development and implantation. Sows ovulate roughly 20–30 follicles, and with a fertilization rate close to 90%, the number of early embryos in sows can theoretically reach 18–27; however, due to different variables, the litter size only reaches half of the embryos [14]. As a result, the survival rate of early embryos has a significant impact on sow litter performance. It is generally agreed that the fully functional corpus luteum (CL) is essential for the establishment of pregnancy and early embryonic development in all mammals [15]. Porcine CL grows rapidly and gains maximum size between day 10 and 12 after ovulation, weighing approximately 7 g in gilts and 13 g in multiparous sows [16]. Luteinizing hormone (LH) has a role in the maintenance of CL [17]. A study found that suboptimal progesterone concentrations as a result of poor pituitary LH support during pregnancy days 12–18 interfere with proper embryo growth and their ability to create signals necessary for pregnancy recognition [18]. CL provides uninterrupted synthesis and the release of progesterone to stimulate the proliferation of endometrial cells and stabilize the uterus for embryo implantation and pregnancy maintenance [19]. Embryos signal their presence by secreting estrogens, which triggers the pregnancy recognition process [16]. Additionally, as estrogen and progesterone levels rise during the early pregnancy stage, tissues become more sensitive to insulin [20]. Moreover, it is necessary for the remodeling of the endometrial lining in order to assist implantation and the delivery of nutrients to the embryos, which improves embryo survival [16].
Sows exhibit overall anabolic features during pregnancy, particularly in the early stages when nutrients tend to accumulate in the maternal. Maternal metabolism is important in the success of early embryonic development because it creates an optimal condition for the establishment and maintenance of pregnancy [21]. In early pregnancy, changes in basal and postprandial glucose metabolism occur gradually, energy reserves build up in the maternal, and insulin sensitivity increases slightly to accommodate the growing nutritional needs of both the maternal and the fetus [22]. Early pregnancy, in particular, is linked to adipocyte hypertrophy, enhanced lipogenesis, and lipid storage, as well as improved insulin sensitivity of white adipose tissue in the maternal [23]. The first 24–48 h after mating are critical for embryo implantation [24], it is necessary to limit the feed intake to avoid an increase in progesterone catabolism produced by increased hepatic blood flow, which reduces embryo survival rate [25]. Glucose has been demonstrated to affect early embryo development and quality [26]. One study found that cultured porcine early embryos with 7 mM of glucose for 48 h generated endoplasmic reticulum stress and oxidative stress, which further impacted embryo quality and development [27]. However, Diego et al. [28] discovered through meta-analysis that feeding diets with more energy than needed for body maintenance have no negative effect on embryonic survival in the majority of trials. Moreover, within 34 days of insemination, the energy intake of sow gilts (Yorkshire x Landrace) as high as 54 MJ/day showed no deleterious influence on embryo development and improved the pregnancy rate [29]. Increasing sows’ energy intake levels after 48 h of pregnancy aids in the recovery of early backfat. Moreover, from day 3 to day 28 of pregnancy, primiparous sows (Landrace × Large White) with higher energy intake (3.75 kg/d of a diet with 2.18 Mcal NE/kg) had a higher number of live-born piglets than those with lower energy intake (3.125 kg/d of a diet with 2.18 Mcal NE/kg) [30]. Therefore, the development of the embryo benefits from the increase in sows’ energy intake during the early stages of pregnancy.
2.2. The Second Trimester of Pregnancy
The direction of nutrient deposition starts to change during the second trimester of pregnancy to support the maternal body reserve restoration. A relatively low energy intake during the second trimester of pregnancy should be preserved to maintain the sow’s optimal body condition and prevent excessive back fat deposition in sows. A greater rate of stillbirths was observed when gestating gilts (Yorkshire x Landrace) were fed a 2.5 kg/d basal diet containing 3265 kcal of ME/kg as compared to feedings of a 2.0 kg/d diet [31]. Moreover, a study found that feeding sows (Landrace × Large White) an extra 2 kg/d of feed during mid-gestation reduced milk production capability and suckling piglet survival rate [32]. The maternal blood glucose rises in the second trimester of pregnancy [33], and the recovery to normal levels after meals is slow, indicating that insulin sensitivity is gradually impaired. The higher maternal glucose levels in the second trimester of gestation were associated with an increased risk of perinatal complications [34]. According to a human study, the normal maternal blood glucose level in the second trimester of pregnancy was 6.2 mmol/L, slightly higher than 5.7 mmol/L in the early pregnancy; however, the maternal blood glucose level in the second trimester of gestation rises to 8.3 mmol/L, which may be associated with adverse pregnancy outcomes [35]. Sows’ energy intake should be adjusted appropriately throughout this period of pregnancy to preserve the optimal body condition of sows.
2.3. Late Pregnancy
Most fetal weight gain occurs in late pregnancy. Sow metabolism increases dramatically in late pregnancy, maternal insulin sensitivity decreases further, and maternal catabolism satisfies the needs of rapid fetal growth. In late pregnancy, maternal fasting blood glucose levels fall [36]. However, the peak blood glucose level after a meal is high, and the rate of glucose clearance reduces as pregnancy progresses [37], indicating that insulin sensitivity declines. Sows in normal condition require more energy to maintain high blood glucose levels, which is conducive to the transmission of glucose to the fetus via the placenta and meets the fetus’s rapid growth in late pregnancy. Dietary energy intake during late gestation influenced the subsequent reproductive performance in multiparous sows [38]. A study has indicated that the piglet birth weight of sows (PIC 1050) with 6.75 Mcal NE/d energy intake in late pregnancy was 0.2–0.4 kg higher than that of sows with 4.5 Mcal NE/d [39]. Sows (Landrace x Yorkshire) fed 9600 kcal ME/d from day 85 of gestation till farrowing had higher body weight on day 110 of gestation and piglet weight than sows fed 8374 kcal ME/d [40]. However, excessive maternal energy intake in late pregnancy also thickened sows’ backfat, which raised the number of piglets born with low birth weight [41]. Furthermore, a study revealed that sows (Landrace × Large White) with higher feed intake (4.0 kg/d, 11.40 Mcal/d ME) had higher levels of reactive oxygen species (ROS) and malondialdehyde (MDA) in plasma at parturition compared to sows with lower feed intake (2.8 kg/d, 7.98 Mcal/d ME), which resulted in maternal oxidative stress [42].
The endocrine system also undergoes significant alterations in late pregnancy in order to support the fetus’ rapid growth and prepare for maternal delivery. Leptin is recognized as an important component linking metabolic status to reproduction, peaking between mid and late pregnancy in humans and mice, controlling peripherally the balance of energy reserves by facilitating pregnancy-specific endocrine responses [43]. The maternal follicle-stimulating hormone (FSH) works in tandem with LH to increase estrogen secretion. A study found that the levels of all three hormones in Bama mini pigs fluctuated continuously throughout the pregnancy period, peaking in the middle and late stages [44]. Saleri et al. [45] discovered that progesterone levels in sows (Large White x Landrace) declined in late pregnancy and were 7.38 ± 0.54 ng/mL at delivery. They also measured blood plasma prolactin levels, which revealed that prolactin levels increased two weeks before farrowing to reach a high at farrowing (67.83 ± 4.91 ng/mL), which is required for normal parturition, advantageous to mammary gland development, and prepares for postpartum lactation [45,46].
2.4. Progressive Oxidative Stress during Pregnancy of Sows
Table 1 summarizes some research findings on oxidative stress indicator levels and antioxidant capacity in sows during various phases of pregnancy. It has been discovered that progressive oxidative stress in sows occurs primarily in late pregnancy. Oxidative stress occurs when the amount of ROS produced exceeds the ability of antioxidants to neutralize it. Excess ROS causes oxidative damage to proteins, lipids, and DNA, and accelerates the pace of telomere shortening in prenatal tissues, and eventually impairs cell and organ functions [47,48]. The level of oxidative stress is determined by the balance between pro-oxidants and antioxidants.

Table 1.
Oxidative stress markers of sows at different pregnancy stages.
As previously stated, the rapid development of the fetus, increase in energy intake of sows, and acceleration of maternal metabolism all culminate in increased ROS generation in late pregnancy. To ensure fetal growth and mammary development, increased rates of maternal digestion, absorption, and tissue mobilization result in an increase in ROS generation [50,53,54]. Furthermore, the antioxidant system of sows in late pregnancy declined, as did plasma antioxidant enzyme activity and total antioxidant capacity [54,55], potentially causing oxidative stress in the maternal. According to a study, the serum levels of ROS, DNA damage markers 8-hydroxy-deoxyguanosine (8-OHdG), and thiobarbituric acid reactive substances (TBARS) in sows were higher at 90 and 109 days of gestation than at 10 and 60 days [53], indicating that sows experienced increased systemic oxidative stress during late gestation. In late pregnancy, the function of the maternal non-enzymatic system also reduces. A study found that the plasma α-tocopherol and retinol concentrations of sows decreased from 7.14 and 1.10 mol/L (30 days of gestation) to 3.07 and 0.57 mol/L (110 days of gestation), respectively [54]. Sows are prone to being overweight in late pregnancy, which causes structural, metabolic, and functional changes in various tissues and organs, aggravating progressive oxidative stress [56], insulin resistance [57], and systemic inflammation [53] with high levels of proinflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), interleukins (IL-1, IL-6, and IL-8), and C-reactive protein (CRP) [58,59], leading to placental lipid toxicity [60]. Excessive back fat in sows during late pregnancy causes a lipid-toxic placental environment characterized by lipid accumulation, increased inflammation and oxidative stress, and insulin sensitivity reduction. These variables influence the expression of nutrition transport-related proteins such as the glucose transporter [61], fatty acid transporter [62], and amino acid transporter [63], which has a negative impact on nutrition transmission between the maternal and fetus, leading to undesirable consequences in pregnancy outcomes. Zhou et al. [41] collected data on 846 farrowing multiparous Yorkshire sows with parity ranging from 3 to 5, and found that the increased backfat thickness of sows at day 109 of gestation exhibited a convex quadratic relationship with litter size, litter weight, and average piglet birth weight. This demonstrates the connection between sows’ back fat thickness and litter performance. According to a study, high back fat sows (≥23 mm) had higher levels of triglycerides, malondialdehyde MDA, and pro-inflammatory factors in the placenta than normal back fat sows (17–22 mm). They also had reduced placental effectiveness on the 107th day of pregnancy, as well as smaller litters and piglets born with lower average weights than typical [64]. Therefore, the litter performance of sows is greatly influenced by the status and function of the placenta.
3. Placenta and Oxidative Stress
3.1. The Structural Characteristics of Placenta
The placenta is critical in transferring maternal nourishment to the fetus and regulating fetal growth and development [65]. The porcine placenta is diffuse, folded, without decidualization, non-invasive, and epitheliochorial, accomplished with interdigitations of the trophectoderm microvilli and surface uterine epithelium [66], which belongs to an epitheliochorial type, with its surface being attached to maternal endometrium, and six layers of tissues to separate the fetus from the maternal blood [67]. Endometrial epithelium, connective tissue, and vascular endothelium are found in the maternal part, while vascular endothelium, intermediate connective tissue, and trophoblast cells are found in the fetal part. At 30 gestation days, the pig placenta is fully developed [67], and a considerable number of microcapillaries begin to appear [66]. The formation and development of the placenta in early pregnancy are critical to the survival and healthy growth of the fetus (20–30% of fetal loss occurs during the early pregnancy) [68]. Placental growth occurs between days 20 and 70 of pregnancy, and by the second trimester (60 to 70 days), the placenta is close to its maximal size [69]. This is followed by rapid angiogenesis, which is a precursor to the rapid fetal growth that occurs in the latter stages of pregnancy [69]. At 90 days of gestation, the weight of the placenta does not grow [70]; however, the epithelial bilayer thins and capillaries indent the plane of each layer, reducing the distance between capillaries and between blood vessels and villi [71], facilitating nutrient exchange between maternal and fetal.
3.2. Mechanism of Oxidative Stress in Placenta
Throughout gestation, the placenta is continually evolving to accommodate the mounting demands of the fetus. In early pregnancy, the placenta and fetus exist in a hypoxic environment, the ROS produced by the placenta is low, and the O2 tension rises sharply at the end of early pregnancy when trophoblast invasion permits the occluded uterine spiral arteries to open [72]. As pregnancy progresses, the invasion of extra-villous trophoblast, the development of the placental vascular system, and the fetus’ metabolic demands increase, resulting in an increase in placental mitochondrial mass and mitochondrial electron chain enzyme activity, which leads to increased ROS production and placental oxidative stress [73]. Because of its high metabolic activity and frequent cell division, the placenta is very sensitive to oxidative stress [74]. The syncytiotrophoblast is especially vulnerable to oxidative stress because it is located on the surface of the villi, which is the first to encounter an increase in intervillous partial pressure of oxygen (PO2). On the other hand, syncytiotrophoblast has substantially lower quantities of antioxidant enzymes than other villous tissues [75,76,77]. Pregnancy is an oxidative stress condition, especially when the maternal is in the stage of rapid fetal growth. At the same time, the decline of maternal antioxidant capacity leads to an increase in ROS production, which aggravates the oxidative stress of the placenta [54,55].
Maternal insulin sensitivity gradually declines as pregnancy progresses, leading to insulin resistance, and increased triglyceride and cholesterol concentrations in late pregnancy produce lipid toxicity [78]. Lipid toxicity begins with lipid accumulation in non-adipose tissue. The placenta, like other organs such as the liver and muscle, is sensitive to obesity-related lipid accumulation [79,80]. Maternal obesity during pregnancy and insulin resistance lead to ectopic deposition of lipids to the placenta, causing placental lipid toxicity [41,81]. Placental lipid toxicity has been demonstrated to inhibit trophoblast invasion and influence placental development and transport, thus affecting fetal developmental pathways [82]. Placental lipid toxicity is also associated with augmented inflammation and oxidative stress. The results of RNA-sequencing performed on term placenta from obese or lean maternal revealed that maternal obesity increased placental lipid content and the expression of genes related to inflammation while decreasing antioxidant capacity [83]. Placental inflammation has been characterized by increased macrophage infiltration and increased cytokine production in the placenta. Studies have shown that lipid toxicity induces a pro-inflammatory response in placental cells that is regulated by JNK and EGR-1, increases the activation of inflammatory NF-κB signaling, increases pro-inflammatory cytokine levels such as IL-1, IL-6, and TNF-α, and decreases the total antioxidant capacity in the placenta [82,84,85]. The IKKα/NF-κB signaling pathway was also found to be implicated in MARK4-activated oxidative stress and mitochondrial dysfunction [86]. Placental oxidative stress increased placental mitochondrial activity and the production of ROS such as superoxide (O2•−), hydroxide (OH−•), and hydrogen peroxide (H2O2), which cause cellular damage and tissue malfunction and have a significant impact on placental function such as trophoblast proliferation and differentiation and vascular reactivity [87,88]. Mitochondria, a vital energy source for placental activity, are also the predominant producer of ROS. Excessive ROS can damage lipids, proteins, and nucleic acids within the mitochondria, resulting in alterations to mitochondrial structure and function. Furthermore, mitochondrial malfunction and superoxide overproduction could be part of a vicious cycle [89,90]. According to research, the consequences of increased oxidative stress on sow reproductive performance are mostly evident in reduced litter size and piglet survival, decreased ability for breastfeeding, and lower sow health status [91]. Increased oxidative stress is responsible for impaired milk production, reproductive performance, and finally, the longevity of sows [50]. During pregnancy, oxidative damage may predispose to embryonic resorption, limit fetal growth, and cause stillbirths [92].
3.3. Effects of Oxidative Stress on Placental Function
The exchange of nutrients, gases, and wastes between maternal and fetal circulation occurs through placental vessels, and a placenta with a high vascular density promotes the fetus’s growth and development. The porcine placenta belongs to an epitheliochorial type, with its surface being attached to the maternal endometrium, and six layers of tissues to separate the fetus from the maternal blood [67]. Increased levels of ROS have been shown to cause vascular endothelial cells to undergo autophagy, malfunction, and apoptosis, which may retard placental vasculature development [93]. The vascular endothelial growth factor receptor system (VEGF/VEGFR) plays a major role in the complex process of angiogenesis [94]. Oxidative stress induced by H2O2 prompted intracellular ROS generation and inhibited the tube formation and migration of porcine vascular endothelial cells (PVECs), as well as the expression of VEGF-A [95]. Placental insufficiency is more just inadequate blood flow; it may also result in diminished transplacental nutrition transfer capacity [96]. Passage across the placenta can occur via simple diffusion, pinocytosis, receptor-mediated uptake, and both the active and facilitative transporters, and substances required for fetal growth, such as glucose, fatty acids, and amino acids, are transported through specific uptake and transport mechanisms that can adapt to supply and demand [97]. Increased oxidative stress reduces GLUT1 expression and glucose transport in the pig placenta [98,99]. In addition, Cocl2-induced oxidative stress reduced mTOR signaling activity and LAT expression in HTR-8/SVneo human trophoblast cells [100]. Furthermore, reduced placental SNAT activity has been seen in pregnancies complicated by fetal growth limitation [101].
4. Effect of Oxidative Stress on Fetus
4.1. Fetal Development during Pregnancy
Early embryonic development in pigs, like in most mammals, consists primarily of different stages. The zygote, formed by fertilization, divides and develops into a blastocyst (embryonic day 0–5.5), the embryo attaches to the uterine wall (embryonic day 12), which is followed by the tissue development and organogenesis phase and embryo growth [102,103]. There are a large number of early embryos in the uterus during the post-implantation stage in pigs, in order to prevent intrauterine growth retardation (IUGR), a significant rise in embryo losses. The embryonic stage is followed by the fetal growth stage after organ differentiation and placental development. The fetal growth rate in sow early pregnancy is slow; during 35 days of gestation, the size of the embryo is roughly 3–4 cm and weighs about 5 g. The fetal length and weight in the second trimester of pregnancy (55 days) are approximately 11–12 cm and 90 g, respectively. At 90 days of gestation, the fetal length is about 25 cm and the weight is about 700 g [104]. Furthermore, in late pregnancy, the fetus enters the rapid development period, with fetal weight gain accounting for two-thirds of the birth weight. As a result, the first 30 days of sow pregnancy are crucial for embryo survival, whereas the latter 30 days of gestation are critical for increasing fetal birth weight. To satisfy the needs of self-maintenance, fetal growth, and development, the mother will undergo a series of severe changes in physiology, hormones, and metabolism [23]. The abnormality of metabolic alterations will have direct consequences for fetal health throughout the pregnancy, leading to fetal intrauterine growth retardation and low birth weight, as well as a long-term influence on offspring growth and development.
4.2. Effects of Maternal Oxidative Stress on Offspring in Sows
According to certain theories, oxidative stress is crucial to the pathophysiological process in offspring [105]. Increasing data suggest that unfavorable pregnancy outcomes are linked to maternal oxidative stress [106]. Zhao et al. [49] found that litter size and litter weight were negatively correlated with maternal oxidative stress indicators. Another study discovered that Large White sows with high backfat (21–25 mm) or low backfat (9–12 mm) at 110 days of gestation compared to medium backfat (13–20 mm) caused high levels of oxidative stress in the maternal, increased the number of stillborn piglets per litter, and decreased the number of piglets per litter, piglets born alive per litter, and litter birth weight [56]. Oxidative stress during gestation will impair sow health by increasing ROS production and buildup. Furthermore, ROS accumulation impairs fetal growth and development via the placenta, resulting in fetal death and intrauterine growth retardation (IUGR), and ultimately impairing reproductive performance [107]. Increased maternal MDA levels in plasma were linked to greater levels of IL-6 and IL-7 in the offspring, suggesting that maternal oxidative stress biomarkers may be associated with changes in cytokine concentrations in offspring [108]. Thus, oxidative stress may affect inflammatory pathways in progeny. IUGR is caused by utero-placental insufficiency; when placental tissue is unable to support appropriate nutrition and oxygen exchange, fetal organs grow slowly [109]. A suboptimal prenatal environment causes the placenta to experience cell stress and death, which compromises fetal growth, and causes metabolic organs to experience cellular stress and dysfunction, including oxidative stress and mitochondrial dysfunction [107]. The antioxidant defense mechanism of the IUGR fetus is impaired, aggravating oxidative stress [110]. Studies have revealed that oxidative stress in sows reduces litter sizes and the number of piglets born alive, which can be increased by 1.81 and 2.37, respectively, once maternal oxidative stress is relieved [111]; hence, decreasing oxidative stress in sows can lower IUGR in piglets [55]. The effect of oxidative stress on the fetus is shown in Figure 1.
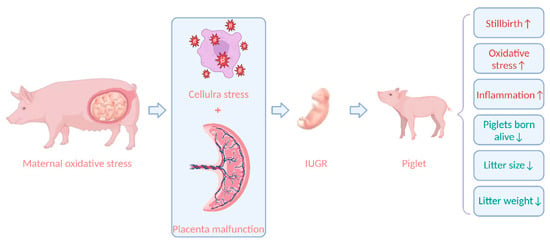
Figure 1.
The effect of oxidative stress on the fetus. IUGR, intrauterine growth retardation. Figure 1 created with BioRender.com URL (https://app.biorender.com/).
5. Conclusions
In conclusion, the maternal, placenta, and fetus are inextricably linked and change dramatically as the pregnancy progresses. The maternal energy metabolism disruption, maternal progressive oxidative stress, and their consequences on placental function throughout pregnancy all have a significant impact on sow reproductive performance. The placenta plays a crucial role in the fetus’ survival and growth during pregnancy as it is a tissue that connects the maternal and the fetus and is susceptible to oxidative stress. The placenta serves as a link between the maternal and the fetus, and their interactions and mutual impacts jointly define mother and fetal health.
Author Contributions
Writing—original draft preparation, X.Y. and R.H.; reviewing-original draft, M.S. and L.W.; writing—final manuscript review and editing, J.H. and S.W.; visualization, J.Y., J.G. and Q.Z.; all authors provided and reviewed the content. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by funds from the National Natural Science Foundation of China (U22A20515, 32102578,) and the Key R&D Program of Hunan Province (2021NK2010).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We appreciate Muhammed Adebayo Arowolo for his help in the English editing.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Guan, R.; Zhou, X.; Cai, H.; Qian, X.; Xin, X.; Li, X. Study on the influence of different production factors on PSY and its correlation. Porc. Health Manag. 2022, 8, 9. [Google Scholar] [CrossRef]
- Lavery, A.; Lawlor, P.G.; Magowan, E.; Miller, H.M.; O’Driscoll, K.; Berry, D.P. An association analysis of sow parity, live-weight and back-fat depth as indicators of sow productivity. Animal 2019, 13, 622–630. [Google Scholar] [CrossRef]
- Oliviero, C.; Junnikkala, S.; Peltoniemi, O. The challenge of large litters on the immune system of the sow and the piglets. Reprod. Domest. Anim. 2019, 54 (Suppl. S3), 12–21. [Google Scholar] [CrossRef] [PubMed]
- Quesnel, H.; Brossard, L.; Valancogne, A.; Quiniou, N. Influence of some sow characteristics on within-litter variation of piglet birth weight. Animal 2008, 2, 1842–1849. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.L.; Zhu, Y.H.; Shi, M.; Li, T.T.; Li, N.; Wu, G.Y.; Bazer, F.W.; Zang, J.J.; Wang, F.L.; Wang, J.J. Within-litter variation in birth weight: Impact of nutritional status in the sow. J. Zhejiang Univ. Sci. B 2015, 16, 417–435. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Guo, C.; Hu, F.; Zhu, W.; Mao, S. Maternal undernutrition induces fetal hepatic lipid metabolism disorder and affects the development of fetal liver in a sheep model. FASEB J. 2019, 33, 9990–10004. [Google Scholar] [CrossRef] [PubMed]
- Furse, S.; Fernandez-Twinn, D.S.; Chiarugi, D.; Koulman, A.; Ozanne, S.E. Lipid Metabolism Is Dysregulated before, during and after Pregnancy in a Mouse Model of Gestational Diabetes. Int. J. Mol. Sci. 2021, 22, 7452. [Google Scholar] [CrossRef]
- Johns, E.C.; Denison, F.C.; Norman, J.E.; Reynolds, R.M. Gestational Diabetes Mellitus: Mechanisms, Treatment, and Complications. Trends Endocrinol. Metab. 2018, 29, 743–754. [Google Scholar] [CrossRef]
- Liu, L.X.; Arany, Z. Maternal cardiac metabolism in pregnancy. Cardiovasc. Res. 2014, 101, 545–553. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Wallace, J.M.; Spencer, T.E. Board-invited review: Intrauterine growth retardation: Implications for the animal sciences. J. Anim. Sci. 2006, 84, 2316–2337. [Google Scholar] [CrossRef]
- Roseboom, T.J.; Painter, R.C.; de Rooij, S.R.; van Abeelen, A.F.M.; Veenendaal, M.V.E.; Osmond, C.; Barker, D.J.P. Effects of famine on placental size and efficiency. Placenta 2011, 32, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Sadovsky, Y.; Mouillet, J.F.; Ouyang, Y.; Bayer, A.; Coyne, C.B. The Function of TrophomiRs and Other MicroRNAs in the Human Placenta. Cold Spring Harb. Perspect. Med. 2015, 5, a023036. [Google Scholar] [CrossRef] [PubMed]
- Tarrade, A.; Panchenko, P.; Junien, C.; Gabory, A. Placental contribution to nutritional programming of health and diseases: Epigenetics and sexual dimorphism. J. Exp. Biol. 2015, 218, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Huang, Z.; Xiong, W.; Ye, H.; Deng, J.; Yin, Y. A review of the amino acid metabolism in placental function response to fetal loss and low birth weight in pigs. J. Anim. Sci. Biotechnol. 2022, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.R.F. Impact of the corpus luteum on survival of the developing embryo and early pregnancy in mares. Theriogenology 2020, 150, 374–381. [Google Scholar] [CrossRef]
- Langendijk, P. Latest Advances in Sow Nutrition during Early Gestation. Animals 2021, 11, 1720. [Google Scholar] [CrossRef]
- Anderson, L.L.; Dyck, G.W.; Mori, H.; Henricks, D.M.; Melampy, R.M. Ovarian function in pigs following hypophysial stalk transection or hypophysectomy. Am. J. Physiol. 1967, 212, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Tast, A.; Love, R.J.; Clarke, I.J.; Evans, G. Effects of active and passive gonadotrophin-releasing hormone immunization on recognition and establishment of pregnancy in pigs. Reprod. Fertil. Dev. 2000, 12, 277–282. [Google Scholar] [CrossRef]
- Qian, Z.; Zhu, H.; Lv, Y.; Liu, H.; Bao, E. Impact of exogenous adrenocorticotropic hormone on gelatinase expression and steroidogenesis in the newly formed corpus luteum in sows. Livest. Sci. 2018, 207, 68–74. [Google Scholar] [CrossRef]
- Leung, K.C.; Xu, A.; Craig, M.E.; Martin, A.; Lam, K.S.; O’Sullivan, A.J. Adiponectin isoform distribution in women--relationship to female sex steroids and insulin sensitivity. Metabolism 2009, 58, 239–245. [Google Scholar] [CrossRef]
- Alminana, C.; Heath, P.R.; Wilkinson, S.; Sanchez-Osorio, J.; Cuello, C.; Parrilla, I.; Gil, M.A.; Vazquez, J.L.; Vazquez, J.M.; Roca, J.; et al. Early developing pig embryos mediate their own environment in the maternal tract. PLoS ONE 2012, 7, e33625. [Google Scholar] [CrossRef] [PubMed]
- Lain, K.Y.; Catalano, P.M. Metabolic changes in pregnancy. Clin. Obstet. Gynecol. 2007, 50, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Napso, T.; Yong, H.E.J.; Lopez-Tello, J.; Sferruzzi-Perri, A.N. The Role of Placental Hormones in Mediating Maternal Adaptations to Support Pregnancy and Lactation. Front. Physiol. 2018, 9, 1091. [Google Scholar] [CrossRef]
- Jindal, R.; Cosgrove, J.R.; Foxcroft, G.R. Progesterone mediates nutritionally induced effects on embryonic survival in gilts. J. Anim. Sci. 1997, 75, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Mattos, F.; Canavessi, A.M.O.; Wiltbank, M.C.; Bastos, M.R.; Lemes, A.P.; Mourao, G.B.; Susin, I.; Coutinho, L.L.; Sartori, R. Investigation of mechanisms involved in regulation of progesterone catabolism using an overfed versus underfed ewe-lamb model. J. Anim. Sci. 2017, 95, 5537–5546. [Google Scholar] [CrossRef] [PubMed]
- Fraser, R.B.; Waite, S.L.; Wood, K.A.; Martin, K.L. Impact of hyperglycemia on early embryo development and embryopathy: In vitro experiments using a mouse model. Hum. Reprod. 2007, 22, 3059–3068. [Google Scholar] [CrossRef]
- Dicks, N.; Gutierrez, K.; Currin, L.; de Macedo, M.P.; Glanzner, W.G.; Mondadori, R.G.; Michalak, M.; Agellon, L.B.; Bordignon, V. Tauroursodeoxycholic acid/TGR5 signaling promotes survival and early development of glucose-stressed porcine embryos. Biol. Reprod. 2021, 105, 76–86. [Google Scholar] [CrossRef]
- Leal, D.F.; Muro, B.B.D.; Nichi, M.; Almond, G.W.; Viana, C.H.C.; Vioti, G.; Carnevale, R.F.; Garbossa, C.A.P. Effects of post-insemination energy content of feed on embryonic survival in pigs: A systematic review. Anim. Reprod. Sci. 2019, 205, 70–77. [Google Scholar] [CrossRef]
- Virolainen, J.V.; Tast, A.; Sorsa, A.; Love, R.J.; Peltoniemi, O.A. Changes in feeding level during early pregnancy affect fertility in gilts. Anim. Reprod. Sci. 2004, 80, 341–352. [Google Scholar] [CrossRef]
- Carrion-Lopez, M.J.; Madrid, J.; Martinez, S.; Hernandez, F.; Orengo, J. Effects of the feeding level in early gestation on body reserves and the productive and reproductive performance of primiparous and multiparous sows. Res. Vet. Sci. 2022, 148, 42–51. [Google Scholar] [CrossRef]
- Piao, L.G.; Ju, W.S.; Long, H.F.; Kim, Y.Y. Effects of Various Feeding Methods for Gestating Gilts on Reproductive Performance and Growth of Their Progeny. Asian Australas J. Anim. Sci. 2010, 23, 1354–1363. [Google Scholar] [CrossRef]
- Cerisuelo, A.; Sala, R.; Gasa, J.; Carrión, D.; Coma, J.; Chapinal, N.; Baucells, M.D. Effects of extra feeding in mid-pregnancy for three successive parities on lean sows’ productive performance and longevity. Can. J. Anim. Sci. 2010, 90, 521–528. [Google Scholar] [CrossRef]
- Scott, E.M.; Feig, D.S.; Murphy, H.R.; Law, G.R.; Grp, C.C. Continuous Glucose Monitoring in Pregnancy: Importance of Analyzing Temporal Profiles to Understand Clinical Outcomes. Diabetes Care 2020, 43, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Hedderson, M.M.; Ferrara, A.; Sacks, D.A. Gestational diabetes mellitus and lesser degrees of pregnancy hyperglycemia: Association with increased risk of spontaneous preterm birth. Obstet. Gynecol. 2003, 102, 850–856. [Google Scholar] [CrossRef]
- Kerssen, A.; de Valk, H.W.; Visser, G.H.A. Increased second trimester maternal glucose levels are related to extremely large-for-gestational-age infants in women with type 1 diabetes. Diabetes Care 2007, 30, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Riskin-Mashiah, S.; Damti, A.; Younes, G.; Auslander, R. Normal fasting plasma glucose levels during pregnancy: A hospital-based study. J. Perinat. Med. 2011, 39, 209–211. [Google Scholar] [CrossRef]
- George, P.B.; England, D.C.; Siers, D.G.; Stanton, H.C. Diabetogenic effects of pregnancy in sows on plasma glucose and insulin release. J. Anim. Sci. 1978, 46, 1694–1700. [Google Scholar] [CrossRef]
- Yang, Y.; Heo, S.; Jin, Z.; Yun, J.; Shinde, P.; Choi, J.; Yang, B.; Chae, B. Effects of dietary energy and lysine intake during late gestation and lactation on blood metabolites, hormones, milk composition and reproductive performance in multiparous sows. Arch. Anim. Nutr. 2008, 62, 10–21. [Google Scholar] [CrossRef]
- Goncalves, M.A.D.; Gourley, K.M.; Dritz, S.S.; Tokach, M.D.; Bello, N.M.; DeRouchey, J.M.; Woodworth, J.C.; Goodband, R.D. Effects of amino acids and energy intake during late gestation of high-performing gilts and sows on litter and reproductive performance under commercial conditions. J. Anim. Sci. 2016, 94, 1993–2003. [Google Scholar] [CrossRef]
- Wang, W.H.; Wang, Z.J.; Ming, D.X.; Huang, C.Y.; Xu, S.; Li, Z.; Wang, Z.Y.; Liu, H.; Zeng, X.F.; Wang, F.L. Effect of maternal dietary starch-to-fat ratio and daily energy intake during late pregnancy on the performance and lipid metabolism of primiparous sows and newborn piglets. J. Anim. Sci. 2022, 100, skac033. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, T.; Cai, A.; Wu, Y.; Wei, H.; Jiang, S.; Peng, J. Excessive backfat of sows at 109 d of gestation induces lipotoxic placental environment and is associated with declining reproductive performance. J. Anim. Sci. 2018, 96, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Zhang, X.M.; Zhou, Y.F.; Wang, C.; Xiong, J.; Guo, L.L.; Wang, L.; Jiang, S.W.; Peng, J. Effect of increasing feed intake during late gestation on piglet performance at parturition in commercial production enterprises. Anim. Reprod. Sci. 2020, 218, 106477. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, M.L.; Spuch, C.; Carro, E.; Senaris, R. Hyperphagia and central mechanisms for leptin resistance during pregnancy. Endocrinology 2011, 152, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Gao, Q.; Zhang, W.; Azad, M.A.K.; Kong, X. Alterations in the Blood Parameters and Fecal Microbiota and Metabolites during Pregnant and Lactating Stages in Bama Mini Pigs as a Model. Mediat. Inflamm. 2020, 2020, 8829072. [Google Scholar] [CrossRef] [PubMed]
- Saleri, R.; Sabbioni, A.; Cavalli, V.; Superchi, P. Monitoring blood plasma leptin and lactogenic hormones in pregnant sows. Animal 2015, 9, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Miller, H.M.; Foxcroft, G.R.; Aherne, F.X. Increasing feed intake in late gestation does not affect plasma progesterone concentration in the sow. Theriogenology 2004, 62, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, S.; Chen, F.; Guan, W.; Zhang, S. Nutritional strategies to alleviate oxidative stress in sows. Anim. Nutr. 2022, 9, 60–73. [Google Scholar] [CrossRef]
- Phillippe, M. Telomeres, oxidative stress, and timing for spontaneous term and preterm labor. Am. J. Obs. Gynecol. 2022, 227, 148–162. [Google Scholar] [CrossRef]
- Zhao, Y.; Kim, S.W. Oxidative stress status and reproductive performance of sows during gestation and lactation under different thermal environments. Asian-Australas J. Anim. Sci. 2020, 33, 722–731. [Google Scholar] [CrossRef]
- Zhao, Y.; Flowers, W.L.; Saraiva, A.; Yeum, K.J.; Kim, S.W. Effect of social ranks and gestation housing systems on oxidative stress status, reproductive performance, and immune status of sows. J. Anim. Sci. 2013, 91, 5848–5858. [Google Scholar] [CrossRef]
- Ostrenko, K.; Nekrasov, R.; Ovcharova, A.; Lemiasheuski, V.; Kutin, I. The Effect of Lithium Salt with Ascorbic Acid on the Antioxidant Status and Productivity of Gestating Sows. Animals 2022, 12, 915. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Wei, H.; Ao, J.; Long, G.; Peng, J. Inclusion of Konjac Flour in the Gestation Diet Changes the Gut Microbiota, Alleviates Oxidative Stress, and Improves Insulin Sensitivity in Sows. Appl. Environ. Microbiol. 2016, 82, 5899–5909. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Wei, H.; Sun, H.; Ao, J.; Long, G.; Jiang, S.; Peng, J. Effects of Dietary Supplementation of Oregano Essential Oil to Sows on Oxidative Stress Status, Lactation Feed Intake of Sows, and Piglet Performance. Biomed. Res. Int. 2015, 2015, 525218. [Google Scholar] [CrossRef] [PubMed]
- Berchieri-Ronchi, C.B.; Kim, S.W.; Zhao, Y.; Correa, C.R.; Yeum, K.J.; Ferreira, A.L. Oxidative stress status of highly prolific sows during gestation and lactation. Animal 2011, 5, 1774–1779. [Google Scholar] [CrossRef]
- Guo, G.; Zhou, T.; Ren, F.; Sun, J.; Deng, D.; Huang, X.; Wassie, T.; Qazi, I.H.; Wu, X. Effect of Maternal Catalase Supplementation on Reproductive Performance, Antioxidant Activity and Mineral Transport in Sows and Piglets. Animals 2022, 12, 828. [Google Scholar] [CrossRef]
- Hu, J.; Yan, P. Effects of Backfat Thickness on Oxidative Stress and Inflammation of Placenta in Large White Pigs. Vet. Sci. 2022, 9, 302. [Google Scholar] [CrossRef]
- Cheng, C.; Wu, X.; Zhang, X.; Zhang, X.; Peng, J. Obesity of Sows at Late Pregnancy Aggravates Metabolic Disorder of Perinatal Sows and Affects Performance and Intestinal Health of Piglets. Animals 2019, 10, 49. [Google Scholar] [CrossRef]
- Pawar, A.S.; Zhu, X.Y.; Eirin, A.; Tang, H.; Jordan, K.L.; Woollard, J.R.; Lerman, A.; Lerman, L.O. Adipose Tissue Remodeling in a Novel Domestic Porcine Model of Diet-Induced Obesity. Obesity 2015, 23, 399–407. [Google Scholar] [CrossRef]
- Fujimori, M.; Franca, E.L.; Morais, T.C.; Fiorin, V.; de Abreu, L.C.; Honorio-Franca, A.C. Cytokine and adipokine are biofactors can act in blood and colostrum of obese mothers. Biofactors 2017, 43, 243–250. [Google Scholar] [CrossRef]
- Jarvie, E.; Hauguel-de-Mouzon, S.; Nelson, S.M.; Sattar, N.; Catalano, P.M.; Freeman, D.J. Lipotoxicity in obese pregnancy and its potential role in adverse pregnancy outcome and obesity in the offspring. Clin. Sci. 2010, 119, 123–129. [Google Scholar] [CrossRef]
- Catalano, P.M. Obesity, insulin resistance, and pregnancy outcome. Reproduction 2010, 140, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Dong, S.S.; Hu, J.; Yao, J.J.; Yan, P.S. The effect of maternal obesity on fatty acid transporter expression and lipid metabolism in the full-term placenta of lean breed swine. J. Anim. Physiol. Anim. Nutr. 2018, 102, e242–e253. [Google Scholar] [CrossRef] [PubMed]
- Fowden, A.L.; Camm, E.J.; Sferruzzi-Perri, A.N. Effects of Maternal Obesity on Placental Phenotype. Curr. Vasc. Pharmacol. 2021, 19, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Hu, J.; Wei, M.; Guo, Y.Y.; Yan, P.S. The Effects of Maternal Obesity on Porcine Placental Efficiency and Proteome. Animals 2019, 9, 546. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Tan, J.; Li, Z.; Wang, L.; Shi, M.; Li, B.; Liu, M.; Yuan, X.; He, J.; Wu, X. Effect of dietary resveratrol on placental function and reproductive performance of late pregnancy sows. Front. Nutr. 2022, 9, 1001031. [Google Scholar] [CrossRef]
- Cristofolini, A.; Fiorimanti, M.; Campos, M.; Sanchis, E.; Diaz, T.; Moschetti, E.; Merkis, C. Morphometric study of the porcine placental vascularization. Reprod. Domest. Anim. 2018, 53, 217–225. [Google Scholar] [CrossRef]
- Leiser, R.; Kaufmann, P. Placental structure: In a comparative aspect. Exp. Clin. Endocrinol. 1994, 102, 122–134. [Google Scholar] [CrossRef]
- Bidarimath, M.; Tayade, C. Pregnancy and spontaneous fetal loss: A pig perspective. Mol. Reprod. Dev. 2017, 84, 856–869. [Google Scholar] [CrossRef]
- Bazer, F.W.; Johnson, G.A. Pig blastocyst-uterine interactions. Differentiation 2014, 87, 52–65. [Google Scholar] [CrossRef]
- Biensen, N.J.; Wilson, M.E.; Ford, S.P. The impact of either a Meishan or Yorkshire uterus on Meishan or Yorkshire fetal and placental development to days 70, 90, and 110 of gestation. J. Anim. Sci. 1998, 76, 2169–2176. [Google Scholar] [CrossRef]
- Vallet, J.L.; Miles, J.R.; Freking, B.A. Development of the pig placenta. Soc. Reprod. Fertil. Suppl. 2009, 66, 265–279. [Google Scholar] [CrossRef]
- Myatt, L.; Cui, X. Oxidative stress in the placenta. Histochem. Cell Biol. 2004, 122, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.D.; De Long, N.E.; Wang, R.C.; Yazdi, F.T.; Holloway, A.C.; Raha, S. Angiogenesis in the placenta: The role of reactive oxygen species signaling. Biomed. Res. Int. 2015, 2015, 814543. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Poston, L.; Burton, G.J. Placental-related diseases of pregnancy: Involvement of oxidative stress and implications in human evolution. Hum. Reprod. Update 2006, 12, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.L.; Skepper, J.N.; Jauniaux, E.; Burton, G.J. Susceptibility of human placental syncytiotrophoblastic mitochondria to oxygen-mediated damage in relation to gestational age. J. Clin. Endocrinol. Metab. 1998, 83, 1697–1705. [Google Scholar] [CrossRef]
- Watson, A.L.; Palmer, M.E.; Jauniaux, E.; Burton, G.J. Variations in expression of copper/zinc superoxide dismutase in villous trophoblast of the human placenta with gestational age. Placenta 1997, 18, 295–299. [Google Scholar] [CrossRef]
- Watson, A.L.; Skepper, J.N.; Jauniaux, E.; Burton, G.J. Changes in concentration, localization and activity of catalase within the human placenta during early gestation. Placenta 1998, 19, 27–34. [Google Scholar] [CrossRef]
- Kampmann, U.; Knorr, S.; Fuglsang, J.; Ovesen, P. Determinants of Maternal Insulin Resistance during Pregnancy: An Updated Overview. J. Diabetes Res. 2019, 2019, 5320156. [Google Scholar] [CrossRef]
- Heerwagen, M.J.; Stewart, M.S.; de la Houssaye, B.A.; Janssen, R.C.; Friedman, J.E. Transgenic increase in N-3/n-6 Fatty Acid ratio reduces maternal obesity-associated inflammation and limits adverse developmental programming in mice. PLoS ONE 2013, 8, e67791. [Google Scholar] [CrossRef]
- Strakovsky, R.S.; Pan, Y.X. A decrease in DKK1, a WNT inhibitor, contributes to placental lipid accumulation in an obesity-prone rat model. Biol. Reprod. 2012, 86, 81. [Google Scholar] [CrossRef]
- Song, T.; Lu, J.; Deng, Z.; Xu, T.; Yang, Y.; Wei, H.; Li, S.; Jiang, S.; Peng, J. Maternal obesity aggravates the abnormality of porcine placenta by increasing N(6)-methyladenosine. Int. J. Obes. 2018, 42, 1812–1820. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Jinglong, X.; Shusheng, D.; Aiyou, W. Maternal obesity stimulates lipotoxicity and up-regulates inflammatory signaling pathways in the full-term swine placenta. Anim. Sci. J. 2018, 89, 1310–1322. [Google Scholar] [CrossRef] [PubMed]
- Saben, J.; Lindsey, F.; Zhong, Y.; Thakali, K.; Badger, T.M.; Andres, A.; Gomez-Acevedo, H.; Shankar, K. Maternal obesity is associated with a lipotoxic placental environment. Placenta 2014, 35, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Saben, J.; Zhong, Y.; Gomez-Acevedo, H.; Thakali, K.M.; Borengasser, S.J.; Andres, A.; Shankar, K. Early growth response protein-1 mediates lipotoxicity-associated placental inflammation: Role in maternal obesity. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1–E14. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, T.; Wu, Y.; Wei, H.; Peng, J. Oxidative Stress and Inflammation in Sows with Excess Backfat: Up-Regulated Cytokine Expression and Elevated Oxidative Stress Biomarkers in Placenta. Animals 2019, 9, 796. [Google Scholar] [CrossRef]
- Tian, L.; Liu, G.; Kang, Z.; Yan, P. Microtubule Affinity-Regulating Kinase 4 Promotes Oxidative Stress and Mitochondrial Dysfunction by Activating NF-kappaB and Inhibiting AMPK Pathways in Porcine Placental Trophoblasts. Biomedicines 2022, 10, 165. [Google Scholar] [CrossRef]
- Xie, C.; Wu, X.; Long, C.; Wang, Q.; Fan, Z.; Li, S.; Yin, Y. Chitosan oligosaccharide affects antioxidant defense capacity and placental amino acids transport of sows. BMC Vet. Res. 2016, 12, 243. [Google Scholar] [CrossRef]
- Myatt, L.; Kossenjans, W.; Sahay, R.; Eis, A.; Brockman, D. Oxidative stress causes vascular dysfunction in the placenta. J. Matern.-Fetal Med. 2000, 9, 79–82. [Google Scholar]
- Mele, J.; Muralimanoharan, S.; Maloyan, A.; Myatt, L. Impaired mitochondrial function in human placenta with increased maternal adiposity. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E419–E425. [Google Scholar] [CrossRef]
- Hastie, R.; Lappas, M. The effect of pre-existing maternal obesity and diabetes on placental mitochondrial content and electron transport chain activity. Placenta 2014, 35, 673–683. [Google Scholar] [CrossRef]
- Prater, M.R.; Laudermilch, C.L.; Liang, C.; Holladay, S.D. Placental oxidative stress alters expression of murine osteogenic genes and impairs fetal skeletal formation. Placenta 2008, 29, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Mutinati, M.; Piccinno, M.; Roncetti, M.; Campanile, D.; Rizzo, A.; Sciorsci, R. Oxidative stress during pregnancy in the sheep. Reprod. Domest. Anim. 2013, 48, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Takino, J.-I.; Nagamine, K.; Nishio, K.; Hori, T. RASGRP2 Suppresses Apoptosis via Inhibition of ROS Production in Vascular Endothelial Cells. Sci. World J. 2019, 2019, 4639165. [Google Scholar] [CrossRef] [PubMed]
- Gourvas, V.; Dalpa, E.; Konstantinidou, A.; Vrachnis, N.; Spandidos, D.A.; Sifakis, S. Angiogenic factors in placentas from pregnancies complicated by fetal growth restriction (review). Mol. Med. Rep. 2012, 6, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wu, Z.; Huang, Z.; Hao, X.; Zhang, L.; Hu, C.; Wei, J.; Deng, J.; Tan, C. Maternal supply of cysteamine alleviates oxidative stress and enhances angiogenesis in porcine placenta. J. Anim. Sci. Biotechnol. 2021, 12, 91. [Google Scholar] [CrossRef]
- Dumolt, J.H.; Powell, T.L.; Jansson, T. Placental Function and the Development of Fetal Overgrowth and Fetal Growth Restriction. Obstet. Gynecol. Clin. North Am. 2021, 48, 247–266. [Google Scholar] [CrossRef]
- Aye, I.L.; Keelan, J.A. Placental ABC transporters, cellular toxicity and stress in pregnancy. Chem. Biol. Interact. 2013, 203, 456–466. [Google Scholar] [CrossRef]
- Hu, C.; Yang, Y.; Deng, M.; Yang, L.; Shu, G.; Jiang, Q.; Zhang, S.; Li, X.; Yin, Y.; Tan, C.; et al. Placentae for Low Birth Weight Piglets Are Vulnerable to Oxidative Stress, Mitochondrial Dysfunction, and Impaired Angiogenesis. Oxidative Med. Cell. Longev. 2020, 2020, 8715412. [Google Scholar] [CrossRef]
- Lappas, M.; Andrikopoulos, S.; Permezel, M. Hypoxanthine-xanthine oxidase down-regulates GLUT1 transcription via SIRT1 resulting in decreased glucose uptake in human placenta. J. Endocrinol. 2012, 213, 49–57. [Google Scholar] [CrossRef]
- Jia, X.; Cao, Y.; Ye, L.; Liu, X.; Huang, Y.; Yuan, X.; Lu, C.; Xu, J.; Zhu, H. Vitamin D stimulates placental L-type amino acid transporter 1 (LAT1) in preeclampsia. Sci. Rep. 2022, 12, 4651. [Google Scholar] [CrossRef] [PubMed]
- Glazier, J.D.; Cetin, I.; Perugino, G.; Ronzoni, S.; Grey, A.M.; Mahendran, D.; Marconi, A.M.; Pardi, G.; Sibley, C.P. Association between the activity of the system A amino acid transporter in the microvillous plasma membrane of the human placenta and severity of fetal compromise in intrauterine growth restriction. Pediatr. Res. 1997, 42, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.A.; Marques, D.B.D.; Costa, T.C.; Oliveira, H.C.; Costa, K.A.; Carrara, E.R.; da Silva, W.; Guimaraes, J.D.; Neves, M.M.; Ibelli, A.M.G.; et al. Transcription Landscape of the Early Developmental Biology in Pigs. Animals 2021, 11, 1443. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Ibeas, P.; Sang, F.; Zhu, Q.; Tang, W.W.C.; Withey, S.; Klisch, D.; Wood, L.; Loose, M.; Surani, M.A.; Alberio, R. Pluripotency and X chromosome dynamics revealed in pig pre-gastrulating embryos by single cell analysis. Nat. Commun. 2019, 10, 500. [Google Scholar] [CrossRef] [PubMed]
- Che, L.; Yang, Z.; Xu, M.; Zhang, Z.; Liu, P.; Xu, S.; Che, L.; Lin, Y.; Fang, Z.; Feng, B.; et al. Dietary energy intake affects fetal survival and development during early and middle pregnancy in Large White and Meishan gilts. Anim. Nutr. (Zhongguo Xu Mu Shou Yi Xue Hui) 2015, 1, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Tao, F.; Xin, L.; Li, Z.; Zhou, X. Effects of maternal serine supplementation on high-fat diet-induced oxidative stress and epigenetic changes in promoters of glutathione synthesis-related genes in offspring. J. Funct. Foods 2018, 47, 316–324. [Google Scholar] [CrossRef]
- Pereira, A.C.; Martel, F. Oxidative stress in pregnancy and fertility pathologies. Cell Biol. Toxicol. 2014, 30, 301–312. [Google Scholar] [CrossRef]
- Hussain, T.; Murtaza, G.; Metwally, E.; Kalhoro, D.H.; Kalhoro, M.S.; Rahu, B.A.; Sahito, R.G.A.; Yin, Y.; Yang, H.S.; Chughtai, M.I.; et al. The Role of Oxidative Stress and Antioxidant Balance in Pregnancy. Mediat. Inflamm. 2021, 2021, 9962860. [Google Scholar] [CrossRef]
- Hernandez-Trejo, M.; Montoya-Estrada, A.; Torres-Ramos, Y.; Espejel-Nunez, A.; Guzman-Grenfell, A.; Morales-Hernandez, R.; Tolentino-Dolores, M.; Laresgoiti-Servitje, E. Oxidative stress biomarkers and their relationship with cytokine concentrations in overweight/obese pregnant women and their neonates. BMC Immunol. 2017, 18, 3. [Google Scholar] [CrossRef]
- Sharma, D.; Shastri, S.; Sharma, P. Intrauterine Growth Restriction: Antenatal and Postnatal Aspects. Clin. Med. Insights. Pediatr. 2016, 10, 67–83. [Google Scholar] [CrossRef]
- Biri, A.; Bozkurt, N.; Turp, A.; Kavutcu, M.; Himmetoglu, O.; Durak, I. Role of oxidative stress in intrauterine growth restriction. Gynecol. Obs. Investig. 2007, 64, 187–192. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Li, J.; Cao, M.; Li, Y.; Zhuo, Y.; Fang, Z.F.; Che, L.Q.; Xu, S.Y.; Feng, B.; Lin, Y.; et al. Dietary supplementation of Bacillus subtilis PB6 improves sow reproductive performance and reduces piglet birth intervals. Anim. Nutr. 2020, 6, 278–287. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).