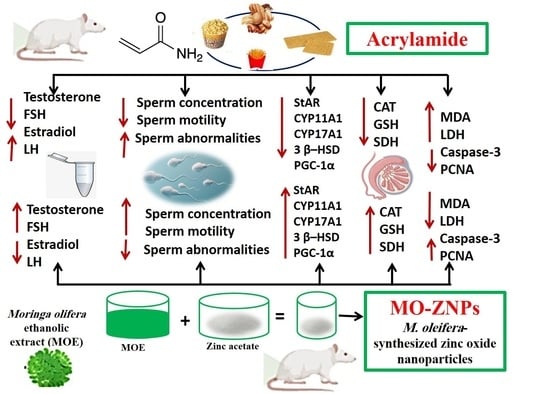
Green Synthesized Zinc Oxide Nanoparticles Using Moringa olifera Ethanolic Extract Lessens Acrylamide-Induced Testicular Damage, Apoptosis, and Steroidogenesis-Related Gene Dysregulation in Adult Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of M. oleifera Ethanol Extracts
2.2. Green Synthesis and Characterization of MO-ZNPs
2.3. Animals and Experimental Design
2.4. Blood and Tissue Sampling
2.5. Semen Analysis
2.6. Male Sex Hormones Measurements
2.7. Testicular Enzymes Evaluations
2.8. Testicular Tissue Oxidative Stress Indices Assessment
2.9. Determination of Testicular Zn Content
2.10. Histopathological Examination
2.11. Immunohistochemistry Evaluation
2.12. Real-Time Quantitative PCR (RT-qPCR) Analysis
2.13. Data Analysis
3. Results
3.1. Changes in Body Weight Gain and Gonadosomatic Index in ACR and/or MO-ZNPs Administered Rats
3.2. Effect of ACR and/or MO-ZNPs Administration on Sperm Quality and Serum Levels of Circulating Reproductive Hormones
3.3. Effect of ACR and/or MO-ZNPs Administration on Testicular Biochemical Indicators and Zn Content
3.4. Histopathological Assessment of Rat’s Testis
3.5. Immunohistochemistry Assessment of Testis
3.6. Effect of ACR and/or MO-ZNPs Administration on Gene Expression Levels of Steroidogenesis-Related Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taeymans, D.; Wood, J.; Ashby, P.; Blank, I.; Studer, A.; Stadler, R.H.; Gondé, P.; Van Eijck, P.; Lalljie, S.; Lingnert, H.; et al. A review of acrylamide: An industry perspective on research, analysis, formation, and control. Crit. Rev. Food Sci. Nutr. 2004, 44, 323–347. [Google Scholar] [CrossRef]
- Jackson, L.S.; Al-Taher, F. Chapter 13—Processing issues: Acrylamide, furan, and trans fatty acids. In Ensuring Global Food Safety, 2nd ed.; Martinović, A., Oh, S., Lelieveld, H., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 229–257. [Google Scholar]
- Cheng, L.; Qin, Y.; Su, Y.; Pan, Y.; Wang, Y.; Liao, R.; Li, Z. Development of a High-Strength and Adhesive Polyacrylamide Gel for Well Plugging. ACS Omega 2022, 7, 6151–6159. [Google Scholar] [CrossRef] [PubMed]
- Mesias, M.; Delgado-Andrade, C.; Holgado, F.; Morales, F.J. Acrylamide content in French fries prepared in food service establishments. LWT 2019, 100, 83–91. [Google Scholar] [CrossRef]
- Bertuzzi, T.; Martinelli, E.; Mulazzi, A.; Rastelli, S. Acrylamide determination during an industrial roasting process of coffee and the influence of asparagine and low molecular weight sugars. Food Chem. 2020, 303, 125372. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.; Kavvadias, D.; Riedel, K.; Scherer, G.; Tricker, A.R. Urinary mercapturic acids and a hemoglobin adduct for the dosimetry of acrylamide exposure in smokers and nonsmokers. Inhal. Toxicol. 2006, 18, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Zamani, E.; Shokrzade, M.; Fallah, M.; Shaki, F. A review of acrylamide toxicity and its mechanism. Pharm. Biomed. Res. 2017, 3, 1–7. [Google Scholar] [CrossRef]
- Goempel, K.; Tedsen, L.; Ruenz, M.; Bakuradze, T.; Schipp, D.; Galan, J.; Eisenbrand, G.; Richling, E. Biomarker monitoring of controlled dietary acrylamide exposure indicates consistent human endogenous background. Arch. Toxicol. 2017, 91, 3551–3560. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-C.; Cheng, Y.-W.; Chen, W.-L.; Fang, W.-H. Negative Association between Acrylamide Exposure and Metabolic Syndrome Markers in Adult Population. Int. J. Environ. Res. Public Health 2021, 18, 11949. [Google Scholar] [CrossRef] [PubMed]
- Kacar, S.; Sahinturk, V.; Kutlu, H.M. Effect of acrylamide on BEAS-2B normal human lung cells: Cytotoxic, oxidative, apoptotic and morphometric analysis. Acta Histochem. 2019, 121, 595–603. [Google Scholar] [CrossRef]
- Shahrzad, E.; Shariati, M.; Naimi, S.; Edalatmanesh, M.A. Protective effect of N-acetylcysteine on changes in serum levels of Pituitary–Gonadal axis hormones and testicular tissue in acrylamide-treated adult rats. Adv. Hum. Biol. 2020, 10, 16. [Google Scholar]
- Ahmed, M.M.; Hammad, A.A.; Orabi, S.H.; Elbaz, H.T.; Elweza, A.E.; Tahoun, E.A.; Elseehy, M.M.; El-Shehawi, A.M.; Mousa, A.A. Reproductive Injury in Male Rats from Acrylamide Toxicity and Potential Protection by Earthworm Methanolic Extract. Animals 2022, 12, 1723. [Google Scholar] [CrossRef]
- Altinoz, E.; Turkoz, Y. The Protective Role Of N-Acetylcysteine Against Acrylamide-Induced Genotoxicity And Oxidative Stress In Rats. Gene Ther. Mol. Biol. 2014, 16, 35–43. [Google Scholar]
- Abdel-Daim, M.M.; Abd Eldaim, M.A.; Hassan, A.G. Trigonella foenum-graecum ameliorates acrylamide-induced toxicity in rats: Roles of oxidative stress, proinflammatory cytokines, and DNA damage. Biochem. Cell Biol. 2015, 93, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, M.; Mombeini, M.A.; Fatemi, I. Neuroprotective effects of Ellagic acid against acrylamide-induced neurotoxicity in rats. Neurol. Res. 2019, 41, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Dahran, N.; Abd-Elhakim, Y.M.; Mohamed, A.A.-R.; Abd-Elsalam, M.M.; Said, E.N.; Metwally, M.M.M.; Abdelhamid, A.E.; Hassan, B.A.; Alsieni, M.; Alosaimi, M.E.; et al. Palliative effect of Moringa olifera-mediated zinc oxide nanoparticles against acrylamide-induced neurotoxicity in rats. Food Chem. Toxicol. 2023, 171, 113537. [Google Scholar] [CrossRef]
- Kandemir, F.M.; Yıldırım, S.; Kucukler, S.; Caglayan, C.; Darendelioğlu, E.; Dortbudak, M.B. Protective effects of morin against acrylamide-induced hepatotoxicity and nephrotoxicity: A multi-biomarker approach. Food Chem. Toxicol. 2020, 138, 111190. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, P.; Lie, T.; Li, J.; Hutz, R.J.; Li, K.; Shi, F. Reproductive toxicity of acrylamide-treated male rats. Reprod. Toxicol. 2010, 29, 225–230. [Google Scholar] [CrossRef]
- Farag, O.M.; Abd-Elsalam, R.M.; El Badawy, S.A. Portulaca oleracea seeds’ extract alleviates acrylamide-induced testicular dysfunction by promoting oxidative status and steroidogenic pathway in rats. BMC Complement. Med. Ther. 2021, 21, 122. [Google Scholar] [CrossRef]
- Yildizbayrak, N.; Erkan, M. Acrylamide disrupts the steroidogenic pathway in Leydig cells: Possible mechanism of action. Toxicol. Environ. Chem. 2018, 100, 235–246. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Manocha, S.; Dhiman, S.; Grewal, A.S.; Guarve, K. Nanotechnology: An approach to overcome bioavailability challenges of nutraceuticals. J. Drug Deliv. Sci. Technol. 2022, 72, 103418. [Google Scholar] [CrossRef]
- Swain, P.S.; Rao, S.B.N.; Rajendran, D.; Dominic, G.; Selvaraju, S. Nano zinc, an alternative to conventional zinc as animal feed supplement: A review. Anim. Nutr. 2016, 2, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Liu, X.; Qu, M. Nanoplastics and Human Health: Hazard Identification and Biointerface. Nanomaterials 2022, 12, 1298. [Google Scholar] [CrossRef]
- Abd El-Hakim, Y.M.; Abdel-Rahman Mohamed, A.; Khater, S.I.; Hamed Arisha, A.; Metwally, M.M.M.; Nassan, M.A.; Hassan, M.E. Chitosan-Stabilized Selenium Nanoparticles and Metformin Synergistically Rescue Testicular Oxidative Damage and Steroidogenesis-Related Genes Dysregulation in High-Fat Diet/Streptozotocin-Induced Diabetic Rats. Antioxidants 2021, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Khater, S.I.; Mohamed, A.A.-R.; Arisha, A.H.; Ebraheim, L.L.M.; El-Mandrawy, S.A.M.; Nassan, M.A.; Mohammed, A.T.; Abdo, S.A. Stabilized-chitosan selenium nanoparticles efficiently reduce renal tissue injury and regulate the expression pattern of aldose reductase in the diabetic-nephropathy rat model. Life Sci. 2021, 279, 119674. [Google Scholar] [CrossRef]
- Surendra, B.; Mallikarjunaswamy, C.; Pramila, S.; Rekha, N. Bio-mediated synthesis of ZnO nanoparticles using Lantana Camara flower extract: Its characterizations, photocatalytic, electrochemical and anti-inflammatory applications. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100442. [Google Scholar]
- Kitture, R.; Chordiya, K.; Gaware, S.; Ghosh, S.; More, P.A.; Kulkarni, P.; Chopade, B.A.; Kale, S. ZnO nanoparticles-red sandalwood conjugate: A promising antidiabetic agent. J. Nanosci. Nanotechnol. 2015, 15, 4046–4051. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.F.H.; Mansour, A.M.; Abo-Youssef, A.M.H.; Elsadek, B.E.; Messiha, B.A.S. Zinc oxide nanoparticles as a novel anticancer approach; In vitro and in vivo evidence. Clin. Exp. Pharmacol. Physiol. 2017, 44, 235–243. [Google Scholar] [CrossRef]
- Kirthi, A.V.; Rahuman, A.A.; Rajakumar, G.; Marimuthu, S.; Santhoshkumar, T.; Jayaseelan, C.; Velayutham, K. Acaricidal, pediculocidal and larvicidal activity of synthesized ZnO nanoparticles using wet chemical route against blood feeding parasites. Parasitol. Res. 2011, 109, 461–472. [Google Scholar] [CrossRef]
- Chelladurai, M.; Sahadevan, R.; Margavelu, G.; Vijayakumar, S.; González-Sánchez, Z.I.; Vijayan, K.; Dharani Balaji, K.C. Anti-skin cancer activity of Alpinia calcarata ZnO nanoparticles: Characterization and potential antimicrobial effects. J. Drug Deliv. Sci. Technol. 2021, 61, 102180. [Google Scholar] [CrossRef]
- Malaikozhundan, B.; Vaseeharan, B.; Vijayakumar, S.; Pandiselvi, K.; Kalanjiam, M.A.R.; Murugan, K.; Benelli, G. Biological therapeutics of Pongamia pinnata coated zinc oxide nanoparticles against clinically important pathogenic bacteria, fungi and MCF-7 breast cancer cells. Microb. Pathog. 2017, 104, 268–277. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Chen, J.; Kalaiselvi, V.; Tungare, K.; Bhori, M.; González-Sánchez, Z.I.; Durán-Lara, E.F. Marine polysaccharide laminarin embedded ZnO nanoparticles and their based chitosan capped ZnO nanocomposites: Synthesis, characterization and in vitro and in vivo toxicity assessment. Environ. Res. 2022, 213, 113655. [Google Scholar] [CrossRef]
- Mirzaei, H.; Darroudi, M. Zinc oxide nanoparticles: Biological synthesis and biomedical applications. Ceram. Int. 2017, 43, 907–914. [Google Scholar] [CrossRef]
- Wang, Z.; Que, B.; Gan, J.; Guo, H.; Chen, Q.; Zheng, L.; Marraiki, N.; Elgorban, A.M.; Zhang, Y. Zinc oxide nanoparticles synthesized from Fraxinus rhynchophylla extract by green route method attenuates the chemical and heat induced neurogenic and inflammatory pain models in mice. J. Photochem. Photobiol. B Biol. 2020, 202, 111668. [Google Scholar] [CrossRef]
- Anwar, F.; Latif, S.; Ashraf, M.; Gilani, A.H. Moringa oleifera: A food plant with multiple medicinal uses. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2007, 21, 17–25. [Google Scholar] [CrossRef]
- González-Romero, J.; Guerra-Hernández, E.J.; Rodríguez-Pérez, C. Chapter 19—Bioactive compounds from Moringa oleifera as promising protectors of in vivo inflammation and oxidative stress processes. In Current Advances for Development of Functional Foods Modulating Inflammation and Oxidative Stress; Hernández-Ledesma, B., Martínez-Villaluenga, C., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 379–399. [Google Scholar]
- Mirone, V.; Napolitano, L.; D’Emmanuele di Villa Bianca, R.; Mitidieri, E.; Sorrentino, R.; Vanelli, A.; Vanacore, D.; Turnaturi, C.; La Rocca, R.; Celentano, G.; et al. A new original nutraceutical formulation ameliorates the effect of Tadalafil on clinical score and cGMP accumulation. Arch. Ital. Di Urol. Androl. Organo Uff. Soc. Ital. Di Ecogr. Urol. E Nefrol. 2021, 93, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman Mohamed, A.; Metwally, M.M.; Khalil, S.R.; Salem, G.A.; Ali, H.A. Moringa oleifera extract attenuates the CoCl2 induced hypoxia of rat’s brain: Expression pattern of HIF-1α, NF-kB, MAO and EPO. Biomed. Pharmacother. 2019, 109, 1688–1697. [Google Scholar] [CrossRef]
- Abd-Elhakim, Y.M.; El Bohi, K.M.; Hassan, S.K.; El Sayed, S.; Abd-Elmotal, S.M. Palliative effects of Moringa olifera ethanolic extract on hemato-immunologic impacts of melamine in rats. Food Chem. Toxicol. 2018, 114, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Abu-Zeid, E.H.; Abdel Fattah, D.M.; Arisha, A.H.; Ismail, T.A.; Alsadek, D.M.; Metwally, M.M.M.; El-Sayed, A.A.; Khalil, A.T. Protective prospects of eco-friendly synthesized selenium nanoparticles using Moringa oleifera or Moringa oleifera leaf extract against melamine induced nephrotoxicity in male rats. Ecotoxicol. Environ. Saf. 2021, 221, 112424. [Google Scholar] [CrossRef]
- Abd-Elhakim, Y.M.; Mohamed, W.A.M.; El Bohi, K.M.; Ali, H.A.; Mahmoud, F.A.; Saber, T.M. Prevention of melamine-induced hepatorenal impairment by an ethanolic extract of Moringa oleifera: Changes in KIM-1, TIMP-1, oxidative stress, apoptosis, and inflammation-related genes. Gene 2021, 764, 145083. [Google Scholar] [CrossRef]
- El-Shehawi, A.M.; Alkafafy, M.; El-Shazly, S.; Sayed, S.; Farouk, S.; Alotaibi, S.; Madkour, D.A.; Khalifa, H.K.; Ahmed, M.M. Moringa oleifera leaves ethanolic extract ameliorates high fat diet-induced obesity in rats. J. King Saud Univ. Sci. 2021, 33, 101552. [Google Scholar] [CrossRef]
- Ghosh, A.; Roychowdhury, T.; Nandi, R.; Maiti, R.; Ghosh, N.N.; Molla, S.A.; Mukhopadhyay, S.; Prodhan, C.; Chaudhury, K.; Das, P.; et al. Inhibitory role of a smart nano-trifattyglyceride of Moringa oleifera root in epithelial ovarian cancer, through attenuation of FSHR—c-Myc axis. J. Tradit. Complement. Med. 2021, 11, 481–492. [Google Scholar] [CrossRef]
- Cuellar-Núñez, M.L.; Gonzalez de Mejia, E.; Loarca-Piña, G. Moringa oleifera leaves alleviated inflammation through downregulation of IL-2, IL-6, and TNF-α in a colitis-associated colorectal cancer model. Food Res. Int. 2021, 144, 110318. [Google Scholar] [CrossRef]
- Akintunde, J.K.; Farai, T.I.; Arogundade, M.R.; Adeleke, J.T. Biogenic zinc-oxide nanoparticles of Moringa oleifera leaves abrogates rotenone induced neuroendocrine toxicity by regulation of oxidative stress and acetylcholinesterase activity. Biochem. Biophys. Rep. 2021, 26, 100999. [Google Scholar] [CrossRef]
- Masserini, M. Nanoparticles for brain drug delivery. ISRN Biochem. 2013, 2013, 238428. [Google Scholar] [CrossRef] [PubMed]
- Okechukwu, P.U.; Okwesili, F.N.; Parker, E.J.; Abubakar, B.; Emmanuel, C.O.; Christian, E.O. Phytochemical and acute toxicity studies of Moringa oleifera ethanol leaf extract. Int. J. Life Sci. Biotechnol. Pharma Res. 2013, 2, 66–71. [Google Scholar]
- Naseer, M.; Aslam, U.; Khalid, B.; Chen, B. Green route to synthesize Zinc Oxide Nanoparticles using leaf extracts of Cassia fistula and Melia azadarach and their antibacterial potential. Sci. Rep. 2020, 10, 9055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hara, S.; Ichinose, H.; Nagashima, D.; Morita, K.; Sakurai, T.; Ichihara, S.; Ichihara, G. Exposure to acrylamide decreases noradrenergic axons in rat brain. NeuroToxicology 2020, 78, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, A.; Oke, B.; Akinloye, A. Characterizing the gonadosomatic index and its relationship with age in greater cane rat (Thryonomys swinderianus, Temminck). J. Vet. Anat. 2009, 2, 53–59. [Google Scholar] [CrossRef]
- Slott, V.L.; Suarez, J.D.; Perreault, S.D. Rat sperm motility analysis: Methodologic considerations. Reprod. Toxicol. 1991, 5, 449–458. [Google Scholar] [CrossRef]
- Robb, G.W.; Amann, R.P.; Killian, G.J. Daily sperm production and epididymal sperm reserves of pubertal and adult rats. J. Reprod. Fertil. 1978, 54, 103–107. [Google Scholar] [CrossRef]
- Knox, R.; Rodriguez-Zas, S.; Roth, S.; Ruggiero, K. Use and accuracy of instruments to estimate sperm concentration: Pros, Cons & Economics. In Proceedings of the American Association of Swine Veterinarians 33rd Annual Meeting, Kansas City, MO, USA, 26 February–1 March 2002; pp. 20–31. [Google Scholar]
- Filler, R. Methods for evaluation of rat epididymal sperm morphology. Methods Toxicol. 1993, 3, 334–343. [Google Scholar]
- Zirkin, B.R.; Chen, H. Regulation of Leydig cell steroidogenic function during aging. Biol. Reprod. 2000, 63, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Buhl, S.N.; Jackson, K.Y. Optimal conditions and comparison of lactate dehydrogenase catalysis of the lactate-to-pyruvate and pyruvate-to-lactate reactions in human serum at 25, 30, and 37 degrees C. Clin. Chem. 1978, 24, 828–831. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.K. Calorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Beutler, E. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Choi, J.; Kim, H.; Kim, P.; Jo, E.; Kim, H.-M.; Lee, M.-Y.; Jin, S.M.; Park, K. Toxicity of zinc oxide nanoparticles in rats treated by two different routes: Single intravenous injection and single oral administration. J. Toxicol. Environ. Health Part A 2015, 78, 226–243. [Google Scholar] [CrossRef]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques E-Book; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Gibson-Corley, K.N.; Olivier, A.K.; Meyerholz, D.K. Principles for valid histopathologic scoring in research. Vet. Pathol. 2013, 50, 1007–1015. [Google Scholar] [CrossRef]
- Johnsen, S.G. Testicular biopsy score count—A method for registration of spermatogenesis in human testes: Normal values and results in 335 hypogonadal males. Hormones 1970, 1, 2–25. [Google Scholar] [CrossRef] [PubMed]
- Saleh, H.; Nassar, A.M.K.; Noreldin, A.E.; Samak, D.; Elshony, N.; Wasef, L.; Elewa, Y.H.A.; Hassan, S.M.A.; Saati, A.A.; Hetta, H.F.; et al. Chemo-Protective Potential of Cerium Oxide Nanoparticles against Fipronil-Induced Oxidative Stress, Apoptosis, Inflammation and Reproductive Dysfunction in Male White Albino Rats. Molecules 2020, 25, 3479. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Buckels, E.J.; Ross, J.M.; Phua, H.H.; Bloomfield, F.H.; Jaquiery, A.L. Whole-slide imaging and a Fiji-based image analysis workflow of immunohistochemistry staining of pancreatic islets. MethodsX 2022, 9, 101856. [Google Scholar] [CrossRef]
- Vis, A.N.; Kranse, R.; Nigg, A.L.; Van Der Kwast, T.H. Quantitative analysis of the decay of immunoreactivity in stored prostate needle biopsy sections. Am. J. Clin. Pathol. 2000, 113, 369–373. [Google Scholar] [CrossRef]
- Arisha, A.H.; Ahmed, M.M.; Kamel, M.A.; Attia, Y.A.; Hussein, M.M.A. Morin ameliorates the testicular apoptosis, oxidative stress, and impact on blood-testis barrier induced by photo-extracellularly synthesized silver nanoparticles. Environ. Sci. Pollut. Res. Int. 2019, 26, 28749–28762. [Google Scholar] [CrossRef] [PubMed]
- Khamis, T.; Abdelalim, A.F.; Abdallah, S.H.; Saeed, A.A.; Edress, N.M.; Arisha, A.H. Early intervention with breast milk mesenchymal stem cells attenuates the development of diabetic-induced testicular dysfunction via hypothalamic Kisspeptin/Kiss1r-GnRH/GnIH system in male rats. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165577. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, X.; Wang, Y.; Song, T.; Li, H.; Xie, L.; Li, L.; Chen, X.; Ma, L.; Chen, Y. Fibroblast growth factor 1 promotes rat stem Leydig cell development. Front. Endocrinol. 2019, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, S.; Asaithambi, K.; Jayaram, P.; Medhamurthy, R. Analysis of 17β-estradiol (E2) role in the regulation of corpus luteum function in pregnant rats: Involvement of IGFBP5 in the E2-mediated actions. Reprod. Biol. Endocrinol. RBE 2016, 14, 19. [Google Scholar] [CrossRef]
- Stojkov, N.; Janjic, M.; Kostic, T.; Andric, S. Orally applied doxazosin disturbed testosterone homeostasis and changed the transcriptional profile of steroidogenic machinery, cAMP/cGMP signalling and adrenergic receptors in Leydig cells of adult rats. Andrology 2013, 1, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Said, A.A.; Nasr, Y.; Galal, A.A.; Abdelhamid, A.E.; Mohamed, H.A.; Metwally, M.M.; Said, M.A.; Nassan, M.A.; Dahran, N.; Mohamed, A.A.-R. Concerns with Male Infertility Induced by Exposure to Titanium Nanoparticles and the Supporting Impact of Pelargonium graveolens Essential Oil: Morphometric Records in Male-Wistar Rats. Life 2022, 12, 639. [Google Scholar] [CrossRef]
- Yang, Z.-Y.; Yuan, C.-X. IL-17A promotes the neuroinflammation and cognitive function in sevoflurane anesthetized aged rats via activation of NF-κB signaling pathway. BMC Anesthesiol. 2018, 18, 1–9. [Google Scholar] [CrossRef]
- Wang, M.; Guckland, A.; Murfitt, R.; Ebeling, M.; Sprenger, D.; Foudoulakis, M.; Koutsaftis, A. Relationship between magnitude of body weight effects and exposure duration in mammalian toxicology studies and implications for ecotoxicological risk assessment. Environ. Sci. Eur. 2019, 31, 38. [Google Scholar] [CrossRef]
- Fadil, H.A.E.; Behairy, A.; Ebraheim, L.L.M.; Abd-Elhakim, Y.M.; Fathy, H.H. The palliative effect of mulberry leaf and olive leaf ethanolic extracts on hepatic CYP2E1 and caspase-3 immunoexpression and oxidative damage induced by paracetamol in male rats. Environ. Sci. Pollut. Res. 2023; ahead of print. [Google Scholar] [CrossRef]
- Abo-El-Sooud, K.; Abd-Elhakim, Y.M.; Hashem, M.M.M.; El-Metwally, A.E.; Hassan, B.A.; El-Nour, H.H.M. Ameliorative effects of quercetin against hepatic toxicity of oral sub-chronic co-exposure to aluminum oxide nanoparticles and lead-acetate in male rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022; ahead of print. [Google Scholar] [CrossRef]
- Rodu, B.; Cole, P.; Mandel, J.S. Evaluation of the national toxicology program report on carcinogens. Regul. Toxicol. Pharmacol. 2012, 64, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Hashem, M.M.; Abo-El-Sooud, K.; Abd El-Hakim, Y.M.; Badr, Y.A.-H.; El-Metwally, A.E.; Bahy-El-Dien, A. The impact of long-term oral exposure to low doses of acrylamide on the hematological indicators, immune functions, and splenic tissue architecture in rats. Int. Immunopharmacol. 2022, 105, 108568. [Google Scholar] [CrossRef] [PubMed]
- Garey, J.; Paule, M.G. Effects of chronic low-dose acrylamide exposure on progressive ratio performance in adolescent rats. NeuroToxicology 2007, 28, 998–1002. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Wang, Y.; Jin, X.-W.; Zhang, Y.; Chen, J.-S.; Ma, W.-W.; Wu, Y.-H.; Wang, D.-C. A urinary metabolomics study of rats after the exposure to acrylamide by ultra performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry. Mol. Biosyst. 2015, 11, 1146–1155. [Google Scholar] [CrossRef]
- Swamy, M.; Subbaiah, K.; Aumau, B.; Kamala, K.; Rao, K.; Raju, K. Toxic effect of acrylamide on body weight, the study of antioxidants and histoarchitecture of heart in the developing chick embryo. Indian J. Appl. Res. 2013, 3, 27–30. [Google Scholar] [CrossRef]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2013, 18, 144. [Google Scholar]
- Vickram, S.; Rohini, K.; Srinivasan, S.; Veenakumari, D.N.; Archana, K.; Anbarasu, K.; Jeyanthi, P.; Thanigaivel, S.; Gulothungan, G.; Rajendiran, N. Role of zinc (Zn) in human reproduction: A journey from initial spermatogenesis to childbirth. Int. J. Mol. Sci. 2021, 22, 2188. [Google Scholar] [CrossRef]
- Bao, B.; Ahmad, A.; Azmi, A.; Li, Y.; Prasad, A.; Sarkar, F.H. The biological significance of zinc in inflammation and aging. In Inflammation, Advancing Age and Nutrition; Elsevier: Amsterdam, The Netherlands, 2014; pp. 15–27. [Google Scholar]
- Imamoğlu, S.; Bereket, A.; Turan, S.; Taga, Y.; Haklar, G. Effect of zinc supplementation on growth hormone secretion, IGF-I, IGFBP-3, somatomedin generation, alkaline phosphatase, osteocalcin and growth in prepubertal children with idiopathic short stature. J. Pediatr. Endocrinol. Metab. JPEM 2005, 18, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Selvaraju, V.; Baskaran, S.; Agarwal, A.; Henkel, R. Environmental contaminants and male infertility: Effects and mechanisms. Andrologia 2021, 53, e13646. [Google Scholar] [CrossRef] [PubMed]
- Ou, C.; Uo, D. Effects of acrylamide on the reproductive hormones and sperm quality in male rats. Int. J. Sci. Res. 2015, 6, 5–8. [Google Scholar]
- Erfani Majd, N.; Hajirahimi, A.; Tabandeh, M.R.; Molaei, R. Protective effects of green and chemical zinc oxide nanoparticles on testis histology, sperm parameters, oxidative stress markers and androgen production in rats treated with cisplatin. Cell Tissue Res. 2021, 384, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Aghajanian, H.K.; Haig-Ladewig, L.A.; Gerton, G.L. Sorbitol can fuel mouse sperm motility and protein tyrosine phosphorylation via sorbitol dehydrogenase. Biol. Reprod. 2009, 80, 124–133. [Google Scholar] [CrossRef]
- El-Kabbani, O.; Darmanin, C.; Chung, R.P. Sorbitol dehydrogenase: Structure, function and ligand design. Curr. Med. Chem. 2004, 11, 465–476. [Google Scholar] [CrossRef]
- Abaspour Aporvari, M.H.; Mamoei, M.; Tabatabaei Vakili, S.; Zareei, M.; Dadashpour Davachi, N. The Effect of Oral Administration of Zinc Oxide Nanoparticles on Quantitative and Qualitative Properties of Arabic Ram Sperm and Some Antioxidant Parameters of Seminal Plasma in the Non-Breeding Season. Arch. Razi Inst. 2018, 73, 121–129. [Google Scholar] [CrossRef]
- Yunsang, C.; Wanxi, Y. Functions of essential nutrition for high quality spermatogenesis. Adv. Biosci. Biotechnol. 2011, 2, 182–197. [Google Scholar]
- Yildrim, S.; Sengul, E.; Aksu, E.H.; Cinar, İ.; Gelen, V.; Tekin, S.; Dag, Y. Effects of Selenium on Some Reproductive Parameters in Acrylamide-Induced Testis Toxicity in Rats. Res. Sq. 2022, 1–25. [Google Scholar] [CrossRef]
- Aydin, Y. Acrylamide and its metabolite glycidamide can affect antioxidant defenses and steroidogenesis in Leydig and Sertoli cells. Toxicol. Environ. Chem. 2018, 100, 247–257. [Google Scholar] [CrossRef]
- Elaidy, S.M.; Tawfik, M.M.; Ameen, A.M.; Hassan, W.A.; El Sherif, I.; Amin, M.K.; Elkholy, S.E. Metformin alleviates the dysregulated testicular steroidogenesis and spermatogenesis induced by carbimazole in levothyroxine-primed rats. Life Sci. 2022, 307, 120904. [Google Scholar] [CrossRef] [PubMed]
- Raslan, M.; Ismail, Z.; Abdel-Wareth, A. Application of zinc oxide nanoparticles on productive performance in rabbit nutrition: A Review. SVU-Int. J. Agric. Sci. 2020, 2, 278–290. [Google Scholar] [CrossRef]
- Mohamed, D.A.; Abdelrahman, S.A. The possible protective role of zinc oxide nanoparticles (ZnONPs) on testicular and epididymal structure and sperm parameters in nicotine-treated adult rats (a histological and biochemical study). Cell Tissue Res. 2019, 375, 543–558. [Google Scholar] [CrossRef] [PubMed]
- Bara, N.; Kaul, G. Enhanced steroidogenic and altered antioxidant response by ZnO nanoparticles in mouse testis Leydig cells. Toxicol. Ind. Health 2018, 34, 571–588. [Google Scholar] [CrossRef]
- Husain, W.M.; Araak, J.K.; Ibrahim, O.M.S. Effect of different doses from znonps on the pituitary-testes axis function in adult male rats. Adv. Anim. Vet. Sci 2019, 7, 550–556. [Google Scholar] [CrossRef]
- Al-Ani, N.K.; Al-Kawaz, U.; Saeed, B.T. Protective influence of zinc on reproductive parameters in male rat treated with cadmium. Am. J. Med. Med. Sci. 2015, 5, 73–81. [Google Scholar]
- Bedwal, S.; Prasad, S.; Nair, N.; Saini, M.R.; Bedwal, R.S. Catalase in testes and epididymidis of wistar rats fed zinc deficient diet. Indian J. Pharm. Sci. 2009, 71, 55–58. [Google Scholar] [CrossRef]
- Yilmaz, B.O.; Yildizbayrak, N.; Aydin, Y.; Erkan, M. Evidence of acrylamide- and glycidamide-induced oxidative stress and apoptosis in Leydig and Sertoli cells. Hum. Exp. Toxicol. 2017, 36, 1225–1235. [Google Scholar] [CrossRef]
- Anan, H.H.; Zidan, R.A.; Abd El-Baset, S.A.; Ali, M.M. Ameliorative effect of zinc oxide nanoparticles on cyclophosphamide induced testicular injury in adult rat. Tissue Cell 2018, 54, 80–93. [Google Scholar] [CrossRef]
- Rahman, H.U.; Qureshi, M.S.; Khan, R.U. Influence of dietary zinc on semen traits and seminal plasma antioxidant enzymes and trace minerals of beetal bucks. Reprod. Domest. Anim. Zuchthyg. 2014, 49, 1004–1007. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.P.; Bhatnagar, A. Role of Thiols in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.S.; Singh, S.P.; Singhal, P.; Horne, D.; Singhal, J.; Awasthi, S. Antioxidant role of glutathione S-transferases: 4-Hydroxynonenal, a key molecule in stress-mediated signaling. Toxicol. Appl. Pharm. 2015, 289, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Celino, F.T.; Yamaguchi, S.; Miura, C.; Ohta, T.; Tozawa, Y.; Iwai, T.; Miura, T. Tolerance of spermatogonia to oxidative stress is due to high levels of Zn and Cu/Zn superoxide dismutase. PLoS ONE 2011, 6, e16938. [Google Scholar] [CrossRef] [PubMed]
- Kabel, A.M. Zinc/alogliptin combination attenuates testicular toxicity induced by doxorubicin in rats: Role of oxidative stress, apoptosis and TGF-β1/NF-κB signaling. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 97, 439–449. [Google Scholar] [CrossRef]
- Maremanda, K.P.; Khan, S.; Jena, G. Zinc protects cyclophosphamide-induced testicular damage in rat: Involvement of metallothionein, tesmin and Nrf2. Biochem. Biophys. Res. Commun. 2014, 445, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Adelakun, S.A.; Akintunde, O.W.; Jeje, S.O.; Alao, O.A. Ameliorating and protective potential of 1-isothiocyanato-4-methyl sulfonyl butane on cisplatin induced oligozoospermia and testicular dysfunction via redox-inflammatory pathway: Histomorphometric and immunohistochemical evaluation using proliferating cell nuclear antigen. Phytomed. Plus 2022, 2, 100268. [Google Scholar]
- Zhao, M.; Wang, P.; Zhu, Y.; Liu, X.; Hu, X.; Chen, F. The chemoprotection of a blueberry anthocyanin extract against the acrylamide-induced oxidative stress in mitochondria: Unequivocal evidence in mice liver. Food Funct. 2015, 6, 3006–3012. [Google Scholar] [CrossRef]
- Tian, L.; Zhu, F.; Ren, H.; Jiang, J.; Li, W. Effects of nano-zinc oxide on antioxidant function in broilers. Chin. J. Anim. Nutr. 2009, 21, 534–539. [Google Scholar]
- Kaufmann, S.H.; Hengartner, M.O. Programmed cell death: Alive and well in the new millennium. Trends Cell Biol. 2001, 11, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.M.; Rahuman, A.A.; Marimuthu, S.; Kirthi, A.V.; Anbarasan, K.; Rajakumar, G. An Investigation of the Cytotoxicity and Caspase-Mediated Apoptotic Effect of Green Synthesized Zinc Oxide Nanoparticles Using Eclipta prostrata on Human Liver Carcinoma Cells. Nanomaterials 2015, 5, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Cierech, M.; Wojnarowicz, J.; Kolenda, A.; Krawczyk-Balska, A.; Prochwicz, E.; Woźniak, B.; Łojkowski, W.; Mierzwińska-Nastalska, E. Zinc Oxide Nanoparticles Cytotoxicity and Release from Newly Formed PMMA-ZnO Nanocomposites Designed for Denture Bases. Nanomaterials 2019, 9, 1318. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Jin, Y.; Li, Y.; Tjong, S.C. Interactions of Zinc Oxide Nanostructures with Mammalian Cells: Cytotoxicity and Photocatalytic Toxicity. Int. J. Mol. Sci. 2020, 21, 6305. [Google Scholar] [CrossRef] [PubMed]
- El-Behery, E.I.; El-Naseery, N.I.; El-Ghazali, H.M.; Elewa, Y.H.A.; Mahdy, E.A.A.; El-Hady, E.; Konsowa, M.M.H. The efficacy of chronic zinc oxide nanoparticles using on testicular damage in the streptozotocin-induced diabetic rat model. Acta Histochem. 2019, 121, 84–93. [Google Scholar] [CrossRef]
- Aktas, C.; Erboga, M.; Fidanol Erboga, Z.; Bozdemir Donmez, Y.; Topcu, B.; Gurel, A. Protective effects of Urtica dioica L. on experimental testicular ischaemia reperfusion injury in rats. Andrologia 2017, 49, e12636. [Google Scholar] [CrossRef] [PubMed]
- Jeremy, M.; Gurusubramanian, G.; Roy, V.K. Vitamin D3 regulates apoptosis and proliferation in the testis of D-galactose-induced aged rat model. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]







| Genes | Primer Sequences | Product Size /bp | GenBank Accession Numbers | References |
|---|---|---|---|---|
| StAR | 5’-CCCAAATGTCAAGGAAATCA-3’ | 187 | NM_031558.3 | [72] |
| 3’-AGGCATCTCCCCAAAGTG-5’ | ||||
| CYP11A1 | 5’-AAGTATCCGTGATGTGGG-3’ | 127 | NM_017286.3 | [72] |
| 3’-TCATACAGTGTCGCCTTTTCT-5’ | ||||
| CYP17A1 | 5’-TGGCTTTCCTGGTGCACAATC-3’ | 90 | NM_012753.2 | [72] |
| 3’-TGAAAGTTGGTGTTCGGCTGAAG-5’ | ||||
| CYP19A1 | 5’-GCTGAGAGACGTGGAGACCTG-3’ | 178 | NM_017085.2 | [73] |
| 3’-CTCTGTCACCAACAACAGTGTGG-5’ | ||||
| HSD17B3 | 5’-AGTGTGTGAGGTTCTCCCGGTACCT-3’ | 161 | NM_054007.1 | [74] |
| 3’-TACAACATTGAGTCCATGTCTGGCCAG-5’ | ||||
| PGC1-α | 5’-ATGTGTCGCCTTCTTGCTCT-3’ | 180 | NM_031347.1 | [75] |
| 3’-ATCTACTGCCTGGGGACCTT-5’ | ||||
| GAPDH | 5’-GGCACAGTCAAGGCTGAGAATG-3’ | 143 | NM_017008.4 | [76] |
| 3’-ATGGTGGTGAAGACGCCAGTA-5’ |
| Experimental Groups | Body Weight Gain (g) | Absolute Weight of Testis (g) | Gonadosomatic Index |
|---|---|---|---|
| Control | 168.33 ± 14.75 | 1.97 ± 0.08 | 1.19 ± 0.12 |
| MO-ZNPs | 190.33 * ± 2.60 | 2.30 ± 0.06 | 1.21 ± 0.01 |
| ACR | 141.67 * ± 7.54 | 1.27 * ± 0.09 | 0.89 ± 0.04 |
| ACR+MO-ZNPs | 164.33 # ± 1.45 | 1.83 # ± 0.20 | 1.12 ± 0.13 |
| Estimated Parameters | Experimental Groups | |||
|---|---|---|---|---|
| Control | MO-ZNPs | ACR | ACR+MO-ZNPs | |
| Sperm parameters | ||||
| Sperm motility (%) | 85.00 ± 2.89 | 94.00± 1.00 | 25.00 * ± 2.89 | 65.00 *# ±2.89 |
| Sperm count (sp.cc/mL × 125 × 104) | 55.67 ± 2.96 | 68.00 * ±1.73 | 14.00 * ± 2.08 | 39.33 *# ±3.48 |
| Sperm abnormalities (%) | 16.78 ± 1.24 | 12.66± 0.67 | 48.34 * ± 3.84 | 27.78 *# ±0.87 |
| Serum hormonal analysis | ||||
| Testosterone (ng/mL) | 2.18 ± 0.06 | 2.33 ± 0.04 | 0.16 * ± 0.03 | 1.71 *# ± 0.12 |
| FSH (ng/mL) | 5.13 ± 0.31 | 5.92 ± 0.35 | 2.60 * ± 0.12 | 3.10 * ± 0.20 |
| Estradiol (pg/mL) | 42.03 ± 0.09 | 42.33 ± 1.20 | 60.00 * ± 4.00 | 46.83 *# ± 3.03 |
| LH (ng/mL) | 1.90 ± 0.16 | 1.61 ± 0.03 | 3.44 * ± 0.35 | 2.19 *# ± 0.24 |
| Estimated Parameters | Experimental Groups | |||
|---|---|---|---|---|
| Control | MO-ZNPs | ACR | ACR+MO-ZNPs | |
| Testicular enzymes | ||||
| LDH (U/L) | 125.18 ± 1.50 | 94.43 ± 5.44 | 209.29 * ± 10.61 | 150.74 # ± 7.05 |
| SDH (ng/mg) | 42.12 ± 2.25 | 43.47 ± 1.95 | 5.88 * ± 1.52 | 30.47 *# ± 0.93 |
| Antioxidant parameters | ||||
| CAT (ng/mg) | 10.29 ± 0.73 | 11.07 ± 0.08 | 0.55 * ± 0.04 | 3.59 *# ± 0.47 |
| GSH (ng/mg) | 130.13 ± 4.33 | 207.53 * ±12.84 | 68.88 * ± 4.71 | 142.04 # ± 4.75 |
| MDA (nmol/mg) | 1.02 ± 0.04 | 0.49 ± 0.04 | 6.17 * ± 0.72 | 1.97 # ± 0.16 |
| Zn residues (ppm) | 11.90 ± 0.44 | 13.09 # ± 0.52 | 10.52 ± 0.06 | 11.33 ± 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostafa-Hedeab, G.; Behairy, A.; Abd-Elhakim, Y.M.; Mohamed, A.A.-R.; Noreldin, A.E.; Dahran, N.; Gaber, R.A.; Alqahtani, L.S.; Essawi, W.M.; Eskandrani, A.A.; et al. Green Synthesized Zinc Oxide Nanoparticles Using Moringa olifera Ethanolic Extract Lessens Acrylamide-Induced Testicular Damage, Apoptosis, and Steroidogenesis-Related Gene Dysregulation in Adult Rats. Antioxidants 2023, 12, 361. https://doi.org/10.3390/antiox12020361
Mostafa-Hedeab G, Behairy A, Abd-Elhakim YM, Mohamed AA-R, Noreldin AE, Dahran N, Gaber RA, Alqahtani LS, Essawi WM, Eskandrani AA, et al. Green Synthesized Zinc Oxide Nanoparticles Using Moringa olifera Ethanolic Extract Lessens Acrylamide-Induced Testicular Damage, Apoptosis, and Steroidogenesis-Related Gene Dysregulation in Adult Rats. Antioxidants. 2023; 12(2):361. https://doi.org/10.3390/antiox12020361
Chicago/Turabian StyleMostafa-Hedeab, Gomaa, Amany Behairy, Yasmina M. Abd-Elhakim, Amany Abdel-Rahman Mohamed, Ahmed E. Noreldin, Naief Dahran, Rasha A. Gaber, Leena S. Alqahtani, Walaa M. Essawi, Areej A. Eskandrani, and et al. 2023. "Green Synthesized Zinc Oxide Nanoparticles Using Moringa olifera Ethanolic Extract Lessens Acrylamide-Induced Testicular Damage, Apoptosis, and Steroidogenesis-Related Gene Dysregulation in Adult Rats" Antioxidants 12, no. 2: 361. https://doi.org/10.3390/antiox12020361
APA StyleMostafa-Hedeab, G., Behairy, A., Abd-Elhakim, Y. M., Mohamed, A. A.-R., Noreldin, A. E., Dahran, N., Gaber, R. A., Alqahtani, L. S., Essawi, W. M., Eskandrani, A. A., & El-Shetry, E. S. (2023). Green Synthesized Zinc Oxide Nanoparticles Using Moringa olifera Ethanolic Extract Lessens Acrylamide-Induced Testicular Damage, Apoptosis, and Steroidogenesis-Related Gene Dysregulation in Adult Rats. Antioxidants, 12(2), 361. https://doi.org/10.3390/antiox12020361