Phenolic Compounds and Antioxidant Status of Cookies Supplemented with Apple Pomace
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Gluten-Free Cookies Preparation
2.2.2. Chemical Composition
2.2.3. Antioxidants Content and Antioxidant/Antiradical Activity
- Extraction procedure
- Total phenolic content (TPC)
- Antioxidant/antiradical activity
- Determination of antiradical activity by synthetic DPPH radical according to Brand-Williams et al. [31]
2.2.4. Determination of Individual Polyphenols by UPLC-PDA-MS/MS
- Extraction:
- Assay:
2.2.5. Physical Properties of Cookies with Apple Pomace
- Texture profile analysis:
- The volume of cookies:
- Instrumental Color Analysis:
2.2.6. Statistical Analysis
3. Results
3.1. Apple Pomace Characteristics
3.2. Characteristics of Cookies with a Different Share of Apple Pomace
3.3. Physical Properties of Gluten-Free Cookies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lyu, F.; Luiz, S.F.; Azeredo, D.R.P.; Cruz, A.G.; Ajlouni, S.; Ranadheera, C.S. Apple pomace as a functional and healthy ingredient in food products: A review. Processes 2020, 8, 319. [Google Scholar] [CrossRef]
- Górnaś, P.; Mišina, I.; Olšteine, A.; Krasnova, I.; Pugajeva, I.; Lācis, G.; Siger, A.; Michalak, M.; Soliven, A.; Segliņa, D. Phenolic compounds in different fruit parts of crab apple: Dihydrochalcones as promising quality markers of industrial apple pomace by-products. Ind. Crops Prod. 2015, 74, 607–612. [Google Scholar] [CrossRef]
- Antonic, B.; Jancikova, S.; Dordevic, D.; Tremlova, B. Apple pomace as food fortification ingredient: A systematic review and meta-analysis. J. Food Sci. 2020, 85, 2977–2985. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.; da Silva, J.A.L.; Pintado, M. Fruit and vegetable by-products’ flours as ingredients: A review on production process, health benefits and technological functionalities. LWT 2022, 154, 112707. [Google Scholar] [CrossRef]
- Bhushan, S.; Kalia, K.; Sharma, M.; Singh, B.; Ahuja, P.S. Processing of apple pomace for bioactive molecules. Crit. Rev. Biotechnol. 2008, 28, 285–296. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Bebak, A. Aktywność biologiczna wybranych wytłoków owocowych oraz warzywnych. Zywnosc Nauka Technol. Jakosc 2012, 19, 55–65. [Google Scholar]
- Grigelmo-Miguel, N.; Martin-Belloso, O. Comparison of dietary fibre from by-products of processing fruits and greens and from cereals. LWT Food Sci. Technol. 1999, 32, 503–508. [Google Scholar] [CrossRef]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2011, 49, 255–266. [Google Scholar] [CrossRef]
- Gazalli, H.; Malik, A.H.; Jalal, H.; Afshan, S.; Mir, A. Proximate composition of carrot powder and apple pomace powder. Int. J. Food Nutr. Saf. 2013, 3, 25–28. [Google Scholar]
- Li, X.; He, X.; Lv, Y.; He, Q. Extraction and functional properties of water-soluble dietary fiber from apple pomace. J. Food Process Eng. 2014, 37, 293–298. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Rigo, A.; Tonon, D.; Mattivi, F. Quantitation of polyphenols in different apple varieties. J. Agric. Food Chem. 2004, 52, 6532–6538. [Google Scholar] [CrossRef] [PubMed]
- Ćetković, G.; Čanadanović-Brunet, J.; Djilas, S.; Savatović, S.; Mandić, A.; Tumbas, V. Assessment of polyphenolic content and in vitro antiradical characteristics of apple pomace. Food Chem. 2008, 109, 340–347. [Google Scholar] [CrossRef]
- Kammerer, D.R.; Kammerer, J.; Valet, R.; Carle, R. Recovery of polyphenols from the by-products of plant food processing and application as valuable food ingredients. Food Res. Int. 2014, 65, 2–12. [Google Scholar] [CrossRef]
- Balasuriya, N.; Rupasinghe, H.V. Antihypertensive properties of flavonoid-rich apple peel extract. Food Chem. 2012, 135, 2320–2325. [Google Scholar] [CrossRef]
- Makarova, E.; Górnaś, P.; Konrade, I.; Tirzite, D.; Cirule, H.; Gulbe, A.; Pugajeva, I.; Seglina, D.; Dambrova, M. Acute anti-hyperglycaemic effects of an unripe apple preparation containing phlorizin in healthy volunteers: A preliminary study. J. Sci. Food Agric. 2014, 95, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Muela, C.; Rodriguez, H.E.; Arzola, C.; Diaz-Plascencia, D.; Ramirez-Godinez, J.A.; Flores-Marinelarena, A.; Mancillas-Flores, P.F.; Corral, G. Antioxidant activity in plasma and rumen papillae development in lambs fed fermented apple pomace. J. Anim. Sci. 2015, 93, 2357–2362. [Google Scholar] [CrossRef]
- Skinner, R.C.; Gigliotti, J.C.; Ku, K.-M.; Tou, J.C. A comprehensive analysis of the composition, health benefits, and safety of apple pomace. Nutr. Rev. 2018, 76, 893–909. [Google Scholar] [CrossRef]
- Aryal, S.; Skinner, T.; Bridges, B.; Weber, J.T. The pathology of Parkinson’s disease and potential benefit of dietary polyphenols. Molecules 2020, 25, 4382. [Google Scholar] [CrossRef]
- Sudha, M.L.; Dharmesh, S.M.; Pynam, H.; Bhimangouder, S.V.; Eipson, S.W.; Somasundaram, R.; Nanjarajurs, S.M. Antioxidant and cyto/DNA protective properties of apple pomace enriched bakery products. J. Food Sci. Technol. 2016, 53, 1909–1918. [Google Scholar] [CrossRef]
- Maluf, S.W.; Filho, D.W.; Parisotto, E.B.; da Silva de Medeiros, G.; Pereira, C.H.J.; Maraslis, F.T.; Schoeller, C.C.D.; da Rosa, J.S.; Fröde, T.S. DNA damage, oxidative stress, and inflammation in children with celiac disease. Genet. Mol. Biol. 2020, 43, e20180390. [Google Scholar] [CrossRef]
- Rowicka, G.; Czaja-Bulsa, G.; Chełchowska, M.; Riahi, A.; Strucińska, M.; Weker, H.; Ambroszkiewicz, J. Oxidative and Antioxidative Status of Children with Celiac Disease Treated with a Gluten Free-Diet. Oxid. Med. Cell Longev. 2018, 2018, 1324820. [Google Scholar] [CrossRef]
- Grindel, A.; Müllner, E.; Brath, H.; Jäger, W.; Henriksen, T.; Poulsen, H.E.; Marko, D.; Wagner, K.-H. Influence of polyphenol-rich apple pomace extract on oxidative damage to DNA in type 2 diabetes mellitus individuals. Cancer Metab. 2014, 2, 1–2. [Google Scholar] [CrossRef]
- Vazhappilly, C.G.; Rupasinghe, H.P.V. Apple flavonoids suppress carcinogen-induced DNA damage in normal human bronchial epithelial cells. Oxid. Med. Cell Longev. 2017, 2017, 1767198. [Google Scholar]
- Christa, K.; Soral-Śmietana, M.; Lewandowicz, G. Buckwheat starch: Structure, functionality and enzyme in vitro susceptibility upon the roasting process. Int. J. Food Sci. Nutr. 2009, 60, 140–154. [Google Scholar] [CrossRef] [PubMed]
- Wierdsma, N.; van Bokhorst-de van der Schueren, M.; Berkenpas, M.; Mulder, C.; van Bodegraven, A. Vitamin and mineral deficiencies are highly prevalent in newly diagnosed celiac disease patients. Nutrients 2013, 5, 3975–3992. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Rockville, MD, USA, 2006. [Google Scholar]
- AACC. American Association of Cereal Chemists. Approved Methods; American Association of Cereal Chemists: Eagan, MN, USA, 2000. [Google Scholar]
- Buksa, K.; Nowotna, A.; Ziobro, R. Application of cross-linked and hydrolyzed arabinoxylans in baking of model rye bread. Food Chem. 2016, 192, 991–996. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; pp. 152–178. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Oszmiański, J.; Kolniak-Ostek, J.; Wojdyło, A. Application of ultra performance liquid chromatography-photodiode detector-quadrupole/time of flight-mass spectrometry (UPLC-PDA-Q/TOF-MS) method for the characterization of phenolic compounds of Lepidium sativum L. sprouts. Eur. Food Res. Technol. 2013, 236, 699–706. [Google Scholar] [CrossRef]
- CIE. Colorimetry, 3rd ed.; International Commission on Illumination: Vienna, Austria, 2004. [Google Scholar]
- Escarpa, A.; González, M. High-performance liquid chromatography with diode-array detection for the determination of phenolic compounds in peel and pulp from different apple varieties. J. Chromatogr. A 1998, 823, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Corral, J.; Quintero-Ramos, A.; Camacho-Dávila, A.; de Jesús Zazueta-Morales, J.; Aguilar-Palazuelos, E.; Ruiz-Gutiérrez, M.G.; Meléndez-Pizarro, C.O.; de Jesús Ruiz-Anchondo, T. Polyphenolic compound stability and antioxidant capacity of apple pomace in an extruded cereal. LWT Food Sci. Technol. 2016, 65, 228–236. [Google Scholar] [CrossRef]
- Rabetafika, H.N.; Bchir, B.; Blecker, C.; Richel, A. Fractionation of apple by-products as source of new ingredients: Current situation and perspectives. Trends Food Sci. Technol. 2014, 40, 99–114. [Google Scholar] [CrossRef]
- Persic, M.; Mikulic-Petkovsek, M.; Slatnar, A.; Veberic, R. Chemical composition of apple fruit, juice and pomace and the correlation between phenolic content, enzymatic activity and browning. LWT Food Sci. Technol. 2017, 82, 23–31. [Google Scholar] [CrossRef]
- Sato, M.F.; Vieira, R.G.; Zardo, D.M.; Falcão, L.D.; Nogueira, A.; Wosiacki, G. Apple pomace from eleven cultivars: An approach to identify sources of bioactive compounds. Acta Sci. Agron. 2010, 32, 29–35. [Google Scholar]
- Pieszka, M.; Gogol, P.; Pietras, M.; Pieszka, M. Valuable components of dried pomaces of chokeberry, black currant, strawberry, apple and carrot as a source of natural antioxidants and nutraceuticals in the animal diet. Ann. Anim. Sci. 2015, 15, 475–491. [Google Scholar] [CrossRef]
- Waldbauer, K.; McKinnon, R.; Kopp, B. Apple pomace as potential source of natural active compounds. Planta Med. 2017, 83, 994–1010. [Google Scholar] [CrossRef]
- Kołodziejczyk, K.; Kosmala, M.; Milala, J.; Sójka, M.; Uczciwek, M.; Król, B.; Markowski, J.; Renard, C.M.G.C. Characterisation of the chemical composition of scab-resistant apple pomaces. J. Hortic. Sci. Biotechnol. 2009, 84, 89–95. [Google Scholar] [CrossRef]
- Singhal, K.; Thakur, S.; Sharma, D. Nutritive value of dried and stored apple pomace and its further processing for improved utilization. Indian J. Anim. Nutr. 1991, 8, 213–216. [Google Scholar]
- Gabriel, L.S.; Prestes, R.A.; Pinheiro, L.A.; Barison, A.; Wosiacki, G. Multivariate analysis of the spectroscopic profile of the sugar fraction of apple pomace. Braz. Arch. Biol. Technol. 2013, 56, 439–446. [Google Scholar] [CrossRef]
- Nicoli, M.; Anese, M.; Parpinel, M. Influence of processing on the antioxidant properties of fruit and vegetables. Trends Food Sci. Technol. 1999, 10, 94–100. [Google Scholar] [CrossRef]
- Lindenmeier, M.; Hofmann, T. Influence of baking conditions and precursor supplementation on the amounts of the antioxidant pronyl-L-lysine in bakery products. J. Agric. Food Chem. 2003, 52, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Sakač, M.; Torbica, A.; Sedej, I.; Hadnađev, M. Influence of breadmaking on antioxidant capacity of gluten free breads based on rice and buckwheat flours. Food Res. Int. 2011, 44, 2806–2813. [Google Scholar] [CrossRef]
- Malinowski, J.; Drzeżdżon, J.; Chmurzyński, L.; Jacewicz, D. Metody in-vivo badania właściwości antyoksydacyjnych związków kompleksowych. Wiadomości Chem. 2019, 73, 7–8. [Google Scholar]
- Arnao, M.B. Some methodological problems in the determination of antioxidant activity using chromogen radicals: A practical case. Trends Food Sci. Technol. 2000, 11, 419–421. [Google Scholar] [CrossRef]
- Mir, S.A.; Bosco, S.J.D.; Shah, M.A.; Santhalakshmy, S.; Mir, M.M. Effect of apple pomace on quality characteristics of brown rice based cracker. J. Saudi Soc. Agric. Sci. 2017, 16, 25–32. [Google Scholar] [CrossRef]
- Šarić, B.; Mišan, A.; Mandić, A.; Nedeljković, N.; Pojić, M.; Pestorić, M.; Ðilas, S. Valorisation of raspberry and blueberry pomace through the formulation of value-added gluten-free cookies. J. Food Sci. Technol. 2015, 53, 1140–1150. [Google Scholar] [CrossRef]
- Maillard, M.-N.; Berset, C. Evolution of antioxidant activity during kilning: Role of insoluble bound phenolic acids of barley and malt. J. Agric. Food Chem. 1995, 43, 1789–1793. [Google Scholar] [CrossRef]
- Zielińska, D.; Zieliński, H. Low molecular weight antioxidants and other biologically active components of buckwheat seeds. Eur. J. Plant Sci. Biotechnol. 2009, 3, 29–38. [Google Scholar]
- Alvarez-Jubete, L.; Arendt, E.; Gallagher, E. Nutritive value of pseudocereals and their increasing use as functional gluten-free ingredients. Trends Food Sci. Technol. 2010, 21, 106–113. [Google Scholar] [CrossRef]
- Sivam, A.S.; Sun-Waterhouse, D.; Quek, S.; Perera, C.O. Properties of bread dough with added fiber polysaccharides and phenolic antioxidants: A review. J. Food Sci. 2010, 75, R163–R174. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Bhushan, S. Apple phenolics as nutraceuticals: Assessment, analysis and application. J. Food Sci. Technol. 2015, 53, 1727–1738. [Google Scholar] [CrossRef] [PubMed]
- Kasai, H.; Fukada, S.; Yamaizumi, Z.; Sugie, S.; Mori, H. Action of chlorogenic acid in vegetables and fruits as an inhibitor of 8-hydroxydeoxyguanosine formation in vitro and in a rat carcinogenesis model. Food Chem. Toxicol. 2000, 38, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Rifaai, R.; El-Tahawy, N.; Saber, E.A.; Ahmed, R. Effect of quercetin on the endocrine pancreas of the experimentally induced diabetes in male albino rats: A histological and immunohistochemical study. J. Diabetes Metab. 2012, 3, 2. [Google Scholar] [CrossRef]
- Ziobro, R.; Gumul, D.; Korus, J.; Korus, A. Starch bread with a share of non-wheat flours as a source of bioactive compounds in gluten-free diet. J. Food Nutr. Res. 2016, 55, 11–21. [Google Scholar]
- O’Shea, N.; Rößle, C.; Arendt, E.; Gallagher, E. Modelling the effects of orange pomace using response surface design for gluten-free bread baking. Food Chem. 2015, 166, 223–230. [Google Scholar] [CrossRef]
- Rupasinghe, H.V.; Wang, L.; Huber, G.M.; Pitts, N.L. Effect of baking on dietary fibre and phenolics of muffins incorporated with apple skin powder. Food Chem. 2007, 107, 1217–1224. [Google Scholar] [CrossRef]
- Ferretti, G.; Turco, I.; Bacchetti, T. Apple as a source of dietary phytonutrients: Bioavailability and evidence of protective effects against human cardiovascular disease. Food Nutr. Sci. 2014, 5, 1234–1246. [Google Scholar] [CrossRef]
- Sakr, A.M.; Hussien, H.A. Nutritional quality of gluten free biscuits supplemented with sweet chickpeas and date palm powder. Int. J. Food Sci. Nutr. 2017, 2, 128–134. [Google Scholar]
- Demirkesen, I. Formulation of chestnut cookies and their rheological and quality characteristics. J. Food Qual. 2016, 39, 264–273. [Google Scholar] [CrossRef]
- Cervini, M.; Frustace, A.; Garrido, G.D.; Rocchetti, G.; Giuberti, G. Nutritional, physical and sensory characteristics of gluten-free biscuits incorporated with a novel resistant starch ingredient. Heliyon 2021, 7, e06562. [Google Scholar] [CrossRef] [PubMed]
- Bolek, S. Olive stone powder: A potential source of fiber and antioxidant and its effect on the rheological characteristics of biscuit dough and quality. Innov. Food Sci. Emerg. Technol. 2020, 64, 102423. [Google Scholar] [CrossRef]
- Jia, M.; Yu, Q.; Chen, J.; He, Z.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. Physical quality and in vitro starch digestibility of biscuits as affected by addition of soluble dietary fiber from defatted rice bran. Food Hydrocoll. 2020, 99, 105349. [Google Scholar] [CrossRef]
- Sudha, M.; Baskaran, V.; Leelavathi, K. Apple pomace as a source of dietary fiber and polyphenols and its effect on the rheological characteristics and cake making. Food Chem. 2007, 104, 686–692. [Google Scholar] [CrossRef]
- Ajila, C.; Leelavathi, K.; Rao, U.P. Improvement of dietary fiber content and antioxidant properties in soft dough biscuits with the incorporation of mango peel powder. J. Cereal Sci. 2008, 48, 319–326. [Google Scholar] [CrossRef]
- Faillie, J.-L. Pharmacological aspects of the safety of gliflozins. Pharmacol. Res. 2017, 118, 71–81. [Google Scholar] [CrossRef]
- Haghighi, M.; Rezaei, K. Designing an all-apple-pomace-based functional dessert formulation. Br. Food J. 2013, 115, 409–424. [Google Scholar] [CrossRef]
- Randhawa, V.; Sharma, P.; Bhushan, S.; Bagler, G. Identification of key nodes of type 2 diabetes mellitus protein interactome and study of their interactions with phloridzin. OMICS J. Integr. Biol. 2013, 17, 302–317. [Google Scholar] [CrossRef]
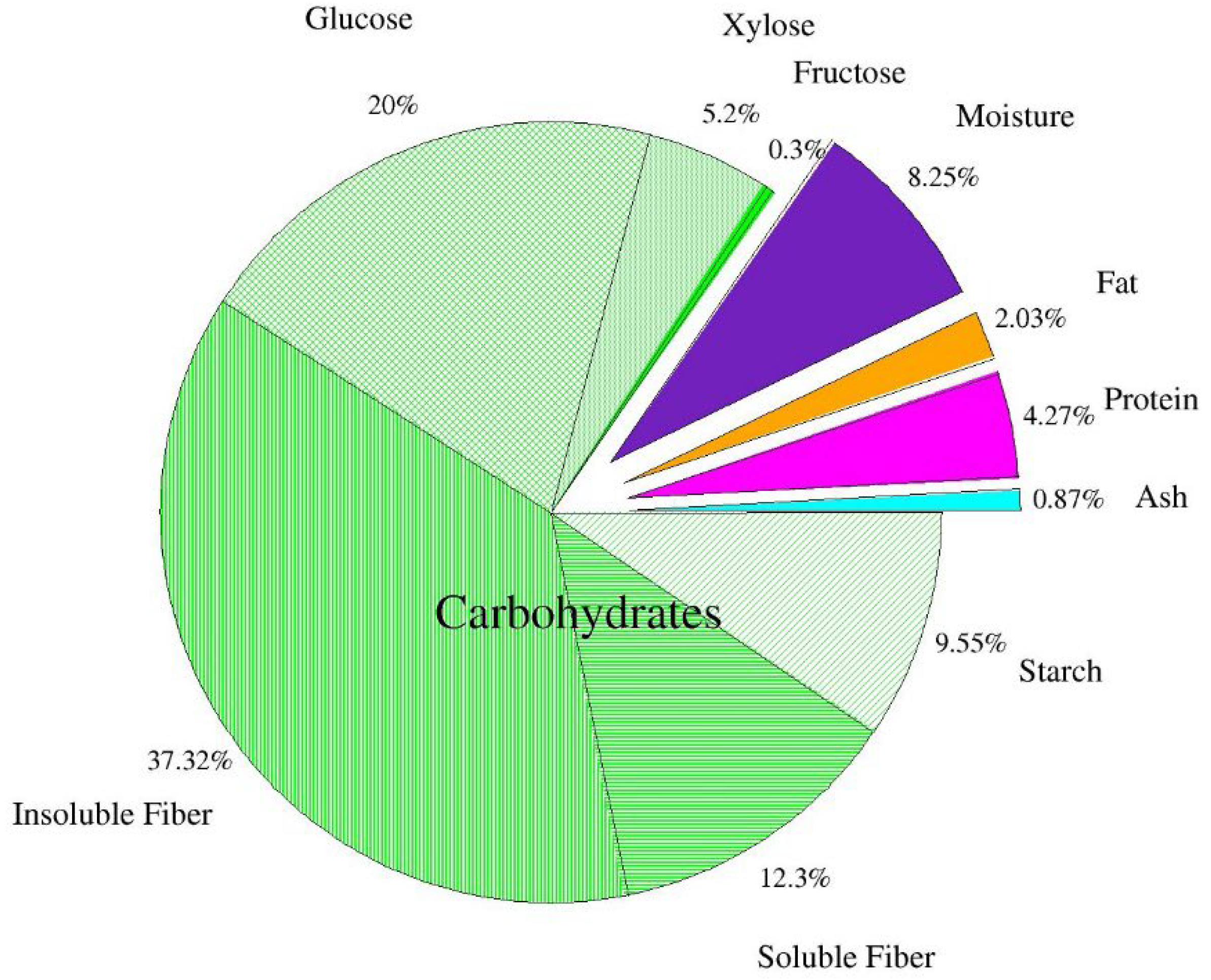
| Sample | Corn Flour | Apple Pomace | Powdered Sugar | Margarine | Egg Mass |
|---|---|---|---|---|---|
| Control * | 364 | 0 | 46 | 208 | 32 |
| B 15AP | 309 | 55 | 46 | 208 | 32 |
| B 30AP | 255 | 109 | 46 | 208 | 32 |
| B 45AP | 200 | 164 | 46 | 208 | 32 |
| B 60AP | 146 | 218 | 46 | 208 | 32 |
| Group of Compounds | Name | Content |
|---|---|---|
| Flavonols | quercetin-O-rutinoside | 4.21 ± 0.17 1 |
| quercetin-3-O-galactoside | 32.63 ± 0.21 | |
| quercetin-3-O-glucoside | 8.40 ± 0.05 | |
| quercetin-3-O-arabinoside | 11.55 ± 0.03 | |
| quercetin-3-O-xyloside | 19.60 ± 0.00 | |
| quercetin-3-O-rhamnoside | 26.80 ± 0.00 | |
| isorhamnetin-3-O-galactoside | 1.30 ± 0.42 | |
| isorhamnetin-3-O-glucoside | 0.90 ± 0.00 | |
| Phenolic acids | chlorogenic acid | 22.70 ± 0.23 |
| cryptochlorogenic acid | 1.20 ± 0.07 | |
| p-coumaroylquinic acid | 1.83 ± 0.20 | |
| Flavan-3-ols | (+) catechin | 1.67 ± 0.03 |
| procyanidin B2 | 3.79 ± 0.00 | |
| (−)epicatechin | 1.35 ± 0.23 | |
| Dihydrochalcones | phloretin-2-O-xylosylglucoside | 1.90 ± 0.11 |
| phloretin 2-O-glucoside (phloridzin) | 21.30 ± 0.08 | |
| Sum | 161.13 | |
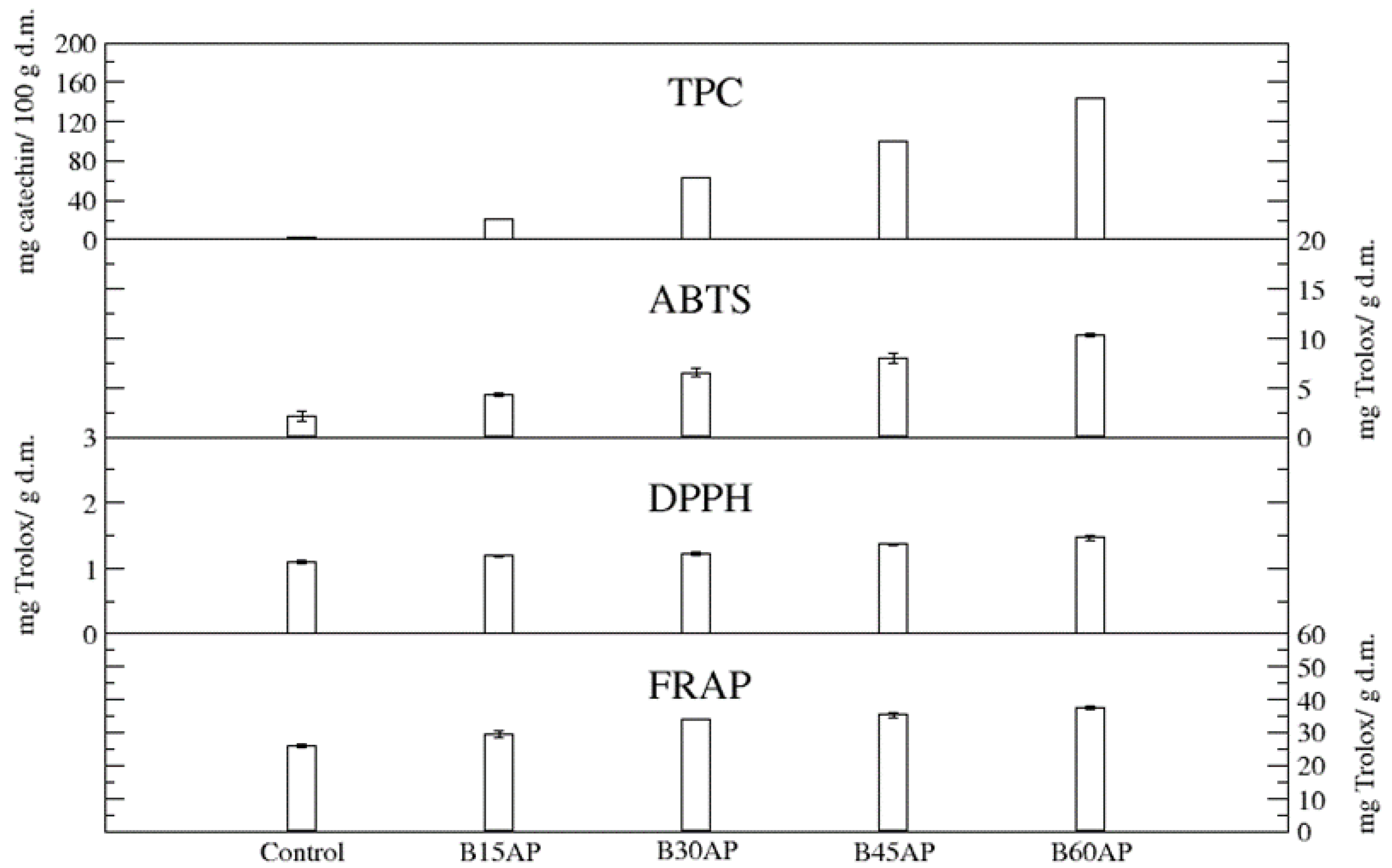
| TPC (mg gallic acid/100 g d.m.) | 470.5 ± 1.5 | |
| ABTS (mg Trolox/g d.m.) | 12.16 ± 0.43 | |
| DPPH (mg Trolox/g d.m.) | 3.09 ± 0.20 | |
| FRAP (mg Trolox/g d.m.) | 27.7 ± 0.0 | |
| Compounds | Control * | B 15AP | B 30AP | B 45AP | B 60AP |
|---|---|---|---|---|---|
| Flavonols (mg/100 g d.m.) | |||||
| isorhamnetin-3-O-galactoside | n.d. | 0.21 ± 0.04 a | 0.23 ± 0.03 a | 0.25 ± 0.02 a | 0.27 ± 0.02 a |
| isorhamnetin-3-O-glucoside | n.d. | 0.27 ± 0.01 a | 0.29 ± 0.03 a | 0.28 ± 0.03 a | 0.30 ± 0.02 a |
| quercetin-O-rutinoside | n.d. | 0.57 ± 0.01 a | 0.74 ± 0.03 b | 1.16 ± 0.07 c | 1.29 ± 0.02 d |
| quercetin -3-O-galactoside | n.d. | 5.27 ± 0.00 a | 6.75 ± 0.05 b | 8.72 ± 0.00 c | 10.53 ± 0.04 d |
| quercetin -3-O-glucoside | n.d. | 1.28 ± 0.00 a | 1.56 ± 0.08 b | 2.30 ± 0.04 c | 3.13 ± 0.03 d |
| quercetin -3-O-arabinoside | n.d. | 2.09 ± 0.02 a | 2.31 ± 0.04 b | 3.04 ± 0.03 c | 4.26 ± 0.07 d |
| quercetin -3-O-xyloside | n.d. | 3.85 ± 0.03 a | 4.00 ± 0.00 b | 5.26 ± 0.02 c | 6.49 ± 0.02 d |
| quercetin -3-O-rhamnoside | n.d. | 4.63 ± 0.04 a | 5.02 ± 0.03 b | 7.35 ± 0.08 c | 9.21 ± 0.06 d |
| luteolin 6-C-hexoside-O-hexoside | n.d. | n.d. | n.d. | n.d. | n.d. |
| luteolin O- hexoside-C-hexoside | n.d. | n.d. | n.d. | n.d. | n.d. |
| Phenolic acids (mg/100 g d.m.) | |||||
| chlorogenic acid | 0.33 ± 0.08 a | 4.34 ± 0.13 b | 6.63 ± 0.09 c | 10.27 ± 0.17 d | 13.57 ± 0.08 e |
| cryptochlorogenic acid | 0.03 ± 0.00 a | 0.24 ± 0.02 b | 0.41 ± 0.07 c | 0.53 ± 0.07 c | 0.78 ± 0.05 d |
| caffeoylquinic acid | n.d. | 0.18 ± 0.03 a | 0.18 ± 0.02 a | 0.19 ± 0.00 a | 0.18 ± 0.00 a |
| p-coumaroylquinic acid | 0.01 ± 0.00 a | 0.25 ± 0.00 b | 0.24 ± 0.02 b | 0.27 ± 0.04 b | 0.28 ± 0.00 b |
| caffeoyl-dihydroxyphenyllactaoyl-tartaric acid | n.d. | 0.56 ± 0.00 a | 0.74 ± 0.15 b | 0.82 ± 0.07 b | 0.93 ± 0.11 b |
| 2-O-p -coumaroylglicerol | n.d. | n.d. | n.d. | n.d. | n.d. |
| 1-O-p-coumaroylglicerol | n.d. | 0.14 ± 0.01 a | 0.19 ± 0.03 b | 0.25 ± 0.02 b | 0.37 ± 0.05 c |
| p-coumaroylspermidin | 0.08 ± 0.00 b | 0.06 ± 0.00 a | 0.03 ± 0.00 a | n.d. | n.d. |
| di-p-coumaroylspermidin | 0.14 ± 0.04 b | 0.10 ± 0.00 a | 0.06 ± 0.00 a | 0.02 ± 0.00 a | n.d. |
| Ferullyquinic acid | 0.09 ± 0.00 a | 0.17 ± 0.00 b | 0.23 ± 0.07 b | 0.31 ± 0.03 b | 0.47 ± 0.06 c |
| Flavan-3-ols (mg/100 g d.m.) | |||||
| (+) catechin | n.d. | 0.41 ± 0.00 a | 0.42 ± 0.03 a | 0.53 ± 0.02 b | 1.24 ± 0.04 c |
| procyanidin B2 | n.d. | 0.48 ± 0.00 a | 0.79 ± 0.05 b | 1.10 ± 0.04 c | 1.42 ± 0.03 d |
| (−)epicatechin | n.d. | 0.28 ± 0.00 a | 0.27 ± 0.03 a | 0.30 ± 0.02 a | 0.31 ± 0.04 a |
| Dihydrochalcones (mg/100 g d.m.) | |||||
| phloretin-2-O-xylosylglucoside | n.d. | 0.28 ± 0.00 a | 0.37 ± 0.00 b | 0.60 ± 0.03 c | 0.73 ± 0.07 d |
| phloretin 2-O-glucoside (phloridzin) | n.d. | 3.29 ± 0.04 a | 4.16 ± 0.09 b | 6.07 ± 0.05 c | 8.16 ± 0.06 d |
| Compounds | Control * | B 15AP | B 30AP | B 45AP | B 60AP |
|---|---|---|---|---|---|
| Moisture | 6.13 ± 0.10 c | 5.46 ± 0.32 b | 4.97 ± 0.21 a | 4.9 ± 0.37 a | 5.02 ± 0.15 a |
| Protein (g/100 g d.m.) | 7.45 ± 0.03 e | 6.97 ± 0.01 d | 6.48 ± 0.03 c | 6.07 ± 0.06 b | 5.78 ± 0.01 a |
| Fat (g/100 g d.m.) | 31.70 ± 0.17 a | 32.20 ± 0.35 a | 32.22 ± 0.27 a | 32.90 ± 0.10 b | 34.25 ± 0.24 c |
| Ash (g/100 g d.m.) | 0.36 ± 0.01 a | 0.41 ± 0.01 b | 0.46 ± 0.01 c | 0.53 ± 0.01 d | 0.57 ± 0.01 e |
| Available carbohydrates (g/100 g d.m.) | 58.55 ± 0.21 e | 51.6 ± 0.37 d | 46.23 ± 0.31 c | 39.8 ± 0.17 b | 31.7 ± 0.26 a |
| Insoluble dietary fiber | 0.63 ± 0.05 a | 5.68 ± 0.02 b | 9.78 ± 0.05 c | 14.62 ± 0.01 d | 19.17 ± 0.04 e |
| Soluble dietary fiber | 1.31 ± 0.02 a | 3.21 ± 0.05 b | 4.83 ± 0.06 c | 6.09 ± 0.01 d | 8.03 ± 0.03 e |
| Total dietary fiber | 1.94 ± 0.02 a | 8.83 ± 0.04 b | 14.61 ± 0.01 c | 20.71 ± 0.01 d | 27.71 ± 0.01 e |
| Samples | Hardness (N) | Volume (cm3/ 100 g) | L* | a* | b* | ΔE | |
|---|---|---|---|---|---|---|---|
| Day 1 | Day 30 | ||||||
| Control 1 | 4.00 ± 0.48 a 2 | 4.10 ± 0.32 a | 111.27 ± 1.32 c | 75.86 ± 0.36 e | 10.01 ± 0.41 a | 51.29 ± 0.40 e | 16.56 ± 0.16 e |
| B 15AP | 5.08 ± 0.40 b | 5.87 ± 0.25 b | 106.34 ± 1.76 b | 51.33 ± 0.39 d | 11.97 ± 0.49 d | 29.40 ± 0.62 d | 13.62 ± 0.07 d |
| B 30AP | 5.51 ± 0.16 c | 6.71 ± 0.68 c | 105.94 ± 0.92 b | 42.45 ± 0.42 c | 11.72 ± 0.14 cd | 22.24 ± 0.35 c | 12.36 ± 0.14 c |
| B 45AP | 6.35 ± 0.35 d | 7.57 ± 3.60 d | 104.12 ± 1.33 a | 38.02 ± 1.29 b | 11.36 ± 0.27 bc | 18.40 ± 1.30 b | 11.64 ± 0.12 b |
| B 60AP | 8.66 ± 1.20 e | 9.45 ± 3.68 e | 105.16 ± 0.82 ab | 35.74 ± 0.43 a | 11.07 ± 0.26 b | 16.65 ± 0.56 a | 11.27 ± 0.09 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruczek, M.; Gumul, D.; Korus, A.; Buksa, K.; Ziobro, R. Phenolic Compounds and Antioxidant Status of Cookies Supplemented with Apple Pomace. Antioxidants 2023, 12, 324. https://doi.org/10.3390/antiox12020324
Kruczek M, Gumul D, Korus A, Buksa K, Ziobro R. Phenolic Compounds and Antioxidant Status of Cookies Supplemented with Apple Pomace. Antioxidants. 2023; 12(2):324. https://doi.org/10.3390/antiox12020324
Chicago/Turabian StyleKruczek, Marek, Dorota Gumul, Anna Korus, Krzysztof Buksa, and Rafał Ziobro. 2023. "Phenolic Compounds and Antioxidant Status of Cookies Supplemented with Apple Pomace" Antioxidants 12, no. 2: 324. https://doi.org/10.3390/antiox12020324
APA StyleKruczek, M., Gumul, D., Korus, A., Buksa, K., & Ziobro, R. (2023). Phenolic Compounds and Antioxidant Status of Cookies Supplemented with Apple Pomace. Antioxidants, 12(2), 324. https://doi.org/10.3390/antiox12020324







