Mitochondrial and Redox Changes in Periodontitis and Type 2 Diabetes Human Blood Mononuclear Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Exclusion Criteria
2.3. Glycated Hemoglobin Determination
2.4. Resistin Levels
2.5. Isolation of Peripheral Blood Mononuclear Cells
2.6. Mitochondrial Oxygen Consumption Rate
2.7. Analysis of H2O2 Production in PBMCs
2.8. Preparation of PBMCs’ Total Extracts
2.9. Evaluation of Enzyme’s Activity
2.10. Sample Preparation and Western Blotting
2.11. Fluorescence-Activated Cell Sorting (FACS)
2.12. Analysis of Nrf2-Target Genes
- SOD1 F: GGTGGGCCAAAGGATGAAGAG/R:CCACAAGCCAAACGACTTCC
- HO-1 F: CCTGAGTTTCAAGTATCC/R:AACAACAGAACACAACAA
- GST F: GGAGGCAAGACCTTCATT/R:ATGGATCAGCAGCAAGTC
- GCLc F:GGCACAAGGACGTTCTCAAGT/R:CAAGACAGGACCAACCGGAC
- GCLm F:AACTCTTCATCATCAACTA/R:AACTCCATCTTCAATAGG
- GAPDH F: ATTCCACCCATGGCAAATTC/R:GGGATTTCCATTGATGACAAGC
2.13. Power and Sample Size
2.14. Statistical Analysis
3. Results
3.1. Evidence for Clinical Markers in Periodontitis and Type 2 Diabetes
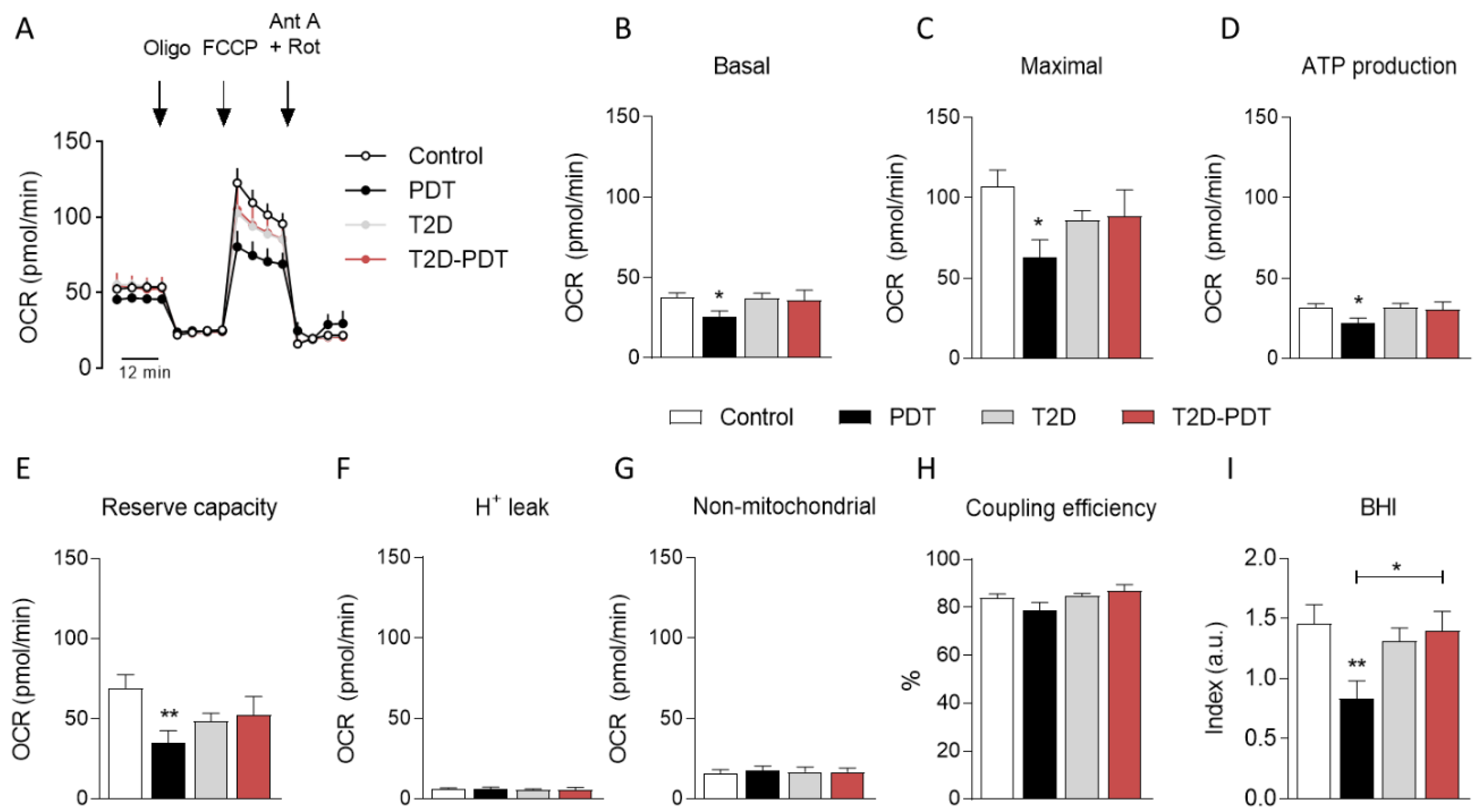
3.2. Decreased Mitochondrial Oxygen Consumption in PBMCs from Periodontitis Patients
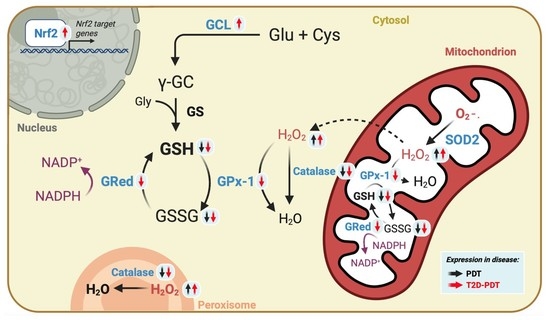
3.3. Enhanced H2O2 Levels and Concomitant Reduced Catalase and Glutathione Cycle in PBMCs from Periodontitis and Periodontitis plus Type 2 Diabetes
3.4. Apparent Compensatory Nrf2 and Glutamate Cysteine Ligase Protein Levels in PBMCs from Periodontitis Plus Type 2 Diabetes Mellitus
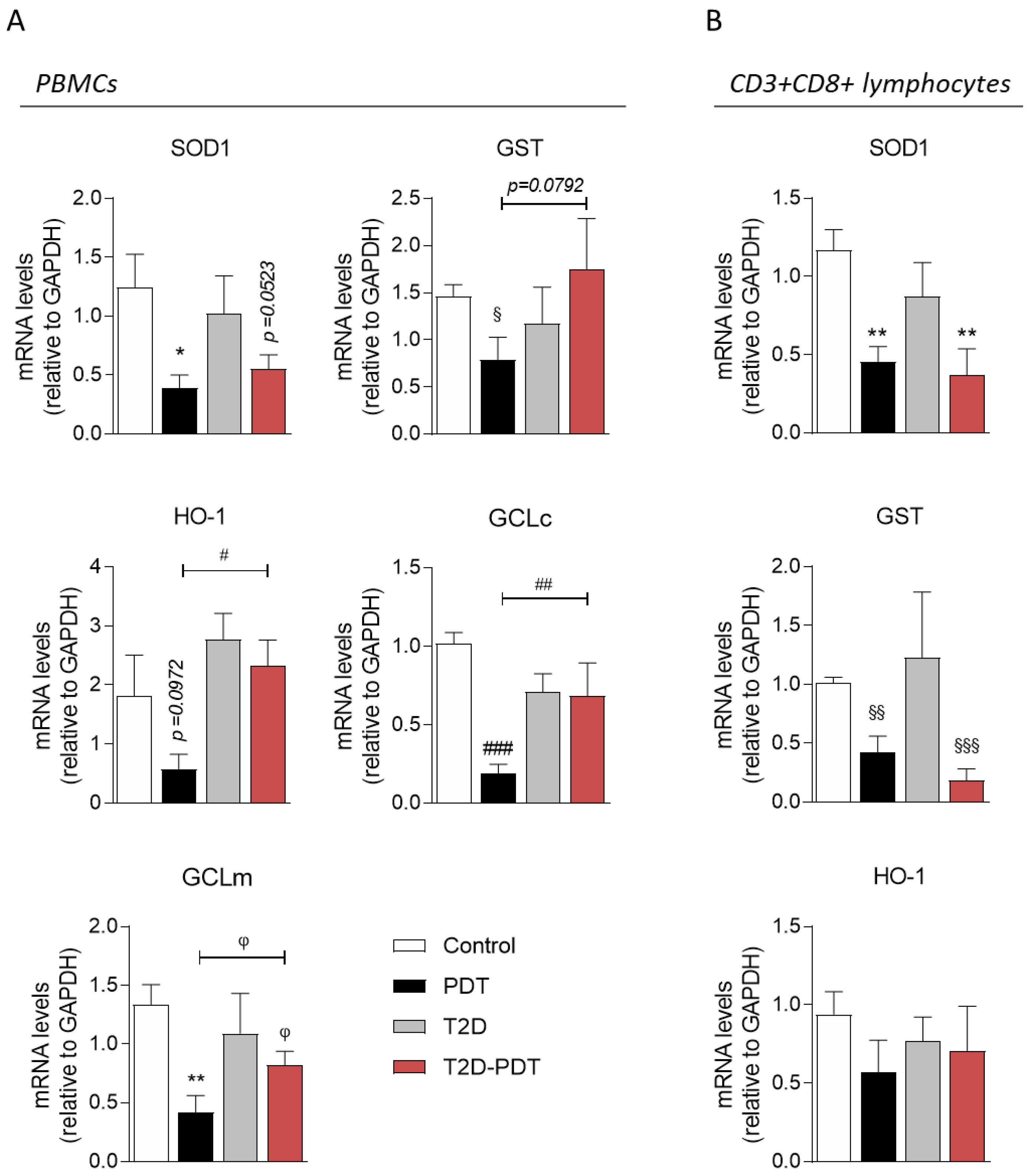
3.5. Expression of Nrf2 Target Genes SOD1, GCLc, GCLm, GST, and HO-1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bartold, P.M.; Van Dyke, T.E. Periodontitis: A host-mediated disruption of microbial homeostasis. Unlearning learned concepts. Periodontol. 2000 2013, 62, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.; Bouchard, P.; Cagetti, M.G.; Campus, G.; Carra, M.C.; Cocco, F.; Schulte, A.G. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases, consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J. Clin. Periodontol. 2017, 44 (Suppl. S18), S39–S51. [Google Scholar] [CrossRef] [PubMed]
- Frencken, J.E.; Sharma, P.; Stenhouse, L.; Green, D.; Laverty, D.; Dietrich, T. Global epidemiology of dental caries and severe periodontitis—A comprehensive review. J. Clin. Periodontol. 2017, 44 (Suppl. S18), S94–S105. [Google Scholar] [CrossRef]
- Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019). Seattle, Institute of Health Metrics and Evaluation (IHME). Available online: http://ghdx.healthdata.org/gbd-results-tool (accessed on 22 December 2022).
- Bernabe, E.; Marcenes, W.; Hernandez, C.R.; Bailey, J.; Abreu, L.G.; Kassebaum, N.J. Global, Regional, and National Levels and Trends in Burden of Oral Conditions from 1990 to 2017, A Systematic Analysis for the Global Burden of Disease 2017 Study. J. Dent. Res. 2020, 99, 362–373. [Google Scholar] [CrossRef]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Liccardo, D.; Cannavo, A.; Spagnuolo, G.; Ferrara, N.; Cittadini, A.; Rengo, C.; Rengo, G. Periodontal disease, a risk factor for diabetes and cardiovascular disease. Int. J. Mol. Sci. 2019, 20, 1414. [Google Scholar] [CrossRef] [PubMed]
- Stöhr, J.; Barbaresko, J.; Neuenschwander, M.; Schlesinger, S. Bidirectional association between periodontal disease and diabetes mellitus, a systematic review and meta-analysis of cohort studies. Sci. Rep. 2021, 11, 13686. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, W.; Liu, X.; Zhang, W.; Li, Y. Interrelationship between diabetes and periodontitis, role of hyperlipidemia. Arch. Oral Biol. 2015, 60, 667–674. [Google Scholar] [CrossRef]
- Graziani, F.; Gennai, S.; Solini, A.; Petrini, M. A systematic review and meta-analysis of epidemiologic observational evidence on the effect of periodontitis on diabetes an update of the EFP-AAP review. J. Clin. Periodontol. 2018, 45, 167–187. [Google Scholar] [CrossRef]
- Pillarisetti, S.; Saxena, U. Role of oxidative stress and inflammation in the origin of type 2 diabetes—A paradigm shift. Expert Opin. Ther. Targets 2004, 8, 401–408. [Google Scholar] [CrossRef]
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Lazar, M.A. The hormone resistin links obesity to diabetes. Nature 2001, 409, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, A.V.B.; Nokhbehsaim, M.; Tekin, S.; De Molon, R.S.; Spolidorio, L.C.; Memmert, S.; Cirelli, J.A. Resistin is increased in periodontal cells and tissues, in vitro and in vivo studies. Mediat. Inflamm. 2020, 2020, 9817095. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.L.; Kelly, B.O.; Neill, L.A.J. Mitochondria are the powerhouses of immunity. Nat. Immunol. 2017, 18, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Hartman, M.L.; Shirihai, O.S.; Holbrook, M.; Xu, G.; Kocherla, M.; Shah, A.; Vita, J.A. Relation of mitochondrial oxygen consumption in peripheral blood mononuclear cells to vascular function in type 2 diabetes mellitus. Vasc. Med. 2014, 19, 67–74. [Google Scholar] [CrossRef]
- Sun, X.; Mao, Y.; Dai, P.; Li, X.; Gu, W.; Wang, H.; Huang, S. Mitochondrial dysfunction is involved in the aggravation of periodontitis by diabetes. J. Clin. Periodontol. 2017, 44, 463–471. [Google Scholar] [CrossRef]
- Li, X.; Sun, X.; Zhang, X.; Mao, Y.; Ji, Y.; Shi, L.; Huang, S. Enhanced oxidative damage and Nrf2 downregulation contribute to the aggravation of periodontitis by diabetes mellitus. Oxid. Med. Cell. Longev. 2018, 2018, 9421019. [Google Scholar] [CrossRef]
- Nicholas, D.; Proctor, E.A.; Raval, F.M.; Ip, B.C.; Habib, C.; Ritou, E.; Nikolajczyk, B.S. Advances in the quantification of mitochondrial function in primary human immune cells through extracellular flux analysis. PLoS ONE 2017, 12, e0170975. [Google Scholar] [CrossRef]
- Portes, J.; Bullón, B.; Quiles, J.L.; Battino, M.; Bullón, P. Diabetes mellitus and periodontitis share intracellular disorders as the main meeting point. Cells 2021, 10, 2411. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis, framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89 (Suppl. S1), S159–S172. [Google Scholar] [CrossRef]
- Ferreira, I.L.; Carmo, C.; Naia, L.; Mota, S.I.; Rego, A.C. Assessing mitochondrial function in in vitro and ex vivo models of Huntington’s disease. In Huntington’s Disease. Methods in Molecular Biology; Precious, S.V., Rosser, A.E., Dunnett, S.B., Eds.; Springer Protocols: New York, NY, USA, 2018; Volume 1780, pp. 415–442. ISBN 978-1-4939-7824-3. [Google Scholar]
- Chacko, B.K.; Kramer, P.A.; Ravi, S.; Benavides, G.A.; Mitchell, T.; Dranka, B.P.; Darley-Usmar, V.M. The bioenergetic health index, a new concept in mitochondrial translational research. Clin. Sci. 2014, 127, 367–373. [Google Scholar] [CrossRef]
- Spinazzi, M.; Casarin, A.; Pertegato, V.; Salviati, L.; Angelini, C. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat. Protoc. 2012, 7, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, L.; Wang, P.; Li, X.; Qiu, D.; Li, L.; Wang, Q. Sirt3-dependent deacetylation of SOD2 plays a protective role against oxidative stress in oocytes from diabetic mice. Cell Cycle. 2017, 16, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Rosenstock, T.R.; Cunha-Oliveira, T.; Ferreira, I.L.; Oliveira, C.R.; Rego, A.C. Glutathione redox cycle dysregulation in Huntington’s disease knock-in striatal cells. Free Radic. Biol. Med. 2012, 53, 1857–1867. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Jiang, A.; Li, X.; Chang, W.; Chen, J.; Ye, F. Metformin alleviates lead-induced mitochondrial fragmentation via AMPK/Nrf2 activation in SH-SY5Y cells. Redox Biol. 2020, 36, 101626. [Google Scholar] [CrossRef]
- Kaser, S.; Kaser, A.; Sandhofer, A.; Ebenbichler, C.F.; Tilg, H.; Patsch, J.R. Resistin messenger-RNA expression is increased by proinflammatory cytokines in vitro. Biochem. Biophys. Res. Commun. 2003, 309, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Handzlik-Orlik, G.; Okopien, B. The role of adipokines in connective tissue diseases. Eur. J. Nutr. 2012, 5, 513–528. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Yoshie, H. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium, consensus report of workgroup 1 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J. Periodontol. 2018, 89 (Suppl. S1), S74–S84. [Google Scholar] [CrossRef]
- Patil, V.S.; Patil, V.P.; Gokhale, N.; Acharya, A.; Kangokar, P. Chronic periodontitis in type 2 diabetes mellitus, oxidative stress as a common factor in periodontal tissue injury. J. Clin. Diagn. Res. 2016, 10, BC12–BC16. [Google Scholar] [CrossRef]
- Baradaran, A.; Nasri, H.; Rafieian-Kopaei, M. Oxidative stress and hypertension: Possibility of hypertension therapy with antioxidants. J. Res. Med. Sci. 2014, 19, 358–367. [Google Scholar]
- Sørensen, A.L.; Hasselbalch, H.C.; Nielsen, C.H.; Poulsen, H.E.; Ellervik, C. Statin treatment, oxidative stress and inflammation in a Danish population. Redox Biol. 2019, 21, 101088. [Google Scholar] [CrossRef]
- Trivedi, S.; Lal, N.; Mahdi, A.A.; Mittal, M.; Singh, B.; Pandey, S. Evaluation of antioxidant enzymes activity and malondialdehyde levels in patients with chronic periodontitis and diabetes mellitus. J. Periodontol. 2014, 85, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Arana, C.; Moreno-Fernández, A.M.; Gómez-Moreno, G.; Morales-Portillo, C.; Serrano-Olmedo, I.; de la Cuesta Mayor, M.C.; Hernández, T.M. Increased salivary oxidative stress parameters in patients with type 2 diabetes, relation with periodontal disease. Endocrinol. Diabetes Nutr. 2017, 64, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Monea, A.; Mezei, T.; Popsor, S.; Monea, M. Oxidative stress, a link between diabetes mellitus and periodontal disease. Int. J. Endocrinol. 2014, 2014, 917631. [Google Scholar] [CrossRef] [PubMed]
- Duarte, P.M.; Napimoga, M.H.; Fagnani, E.C.; Santos, V.R.; Bastos, M.F.; Ribeiro, F.V.; Demasi, A.P.D. The expression of antioxidant enzymes in the gingivae of type 2 diabetics with chronic periodontitis. Arch. Oral Biol. 2012, 57, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Xiong, Y.; Zhang, W.; Ma, X.; Xu, X. Metformin promotes osteogenic differentiation and protects against oxidative stress-induced damage in periodontal ligament stem cells via activation of the Akt/Nrf2 signaling pathway. Exp. Cell Res. 2020, 386, 111717. [Google Scholar] [CrossRef]
- Sima, C.; Aboodi, G.M.; Lakschevitz, F.S.; Sun, C.; Goldberg, M.B.; Glogauer, M. Nuclear factor erythroid 2-related factor 2 down-regulation in oral neutrophils is associated with periodontal oxidative damage and severe chronic periodontitis. Am. J. Pathology. 2016, 186, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Seymour, G.J.; Gemmell, E.; Walsh, L.J.; Powell, R.N. Immunohistological analysis of experimental gingivitis in humans. Clin. Exp. Immunol. 1988, 71, 132–137. [Google Scholar] [PubMed]
- Stoufi, E.D.; Taubman, M.A.; Ebersole, J.L.; Smith, D.J.; Stashenko, P.P. Phenotypic analyses of mononuclear cells recovered from healthy and diseased human periodontal tissues. J. Clin. Immunol. 1987, 7, 235–245. [Google Scholar] [CrossRef]
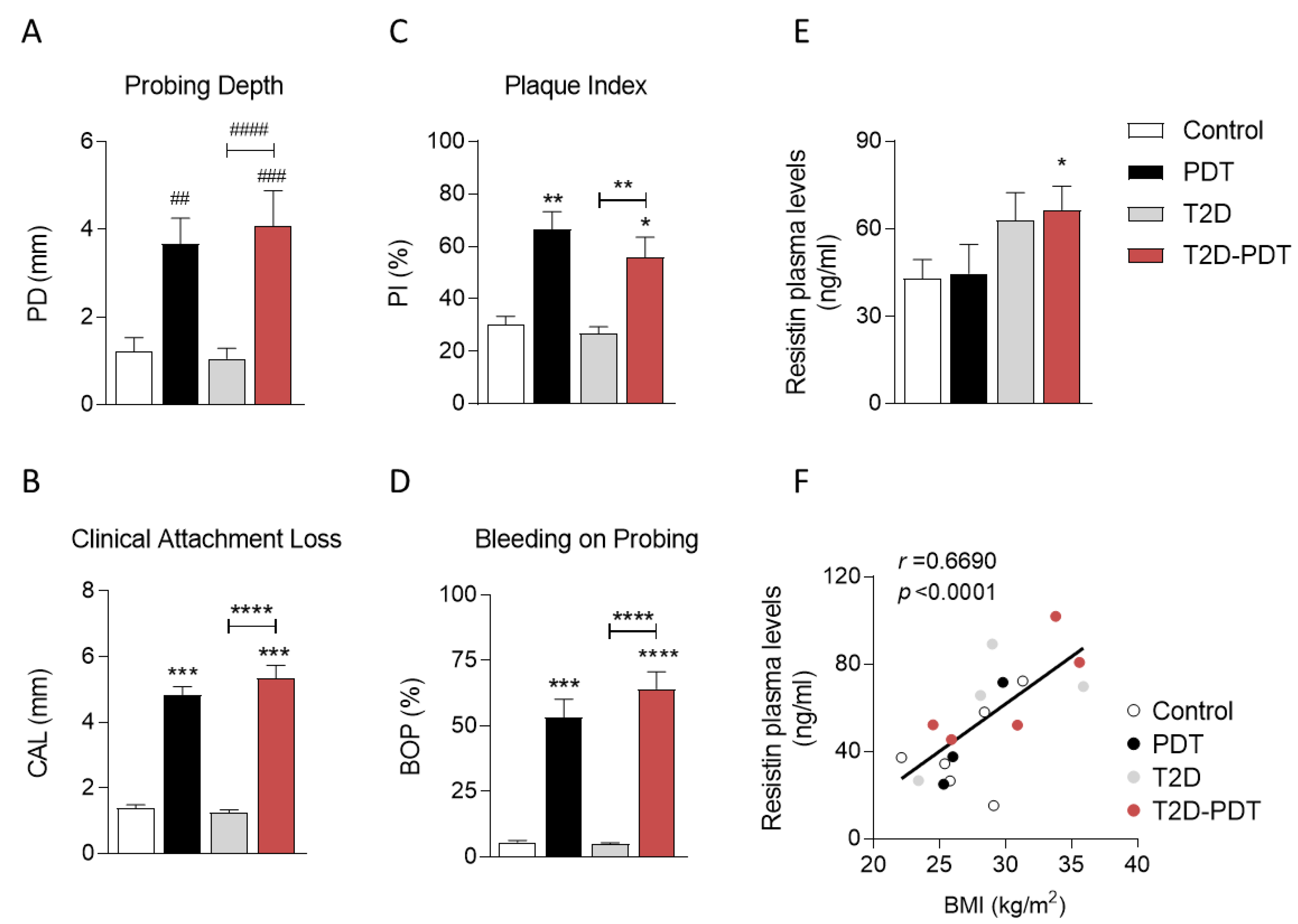

| Subject | Age | Males/Females | HbA1c | BMI | Smokers |
|---|---|---|---|---|---|
| (Years) | (Number) | (%) | (kg/m2) | (Number) | |
| Control | 59.5 ± 1.7 | 1/9 | 5.74 ± 0.06 | 26.85 ± 0.96 | 0 |
| PDT | 56.0 ± 2.1 | 7/3 | 5.72 ± 0.12 | 27.20 ± 1.11 | 3 |
| T2D | 49.6 ± 10.4 | 4/6 | 6.41 ± 0.19 ## | 28.11 ± 1.51 | 0 |
| T2D-PDT | 53.6 ± 7.7 | 8/2 | 7.08 ± 0.22 #### | 29.18 ± 1.40 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, I.L.; Costa, S.; Moraes, B.J.; Costa, A.; Fokt, O.; Marinho, D.; Alves, V.; Baptista, I.P.; Rego, A.C. Mitochondrial and Redox Changes in Periodontitis and Type 2 Diabetes Human Blood Mononuclear Cells. Antioxidants 2023, 12, 226. https://doi.org/10.3390/antiox12020226
Ferreira IL, Costa S, Moraes BJ, Costa A, Fokt O, Marinho D, Alves V, Baptista IP, Rego AC. Mitochondrial and Redox Changes in Periodontitis and Type 2 Diabetes Human Blood Mononuclear Cells. Antioxidants. 2023; 12(2):226. https://doi.org/10.3390/antiox12020226
Chicago/Turabian StyleFerreira, Ildete L., Solange Costa, Bruno J. Moraes, Ana Costa, Olga Fokt, Daniela Marinho, Vera Alves, Isabel P. Baptista, and A. Cristina Rego. 2023. "Mitochondrial and Redox Changes in Periodontitis and Type 2 Diabetes Human Blood Mononuclear Cells" Antioxidants 12, no. 2: 226. https://doi.org/10.3390/antiox12020226
APA StyleFerreira, I. L., Costa, S., Moraes, B. J., Costa, A., Fokt, O., Marinho, D., Alves, V., Baptista, I. P., & Rego, A. C. (2023). Mitochondrial and Redox Changes in Periodontitis and Type 2 Diabetes Human Blood Mononuclear Cells. Antioxidants, 12(2), 226. https://doi.org/10.3390/antiox12020226