Betula pendula Leaf Extract Targets the Interplay between Brain Oxidative Stress, Inflammation, and NFkB Pathways in Amyloid Aβ1-42-Treated Rats
Abstract
:1. Introduction
2. Material and Methods
2.1. Reagents
2.2. Vegetal Material
2.3. Extraction Procedure and Sample Preparation
2.4. Spectrophotometric Assays
2.4.1. Total Polyphenolic Content (TPC)
2.4.2. Total Flavonoid Content (TFC)
2.5. LC/MS Analysis
2.6. Antioxidant Capacity Assays
2.6.1. The DPPH Method
2.6.2. FRAP Method
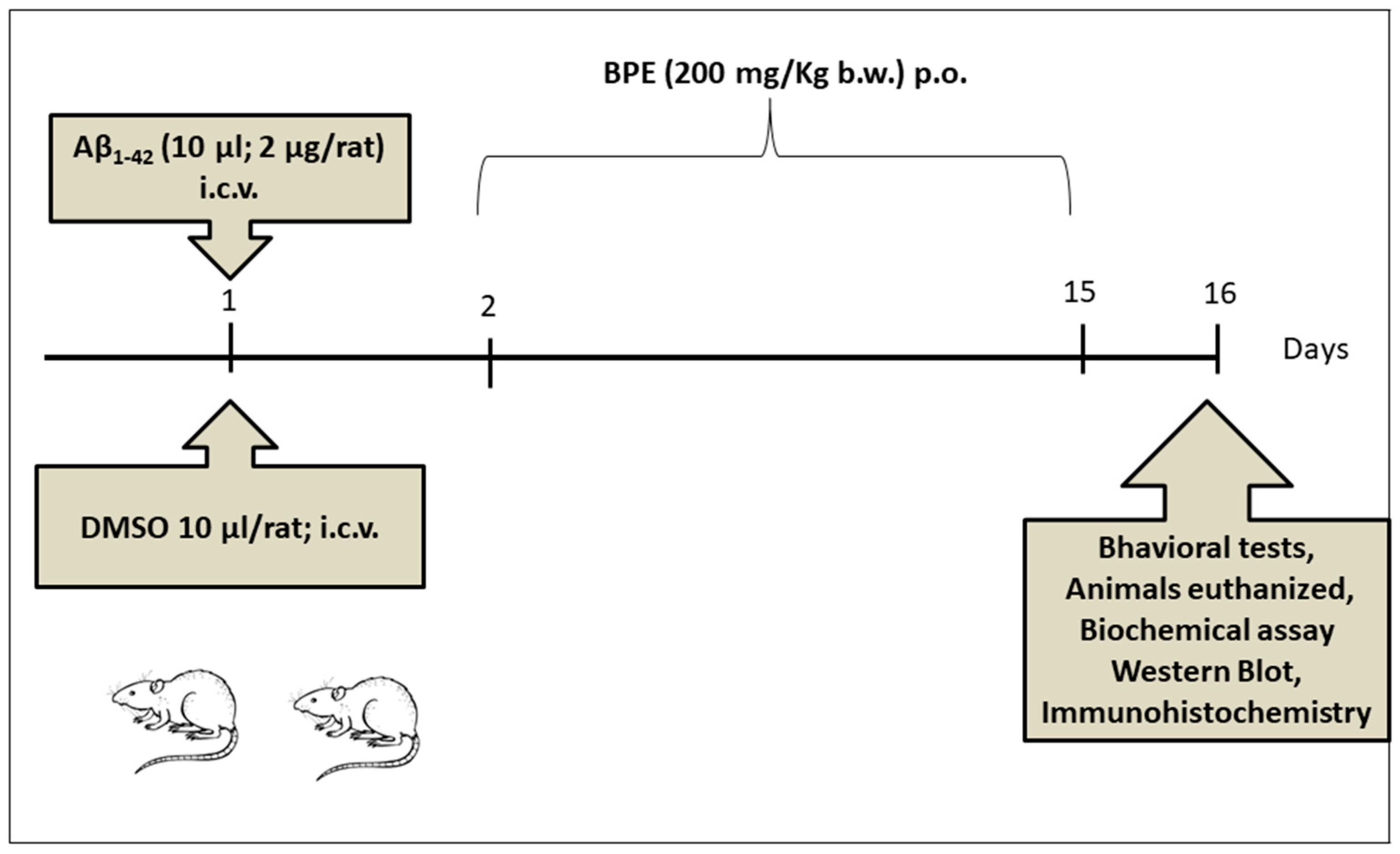
2.7. Animals and Experimental Design
2.8. Behavioral Testing
2.9. Biochemical Investigations of Oxidative Stress
2.10. Quantitative Estimation of Tumor Necrosis Factor-α (TNF-α) and Interleukin 1β (IL-1β) Levels Using ELISA Technique
2.11. Evaluation of Phospho–Tau (Ser 404 and S396), Synaptophysin, Cyclooxygenase-2 (COX 2), NFkB, and pNFkB Protein Expressions
2.12. Histological and Immunohistochemical Investigation of the Brain
2.13. Statistical Analysis
3. Results
3.1. TPC and TFC
3.2. LC/MS Analysis of BPE
3.3. Antioxidant Assays
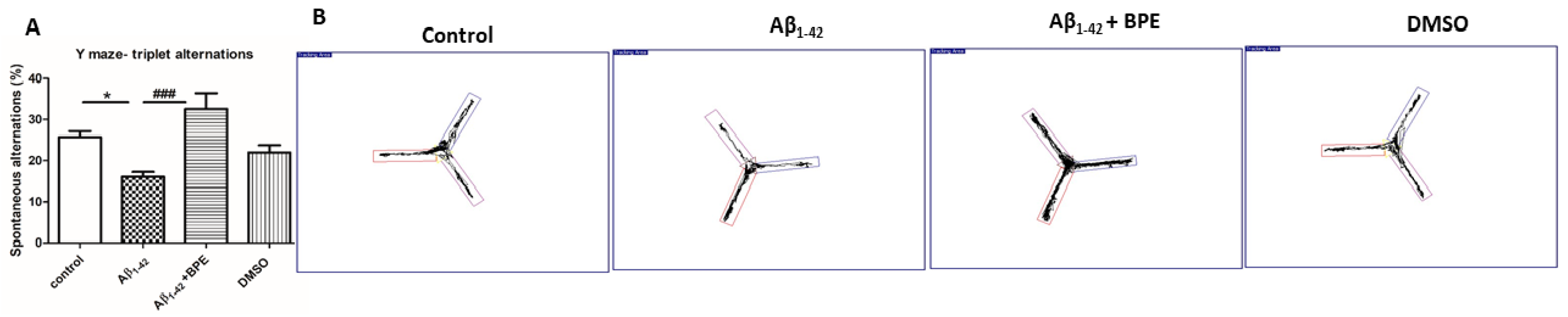
3.4. Behavioral Studies

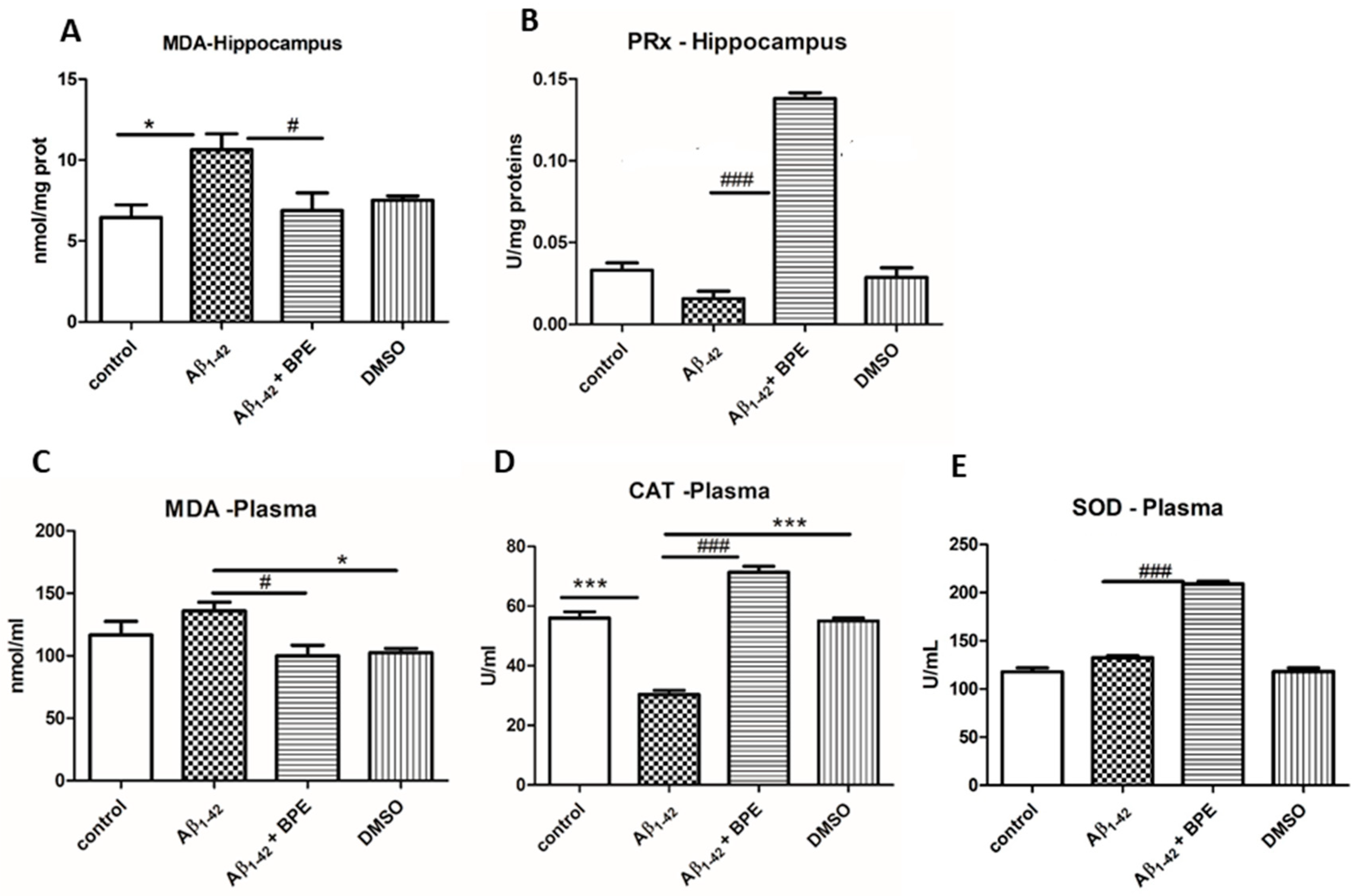
3.5. Oxidative Stress Assessment in Plasma and Brain
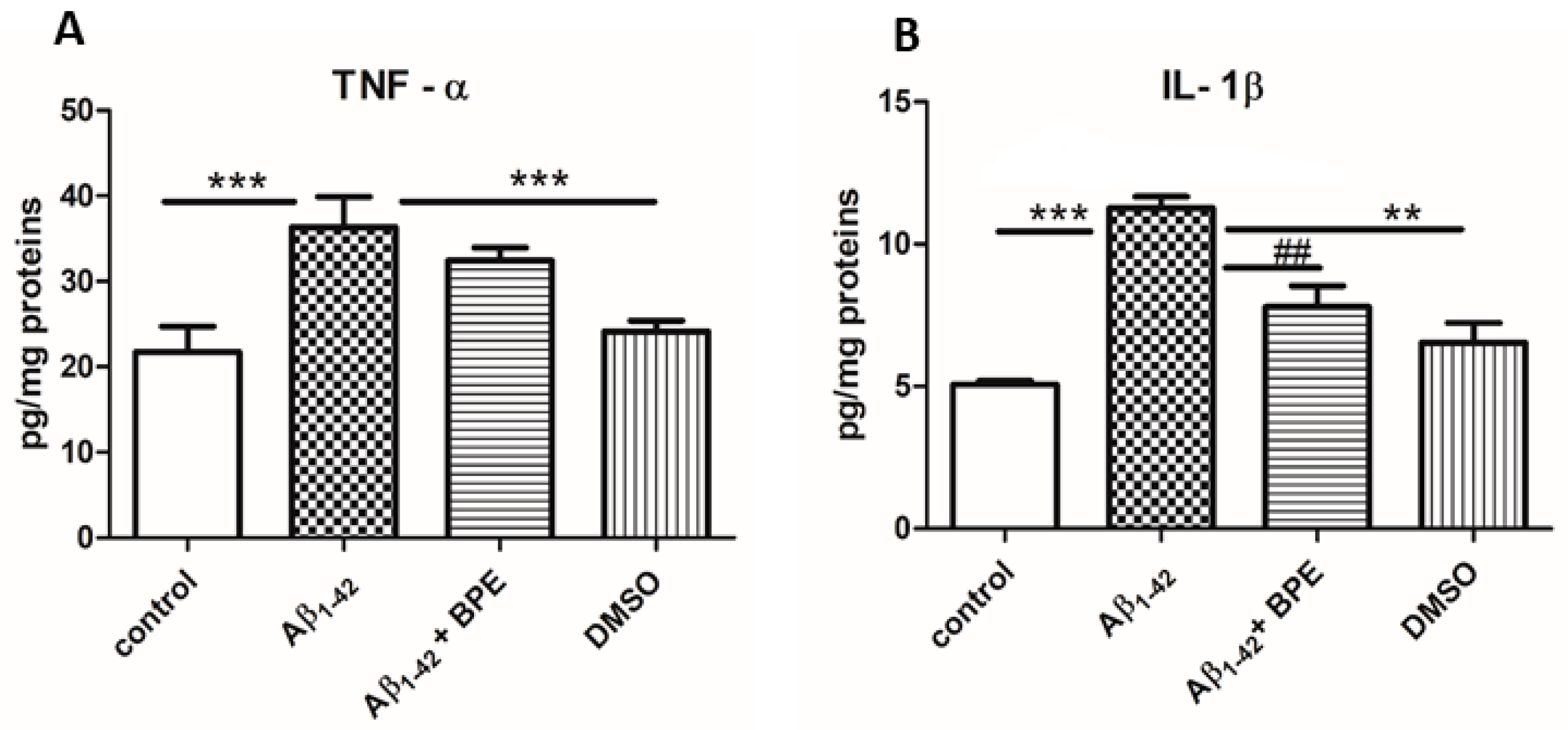
3.6. Quantitative Estimation of TNF-α and IL-1β Levels Using ELISA Technique
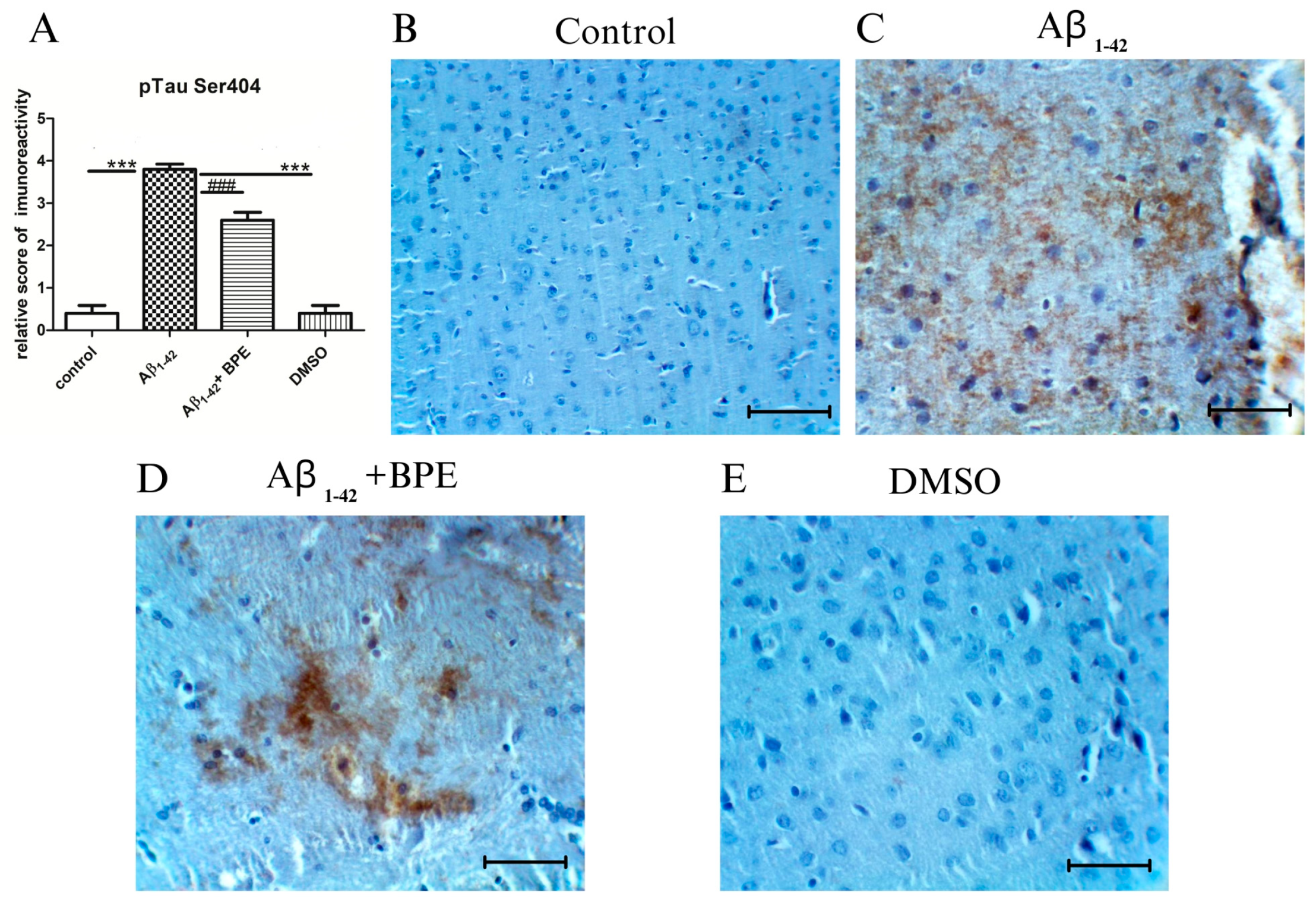
3.7. Evaluation of Tau, Synaptophysin, COX 2, NFkB, and pNFkB Protein Expressions
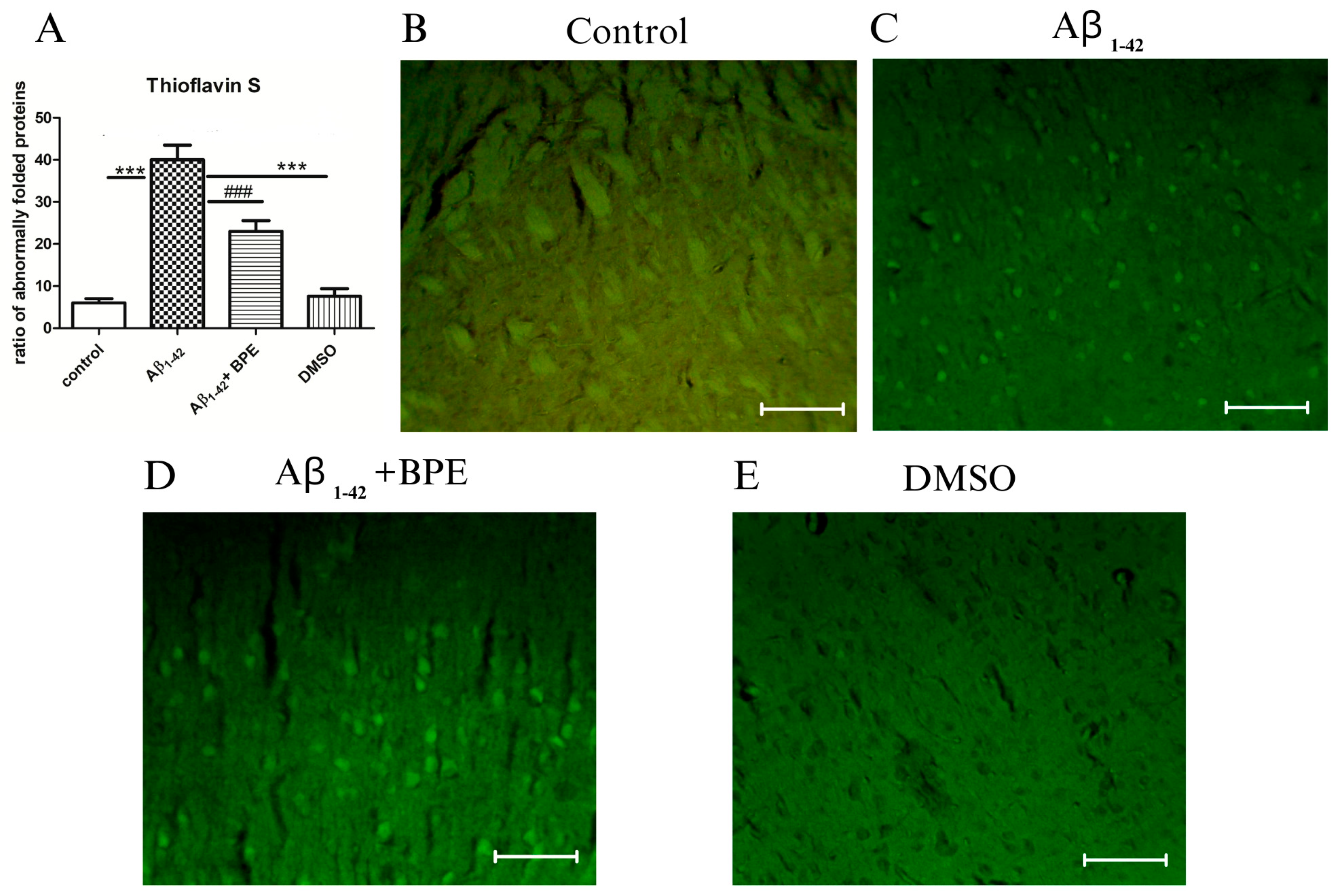
3.8. Histological and Immunohistochemical Investigation of the Hippocampal Formation
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hippius, H.; Neundörfer, G. The discovery of Alzheimer’s disease. Dialogues Clin. Neurosci. 2003, 5, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Sadigh-Eteghad, S.; Sabermarouf, B.; Majdi, A.; Talebi, M.; Farhoudi, M.; Mahmoudi, J. Amyloid-beta: A crucial factor in Alzheimer’s disease. Med. Princ. Pract. 2015, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://picower.mit.edu/about/aging-brain-initiative (accessed on 29 September 2023).
- Zakaria, R.; Wan Yaacob, W.M.; Othman, Z.; Long, I.; Ahmad, A.H.; Al-Rahbi, B. Lipopolysaccharide-induced memory impairment in rats: A model of Alzheimer’s disease. Physiol. Res. 2017, 66, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Nan, S.; Wang, P.; Zhang, Y.; Fan, J. Epigallocatechin-3-Gallate Provides Protection Against Alzheimer’s Disease-Induced Learning and Memory Impairments in Rats. Drug Des. Devel Ther. 2021, 15, 2013–2024. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Guo, J.; Ye, X.-Y.; Xie, Y.; Xie, T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 2022, V77, 101619. [Google Scholar] [CrossRef] [PubMed]
- Neddens, J.; Temmel, M.; Flunkert, S.; Kerschbaumer, B.; Hoeller, C.; Loeffler, T.; Niederkofler, V.; Daum, G.; Attems, J.; Hutter-Paier, B. Phosphorylation of different tau sites during progression of Alzheimer’s disease. Acta Neuropathol. Commun. 2018, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- d’Errico, P.; Meyer-Luehmann, M. Mechanisms of Pathogenic Tau and Aβ Protein Spreading in Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 265. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, W.; Zhao, M.; Ma, L.; Jiang, X.; Pei, H.; Cao, Y.; Li, H. Interaction between Aβ and Tau in the Pathogenesis of Alzheimer’s Disease. Int. J. Biol. Sci. 2021, 17, 2181–2192. [Google Scholar] [CrossRef]
- Buccellato, F.R.; D’Anca, M.; Serpente, M.; Arighi, A.; Galimberti, D. The Role of Glymphatic System in Alzheimer’s and Parkinson’s Disease Pathogenesis. Biomedicines. 2022, 10, 2261. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef]
- Koppula, S.; Kumar, H.; Kim, I.S.; Choi, D.K. Reactive Oxygen Species and Inhibitors of Inflammatory Enzymes, NADPH Oxidase, and iNOS in Experimental Models of Parkinson’s Disease. Hindawi Publ. Corp. Mediat. Inflamm. 2012, 2012, 823902. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Duszenko, M.; Gospodaryov, D.V.; Garaschuk, O. Oxidative Stress and Energy Metabolism in the Brain: Midlife as a Turning Point. Antioxidants 2021, 10, 1715. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Lan, Z.; Xie, G.; Wei, M.; Wang, P.; Chen, L. The protective effect of Epimedii Folium and Curculiginis Rhizoma on Alzheimer’s disease by the inhibitions of NF-κB/MAPK pathway and NLRP3 inflammasome. Oncotarget 2017, 8, 43709–43720. [Google Scholar] [CrossRef]
- Wang, C.; Zong, S.; Cui, X.; Wang, X.; Wu, S.; Wang, L.; Liu, Y.; Lu, Z. The effects of microglia-associated neuroinflammation on Alzheimer’s disease. Front. Immunol. 2023, 14, 1117172. [Google Scholar] [CrossRef]
- Sepulcre, J.; Schultz, A.P.; Sabuncu, M.; Gomez-Isla, T.; Chhatwal, J.; Becker, A.; Sperling, R.; Johnson, K.A. In vivo tau, amyloid, and gray matter profiles in the aging brain. J. Neurosci. 2016, 36, 7364–7374. [Google Scholar] [CrossRef]
- Schneider, L.S.; Sano, M. Current Alzheimer’s disease clinical trials: Methods and placebo outcomes. Alzheimers Dement. 2009, 5, 388–397. [Google Scholar] [CrossRef]
- Cummings, J.; Aisen, P.S.; DuBois, B.; Frolich, L.; Jack, C.R., Jr.; Jones, R.W.; Morris, J.C.; Raskin, J.; Dowsett, S.A.; Scheltens, P. Drug development in Alzheimer’s disease: The path to 2025. Alzheimers Res. Ther. 2016, 8, 39. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Shi, Z.; Wang, D.; Sun, L.; Wang, J.; Zhao, M.; Zhang, S. Chemical constituents from the bark of Betula pendula and their chemotaxonomic significance. Biochem. Syst. Ecol. 2023, 109, 104677. [Google Scholar] [CrossRef]
- Germanò, M.P.; Cacciola, F.; Donato, P.; Dugo, P.; Certo, G.; D’Angelo, V.; Mondello, L.; Rapisarda, A. Betula pendula leaves: Polyphenolic characterization and potential innovative use in skin whitening products. Fitoterapia 2012, 83, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Penkov, D.; Andonova, V.; Delev, D.; Kostadinov, I.; Kassarova, M. Antioxidant activity of dry birch (Betula Pendula) leaves extract. Folia Med. 2018, 60, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Gründemann, C.; Gruber, C.W.; Hertrampf, A.; Zehl, M.; Kopp, B.; Huber, R. An aqueous birch leaf extract of Betula pendula inhibits the growth and cell division of inflammatory lymphocytes. J. Ethnopharmacol. 2011, 136, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Germanò, M.P.; Cacciola, F.; Donato, P.; Dugo, P.; Certo, G.; D’Angelo, V.; Mondello, L.; Rapisarda, A. Betula pendula Roth leaves: Gastroprotective effects of an HPLC-fingerprinted methanolic extract. Nat. Prod. Res. Former. Nat. Prod. Lett. 2013, 27, 1569–1575. [Google Scholar] [CrossRef]
- Azman, N.A.M.; Skowyra, M.; Muhammad, K.; Gallego, M.G.; Almajano, M.P. Evaluation of the antioxidant activity of Betula pendula leaves extract and its effects on model foods. Pharm. Biol. 2017, 55, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Duric, K.; Kovac-Besovic, E.; Niksic, H.; Sofic, E. Antibacterial Activity of Methanolic Extracts, Decoction and Isolated Triterpene Products From Different Parts of Birch, Betula pendula, Roth. J. Plant Stud. 2013, 2, 2. [Google Scholar] [CrossRef]
- Rastogi, S.; Pandey, M.M.; Rawat, A.K.S. Medicinal plants of the genus Betula—Traditional uses and a phytochemical–pharmacological review. J. Ethnopharmacol. 2015, 159, 62–83. [Google Scholar] [CrossRef] [PubMed]
- Committee on Herbal Medicinal Products (HMPC). Assessment Report on Betula Pendula Roth and/or Betula Pubescens Ehrh. as well as Hybrids of Both Species, Folium EMA/HMPC/482160/2015. Available online: https://www.ema.europa.eu/en/medicines/herbal/betulae-folium (accessed on 22 October 2023).
- Ielciu, I.; Sevastre, B.; Olah, N.-K.; Turdean, A.; Chis, E.; Marica, R.; Oniga, I.; Uifalean, A.; Sevastre-Berghian, A.C.; Niculae, M.; et al. Evaluation of Hepatoprotective Activity and Oxidative Stress Reduction of Rosmarinus officinalis L. Shoots Tincture in Rats with Experimentally Induced Hepatotoxicity. Molecules 2021, 26, 1737. [Google Scholar] [CrossRef]
- Simea, S.; Ielciu, I.; Hanganu, D.; Niculae, M.; Pall, E.; Burtescu, R.F.; Olah, N.-K.; Cenariu, M.; Oniga, I.; Benedec, D.; et al. Evaluation of the Cytotoxic, Antioxidative and Antimicrobial Effects of Dracocephalum moldavica L. Cultivars. Molecules 2023, 28, 1604. [Google Scholar] [CrossRef]
- Buza, V.; Niculae, M.; Hanganu, D.; Pall, E.; Burtescu, R.F.; Olah, N.-K.; Matei-Lațiu, M.-C.; Vlasiuc, I.; Iozon, I.; Szakacs, A.R.; et al. Biological Activities and Chemical Profile of Gentiana asclepiadea and Inula helenium Ethanolic Extracts. Molecules 2022, 27, 3560. [Google Scholar] [CrossRef]
- Ielciu, I.; Niculae, M.; Pall, E.; Barb Alata, C.; Tomuta, I.; Olah, N.-K.; Burtescu, R.F.; Benedec, D.; Oniga, I.; Hanganu, D. Antiproliferative and Antimicrobial Effects of Rosmarinus officinalis L. Loaded Liposomes. Molecules 2022, 27, 3988. [Google Scholar] [CrossRef]
- Ielciu, I.; Hanganu, D.; Păltinean, R.; Vlase, L.; Frédérich, M.; Gheldiu, A.M.; Benedec, D.; Crişan, G. Antioxidant capacity and polyphenolic content of the Echinocystis lobata (Michx.) Torr. et A.Gray flowers. Pak. J. Pharm. Sci. 2018, 31 (Suppl. 2), 677–683. [Google Scholar]
- Ielciu, I.; Vlase, L.; Frédérich, M.; Hanganu, D.; Păltinean, R.; Cieckiewicz, E.; Olah, N.-K.; Gheldiu, A.M.; Crişan, G. Polyphenolic profile and biological activities of the leaves and aerial parts of Echinocystis lobata (Michx.) Torr. et A.Gray (Cucurbitaceae). Farmacia 2017, 65, 2. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 30. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Chromy, B.A.; Nowak, R.J.; Lambert, M.P.; Viola, K.L.; Chang, L.; Velasco, P.T.; Jones, B.W.; Fernandez, S.J.; Lacor, P.N.; Horowitz, P.; et al. Self-assemblyof Aβ1-42 into globular neurotoxins. Biochemistry 2003, 42, 12749–12760. [Google Scholar] [CrossRef]
- Morroni, F.; Sita, G.; Tarozzi, A.; Rimondini, R.; Hrelia, P. Early effects of Aβ1-42 oligomers injection in mice:involvement of PI3K/Akt/GSK3 and MAPK/ERK1/2 pathways. Behav. Brain Res. 2016, 314, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Sheng, C.; Xu, P.; Zhou, K.; Deng, D.; Zhang, C.; Wang, Z. Icariin Attenuates Synaptic and Cognitive Deficits in an Aβ1-42-Induced Rat Model of Alzheimer’s Disease. Biomed. Res. Int. 2017, 2017, 7464872. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, S.; Sarkaki, A.; Farbood, Y.; Eidi, A.; Mortazavi, P.; Valizadeh, Z. Gallic acid effect on dementia type of alzheimer disease. Basic Clin. Neurosci. 2016, 7, 97–106. [Google Scholar] [PubMed]
- Walf, A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef]
- Gamberini, M.T.; Rodrigues, D.S.; Rodrigues, D.; Pontes, C.V. Effects of the aqueous extract of Pimpinella anisum L. seeds on exploratory activity and emotional behavior in rats using the open field and elevated plus maze tests. J. Ethnopharmacol. 2015, 168, 45–49. [Google Scholar] [CrossRef]
- Sevastre-Berghian, A.C.; Făgărăsan, V.; Toma, V.A.; Bâldea, I.; Olteanu, D.; Moldovan, R.; Decea, N.; Filip, G.A.; Clichici, S.V. Curcumin reverses the Diazepam-induced cognitive impairment by modulation of oxidative stress and ERK 1/2/NF-κB pathway in brain. Oxidative Med. Cell. Longev. 2017, 2017, 1–16. [Google Scholar] [CrossRef]
- Kim, D.H.; Yoon, B.H.; Kim, Y.W.; Lee, S.; Shin, B.Y.; Jung, J.W.; Kim, H.J.; Lee, Y.S.; Choi, J.S.; Kim, S.Y.; et al. The seed extract of Cassia obtusifolia ameliorates learning and memory impairments induced by scopolamine or transient cerebral hypoperfusion in mice. J. Pharmacol. Sci. 2007, 105, 82–93. [Google Scholar] [CrossRef]
- Harquin Simplice, F.; Emery, T.D.; Hervé Hervé, N.A. Enhancing spatial memory: Anxiolytic and antidepressant effects of Tapinanthus dodoneifolius (DC) Danser in mice. Neurol. Res. Int. 2014, 2014, 974308. [Google Scholar] [CrossRef]
- Conti, M.; Morand, P.C.; Levillain, P.; Lemonnier, A. Improved fluorometric determination of malonaldehyde. Clin. Chem. 1991, 37, 1273–1275. [Google Scholar] [CrossRef]
- Pippenger, C.E.; Browne, R.W.; Armstrong, D. Regulatory antioxidant enzymes. Methods Mol. Biol. 1998, 108, 299–313. [Google Scholar]
- Fridovich, I. Superoxide dismutases. An adaptation to a paramagnetic gas. J. Biol. Chem. 1989, 264, 7761–7764. [Google Scholar] [CrossRef] [PubMed]
- Baldea, I.; Costin, G.-E.; Shellman, Y.; Kechris, K.; Olteanu, E.D.; Filip, A.; Cosgarea, M.R.; Norris, D.A.; Birlea, S.A. Biphasic Pro Melanogenic and pro-Apoptotic Effects of All-Trans-Retinoic Acid (ATRA) on Human Melanocytes: Time-Course Study. J. Dermatol. Sci. 2013, 72, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.urmc.rochester.edu/urmclabs/pathology/stainsmanual/index.html?THIOFLAVINSMETHODFORAMYLOIDINNEUROFIBRILLARYPLAQUES (accessed on 29 September 2023).
- Schwartz, P. Amyloid degeneration and tuberculosis in the aged. Gerontologia 1972, 18, 321–362. [Google Scholar] [CrossRef] [PubMed]
- Vassar, P.S.; Culling, C.F. Fluorescent stains, with special reference to amyloid and connective tissues. Arch. Pathol. 1959, 68, 487–498. [Google Scholar]
- Toma, V.A.; Farcas, A.D.; Parvu, M.; Silaghi-Dumitrescu, R.; Roman, I. CA3 hippocampal field: Cellular changes and its relation with blood nitro-oxidative stress reveal a balancing function of CA3 area in rats exposed to repeated restraint stress. Brain Res. Bull. 2017, 130, 10–17. [Google Scholar] [CrossRef]
- Ma, C.; Hong, F.; Yang, S. Amyloidosis in Alzheimer’s Disease: Pathogeny, Etiology, and Related Therapeutic Directions. Molecules 2022, 27, 1210. [Google Scholar] [CrossRef]
- Hillen, H. The Beta Amyloid Dysfunction (BAD) Hypothesis for Alzheimer’s Disease. Front. Neurosci. 2019, 13, 1154. [Google Scholar] [CrossRef]
- Forloni, G.; Artuso, V.; La Vitola, P.; Balducci, C. Oligomeropathies and pathogenesis of Alzheimer and Parkinson’s diseases. Mov. Disord. 2016, 31, 771–781. [Google Scholar] [CrossRef]
- Chen, X.; Guo, C.; Kong, J. Oxidative stress in neurodegenerative diseases. Neural Regen. Res. 2012, 7, 376–385. [Google Scholar] [CrossRef]
- Jellinger, K.A. Basic mechanisms of neurodegeneration: A critical update. J. Cell Mol. Med. 2010, 14, 457–487. [Google Scholar] [CrossRef]
- Hajialyani, M.; Hosein Farzaei, M.; Echeverría, J.; Nabavi, S.M.; Uriarte, E.; Sobarzo-Sánchez, E. Hesperidin as a Neuroprotective Agent: A Review of Animal and Clinical Evidence. Molecules 2019, 24, 648. [Google Scholar] [CrossRef]
- Yin, R.; Xue, J.; Tan, Y.; Fang, C.; Hu, C.; Yang, Q.; Mei, X.; Qi, D. The Positive Role and Mechanism of Herbal Medicine in Parkinson’s Disease. Oxidative Med. Cell. Longev. 2021, 2021, 9923331. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.; Terzo, S.; Mulè, F. Natural Compounds as Beneficial Antioxidant Agents in Neurodegenerative Disorders: A Focus on Alzheimer’s Disease. Antioxidants 2019, 8, 608. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Wang, G.; Wang, X.; Tang, J.; Yu, Q.; Zhang, X.; Wang, S. Neuro-protection of Chlorogenic acid against Al-induced apoptosis in PC12 cells via modulation of Al metabolism and Akt/GSK-3β pathway. J. Funct. Foods 2020, 70, 103984. [Google Scholar] [CrossRef]
- Zhang, X.-W.; Chen, J.-Y.; Ouyang, D.; Lu, J.-H. Quercetin in Animal Models of Alzheimer’s Disease: A Systematic Review of Preclinical Studies. Int. J. Mol. Sci. 2020, 21, 493. [Google Scholar] [CrossRef]
- Vladimirov, M.S.; Nikolić, V.D.; Stanojević, L.P.; Nikolić, L.B.; Dinić, A. Common birch (Betula pendula Roth.): Chemical composition and biological activity of isolates. Adv. Technol. 2019, 8, 65–77. [Google Scholar] [CrossRef]
- Ossipov, V.; Nurmi, K.; Loponen, J.; Haukioja, E.; Pihlaja, K. High-performance liquid chromatographic separation and identification of phenolic compounds from leaves of Betula pubescens and Betula pendula. J. Chromatogr. A 1996, 721, 59–68. [Google Scholar] [CrossRef]
- Calliste, C.A.; Trouillas, P.; Allais, D.P.; Simon, A.; Duroux, J.L. Free radical scavenging activities measured by electron spin resonance spectroscopy and B16 cell antiproliferative behaviors of seven plants. J. Agric. Food Chem. 2001, 49, 3321–3327. [Google Scholar] [CrossRef]
- Ghimire, B.K.; Tamang, J.P.; Yu, C.Y.; Jung, S.J.; Chung, I.M. Antioxidant, antimicrobial activity and inhibition of α-glucosidase activity by Betula alnoides Buch. bark extract and their relationship with polyphenolic compounds concentration. Immunopharmacol. Immunotoxicol. 2012, 34, 824–831. [Google Scholar] [CrossRef]
- Raudonė, L.; Raudonis, R.; Janulis, V.; Viškelis, P. Quality evaluation of different preparations of dry extracts of birch (Betula pendula Roth) leaves. Nat. Prod. Res. 2014, 28, 1645–1648. [Google Scholar] [CrossRef]
- Iqbal, K.; Liu, F.; Gong, C.X.; Grundke-Iqbal, I. Tau in Alzheimer disease and related tauopathies. Curr. Alzheimer Res. 2010, 7, 656–664. [Google Scholar] [CrossRef]
- Naseri, N.N.; Wang, H.; Guo, J.; Sharma, M.; Luo, W. The complexity of tau in Alzheimer’s disease. Neurosci. Lett. 2019, 13, 183–194. [Google Scholar] [CrossRef]
- Drummond, E.; Pires, G.; MacMurray, C.; Askenazi, M.; Nayak, S.; Bourdon, M.; Safar, J.; Ueberheide, B.; Wisniewski, T. Phosphorylated tau interactome in the human Alzheimer’s disease brain. Brain 2020, 143, 2803–2817. [Google Scholar] [CrossRef]
- Medeiros, R.; Baglietto-Vargas, D.; LaFer, F.M. The role of tau in Alzheimer’s disease and related disorders. CNS Neurosci. Ther. 2011, 17, 514–524. [Google Scholar] [CrossRef]
- Foidl, B.M.; Humpel, C. Differential Hyperphosphorylation of Tau-S199, -T231 and -S396 in Organotypic Brain Slices of Alzheimer Mice. A Model to Study Early Tau Hyperphosphorylation Using Okadaic Acid. Front. Aging Neurosci. 2018, 10, 113. [Google Scholar] [CrossRef]
- Mondragón-Rodríguez, S.; Perry, G.; Luna-Muñoz, J.; Acevedo-Aquino, M.C.; Williams, S. Phosphorylation of tau protein at sites Ser(396-404) is one of the earliest events in Alzheimer’s disease and Down syndrome. Neuropathol. Appl. Neurobiol. 2014, 40, 121–135. [Google Scholar] [CrossRef] [PubMed]
- SantaCruz, K.; Lewis, J.; Spires, T.; Paulson, J.; Kotilinek, L.; Ingelsson, M.; Guimaraes, A.; DeTure, M.; Ramsden, M.; McGowan, E.; et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science 2005, 309, 476–481. [Google Scholar] [CrossRef]
- Newcomer, J.W.; Farber, N.B.; Olney, J.W. NMDA receptor function, memory, and brain aging. Dialogues Clin. Neurosci. 2000, 2, 219–232. [Google Scholar] [CrossRef]
- Cassidy, L.; Fernandez, F.; Johnson, J.B.; Naiker, M.; Owoola, A.G.; Broszczak, D.A. Oxidative stress in alzheimer’s disease: A review on emergent natural polyphenolic therapeutics. Complement. Ther. Med. 2020, 49, 102294. [Google Scholar] [CrossRef]
- Baram, T.Z.; Donato, F.; Holmes, G.L. Construction and disruption of spatial memory networks during development. Learn. Mem. 2019, 26, 206–218. [Google Scholar] [CrossRef]
- Proskauer Pena, S.L.; Mallouppas, K.; Oliveira, A.M.G.; Zitricky, F.; Nataraj, A.; Jezek, K. Early Spatial Memory Impairment in a Double Transgenic Model of Alzheimer’s Disease TgF-344 AD. Brain Sci. 2021, 11, 1300. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, G.; Laczó, J.; Hort, J.; Minihane, A.-M.; Hornberger, M. Spatial Navigation Deficits—Overlooked Cognitive Marker for Preclinical Alzheimer Disease? Nat. Rev. Neurol. 2018, 14, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Utz, J.; Berner, J.; Muñoz, L.E.; Oberstein, T.J.; Kornhuber, J.; Herrmann, M.; Maler, J.M.; Spitzer, P. Cerebrospinal Fluid of Patients With Alzheimer’s Disease Contains Increased Percentages of Synaptophysin-Bearing Microvesicles. Front. Aging Neurosci. 2021, 13, 682115. [Google Scholar] [CrossRef] [PubMed]
- Tannenberg, R.K.; Dodd, P.R. Cell Damage/Excitotoxicity. Excitotoxicity and Neurodegenerative Disease. Encyclopedia of Basic Epilepsy Research; Academic Press: Cambridge, MA, USA, 2009; pp. 114–119. [Google Scholar] [CrossRef]
- Jones, S.V.; Kounatidis, I. Nuclear Factor-Kappa B and Alzheimer Disease, Unifying Genetic and Environmental Risk Factors from Cell to Humans. Front. Immunol. 2017, 8, 1805. [Google Scholar] [CrossRef] [PubMed]
- Thawkar, B.S.; Kaur, G. Inhibitors of NF-κB and P2X7/NLRP3/Caspase 1 pathway in microglia: Novel therapeutic opportunities in neuroinflammation induced early-stage Alzheimer’s disease. J. Neuroimmun. 2019, 326, 62–74. [Google Scholar] [CrossRef]
- Chiarini, A.; Armato, U.; Hu, P.; Prà, I.D. Danger-Sensing/Patten Recognition Receptors and Neuroinflammation in Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 9036. [Google Scholar] [CrossRef] [PubMed]
- Sun, E.; Motolani, A.; Campos, L.; Lu, T. The Pivotal Role of NF-kB in the Pathogenesis and Therapeutics of Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 8972. [Google Scholar] [CrossRef] [PubMed]
- Tamagno, E.; Guglielmotto, M.; Vasciaveo, V.; Tabaton, M. Oxidative Stress and Beta Amyloid in Alzheimer’s Disease. Which Comes First: The Chicken or the Egg? Antioxidants 2021, 10, 1479. [Google Scholar] [CrossRef]
- Snow, W.M.; Albensi, B.C. Neuronal gene targets of NF-κB and their dysregulation in Alzheimer’s disease. Front. Mol. Neurosci. 2016, 9, 118. [Google Scholar] [CrossRef]
- Jha, N.K.; Jha, S.K.; Kar, R.; Nand, P.; Swati, K.; Goswami, V.K. Nuclear factor-kappa β as a therapeutic target for Alzheimer’s disease. J. Neurochem. 2019, 150, 113–137. [Google Scholar] [CrossRef]
- Shih, R.H.; Wang, C.Y.; Yang, C.M. NF-kappaB Signaling Pathways in Neurological Inflammation: A Mini Review. Front. Mol. Neurosci. 2015, 8, 77. [Google Scholar] [CrossRef]
- Gagliardi, S.; Franco, V.; Sorrentino, S.; Zucca, S.; Pandini, C.; Rota, P.; Bernuzzi, S.; Costa, A.; Sinforiani, E.; Pansarasa, O.; et al. Curcumin and Novel Synthetic Analogs in Cell-Based Studies of Alzheimer’s Disease. Front. Pharmacol. 2018, 9, 1404. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Y.; Mueller-Steiner, S.; Chen, L.F.; Kwon, H.; Yi, S.; Mucke, L.; Gan, L. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-κB signaling. J. Biol. Chem. 2005, 280, 40364–40374. [Google Scholar] [CrossRef]

| Sample | TPC (g GAE/100 mL Extract) | TFC (g RE/100 mL Extract) |
|---|---|---|
| BPE | 2.07 ± 0.16 | 0.73 ± 0.07 |
| Compound | Retention Time (min) | m/z and Main Transition | Concentration (μg/mL) | ||
|---|---|---|---|---|---|
| Reference | Separated Compound | Reference | Separated Compound | ||
| Caffeic acid | 13.8 | 13.6 | 179.0 > 135.0 | 179.0 > 135.0 | 35 ± 0.003 |
| Chlorogenic acid | 12.0 | 12.0 | 353.0 > 191.0 | 353.0 > 191.0 | 125 ± 0.014 |
| Apigenin | 28.2 | 28.1 | 269.0 > 117.0 | 269.0 > 117.0 | 13 ± 0.001 |
| Chrysin | 29.7 | 30.0 | 253.0 > 143.0 | 253.0 > 143.0 | 1 ± 0.001 |
| Luteolin | 26.9 | 26.8 | 287.0 > 153.0 | 287.0 > 153.0 | 1 ± 0.002 |
| Luteolin-7-O-glucoside | 19.9 | 19.8 | 447.0 > 284.9 | 447.0 > 284.9 | 1 ± 0.05 |
| Quercetin | 25.7 | 27.0 | 300.9 > 151.0 | 300.9 > 151.0 | 40 ± 0.003 |
| Naringenin | 26.3 | 26.9 | 271.0 > 119.0 | 271.0 > 119.0 | 3 ± 0.007 |
| Gallic acid | 7.0 | 7.0 | 168.9 > 125.0 | 447.0 > 229.9 | 72 ± 0.002 |
| Ferulic acid | 18.4 | 18.2 | 193.0 > 134.0 | 193.0 > 178.0 | 20.1 ± 0.01 |
| Trans-p-coumaric acid | 17.5 | 17.9 | 163.0 > 119.0 | 163.0 > 93.0 | 112 ± 0.009 |
| Catechin | 10.3 | 10.3 | 289.0 > 202.9 | 289.0 > 202.9 | 0.4 ± 0.0002 |
| Carnosol | 30.6 | 31.0 | 329.1 > 285.1 | 329.1 > 285.1 | 0.28 ± 0.001 |
| Hyperoside | 20.3 | 20.2 | 463.1 > 300.0 | 463.1 > 300.0 | 2 ± 0.001 |
| Quercetin | 25.4 | 25.1 | 300.9 > 151.0 | 300.9 > 121.0 | 40 ± 0.003 |
| Quercitrin | 22.1 | 22.1 | 447.0 > 229.9 | 447.0 > 229.9 | 99 ± 0.009 |
| Salicylic acid | 23.5 | 23.3 | 137.0 > 93.0 | 137.0 > 75.0 | 14 ± 0.002 |
| Sample | DPPH IC50 (µg/mL) | FRAP µmol TE/100 mL Extract |
|---|---|---|
| BPE | 31.92 ± 0.0087 | 9.32 ± 0.0059 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sevastre-Berghian, A.-C.; Ielciu, I.; Bab, T.; Olah, N.-K.; Neculicioiu, V.S.; Toma, V.A.; Sevastre, B.; Mocan, T.; Hanganu, D.; Bodoki, A.E.; et al. Betula pendula Leaf Extract Targets the Interplay between Brain Oxidative Stress, Inflammation, and NFkB Pathways in Amyloid Aβ1-42-Treated Rats. Antioxidants 2023, 12, 2110. https://doi.org/10.3390/antiox12122110
Sevastre-Berghian A-C, Ielciu I, Bab T, Olah N-K, Neculicioiu VS, Toma VA, Sevastre B, Mocan T, Hanganu D, Bodoki AE, et al. Betula pendula Leaf Extract Targets the Interplay between Brain Oxidative Stress, Inflammation, and NFkB Pathways in Amyloid Aβ1-42-Treated Rats. Antioxidants. 2023; 12(12):2110. https://doi.org/10.3390/antiox12122110
Chicago/Turabian StyleSevastre-Berghian, Alexandra-Cristina, Irina Ielciu, Timea Bab, Neli-Kinga Olah, Vlad Sever Neculicioiu, Vlad Alexandru Toma, Bogdan Sevastre, Teodora Mocan, Daniela Hanganu, Andreea Elena Bodoki, and et al. 2023. "Betula pendula Leaf Extract Targets the Interplay between Brain Oxidative Stress, Inflammation, and NFkB Pathways in Amyloid Aβ1-42-Treated Rats" Antioxidants 12, no. 12: 2110. https://doi.org/10.3390/antiox12122110
APA StyleSevastre-Berghian, A.-C., Ielciu, I., Bab, T., Olah, N.-K., Neculicioiu, V. S., Toma, V. A., Sevastre, B., Mocan, T., Hanganu, D., Bodoki, A. E., Roman, I., Lucaciu, R. L., Hangan, A. C., Hașaș, A.-D., Decea, R. M., & Băldea, I. (2023). Betula pendula Leaf Extract Targets the Interplay between Brain Oxidative Stress, Inflammation, and NFkB Pathways in Amyloid Aβ1-42-Treated Rats. Antioxidants, 12(12), 2110. https://doi.org/10.3390/antiox12122110










.jpg)


