Essential Oils in Cervical Cancer: Narrative Review on Current Insights and Future Prospects
Abstract
:1. Introduction
2. Literature Research Methodology
3. Human Papillomavirus as Risk Factors of Cervical Cancer
4. Conventional Treatment of Cervical Cancer
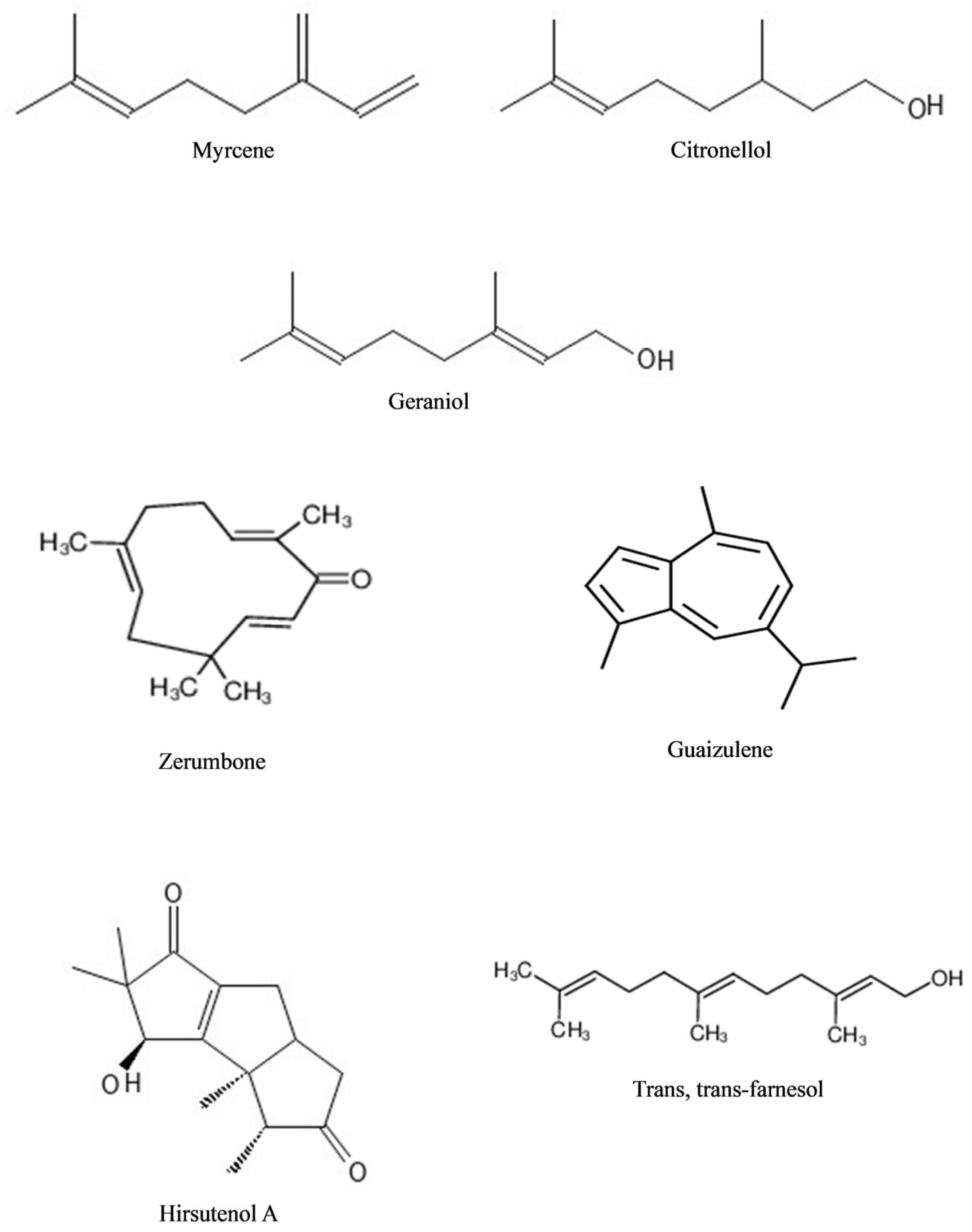
5. Essential Oils: Composition and Their Potential Benefits
6. Role of Essential Oils in Cervical Cancer: Evidence from Laboratory
6.1. Essential Oils with High Monoterpenes
6.2. Essential Oils with High Sesquiterpenes
6.3. Essential Oils with High Monoterpenoids
6.4. Essential Oils with High Sesquiterpenoids
| Essential Oil | Major Constituent | Study Model, Treatment Regimen | Important Findings | References |
|---|---|---|---|---|
| Achillea millefolium (Yarrow) | 1,8-Cineole (27.3%) Camphor (24.3%) β-Eudesmol (18.7%) | HeLa human cervical epithelioid carcinoma cells | Reduced cell viability (IC50 ND). Blocked cells in G0/G1 phase. | [89] |
| Acorus calamus (Sweet flag) | β-Asarone (31.56–91.27%) α-Asarone (1.05–52.96%) | SiHa human cervical cancer cells | Reduced cell viability (IC50 55.5%) at 300 µg/mL. | [121] |
| Aegle marmelos (L.) Correa (Bael tree) | p-Mentha-1,4(8)-diene (33.2%) Limonene (13.1%) p-Cymen-α-ol (9.5%) γ-Gurjunene- (7.9%) β-Phellandrene (4.3%) | HeLa cells | Reduced cell viability (IC50 85.6 μg/mL). Effective in suppressing ROS. | [102] |
| Aloysia citriodora (Lemon verbena) | α-Citral (43.46–47.62%) α-Curcumene (11.35–14.39%) trans-1,2-Bis-(1-methylethenyl)cyclobutane (10.08–15.07%) | HeLa cells | Reduced cell viability (IC50 84.5 and 33.31 μg/mL, vs. IC50 22.01 μg/mL (Doxorubicin)). Inhibited COX-1 and COX-2 enzymes. | [103] |
| Alpinia nigra (Gaertn.) Burtt (Black Galangal) | Leaves: β-pinene (56.27%) α-Farnesene (7.92%) Caryophyllene (6.46%) Rhizomes: β-pinene (38.03%) myrtenol (9.35%) α-Humulene (7.82%) Humulene epoxide II (6.00%) | HeLa cells | Inhibited 60% (leaves EO) and 79% (rhizomes EO) proliferations at 20 µg/mL. | [96] |
| Artemisia arborescens (Vaill.) L. (Silver wormwood) | β-Thujone (79.16–89.64%) Camphor (5.34–6.58%) β-Pinene (2.01%) Sabinene (3.44%) | HeLa cells | Reduced cell viability (IC50 326 and 467 μg/mL). Inhibit COX-1 and COX-2. | [100] |
| Cephalotaxus griffithii Hook. f. (Griffith’s plum yew) | ND | HeLa cells | Reduced cell viability (IC50 ND). Induced apoptosis (up-regulated caspase-3 expression, reduced cell volume, increased cytoplasmic membrane blebbing, nuclear contraction, nuclear fragmentation, and formation of apoptotic bodies). Inhibit cell migration. | [87] |
| Chenopodium botrys L. (Jerusalem-oak) | α-Eudesmol (16.81%) Elemol acetate (13.2%) Elemol (9.0%) α-Chenopodiol-6-acetate (7.9%) | HeLa cells | Reduced cell viability (IC50 79.62 µg/mL). Increased numbers of apoptotic cells. Induced cell cycle arrest in G1 phase. Increased p21, p53, Bax, and caspase-3 expressions. | [90] |
| Cinnamomum zeylanicum Blume (Cinnamon) | Cinnamaldehyde (77.34%) trans-Cinnamyl acetate (4.98%) Benzene dicarboxylic acid (3.55%) α-Pinene (2.6%) | HeLa cells | Reduced cell viability (IC50 0.13 μg/mL). | [80] |
| Crassocephalum crepidioides (Thickhead weed) | β-Myrcene (65.9%) β-Phellandrene (8.8%) α-Pinene (3.1%) α-Copaene (1.5%) | SiHa cells | Reduced cell viability (IC50 45.9 µg/mL). | [104] |
| Curcuma aromatica (Wild turmeric) | ar-Tumerone (ND) | HeLa cells | Reduced cell viability (IC50 72.02 µg/mL). | [63] |
| C. longa L. (Turmeric) | ar-Tumerone (ND) | HeLa cells | Reduced cell viability (IC50 24.82 µg/mL). | [63] |
| ar-Turmerone (33.2%) α-Turmerone (23.5%) β-Turmerone (22.7%) | HeLa cells | Reduced cell viability (IC50 36.6 µg/mL). HeLa cell morphology showed condensation of chromatin, loss of cell membrane integrity with protrusions, and cell content leakage. | [64] | |
| C. zedoaria (Christm.) Roscoe (White turmeric) | Zerumbone (17.2%) Camphor (17.56%) Curzerenone (10.2%) Isovelleral (6.6%) | HeLa and SiHa cells | Reduced HeLa cell viability (IC50 6.4 μg/mL vs. IC50 6.5 μg/mL (doxorubicin)). Reduced SiHa cell viability (IC50 9.8 μg/mL vs. IC50 7.8 μg/mL (doxorubicin)). | [65] |
| Cymbopogon nardus (Citronella grass) | Citronellal (33.06%) Geraniol (28.40%) Nerol (10.94%) Elemol (5.25%) | HeLa cells | Reduced cell viability (IC50 142 μg/mL). | [118] |
| Dittrichia viscosa (L.) Greuter (Yellow fleabane) | 1,8-Cineole (16.41%) Caryophyllene oxide (15.14%) α-Terpinyl acetate (13.92) α-Muurolol (13.75%) | HeLa cells | Reduced cell viability (IC50 660 μg/mL). | [113] |
| Ephedra intermedia Schrenk and Mey | 2-Ethyl-pyrazine (67.37%) γ-Elemene (9.21%) Benzyl acetate (9.10%) 2-Methyl-butyl acetate (5.28%) | HeLa cells | Reduced cell viability (IC50 423.22 μg/mL). | [81] |
| Erigeron canadensis L. (Canadian horseweed) | Limonene (65.68%) (Z)-β-ocimene (6.87%) β-Pinene (6.29%) Germacrene (4.03%) | HeLa cells | Reduced cell viability (IC50 6780 μg/mL vs IC50 1120 μg/mL (Limonene alone)). Cells showed nuclear pyknosis and abnormal chromatin condensation after EO treatment. Decreased G1 phase cells and increased G2/M phase cells. Increased expression of Caspase-3, -9, and -12 proteins. Inhibited mitochondrial membrane potential. | [88] |
| Ferula tingitana (L.) Apiaceae (Giant Tangier fennel) | Leaves: δ-Cadinol (13.8%) γ-Eudesmol (9.7%) 7-α-Eudesma-3,5-diene (9.0%) Elemol (8.3%) Fruits: 3-Carene (13.9%) α-Thujene (13.5%) Elemol (8.9%) Myrcene (8.1%) | HeLa cells | Reduced cell viability (IC50 10.9 µg/mL (leaves EO), IC50 8.6 µg/mL (fruits EO) vs. IC50 4.7 µg/mL (doxorubicin)). | [101] |
| Foeniculum vulgare (Fennel) | trans-Anethole (36.8%) p-Anisaldehyde (7.7%) α-Ehyl-p-methoxybenzyl alcohol (9.1%) | HeLa cells | Reduced cell viability (IC50 207 mg/L). | [66] |
| trans-Anethole (80.63%) L-Fenchone (11.57%) Estragole (3.67%) Limonene (2.68%) | HeLa cells | Reduced cell viability (IC50 1.26 μg/mL). | [67] | |
| trans-Anethole (88.28%) Estragole (4.25%) D-Limonene (2.04%) Fenchone (2.03%) | HeLa cells | Reduced cell viability (IC50 56.43 µg/mL vs. 17.95 µg/mL (Paclitaxel)). | [68] | |
| Ganoderma applanatum (Artist’s conk) | γ-Terpinene (30.3%) d-Limonene (23.6%) Cis-2-methyl-4-pentylthiane-s,s-dioxide (15.3%) Cymene (12.7%) | HEp-2 cervical cancer cells | Reduced cell viability (IC50 43.2 μg/mL). | [97] |
| Hedychium coronarium (White ginger lily) | 1,8-Cineole (ND) | HeLa cells | Reduced cell viability (IC50 87.98 µg/mL). | [63] |
| Helichrysum italicum (Roth) G. Don (Curry plant) | α-pinene (21.6%) γ-curcumene (21.6%) Neryl acetate (7.9%) | HeLa cells | Reduced cell viability (IC50 7.5 µg/mL). No effect on cell cycle. Induced apoptosis. | [85] |
| Hyptis suaveolens (L.) Poit. (Pignut) | Sabinene (14.03%) Eucalyptol (12.78%) β-Caryophyllene (11.27%) Bicyclogermacrene (8.08%) | HeLa cells | Reduced cell viability (IC50 181.37 µg/mL vs. IC50 4.32 (cisplatin)). Induced G0/G1 cell cycle arrest and a decreased G2/M phase. | [91] |
| Inula graveolens (Linnaeus) Desf (Stinkwort) | Bornyl acetate (69.15%) Camphene (11.11%) | HeLa cells | Reduced cell viability (IC50 64.1 µg/mL, IC50 72.0 µg/mL (bornyl acetate) vs. IC50 126.75 µg/mL (cisplatin)). | [82] |
| Juniperus communis (Common Juniper) | α-Pinene, limonene, and sabinene (49.1–82.8%) 4-Terpineol | SiHa cells | Reduced cell viability (IC50 150.6 µg/mL). | [95] |
| Kaempferia galanga (Sand ginger) | Ethyl p-methoxycinnamate (ND) | HeLa cells | Reduced cell viability (IC50 44.18 µg/mL). | [63] |
| Lantana camara Linn. (Common Lantana) | Sabinene (20.38%) β-Caryophyllene (17.88%) Eucalyptol (10.56%) α-Humulene (6.68%) | HeLa cells | Reduced cell viability (IC50 229.27 µg/mL). | [98] |
| Lavandula pubescens Decne. (Downy Lavender) | Carvacrol (72.7%) Carvacrol methyl ether (7.0%) Caryophyllene oxide (5.9%) | HeLa cells | Reduced cell viability (IC50 < 10 µg/mL). | [117] |
| Litsea cubeba (Mountain pepper) | Geranial (37.67%) Neral (32.75%) Limonene (10.55%) | HeLa cells | Reduced cell viability (IC50 67.7 µg/mL). | [105] |
| Melaleuca alternifolia (Maiden and Betche) Cheel (Tea tree) | Terpinen-4-ol (41.9%) γ-Terpinene (17.8%) α-Terpinene (8%) p-Cymene (4.6%) | HeLa cells | Reduced cell viability (IC50 ND). | [74] |
| Mentha piperita L. (Peppermint) | Menthol (43.9%) Menthone (23.1%) 1,8-Cineole (6.6%) Menthyl acetate (4.9%) | HeLa cells | Reduced cell viability (IC50 ND). | [74] |
| Mikania micrantha Kunth (Bittervine) | Isoledene (16%) δ-Cadinene (11.2%) Debromofiliformin (9.4%) trans-Caryophyllene (9.1%) | HeLa cells | Reduced cell viability (IC50 5.44 μg/mL). | [112] |
| Moringa oleifera Lam. (Horseradish tree) | ND | HeLa cells | Reduced cell viability (IC50 422.8 µg/mL). | [78] |
| Moringa peregrina (Ben tree) | ND | HeLa cells | Reduced cell viability (IC50 366.3 µg/mL). | [79] |
| Nectandra leucantha Nees and Mart. | Bicyclogermacrene (28.44%) Germacrene A (7.34%) Spathulenol (5.82%) Globulol (5.25%) | HeLa & SiHa cells | Reduced cell viability (IC50 60 µg/mL, 12.4 µg/mL (Bicyclogermacrene), vs. 20 µg/mL (cisplatin)). | [111] |
| Nepeta curviflora Boiss (Syrian catmint) | 1,6-Dimethyl spiro [4.5] decane (27.5%) Caryophyllene oxide (20.1%) β-caryophyllene (18.3%) | HeLa cells | Reduced cell viability (IC50 746.9 and 453.1 µg/mL). Inhibit cell migration. | [69] |
| Nepeta rtanjensis Diklić and Milojević (Rtanj catmint) | trans,cis-Nepetalactone (71.66%) cis,trans-Nepetalactone (17.21%) α-Pinene (3.28%) | HeLa cells | Reduced cell viability (IC50 0.050 μL/mL). Cells demonstrated cytoplasmic shrinkage and nuclear condensation and fragmentation, cell membrane blebbing, and occurrence of apoptotic bodies. Induced cell cycle perturbations. Increased number of cells with fragmented DNA. Up-regulated Bax and p53 expressions. Down-regulated Bcl-2 and Skp2 expressions. | [70] |
| Nepeta sintenisii Bornm. | 4aα,7α,7aβ-Nepetalactone (51.74%) β-Farnesene (12.26%) 4aα,7α,7aα-Nepetalactone (8.01%) Germacrene-D (5.01%) | HeLa cells | Reduced cell viability (IC50 20.37 µg/mL). | [71] |
| Origanum acutidens (Hand-Mazz.) Ietswaart | Carvacrol (61.69%) p-Cymene (17.32%) γ-Terpinene (4.05%) Borneol (3.96%) | HeLa cells | Reduced cell viability (IC50 < 10 µg/mL). | [115] |
| Perilla frutescens (L.) Britt. | Perilla ketone (80.88%) Apiol (1.77%) β-Caryophyllene (1.59%) | HeLa cells | Reduced cell viability (IC50 34.58 μg/mL). | [83] |
| Peucedanum dhana A. Ham | trans-Piperitol (51.2%) o-Cymene (11.1%) γ-Terpinene (9.2%) | HeLa cells | Reduced cell viability (IC50 56.63 μg/mL vs 7.07 μg/mL (trans-piperitol)). | [119] |
| Pinus eldarica (Eldar pine) | β-Caryophyllene (14.8%) Germacrene D (12.9%) α-Terpinenyl acetate (8.15%) α-Pinene (5.7%) | HeLa cells | Reduced cell viability (IC50 ND). | [110] |
| Pistacia lentiscus var. chia (Mastic tree) | Wild plant: α-Pinene (56.2%, 51.9%) Myrcene (20.1%, 18.6%) β-Pinene (2.7%, 3.1%) Cultivated plant: α-Pinene (70.8%) β-Pinene (5.7%) Myrcene (2.5%) | HeLa cells | Reduced cell viability (IC50 20.11–18.81 µg/mL, IC50 7.62 µg/mL vs. IC50 2.14 µg/mL (Doxorubicin)). | [99] |
| Piper cernuum Vell. (Pariparoba) | β-Elemene (30.0%) Bicyclogermacrene (19.9%) (E)-Caryophyllene (16.3%) Germacrene D (12.7%) | HeLa cells | Reduced cell viability (IC50 23 µg/mL, 17 µg/mL (β-elemene), 12.4 µg/mL (bicyclogermacrene), 10 µg/mL ((E)-caryophyllene), 14.5 µg/mL (germacrene D)) | [108] |
| Piper regnellii (Miq) C. DC. var. regnellii (C. DC.) Yunck | Germacrene D (45.6–51.4%) α-Chamigrene (8.9–11.3%) β-Caryophyllene (8.2–9.5%) | HeLa cells | Reduced cell viability (IC50 7 µg/mL (germacrene D), 11 µg/mL (α-chamigrene), 32 µg/mL (β-caryophyllene) vs. 20 µg/mL (cisplatin)). | [109] |
| Rosmarinus officinalis L. (Rosemary) | 1,8-Cineole (23.56%) Camphene (12.78%) Camphor (12.55%) β-pinene (12.3%) | HeLa cells | Reduced cell viability (IC50 0.011 µg/mL). | [114] |
| Salvia officinalis L. (Garden sage) | α-Thujone (ND) 1,8-Cineole (ND) Camphor (ND) | HeLa cells | Reduced cell viability (IC50 ND). | [72] |
| Salvia sclarea L. (Clary sage) | ND | HeLa cells | Reduced cell viability (IC50 80.69 μg/mL). Cells demonstrated apoptotic bodies, e.g., blebbing, cell breakage, and chromatin condensation. | [73] |
| Satureja boissieri Hausskn. Ex Boiss. (Catri/Kekik) | p-Cymene (23.15%) γ-Terpinene (22.84%) Carvacrol (21.25%) Thymol (18.96%) | HeLa cells | p-Cymene, thymol, and carvacrol inhibited cell viability. | [116] |
| Siegesbeckia pubescens | Caryophyllene oxide (21.89%) trans-longipinocarveol (5.87%) dehydrosaussurea lactone (4.85%) | HeLa cells | Reduced cell viability (IC50 37.72 μg/mL). | [120] |
| Syzygium aromaticum (L.) Merr. and L.M. Perry (Clove] | Eugenol (85.2%) (E)-β-Caryophyllene (9.9%) | HeLa cells | Reduced cell viability (IC50 ND). | [74] |
| Tagetes ostenii Hicken | Dihydro-tagetone (64.2%) (E)-Ocimenone (39.9%) (Z)-β-Ocimene (26.1%) (Z)-Ocimenone (17.5%) | SiHa cells | Reduced cell viability (IC50 0.072–0.083 µg/mL). Inhibit the adhesion process and clonogenic ability after 24 h of treatment. | [86] |
| Thymus vulgaris L. (Thyme) | Thymol (48.6%) p-Cymene (18.4%) γ-Terpinene (8.8%) Carvacrol (5.5%) | HeLa cells | Reduced cell viability (IC50 ND). | [74] |
| Thymus bovei Benth. (Thyme) | Geraniol (32.3%) α-Citral (27.7%) β-Citral (12.4%) Thymol (3.8%) | HeLa cells | Reduced cell viability (IC50 7.22 µg/mL vs. IC50 4.24 µg/mL (cisplatin)). | [75] |
| Zataria multiflora (Shirazi thyme) | Carvacrol (52.2%) γ-Terpinene (12.4%) Carvacrol methyl ether (10.23%) p-Cymen (4.3%) | TC1 mouse cervical cancer cells | Reduced cell viability (IC50 15.62–66.52 µg/mL). Promoted apoptosis through caspase-3 dependent pathway. | [84] |
| Subcutaneous inoculation of TC1 cells (8 × 105) in C57BL/6 mice; 500 mg/kg, 7 days, intraperitoneally | Decreased tumor weight. Increased secretion of TNF-α, IFN-γ, and IL-2. Decreased secretion of IL-4. | |||
| Zingiber officinale Roscoe (Ginger) | α-Zingiberene (35%) ar-Curcumene (15.3%) β-Sesquiphellandrene (12.3%) | SiHa cells | Reduced cell viability (IC50 38.6 µg/mL and 46.2 µg/mL). Produced nucleosomal DNA fragmentation. Increased cytochrome C release and caspase-3 activation. | [76] |
| Camphene (16.4%) Geranial (9.9%) 1.8-Cineole (8.9%) β-Phellandrene (8.8%) | HeLa cells | Reduced cell viability (IC50 129.9 µg/mL). HeLa cell morphology showed condensation of chromatin, loss of cell membrane integrity with protrusions, and cell content leakage. | [64] | |
| Zingiber ottensii (Malaysian Ginger) | ND | HeLa cells | Reduced cell viability (IC50 1:3000 dilutions). Induced apoptosis. Activated intrinsic apoptotic pathway via caspase and PARP pathway. Decreased IL-6 in a dilution-dependent manner. | [77] |
6.5. Essential Oils Clinical Trials in Cervical Cancer and HPV Infection
7. Future Directions and Challenges
7.1. Innovation in the Delivery of Essential Oils
| Essential Oils | Nanocarrier (Particle Size) | Study Model | Important Findings | References |
|---|---|---|---|---|
| Eucalyptus globulus L. (Eucalyptus) | SLN (ND) | HeLa cells | IC50 33.20 μg/mL (EO) IC50 21.30 μg/mL (SLN) IC50 0.24 μg/mL (Doxorubicin) | [129] |
| Thymoquinone (Main constituent of Nigella sativa EO) | SLN (35.66 nm) | HeLa and SiHa cells | SiHa cells: IC50 19.42 (24 h), 10.42 (48 h), 8.50 μg/mL (72 h) HeLa cells: IC50 23.00 (24 h), 18.17 (48 h), 15.58 μg/mL (72 h) | [130] |
| Eugenol (Main constituent of Syzygium aromaticum EO) | NP encapsulation with chitosan (250–351 nm) | HeLa cells | Greater inhibition of cell viability compared to EO (IC50 ND). Increased cells in G0/G1 interphase. Decreased cells in G2/M phase. | [131] |
| Rosa damascene (Rose) | Nanoemulsion (30–50 nm) | HeLa cells | IC50 4.6 µg/mL (nanoemulsion) | [133] |
| Melaleuca alternifolia (Tea tree) | Nanoemulsion (300 nm) | HeLa cells | Stable under centrifugal, freeze thaw stress and long-term storage (50 days). Greater inhibition of cell viabilitycompared to paclitaxel (IC50 ND). | [134] |
| Zingiber ottensii (Malaysian Ginger) | Nanoemulsion (13.8 nm) Microemulsion (21.2 nm) Nanoemulgel (99.5 nm) Microemugel (99.2 nm) | HeLa cells | IC50 23.25 μg/mL (EO) IC50 5.81 μg/mL (Nanoemulsion) IC50 7.24 μg/mL (Microemulsion) IC50 8.88 μg/mL (Nanoemulgel) IC50 11.88 μg/mL (Microemulgel) | [137] |
| Coriandrum sativum (Cilantro) | Nanoemulgel (<200 nm) | HeLa cells | IC50 67.60 µg/mL (EO) IC50 24.54 µg/mL (Nanoemulgel) IC50 10.11 µg/mL (Doxorubicin) | [136] |
| Eugenia brejoensis | Coprecipitation with β-CD (ND) | HeLa cells | Increased thermal stability of EO. CC50 4460.55 µg/mL (β-CD) CC50 63.20 µg/mL (EO) CC50 886.71 µg/mL (β-CD/EO) | [140] |
| Syzygium aromaticum (Clove) | Kneading with β-CD (ND) | HeLa cells | IC50 > 500 µg/mL (β-CD) IC50 190.0 µg/mL (EO) IC50 12.5 µg/mL (β-CD/EO) | [139] |
7.2. Combining Essential Oils with Conventional Treatments
7.3. Challenges of Using Essential Oils in Cervical Cancer Treatment
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- D’Oria, O.; Corrado, G.; Lagana, A.S.; Chiantera, V.; Vizza, E.; Giannini, A. New advances in cervical cancer: From bench to bedside. Int. J. Environ. Res. Public Health 2022, 19, 7094. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 20 October 2023).
- Anderson, D.M.; Lee, J.; Elkas, J.C. Cervical and Vaginal Cancer. In Berek and Novak’s Gynecology; Berek, J.S., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2019. [Google Scholar]
- Park, J.; Kim, Y.J.; Song, M.K.; Nam, J.H.; Park, S.Y.; Kim, Y.S.; Kim, J.Y. Definitive chemoradiotherapy versus radical hysterectomy followed by tailored adjuvant therapy in women with early-stage cervical cancer presenting with pelvic lymph node metastasis on pretreatment evaluation: A propensity score matching analysis. Cancers 2021, 13, 3703. [Google Scholar] [CrossRef] [PubMed]
- Abd Rashid, N.; Hussan, F.; Hamid, A.; Adib Ridzuan, N.R.; Halim, S.; Abdul Jalil, N.A.; Najib, N.H.M.; Teoh, S.L.; Budin, S.B. Polygonum minus essential oil modulates cisplatin-induced hepatotoxicity through inflammatory and apoptotic pathways. EXCLI J. 2020, 19, 1246–1265. [Google Scholar] [CrossRef] [PubMed]
- Ishak, S.F.; Rajab, N.F.; Basri, D.F. Antiproliferative activities of acetone extract from Canarium odontophyllum (Dabai) stem bark against human colorectal cancer cells. Dose-Response 2023, 21, 15593258221098980. [Google Scholar] [CrossRef]
- Ni, Z.; Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.; Hu, F.; Wei, Z. Recent updates on the chemistry, bioactivities, mode of action, and industrial applications of plant essential oils. Trends Food Sci. 2021, 110, 78–89. [Google Scholar] [CrossRef]
- Leon-Mendez, G.; Pajaro-Castro, N.; Pajaro-Castro, E.; Torrenegra-Alarcon, M.; Herrera-Barros, A. Essential oils as a source of bioactive molecules. Rev. Colomb. Cienc. Quím. Farm. 2019, 48, 80–93. [Google Scholar] [CrossRef]
- Burd, E.M. Human papillomavirus and cervical cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef]
- Dretcanu, G.; Iuhas, C.I.; Diaconeasa, Z. The involvement of natural polyphenols in the chemoprevention of cervical cancer. Int. J. Mol. Sci. 2021, 22, 8812. [Google Scholar] [CrossRef]
- Bansal, A.; Singh, M.P.; Rai, B. Human papillomavirus-associated cancers: A growing global problem. Int. J. Appl. Basic Med. Res. 2016, 6, 84–89. [Google Scholar] [CrossRef]
- Kamolratanakul, S.; Pitisuttithum, P. Human papillomavirus vaccine efficacy and effectiveness against cancer. Vaccines 2021, 9, 1413. [Google Scholar] [CrossRef] [PubMed]
- Hemmat, N.; Bannazadeh Baghi, H. Association of human papillomavirus infection and inflammation in cervical cancer. Pathog. Dis. 2019, 77, ftz048. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Orang’o, E.; Nakalembe, M.; Tonui, P.; Itsura, P.; Muthoka, K.; Titus, M.; Kiptoo, S.; Mwangi, A.; Ong’echa, J.; et al. The East Africa Consortium for human papillomavirus and cervical cancer in women living with HIV/AIDS. Ann. Med. 2022, 54, 1202–1211. [Google Scholar] [CrossRef]
- Bhuvanendran Pillai, A.; Mun Wong, C.; Dalila Inche Zainal Abidin, N.; Fazlinda Syed Nor, S.; Fathulzhafran Mohamed Hanan, M.; Rasidah Abd Ghani, S.; Afzan Aminuddin, N.; Safian, N. Chlamydia infection as a risk factor for cervical cancer: A systematic review and meta-analysis. Iran J. Public Health 2022, 51, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, P.; Luo, G.; Liu, D.; Zou, H. Cancer attributable to human papillomavirus infection in China: Burden and trends. Cancer 2020, 126, 3719–3732. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, M.; Mena, M.; Maffini, F.; Gheit, T.; Quiros Blasco, B.; Holzinger, D.; Tous, S.; Scelsi, D.; Riva, D.; Grosso, E.; et al. Role of human papillomavirus infection in head and neck cancer in Italy: The HPV-AHEAD study. Cancers 2020, 12, 3567. [Google Scholar] [CrossRef]
- Kjaer, S.K.; Dehlendorff, C.; Belmonte, F.; Baandrup, L. Real-world effectiveness of human papillomavirus vaccination against cervical cancer. J. Natl. Cancer Inst. 2021, 113, 1329–1335. [Google Scholar] [CrossRef]
- Regalado Porras, G.O.; Chavez Nogueda, J.; Poitevin Chacon, A. Chemotherapy and molecular therapy in cervical cancer. Rep. Pract. Oncol. Radiother. 2018, 23, 533–539. [Google Scholar] [CrossRef]
- Chizenga, E.P.; Abrahamse, H. Biological therapy with complementary and alternative medicine in innocuous integrative oncology: A case of cervical cancer. Pharmaceutics 2021, 13, 626. [Google Scholar] [CrossRef]
- Burmeister, C.A.; Khan, S.F.; Schafer, G.; Mbatani, N.; Adams, T.; Moodley, J.; Prince, S. Cervical cancer therapies: Current challenges and future perspectives. Tumour Virus Res. 2022, 13, 200238. [Google Scholar] [CrossRef]
- Pfaendler, K.S.; Wenzel, L.; Mechanic, M.B.; Penner, K.R. Cervical cancer survivorship: Long-term quality of life and social support. Clin. Ther. 2015, 37, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Bjelic-Radisic, V.; Jensen, P.T.; Vlasic, K.K.; Waldenstrom, A.C.; Singer, S.; Chie, W.; Nordin, A.; Greimel, E. Quality of life characteristics inpatients with cervical cancer. Eur. J. Cancer 2012, 48, 3009–3018. [Google Scholar] [CrossRef] [PubMed]
- Eifel, P.J. Chemoradiotherapy in the treatment of cervical cancer. Semin. Radiat. Oncol. 2006, 16, 177–185. [Google Scholar] [CrossRef]
- Mauricio, D.; Zeybek, B.; Tymon-Rosario, J.; Harold, J.; Santin, A.D. Immunotherapy in cervical cancer. Curr. Oncol. Rep. 2021, 23, 61. [Google Scholar] [CrossRef]
- Chaitosa, R.; Maskasame, W. Study of side effects of patients receiving cisplatin in the treatment of cervical cancer by age group. Ramathibodi Med. J. 2020, 43, 28–38. [Google Scholar] [CrossRef]
- Waseem, M.; Tabassum, H.; Bhardwaj, M.; Parvez, S. Ameliorative efficacy of quercetin against cisplatin-induced mitochondrial dysfunction: Study on isolated rat liver mitochondria. Mol. Med. Rep. 2017, 16, 2939–2945. [Google Scholar] [CrossRef] [PubMed]
- Cheaib, B.; Auguste, A.; Leary, A. The PI3K/Akt/mTOR pathway in ovarian cancer: Therapeutic opportunities and challenges. Chin. J. Cancer 2015, 34, 4–16. [Google Scholar] [CrossRef]
- Liu, L.; Wang, M.; Li, X.; Yin, S.; Wang, B. An overview of novel agents for cervical cancer treatment by inducing apoptosis: Emerging drugs ongoing clinical trials and preclinical studies. Front. Med. 2021, 8, 682366. [Google Scholar] [CrossRef]
- Abd Rashid, N.; Hussan, F.; Hamid, A.; Adib Ridzuan, N.R.; Teoh, S.L.; Budin, S.B. Preventive Effects of Polygonum minus essential oil on cisplatin-induced hepatotoxicity in sprague dawley rats. Sains Malays. 2019, 48, 1975–1988. [Google Scholar] [CrossRef]
- Kadir, N.H.A.; Salleh, W.M.N.H.W.; Ghani, N.A.; Khamis, S.; Juhari, M.A.A. Chemical composition and acetylcholinesterase activity of Syzygium pyrifolium (Blume) DC. essential oil. Lat. Am. Appl. Res. 2023, 53, 357–359. [Google Scholar] [CrossRef]
- Lim, A.C.; Tang, S.G.H.; Zin, N.M.; Maisarah, A.M.; Ariffin, I.A.; Ker, P.J.; Mahlia, T.M.I. Chemical composition, antioxidant, antibacterial, and antibiofilm activities of Backhousia citriodora essential oil. Molecules 2022, 27, 4895. [Google Scholar] [CrossRef]
- de Almeida, R.N.; Agra Mde, F.; Maior, F.N.; de Sousa, D.P. Essential oils and their constituents: Anticonvulsant activity. Molecules 2011, 16, 2726–2742. [Google Scholar] [CrossRef]
- Hanif, M.A.; Nisar, S.; Khan, G.S.; Mushtaq, Z.; Zubair, M. Essential oils. In Essential Oil Research; Malik, S., Ed.; Springer: Cham, Switzerlnad, 2019. [Google Scholar]
- Pichersky, E.; Raguso, R.A. Why do plants produce so many terpenoid compounds? New Phytol. 2018, 220, 692–702. [Google Scholar] [CrossRef]
- Ozbek, H.; Kırmızı, N.I.; Cengiz, N.; Erdogan, E. Hepatoprotective effects of Coriandrum sativum essential oil against acute hepatotoxicity induced by carbon tetrachloride on rats. Acta Pharm. Sci. 2016, 54, 35–40. [Google Scholar] [CrossRef]
- Biltekin, S.N.; Karadag, A.E.; Demirci, F.; Demirci, B. In vitro anti-inflammatory and anticancer evaluation of Mentha spicata L. and Matricaria chamomilla L. essential oils. ACS Omega 2023, 8, 17143–17150. [Google Scholar] [CrossRef]
- Jaradat, N. Phytochemical profile and in vitro antioxidant, antimicrobial, vital physiological enzymes inhibitory and cytotoxic effects of Artemisia jordanica leaves essential oil from Palestine. Molecules 2021, 26, 2831. [Google Scholar] [CrossRef]
- Eldahshan, O.A.; Halim, A.F. Comparison of the composition and antimicrobial activities of the essential oils of green branches and leaves of Egyptian navel orange (Citrus sinensis (L.) Osbeck var. malesy). Chem. Biodivers. 2016, 13, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Del Prado-Audelo, M.L.; Cortes, H.; Caballero-Floran, I.H.; Gonzalez-Torres, M.; Escutia-Guadarrama, L.; Bernal-Chavez, S.A.; Giraldo-Gomez, D.M.; Magana, J.J.; Leyva-Gomez, G. Therapeutic applications of terpenes on inflammatory diseases. Front. Pharmacol. 2021, 12, 704197. [Google Scholar] [CrossRef]
- Jiang, J.; Xu, H.; Wang, H.; Zhang, Y.; Ya, P.; Yang, C.; Li, F. Protective effects of lemongrass essential oil against benzo(a)pyrene-induced oxidative stress and DNA damage in human embryonic lung fibroblast cells. Toxicol. Mech. Methods 2017, 27, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Al-Dabbagh, B.; Elhaty, I.A.; Elhaw, M.; Murali, C.; Al Mansoori, A.; Awad, B.; Amin, A. Antioxidant and anticancer activities of chamomile (Matricaria recutita L.). BMC Res. Notes 2019, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Trang, D.T.; Hoang, T.K.V.; Nguyen, T.T.M.; Van Cuong, P.; Dang, N.H.; Dang, H.D.; Nguyen Quang, T.; Dat, N.T. Essential oils of lemongrass (Cymbopogon citratus Stapf) induces apoptosis and cell cycle arrest in A549 lung cancer cells. Biomed. Res. Int. 2020, 2020, 5924856. [Google Scholar] [CrossRef] [PubMed]
- Al-Oqail, M.M.; Al-Sheddi, E.S.; Al-Massarani, S.M.; Siddiqui, M.A.; Ahmad, J.; Musarrat, J.; Al-Khedhairy, A.A.; Farshori, N.N. Nigella sativa seed oil suppresses cell proliferation and induces ROS dependent mitochondrial apoptosis through p53 pathway in hepatocellular carcinoma cells. S. Afr. J. Bot. 2017, 112, 70–78. [Google Scholar] [CrossRef]
- Di Martile, M.; Garzoli, S.; Sabatino, M.; Valentini, E.; D’Aguanno, S.; Ragno, R.; Del Bufalo, D. Antitumor effect of Melaleuca alternifolia essential oil and its main component terpinen-4-ol in combination with target therapy in melanoma models. Cell. Death Discov. 2021, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, J.; Pachaiappan, P.; Subramaniyan, S. Dose-dependent chemopreventive effects of citronellol in DMBA-induced breast cancer among rats. Drug Dev. Res. 2019, 80, 867–876. [Google Scholar] [CrossRef]
- Singh, H.P.; Kaur, S.; Negi, K.; Kumari, S.; Saini, V.; Batish, D.R.; Kohli, R.K. Assessment of in vitro antioxidant activity of essential oil of Eucalyptus citriodora (lemon-scented Eucalypt; Myrtaceae) and its major constituents. LWT-Food Sci. Technol. 2012, 48, 237–241. [Google Scholar] [CrossRef]
- Giovannini, D.; Gismondi, A.; Basso, A.; Canuti, L.; Braglia, R.; Canini, A.; Mariani, F.; Cappelli, G. Lavandula angustifolia Mill. essential oil exerts antibacterial and anti-inflammatory effect in macrophage mediated immune response to Staphylococcus aureus. Immunol. Investig. 2016, 45, 11–28. [Google Scholar] [CrossRef]
- Amorim, J.L.; Simas, D.L.; Pinheiro, M.M.; Moreno, D.S.; Alviano, C.S.; da Silva, A.J.; Fernandes, P.D. Anti-inflammatory properties and chemical characterization of the essential oils of four Citrus species. PLoS ONE 2016, 11, e0153643. [Google Scholar] [CrossRef]
- Arranz, E.; Jaime, L.; López de las Hazas, M.C.; Reglero, G.; Santoyo, S. Supercritical fluid extraction as an alternative process to obtain essential oils with anti-inflammatory properties from marjoram and sweet basil. Ind. Crops Prod. 2015, 67, 121–129. [Google Scholar] [CrossRef]
- Ye, Z.; Liang, Z.; Mi, Q.; Guo, Y. Limonene terpenoid obstructs human bladder cancer cell (T24 cell line) growth by inducing cellular apoptosis, caspase activation, G2/M phase cell cycle arrest and stops cancer metastasis. J. BUON 2020, 25, 280–285. [Google Scholar]
- Mandal, D.; Patel, P.; Verma, S.K.; Sahu, B.R.; Parija, T. Proximal discrepancy in intrinsic atomic interaction arrests G2/M phase by inhibiting Cyclin B1/CDK1 to infer molecular and cellular biocompatibility of D-limonene. Sci. Rep. 2022, 12, 18184. [Google Scholar] [CrossRef]
- Falleh, H.; Ben Jemaa, M.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef]
- Fotsing Yannick Stephane, F.; Kezetas Jean Jules, B. Terpenoids as important bioactive constituents of essential oils. In Essential Oils-Bioactive Compounds, New Perspectives and Applications; de Oliveira, M.S., da Costa, W.A., Silva, S.G., Eds.; IntechOpen: London, UK, 2020; p. 222. [Google Scholar]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Aljaafari, M.N.; Alkhoori, M.A.; Hag-Ali, M.; Cheng, W.H.; Lim, S.H.; Loh, J.Y.; Lai, K.S. Contribution of aldehydes and their derivatives to antimicrobial and immunomodulatory activities. Molecules 2022, 27, 3589. [Google Scholar] [CrossRef] [PubMed]
- Ghaffar, A.; Yameen, M.; Kiran, S.; Kamal, S.; Jalal, F.; Munir, B.; Saleem, S.; Rafiq, N.; Ahmad, A.; Saba, I.; et al. Chemical composition and in-vitro evaluation of the antimicrobial and antioxidant activities of essential oils extracted from seven Eucalyptus species. Molecules 2015, 20, 20487–20498. [Google Scholar] [CrossRef] [PubMed]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Vallverdu-Queralt, A.; Regueiro, J.; Martinez-Huelamo, M.; Rinaldi Alvarenga, J.F.; Leal, L.N.; Lamuela-Raventos, R.M. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014, 154, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Sarkic, A.; Stappen, I. Essential oils and their single compounds in cosmetics—A critical review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef]
- Suffness, M.; Pezzuto, J. Assays related to cancer drug discovery. In Methods Plant Biochemistry: Assays for Bioactivity; Hostettmann, K., Ed.; Academic Press: London, UK, 1990; pp. 71–133. [Google Scholar]
- Parida, R.; Nayak, S. Anti-proliferative activity of in vitro Zingiberaceae essential oil against human cervical cancer (HeLa) cell line. Res. J. Pharm. Technol. 2022, 15, 325–328. [Google Scholar] [CrossRef]
- Santos, P.A.; Avanco, G.B.; Nerilo, S.B.; Marcelino, R.I.; Janeiro, V.; Valadares, M.C.; Machinski, M. Assessment of cytotoxic activity of rosemary (Rosmarinus officinalis L.), turmeric (Curcuma longa L.), and ginger (Zingiber officinale R.) essential oils in cervical cancer cells (HeLa). Sci. World J. 2016, 2016, 9273078. [Google Scholar] [CrossRef]
- Syamsir, D.S.; Sivasothy, Y.; Hazni, H.; Abdul Malek, S.N.; Nagoor, N.H.; Ibrahim, H.; Awang, K. Chemical constituents and evaluation of cytotoxic activities of Curcuma zedoaria (Christm.) Roscoe oils from Malaysia and Indonesia. J. Essent. Oil-Bear Plants 2017, 20, 972–982. [Google Scholar] [CrossRef]
- Sharopov, F.; Valiev, A.; Satyal, P.; Gulmurodov, I.; Yusufi, S.; Setzer, W.N.; Wink, M. Cytotoxicity of the essential oil of fennel (Foeniculum vulgare) from Tajikistan. Foods 2017, 6, 73. [Google Scholar] [CrossRef]
- Akhbari, M.; Kord, R.; Jafari Nodooshan, S.; Hamedi, S. Analysis and evaluation of the antimicrobial and anticancer activities of the essential oil isolated from Foeniculum vulgare from Hamedan, Iran. Nat. Prod. Res. 2019, 33, 1629–1632. [Google Scholar] [CrossRef]
- Chen, F.; Guo, Y.; Kang, J.; Yang, X.; Zhao, Z.; Liu, S.; Ma, Y.; Gao, W.; Luo, D. Insight into the essential oil isolation from Foeniculum vulgare Mill. fruits using double-condensed microwave-assisted hydrodistillation and evaluation of its antioxidant, antifungal and cytotoxic activity. Ind. Crops Prod. 2020, 144, 112052. [Google Scholar] [CrossRef]
- Jaradat, N.; Al-Maharik, N.; Abdallah, S.; Shawahna, R.; Mousa, A.; Qtishat, A. Nepeta curviflora essential oil: Phytochemical composition, antioxidant, anti-proliferative and anti-migratory efficacy against cervical cancer cells, and α-glucosidase, α-amylase and porcine pancreatic lipase inhibitory activities. Ind. Crops Prod. 2020, 158, 112946. [Google Scholar] [CrossRef]
- Skorić, M.; Gligorijević, N.M.; Todorović, S.; Janković, R.; Ristić, M.; Mišić, D.; Radulović, S. Cytotoxic activity of Nepeta rtanjensis Diklić & Milojević essential oil and its mode of action. Ind. Crops Prod. 2017, 100, 163–170. [Google Scholar] [CrossRef]
- Shakeri, A.; Khakdan, F.; Soheili, V.; Sahebkar, A.; Shaddel, R.; Asili, J. Volatile composition, antimicrobial, cytotoxic and antioxidant evaluation of the essential oil from Nepeta sintenisii Bornm. Ind. Crops Prod. 2016, 84, 224–229. [Google Scholar] [CrossRef]
- Privitera, G.; Luca, T.; Castorina, S.; Passanisi, R.; Ruberto, G.; Napoli, E. Anticancer activity of Salvia officinalis essential oil and its principal constituents against hormone-dependent tumour cells. Asian Pac. J. Trop. Biomed. 2019, 9, 24–28. [Google Scholar] [CrossRef]
- Durgha, H.; Thirugnanasampandan, R.; Ramya, G.; Ramanth, M.G. Inhibition of inducible nitric oxide synthase gene expression (iNOS) and cytotoxic activity of Salvia sclarea L. essential oil. J. King Saud. Univ. Sci. 2016, 28, 390–395. [Google Scholar] [CrossRef]
- Rajkowska, K.; Nowak, A.; Kunicka-Styczyńska, A.; Siadura, A. Biological effects of various chemically characterized essential oils: Investigation of the mode of action against Candida albicans and HeLa cells. RSC Adv. 2016, 6, 97199–97207. [Google Scholar] [CrossRef]
- Hassan, S.T.S.; Berchova-Bimova, K.; Sudomova, M.; Malanik, M.; Smejkal, K.; Rengasamy, K.R.R. In vitro study of multi-therapeutic properties of Thymus bovei Benth. essential oil and its main component for promoting their use in clinical practice. J. Clin. Med. 2018, 7, 283. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y. Cytotoxicity evaluation of essential oil and its component from Zingiber officinale Roscoe. Toxicol. Res. 2016, 32, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Ruttanapattanakul, J.; Wikan, N.; Chinda, K.; Jearanaikulvanich, T.; Krisanuruks, N.; Muangcha, M.; Okonogi, S.; Potikanond, S.; Nimlamool, W. Essential oil from Zingiber ottensii induces human cervical cancer cell apoptosis and inhibits MAPK and PI3K/AKT signaling cascades. Plants 2021, 10, 1419. [Google Scholar] [CrossRef]
- Elsayed, E.A.; Sharaf-Eldin, M.A.; Wadaan, M. In vitro evaluation of cytotoxic activities of essential oil from Moringa oleifera seeds on HeLa, HepG2, MCF-7, CACO-2 and L929 cell lines. Asian Pac. J. Cancer Prev. 2015, 16, 4671–4675. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, E.A.; Sharaf-Eldin, M.A.; El-Enshasy, H.A.; Wadaan, M. In vitro assessment of anticancer properties of Moringa peregrina essential seed oil on different cell lines. Pakistan J. Zool. 2016, 48, 853–859. [Google Scholar]
- Kallel, I.; Hadrich, B.; Gargouri, B.; Chaabane, A.; Lassoued, S.; Gdoura, R.; Bayoudh, A.; Ben Messaoud, E. Optimization of cinnamon (Cinnamomum zeylanicum Blume) essential oil extraction: Evaluation of antioxidant and antiproliferative effects. Evid.-Based Complement. Altern. Med. 2019, 2019, 6498347. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Mahdavi, B.; Ghezi, S. Chemical composition, antimicrobial, hemolytic, and antiproliferative activity of essential oils from Ephedra intermedia Schrenk & Mey. J. Essent. Oil-Bear Plants 2019, 22, 1562–1570. [Google Scholar] [CrossRef]
- Karan, T.; Yildiz, I.; Aydin, A.; Erenler, R. Inhibition of various cancer cells proliferation of bornyl acetate and essential oil from Inula graveolens (Linnaeus) Desf. Rec. Nat. Prod. 2018, 12, 273–283. [Google Scholar] [CrossRef]
- Chen, F.; Liu, S.; Zhao, Z.; Gao, W.; Ma, Y.; Wang, X.; Yan, S.; Luo, D. Ultrasound pre-treatment combined with microwave-assisted hydrodistillation of essential oils from Perilla frutescens (L.) Britt. leaves and its chemical composition and biological activity. Ind. Crops Prod. 2020, 143, 111908. [Google Scholar] [CrossRef]
- Azadi, M.; Jamali, T.; Kianmehr, Z.; Kavoosi, G.; Ardestani, S.K. In-vitro (2D and 3D cultures) and in-vivo cytotoxic properties of Zataria multiflora essential oil (ZEO) emulsion in breast and cervical cancer cells along with the investigation of immunomodulatory potential. J. Ethnopharmacol. 2020, 257, 112865. [Google Scholar] [CrossRef]
- Staver, M.M.; Gobin, I.; Ratkaj, I.; Petrovic, M.; Vulinovic, A.; Dinarina-Sablic, M.; Broznic, D. In vitro antiproliferative and antimicrobial activity of the essential oil from the flowers and leaves of Helichrysum italicum (Roth) G. Don Growing in Central Dalmatia (Croatia). J. Essent. Oil-Bear Plants 2018, 21, 77–91. [Google Scholar] [CrossRef]
- Nunez, J.G.; Pinheiro, J.S.; Padilha, G.L.; Garcia, H.O.; Porta, V.; Apel, M.A.; Bruno, A.N. Antineoplastic potential and chemical evaluation of essential oils from leaves and flowers of Tagetes ostenii Hicken. An. Acad. Bras. Cienc. 2020, 92, e20191143. [Google Scholar] [CrossRef] [PubMed]
- Moirangthem, D.S.; Laishram, S.; Rana, V.S.; Borah, J.C.; Talukdar, N.C. Essential oil of Cephalotaxus griffithii needle inhibits proliferation and migration of human cervical cancer cells: Involvement of mitochondria-initiated and death receptor-mediated apoptosis pathways. Nat. Prod. Res. 2015, 29, 1161–1165. [Google Scholar] [CrossRef]
- Si, C.; Ou, Y.; Ma, D.; Hei, L.; Wang, X.; Du, R.; Yang, H.; Liao, Y.; Zhao, J. Cytotoxic effect of the essential oils from Erigeron canadensis L. on human cervical cancer HeLa cells in vitro. Chem. Biodivers. 2022, 19, e202200436. [Google Scholar] [CrossRef]
- Acar, M.; İbiş, E.; Şimşek, A.; Vural, C.; Tez, C.; Özcan, S. Evaluation of Achillea millefolium essential oil compounds and biological effects on cervix cancer HeLa cell line. EuroBiotech J. 2020, 4, 17–24. [Google Scholar] [CrossRef]
- Rezaieseresht, H.; Shobeiri, S.S.; Kaskani, A. Chenopodium botrys essential oil as a source of sesquiterpenes to induce apoptosis and G1 cell cycle arrest in cervical cancer cells. Iran J. Pharm. Res. 2020, 19, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Bayala, B.; Nadembega, C.; Guenne, S.; Bunay, J.; Mahoukede Zohoncon, T.; Wendkuuni Djigma, F.; Yonli, A.; Baron, S.; Figueredo, G.; Jean-Marc, A.; et al. Chemical composition, antioxidant and cytotoxic activities of Hyptis suaveolens (L.) Poit. essential oil on prostate and cervical cancers cells. Pak. J. Biol. Sci. 2020, 23, 1184–1192. [Google Scholar] [CrossRef]
- Suntar, I. Importance of ethnopharmacological studies in drug discovery: Role of medicinal plants. Phytochem. Rev. 2020, 19, 1199–1209. [Google Scholar] [CrossRef]
- Machado, T.Q.; da Fonseca, A.C.C.; Duarte, A.B.S.; Robbs, B.K.; de Sousa, D.P. A narrative review of the antitumor activity of monoterpenes from essential oils: An update. Biomed. Res. Int. 2022, 2022, 6317201. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Rudkowska, M.; Kasprzak-Drozd, K.; Oniszczuk, A.; Borowicz-Reutt, K. Activity of selected group of monoterpenes in Alzheimer’s disease symptoms in experimental model studies-A non-systematic review. Int. J. Mol. Sci. 2021, 22, 7366. [Google Scholar] [CrossRef]
- Maurya, A.K.; Devi, R.; Kumar, A.; Koundal, R.; Thakur, S.; Sharma, A.; Kumar, D.; Kumar, R.; Padwad, Y.S.; Chand, G.; et al. Chemical composition, cytotoxic and antibacterial activities of essential oils of cultivated clones of Juniperus communis and wild Juniperus species. Chem. Biodivers. 2018, 15, e1800183. [Google Scholar] [CrossRef]
- Sahoo, S.; Kar, B.; Dash, S.; Ray, M.; Acharya, K.G.; Singh, S.; Nayak, S. Anticancerous and immunomodulatory activities of Alpinia nigra (Gaertn.) Burtt. J. Essent. Oil-Bear Plants 2018, 21, 869–875. [Google Scholar] [CrossRef]
- Hakkim, F.L.; Al-Buloshi, M.; Achankunju, J. Chemical composition and anti-proliferative effect of Oman’s Ganoderma applanatum on breast cancer and cervical cancer cells. J. Taibah. Univ. Med. Sci. 2016, 11, 145–151. [Google Scholar] [CrossRef]
- Coulibaly, L.L.; Bayala, B.; Zongo, L.; Zongo, P.F.I.; Ouedraogo, E.; Djigma, F.W.; Yonli, A.; Baron, S.; Figueredo, G.; Lobaccaro, J.M.; et al. Chemical composition and antiproliferative activity on prostate and cervical cancer cell lines of Lantana camara Linn. essential oil. Int. J. Biol. Chem. Sci. 2023, 17, 293–303. [Google Scholar] [CrossRef]
- Tabanca, N.; Nalbantsoy, A.; Kendra, P.E.; Demirci, F.; Demirci, B. Chemical characterization and biological activity of the mastic gum essential oils of Pistacia lentiscus Var. Chia from Turkey. Molecules 2020, 25, 2136. [Google Scholar] [CrossRef] [PubMed]
- Jaradat, N.; Qneibi, M.; Hawash, M.; Al-Maharik, N.; Qadi, M.; Abualhasan, M.N.; Ayesh, O.; Bsharat, J.; Khadir, M.; Morshed, R.; et al. Assessing Artemisia arborescens essential oil compositions, antimicrobial, cytotoxic, anti-inflammatory, and neuroprotective effects gathered from two geographic locations in Palestine. Ind. Crops Prod. 2022, 176, 114360. [Google Scholar] [CrossRef]
- Elghwaji, W.; El-Sayed, A.M.; El-Deeb, K.S.; ElSayed, A.M. Chemical composition, antimicrobial and antitumor potentiality of essential oil of Ferula tingitana L. Apiaceae grow in Libya. Pharmacogn. Mag. 2017, 13, S446–S451. [Google Scholar] [CrossRef] [PubMed]
- Poonkodi, K.; Vimaladevi, K.; Suganthi, M.; Gayathri, N. Essential oil composition and biological activities of Aegle marmelos (L.) Correa grown in Western Ghats region-South India. J. Essent. Oil-Bear Plants 2019, 22, 1013–1021. [Google Scholar] [CrossRef]
- Jaradat, N.; Hawash, M.; Abualhasan, M.N.; Qadi, M.; Ghanim, M.; Massarwy, E.; Ammar, S.A.; Zmero, N.; Arar, M.; Hussein, F.; et al. Spectral characterization, antioxidant, antimicrobial, cytotoxic, and cyclooxygenase inhibitory activities of Aloysia citriodora essential oils collected from two Palestinian regions. BMC Complement. Med. Ther. 2021, 21, 143. [Google Scholar] [CrossRef]
- Thakur, S.; Koundal, R.; Kumar, D.; Maurya, A.K.; Padwad, Y.S.; Lal, B.; Agnihotri, V.K. Volatile composition and cytotoxic activity of aerial parts of Crassocephalum crepidioides growing in Western Himalaya, India. Indian J. Pharm. Sci. 2019, 81, 167–172. [Google Scholar] [CrossRef]
- Pante, G.C.; Castro, J.C.; Lini, R.S.; Romoli, J.C.Z.; Almeida, R.T.R.; Garcia, F.P.; Nakamura, C.V.; Pilau, E.J.; Abreu Filho, B.A.; Machinski, M. Litsea cubeba essential oil: Chemical profile, antioxidant activity, cytotoxicity, effect against Fusarium verticillioides and fumonisins production. J. Environ. Sci. Health B 2021, 56, 387–395. [Google Scholar] [CrossRef]
- Hajaji, S.; Sifaoui, I.; Lopez-Arencibia, A.; Reyes-Batlle, M.; Valladares, B.; Pinero, J.E.; Lorenzo-Morales, J.; Akkari, H. Amoebicidal activity of α-bisabolol, the main sesquiterpene in chamomile (Matricaria recutita L.) essential oil against the trophozoite stage of Acanthamoeba castellani Neff. Acta Parasitol. 2017, 62, 290–295. [Google Scholar] [CrossRef]
- Carneiro, S.B.; Costa Duarte, F.I.; Heimfarth, L.; Siqueira Quintans, J.S.; Quintans-Junior, L.J.; Veiga Junior, V.F.D.; Neves de Lima, A.A. Cyclodextrin-drug inclusion complexes: In vivo and in vitro approaches. Int. J. Mol. Sci. 2019, 20, 642. [Google Scholar] [CrossRef] [PubMed]
- Capello, T.M.; Martins, E.G.A.; De Farias, C.F.; Figueiredo, C.R.; Matsuo, A.L.; Passero, L.F.D.; Oliveira-Silva, D.; Sartorelli, P.; Lago, J.H.G. Chemical composition and in vitro cytotoxic and antileishmanial activities of extract and essential oil from leaves of Piper cernuum. Nat. Prod. Commun. 2015, 10, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.R.; Girola, N.; Figueiredo, C.R.; Londero, V.S.; Lago, J.H.G. Circadian variation and in vitro cytotoxic activity evaluation of volatile compounds from leaves of Piper regnellii (Miq) C. DC. var. regnellii (C. DC.) Yunck (Piperaceae). Nat. Prod. Res. 2018, 32, 859–862. [Google Scholar] [CrossRef] [PubMed]
- Sarvmeili, N.; Jafarian-Dehkordi, A.; Zolfaghari, B. Cytotoxic effects of Pinus eldarica essential oil and extracts on HeLa and MCF-7 cell lines. Res. Pharm. Sci. 2016, 11, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Grecco Sdos, S.; Martins, E.G.; Girola, N.; de Figueiredo, C.R.; Matsuo, A.L.; Soares, M.G.; Bertoldo Bde, C.; Sartorelli, P.; Lago, J.H. Chemical composition and in vitro cytotoxic effects of the essential oil from Nectandra leucantha leaves. Pharm. Biol. 2015, 53, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Saikia, S.; Tamuli, K.J.; Narzary, B.; Banik, D.; Bordoloi, M. Chemical characterization, antimicrobial activity, and cytotoxic activity of Mikania micrantha Kunth flower essential oil from North East India. Chem. Pap. 2020, 74, 2515–2528. [Google Scholar] [CrossRef]
- Vuko, E.; Dunkic, V.; Maravic, A.; Ruscic, M.; Nazlic, M.; Radan, M.; Ljubenkov, I.; Soldo, B.; Fredotovic, Z. Not only a weed plant-Biological activities of essential oil and hydrosol of Dittrichia viscosa (L.) Greuter. Plants 2021, 10, 1837. [Google Scholar] [CrossRef]
- Jardak, M.; Elloumi-Mseddi, J.; Aifa, S.; Mnif, S. Chemical composition, anti-biofilm activity and potential cytotoxic effect on cancer cells of Rosmarinus officinalis L. essential oil from Tunisia. Lipids Health Dis. 2017, 16, 190. [Google Scholar] [CrossRef]
- Oke Altuntas, F.; Demirtas, I. Real-time cell analysis of the cytotoxicity of Origanum acutidens essential oil on HT-29 and HeLa cell lines. Turk. J. Pharm. Sci. 2017, 14, 29–33. [Google Scholar] [CrossRef]
- Oke-Altuntas, F.; Demirtas, I.; Tufekci, A.R.; Koldas, S.; Gul, F.; Behcet, L.; Gecibesler, H.I. Inhibitory effects of the active components isolated from Satureja Boissieri Hausskn. Ex Boiss. on human cervical cancer cell line. J. Food Biochem. 2016, 40, 499–506. [Google Scholar] [CrossRef]
- Al-Badani, R.N.; da Silva, J.K.R.; Mansi, I.; Muharam, B.A.; Setzer, W.N.; Ali, N.A.A. Chemical composition and biological activity of Lavandula pubescens essential oil from Yemen. J. Essent. Oil-Bear Plants 2017, 20, 509–515. [Google Scholar] [CrossRef]
- Bayala, B.; Coulibaly, A.Y.; Djigma, F.W.; Nagalo, B.M.; Baron, S.; Figueredo, G.; Lobaccaro, J.A.; Simpore, J. Chemical composition, antioxidant, anti-inflammatory and antiproliferative activities of the essential oil of Cymbopogon nardus, a plant used in traditional medicine. Biomol. Concepts 2020, 11, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Khruengsai, S.; Sripahco, T.; Rujanapun, N.; Charoensup, R.; Pripdeevech, P. Chemical composition and biological activity of Peucedanum dhana A. Ham essential oil. Sci. Rep. 2021, 11, 19079. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wei, J.; Hong, L.; Fan, S.; Hu, G.; Jia, J. Comparative analysis of chemical composition, anti-inflammatory activity and antitumor activity in essential oils from Siegesbeckia orientalis, S. glabrescens and S. pubescens with an ITS sequence analysis. Molecules 2018, 23, 2185. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, S.; Sharma, S.; Kumari, A.; Kumar, D.; Nadda, G.; Padwad, Y.; Ogra, R.K.; Kumar, N. Chemical composition, cytotoxicity and insecticidal activities of Acorus calamus accessions from the western Himalayas. Ind. Crops Prod. 2016, 94, 520–527. [Google Scholar] [CrossRef]
- Blackburn, L.; Hill, C.; Lindsey, A.L.; Sinnott, L.T.; Thompson, K.; Quick, A. Effect of foot reflexology and aromatherapy on anxiety and pain during brachytherapy for cervical cancer. Oncol. Nurs. Forum. 2021, 48, 265–276. [Google Scholar] [CrossRef]
- Sriningsih, I.; Elisa, E.; Lestari, K.P. Aromatherapy ginger use in patients with nausea & vomiting on post cervical cancer chemotherapy. KEMAS 2017, 13, 59–68. [Google Scholar] [CrossRef]
- Nikakhtar, Z.; Hasanzadeh, M.; Hamedi, S.S.; Najafi, M.N.; Tavassoli, A.P.; Feyzabadi, Z.; Meshkat, Z.; Saki, A. The efficacy of vaginal suppository based on myrtle in patients with cervicovaginal human papillomavirus infection: A randomized, double-blind, placebo trial. Phytother. Res. 2018, 32, 2002–2008. [Google Scholar] [CrossRef]
- Baleka Mutombo, A.; Tozin, R.; Kanyiki, H.; Van Geertruyden, J.P.; Jacquemyn, Y. Impact of antiviral AV2 in the topical treatment of HPV-associated lesions of the cervix: Results of a phase III randomized placebo-controlled trial. Contemp. Clin. Trials Commun. 2019, 15, 100377. [Google Scholar] [CrossRef]
- Tang, G.; Zhou, Z.; Zhang, X.; Liu, Y.; Yan, G.; Wang, H.; Li, X.; Huang, Y.; Wang, J.; Cao, Y. Fabrication of supramolecular self-assembly of the Schiff base complex for improving bioavailability of aldehyde-containing plant essential oil. Chem. Eng. J. 2023, 471, 144471. [Google Scholar] [CrossRef]
- AbouAitah, K.; Lojkowski, W. Nanomedicine as an emerging technology to foster application of essential oils to fight cancer. Pharmaceuticals 2022, 15, 793. [Google Scholar] [CrossRef] [PubMed]
- Subroto, E.; Andoyo, R.; Indiarto, R. Solid lipid nanoparticles: Review of the current research on encapsulation and delivery systems for active and antioxidant compounds. Antioxidants 2023, 12, 633. [Google Scholar] [CrossRef] [PubMed]
- Azadmanesh, R.; Tatari, M.; Asgharzade, A.; Taghizadeh, S.F.; Shakeri, A. GC/MS profiling and biological traits of Eucalyptus globulus L. essential oil exposed to solid lipid nanoparticle (SLN). J. Essent. Oil-Bear Plants 2021, 24, 863–878. [Google Scholar] [CrossRef]
- Ng, W.K.; Saiful Yazan, L.; Yap, L.H.; Wan Nor Hafiza, W.A.; How, C.W.; Abdullah, R. Thymoquinone-loaded nanostructured lipid carrier exhibited cytotoxicity towards breast cancer cell lines (MDA-MB-231 and MCF-7) and cervical cancer cell lines (HeLa and SiHa). Biomed. Res. Int. 2015, 2015, 263131. [Google Scholar] [CrossRef] [PubMed]
- Happy Kurnia, P.; Riz’q Threevisca, C.; Dhanang Puruhita, T.R.; Haykal, M.N. Eugenol nanoparticle encapsulated chitosan enhances cell cycle arrest in HeLa human cervical cancer cells. Sys. Rev. Pharm. 2021, 12, 692–699. [Google Scholar]
- Gledovic, A.; Janosevic-Lezaic, A.; Tamburic, S.; Savic, S. Red raspberry seed oil low energy nanoemulsions: Influence of surfactants, antioxidants, and temperature on oxidative stability. Antioxidants 2022, 11, 1898. [Google Scholar] [CrossRef]
- Saffari, I.; Moghanjoghi, A.M.; Chaleshtori, R.S.; Ataee, M.; Khaledi, A. Nanoemulsification of rose (Rosa damascena) essential oil: Characterization, anti-Salmonella, in vitro cytotoxicity to cancer cells, and advantages in sheep meat application. J. Food Qual. 2023, 2023, 6665799. [Google Scholar] [CrossRef]
- Sharma, A.D.; Chhabra, R.; Jain, P.; Kaur, I.; Amrita; Bhawna. Nanoemulsions (O/W) prepared from essential oil extracted from Melaleuca alternifolia: Synthesis, characterization, stability and evaluation of anticancerous, anti-oxidant, anti-inflammatory and antidiabetic activities. J. Biomater. Sci. Polym. Ed. 2023, 34, 2438–2461. [Google Scholar] [CrossRef]
- Aithal, G.C.; Narayan, R.; Nayak, U.Y. Nanoemulgel: A promising phase in drug delivery. Curr. Pharm. Des. 2020, 26, 279–291. [Google Scholar] [CrossRef]
- Eid, A.M.; Issa, L.; Al-Kharouf, O.; Jaber, R.; Hreash, F. Development of Coriandrum sativum oil nanoemulgel and evaluation of its antimicrobial and anticancer activity. Biomed. Res. Int. 2021, 2021, 5247816. [Google Scholar] [CrossRef] [PubMed]
- Panyajai, P.; Chueahongthong, F.; Viriyaadhammaa, N.; Nirachonkul, W.; Tima, S.; Chiampanichayakul, S.; Anuchapreeda, S.; Okonogi, S. Anticancer activity of Zingiber ottensii essential oil and its nanoformulations. PLoS ONE 2022, 17, e0262335. [Google Scholar] [CrossRef]
- Kringel, D.H.; Antunes, M.D.; Klein, B.; Crizel, R.L.; Wagner, R.; de Oliveira, R.P.; Dias, A.R.G.; Zavareze, E.D.R. Production, characterization, and stability of orange or Eucalyptus essential oil/β-cyclodextrin inclusion complex. J. Food Sci. 2017, 82, 2598–2605. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, J.H.; Castro, J.C.; Fenelon, V.C.; Garcia, F.P.; Nakamura, C.V.; Nogueira, A.C.; Ueda-Nakamura, T.; de Souza, H.M.; Mangolim, C.S.; Moura-Costa, G.F.; et al. Essential oil characterization of Ocimum basilicum and Syzygium aromaticum free and complexed with β-cyclodextrin. Determination of its antioxidant, antimicrobial, and antitumoral activities. J. Incl. Phenom. Macrocycl. Chem. 2022, 102, 117–132. [Google Scholar] [CrossRef]
- de Santana, N.A.; da Silva, R.C.S.; Fourmentin, S.; dos Anjos, K.F.L.; Ootan, M.A.; da Silva, A.G.; Araújo, B.G.P.; dos Santos Correia, M.T.; da Silva, M.V.; Machado, G. Synthesis, characterization and cytotoxicity of the Eugenia brejoensis essential oil inclusion complex with β-cyclodextrin. J. Drug Deliv. Sci. Technol. 2020, 60, 101876. [Google Scholar] [CrossRef]
- Alghamdi, R.S.; Alkhatib, M.H.; Balamash, K.S.; Khojah, S.M. Apoptotic effect of bleomycin formulated in cinnamon oil nanoemulsion on HeLa cervical cancer cells. Asian J. Pharm. 2020, 14, 356–361. [Google Scholar] [CrossRef]
- Flores-Villasenor, S.E.; Peralta-Rodriguez, R.D.; Padilla-Vaca, F.; Meléndez-Ortiz, H.I.; Ramirez-Contreras, J.C.; Franco, B. Preparation of peppermint oil-based nanodevices loaded with paclitaxel: Cytotoxic and apoptosis studies in HeLa cells. AAPS PharmSciTech 2019, 20, 198. [Google Scholar] [CrossRef]
- AlMotwaa, S.M. Coupling Ifosfamide to nanoemulsion-based clove oil enhances its toxicity on malignant breast cancer and cervical cancer cells. Pharmacia 2021, 68, 779–787. [Google Scholar] [CrossRef]
- Alkhatib, M.H.; AlMotwaa, S.M.; Alkreathy, H.M. Incorporation of ifosfamide into various essential oils -based nanoemulsions ameliorates its apoptotic effect in the cancers cells. Sci. Rep. 2019, 9, 695. [Google Scholar] [CrossRef]
- Alkhatib, M.H.; Al-Otaibi, W.A.; Wali, A.N. Antineoplastic activity of mitomycin C formulated in nanoemulsions-based essential oils on HeLa cervical cancer cells. Chem. Biol. Interact. 2018, 291, 72–80. [Google Scholar] [CrossRef]
- Al-Otaibi, W.A.; Alkhatib, M.H.; Wali, A.N. Cytotoxicity and apoptosis enhancement in breast and cervical cancer cells upon coadministration of mitomycin C and essential oils in nanoemulsion formulations. Biomed. Pharmacother. 2018, 106, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Akhlaq, A.; Ashraf, M.; Omer, M.O.; Altaf, I. Carvacrol-fabricated chitosan nanoparticle synergistic potential with topoisomerase inhibitors on breast and cervical cancer cells. ACS Omega 2023, 8, 31826–31838. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Souza, F.; Magalhães, I.F.B.; Guedes, A.C.; Santana, V.M.; Teles, A.M.; Mouchrek, A.N.; Calabrese, K.S.; Abreu-Silva, A.L. Safety assessment of essential oil as a food ingredient. In Essential Oils; de Oliveira, M.S., Ed.; Springer: Cham, Switzerland, 2022; pp. 123–171. [Google Scholar]
- Escobar, F.M.; Sabini, M.C.; Cariddi, L.N.; Sabini, L.I.; Manas, F.; Cristofolini, A.; Bagnis, G.; Gallucci, M.N.; Cavaglieri, L.R. Safety assessment of essential oil from Minthostachys verticillata (Griseb.) Epling (peperina): 90-days oral subchronic toxicity study in rats. Regul. Toxicol. Pharmacol. 2015, 71, 1–7. [Google Scholar] [CrossRef] [PubMed]
| Type of Treatment | Side Effects | References |
|---|---|---|
| Surgery | Lymphoedema, sexual and vaginal dysfunction | [24] |
| Chemotherapy | Vomiting, diarrhea | [25] |
| Immunotherapy | Hypothyroidism, liver toxicity | [26] |
| Radiotherapy | Rectal bleeding, constipation, hematuria, dysuria | [28] |
| Essential Oils | Study Design | Important Findings | References |
|---|---|---|---|
| Foot reflexology and aromatherapy | Randomized controlled trial of 41 locally advanced cervical cancer patients who received intracavitary brachytherapy | Average pain scores were lower for the intervention group. Average anxiety scores were lower for the intervention group. | [122] |
| Ginger aromatherapy | Pre-test post-test control group design of 60 post-cervical cancer chemotherapy patients | Improved nausea and vomiting frequency in the intervention group. | [123] |
| Myrtus communis L.-based vaginal suppository | Randomized double-blind placebo trial of 60 patients with cervicovaginal HPV infection | Increased HPV test negative results in the intervention group. Reduced cervical lesion size in the intervention group. | [124] |
| Antiviral AV2® (mixture of eugenol, carvone, nerolidol, and geraniol in olive oil) | Randomized placebo-controlled clinical trial of 327 visual inspection of cervix with acetic acid-positive patients | No differences in regression of lesion and HPV clearance rate between intervention and control groups. | [125] |
| Conventional Drug | Essential Oils | Study Model | Important Findings | References |
|---|---|---|---|---|
| Bleomycin | Cinnamon oil nanoemulsion | HeLa cells | IC50 10 μM Bleomycin IC50 0.2 μM EO + Bleomycin Increased apoptotic effect compared to bleomycin alone. | [141] |
| Paclitaxel | Peppermint oil microemulsion | HeLa cells | Stable under centrifugal and freeze thaw stress. ≈90% of paclitaxel released in the first 48 h. Showed 70% and 90% viability reduction in HeLa cells after 24 and 48 h of exposure (greater than paclitaxel and EO alone). | [142] |
| Ifosfamide | Clove EO nanoemulsion | HeLa cells | IC50 210 μM EO IC50 140 μM EO + Ifosfamide | [143] |
| Lemon EO nanoemulsion | HeLa cells | IC50 7690 μM Ifosfamide IC50 219 μM EO IC50 165 μM EO + Ifosfamide | [144] | |
| Salvia EO nanoemulsion | HeLa cells | IC50 7690 μM Ifosfamide IC50 250 μM EO IC50 141 μM EO + Ifosfamide | [144] | |
| Mitomycin C | Chamomile EO nanoemulsion | HeLa cells | IC50 29.8 μM Mitomycin C IC50 1.4 μM EO IC50 0.7 μM EO + Mitomycin C | [145] |
| Frankincense EO nanoemulsion | HeLa cells | IC50 10.59 μg/mL Mitomycin C IC50 0.24 μg/mL EO + Mitomycin C | [146] | |
| Garlic EO nanoemulsion | HeLa cells | IC50 29.8 μM Mitomycin C IC50 1.8 μM EO IC50 1.49 μM EO + Mitomycin C | [145] | |
| Ginger EO nanoemulsion | HeLa cells | IC50 10.59 μg/mL Mitomycin C IC50 0.36 μg/mL EO + Mitomycin C | [146] | |
| Doxorubicin | Carvacrol-loaded chitosan NP | HeLa cells | IC50 6.30 μg/mL Doxorubicin IC50 2.98 μg/mL Carvacrol NP IC50 5.66 μg/mL Carvacrol NP + Doxorubicin | [147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd Rashid, N.; Mohamad Najib, N.H.; Abdul Jalil, N.A.; Teoh, S.L. Essential Oils in Cervical Cancer: Narrative Review on Current Insights and Future Prospects. Antioxidants 2023, 12, 2109. https://doi.org/10.3390/antiox12122109
Abd Rashid N, Mohamad Najib NH, Abdul Jalil NA, Teoh SL. Essential Oils in Cervical Cancer: Narrative Review on Current Insights and Future Prospects. Antioxidants. 2023; 12(12):2109. https://doi.org/10.3390/antiox12122109
Chicago/Turabian StyleAbd Rashid, Norhashima, Nor Haliza Mohamad Najib, Nahdia Afiifah Abdul Jalil, and Seong Lin Teoh. 2023. "Essential Oils in Cervical Cancer: Narrative Review on Current Insights and Future Prospects" Antioxidants 12, no. 12: 2109. https://doi.org/10.3390/antiox12122109
APA StyleAbd Rashid, N., Mohamad Najib, N. H., Abdul Jalil, N. A., & Teoh, S. L. (2023). Essential Oils in Cervical Cancer: Narrative Review on Current Insights and Future Prospects. Antioxidants, 12(12), 2109. https://doi.org/10.3390/antiox12122109







