Lipofuscin, Its Origin, Properties, and Contribution to Retinal Fluorescence as a Potential Biomarker of Oxidative Damage to the Retina
Abstract
:1. Introduction
2. Lipofuscin: Formation, Composition, and Potentially Harmful Effects
2.1. Lipofuscin Formation
2.2. Deleterious Effects of Lipofuscin and Potential Mechanisms Involved
3. Lipofuscin Fluorescence
4. Lipofuscin in the Retina
4.1. Retinal Pigment Epithelium (RPE) Is the Major Site of Lipofuscin Accumulation in the Retina
4.2. The Major Source of RPE of Lipofuscin Is Phagocytosis of Photoreceptor Outer Segments
4.3. Oxidative Stress, Lysosomal Dysfunction, and Vitamin A Derivatives as Contributors to the Accumulation of RPE Lipofuscin
4.3.1. Role of Retinaldehydes and Lipids in Lipofuscin Formation
4.3.2. Role of Vitamin A Depletion and Inhibition of Synthesis of 11-cis-Retinaldehyde in Lipofuscin Formation
4.3.3. Role of Inhibition of Lysosomal Degradation by A2E, Products of Lipid Peroxidation, and Complement Activation in Lipofuscin Formation
4.3.4. The Increased Length of Rod Outer Segments in the Para- and Perifovea May Cause Their Increased Susceptibility to Oxidation and Decreased Susceptibility to Lysosomal Degradation
4.4. Structure and Composition of RPE Lipofuscin
4.5. Distribution of Lipofuscin in the Human RPE
4.5.1. Age-Related Changes in the Topographical Distribution of RPE Lipofuscin in the Human Retina
4.5.2. Association of Lipofuscin Accumulation with Retinal Degenerations
4.6. Effects of RPE Lipofuscin on the Function and Viability of RPE Cells and Photoreceptors
4.6.1. Effects of RPE Lipofuscin on Cultured RPE Cells
4.6.2. Susceptibility to Autooxidation and Photosensitizing Properties of RPE Lipofuscin
4.6.3. Negligible Contribution of A2E to the Photosensitizing Properties of Lipofuscin
4.6.4. Lack of Evidence of the Deleterious Effect of A2E to Cultured Cells While Incorporated into Lipofuscin
4.6.5. Potential Role of Oxidized DHA in Photosensitizing Properties of Lipofuscin
4.6.6. Neglected Components of Lipofuscin Exhibiting High Photoreactivity
4.6.7. Circumstantial Pieces of Evidence Suggesting That Lipofuscin Contributes to Retinal Phototoxicity In Vivo
4.6.8. Circumstantial Pieces of Evidence Suggesting That Lipofuscin Contributes to Retinal Degeneration In Vivo in Dark-Reared abca4(-/-)rdh8(-/-) Double Knockout Mice
4.6.9. Protective Effect of Deuterated Vitamin A on A2E and Lipofuscin Accumulation, Complement Activation and Retinal Degeneration in Mice, and on Slowing Down Geographic Atrophy Progression in Stargardt’s Disease Patients
4.6.10. Is There an Association between Light Exposure and the Development or Progression of AMD?
4.6.11. How Much Sunlight Reaches the Retina?
4.6.12. Does Lipofuscin Contribute to Light-Induced Injury of the Retina In Vivo?
5. Fluorescence of RPE Lipofuscin
6. Fluorescence of the Retina
6.1. Sources of Fluorescence in the Retina
6.2. Imaging of Fluorescence in the Retina
6.3. Age-Related Changes in Retinal Fluorescence
| Study on Normal Human Eyes In Vivo and Ex Vivo | Excitation (nm) | Emission (nm) | Age-Related Changes in Fluorescence Intensity or Spectra |
|---|---|---|---|
| Cross-sections from eyes of 19 White donors, 2 weeks–88 years of age, and 19 Black donors, 6.5–90 years old; 5 sites per eye: fovea, parafovea (half a distance from the fovea to the disc and on the other side, two equatorial sites [182] | 365 | 470 | Age-related increase for Whites No correlation with age for Blacks |
| Cross-sections from 44 human eyes from 35 donors, 6-week premature newborn–88 years; from ora serrata via optic disc and fovea to ora serrata on opposite site; lipofuscin from all the length of RPE was quantified in 29 eyes [181] | 380 | 460–480 | A fast increase in the first and second decades of life, then slowing down followed by an increase in people above the age of 60 years; about a 40% increase in fluorescence emission intensity in the oldest age group 61–88 in comparison with 31–60 years group |
| 30 participants, 21–67 years of age; excitation area of 3° in diameter; fluorescence measured at the fovea and at 7° temporal to the fovea from an area of 2° in diameter [379] | 430 | 620 | No significant correlation with age |
| Sections from formalin-fixed 8 mm in diameter circles centred on the fovea of 88 donors ranging in age from 1–98 years [438] | 450–490 | >520 | A linear increase up to the age of 60 years, followed by a plateau; supported by TEM quantification of lipofuscin |
| RPE-Bruch’s membrane flat-mounts about 20 × 20 mm including optic disc and macula from 20 donors divided into two age groups: 16–51 years of age (10 donors, average age of 40 years), and 82–90 years of age (10 donors, average age of 85 years) [435] | 460–490 | >505 | Increased in the 82–90 year-old group in comparison with the 16–51 year-old group |
| 145 participants, 15–80 years of age; retinal field of 13° circle centred on the fovea and quantified at the fovea and at 7° eccentricity temporal to the fovea; individually corrected for the absorption of light by the lens [434] | 470 | >520 | Intensities reached a maximum for the age group in their 7th decade and remained at the same level in the 8th decade |
| 33 White participants 6–78 years of age; fluorescence imaged over 40° field-of-view and quantified at the fovea and at the site of maximum intensity 7–15° of eccentricity [385] | 488 | >521 | A linear increase with age from 6 to about 60 years, above 60 the emission appears to plateau |
| 277 participants of different ethnicities from 5–60 years of age; fluorescence imaged over 30° × 30° and quantified in a ring at about 8.4° of eccentricity [439] | 488 | 500–680 | The age-related increase in fundus fluorescence was the greatest for Whites, followed by Indogenous Americans, Hispanics, Blacks, and Asians |
| 53 White participants, 5–18 years of age [437] and 103 White participants, 18–77 years of age [436]; fluorescence imaged over 30° × 30° area centred on the fovea | 488 | 500–750 | Overall a monotonic increase with age in the fovea and extrafoveal circle extending to the optic nerve head, with an initial rapid linear increase up to the age of 20 years, possibly reaching a plateau around the age of 60 and further increase after the age of 65 |
| 30 participants, 21–67 years of age; other details as for excitation with 430 nm [379] | 470, 510 or 550 | 620 | Positive correlation with age |
| 145 participants, 15–80 years of age; other details as for excitation with 470 nm [434] | 550 | 650–750 | A linear increase in fluorescence occurred up to the age of 70 years, followed by a steep decrease |
| 44 participants below 40 years of age (average age of 24 years) and 18 participants above 40 years of age (average age of 67.5 years); calculated emission maxima based on emission of fluorescence in two spectral channels [441] | 473 | 498–560 and 560–720 | For the younger group, the emission maxima were at 602 ± 16, 614 ± 12, and 621 ± 11 nm for the fovea (1 mm in diameter), inner ring (1–3 mm in diameter) and outer ring (3–6 mm in diameter), respectively. For the older group the emission maxima were at 599 ± 17, 611 ± 11, and 614 ± 11, respectively |
6.4. Fundus Autofluorescence in Age-Related Macular Degeneration (AMD)
6.4.1. Sources of Fluorescence in the AMD Retina Examined Ex Vivo
6.4.2. Fluorescence Characteristics of AMD Retina In Vivo
6.4.3. Current Evidence for the Prognostic Value of Fundus Fluorescence Characteristics for AMD Progression
7. Retinal Spectral Fluorescence Characteristics as a Potential In Vivo Biomarker of Oxidative Damage and Efficacy of Potential Antioxidant Therapies
7.1. Current Evidence for Photooxidation of Lipofuscin In Vivo
7.2. Age-Related Changes of A2E Content in the Macula and Periphery
7.3. Are There Spectral Changes in Retinal Fluorescence with Age?
7.4. Current Evidence for Increased Oxidative Stress and Oxidative Damage in AMD or Stargardt’s Retina
7.5. RPE Lipofuscin Fluorescence: Intensity and Spectral Characteristics as a Potential Biomarker of Oxidative Damage to the Retina In Vivo
8. Conclusions
9. Future Research Directions
9.1. Elucidation of the Role of Oxidized DHA in Photosensitizing and Fluorescence Properties of Lipofuscin
9.2. Relative Contribution of Retinaldehydes and Lipofuscin to Light-Induced Retinal Injury
9.3. Determination of Topography of Retinal Irradiance under Various Daily Activities in Different Geographical Locations and Atmospheric Conditions
9.4. Comparison of the Effects of Deuterated Vitamin A and Deuterated DHA on Lipofuscin Accumulation, Susceptibility to Light-Induced Retinal Injury, and Progression of Geographic Atrophy in Animal Models of Stargardt’s Disease and AMD
9.5. Stimulation of Lipofuscin Removal by Light
9.6. Lipofuscin Fluorescence as a Way of Monitoring Oxidative Damage in RPE
9.7. Is RPE Lipofuscin Really So Much Different from Lipofuscins from Other Cells?
Funding
Conflicts of Interest
Abbreviations
References
- Yin, D. Biochemical basis of lipofuscin, ceroid, and age pigment-like fluorophores. Free Radic. Biol. Med. 1996, 21, 871–888. [Google Scholar] [CrossRef] [PubMed]
- Terman, A.; Gustafsson, B.; Brunk, U.T. Autophagy, organelles and ageing. J. Pathol. 2007, 211, 134–143. [Google Scholar] [CrossRef]
- Jung, T.; Bader, N.; Grune, T. Lipofuscin: Formation, distribution, and metabolic consequences. Ann. N. Y. Acad. Sci. 2007, 1119, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Terman, A.; Kurz, T.; Navratil, M.; Arriaga, E.A.; Brunk, U.T. Mitochondrial turnover and aging of long-lived postmitotic cells: The mitochondrial-lysosomal axis theory of aging. Antioxid. Redox Signal. 2010, 12, 503–535. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Wang, Q.; Hu, H.; Shen, Y.; Fan, C.; Chen, P.; Ma, Y.; Wu, H.; Xiang, M. Restoring autophagic flux attenuates cochlear spiral ganglion neuron degeneration by promoting TFEB nuclear translocation via inhibiting MTOR. Autophagy 2019, 15, 998–1016. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.P.; Gugiu, B.; Renganathan, K.; Davies, M.W.; Gu, X.; Crabb, J.S.; Kim, S.R.; Rozanowska, M.B.; Bonilha, V.L.; Rayborn, M.E.; et al. Retinal pigment epithelium lipofuscin proteomics. Mol. Cell. Proteom. 2008, 7, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Krog, S.; Ludvigsen, T.P.; Nielsen, O.L.; Kirk, R.K.; Lykkegaard, K.; Wulff, E.M.; Moller, J.E.; Pedersen, H.D.; Olsen, L.H. Myocardial Changes in Diabetic and Nondiabetic Nonhuman Primates. Vet. Pathol. 2020, 57, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Kakimoto, Y.; Okada, C.; Kawabe, N.; Sasaki, A.; Tsukamoto, H.; Nagao, R.; Osawa, M. Myocardial lipofuscin accumulation in ageing and sudden cardiac death. Sci. Rep. 2019, 9, 3304. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Garcia, A.; Kun, A.; Calero, O.; Medina, M.; Calero, M. An Overview of the Role of Lipofuscin in Age-Related Neurodegeneration. Front. Neurosci. 2018, 12, 464. [Google Scholar] [CrossRef]
- Couve, E.; Schmachtenberg, O. Autophagic activity and aging in human odontoblasts. J. Dent. Res. 2011, 90, 523–528. [Google Scholar] [CrossRef]
- Sulzer, D.; Mosharov, E.; Talloczy, Z.; Zucca, F.A.; Simon, J.D.; Zecca, L. Neuronal pigmented autophagic vacuoles: Lipofuscin, neuromelanin, and ceroid as macroautophagic responses during aging and disease. J. Neurochem. 2008, 106, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.; Hohn, A.; Grune, T. Lipofuscin: Detection and quantification by microscopic techniques. Methods Mol. Biol. 2010, 594, 173–193. [Google Scholar] [CrossRef] [PubMed]
- Simonati, A.; Williams, R.E. Neuronal Ceroid Lipofuscinosis: The Multifaceted Approach to the Clinical Issues, an Overview. Front. Neurol. 2022, 13, 811686. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Nelvagal, H.R.; Lange, J.; Cooper, J.D. Glial Dysfunction and Its Contribution to the Pathogenesis of the Neuronal Ceroid Lipofuscinoses. Front. Neurol. 2022, 13, 886567. [Google Scholar] [CrossRef] [PubMed]
- Nelvagal, H.R.; Lange, J.; Takahashi, K.; Tarczyluk-Wells, M.A.; Cooper, J.D. Pathomechanisms in the neuronal ceroid lipofuscinoses. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165570. [Google Scholar] [CrossRef] [PubMed]
- Valdez, C.; Wong, Y.C.; Schwake, M.; Bu, G.; Wszolek, Z.K.; Krainc, D. Progranulin-mediated deficiency of cathepsin D results in FTD and NCL-like phenotypes in neurons derived from FTD patients. Hum. Mol. Genet. 2017, 26, 4861–4872. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Banerjee, K.; Lehmann, G.L.; Almeida, D.; Hajjar, K.A.; Benedicto, I.; Jiang, Z.; Radu, R.A.; Thompson, D.H.; Rodriguez-Boulan, E.; et al. Lipofuscin causes atypical necroptosis through lysosomal membrane permeabilization. Proc. Natl. Acad. Sci. USA 2021, 118, e2100122118. [Google Scholar] [CrossRef]
- Klein, Z.A.; Takahashi, H.; Ma, M.; Stagi, M.; Zhou, M.; Lam, T.T.; Strittmatter, S.M. Loss of TMEM106B Ameliorates Lysosomal and Frontotemporal Dementia-Related Phenotypes in Progranulin-Deficient Mice. Neuron 2017, 95, 281–296.e286. [Google Scholar] [CrossRef]
- Kohlschutter, A.; Schulz, A. CLN2 Disease (Classic Late Infantile Neuronal Ceroid Lipofuscinosis). Pediatr. Endocrinol. Rev. 2016, 13 (Suppl. S1), 682–688. [Google Scholar] [PubMed]
- Ach, T.; Tolstik, E.; Messinger, J.D.; Zarubina, A.V.; Heintzmann, R.; Curcio, C.A. Lipofuscin redistribution and loss accompanied by cytoskeletal stress in retinal pigment epithelium of eyes with age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3242–3252. [Google Scholar] [CrossRef]
- Nilsson, M.I.; MacNeil, L.G.; Kitaoka, Y.; Suri, R.; Young, S.P.; Kaczor, J.J.; Nates, N.J.; Ansari, M.U.; Wong, T.; Ahktar, M.; et al. Combined aerobic exercise and enzyme replacement therapy rejuvenates the mitochondrial-lysosomal axis and alleviates autophagic blockage in Pompe disease. Free Radic. Biol. Med. 2015, 87, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Simonati, A.; Pezzini, F.; Moro, F.; Santorelli, F.M. Neuronal Ceroid Lipofuscinosis: The Increasing Spectrum of an Old Disease. Curr. Mol. Med. 2014, 14, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Donet, J.M.; Carcel-Trullols, J.; Casanova, B.; Aguado, C.; Knecht, E. Alterations in ROS activity and lysosomal pH account for distinct patterns of macroautophagy in LINCL and JNCL fibroblasts. PLoS ONE 2013, 8, e55526. [Google Scholar] [CrossRef] [PubMed]
- Piyanova, A.; Albayram, O.; Rossi, C.A.; Farwanah, H.; Michel, K.; Nicotera, P.; Sandhoff, K.; Bilkei-Gorzo, A. Loss of CB1 receptors leads to decreased cathepsin D levels and accelerated lipofuscin accumulation in the hippocampus. Mech. Ageing Dev. 2013, 134, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Guha, S.; Baltazar, G.C.; Coffey, E.E.; Tu, L.A.; Lim, J.C.; Beckel, J.M.; Patel, S.; Eysteinsson, T.; Lu, W.; O’Brien-Jenkins, A.; et al. Lysosomal alkalinization, lipid oxidation, and reduced phagosome clearance triggered by activation of the P2X7 receptor. FASEB J. 2013, 27, 4500–4509. [Google Scholar] [CrossRef]
- Kohan, R.; Cismondi, I.A.; Oller-Ramirez, A.M.; Guelbert, N.; Anzolini, T.V.; Alonso, G.; Mole, S.E.; de Kremer, D.R.; de Halac, N.I. Therapeutic approaches to the challenge of neuronal ceroid lipofuscinoses. Curr. Pharm. Biotechnol. 2011, 12, 867–883. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.L.; Robison, W.G., Jr. What is lipofuscin? Defining characteristics and differentiation from other autofluorescent lysosomal storage bodies. Arch. Gerontol. Geriatr. 2002, 34, 169–184. [Google Scholar] [CrossRef]
- Katz, M.L.; Shanker, M.J. Development of lipofuscin-like fluorescence in the retinal pigment epithelium in response to protease inhibitor treatment. Mech. Ageing Dev. 1989, 49, 23–40. [Google Scholar] [CrossRef]
- Ivy, G.O.; Schottler, F.; Wenzel, J.; Baudry, M.; Lynch, G. Inhibitors of lysosomal enzymes: Accumulation of lipofuscin-like dense bodies in the brain. Science 1984, 226, 985–987. [Google Scholar] [CrossRef]
- Kang, H.T.; Lee, K.B.; Kim, S.Y.; Choi, H.R.; Park, S.C. Autophagy impairment induces premature senescence in primary human fibroblasts. PLoS ONE 2011, 6, e23367. [Google Scholar] [CrossRef]
- Lee, S.J.; Park, M.H.; Kim, H.J.; Koh, J.Y. Metallothionein-3 regulates lysosomal function in cultured astrocytes under both normal and oxidative conditions. Glia 2010, 58, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Stroikin, Y.; Dalen, H.; Loof, S.; Terman, A. Inhibition of autophagy with 3-methyladenine results in impaired turnover of lysosomes and accumulation of lipofuscin-like material. Eur. J. Cell Biol. 2004, 83, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Hohn, A.; Sittig, A.; Jung, T.; Grimm, S.; Grune, T. Lipofuscin is formed independently of macroautophagy and lysosomal activity in stress-induced prematurely senescent human fibroblasts. Free Radic. Biol. Med. 2012, 53, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, U.; Nagy, M.; Fenton, W.A.; Horwich, A.L. Absence of lipofuscin in motor neurons of SOD1-linked ALS mice. Proc. Natl. Acad. Sci. USA 2014, 111, 11055–11060. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Cisuelo, V.; Gomez, J.; Garcia-Junceda, I.; Naudi, A.; Cabre, R.; Mota-Martorell, N.; Lopez-Torres, M.; Gonzalez-Sanchez, M.; Pamplona, R.; Barja, G. Rapamycin reverses age-related increases in mitochondrial ROS production at complex I, oxidative stress, accumulation of mtDNA fragments inside nuclear DNA, and lipofuscin level, and increases autophagy, in the liver of middle-aged mice. Exp. Gerontol. 2016, 83, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Tzekov, R.; Li, H.; McDowell, J.H.; Gao, G.; Smith, W.C.; Tang, S.; Kaushal, S. Inhibition or Stimulation of Autophagy Affects Early Formation of Lipofuscin-Like Autofluorescence in the Retinal Pigment Epithelium Cell. Int. J. Mol. Sci. 2017, 18, 728. [Google Scholar] [CrossRef]
- Ramachandra Rao, S.; Fliesler, S.J. Monitoring basal autophagy in the retina utilizing CAG-mRFP-EGFP-MAP1LC3B reporter mouse: Technical and biological considerations. Autophagy 2022, 18, 1187–1201. [Google Scholar] [CrossRef]
- Mei, L.; Yu, M.; Liu, Y.; Weh, E.; Pawar, M.; Li, L.; Besirli, C.G.; Schwendeman, A.A. Synthetic high-density lipoprotein nanoparticles delivering rapamycin for the treatment of age-related macular degeneration. Nanomedicine 2022, 44, 102571. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Blasiak, J.; Liton, P.; Boulton, M.; Klionsky, D.J.; Sinha, D. Autophagy in age-related macular degeneration. Autophagy 2022, 19, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Li, W.W.; Wang, H.J.; Tan, Y.Z.; Wang, Y.L.; Yu, S.N.; Li, Z.H. Reducing lipofuscin accumulation and cardiomyocytic senescence of aging heart by enhancing autophagy. Exp. Cell Res. 2021, 403, 112585. [Google Scholar] [CrossRef]
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in healthy aging and disease. Nat. Aging 2021, 1, 634–650. [Google Scholar] [CrossRef]
- Bajaj, L.; Lotfi, P.; Pal, R.; Ronza, A.D.; Sharma, J.; Sardiello, M. Lysosome biogenesis in health and disease. J. Neurochem. 2019, 148, 573–589. [Google Scholar] [CrossRef] [PubMed]
- Hakvoort, K.; Otto, L.; Haeren, R.; Hoogland, G.; Schijns, O.; Vink, H.; Klein, D.; van Zandvoort, M.; Rijkers, K. Shedding light on human cerebral lipofuscin: An explorative study on identification and quantification. J. Comp. Neurol. 2021, 529, 605–615. [Google Scholar] [CrossRef] [PubMed]
- McElnea, E.M.; Hughes, E.; McGoldrick, A.; McCann, A.; Quill, B.; Docherty, N.; Irnaten, M.; Farrell, M.; Clark, A.F.; O’Brien, C.J.; et al. Lipofuscin accumulation and autophagy in glaucomatous human lamina cribrosa cells. BMC Ophthalmol. 2014, 14, 153. [Google Scholar] [CrossRef] [PubMed]
- Kurz, T.; Terman, A.; Gustafsson, B.; Brunk, U.T. Lysosomes and oxidative stress in aging and apoptosis. Biochim. Biophys. Acta 2008, 1780, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Liton, P.B.; Lin, Y.; Luna, C.; Li, G.; Gonzalez, P.; Epstein, D.L. Cultured porcine trabecular meshwork cells display altered lysosomal function when subjected to chronic oxidative stress. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3961–3969. [Google Scholar] [CrossRef] [PubMed]
- Marzabadi, M.R.; Sohal, R.S.; Brunk, U.T. Effect of alpha-tocopherol and some metal chelators on lipofuscin accumulation in cultured neonatal rat cardiac myocytes. Anal. Cell. Pathol. 1990, 2, 333–346. [Google Scholar] [PubMed]
- Marzabadi, M.R.; Sohal, R.S.; Brunk, U.T. Effect of ferric iron and desferrioxamine on lipofuscin accumulation in cultured rat heart myocytes. Mech. Ageing Dev. 1988, 46, 145–157. [Google Scholar] [CrossRef]
- Brunk, U.T.; Jones, C.B.; Sohal, R.S. A novel hypothesis of lipofuscinogenesis and cellular aging based on interactions between oxidative stress and autophagocytosis. Mutat. Res. 1992, 275, 395–403. [Google Scholar] [CrossRef]
- Shevtsova, Z.; Garrido, M.; Weishaupt, J.; Saftig, P.; Bahr, M.; Luhder, F.; Kugler, S. CNS-expressed cathepsin D prevents lymphopenia in a murine model of congenital neuronal ceroid lipofuscinosis. Am. J. Pathol. 2010, 177, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Reeg, S.; Grune, T. Protein Oxidation in Aging: Does It Play a Role in Aging Progression? Antioxid. Redox Signal. 2015, 23, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Stroikin, Y.; Dalen, H.; Brunk, U.T.; Terman, A. Testing the “garbage” accumulation theory of ageing: Mitotic activity protects cells from death induced by inhibition of autophagy. Biogerontology 2005, 6, 39–47. [Google Scholar] [CrossRef] [PubMed]
- von Zglinicki, T.; Nilsson, E.; Docke, W.D.; Brunk, U.T. Lipofuscin accumulation and ageing of fibroblasts. Gerontology 1995, 41 (Suppl. S2), 95–108. [Google Scholar] [CrossRef] [PubMed]
- Terman, A.; Kurz, T.; Gustafsson, B.; Brunk, U.T. The involvement of lysosomes in myocardial aging and disease. Curr. Cardiol. Rev. 2008, 4, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Epstein, D.L.; Liton, P.B. Intralysosomal iron induces lysosomal membrane permeabilization and cathepsin D-mediated cell death in trabecular meshwork cells exposed to oxidative stress. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6483–6495. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Gomez, N.M.; Lim, J.C.; Guha, S.; O’Brien-Jenkins, A.; Coffey, E.E.; Campagno, K.E.; McCaughey, S.A.; Laties, A.M.; Carlsson, L.G.; et al. The P2Y12 Receptor Antagonist Ticagrelor Reduces Lysosomal pH and Autofluorescence in Retinal Pigmented Epithelial Cells from the ABCA4−/− Mouse Model of Retinal Degeneration. Front. Pharmacol. 2018, 9, 242. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, C.; Kravic, B.; Meyer, H. Repair or Lysophagy: Dealing with Damaged Lysosomes. J. Mol. Biol. 2020, 432, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Kurz, T.; Eaton, J.W.; Brunk, U.T. Redox activity within the lysosomal compartment: Implications for aging and apoptosis. Antioxid. Redox Signal. 2010, 13, 511–523. [Google Scholar] [CrossRef]
- Hohn, A.; Jung, T.; Grimm, S.; Grune, T. Lipofuscin-bound iron is a major intracellular source of oxidants: Role in senescent cells. Free Radic. Biol. Med. 2010, 48, 1100–1108. [Google Scholar] [CrossRef]
- Kurz, T.; Terman, A.; Gustafsson, B.; Brunk, U.T. Lysosomes in iron metabolism, ageing and apoptosis. Histochem. Cell Biol. 2008, 129, 389–406. [Google Scholar] [CrossRef]
- Grubman, A.; Pollari, E.; Duncan, C.; Caragounis, A.; Blom, T.; Volitakis, I.; Wong, A.; Cooper, J.; Crouch, P.J.; Koistinaho, J.; et al. Deregulation of biometal homeostasis: The missing link for neuronal ceroid lipofuscinoses? Metallomics 2014, 6, 932–943. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, Y.; Park, M.K.; Mori, T.; Kawashima, S. The difference in autofluorescence features of lipofuscin between brain and adrenal. Zool. Sci. 1995, 12, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.L.; Robison, W.G., Jr.; Herrmann, R.K.; Groome, A.B.; Bieri, J.G. Lipofuscin accumulation resulting from senescence and vitamin E deficiency: Spectral properties and tissue distribution. Mech. Ageing Dev. 1984, 25, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Boulton, M.; Docchio, F.; Dayhaw-Barker, P.; Ramponi, R.; Cubeddu, R. Age-related changes in the morphology, absorption and fluorescence of melanosomes and lipofuscin granules of the retinal pigment epithelium. Vision Res. 1990, 30, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Kikugawa, K.; Beppu, M.; Sato, A.; Kasai, H. Separation of multiple yellow fluorescent lipofuscin components in rat kidney and their characterization. Mech. Ageing Dev. 1997, 97, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Eldred, G.E.; Miller, G.V.; Stark, W.S.; Feeney-Burns, L. Lipofuscin: Resolution of discrepant fluorescence data. Science 1982, 216, 757–759. [Google Scholar] [CrossRef] [PubMed]
- Warburton, S.; Southwick, K.; Hardman, R.M.; Secrest, A.M.; Grow, R.K.; Xin, H.; Woolley, A.T.; Burton, G.F.; Thulin, C.D. Examining the proteins of functional retinal lipofuscin using proteomic analysis as a guide for understanding its origin. Mol. Vis. 2005, 11, 1122–1134. [Google Scholar]
- Kikugawa, K.; Beppu, M.; Kato, T.; Yamaki, S.; Kasai, H. Accumulation of autofluorescent yellow lipofuscin in rat tissues estimated by sodium dodecylsulfate extraction. Mech. Ageing Dev. 1994, 74, 135–148. [Google Scholar] [CrossRef]
- Kikugawa, K.; Beppu, M. Involvement of lipid oxidation products in the formation of fluorescent and cross-linked proteins. Chem. Phys. Lipids 1987, 44, 277–296. [Google Scholar] [CrossRef] [PubMed]
- Kikugawa, K.; Kato, T.; Beppu, M.; Hayasaka, A. Fluorescent and cross-linked proteins formed by free radical and aldehyde species generated during lipid oxidation. Adv. Exp. Med. Biol. 1989, 266, 345–356; discussion 357. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, F.J.; Zamora, R. Modification of bovine serum albumin structure following reaction with 4,5(E)-epoxy-2(E)-heptenal. Chem. Res. Toxicol. 2000, 13, 501–508. [Google Scholar] [CrossRef] [PubMed]
- d’Ischia, M.; Costantini, C.; Prota, G. Lipofuscin-like pigments by autoxidation of polyunsaturated fatty acids in the presence of amine neurotransmitters: The role of malondialdehyde. Biochim. Biophys. Acta 1996, 1290, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Eldred, G.E.; Katz, M.L. The autofluorescent products of lipid peroxidation may not be lipofuscin-like. Free Radic. Biol. Med. 1989, 7, 157–163. [Google Scholar] [CrossRef]
- Yin, D.Z.; Brunk, U.T. Microfluorometric and fluorometric lipofuscin spectral discrepancies: A concentration-dependent metachromatic effect? Mech. Ageing Dev. 1991, 59, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Rozanowska, M.B.; Rozanowski, B. Photodegradation of Lipofuscin in Suspension and in ARPE-19 Cells and the Similarity of Fluorescence of the Photodegradation Product with Oxidized Docosahexaenoate. Int. J. Mol. Sci. 2022, 23, 922. [Google Scholar] [CrossRef] [PubMed]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [PubMed]
- Feeney-Burns, L.; Hilderbrand, E.S.; Eldridge, S. Aging human RPE: Morphometric analysis of macular, equatorial, and peripheral cells. Investig. Ophthalmol. Vis. Sci. 1984, 25, 195–200. [Google Scholar]
- Hayasaka, S. Aging changes in lipofuscin, lysosomes and melanin in the macular area of human retina and choroid. Jpn. J. Ophthalmol. 1989, 33, 36–42. [Google Scholar] [PubMed]
- Artigas, J.M.; Felipe, A.; Navea, A.; Fandino, A.; Artigas, C. Spectral transmission of the human crystalline lens in adult and elderly persons: Color and total transmission of visible light. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4076–4084. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.G., Jr.; Richey, E.O. Eclipse burns in humans and laboratory threshold measurements in rabbits. SAM-TR-66-45. Tech. Rep. SAM-TR 1966, 1–5. [Google Scholar]
- Rozanowska, M. Properties and Functions of Ocular Melanins and Melanosomes. In Melanins and Melanosomes: Biosynthesis, Biogenesis, Physiological and Pathological Functions; Borovansky, J., Riley, P.A., Eds.; Wiley-Blackwell: Singapore, 2011; pp. 187–224. [Google Scholar]
- Yanoff, M.; Duker, J.S. Ophthalmology: Expert Consult: Online and Print; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Lewandowski, D.; Sander, C.L.; Tworak, A.; Gao, F.; Xu, Q.; Skowronska-Krawczyk, D. Dynamic lipid turnover in photoreceptors and retinal pigment epithelium throughout life. Prog. Retin. Eye Res. 2022, 89, 101037. [Google Scholar] [CrossRef] [PubMed]
- Boulton, M.; McKechnie, N.M.; Breda, J.; Bayly, M.; Marshall, J. The formation of autofluorescent granules in cultured human RPE. Investig. Ophthalmol. Vis. Sci. 1989, 30, 82–89. [Google Scholar]
- Katz, M.L.; Drea, C.M.; Eldred, G.E.; Hess, H.H.; Robison, W.G., Jr. Influence of early photoreceptor degeneration on lipofuscin in the retinal pigment epithelium. Exp. Eye Res. 1986, 43, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Feeney-Burns, L.; Eldred, G.E. The fate of the phagosome: Conversion to ‘age pigment’ and impact in human retinal pigment epithelium. Trans. Ophthalmol. Soc. U. K. 1983, 103 Pt 4, 416–421. [Google Scholar] [PubMed]
- Feeney, L. Lipofuscin and melanin of human retinal pigment epithelium. Fluorescence, enzyme cytochemical, and ultrastructural studies. Investig. Ophthalmol. Vis. Sci. 1978, 17, 583–600. [Google Scholar]
- Pugh, E.N., Jr.; Lamb, T.D. Amplification and kinetics of the activation steps in phototransduction. Biochim. Biophys. Acta 1993, 1141, 111–149. [Google Scholar] [CrossRef]
- Rodieck, R.W. The First Steps in Seeing; Sinauer Associates Inc.: Sunderland, MA, USA, 1998. [Google Scholar]
- Kevany, B.M.; Palczewski, K. Phagocytosis of retinal rod and cone photoreceptors. Physiology 2010, 25, 8–15. [Google Scholar] [CrossRef]
- Mustafi, D.; Engel, A.H.; Palczewski, K. Structure of cone photoreceptors. Prog. Retin. Eye Res. 2009, 28, 289–302. [Google Scholar] [CrossRef]
- de Araujo, M.E.G.; Liebscher, G.; Hess, M.W.; Huber, L.A. Lysosomal size matters. Traffic 2020, 21, 60–75. [Google Scholar] [CrossRef]
- Snodderly, D.M.; Sandstrom, M.M.; Leung, I.Y.; Zucker, C.L.; Neuringer, M. Retinal pigment epithelial cell distribution in central retina of rhesus monkeys. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2815–2818. [Google Scholar]
- Rozanowska, M.; Rozanowski, B.; Boulton, M. Photobiology of the retina: Light damage to the retina. In Photobiological Sciences; Smith, K.C., Ed.; American Society for Photobiology: Herndon, VA, USA, 2009; Available online: http://www.photobiology.info (accessed on 23 March 2023).
- Katz, M.L.; Gao, C.L. Vitamin A incorporation into lipofuscin-like inclusions in the retinal pigment epithelium. Mech. Ageing Dev. 1995, 84, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.L.; Rice, L.M.; Gao, C.L. Reversible accumulation of lipofuscin-like inclusions in the retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 1999, 40, 175–181. [Google Scholar]
- Ivy, G.O.; Ihara, Y.; Kitani, K. The protease inhibitor leupeptin induces several signs of aging in brain, retina and internal organs of young rats. Arch. Gerontol. Geriatr. 1991, 12, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.L.; Stientjes, H.J.; Gao, C.L.; Christianson, J.S. Iron-induced accumulation of lipofuscin-like fluorescent pigment in the retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 1993, 34, 3161–3171. [Google Scholar]
- Katz, M.L.; Christianson, J.S.; Gao, C.L.; Handelman, G.J. Iron-induced fluorescence in the retina: Dependence on vitamin A. Investig. Ophthalmol. Vis. Sci. 1994, 35, 3613–3624. [Google Scholar]
- Liu, Y.; Bell, B.A.; Song, Y.; Kim, H.J.; Sterling, J.K.; Kim, B.J.; Poli, M.; Guo, M.; Zhang, K.; Rao, A.; et al. Intraocular iron injection induces oxidative stress followed by elements of geographic atrophy and sympathetic ophthalmia. Aging Cell 2021, 20, e13490. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.L.; Stone, W.L.; Dratz, E.A. Fluorescent pigment accumulation in retinal pigment epithelium of antioxidant-deficient rats. Investig. Ophthalmol. Vis. Sci. 1978, 17, 1049–1058. [Google Scholar]
- Katz, M.L.; Drea, C.M.; Robison, W.G., Jr. Relationship between dietary retinol and lipofuscin in the retinal pigment epithelium. Mech. Ageing Dev. 1986, 35, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Hayes, K.C. Retinal degeneration in monkeys induced by deficiencies of vitamin E or A. Investig. Ophthalmol. 1974, 13, 499–510. [Google Scholar]
- Rozanowska, M.B.; Czuba-Pelech, B.; Rozanowski, B. Is There an Optimal Combination of AREDS2 Antioxidants Zeaxanthin, Vitamin E and Vitamin C on Light-Induced Toxicity of Vitamin A Aldehyde to the Retina? Antioxidants 2022, 11, 1132. [Google Scholar] [CrossRef] [PubMed]
- Rozanowska, M.B.; Czuba-Pelech, B.; Landrum, J.T.; Rozanowski, B. Comparison of Antioxidant Properties of Dehydrolutein with Lutein and Zeaxanthin, and their Effects on Cultured Retinal Pigment Epithelial Cells. Antioxidants 2021, 10, 753. [Google Scholar] [CrossRef] [PubMed]
- Rozanowska, M.; Handzel, K.; Boulton, M.E.; Rozanowski, B. Cytotoxicity of all-trans-retinal increases upon photodegradation. Photochem. Photobiol. 2012, 88, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Rozanowska, M.; Sarna, T. Light-induced damage to the retina: Role of rhodopsin chromophore revisited. Photochem. Photobiol. 2005, 81, 1305–1330. [Google Scholar] [CrossRef] [PubMed]
- Boulton, M.; Rozanowska, M.; Rozanowski, B. Retinal photodamage. J. Photochem. Photobiol. B Biol. 2001, 64, 144–161. [Google Scholar] [CrossRef] [PubMed]
- Kiser, P.D.; Golczak, M.; Palczewski, K. Chemistry of the Retinoid (Visual) Cycle. Chem. Rev. 2014, 114, 194–232. [Google Scholar] [CrossRef] [PubMed]
- Bazan, H.E.; Bazan, N.G.; Feeney-Burns, L.; Berman, E.R. Lipids in human lipofuscin-enriched subcellular fractions of two age populations. Comparison with rod outer segments and neural retina. Investig. Ophthalmol. Vis. Sci. 1990, 31, 1433–1443. [Google Scholar]
- Bernoud-Hubac, N.; Davies, S.S.; Boutaud, O.; Montine, T.J.; Roberts, L.J., 2nd. Formation of highly reactive gamma-ketoaldehydes (neuroketals) as products of the neuroprostane pathway. J. Biol. Chem. 2001, 276, 30964–30970. [Google Scholar] [CrossRef]
- Bernoud-Hubac, N.; Roberts, L.J., 2nd. Identification of oxidized derivatives of neuroketals. Biochemistry 2002, 41, 11466–11471. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.J., 2nd; Morrow, J.D. Products of the isoprostane pathway: Unique bioactive compounds and markers of lipid peroxidation. Cell. Mol. Life Sci. 2002, 59, 808–820. [Google Scholar] [CrossRef]
- Davies, S.S.; Amarnath, V.; Montine, K.S.; Bernoud-Hubac, N.; Boutaud, O.; Montine, T.J.; Roberts, L.J., 2nd. Effects of reactive gamma-ketoaldehydes formed by the isoprostane pathway (isoketals) and cyclooxygenase pathway (levuglandins) on proteasome function. FASEB J. 2002, 16, 715–717. [Google Scholar] [CrossRef]
- Poliakov, E.; Meer, S.G.; Roy, S.C.; Mesaros, C.; Salomon, R.G. Iso[7]LGD2-protein adducts are abundant in vivo and free radical-induced oxidation of an arachidonyl phospholipid generates this D series isolevuglandin in vitro. Chem. Res. Toxicol. 2004, 17, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Montuschi, P.; Barnes, P.J.; Roberts, L.J., 2nd. Isoprostanes: Markers and mediators of oxidative stress. FASEB J. 2004, 18, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Salomon, R.G. Levuglandins and isolevuglandins: Stealthy toxins of oxidative injury. Antioxid. Redox Signal. 2005, 7, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Else, P.L.; Kraffe, E. Docosahexaenoic and arachidonic acid peroxidation: It’s a within molecule cascade. Biochim. Biophys. Acta 2015, 1848, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, M.; de Oliveira, E.; Odena, M.A.; Portero, M.; Pamplona, R.; Ferrer, I. Redox proteomic profiling of neuroketal-adducted proteins in human brain: Regional vulnerability at middle age increases in the elderly. Free Radic. Biol. Med. 2016, 95, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sousa, B.C.; Pitt, A.R.; Spickett, C.M. Chemistry and analysis of HNE and other prominent carbonyl-containing lipid oxidation compounds. Free Radic. Biol. Med. 2017, 111, 294–308. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.N.; Warren, E.; Liang, L.P.; Roberts, L.J., 2nd; Patel, M. Scavenging of highly reactive gamma-ketoaldehydes attenuates cognitive dysfunction associated with epileptogenesis. Neurobiol. Dis. 2017, 98, 88–99. [Google Scholar] [CrossRef]
- Sun, H.; Nathans, J. ABCR, the ATP-binding cassette transporter responsible for Stargardt macular dystrophy, is an efficient target of all-trans-retinal-mediated photooxidative damage in vitro. Implications for retinal disease. J. Biol. Chem. 2001, 276, 11766–11774. [Google Scholar] [CrossRef] [PubMed]
- Molday, R.S.; Garces, F.A.; Scortecci, J.F.; Molday, L.L. Structure and function of ABCA4 and its role in the visual cycle and Stargardt macular degeneration. Prog. Retin. Eye Res. 2022, 89, 101036. [Google Scholar] [CrossRef] [PubMed]
- Lenis, T.L.; Hu, J.; Ng, S.Y.; Jiang, Z.; Sarfare, S.; Lloyd, M.B.; Esposito, N.J.; Samuel, W.; Jaworski, C.; Bok, D.; et al. Expression of ABCA4 in the retinal pigment epithelium and its implications for Stargardt macular degeneration. Proc. Natl. Acad. Sci. USA 2018, 115, E11120–E11127. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.D.; Salom, D.; Kochman, M.A.; Kubas, A.; Kiser, P.D.; Palczewski, K. Chromophore hydrolysis and release from photoactivated rhodopsin in native membranes. Proc. Natl. Acad. Sci. USA 2022, 119, e2213911119. [Google Scholar] [CrossRef]
- Zhao, J.; Kim, H.J.; Ueda, K.; Zhang, K.; Montenegro, D.; Dunaief, J.L.; Sparrow, J.R. A vicious cycle of bisretinoid formation and oxidation relevant to recessive Stargardt disease. J. Biol. Chem. 2021, 296, 100259. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Sparrow, J.R. Bisretinoid phospholipid and vitamin A aldehyde: Shining a light. J. Lipid Res. 2021, 62, 100042. [Google Scholar] [CrossRef]
- Kim, H.J.; Montenegro, D.; Zhao, J.; Sparrow, J.R. Bisretinoids of the Retina: Photo-Oxidation, Iron-Catalyzed Oxidation, and Disease Consequences. Antioxidants 2021, 10, 1382. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.; Elliott, M.H.; Floor, E.; Truscott, T.G.; Zareba, M.; Sarna, T.; Shamsi, F.A.; Boulton, M.E. Photocytotoxicity of lipofuscin in human retinal pigment epithelial cells. Free Radic. Biol. Med. 2001, 31, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Avalle, L.B.; Wang, Z.; Dillon, J.P.; Gaillard, E.R. Observation of A2E oxidation products in human retinal lipofuscin. Exp. Eye Res. 2004, 78, 895–898. [Google Scholar] [CrossRef]
- Jang, Y.P.; Matsuda, H.; Itagaki, Y.; Nakanishi, K.; Sparrow, J.R. Characterization of peroxy-A2E and furan-A2E photooxidation products and detection in human and mouse retinal pigment epithelial cell lipofuscin. J. Biol. Chem. 2005, 280, 39732–39739. [Google Scholar] [CrossRef] [PubMed]
- Ablonczy, Z.; Higbee, D.; Anderson, D.M.; Dahrouj, M.; Grey, A.C.; Gutierrez, D.; Koutalos, Y.; Schey, K.L.; Hanneken, A.; Crouch, R.K. Lack of correlation between the spatial distribution of A2E and lipofuscin fluorescence in the human retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5535–5542. [Google Scholar] [CrossRef] [PubMed]
- Adler, L.T.; Boyer, N.P.; Anderson, D.M.; Spraggins, J.M.; Schey, K.L.; Hanneken, A.; Ablonczy, Z.; Crouch, R.K.; Koutalos, Y. Determination of N-retinylidene-N-retinylethanolamine (A2E) levels in central and peripheral areas of human retinal pigment epithelium. Photochem. Photobiol. Sci. 2015, 14, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Kotnala, A.; Senthilkumari, S.; Wu, G.; Stewart, T.G.; Curcio, C.A.; Halder, N.; Singh, S.B.; Kumar, A.; Velpandian, T. Retinal Pigment Epithelium in Human Donor Eyes Contains Higher Levels of Bisretinoids Including A2E in Periphery than Macula. Investig. Ophthalmol. Vis. Sci. 2022, 63, 6. [Google Scholar] [CrossRef] [PubMed]
- Fite, K.V.; Bengston, L.; Donaghey, B. Experimental light damage increases lipofuscin in the retinal pigment epithelium of Japanese quail (Coturnix coturnix japonica). Exp. Eye Res. 1993, 57, 449–460. [Google Scholar] [CrossRef]
- Boyer, N.P.; Thompson, D.A.; Koutalos, Y. Relative Contributions of All-Trans and 11-Cis Retinal to Formation of Lipofuscin and A2E Accumulating in Mouse Retinal Pigment Epithelium. Investig. Ophthalmol. Vis. Sci. 2021, 62, 1. [Google Scholar] [CrossRef] [PubMed]
- Adler, L.T.; Boyer, N.P.; Chen, C.; Ablonczy, Z.; Crouch, R.K.; Koutalos, Y. The 11-cis Retinal Origins of Lipofuscin in the Retina. Prog. Mol. Biol. Transl. Sci. 2015, 134, e1–e12. [Google Scholar] [CrossRef] [PubMed]
- Boyer, N.P.; Higbee, D.; Currin, M.B.; Blakeley, L.R.; Chen, C.; Ablonczy, Z.; Crouch, R.K.; Koutalos, Y. Lipofuscin and N-retinylidene-N-retinylethanolamine (A2E) accumulate in retinal pigment epithelium in absence of light exposure: Their origin is 11-cis-retinal. J. Biol. Chem. 2012, 287, 22276–22286. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Golczak, M.; Maeda, T.; Palczewski, K. Limited roles of Rdh8, Rdh12, and Abca4 in all-trans-retinal clearance in mouse retina. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5435–5443. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Maeda, T.; Golczak, M.; Palczewski, K. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J. Biol. Chem. 2008, 283, 26684–26693. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Maeda, T.; Sun, W.; Zhang, H.; Baehr, W.; Palczewski, K. Redundant and unique roles of retinol dehydrogenases in the mouse retina. Proc. Natl. Acad. Sci. USA 2007, 104, 19565–19570. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Maeda, T.; Imanishi, Y.; Kuksa, V.; Alekseev, A.; Bronson, J.D.; Zhang, H.; Zhu, L.; Sun, W.; Saperstein, D.A.; et al. Role of photoreceptor-specific retinol dehydrogenase in the retinoid cycle in vivo. J. Biol. Chem. 2005, 280, 18822–18832. [Google Scholar] [CrossRef]
- Mata, N.L.; Tzekov, R.T.; Liu, X.; Weng, J.; Birch, D.G.; Travis, G.H. Delayed dark-adaptation and lipofuscin accumulation in abcr+/− mice: Implications for involvement of ABCR in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1685–1690. [Google Scholar]
- Weng, J.; Mata, N.L.; Azarian, S.M.; Tzekov, R.T.; Birch, D.G.; Travis, G.H. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt’s disease from the phenotype in abcr knockout mice. Cell 1999, 98, 13–23. [Google Scholar] [CrossRef]
- Eldred, G.E. Vitamins A and E in RPE lipofuscin formation and implications for age-related macular degeneration. Prog. Clin. Biol. Res. 1989, 314, 113–129. [Google Scholar] [PubMed]
- Katz, M.L.; Norberg, M. Influence of dietary vitamin A on autofluorescence of leupeptin-induced inclusions in the retinal pigment epithelium. Exp. Eye Res. 1992, 54, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.L.; Norberg, M.; Stientjes, H.J. Reduced phagosomal content of the retinal pigment epithelium in response to retinoid deprivation. Investig. Ophthalmol. Vis. Sci. 1992, 33, 2612–2618. [Google Scholar]
- Dobri, N.; Qin, Q.; Kong, J.; Yamamoto, K.; Liu, Z.; Moiseyev, G.; Ma, J.X.; Allikmets, R.; Sparrow, J.R.; Petrukhin, K. A1120, a nonretinoid RBP4 antagonist, inhibits formation of cytotoxic bisretinoids in the animal model of enhanced retinal lipofuscinogenesis. Investig. Ophthalmol. Vis. Sci. 2013, 54, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Golczak, M.; Chen, Y.; Okano, K.; Kohno, H.; Shiose, S.; Ishikawa, K.; Harte, W.; Palczewska, G.; Maeda, T.; et al. Primary amines protect against retinal degeneration in mouse models of retinopathies. Nat. Chem. Biol. 2011, 8, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Maeda, A.; Matosky, M.; Okano, K.; Roos, S.; Tang, J.; Palczewski, K. Evaluation of potential therapies for a mouse model of human age-related macular degeneration caused by delayed all-trans-retinal clearance. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4917–4925. [Google Scholar] [CrossRef] [PubMed]
- Golczak, M.; Maeda, A.; Bereta, G.; Maeda, T.; Kiser, P.D.; Hunzelmann, S.; von Lintig, J.; Blaner, W.S.; Palczewski, K. Metabolic basis of visual cycle inhibition by retinoid and nonretinoid compounds in the vertebrate retina. J. Biol. Chem. 2008, 283, 9543–9554. [Google Scholar] [CrossRef] [PubMed]
- Radu, R.A.; Han, Y.; Bui, T.V.; Nusinowitz, S.; Bok, D.; Lichter, J.; Widder, K.; Travis, G.H.; Mata, N.L. Reductions in serum vitamin A arrest accumulation of toxic retinal fluorophores: A potential therapy for treatment of lipofuscin-based retinal diseases. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4393–4401. [Google Scholar] [CrossRef]
- Golczak, M.; Kuksa, V.; Maeda, T.; Moise, A.R.; Palczewski, K. Positively charged retinoids are potent and selective inhibitors of the trans-cis isomerization in the retinoid (visual) cycle. Proc. Natl. Acad. Sci. USA 2005, 102, 8162–8167. [Google Scholar] [CrossRef]
- Radu, R.A.; Mata, N.L.; Nusinowitz, S.; Liu, X.; Sieving, P.A.; Travis, G.H. Treatment with isotretinoin inhibits lipofuscin accumulation in a mouse model of recessive Stargardt’s macular degeneration. Proc. Natl. Acad. Sci. USA 2003, 100, 4742–4747. [Google Scholar] [CrossRef]
- Radu, R.A.; Yuan, Q.; Hu, J.; Peng, J.H.; Lloyd, M.; Nusinowitz, S.; Bok, D.; Travis, G.H. Accelerated accumulation of lipofuscin pigments in the RPE of a mouse model for ABCA4-mediated retinal dystrophies following Vitamin A supplementation. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3821–3829. [Google Scholar] [CrossRef] [PubMed]
- Crabb, J.W.; O’Neil, J.; Miyagi, M.; West, K.; Hoff, H.F. Hydroxynonenal inactivates cathepsin B by forming Michael adducts with active site residues. Protein Sci. 2002, 11, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Bermann, M.; Schutt, F.; Holz, F.G.; Kopitz, J. Does A2E, a retinoid component of lipofuscin and inhibitor of lysosomal degradative functions, directly affect the activity of lysosomal hydrolases? Exp. Eye Res. 2001, 72, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Lenis, T.L.; Sarfare, S.; Jiang, Z.; Lloyd, M.B.; Bok, D.; Radu, R.A. Complement modulation in the retinal pigment epithelium rescues photoreceptor degeneration in a mouse model of Stargardt disease. Proc. Natl. Acad. Sci. USA 2017, 114, 3987–3992. [Google Scholar] [CrossRef] [PubMed]
- Young, R.W. The renewal of rod and cone outer segments in the rhesus monkey. J. Cell Biol. 1971, 49, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Qiao-Grider, Y.; Hung, L.F.; Kee, C.S.; Ramamirtham, R.; Smith, E.L., 3rd. Normal ocular development in young rhesus monkeys (Macaca mulatta). Vision Res. 2007, 47, 1424–1444. [Google Scholar] [CrossRef] [PubMed]
- Curcio, C.A.; Sloan, K.R.; Kalina, R.E.; Hendrickson, A.E. Human photoreceptor topography. J. Comp. Neurol. 1990, 292, 497–523. [Google Scholar] [CrossRef]
- Bernstein, P.S.; Delori, F.C.; Richer, S.; van Kuijk, F.J.; Wenzel, A.J. The value of measurement of macular carotenoid pigment optical densities and distributions in age-related macular degeneration and other retinal disorders. Vision Res. 2010, 50, 716–728. [Google Scholar] [CrossRef] [PubMed]
- Różanowska, M.; Różanowski, B. Uptake and photoprotection in cultured RPE cells. In Carotenoids: Physical, Chemical, and Biological Functions and Properties; Landrum, J.T., Ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 309–364. [Google Scholar]
- Werner, J.S.; Donnelly, S.K.; Kliegl, R. Aging and human macular pigment density. Appended with translations from the work of Max Schultze and Ewald Hering. Vision Res. 1987, 27, 257–268. [Google Scholar] [CrossRef]
- Warburton, S.; Davis, W.E.; Southwick, K.; Xin, H.; Woolley, A.T.; Burton, G.F.; Thulin, C.D. Proteomic and phototoxic characterization of melanolipofuscin: Correlation to disease and model for its origin. Mol. Vis. 2007, 13, 318–329. [Google Scholar]
- Schutt, F.; Bergmann, M.; Holz, F.G.; Kopitz, J. Proteins modified by malondialdehyde, 4-hydroxynonenal, or advanced glycation end products in lipofuscin of human retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3663–3668. [Google Scholar] [CrossRef] [PubMed]
- Schutt, F.; Ueberle, B.; Schnolzer, M.; Holz, F.G.; Kopitz, J. Proteome analysis of lipofuscin in human retinal pigment epithelial cells. FEBS Lett. 2002, 528, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Sinha, T.; Naash, M.I.; Al-Ubaidi, M.R. The Symbiotic Relationship between the Neural Retina and Retinal Pigment Epithelium Is Supported by Utilizing Differential Metabolic Pathways. iScience 2020, 23, 101004. [Google Scholar] [CrossRef] [PubMed]
- Kanow, M.A.; Giarmarco, M.M.; Jankowski, C.S.; Tsantilas, K.; Engel, A.L.; Du, J.; Linton, J.D.; Farnsworth, C.C.; Sloat, S.R.; Rountree, A.; et al. Biochemical adaptations of the retina and retinal pigment epithelium support a metabolic ecosystem in the vertebrate eye. eLife 2017, 6, e28899. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Reveles, J.; Dhingra, A.; Alexander, D.; Bragin, A.; Philp, N.J.; Boesze-Battaglia, K. Phagocytosis-dependent ketogenesis in retinal pigment epithelium. J. Biol. Chem. 2017, 292, 8038–8047. [Google Scholar] [CrossRef] [PubMed]
- Porter, N.A. A perspective on free radical autoxidation: The physical organic chemistry of polyunsaturated fatty acid and sterol peroxidation. J. Org. Chem. 2013, 78, 3511–3524. [Google Scholar] [CrossRef]
- Rozanowska, M.; Pawlak, A.; Rozanowski, B.; Skumatz, C.; Zareba, M.; Boulton, M.E.; Burke, J.M.; Sarna, T.; Simon, J.D. Age-related changes in the photoreactivity of retinal lipofuscin granules: Role of chloroform-insoluble components. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Różanowska, M.; Różanowski, B. Visual transduction and age-related changes in lipofuscin. In Ophthalmology Research: The Visual Transduction Cascade; Tombran-Tink, J., Barnstable, C.J., Eds.; The Humana Press Inc.: Totowa, NJ, USA, 2008; pp. 405–446. [Google Scholar]
- Guan, Z.; Li, Y.; Jiao, S.; Yeasmin, N.; Rosenfeld, P.J.; Dubovy, S.R.; Lam, B.L.; Wen, R. A2E Distribution in RPE Granules in Human Eyes. Molecules 2020, 25, 1413. [Google Scholar] [CrossRef]
- Bhosale, P.; Serban, B.; Bernstein, P.S. Retinal carotenoids can attenuate formation of A2E in the retinal pigment epithelium. Arch. Biochem. Biophys. 2009, 483, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.M.R.; Krogmeier, J.R.; Pawlak, A.; Rozanowska, M.; Sarna, T.; Dunn, R.C.; Simon, J.D. Atomic force microscopy and near-field scanning optical microscopy measurements of single human retinal lipofuscin granules. J. Phys. Chem. B 2000, 104, 12098–12101. [Google Scholar] [CrossRef]
- Krogmeier, J.R.; Clancy, C.M.; Pawlak, A.; Rozanowska, M.; Sarna, T.; Simon, J.D.; Dunn, R.C. Mapping the distribution of emissive molecules in human ocular lipofuscin granules with near-field scanning optical microscopy. J. Microsc. 2001, 202, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Gouras, P.; Brown, K.R.; Mattison, J.A.; Neuringer, M.; Nagasaki, T.; Ivert, L. The Ultrastructure, Spatial Distribution, and Osmium Tetroxide Binding of Lipofuscin and Melanosomes in Aging Monkey Retinal Epithelium. Curr. Eye Res. 2018, 43, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Haralampus-Grynaviski, N.M.; Lamb, L.E.; Clancy, C.M.; Skumatz, C.; Burke, J.M.; Sarna, T.; Simon, J.D. Spectroscopic and morphological studies of human retinal lipofuscin granules. Proc. Natl. Acad. Sci. USA 2003, 100, 3179–3184. [Google Scholar] [CrossRef] [PubMed]
- Petrukhin, A.N.; Astaf’ev, A.A.; Zolotavin, P.N.; Fel’dman, T.B.; Dontsov, A.E.; Sarkisov, O.M.; Ostrovsky, M.A. Heterogeneity of structure and fluorescence of single lipofuscin granule from retinal pigment epithelium of human donor eyes: Study with the use of atomic force microscopy and near-field microscopy. Dokl. Biochem. Biophys. 2005, 405, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Wing, G.L.; Blanchard, G.C.; Weiter, J.J. The topography and age relationship of lipofuscin concentration in the retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 1978, 17, 601–607. [Google Scholar]
- Weiter, J.J.; Delori, F.C.; Wing, G.L.; Fitch, K.A. Retinal pigment epithelial lipofuscin and melanin and choroidal melanin in human eyes. Investig. Ophthalmol. Vis. Sci. 1986, 27, 145–152. [Google Scholar]
- Gliem, M.; Muller, P.L.; Birtel, J.; Herrmann, P.; McGuinness, M.B.; Holz, F.G.; Charbel Issa, P. Quantitative Fundus Autofluorescence and Genetic Associations in Macular, Cone, and Cone-Rod Dystrophies. Ophthalmol. Retina 2020, 4, 737–749. [Google Scholar] [CrossRef]
- Bakall, B.; Radu, R.A.; Stanton, J.B.; Burke, J.M.; McKay, B.S.; Wadelius, C.; Mullins, R.F.; Stone, E.M.; Travis, G.H.; Marmorstein, A.D. Enhanced accumulation of A2E in individuals homozygous or heterozygous for mutations in BEST1 (VMD2). Exp. Eye Res. 2007, 85, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Small, K.W.; Grossniklaus, H.E. Clinicopathologic findings in Best vitelliform macular dystrophy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 745–751. [Google Scholar] [CrossRef]
- Jauregui, R.; Chan, L.; Oh, J.K.; Cho, A.; Sparrow, J.R.; Tsang, S.H. Disease asymmetry and hyperautofluorescent ring shape in retinitis pigmentosa patients. Sci. Rep. 2020, 10, 3364. [Google Scholar] [CrossRef]
- Jauregui, R.; Park, K.S.; Duong, J.K.; Sparrow, J.R.; Tsang, S.H. Quantitative Comparison of Near-infrared Versus Short-wave Autofluorescence Imaging in Monitoring Progression of Retinitis Pigmentosa. Am. J. Ophthalmol. 2018, 194, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Gliem, M.; Muller, P.L.; Finger, R.P.; McGuinness, M.B.; Holz, F.G.; Charbel Issa, P. Quantitative Fundus Autofluorescence in Early and Intermediate Age-Related Macular Degeneration. JAMA Ophthalmol. 2016, 134, 817–824. [Google Scholar] [CrossRef]
- Bermond, K.; von der Emde, L.; Tarau, I.S.; Bourauel, L.; Heintzmann, R.; Holz, F.G.; Curcio, C.A.; Sloan, K.R.; Ach, T. Autofluorescent Organelles within the Retinal Pigment Epithelium in Human Donor Eyes with and without Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2022, 63, 23. [Google Scholar] [CrossRef] [PubMed]
- Dorey, C.K.; Wu, G.; Ebenstein, D.; Garsd, A.; Weiter, J.J. Cell loss in the aging retina. Relationship to lipofuscin accumulation and macular degeneration. Investig. Ophthalmol. Vis. Sci. 1989, 30, 1691–1699. [Google Scholar]
- Curcio, C.A. Photoreceptor topography in ageing and age-related maculopathy. Eye 2001, 15, 376–383. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Sakmar, T.P. Interaction of A2E with model membranes. Implications to the pathogenesis of age-related macular degeneration. J. Gen. Physiol. 2002, 120, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Schutt, F.; Bergmann, M.; Holz, F.G.; Kopitz, J. Isolation of intact lysosomes from human RPE cells and effects of A2-E on the integrity of the lysosomal and other cellular membranes. Graefe’s Arch. Clin. Exp. Ophthalmol. 2002, 240, 983–988. [Google Scholar] [CrossRef]
- Bergmann, M.; Schutt, F.; Holz, F.G.; Kopitz, J. Inhibition of the ATP-driven proton pump in RPE lysosomes by the major lipofuscin fluorophore A2-E may contribute to the pathogenesis of age-related macular degeneration. FASEB J. 2004, 18, 562–564. [Google Scholar] [CrossRef] [PubMed]
- Mitter, S.K.; Song, C.; Qi, X.; Mao, H.; Rao, H.; Akin, D.; Lewin, A.; Grant, M.; Dunn, W., Jr.; Ding, J.; et al. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy 2014, 10, 1989–2005. [Google Scholar] [CrossRef]
- Boulton, M.; Marshall, J. Repigmentation of human retinal pigment epithelial cells in vitro. Exp. Eye Res. 1985, 41, 209–218. [Google Scholar] [CrossRef]
- Rakoczy, P.; Kennedy, C.; Thompson-Wallis, D.; Mann, K.; Constable, I. Changes in retinal pigment epithelial cell autofluorescence and protein expression associated with phagocytosis of rod outer segments in vitro. Biol. Cell 1992, 76, 49–54. [Google Scholar] [CrossRef]
- Brunk, U.T.; Wihlmark, U.; Wrigstad, A.; Roberg, K.; Nilsson, S.E. Accumulation of lipofuscin within retinal pigment epithelial cells results in enhanced sensitivity to photo-oxidation. Gerontology 1995, 41 (Suppl. S2), 201–212. [Google Scholar] [CrossRef] [PubMed]
- Wassell, J.; Ellis, S.; Burke, J.; Boulton, M. Fluorescence properties of autofluorescent granules generated by cultured human RPE cells. Investig. Ophthalmol. Vis. Sci. 1998, 39, 1487–1492. [Google Scholar]
- Boulton, M.E. Studying melanin and lipofuscin in RPE cell culture models. Exp. Eye Res. 2014, 126, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Presswalla, F.; Calton, M.; Charniga, C.; Stern, J.; Temple, S.; Vollrath, D.; Zacks, D.N.; Ali, R.R.; Thompson, D.A.; et al. Highly Differentiated Human Fetal RPE Cultures Are Resistant to the Accumulation and Toxicity of Lipofuscin-Like Material. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3468–3479. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.O. Phagocytosis of light- and dark-adapted rod outer segments by cultured pigment epithelium. Science 1978, 202, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.E.; Sundelin, S.P.; Wihlmark, U.; Brunk, U.T. Aging of cultured retinal pigment epithelial cells: Oxidative reactions, lipofuscin formation and blue light damage. Doc. Ophthalmol. 2003, 106, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Tzekov, R.; McDowell, J.H.; Smith, W.C.; Tang, S.; Kaushal, S. Formation of lipofuscin-like material in the RPE Cell by different components of rod outer segments. Exp. Eye Res. 2013, 112, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Kaemmerer, E.; Schutt, F.; Krohne, T.U.; Holz, F.G.; Kopitz, J. Effects of lipid peroxidation-related protein modifications on RPE lysosomal functions and POS phagocytosis. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1342–1347. [Google Scholar] [CrossRef]
- Krohne, T.U.; Stratmann, N.K.; Kopitz, J.; Holz, F.G. Effects of lipid peroxidation products on lipofuscinogenesis and autophagy in human retinal pigment epithelial cells. Exp. Eye Res. 2010, 90, 465–471. [Google Scholar] [CrossRef]
- Rakoczy, P.E.; Baines, M.; Kennedy, C.J.; Constable, I.J. Correlation between autofluorescent debris accumulation and the presence of partially processed forms of cathepsin D in cultured retinal pigment epithelial cells challenged with rod outer segments. Exp. Eye Res. 1996, 63, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Escrevente, C.; Falcao, A.S.; Hall, M.J.; Lopes-da-Silva, M.; Antas, P.; Mesquita, M.M.; Ferreira, I.S.; Cardoso, M.H.; Oliveira, D.; Fradinho, A.C.; et al. Formation of Lipofuscin-Like Autofluorescent Granules in the Retinal Pigment Epithelium Requires Lysosome Dysfunction. Investig. Ophthalmol. Vis. Sci. 2021, 62, 39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hui, Y.N.; Wang, Y.S.; Ma, J.X.; Wang, J.B.; Ma, L.N. Calcium overload is associated with lipofuscin formation in human retinal pigment epithelial cells fed with photoreceptor outer segments. Eye 2011, 25, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Maeda, T.; Golczak, M.; Chou, S.; Desai, A.; Hoppel, C.L.; Matsuyama, S.; Palczewski, K. Involvement of all-trans-retinal in acute light-induced retinopathy of mice. J. Biol. Chem. 2009, 284, 15173–15183. [Google Scholar] [CrossRef] [PubMed]
- Wiktor, A.; Sarna, M.; Wnuk, D.; Sarna, T. Lipofuscin-mediated photodynamic stress induces adverse changes in nanomechanical properties of retinal pigment epithelium cells. Sci. Rep. 2018, 8, 17929. [Google Scholar] [CrossRef] [PubMed]
- Olchawa, M.M.; Furso, J.A.; Szewczyk, G.M.; Sarna, T.J. Lipofuscin-mediated photic stress inhibits phagocytic activity of ARPE-19 cells; effect of donors’ age and antioxidants. Free Radic. Res. 2017, 51, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Zareba, M.; Skumatz, C.M.; Sarna, T.J.; Burke, J.M. Photic injury to cultured RPE varies among individual cells in proportion to their endogenous lipofuscin content as modulated by their melanosome content. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4982–4990. [Google Scholar] [CrossRef] [PubMed]
- Godley, B.F.; Shamsi, F.A.; Liang, F.Q.; Jarrett, S.G.; Davies, S.; Boulton, M. Blue light induces mitochondrial DNA damage and free radical production in epithelial cells. J. Biol. Chem. 2005, 280, 21061–21066. [Google Scholar] [CrossRef]
- Shamsi, F.A.; Boulton, M. Inhibition of RPE lysosomal and antioxidant activity by the age pigment lipofuscin. Investig. Ophthalmol. Vis. Sci. 2001, 42, 3041–3046. [Google Scholar]
- Sliney, D.H. Exposure geometry and spectral environment determine photobiological effects on the human eye. Photochem. Photobiol. 2005, 81, 483–489. [Google Scholar] [CrossRef]
- Ham, W.T., Jr.; Ruffolo, J.J., Jr.; Mueller, H.A.; Clarke, A.M.; Moon, M.E. Histologic analysis of photochemical lesions produced in rhesus retina by short-wave-length light. Investig. Ophthalmol. Vis. Sci. 1978, 17, 1029–1035. [Google Scholar]
- Friedman, E.; Kuwabara, T. The retinal pigment epithelium. IV. The damaging effects of radiant energy. Arch. Ophthalmol. 1968, 80, 265–279. [Google Scholar] [CrossRef]
- Ham, W.T., Jr.; Mueller, H.A.; Ruffolo, J.J., Jr.; Guerry, D., 3rd; Guerry, R.K. Action spectrum for retinal injury from near-ultraviolet radiation in the aphakic monkey. Am. J. Ophthalmol. 1982, 93, 299–306. [Google Scholar] [CrossRef]
- Rozanowska, M.; Jarvis-Evans, J.; Korytowski, W.; Boulton, M.E.; Burke, J.M.; Sarna, T. Blue light-induced reactivity of retinal age pigment. In vitro generation of oxygen-reactive species. J. Biol. Chem. 1995, 270, 18825–18830. [Google Scholar] [CrossRef] [PubMed]
- Boulton, M.; Dontsov, A.; Jarvis-Evans, J.; Ostrovsky, M.; Svistunenko, D. Lipofuscin is a photoinducible free radical generator. J. Photochem. Photobiol. B 1993, 19, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Rozanowska, M.; Korytowski, W.; Rozanowski, B.; Skumatz, C.; Boulton, M.E.; Burke, J.M.; Sarna, T. Photoreactivity of aged human RPE melanosomes: A comparison with lipofuscin. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2088–2096. [Google Scholar]
- Rozanowska, M.; Wessels, J.; Boulton, M.; Burke, J.M.; Rodgers, M.A.J.; Truscott, T.G.; Sarna, T. Blue light-induced singlet oxygen generation by retinal lipofuscin in non-polar media. Free Radic. Biol. Med. 1998, 24, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, E.R.; Atherton, S.J.; Eldred, G.; Dillon, J. Photophysical studies on human retinal lipofuscin. Photochem. Photobiol. 1995, 61, 448–453. [Google Scholar] [CrossRef]
- Pawlak, A.; Wrona, M.; Rozanowska, M.; Zareba, M.; Lamb, L.E.; Roberts, J.E.; Simon, J.D.; Sarna, T. Comparison of the aerobic photoreactivity of A2E with its precursor retinal. Photochem. Photobiol. 2003, 77, 253–258. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Boulton, M. RPE lipofuscin and its role in retinal pathobiology. Exp. Eye Res. 2005, 80, 595–606. [Google Scholar] [CrossRef]
- Zhou, J.; Jang, Y.P.; Kim, S.R.; Sparrow, J.R. Complement activation by photooxidation products of A2E, a lipofuscin constituent of the retinal pigment epithelium. Proc. Natl. Acad. Sci. USA 2006, 103, 16182–16187. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shabat, S.; Itagaki, Y.; Jockusch, S.; Sparrow, J.R.; Turro, N.J.; Nakanishi, K. Formation of a nonaoxirane from A2E, a lipofuscin fluorophore related to macular degeneration, and evidence of singlet oxygen involvement. Angew. Chem. Int. Ed. Engl. 2002, 41, 814–817. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, J.R.; Vollmer-Snarr, H.R.; Zhou, J.; Jang, Y.P.; Jockusch, S.; Itagaki, Y.; Nakanishi, K. A2E-epoxides damage DNA in retinal pigment epithelial cells. Vitamin E and other antioxidants inhibit A2E-epoxide formation. J. Biol. Chem. 2003, 278, 18207–18213. [Google Scholar] [CrossRef] [PubMed]
- Radu, R.A.; Mata, N.L.; Bagla, A.; Travis, G.H. Light exposure stimulates formation of A2E oxiranes in a mouse model of Stargardt’s macular degeneration. Proc. Natl. Acad. Sci. USA 2004, 101, 5928–5933. [Google Scholar] [CrossRef] [PubMed]
- Yakovleva, M.A.; Sakina, N.L.; Kononikhin, A.S.; Feldman, T.B.; Nikolaev, E.N.; Dontsov, A.E.; Ostrovsky, M.A. Detection and study of the products of photooxidation of N-retinylidene-N-retinylethanolamine (A2E), the fluorophore of lipofuscin granules from retinal pigment epithelium of human donor eyes. Dokl. Biochem. Biophys. 2006, 409, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Keller, L.M.; Dillon, J.; Gaillard, E.R. Oxidation of A2E results in the formation of highly reactive aldehydes and ketones. Photochem. Photobiol. 2006, 82, 1251–1257. [Google Scholar] [CrossRef]
- Gaillard, E.R.; Avalle, L.B.; Keller, L.M.; Wang, Z.; Reszka, K.J.; Dillon, J.P. A mechanistic study of the photooxidation of A2E, a component of human retinal lipofuscin. Exp. Eye Res. 2004, 79, 313–319. [Google Scholar] [CrossRef]
- Dillon, J.; Wang, Z.; Avalle, L.B.; Gaillard, E.R. The photochemical oxidation of A2E results in the formation of a 5,8,5′,8′-bis-furanoid oxide. Exp. Eye Res. 2004, 79, 537–542. [Google Scholar] [CrossRef]
- Ablonczy, Z.; Higbee, D.; Grey, A.C.; Koutalos, Y.; Schey, K.L.; Crouch, R.K. Similar molecules spatially correlate with lipofuscin and N-retinylidene-N-retinylethanolamine in the mouse but not in the human retinal pigment epithelium. Arch. Biochem. Biophys. 2013, 539, 196–202. [Google Scholar] [CrossRef]
- Kim, H.J.; Sparrow, J.R. Bisretinoids: More than Meets the Eye. Adv. Exp. Med. Biol. 2019, 1185, 341–346. [Google Scholar] [CrossRef]
- Ueda, K.; Kim, H.J.; Zhao, J.; Sparrow, J.R. Bisretinoid Photodegradation Is Likely Not a Good Thing. Adv. Exp. Med. Biol. 2018, 1074, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Murdaugh, L.S.; Avalle, L.B.; Mandal, S.; Dill, A.E.; Dillon, J.; Simon, J.D.; Gaillard, E.R. Compositional studies of human RPE lipofuscin. J. Mass. Spectrom. 2010, 45, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Murdaugh, L.S.; Mandal, S.; Dill, A.E.; Dillon, J.; Simon, J.D.; Gaillard, E.R. Compositional studies of human RPE lipofuscin: Mechanisms of molecular modifications. J. Mass. Spectrom. 2011, 46, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Rózanowska, M.B.; Pawlak, A.; Rózanowski, B. Products of docosahexaenoate oxidation as contributors to photosensitising properties of retinal lipofuscin. Int. J. Mol. Sci. 2021, 22, 3525. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Braun, R.D.; Dunn, R., Jr.; Linsenmeier, R.A. Oxygen distribution in the macaque retina. Investig. Ophthalmol. Vis. Sci. 1993, 34, 516–521. [Google Scholar]
- Organisciak, D.T.; Darrow, R.M.; Barsalou, L.; Darrow, R.A.; Kutty, R.K.; Kutty, G.; Wiggert, B. Light history and age-related changes in retinal light damage. Investig. Ophthalmol. Vis. Sci. 1998, 39, 1107–1116. [Google Scholar]
- Maeda, A.; Maeda, T.; Golczak, M.; Imanishi, Y.; Leahy, P.; Kubota, R.; Palczewski, K. Effects of potent inhibitors of the retinoid cycle on visual function and photoreceptor protection from light damage in mice. Mol. Pharmacol. 2006, 70, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Maeda, T.; Imanishi, Y.; Sun, W.; Jastrzebska, B.; Hatala, D.A.; Winkens, H.J.; Hofmann, K.P.; Janssen, J.J.; Baehr, W.; et al. Retinol dehydrogenase (RDH12) protects photoreceptors from light-induced degeneration in mice. J. Biol. Chem. 2006, 281, 37697–37704. [Google Scholar] [CrossRef] [PubMed]
- Shiose, S.; Chen, Y.; Okano, K.; Roy, S.; Kohno, H.; Tang, J.; Pearlman, E.; Maeda, T.; Palczewski, K.; Maeda, A. Toll-like receptor 3 is required for development of retinopathy caused by impaired all-trans-retinal clearance in mice. J. Biol. Chem. 2011, 286, 15543–15555. [Google Scholar] [CrossRef]
- Chen, Y.; Okano, K.; Maeda, T.; Chauhan, V.; Golczak, M.; Maeda, A.; Palczewski, K. Mechanism of all-trans-retinal toxicity with implications for stargardt disease and age-related macular degeneration. J. Biol. Chem. 2012, 287, 5059–5069. [Google Scholar] [CrossRef]
- Okano, K.; Maeda, A.; Chen, Y.; Chauhan, V.; Tang, J.; Palczewska, G.; Sakai, T.; Tsuneoka, H.; Palczewski, K.; Maeda, T. Retinal cone and rod photoreceptor cells exhibit differential susceptibility to light-induced damage. J. Neurochem. 2012, 121, 146–156. [Google Scholar] [CrossRef]
- Maeda, T.; Golczak, M.; Maeda, A. Retinal photodamage mediated by all-trans-retinal. Photochem. Photobiol. 2012, 88, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sawada, O.; Kohno, H.; Le, Y.Z.; Subauste, C.; Maeda, T.; Maeda, A. Autophagy protects the retina from light-induced degeneration. J. Biol. Chem. 2013, 288, 7506–7518. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Palczewska, G.; Mustafi, D.; Golczak, M.; Dong, Z.; Sawada, O.; Maeda, T.; Maeda, A.; Palczewski, K. Systems pharmacology identifies drug targets for Stargardt disease-associated retinal degeneration. J. Clin. Investig. 2013, 123, 5119–5134. [Google Scholar] [CrossRef] [PubMed]
- Kohno, H.; Chen, Y.; Kevany, B.M.; Pearlman, E.; Miyagi, M.; Maeda, T.; Palczewski, K.; Maeda, A. Photoreceptor proteins initiate microglial activation via Toll-like receptor 4 in retinal degeneration mediated by all-trans-retinal. J. Biol. Chem. 2013, 288, 15326–15341. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Palczewska, G.; Golczak, M.; Kohno, H.; Dong, Z.; Maeda, T.; Palczewski, K. Two-photon microscopy reveals early rod photoreceptor cell damage in light-exposed mutant mice. Proc. Natl. Acad. Sci. USA 2014, 111, E1428–E1437. [Google Scholar] [CrossRef] [PubMed]
- Sawada, O.; Perusek, L.; Kohno, H.; Howell, S.J.; Maeda, A.; Matsuyama, S.; Maeda, T. All-trans-retinal induces Bax activation via DNA damage to mediate retinal cell apoptosis. Exp. Eye Res. 2014, 123, 27–36. [Google Scholar] [CrossRef]
- Wu, X.; Yu, G.; Luo, C.; Maeda, A.; Zhang, N.; Sun, D.; Zhou, Z.; Puntel, A.; Palczewski, K.; Lu, Z.R. Synthesis and evaluation of a nanoglobular dendrimer 5-aminosalicylic Acid conjugate with a hydrolyzable schiff base spacer for treating retinal degeneration. ACS Nano 2014, 8, 153–161. [Google Scholar] [CrossRef]
- Yu, G.; Wu, X.; Ayat, N.; Maeda, A.; Gao, S.Q.; Golczak, M.; Palczewski, K.; Lu, Z.R. Multifunctional PEG retinylamine conjugate provides prolonged protection against retinal degeneration in mice. Biomacromolecules 2014, 15, 4570–4578. [Google Scholar] [CrossRef]
- Puntel, A.; Maeda, A.; Golczak, M.; Gao, S.Q.; Yu, G.; Palczewski, K.; Lu, Z.R. Prolonged prevention of retinal degeneration with retinylamine loaded nanoparticles. Biomaterials 2015, 44, 103–110. [Google Scholar] [CrossRef]
- Parmar, T.; Parmar, V.M.; Arai, E.; Sahu, B.; Perusek, L.; Maeda, A. Acute Stress Responses Are Early Molecular Events of Retinal Degeneration in Abca4−/−Rdh8−/− Mice After Light Exposure. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3257–3267. [Google Scholar] [CrossRef] [PubMed]
- Schur, R.M.; Gao, S.; Yu, G.; Chen, Y.; Maeda, A.; Palczewski, K.; Lu, Z.R. New GABA modulators protect photoreceptor cells from light-induced degeneration in mouse models. FASEB J. 2018, 32, 3289–3300. [Google Scholar] [CrossRef] [PubMed]
- Parmar, T.; Parmar, V.M.; Perusek, L.; Georges, A.; Takahashi, M.; Crabb, J.W.; Maeda, A. Lipocalin 2 Plays an Important Role in Regulating Inflammation in Retinal Degeneration. J. Immunol. 2018, 200, 3128–3141. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Tschulakow, A.; Taubitz, T.; Illing, B.; Biesemeier, A.; Julien-Schraermeyer, S.; Radu, R.A.; Jiang, Z.; Schraermeyer, U. Fundus autofluorescence, spectral-domain optical coherence tomography, and histology correlations in a Stargardt disease mouse model. FASEB J. 2020, 34, 3693–3714. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Gao, S.Q.; Dong, Z.; Sheng, L.; Sun, D.; Zhang, N.; Zhang, J.; Margeivicus, S.; Fu, P.; Golczak, M.; et al. Peptide Derivatives of Retinylamine Prevent Retinal Degeneration with Minimal Side Effects on Vision in Mice. Bioconjug. Chem. 2021, 32, 572–583. [Google Scholar] [CrossRef]
- Fang, Y.; Taubitz, T.; Tschulakow, A.V.; Heiduschka, P.; Szewczyk, G.; Burnet, M.; Peters, T.; Biesemeier, A.; Sarna, T.; Schraermeyer, U.; et al. Removal of RPE lipofuscin results in rescue from retinal degeneration in a mouse model of advanced Stargardt disease: Role of reactive oxygen species. Free. Radic. Biol. Med. 2022, 182, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.I.; Hunter, J.J.; Masella, B.; Wolfe, R.; Gray, D.C.; Merigan, W.H.; Delori, F.C.; Williams, D.R. Light-induced retinal changes observed with high-resolution autofluorescence imaging of the retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3715–3729. [Google Scholar] [CrossRef]
- Morgan, J.I.; Hunter, J.J.; Merigan, W.H.; Williams, D.R. The reduction of retinal autofluorescence caused by light exposure. Investig. Ophthalmol. Vis. Sci. 2009, 50, 6015–6022. [Google Scholar] [CrossRef]
- Hunter, J.J.; Morgan, J.I.; Merigan, W.H.; Sliney, D.H.; Sparrow, J.R.; Williams, D.R. The susceptibility of the retina to photochemical damage from visible light. Prog. Retin. Eye Res. 2012, 31, 28–42. [Google Scholar] [CrossRef]
- Zhang, J.; Sabarinathan, R.; Bubel, T.; Williams, D.R.; Hunter, J.J. Action spectrum for photochemical retinal pigment epithelium (RPE) disruption in an in vivo monkey model. Opt. Interact. Tissue Cells XXVII 2016, 9706, 273–278. [Google Scholar] [CrossRef]
- Wu, L.; Ueda, K.; Nagasaki, T.; Sparrow, J.R. Light damage in Abca4 and Rpe65rd12 mice. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1910–1918. [Google Scholar] [CrossRef] [PubMed]
- Teussink, M.M.; Lee, M.D.; Smith, R.T.; van Huet, R.A.; Klaver, C.C.; Klevering, B.J.; Theelen, T.; Hoyng, C.B. The effect of light deprivation in patients with Stargardt disease. Am. J. Ophthalmol. 2015, 159, 964–972.e962. [Google Scholar] [CrossRef] [PubMed]
- Aits, S.; Kricker, J.; Liu, B.; Ellegaard, A.M.; Hamalisto, S.; Tvingsholm, S.; Corcelle-Termeau, E.; Hogh, S.; Farkas, T.; Holm Jonassen, A.; et al. Sensitive detection of lysosomal membrane permeabilization by lysosomal galectin puncta assay. Autophagy 2015, 11, 1408–1424. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Kovalenko, A.; Bogdanov, K.; Wallach, D. MLKL, the Protein that Mediates Necroptosis, also Regulates Endosomal Trafficking and Extracellular Vesicle Generation. Immunity 2017, 47, 51–65.e57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Robinson, K.; Washington, I. C20D3-Vitamin A Prevents Retinal Pigment Epithelium Atrophic Changes in a Mouse Model. Transl. Vis. Sci. Technol. 2021, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Charbel Issa, P.; Barnard, A.R.; Herrmann, P.; Washington, I.; MacLaren, R.E. Rescue of the Stargardt phenotype in Abca4 knockout mice through inhibition of vitamin A dimerization. Proc. Natl. Acad. Sci. USA 2015, 112, 8415–8420. [Google Scholar] [CrossRef] [PubMed]
- Allingham, M.J.; Loksztejn, A.; Cousins, S.W.; Mettu, P.S. Immunological Aspects of Age-Related Macular Degeneration. In Age-Related Macular Degeneration; Chew, E.Y., Swaroop, A., Eds.; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2021; Volume 1256, pp. 143–190. [Google Scholar]
- Anderson, D.H.; Radeke, M.J.; Gallo, N.B.; Chapin, E.A.; Johnson, P.T.; Curletti, C.R.; Hancox, L.S.; Hu, J.; Ebright, J.N.; Malek, G.; et al. The pivotal role of the complement system in aging and age-related macular degeneration: Hypothesis re-visited. Prog. Retin. Eye Res. 2010, 29, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Scholl, H.P.; DeBartolomeo, G.; Washington, I.; Saad, L. ALK-001 (C20-D3-Vitamin A) slows the growth of atrophic lesions in ABCA4-related Stargardt Disease: Results of a Phase 2 placebo-controlled clinical trial (TEASE study). Investig. Ophthalmol. Vis. Sci. 2022, 63, 38. [Google Scholar]
- Zhou, H.; Zhang, H.; Yu, A.; Xie, J. Association between sunlight exposure and risk of age-related macular degeneration: A meta-analysis. BMC Ophthalmol. 2018, 18, 331. [Google Scholar] [CrossRef] [PubMed]
- Schick, T.; Ersoy, L.; Lechanteur, Y.T.; Saksens, N.T.; Hoyng, C.B.; den Hollander, A.I.; Kirchhof, B.; Fauser, S. History of Sunlight Exposure Is a Risk Factor for Age-Related Macular Degeneration. Retina 2016, 36, 787–790. [Google Scholar] [CrossRef]
- Huang, E.J.; Wu, S.H.; Lai, C.H.; Kuo, C.N.; Wu, P.L.; Chen, C.L.; Chen, C.Y.; King, Y.C.; Wu, P.C. Prevalence and risk factors for age-related macular degeneration in the elderly Chinese population in south-western Taiwan: The Puzih eye study. Eye 2014, 28, 705–714. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, J.H.; Woo, S.J.; Ahn, J.; Shin, J.P.; Song, S.J.; Kang, S.W.; Park, K.H.; Epidemiologic Survey Committee of the Korean Ophthalmologic Society. Age-related macular degeneration: Prevalence and risk factors from Korean National Health and Nutrition Examination Survey, 2008 through 2011. Ophthalmology 2014, 121, 1756–1765. [Google Scholar] [CrossRef] [PubMed]
- Ristau, T.; Ersoy, L.; Hahn, M.; den Hollander, A.I.; Kirchhof, B.; Liakopoulos, S.; Fauser, S. Nongenetic risk factors for neovascular age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5228–5232. [Google Scholar] [CrossRef] [PubMed]
- Nano, M.E.; Lansingh, V.C.; Pighin, M.S.; Zarate, N.; Nano, H.; Carter, M.J.; Furtado, J.M.; Nano, C.C.; Vernengo, L.F.; Luna, J.D.; et al. Risk factors of age-related macular degeneration in Argentina. Arq. Bras. Oftalmol. 2013, 76, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.L.; Lee, E.T.; Klein, R.; Russell, D.; Ogola, G.; Warn, A.; Kingsley, R.M.; Yeh, J. Prevalence and risks factors of age-related macular degeneration in Oklahoma Indians: The Vision Keepers Study. Ophthalmology 2011, 118, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, A.E.; Bentham, G.C.; Agnew, M.; Young, I.S.; Augood, C.; Chakravarthy, U.; de Jong, P.T.; Rahu, M.; Seland, J.; Soubrane, G.; et al. Sunlight exposure, antioxidants, and age-related macular degeneration. Arch. Ophthalmol. 2008, 126, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.R.; West, S.; Munoz, B.; Rosenthal, F.S.; Bressler, S.B.; Bressler, N.M. The long-term effects of visible light on the eye. Arch. Ophthalmol. 1992, 110, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.R.; Munoz, B.; West, S.; Bressler, N.M.; Bressler, S.B.; Rosenthal, F.S. Visible light and risk of age-related macular degeneration. Trans. Am. Ophthalmol. Soc. 1990, 88, 163–173; discussion 173–168. [Google Scholar] [PubMed]
- Lazreg, S.; Delcourt, C.; Zeggane, S.; Sanchez, A.; Ziani, A.; Daghbouche, M.; Benmoussa, S.; Mokrani, K.; Billah Mekki, M.; Renault, D.; et al. Age-Related Macular Degeneration and Its Risk Factors in North Africans Living in Algeria and Italy. Ophthalmic Res. 2016, 56, 145–154. [Google Scholar] [CrossRef]
- Bai, Z.L.; Ren, B.-C.; Yagi, J.-G.; He, Y.; Chen, L.; Sun, N.-X. Epidemiological investigation on age related macular degeneration in rural area of Shaanxi Province, China. Int. J. Ophthalmol. 2008, 1, 77–84. [Google Scholar]
- Khan, J.C.; Shahid, H.; Thurlby, D.A.; Bradley, M.; Clayton, D.G.; Moore, A.T.; Bird, A.C.; Yates, J.R. Age related macular degeneration and sun exposure, iris colour, and skin sensitivity to sunlight. Br. J. Ophthalmol. 2006, 90, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Tomany, S.C.; Cruickshanks, K.J.; Klein, R.; Klein, B.E.; Knudtson, M.D. Sunlight and the 10-year incidence of age-related maculopathy: The Beaver Dam Eye Study. Arch. Ophthalmol. 2004, 122, 750–757. [Google Scholar] [CrossRef]
- Delcourt, C.; Carriere, I.; Ponton-Sanchez, A.; Fourrey, S.; Lacroux, A.; Papoz, L.; Group, P.S. Light exposure and the risk of age-related macular degeneration: The Pathologies Oculaires Liees a l’Age (POLA) study. Arch. Ophthalmol. 2001, 119, 1463–1468. [Google Scholar] [CrossRef]
- Group, T.E.D.C.-C.S. Risk factors for neovascular age-related macular degeneration. The Eye Disease Case-Control Study Group. Arch. Ophthalmol. 1992, 110, 1701–1708. [Google Scholar] [CrossRef]
- Sui, G.Y.; Liu, G.C.; Liu, G.Y.; Gao, Y.Y.; Deng, Y.; Wang, W.Y.; Tong, S.H.; Wang, L. Is sunlight exposure a risk factor for age-related macular degeneration? A systematic review and meta-analysis. Br. J. Ophthalmol. 2013, 97, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, U.J.; Lee, Y.; Han, E.; Ham, S.; Lee, W.; Choi, W.J.; Kang, S.K. Sunlight exposure and eye disorders in an economically active population: Data from the KNHANES 2008–2012. Ann. Occup. Environ. Med. 2021, 33, e24. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, M.; Tanaka, M.; Tanaka, Y.; Okubo, A.; Koriyama, C.; Tsuji, M.; Akiba, S.; Miyamoto, K.; Hillebrand, G.; Yamashita, T.; et al. Age-related maculopathy and sunlight exposure evaluated by objective measurement. Br. J. Ophthalmol. 2008, 92, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Vojnikovic, B.; Njiric, S.; Coklo, M.; Spanjol, J. Ultraviolet sun radiation and incidence of age-related macular degeneration on Croatian Island Rab. Coll. Antropol. 2007, 31 (Suppl. S1), 43–44. [Google Scholar] [PubMed]
- Plestina-Borjan, I.; Klinger-Lasic, M. Long-term exposure to solar ultraviolet radiation as a risk factor for age-related macular degeneration. Coll. Antropol. 2007, 31 (Suppl. S1), 33–38. [Google Scholar] [PubMed]
- Cruickshanks, K.J.; Klein, R.; Klein, B.E.; Nondahl, D.M. Sunlight and the 5-year incidence of early age-related maculopathy: The beaver dam eye study. Arch. Ophthalmol. 2001, 119, 246–250. [Google Scholar] [PubMed]
- Delcourt, C.; Cougnard-Gregoire, A.; Boniol, M.; Carriere, I.; Dore, J.F.; Delyfer, M.N.; Rougier, M.B.; Le Goff, M.; Dartigues, J.F.; Barberger-Gateau, P.; et al. Lifetime exposure to ambient ultraviolet radiation and the risk for cataract extraction and age-related macular degeneration: The Alienor Study. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7619–7627. [Google Scholar] [CrossRef] [PubMed]
- Morrison, M.A.; Silveira, A.C.; Huynh, N.; Jun, G.; Smith, S.E.; Zacharaki, F.; Sato, H.; Loomis, S.; Andreoli, M.T.; Adams, S.M.; et al. Systems biology-based analysis implicates a novel role for vitamin D metabolism in the pathogenesis of age-related macular degeneration. Hum. Genomics 2011, 5, 538–568. [Google Scholar] [CrossRef]
- Millen, A.E.; Voland, R.; Sondel, S.A.; Parekh, N.; Horst, R.L.; Wallace, R.B.; Hageman, G.S.; Chappell, R.; Blodi, B.A.; Klein, M.L.; et al. Vitamin D status and early age-related macular degeneration in postmenopausal women. Arch. Ophthalmol. 2011, 129, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Darzins, P.; Mitchell, P.; Heller, R.F. Sun exposure and age-related macular degeneration. An Australian case-control study. Ophthalmology 1997, 104, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Organisciak, D.T.; Jiang, Y.L.; Wang, H.M.; Pickford, M.; Blanks, J.C. Retinal light damage in rats exposed to intermittent light. Comparison with continuous light exposure. Investig. Ophthalmol. Vis. Sci. 1989, 30, 795–805. [Google Scholar]
- Organisciak, D.T.; Xie, A.; Wang, H.M.; Jiang, Y.L.; Darrow, R.M.; Donoso, L.A. Adaptive changes in visual cell transduction protein levels: Effect of light. Exp. Eye Res. 1991, 53, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Cao, W.; Anderson, R.E. Protection of photoreceptor cells in adult rats from light-induced degeneration by adaptation to bright cyclic light. Exp. Eye Res. 2001, 73, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Cao, W.; Anderson, R.E. Alleviation of constant-light-induced photoreceptor degeneration by adaptation of adult albino rat to bright cyclic light. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4968–4975. [Google Scholar] [CrossRef]
- Organisciak, D.T.; Noell, W.K. The rod outer segment phospholipid/opsin ratio of rats maintained in darkness or cyclic light. Investig. Ophthalmol. Vis. Sci. 1977, 16, 188–190. [Google Scholar]
- Penn, J.S.; Williams, T.P. Photostasis: Regulation of daily photon-catch by rat retinas in response to various cyclic illuminances. Exp. Eye Res. 1986, 43, 915–928. [Google Scholar] [CrossRef]
- Penn, J.S.; Anderson, R.E. Effect of light history on rod outer-segment membrane composition in the rat. Exp. Eye Res. 1987, 44, 767–778. [Google Scholar] [CrossRef]
- Schremser, J.L.; Williams, T.P. Rod outer segment (ROS) renewal as a mechanism for adaptation to a new intensity environment. II. Rhodopsin synthesis and packing density. Exp. Eye Res. 1995, 61, 25–32. [Google Scholar] [CrossRef]
- Schremser, J.L.; Williams, T.P. Rod outer segment (ROS) renewal as a mechanism for adaptation to a new intensity environment. I. Rhodopsin levels and ROS length. Exp. Eye Res. 1995, 61, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Rakshit, T.; Senapati, S.; Parmar, V.M.; Sahu, B.; Maeda, A.; Park, P.S. Adaptations in rod outer segment disc membranes in response to environmental lighting conditions. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1691–1702. [Google Scholar] [CrossRef] [PubMed]
- Dillon, J.; Skonieczna, M.; Mandal, K.; Paik, D. The photochemical attachment of the O-glucoside of 3-hydroxykynurenine to alpha-crystallin: A model for lenticular aging. Photochem. Photobiol. 1999, 69, 248–253. [Google Scholar] [PubMed]
- Ellozy, A.R.; Wang, R.H.; Dillon, J. Model studies on the photochemical production of lenticular fluorophores. Photochem. Photobiol. 1994, 59, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Dillon, J. The photophysics and photobiology of the eye. J. Photochem. Photobiol. B 1991, 10, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Dillon, J.; Wang, R.H.; Atherton, S.J. Photochemical and photophysical studies on human lens constituents. Photochem. Photobiol. 1990, 52, 849–854. [Google Scholar] [CrossRef]
- Reins, R.Y.; McDermott, A.M. Vitamin D: Implications for ocular disease and therapeutic potential. Exp. Eye Res. 2015, 134, 101–110. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Pawlowska, E.; Szczepanska, J.; Jablkowska, A.; Blasiak, J. Can vitamin D protect against age-related macular degeneration or slow its progression? Acta Biochim. Pol. 2019, 66, 147–158. [Google Scholar] [CrossRef]
- Parekh, N.; Chappell, R.J.; Millen, A.E.; Albert, D.M.; Mares, J.A. Association between vitamin D and age-related macular degeneration in the Third National Health and Nutrition Examination Survey, 1988 through 1994. Arch. Ophthalmol. 2007, 125, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Seddon, J.M.; Reynolds, R.; Shah, H.R.; Rosner, B. Smoking, dietary betaine, methionine, and vitamin D in monozygotic twins with discordant macular degeneration: Epigenetic implications. Ophthalmology 2011, 118, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- McKay, G.J.; Young, I.S.; McGinty, A.; Bentham, G.C.; Chakravarthy, U.; Rahu, M.; Seland, J.; Soubrane, G.; Tomazzoli, L.; Topouzis, F.; et al. Associations between Serum Vitamin D and Genetic Variants in Vitamin D Pathways and Age-Related Macular Degeneration in the European Eye Study. Ophthalmology 2017, 124, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Merle, B.M.J.; Silver, R.E.; Rosner, B.; Seddon, J.M. Associations between Vitamin D Intake and Progression to Incident Advanced Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4569–4578. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.L.; Park, S.P. Association between serum vitamin D deficiency and age-related macular degeneration in Koreans: Clinical case-control pilot study. Medicine 2018, 97, e11908. [Google Scholar] [CrossRef] [PubMed]
- Kan, E.; Kan, E.K.; Yucel, O.E. The Possible Link Between Vitamin D Levels and Exudative Age-related Macular Degeneration. Oman Med. J. 2020, 35, e83. [Google Scholar] [CrossRef] [PubMed]
- Perez Serena, A.; Martinez Betancourt, D.P.; Gonzalez Del Valle, F.; Ruiz-Moreno, J.M. Serum 25-hydroxy vitamin D levels in age-related macular degeneration. Int. J. Retina Vitr. 2022, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Silva, N.; Furtado, M.J.; Carneiro, A.; Lume, M.; Andrade, J.P. Serum vitamin D and age-related macular degeneration: Systematic review and meta-analysis. Surv. Ophthalmol. 2021, 66, 183–197. [Google Scholar] [CrossRef]
- Lee, J.S.; Li, P.R.; Hou, C.H.; Lin, K.K.; Kuo, C.F.; See, L.C. Effect of Blue Light-Filtering Intraocular Lenses on Age-Related Macular Degeneration: A Nationwide Cohort Study with 10-Year Follow-up. Am. J. Ophthalmol. 2022, 234, 138–146. [Google Scholar] [CrossRef]
- Achiron, A.; Elbaz, U.; Hecht, I.; Spierer, O.; Einan-Lifshitz, A.; Karesvuo, P.; Laine, I.; Tuuminen, R. The Effect of Blue-Light Filtering Intraocular Lenses on the Development and Progression of Neovascular Age-Related Macular Degeneration. Ophthalmology 2021, 128, 410–416. [Google Scholar] [CrossRef]
- Hamel, T.; Rheault, J.; Simonyan, D.; Bourgault, S.; Rochette, P.J. The Influence of Blue-Filtering Intraocular Lenses Implant on Exudative Age-Related Macular Degeneration: A Case-Control Study. Clin. Ophthalmol. 2021, 15, 2287–2292. [Google Scholar] [CrossRef] [PubMed]
- Pipis, A.; Touliou, E.; Pillunat, L.E.; Augustin, A.J. Effect of the blue filter intraocular lens on the progression of geographic atrophy. Eur. J. Ophthalmol. 2015, 25, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Hirano, Y.; Yasukawa, T.; Morita, H.; Nozaki, M.; Wolf-Schnurrbusch, U.; Wolf, S.; Ogura, Y. Prevention of increased abnormal fundus autofluorescence with blue light-filtering intraocular lenses. J. Cataract Refract. Surg. 2015, 41, 1855–1859. [Google Scholar] [CrossRef] [PubMed]
- Altay, L.; Liakopoulos, S.; Berghold, A.; Rosenberger, K.D.; Ernst, A.; de Breuk, A.; den Hollander, A.I.; Fauser, S.; Schick, T. Genetic and environmental risk factors for reticular pseudodrusen in the EUGENDA study. Mol. Vis. 2021, 27, 757–767. [Google Scholar] [PubMed]
- Keenan, T.D.L.; Cukras, C.A.; Chew, E.Y. Age-Related Macular Degeneration: Epidemiology and Clinical Aspects. In Age-Related Macular Degeneration, Advances in Experimental Medicine and Biology; Chew, E.Y., Swaroop, A., Eds.; Advances in Experimental Medicine and Biology; Springer Nature Switzerland AG: Cham, Switzerland, 2021; Volume 1256, pp. 1–31. [Google Scholar]
- Foster, R.G.; Hughes, S.; Peirson, S.N. Circadian Photoentrainment in Mice and Humans. Biology 2020, 9, 180. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Merrow, M. The Circadian Clock and Human Health. Curr. Biol. 2016, 26, R432–R443. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, G.W.; Rylander, H.G. Photocoagulation of the fovea. Trans. Am. Ophthalmol. Soc. 1978, 76, 278–295. [Google Scholar]
- Pflibsen, K.P.; Pomerantzeff, O.; Ross, R.N. Retinal illuminance using a wide-angle model of the eye. J. Opt. Soc. Am. A 1988, 5, 146–150. [Google Scholar] [CrossRef]
- Marshall, J.; Grindle, J.; Ansell, P.L.; Borwein, B. Convolution in human rods: An ageing process. Br. J. Ophthalmol. 1979, 63, 181–187. [Google Scholar] [CrossRef]
- Rozanowska, M. Adaptation of Rod Photoreceptors to Light and Dark. In Photobiological Sciences; Smith, K.C., Ed.; American Society for Photobiology: Herndon, VA, USA, 2014; Available online: http://photobiology.info/Rozanowska2.html (accessed on 8 August 2023).
- Owsley, C. Vision and Aging. Annu. Rev. Vis. Sci. 2016, 2, 255–271. [Google Scholar] [CrossRef]
- Owsley, C. Aging and vision. Vision Res. 2011, 51, 1610–1622. [Google Scholar] [CrossRef] [PubMed]
- Haralampus-Grynaviski, N.M.; Lamb, L.E.; Simon, J.D.; Krogmeier, J.R.; Dunn, R.C.; Pawlak, A.; Rozanowska, M.; Sarna, T.; Burke, J.M. Probing the spatial dependence of the emission spectrum of single human retinal lipofuscin granules using near-field scanning optical microscopy. Photochem. Photobiol. 2001, 74, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, J.R.; Gregory-Roberts, E.; Yamamoto, K.; Blonska, A.; Ghosh, S.K.; Ueda, K.; Zhou, J. The bisretinoids of retinal pigment epithelium. Prog. Retin. Eye Res. 2012, 31, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Eldred, G.E.; Lasky, M.R. Retinal age pigments generated by self-assembling lysosomotropic detergents. Nature 1993, 361, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Ragauskaite, L.; Heckathorn, R.C.; Gaillard, E.R. Environmental effects on the photochemistry of A2-E, a component of human retinal lipofuscin. Photochem. Photobiol. 2001, 74, 483–488. [Google Scholar] [CrossRef]
- Lamb, L.E.; Ye, T.; Haralampus-Grynaviski, N.M.; Williams, T.R.; Pawlak, A.; Sarna, T.; Simon, J.D. Primary photophysical properties of A2E in solution. J. Phys. Chem. B 2001, 105, 11507–11512. [Google Scholar] [CrossRef]
- Reszka, K.; Eldred, G.E.; Wang, R.H.; Chignell, C.; Dillon, J. The photochemistry of human retinal lipofuscin as studied by EPR. Photochem. Photobiol. 1995, 62, 1005–1008. [Google Scholar] [CrossRef]
- Kopitz, J.; Holz, F.G.; Kaemmerer, E.; Schutt, F. Lipids and lipid peroxidation products in the pathogenesis of age-related macular degeneration. Biochimie 2004, 86, 825–831. [Google Scholar] [CrossRef]
- Dontsov, A.E.; Sakina, N.L.; Golubkov, A.M.; Ostrovsky, M.A. Light-induced release of A2E photooxidation toxic products from lipofuscin granules of human retinal pigment epithelium. Dokl. Biochem. Biophys. 2009, 425, 98–101. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Szmacinski, H.; Nowaczyk, K.; Johnson, M.L. Fluorescence lifetime imaging of free and protein-bound NADH. Proc. Natl. Acad. Sci. USA 1992, 89, 1271–1275. [Google Scholar] [CrossRef]
- Croce, A.C.; Bottiroli, G. Autofluorescence spectroscopy and imaging: A tool for biomedical research and diagnosis. Eur. J. Histochem. 2014, 58, 320–337. [Google Scholar] [CrossRef] [PubMed]
- Croce, A.C.; Ferrigno, A.; Bottiroli, G.; Vairetti, M. Autofluorescence-based optical biopsy: An effective diagnostic tool in hepatology. Liver Int. 2018, 38, 1160–1174. [Google Scholar] [CrossRef] [PubMed]
- Layer, G.; Reichelt, J.; Jahn, D.; Heinz, D.W. Structure and function of enzymes in heme biosynthesis. Protein Sci. 2010, 19, 1137–1161. [Google Scholar] [CrossRef] [PubMed]
- Palczewska, G.; Wojtkowski, M.; Palczewski, K. From mouse to human: Accessing the biochemistry of vision in vivo by two-photon excitation. Prog. Retin. Eye Res. 2023, 93, 101170. [Google Scholar] [CrossRef] [PubMed]
- Palczewska, G.; Maeda, T.; Imanishi, Y.; Sun, W.; Chen, Y.; Williams, D.R.; Piston, D.W.; Maeda, A.; Palczewski, K. Noninvasive multiphoton fluorescence microscopy resolves retinol and retinal condensation products in mouse eyes. Nat. Med. 2010, 16, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Szweda, L.I. Age-related increase in liver retinyl palmitate. Relationship to lipofuscin. J. Biol. Chem. 1994, 269, 8712–8715. [Google Scholar] [CrossRef] [PubMed]
- Docchio, F.; Boulton, M.; Cubeddu, R.; Ramponi, R.; Barker, P.D. Age-related changes in the fluorescence of melanin and lipofuscin granules of the retinal pigment epithelium: A time-resolved fluorescence spectroscopy study. Photochem. Photobiol. 1991, 54, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Pollreisz, A.; Messinger, J.D.; Sloan, K.R.; Mittermueller, T.J.; Weinhandl, A.S.; Benson, E.K.; Kidd, G.J.; Schmidt-Erfurth, U.; Curcio, C.A. Visualizing melanosomes, lipofuscin, and melanolipofuscin in human retinal pigment epithelium using serial block face scanning electron microscopy. Exp. Eye Res. 2018, 166, 131–139. [Google Scholar] [CrossRef]
- Bermond, K.; Wobbe, C.; Tarau, I.S.; Heintzmann, R.; Hillenkamp, J.; Curcio, C.A.; Sloan, K.R.; Ach, T. Autofluorescent Granules of the Human Retinal Pigment Epithelium: Phenotypes, Intracellular Distribution, and Age-Related Topography. Investig. Ophthalmol. Vis. Sci. 2020, 61, 35. [Google Scholar] [CrossRef] [PubMed]
- Taubitz, T.; Fang, Y.; Biesemeier, A.; Julien-Schraermeyer, S.; Schraermeyer, U. Age, lipofuscin and melanin oxidation affect fundus near-infrared autofluorescence. EBioMedicine 2019, 48, 592–604. [Google Scholar] [CrossRef]
- Kayatz, P.; Thumann, G.; Luther, T.T.; Jordan, J.F.; Bartz-Schmidt, K.U.; Esser, P.J.; Schraermeyer, U. Oxidation causes melanin fluorescence. Investig. Ophthalmol. Vis. Sci. 2001, 42, 241–246. [Google Scholar]
- Katz, M.L.; Eldred, G.E.; Robison, W.G., Jr. Lipofuscin autofluorescence: Evidence for vitamin A involvement in the retina. Mech. Ageing Dev. 1987, 39, 81–90. [Google Scholar] [CrossRef] [PubMed]
- D’Cruz, P.M.; Yasumura, D.; Weir, J.; Matthes, M.T.; Abderrahim, H.; LaVail, M.M.; Vollrath, D. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum. Mol. Genet. 2000, 9, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Hamm, G.; Maglennon, G.; Williamson, B.; Macdonald, R.; Doherty, A.; Jones, S.; Harris, J.; Blades, J.; Harmer, A.R.; Barton, P.; et al. Pharmacological inhibition of MERTK induces in vivo retinal degeneration: A multimodal imaging ocular safety assessment. Arch. Toxicol. 2022, 96, 613–624. [Google Scholar] [CrossRef]
- Zihni, C.; Georgiadis, A.; Ramsden, C.M.; Sanchez-Heras, E.; Haas, A.J.; Nommiste, B.; Semenyuk, O.; Bainbridge, J.W.B.; Coffey, P.J.; Smith, A.J.; et al. Spatiotemporal control of actomyosin contractility by MRCKbeta signaling drives phagocytosis. J. Cell Biol. 2022, 221, e202012042. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Jia, J.; Chen, Y.; Liu, R.; Cao, R.; Duan, M.; Zhang, M.; Xu, Y. Pentoxifylline alleviates ischemic white matter injury through up-regulating Mertk-mediated myelin clearance. J. Neuroinflamm. 2022, 19, 128. [Google Scholar] [CrossRef] [PubMed]
- Audo, I.; Mohand-Said, S.; Boulanger-Scemama, E.; Zanlonghi, X.; Condroyer, C.; Demontant, V.; Boyard, F.; Antonio, A.; Mejecase, C.; El Shamieh, S.; et al. MERTK mutation update in inherited retinal diseases. Hum. Mutat. 2018, 39, 887–913. [Google Scholar] [CrossRef] [PubMed]
- Kaga, T.; Fonseca, R.A.; Dantas, M.A.; Yannuzzi, L.A.; Spaide, R.F. Optical coherence tomography of bleb-like subretinal lesions after retinal reattachment surgery. Am. J. Ophthalmol. 2001, 132, 120–121. [Google Scholar] [CrossRef]
- Spaide, R.F.; Klancnik, J.M., Jr. Fundus autofluorescence and central serous chorioretinopathy. Ophthalmology 2005, 112, 825–833. [Google Scholar] [CrossRef]
- Spaide, R.F.; Noble, K.; Morgan, A.; Freund, K.B. Vitelliform macular dystrophy. Ophthalmology 2006, 113, 1392–1400. [Google Scholar] [CrossRef]
- Laud, K.; Visaetsilpanonta, S.; Yannuzzi, L.A.; Spaide, R.F. Autofluorescence imaging of optic pit maculopathy. Retina 2007, 27, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Fathi, P.; Roslend, A.; Mehta, K.; Moitra, P.; Zhang, K.; Pan, D. UV-trained and metal-enhanced fluorescence of biliverdin and biliverdin nanoparticles. Nanoscale 2021, 13, 4785–4798. [Google Scholar] [CrossRef] [PubMed]
- Sawa, M.; Ober, M.D.; Spaide, R.F. Autofluorescence and retinal pigment epithelial atrophy after subretinal hemorrhage. Retina 2006, 26, 119–120. [Google Scholar] [CrossRef]
- Piccolino, F.C.; Borgia, L.; Zinicola, E.; Iester, M.; Torrielli, S. Pre-injection fluorescence in indocyanine green angiography. Ophthalmology 1996, 103, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Glushko, V.; Thaler, M.; Ros, M. The fluorescence of bilirubin upon interaction with human erythrocyte ghosts. Biochim. Biophys. Acta 1982, 719, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Marmorstein, A.D.; Marmorstein, L.Y.; Sakaguchi, H.; Hollyfield, J.G. Spectral profiling of autofluorescence associated with lipofuscin, Bruch’s Membrane, and sub-RPE deposits in normal and AMD eyes. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2435–2441. [Google Scholar]
- Hammer, M.; Konigsdorffer, E.; Liebermann, C.; Framme, C.; Schuch, G.; Schweitzer, D.; Strobel, J. Ocular fundus auto-fluorescence observations at different wavelengths in patients with age-related macular degeneration and diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 246, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Schultz, R.; Gamage, K.; Messinger, J.D.; Curcio, C.A.; Hammer, M. Fluorescence Lifetimes and Spectra of RPE and Sub-RPE Deposits in Histology of Control and AMD Eyes. Investig. Ophthalmol. Vis. Sci. 2020, 61, 9. [Google Scholar] [CrossRef] [PubMed]
- Schmitz-Valckenberg, S.; Pfau, M.; Fleckenstein, M.; Staurenghi, G.; Sparrow, J.R.; Bindewald-Wittich, A.; Spaide, R.F.; Wolf, S.; Sadda, S.R.; Holz, F.G. Fundus autofluorescence imaging. Prog. Retin. Eye Res. 2021, 81, 100893. [Google Scholar] [CrossRef]
- Delori, F.C.; Dorey, C.K.; Staurenghi, G.; Arend, O.; Goger, D.G.; Weiter, J.J. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Investig. Ophthalmol. Vis. Sci. 1995, 36, 718–729. [Google Scholar]
- Sarna, T.; Burke, J.M.; Korytowski, W.; Rozanowska, M.; Skumatz, C.M.; Zareba, A.; Zareba, M. Loss of melanin from human RPE with aging: Possible role of melanin photooxidation. Exp. Eye Res. 2003, 76, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.T.; Post, R.; Johri, A.; Lee, M.D.; Ablonczy, Z.; Curcio, C.A.; Ach, T.; Sajda, P. Simultaneous decomposition of multiple hyperspectral data sets: Signal recovery of unknown fluorophores in the retinal pigment epithelium. Biomed. Opt. Express 2014, 5, 4171–4185. [Google Scholar] [CrossRef] [PubMed]
- Bermond, K.; Berlin, A.; Tarau, I.S.; Wobbe, C.; Heintzmann, R.; Curcio, C.A.; Sloan, K.R.; Ach, T. Characteristics of normal human retinal pigment epithelium cells with extremes of autofluorescence or intracellular granule count. Ann. Eye Sci. 2021, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Webb, R.H.; Hughes, G.W.; Delori, F.C. Confocal scanning laser ophthalmoscope. Appl. Opt. 1987, 26, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- von Ruckmann, A.; Fitzke, F.W.; Bird, A.C. Distribution of fundus autofluorescence with a scanning laser ophthalmoscope. Br. J. Ophthalmol. 1995, 79, 407–412. [Google Scholar] [CrossRef] [PubMed]
- von Ruckmann, A.; Fitzke, F.W.; Bird, A.C. Fundus autofluorescence in age-related macular disease imaged with a laser scanning ophthalmoscope. Investig. Ophthalmol. Vis. Sci. 1997, 38, 478–486. [Google Scholar]
- von Ruckmann, A.; Fitzke, F.W.; Bird, A.C. Distribution of pigment epithelium autofluorescence in retinal disease state recorded in vivo and its change over time. Graefe’s Arch. Clin. Exp. Ophthalmol. 1999, 237, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Reiter, G.S.; Told, R.; Baratsits, M.; Hecht, A.; Schlanitz, F.G.; Sacu, S.; Schmidt-Erfurth, U. Repeatability and reliability of quantitative fundus autofluorescence imaging in patients with early and intermediate age-related macular degeneration. Acta Ophthalmol. 2019, 97, E526–E532. [Google Scholar] [CrossRef]
- Fischer, J.; Otto, T.; Delori, F.; Pace, L.; Staurenghi, G. Scanning Laser Ophthalmoscopy (SLO). In High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics; Bille, J.F., Ed.; Springer: Cham, Switzerland, 2019; pp. 35–57. [Google Scholar]
- Orellana-Rios, J.; Yokoyama, S.; Bhuiyan, A.; Gao, L.; Otero-Marquez, O.; Smith, R.T. Translational Retinal Imaging. Asia Pac. J. Ophthalmol. 2020, 9, 269–277. [Google Scholar] [CrossRef]
- Borrelli, E.; Battista, M.; Zuccaro, B.; Sacconi, R.; Brambati, M.; Querques, L.; Prascina, F.; Sadda, S.R.; Bandello, F.; Querques, G. Spectrally Resolved Fundus Autofluorescence in Healthy Eyes: Repeatability and Topographical Analysis of the Green-Emitting Fluorophores. J. Clin. Med. 2020, 9, 2388. [Google Scholar] [CrossRef]
- Borrelli, E.; Lei, J.; Balasubramanian, S.; Uji, A.; Cozzi, M.; Sarao, V.; Lanzetta, P.; Staurenghi, G.; Sadda, S.R. Green emission fluorophores in eyes with atrophic age-related macular degeneration: A colour fundus autofluorescence pilot study. Br. J. Ophthalmol. 2018, 102, 827–832. [Google Scholar] [CrossRef]
- Dysli, C.; Muller, P.L.; Birtel, J.; Holz, F.G.; Herrmann, P. Spectrally Resolved Fundus Autofluorescence in ABCA4-Related Retinopathy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 274–281. [Google Scholar] [CrossRef]
- Muller, P.L.; Dysli, C.; Hess, K.; Holz, F.G.; Herrmann, P. Spectral Fundus Autofluorescence Excitation and Emission in Abca4-Related Retinopathy. Retina 2020, 40, 2332–2342. [Google Scholar] [CrossRef]
- Gao, L.; Smith, R.T.; Tkaczyk, T.S. Snapshot hyperspectral retinal camera with the Image Mapping Spectrometer (IMS). Biomed. Opt. Express 2012, 3, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Boguslawski, J.; Palczewska, G.; Tomczewski, S.; Milkiewicz, J.; Kasprzycki, P.; Stachowiak, D.; Komar, K.; Marzejon, M.J.; Sikorski, B.L.; Hudzikowski, A.; et al. In vivo imaging of the human eye using a 2-photon-excited fluorescence scanning laser ophthalmoscope. J. Clin. Investig. 2022, 132, e154218. [Google Scholar] [CrossRef]
- Tong, Y.; Ach, T.; Curcio, C.A.; Smith, R.T. Hyperspectral autofluorescence characterization of drusen and sub-RPE deposits in age-related macular degeneration. Ann. Eye Sci. 2021, 6, 4. [Google Scholar] [CrossRef]
- Granger, C.E.; Yang, Q.; Song, H.; Saito, K.; Nozato, K.; Latchney, L.R.; Leonard, B.T.; Chung, M.M.; Williams, D.R.; Rossi, E.A. Human Retinal Pigment Epithelium: In Vivo Cell Morphometry, Multispectral Autofluorescence, and Relationship to Cone Mosaic. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5705–5716. [Google Scholar] [CrossRef] [PubMed]
- Schultz, R.; Hasan, S.; Curcio, C.A.; Smith, R.T.; Meller, D.; Hammer, M. Spectral and lifetime resolution of fundus autofluorescence in advanced age-related macular degeneration revealing different signal sources. Acta Ophthalmol. 2022, 100, e841–e846. [Google Scholar] [CrossRef] [PubMed]
- Semenov, A.N.; Maksimov, E.G.; Moysenovich, A.M.; Yakovleva, M.A.; Tsoraev, G.V.; Ramonova, A.A.; Shirshin, E.A.; Sluchanko, N.N.; Feldman, T.B.; Rubin, A.B.; et al. Protein-Mediated Carotenoid Delivery Suppresses the Photoinducible Oxidation of Lipofuscin in Retinal Pigment Epithelial Cells. Antioxidants 2023, 12, 413. [Google Scholar] [CrossRef] [PubMed]
- Dysli, C.; Wolf, S.; Berezin, M.Y.; Sauer, L.; Hammer, M.; Zinkernagel, M.S. Fluorescence lifetime imaging ophthalmoscopy. Prog. Retin. Eye Res. 2017, 60, 120–143. [Google Scholar] [CrossRef] [PubMed]
- Palczewska, G.; Boguslawski, J.; Stremplewski, P.; Kornaszewski, L.; Zhang, J.; Dong, Z.; Liang, X.X.; Gratton, E.; Vogel, A.; Wojtkowski, M.; et al. Noninvasive two-photon optical biopsy of retinal fluorophores. Proc. Natl. Acad. Sci. USA 2020, 117, 22532–22543. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.; Brauer, J.L.; Meller, D.; Hammer, M. Impact of cataract on the spectral measurement of fundus autofluorescence. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 2057–2059. [Google Scholar] [CrossRef] [PubMed]
- Berlin, A.; Clark, M.E.; Swain, T.A.; Fischer, N.A.; McGwin, G., Jr.; Sloan, K.R.; Owsley, C.; Curcio, C.A. Impact of the Aging Lens and Posterior Capsular Opacification on Quantitative Autofluorescence Imaging in Age-Related Macular Degeneration. Transl. Vis. Sci. Technol. 2022, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Reiter, G.S.; Schwarzenbacher, L.; Schartmuller, D.; Roggla, V.; Leydolt, C.; Menapace, R.; Schmidt-Erfurth, U.; Sacu, S. Influence of lens opacities and cataract severity on quantitative fundus autofluorescence as a secondary outcome of a randomized clinical trial. Sci. Rep. 2021, 11, 12685. [Google Scholar] [CrossRef]
- Brauer, J.L.; Schultz, R.; Klemm, M.; Hammer, M. Influence of Lens Fluorescence on Fluorescence Lifetime Imaging Ophthalmoscopy (FLIO) Fundus Imaging and Strategies for Its Compensation. Transl. Vis. Sci. Technol. 2020, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- van de Kraats, J.; van Norren, D. Optical density of the aging human ocular media in the visible and the UV. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2007, 24, 1842–1857. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.J.; Masella, B.; Dubra, A.; Sharma, R.; Yin, L.; Merigan, W.H.; Palczewska, G.; Palczewski, K.; Williams, D.R. Images of photoreceptors in living primate eyes using adaptive optics two-photon ophthalmoscopy. Biomed. Opt. Express 2010, 2, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Palczewska, G.; Golczak, M.; Williams, D.R.; Hunter, J.J.; Palczewski, K. Endogenous fluorophores enable two-photon imaging of the primate eye. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4438–4447. [Google Scholar] [CrossRef] [PubMed]
- Palczewska, G.; Dong, Z.; Golczak, M.; Hunter, J.J.; Williams, D.R.; Alexander, N.S.; Palczewski, K. Noninvasive two-photon microscopy imaging of mouse retina and retinal pigment epithelium through the pupil of the eye. Nat. Med. 2014, 20, 785–789. [Google Scholar] [CrossRef]
- Sharma, R.; Yin, L.; Geng, Y.; Merigan, W.H.; Palczewska, G.; Palczewski, K.; Williams, D.R.; Hunter, J.J. In vivo two-photon imaging of the mouse retina. Biomed. Opt. Express 2013, 4, 1285–1293. [Google Scholar] [CrossRef]
- Sharma, R.; Schwarz, C.; Williams, D.R.; Palczewska, G.; Palczewski, K.; Hunter, J.J. In Vivo Two-Photon Fluorescence Kinetics of Primate Rods and Cones. Investig. Ophthalmol. Vis. Sci. 2016, 57, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, C.; Sharma, R.; Fischer, W.S.; Chung, M.; Palczewska, G.; Palczewski, K.; Williams, D.R.; Hunter, J.J. Safety assessment in macaques of light exposures for functional two-photon ophthalmoscopy in humans. Biomed. Opt. Express 2016, 7, 5148–5169. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Williams, D.R.; Palczewska, G.; Palczewski, K.; Hunter, J.J. Two-Photon Autofluorescence Imaging Reveals Cellular Structures Throughout the Retina of the Living Primate Eye. Investig. Ophthalmol. Vis. Sci. 2016, 57, 632–646. [Google Scholar] [CrossRef] [PubMed]
- Marcos, S.; Werner, J.S.; Burns, S.A.; Merigan, W.H.; Artal, P.; Atchison, D.A.; Hampson, K.M.; Legras, R.; Lundstrom, L.; Yoon, G.; et al. Vision science and adaptive optics, the state of the field. Vision Res. 2017, 132, 3–33. [Google Scholar] [CrossRef]
- Sharma, R.; Schwarz, C.; Hunter, J.J.; Palczewska, G.; Palczewski, K.; Williams, D.R. Formation and Clearance of All-Trans-Retinol in Rods Investigated in the Living Primate Eye with Two-Photon Ophthalmoscopy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Murashova, G.A.; Mancuso, C.A.; Sakami, S.; Palczewski, K.; Palczewska, G.; Dantus, M. Epi-direction detected multimodal imaging of an unstained mouse retina with a Yb-fiber laser. Proc. SPIE Int. Soc. Opt. Eng. 2017, 10069, 357–361. [Google Scholar] [CrossRef]
- Murashova, G.A.; Mancuso, C.A.; Canfield, J.L.; Sakami, S.; Palczewski, K.; Palczewska, G.; Dantus, M. Multimodal nonlinear optical imaging of unstained retinas in the epi-direction with a sub-40 fs Yb-fiber laser. Biomed. Opt. Express 2017, 8, 5228–5242. [Google Scholar] [CrossRef] [PubMed]
- Palczewska, G.; Stremplewski, P.; Suh, S.; Alexander, N.; Salom, D.; Dong, Z.; Ruminski, D.; Choi, E.H.; Sears, A.E.; Kern, T.S.; et al. Two-photon imaging of the mammalian retina with ultrafast pulsing laser. JCI Insight 2018, 3, e121555. [Google Scholar] [CrossRef]
- Palczewska, G.; Kern, T.S.; Palczewski, K. Noninvasive Two-Photon Microscopy Imaging of Mouse Retina and Retinal Pigment Epithelium. Methods Mol. Biol. 2019, 1834, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.A.H.; Granger, C.E.; Kunala, K.; Parkins, K.; Huynh, K.T.; Bowles-Johnson, K.; Yang, Q.; Hunter, J.J. Adaptive optics fluorescence lifetime imaging ophthalmoscopy of in vivo human retinal pigment epithelium. Biomed. Opt. Express 2022, 13, 1737–1754. [Google Scholar] [CrossRef] [PubMed]
- Boguslawski, J.; Tomczewski, S.; Dabrowski, M.; Komar, K.; Milkiewicz, J.; Palczewska, G.; Palczewski, K.; Wojtkowski, M. In vivo imaging of the human retina using a two-photon excited fluorescence ophthalmoscope. STAR Protoc. 2023, 4, 102225. [Google Scholar] [CrossRef] [PubMed]
- Wolf-Schnurrbusch, U.E.; Wittwer, V.V.; Ghanem, R.; Niederhaeuser, M.; Enzmann, V.; Framme, C.; Wolf, S. Blue-light versus green-light autofluorescence: Lesion size of areas of geographic atrophy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9497–9502. [Google Scholar] [CrossRef] [PubMed]
- Burke, T.R.; Duncker, T.; Woods, R.L.; Greenberg, J.P.; Zernant, J.; Tsang, S.H.; Smith, R.T.; Allikmets, R.; Sparrow, J.R.; Delori, F.C. Quantitative fundus autofluorescence in recessive Stargardt disease. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2841–2852. [Google Scholar] [CrossRef] [PubMed]
- Duncker, T.; Greenberg, J.P.; Ramachandran, R.; Hood, D.C.; Smith, R.T.; Hirose, T.; Woods, R.L.; Tsang, S.H.; Delori, F.C.; Sparrow, J.R. Quantitative fundus autofluorescence and optical coherence tomography in best vitelliform macular dystrophy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Duncker, T.; Stein, G.E.; Lee, W.; Tsang, S.H.; Zernant, J.; Bearelly, S.; Hood, D.C.; Greenstein, V.C.; Delori, F.C.; Allikmets, R.; et al. Quantitative Fundus Autofluorescence and Optical Coherence Tomography in ABCA4 Carriers. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7274–7285. [Google Scholar] [CrossRef]
- Duncker, T.; Tsang, S.H.; Lee, W.; Zernant, J.; Allikmets, R.; Delori, F.C.; Sparrow, J.R. Quantitative fundus autofluorescence distinguishes ABCA4-associated and non-ABCA4-associated bull’s-eye maculopathy. Ophthalmology 2015, 122, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Duncker, T.; Tsang, S.H.; Woods, R.L.; Lee, W.; Zernant, J.; Allikmets, R.; Delori, F.C.; Sparrow, J.R. Quantitative Fundus Autofluorescence and Optical Coherence Tomography in PRPH2/RDS- and ABCA4-Associated Disease Exhibiting Phenotypic Overlap. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3159–3170. [Google Scholar] [CrossRef]
- Marsiglia, M.; Lee, W.; Mahajan, V.B.; Zernant, J.; Delori, F.C.; Tsang, S.H.; Sparrow, J.R. Quantitative autofluorescence as a clinical tool for expedited differential diagnosis of retinal degeneration. JAMA Ophthalmol. 2015, 133, 219–220. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Duncker, T.; Woods, R.; Delori, F.C. Quantitative Fundus Autofluorescence in Best Vitelliform Macular Dystrophy: RPE Lipofuscin is not Increased in Non-Lesion Areas of Retina. Adv. Exp. Med. Biol. 2016, 854, 285–290. [Google Scholar] [CrossRef]
- Pfau, M.; Goerdt, L.; Schmitz-Valckenberg, S.; Mauschitz, M.M.; Mishra, D.K.; Holz, F.G.; Lindner, M.; Fleckenstein, M. Green-Light Autofluorescence Versus Combined Blue-Light Autofluorescence and Near-Infrared Reflectance Imaging in Geographic Atrophy Secondary to Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO121–BIO130. [Google Scholar] [CrossRef]
- Schuerch, K.; Woods, R.L.; Lee, W.; Duncker, T.; Delori, F.C.; Allikmets, R.; Tsang, S.H.; Sparrow, J.R. Quantifying Fundus Autofluorescence in Patients with Retinitis Pigmentosa. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Messinger, J.D.; Ferrara, D.; Freund, K.B.; Curcio, C.A. Fundus Autofluorescence in Neovascular Age-Related Macular Degeneration: A Clinicopathologic Correlation Relevant to Macular Atrophy. Ophthalmol. Retina 2021, 5, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Salcedo-Villanueva, G.; Lopez-Contreras, Y.; Gonzalez, H.L.A.; Romo-Aguas, J.C.; Garcia-Aguirre, G.; Cernichiaro-Espinosa, L.A.; Martinez-Castellanos, M.A.; Quiroz-Mercado, H. Fundus autofluorescence in premature infants. Sci. Rep. 2021, 11, 8823. [Google Scholar] [CrossRef] [PubMed]
- Delori, F.C.; Goger, D.G.; Dorey, C.K. Age-related accumulation and spatial distribution of lipofuscin in RPE of normal subjects. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1855–1866. [Google Scholar]
- Ach, T.; Huisingh, C.; McGwin, G., Jr.; Messinger, J.D.; Zhang, T.; Bentley, M.J.; Gutierrez, D.B.; Ablonczy, Z.; Smith, R.T.; Sloan, K.R.; et al. Quantitative autofluorescence and cell density maps of the human retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4832–4841. [Google Scholar] [CrossRef] [PubMed]
- Kleefeldt, N.; Bermond, K.; Tarau, I.S.; Hillenkamp, J.; Berlin, A.; Sloan, K.R.; Ach, T. Quantitative Fundus Autofluorescence: Advanced Analysis Tools. Transl. Vis. Sci. Technol. 2020, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Probster, C.; Tarau, I.S.; Berlin, A.; Kleefeldt, N.; Hillenkamp, J.; Nentwich, M.M.; Sloan, K.R.; Ach, T. Quantitative Fundus Autofluorescence in the Developing and Maturing Healthy Eye. Transl. Vis. Sci. Technol. 2021, 10, 15. [Google Scholar] [CrossRef]
- Okubo, A.; Rosa, R.H., Jr.; Bunce, C.V.; Alexander, R.A.; Fan, J.T.; Bird, A.C.; Luthert, P.J. The relationships of age changes in retinal pigment epithelium and Bruch’s membrane. Investig. Ophthalmol. Vis. Sci. 1999, 40, 443–449. [Google Scholar]
- Greenberg, J.P.; Duncker, T.; Woods, R.L.; Smith, R.T.; Sparrow, J.R.; Delori, F.C. Quantitative fundus autofluorescence in healthy eyes. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5684–5693. [Google Scholar] [CrossRef]
- Ben Ami, T.; Tong, Y.; Bhuiyan, A.; Huisingh, C.; Ablonczy, Z.; Ach, T.; Curcio, C.A.; Smith, R.T. Spatial and Spectral Characterization of Human Retinal Pigment Epithelium Fluorophore Families by Ex Vivo Hyperspectral Autofluorescence Imaging. Transl. Vis. Sci. Technol. 2016, 5, 5. [Google Scholar] [CrossRef]
- Schultz, R.; Schwanengel, L.; Klemm, M.; Meller, D.; Hammer, M. Spectral fundus autofluorescence peak emission wavelength in ageing and AMD. Acta Ophthalmol. 2022, 100, e1223–e1231. [Google Scholar] [CrossRef] [PubMed]
- Schultz, R.; Klemm, M.; Meller, D.; Hammer, M. Spectral calibration of fluorescence lifetime imaging ophthalmoscopy. Acta Ophthalmol. 2022, 100, e612–e613. [Google Scholar] [CrossRef] [PubMed]
- Zuclich, J.A.; Previc, F.H.; Novar, B.J.; Edsall, P.R. Near-UV/blue light-induced fluorescence in the human lens: Potential interference with visual function. J. Biomed. Opt. 2005, 10, 44021. [Google Scholar] [CrossRef] [PubMed]
- Rein, D.B.; Wittenborn, J.S.; Burke-Conte, Z.; Gulia, R.; Robalik, T.; Ehrlich, J.R.; Lundeen, E.A.; Flaxman, A.D. Prevalence of Age-Related Macular Degeneration in the US in 2019. JAMA Ophthalmol. 2022, 140, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Creuzot-Garcher, C.P.; Srour, M.; Baudin, F.; Daien, V.; Dot, C.; Nghiem-Buffet, S.; Girmens, J.F.; Coulombel, N.; Ponthieux, A.; Delcourt, C. Incidence and Prevalence of Neovascular Age-Related Macular Degeneration in France between 2008 and 2018: The LAND-SCAPE Study. Ophthalmol. Sci. 2022, 2, 100114. [Google Scholar] [CrossRef] [PubMed]
- Pennington, K.L.; DeAngelis, M.M. Epidemiology of age-related macular degeneration (AMD): Associations with cardiovascular disease phenotypes and lipid factors. Eye Vis. 2016, 3, 34. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Agron, E.; Domalpally, A.; Cukras, C.A.; Clemons, T.E.; Chen, Q.; Lu, Z.; Chew, E.Y.; Keenan, T.D.L.; AREDS and AREDS2 Research Groups. R. Reticular Pseudodrusen: The Third Macular Risk Feature for Progression to Late Age-Related Macular Degeneration: Age-Related Eye Disease Study 2 Report 30. Ophthalmology 2022, 129, 1107–1119. [Google Scholar] [CrossRef]
- Rudolf, M.; Vogt, S.D.; Curcio, C.A.; Huisingh, C.; McGwin, G., Jr.; Wagner, A.; Grisanti, S.; Read, R.W. Histologic basis of variations in retinal pigment epithelium autofluorescence in eyes with geographic atrophy. Ophthalmology 2013, 120, 821–828. [Google Scholar] [CrossRef]
- Zanzottera, E.C.; Ach, T.; Huisingh, C.; Messinger, J.D.; Freund, K.B.; Curcio, C.A. Visualizing Retinal Pigment Epithelium Phenotypes in the Transition to Atrophy in Neovascular Age-Related Macular Degeneration. Retina 2016, 36 (Suppl. S1), S26–S39. [Google Scholar] [CrossRef]
- Bonilha, V.L.; Bell, B.A.; Hu, J.; Milliner, C.; Pauer, G.J.; Hagstrom, S.A.; Radu, R.A.; Hollyfield, J.G. Geographic Atrophy: Confocal Scanning Laser Ophthalmoscopy, Histology, and Inflammation in the Region of Expanding Lesions. Investig. Ophthalmol. Vis. Sci. 2020, 61, 15. [Google Scholar] [CrossRef]
- Zanzottera, E.C.; Messinger, J.D.; Ach, T.; Smith, R.T.; Freund, K.B.; Curcio, C.A. The Project MACULA Retinal Pigment Epithelium Grading System for Histology and Optical Coherence Tomography in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3253–3268. [Google Scholar] [CrossRef] [PubMed]
- Gambril, J.A.; Sloan, K.R.; Swain, T.A.; Huisingh, C.; Zarubina, A.V.; Messinger, J.D.; Ach, T.; Curcio, C.A. Quantifying Retinal Pigment Epithelium Dysmorphia and Loss of Histologic Autofluorescence in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2481–2493. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Ben Ami, T.; Hong, S.; Heintzmann, R.; Gerig, G.; Ablonczy, Z.; Curcio, C.A.; Ach, T.; Smith, R.T. Hyperspectral Autofluorescence Imaging of Drusen and Retinal Pigment Epithelium in Donor Eyes with Age-Related Macular Degeneration. Retina 2016, 36 (Suppl. S1), S127–S136. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, T.; Tong, Y.; Agee, J.; Challa, N.; Heintzmann, R.; Hammer, M.; Curcio, C.A.; Ach, T.; Ablonczy, Z.; Smith, R.T. Ex Vivo Hyperspectral Autofluorescence Imaging and Localization of Fluorophores in Human Eyes with Age-Related Macular Degeneration. Vision 2018, 2, 38. [Google Scholar] [CrossRef] [PubMed]
- Rozanowski, B.; Cuenco, J.; Davies, S.; Shamsi, F.A.; Zadlo, A.; Dayhaw-Barker, P.; Rozanowska, M.; Sarna, T.; Boulton, M.E. The phototoxicity of aged human retinal melanosomes. Photochem. Photobiol. 2008, 84, 650–657. [Google Scholar] [CrossRef]
- Rozanowski, B.; Burke, J.M.; Boulton, M.E.; Sarna, T.; Rozanowska, M. Human RPE melanosomes protect from photosensitized and iron-mediated oxidation but become pro-oxidant in the presence of iron upon photodegradation. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2838–2847. [Google Scholar] [CrossRef]
- Teussink, M.M.; Lambertus, S.; de Mul, F.F.; Rozanowska, M.B.; Hoyng, C.B.; Klevering, B.J.; Theelen, T. Lipofuscin-associated photo-oxidative stress during fundus autofluorescence imaging. PLoS ONE 2017, 12, e0172635. [Google Scholar] [CrossRef]
- Arend, O.; Weiter, J.J.; Goger, D.G.; Delori, F.C. In vivo fundus fluorescence measurements in patients with age related macular degeneration. Ophthalmologe 1995, 92, 647–653. [Google Scholar]
- Delori, F.C.; Fleckner, M.R.; Goger, D.G.; Weiter, J.J.; Dorey, C.K. Autofluorescence distribution associated with drusen in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2000, 41, 496–504. [Google Scholar]
- Hammer, M.; Schultz, R.; Hasan, S.; Sauer, L.; Klemm, M.; Kreilkamp, L.; Zweifel, L.; Augsten, R.; Meller, D. Fundus Autofluorescence Lifetimes and Spectral Features of Soft Drusen and Hyperpigmentation in Age-Related Macular Degeneration. Transl. Vis. Sci. Technol. 2020, 9, 20. [Google Scholar] [CrossRef]
- Weber, S.; Simon, R.; Schwanengel, L.S.; Curcio, C.A.; Augsten, R.; Meller, D.; Hammer, M. Fluorescence Lifetime and Spectral Characteristics of Subretinal Drusenoid Deposits and Their Predictive Value for Progression of Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2022, 63, 23. [Google Scholar] [CrossRef]
- Borrelli, E.; Nittala, M.G.; Abdelfattah, N.S.; Lei, J.; Hariri, A.H.; Shi, Y.; Fan, W.; Cozzi, M.; Sarao, V.; Lanzetta, P.; et al. Comparison of short-wavelength blue-light autofluorescence and conventional blue-light autofluorescence in geographic atrophy. Br. J. Ophthalmol. 2018, 103, 610–616. [Google Scholar] [CrossRef]
- Hammer, M.; Quick, S.; Klemm, M.; Schenke, S.; Mata, N.; Eitner, A.; Schweitzer, D. In-vivo and in-vitro investigations of retinal fluorophores in age-related macular degeneration by fluorescence lifetime imaging. Multiphoton Microsc. Biomed. Sci. IX 2009, 7183, 525–536. [Google Scholar] [CrossRef]
- Schweitzer, D.; Quick, S.; Schenke, S.; Klemm, M.; Gehlert, S.; Hammer, M.; Jentsch, S.; Fischer, J. Comparison of parameters of time-resolved autofluorescence between healthy subjects and patients suffering from early AMD. Ophthalmologe 2009, 106, 714–722. [Google Scholar] [CrossRef]
- Reiter, G.S.; Hacker, V.; Told, R.; Schranz, M.; Krotka, P.; Schlanitz, F.G.; Sacu, S.; Pollreisz, A.; Schmidt-Erfurth, U. Longitudinal Changes in Quantitative Autofluorescence during Progression from Intermediate to Late Age-Related Macular Degeneration. Retina J. Retin. Vitr. Dis. 2021, 41, 1236–1241. [Google Scholar] [CrossRef]
- Reiter, G.S.; Told, R.; Baumann, L.; Sacu, S.; Schmidt-Erfurth, U.; Pollreisz, A. Investigating a Growth Prediction Model in Advanced Age-Related Macular Degeneration with Solitary Geographic Atrophy Using Quantitative Autofluorescence. Retina J. Retin. Vitr. Dis. 2020, 40, 1657–1664. [Google Scholar] [CrossRef]
- Schwanengel, L.S.; Weber, S.; Simon, R.; Lehmann, T.; Augsten, R.; Meller, D.; Hammer, M. Changes in drusen-associated autofluorescence over time observed by fluorescence lifetime imaging ophthalmoscopy in age-related macular degeneration. Acta Ophthalmol. 2022, 101, e154–e166. [Google Scholar] [CrossRef]
- Chen, L.; Yang, P.; Curcio, C.A. Visualizing lipid behind the retina in aging and age-related macular degeneration, via indocyanine green angiography (ASHS-LIA). Eye 2022, 36, 1735–1746. [Google Scholar] [CrossRef]
- Klein, R.; Myers, C.E.; Lee, K.E.; Gangnon, R.E.; Sivakumaran, T.A.; Iyengar, S.K.; Klein, B.E. Small Drusen and Age-Related Macular Degeneration: The Beaver Dam Eye Study. J. Clin. Med. 2015, 4, 424–440. [Google Scholar] [CrossRef]
- Feldman, T.B.; Yakovleva, M.A.; Arbukhanova, P.M.; Borzenok, S.A.; Kononikhin, A.S.; Popov, I.A.; Nikolaev, E.N.; Ostrovsky, M.A. Changes in spectral properties and composition of lipofuscin fluorophores from human-retinal-pigment epithelium with age and pathology. Anal. Bioanal. Chem. 2015, 407, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Yakovleva, M.A.; Feldman, T.B.; Arbukhanova, P.M.; Borzenok, S.A.; Kuzmin, V.A.; Ostrovsky, M.A. The fluorescence lifetime of lipofuscin granule fluorophores contained in the retinal pigment epithelium cells from human cadaver eyes in normal state and in the case of visualized pathology. Dokl. Biochem. Biophys. 2017, 474, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Feldman, T.B.; Yakovleva, M.A.; Larichev, A.V.; Arbukhanova, P.M.; Radchenko, A.S.; Borzenok, S.A.; Kuzmin, V.A.; Ostrovsky, M.A. Spectral analysis of fundus autofluorescence pattern as a tool to detect early stages of degeneration in the retina and retinal pigment epithelium. Eye 2018, 32, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Yakovleva, M.A.; Radchenko, A.S.; Feldman, T.B.; Kostyukov, A.A.; Arbukhanova, P.M.; Borzenok, S.A.; Kuzmin, V.A.; Ostrovsky, M.A. Fluorescence characteristics of lipofuscin fluorophores from human retinal pigment epithelium. Photochem. Photobiol. Sci. 2020, 19, 920–930. [Google Scholar] [CrossRef] [PubMed]
- Mata, N.L.; Weng, J.; Travis, G.H. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc. Natl. Acad. Sci. USA 2000, 97, 7154–7159. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Zhao, J.; Kim, H.J.; Sparrow, J.R. Photodegradation of retinal bisretinoids in mouse models and implications for macular degeneration. Proc. Natl. Acad. Sci. USA 2016, 113, 6904–6909. [Google Scholar] [CrossRef]
- Ueda, K.; Kim, H.J.; Zhao, J.; Song, Y.; Dunaief, J.L.; Sparrow, J.R. Iron promotes oxidative cell death caused by bisretinoids of retina. Proc. Natl. Acad. Sci. USA 2018, 115, 4963–4968. [Google Scholar] [CrossRef]
- Pandya, V.B.; Ho, I.V.; Hunyor, A.P. Does unintentional macular translocation after retinal detachment repair influence visual outcome? Clin. Exp. Ophthalmol. 2012, 40, 88–92. [Google Scholar] [CrossRef]
- Shiragami, C.; Shiraga, F.; Yamaji, H.; Fukuda, K.; Takagishi, M.; Morita, M.; Kishikami, T. Unintentional displacement of the retina after standard vitrectomy for rhegmatogenous retinal detachment. Ophthalmology 2010, 117, 86–92.e81. [Google Scholar] [CrossRef]
- Gaillard, E.R.; Zheng, L.; Merriam, J.C.; Dillon, J. Age-related changes in the absorption characteristics of the primate lens. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1454–1459. [Google Scholar]
- Hollyfield, J.G. Age-related macular degeneration: The molecular link between oxidative damage, tissue-specific inflammation and outer retinal disease: The Proctor lecture. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.X.; Stiles, T.; Douglas, C.; Ho, D.; Fan, W.; Du, H.; Xiao, X. Oxidative stress, innate immunity, and age-related macular degeneration. AIMS Mol. Sci. 2016, 3, 196–221. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Handa, J.T.; Cano, M.; Wang, L.; Datta, S.; Liu, T. Lipids, oxidized lipids, oxidation-specific epitopes, and Age-related Macular Degeneration. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.; Datta, S.; Wang, L.; Pegany, R.; Cano, M.; Handa, J.T. The impact of lipids, lipid oxidation, and inflammation on AMD, and the potential role of miRNAs on lipid metabolism in the RPE. Exp. Eye Res. 2019, 181, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Karunadharma, P.P.; Kapphahn, R.J.; Stahl, M.R.; Olsen, T.W.; Ferrington, D.A. Dissecting Regulators of Aging and Age-Related Macular Degeneration in the Retinal Pigment Epithelium. Oxidative Med. Cell. Longev. 2022, 2022, 6009787. [Google Scholar] [CrossRef] [PubMed]
- Hahn, P.; Milam, A.H.; Dunaief, J.L. Maculas affected by age-related macular degeneration contain increased chelatable iron in the retinal pigment epithelium and Bruch’s membrane. Arch. Ophthalmol. 2003, 121, 1099–1105. [Google Scholar] [CrossRef]
- Liu, Y.; Bell, B.A.; Song, Y.; Zhang, K.; Anderson, B.; Axelsen, P.H.; Bohannan, W.; Agbaga, M.P.; Park, H.G.; James, G.; et al. Deuterated docosahexaenoic acid protects against oxidative stress and geographic atrophy-like retinal degeneration in a mouse model with iron overload. Aging Cell 2022, 21, e13579. [Google Scholar] [CrossRef]
- von Krusenstiern, A.N.; Robson, R.N.; Qian, N.; Qiu, B.; Hu, F.; Reznik, E.; Smith, N.; Zandkarimi, F.; Estes, V.M.; Dupont, M.; et al. Identification of essential sites of lipid peroxidation in ferroptosis. Nat. Chem. Biol. 2023, 19, 719–730. [Google Scholar] [CrossRef]
- Shchepinov, M.S. Polyunsaturated Fatty Acid Deuteration against Neurodegeneration. Trends Pharmacol. Sci. 2020, 41, 236–248. [Google Scholar] [CrossRef]
- Firsov, A.M.; Fomich, M.A.; Bekish, A.V.; Sharko, O.L.; Kotova, E.A.; Saal, H.J.; Vidovic, D.; Shmanai, V.V.; Pratt, D.A.; Antonenko, Y.N.; et al. Threshold protective effect of deuterated polyunsaturated fatty acids on peroxidation of lipid bilayers. FEBS J. 2019, 286, 2099–2117. [Google Scholar] [CrossRef] [PubMed]
- Rosell, M.; Giera, M.; Brabet, P.; Shchepinov, M.S.; Guichardant, M.; Durand, T.; Vercauteren, J.; Galano, J.M.; Crauste, C. Bis-allylic Deuterated DHA Alleviates Oxidative Stress in Retinal Epithelial Cells. Antioxidants 2019, 8, 447. [Google Scholar] [CrossRef] [PubMed]
- Sterling, J.K.; Baumann, B.; Foshe, S.; Voigt, A.; Guttha, S.; Alnemri, A.; McCright, S.J.; Li, M.; Zauhar, R.J.; Montezuma, S.R.; et al. Inflammatory adipose activates a nutritional immunity pathway leading to retinal dysfunction. Cell Rep. 2022, 39, 110942. [Google Scholar] [CrossRef] [PubMed]
- Lukiw, W.J.; Mukherjee, P.K.; Cui, J.G.; Bazan, N.G. A2E selectively induces cox-2 in ARPE-19 and human neural cells. Curr. Eye Res. 2006, 31, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Chahal, S.; Rani, P.; Kiran; Sindhu, J.; Joshi, G.; Ganesan, A.; Kalyaanamoorthy, S.; Mayank; Kumar, P.; Singh, R.; et al. Design and Development of COX-II Inhibitors: Current Scenario and Future Perspective. ACS Omega 2023, 8, 17446–17498. [Google Scholar] [CrossRef]
- Crabb, J.W.; Miyagi, M.; Gu, X.; Shadrach, K.; West, K.A.; Sakaguchi, H.; Kamei, M.; Hasan, A.; Yan, L.; Rayborn, M.E.; et al. Drusen proteome analysis: An approach to the etiology of age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2002, 99, 14682–14687. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Meer, S.G.; Miyagi, M.; Rayborn, M.E.; Hollyfield, J.G.; Crabb, J.W.; Salomon, R.G. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J. Biol. Chem. 2003, 278, 42027–42035. [Google Scholar] [CrossRef]
- Renganathan, K.; Ebrahem, Q.; Vasanji, A.; Gu, X.; Lu, L.; Sears, J.; Salomon, R.G.; Anand-Apte, B.; Crabb, J.W. Carboxyethylpyrrole adducts, age-related macular degeneration and neovascularization. Adv. Exp. Med. Biol. 2008, 613, 261–267. [Google Scholar] [CrossRef]
- Ebrahem, Q.; Renganathan, K.; Sears, J.; Vasanji, A.; Gu, X.; Lu, L.; Salomon, R.G.; Crabb, J.W.; Anand-Apte, B. Carboxyethylpyrrole oxidative protein modifications stimulate neovascularization: Implications for age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2006, 103, 13480–13484. [Google Scholar] [CrossRef]
- Ayalasomayajula, S.P.; Kompella, U.B. Induction of vascular endothelial growth factor by 4-hydroxynonenal and its prevention by glutathione precursors in retinal pigment epithelial cells. Eur. J. Pharmacol. 2002, 449, 213–220. [Google Scholar] [CrossRef]
- Zhou, J.; Cai, B.; Jang, Y.P.; Pachydaki, S.; Schmidt, A.M.; Sparrow, J.R. Mechanisms for the induction of HNE- MDA- and AGE-adducts, RAGE and VEGF in retinal pigment epithelial cells. Exp. Eye Res. 2005, 80, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, Y.; Inoue, Y.; Iriyama, A.; Jang, W.D. Effects of yellow intraocular lenses on light-induced upregulation of vascular endothelial growth factor. J. Cataract. Refract. Surg. 2006, 32, 1540–1544. [Google Scholar] [CrossRef] [PubMed]
- Ung, C.; Lains, I.; Miller, J.W.; Kim, I.K. Current Management of Age-Related Macular Degeneration. In Age-Related Macular Degeneration from Clinic to Genes and Back to Patient Management; Chew, E.Y., Swaroop, A., Eds.; Advances in Experimental Medicine and Biology; Springer Nature Switzerland AG: Cham, Switzerland, 2019; Volume 1256, pp. 295–314. [Google Scholar]
- Higgins, G.T.; Wang, J.H.; Dockery, P.; Cleary, P.E.; Redmond, H.P. Induction of angiogenic cytokine expression in cultured RPE by ingestion of oxidized photoreceptor outer segments. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1775–1782. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.; Segura, R.M.; Fonollosa, A.; Carrasco, E.; Francisco, G.; Simo, R. Interleukin-8, monocyte chemoattractant protein-1 and IL-10 in the vitreous fluid of patients with proliferative diabetic retinopathy. Diabet. Med. 2005, 22, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cano, M.; Datta, S.; Wei, H.; Ebrahimi, K.B.; Gorashi, Y.; Garlanda, C.; Handa, J.T. Pentraxin 3 recruits complement factor H to protect against oxidative stress-induced complement and inflammasome overactivation. J. Pathol. 2016, 240, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, M.; Raisler, B.J.; Sakurai, E.; Sarma, J.V.; Barnum, S.R.; Lambris, J.D.; Chen, Y.; Zhang, K.; Ambati, B.K.; Baffi, J.Z.; et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc. Natl. Acad. Sci. USA 2006, 103, 2328–2333. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.S.Y.; Kady, N.; Hu, J.; Dave, A.; Jiang, Z.; Pei, J.; Gorin, M.B.; Matynia, A.; Radu, R.A. Membrane Attack Complex Mediates Retinal Pigment Epithelium Cell Death in Stargardt Macular Degeneration. Cells 2022, 11, 3462. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Pauer, G.J.; Hagstrom, S.A.; Bok, D.; DeBenedictis, M.J.; Bonilha, V.L.; Hollyfield, J.G.; Radu, R.A. Evidence of complement dysregulation in outer retina of Stargardt disease donor eyes. Redox Biol. 2020, 37, 101787. [Google Scholar] [CrossRef]
- Ferrington, D.A.; Fisher, C.R.; Kowluru, R.A. Mitochondrial Defects Drive Degenerative Retinal Diseases. Trends Mol. Med. 2020, 26, 105–118. [Google Scholar] [CrossRef]
- Ebeling, M.C.; Polanco, J.R.; Qu, J.; Tu, C.; Montezuma, S.R.; Ferrington, D.A. Improving retinal mitochondrial function as a treatment for age-related macular degeneration. Redox Biol. 2020, 34, 101552. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Uusitalo, H.; Blasiak, J.; Felszeghy, S.; Kannan, R.; Kauppinen, A.; Salminen, A.; Sinha, D.; Ferrington, D. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog. Retin. Eye Res. 2020, 79, 100858. [Google Scholar] [CrossRef] [PubMed]
- Ferrington, D.A.; Kapphahn, R.J.; Leary, M.M.; Atilano, S.R.; Terluk, M.R.; Karunadharma, P.; Chen, G.K.; Ratnapriya, R.; Swaroop, A.; Montezuma, S.R.; et al. Increased retinal mtDNA damage in the CFH variant associated with age-related macular degeneration. Exp. Eye Res. 2016, 145, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Terluk, M.R.; Kapphahn, R.J.; Soukup, L.M.; Gong, H.; Gallardo, C.; Montezuma, S.R.; Ferrington, D.A. Investigating mitochondria as a target for treating age-related macular degeneration. J. Neurosci. 2015, 35, 7304–7311. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Xu, H.; Liang, F.Q.; Liang, H.; Gupta, P.; Havey, A.N.; Boulton, M.E.; Godley, B.F. Mitochondrial DNA damage and repair in RPE associated with aging and age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3521–3529. [Google Scholar] [CrossRef] [PubMed]
- Karunadharma, P.P.; Nordgaard, C.L.; Olsen, T.W.; Ferrington, D.A. Mitochondrial DNA damage as a potential mechanism for age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5470–5479. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Kapphahn, R.J.; Zhang, M.; Qian, S.; Montezuma, S.R.; Shang, P.; Ferrington, D.A.; Qu, J. Quantitative Proteomics of Human Retinal Pigment Epithelium Reveals Key Regulators for the Pathogenesis of Age-Related Macular Degeneration. Int. J. Mol. Sci. 2023, 24, 3252. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.Y.; Clemons, T.E.; Agron, E.; Domalpally, A.; Keenan, T.D.L.; Vitale, S.; Weber, C.; Smith, D.C.; Christen, W.; Group, A.R. Long-term Outcomes of Adding Lutein/Zeaxanthin and omega-3 Fatty Acids to the AREDS Supplements on Age-Related Macular Degeneration Progression: AREDS2 Report 28. JAMA Ophthalmol. 2022, 140, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.R.; Lawrenson, J.G. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst. Rev. 2017, 7, CD000254. [Google Scholar] [CrossRef]
- The Age-Related Eye Disease Study 2 (AREDS2) Research Group. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol. 2014, 132, 142–149. [Google Scholar] [CrossRef]
- Group, A.R.; Chew, E.Y.; Clemons, T.; SanGiovanni, J.P.; Danis, R.; Domalpally, A.; McBee, W.; Sperduto, R.; Ferris, F.L. The Age-Related Eye Disease Study 2 (AREDS2): Study design and baseline characteristics (AREDS2 report number 1). Ophthalmology 2012, 119, 2282–2289. [Google Scholar] [CrossRef]
- Bassetto, M.; Zaluski, J.; Li, B.; Zhang, J.; Badiee, M.; Kiser, P.D.; Tochtrop, G.P. Tuning the Metabolic Stability of Visual Cycle Modulators through Modification of an RPE65 Recognition Motif. J. Med. Chem. 2023, 66, 8140–8158. [Google Scholar] [CrossRef] [PubMed]
- Milne, G.L.; Yin, H.; Hardy, K.D.; Davies, S.S.; Roberts, L.J., 2nd. Isoprostane generation and function. Chem. Rev. 2011, 111, 5973–5996. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.W.; Furman, R.; Axelsen, P.H.; Shchepinov, M.S. Free Radical Chain Reactions and Polyunsaturated Fatty Acids in Brain Lipids. ACS Omega 2022, 7, 25337–25345. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.A.; Gibert, J.C.; Mukherjee, A. Light distributions on the retina: Relevance to macular pigment photoprotection. Acta Biochim. Pol. 2012, 59, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.A.; Gibert, J.C.; Mukherjee, A. Light distribution on the retina Implications for age-related macular degeneration. In Carotenoids and Retinal Disease; Landrum, J.T., Nolan, J., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2013. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the Tolerable Upper Intake Level of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA). EFSA J. 2012, 10, 2815. [Google Scholar]
- Kris-Etherton, P.M.; Grieger, J.A.; Etherton, T.D. Dietary reference intakes for DHA and EPA. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 99–104. [Google Scholar] [CrossRef]
- Zhang, Z.; Fulgoni, V.L.; Kris-Etherton, P.M.; Mitmesser, S.H. Dietary Intakes of EPA and DHA Omega-3 Fatty Acids among US Childbearing-Age and Pregnant Women: An Analysis of NHANES 2001–2014. Nutrients 2018, 10, 416. [Google Scholar] [CrossRef] [PubMed]
- Burns, R.P.; Feeney-Burns, L. Clinico-morphologic correlations of drusen of Bruch’s membrane. Trans. Am. Ophthalmol. Soc. 1980, 78, 206–225. [Google Scholar]
- Gouras, P.; Ivert, L.; Mattison, J.A.; Ingram, D.K.; Neuringer, M. Drusenoid maculopathy in rhesus monkeys: Autofluorescence, lipofuscin and drusen pathogenesis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 246, 1403–1411. [Google Scholar] [CrossRef]
- Gouras, P.; Ivert, L.; Neuringer, M.; Mattison, J.A. Topographic and age-related changes of the retinal epithelium and Bruch’s membrane of rhesus monkeys. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 248, 973–984. [Google Scholar] [CrossRef]
- Julien, S.; Schraermeyer, U. Lipofuscin can be eliminated from the retinal pigment epithelium of monkeys. Neurobiol. Aging 2012, 33, 2390–2397. [Google Scholar] [CrossRef] [PubMed]
- Julien-Schraermeyer, S.; Illing, B.; Tschulakow, A.; Taubitz, T.; Guezguez, J.; Burnet, M.; Schraermeyer, U. Penetration, distribution, and elimination of remofuscin/soraprazan in Stargardt mouse eyes following a single intravitreal injection using pharmacokinetics and transmission electron microscopic autoradiography: Implication for the local treatment of Stargardt’s disease and dry age-related macular degeneration. Pharmacol. Res. Perspect. 2020, 8, e00683. [Google Scholar] [CrossRef] [PubMed]
- Findlay, Q.; Jobling, A.I.; Vessey, K.A.; Greferath, U.; Phipps, J.A.; Guymer, R.H.; Fletcher, E.L. Prophylactic laser in age-related macular degeneration: The past, the present and the future. Eye 2018, 32, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Povazay, B.; Brinkmann, R.; Stoller, M.; Kessler, R. Selective Retina Therapy. In High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics; Bille, J.F., Ed.; Springer: Cham, Switzerland, 2019; pp. 237–259. [Google Scholar]
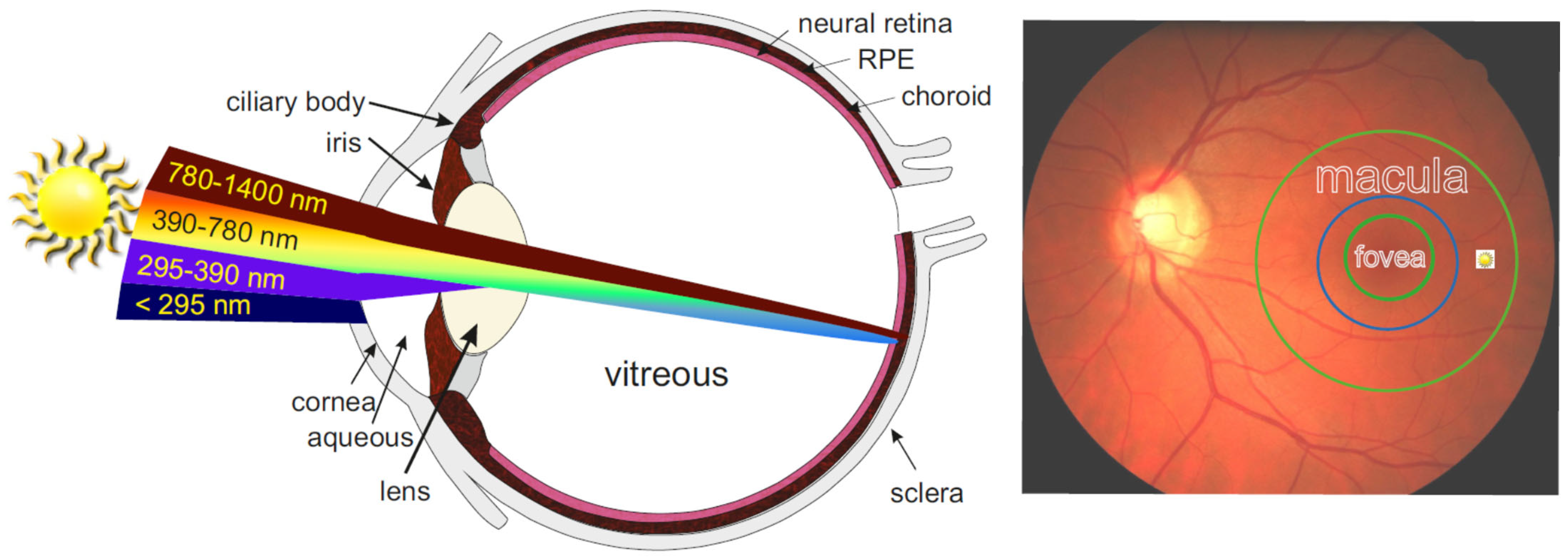
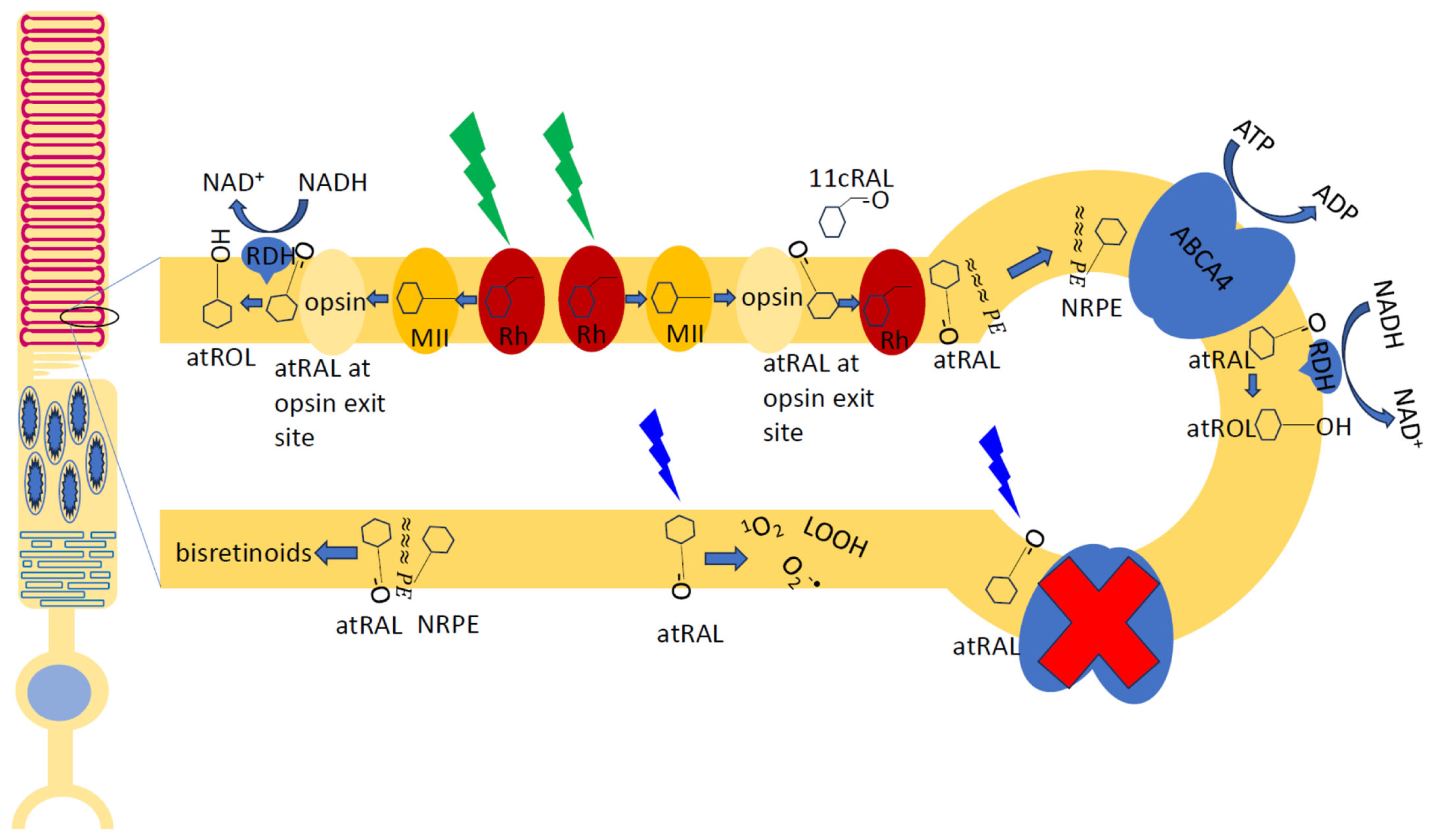

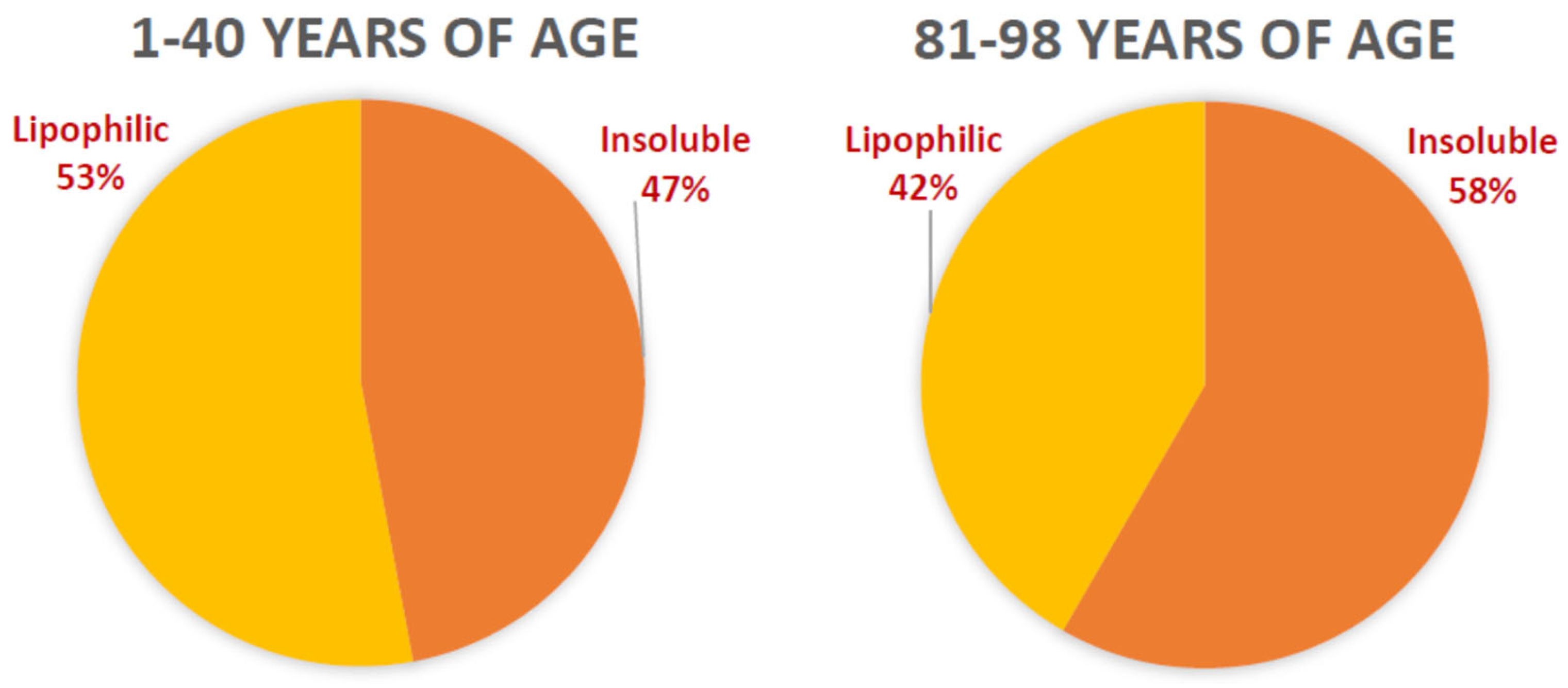
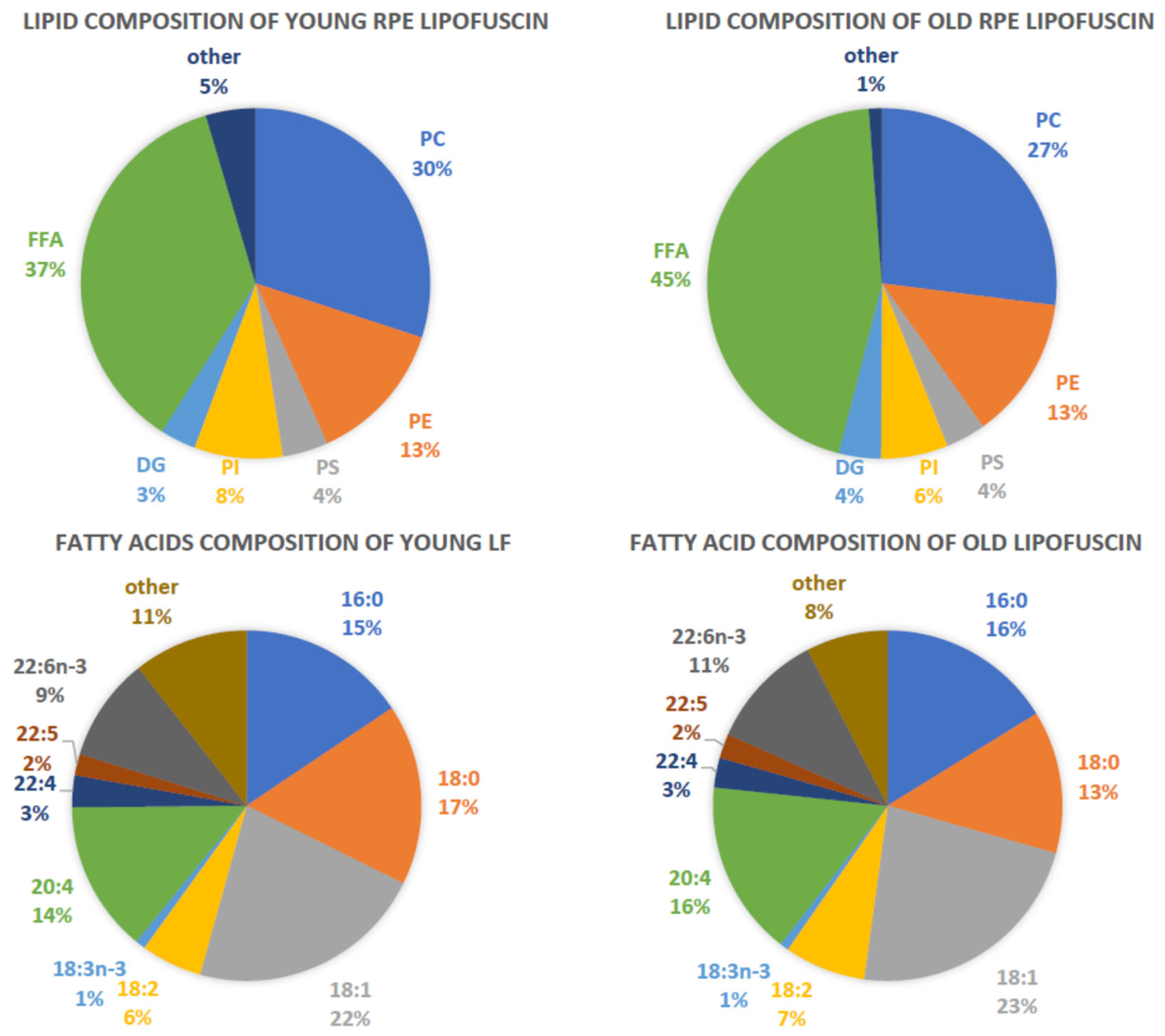
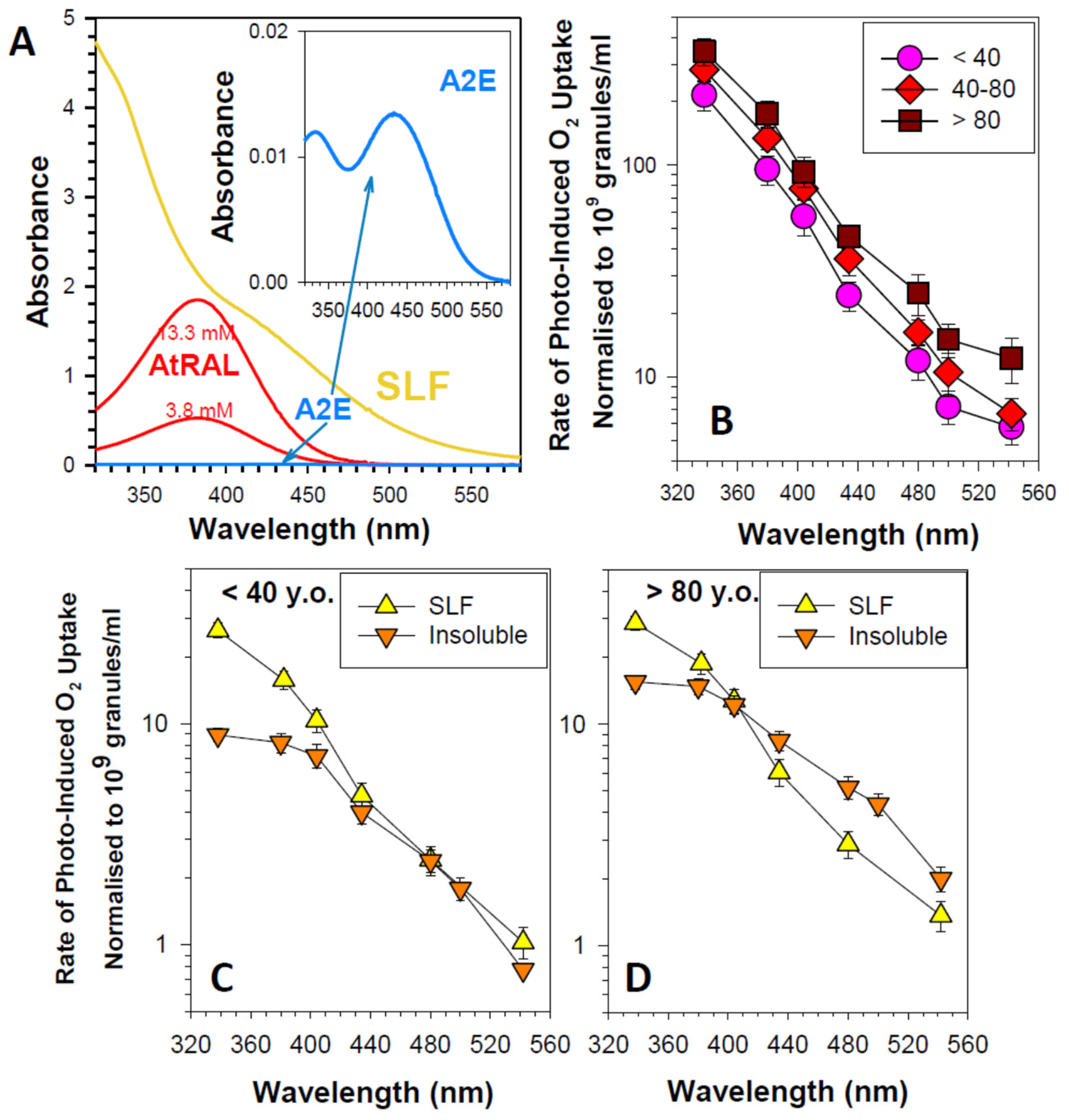
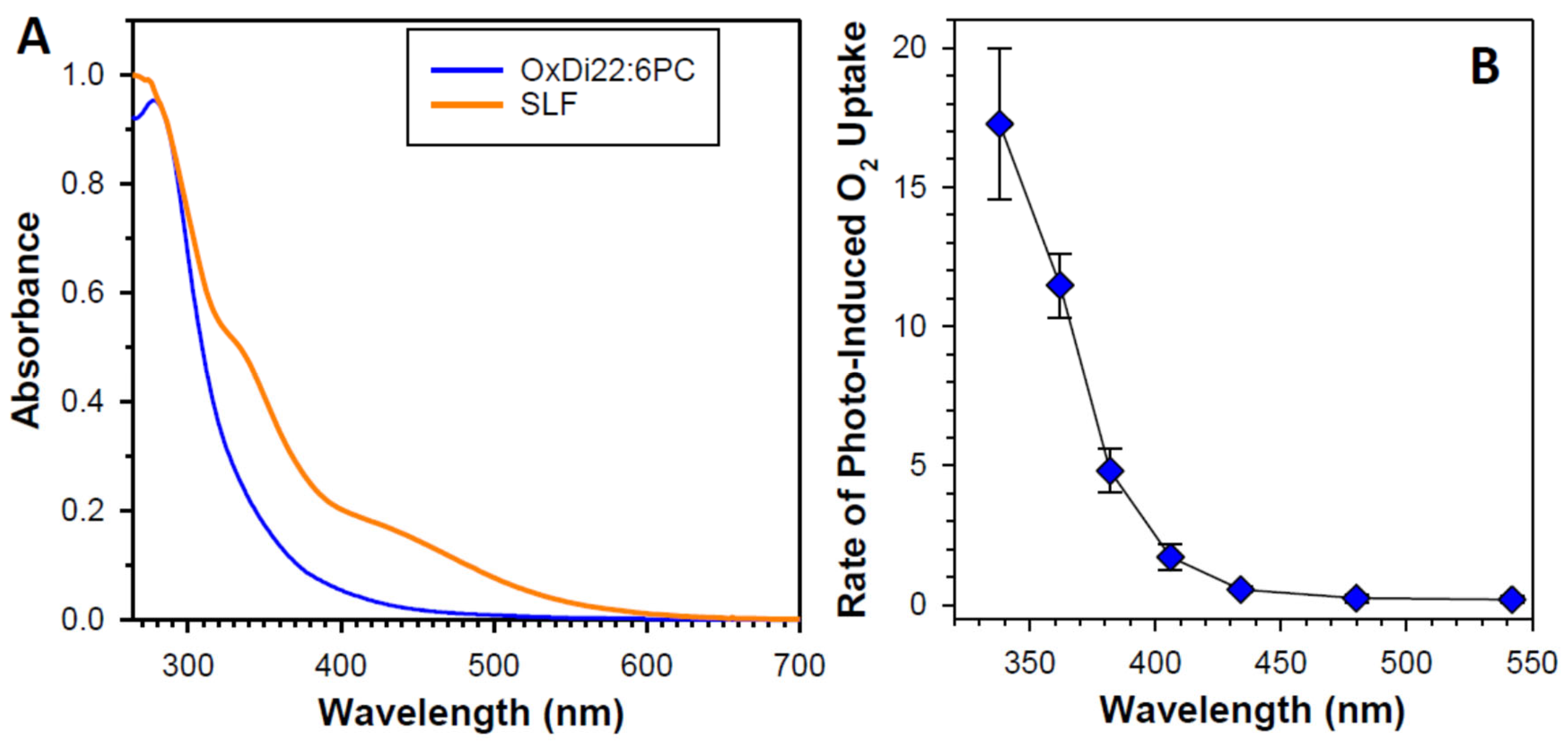
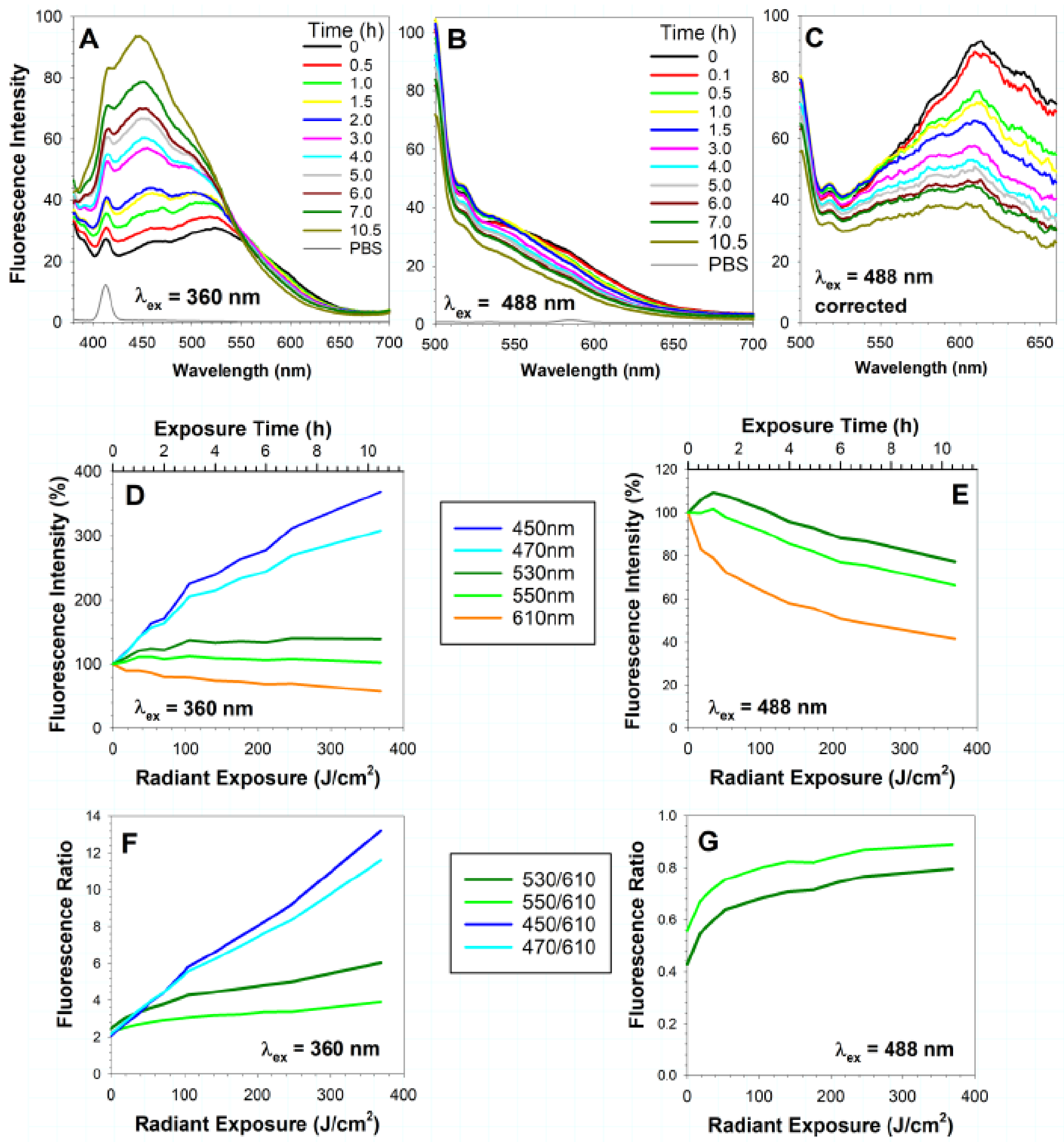

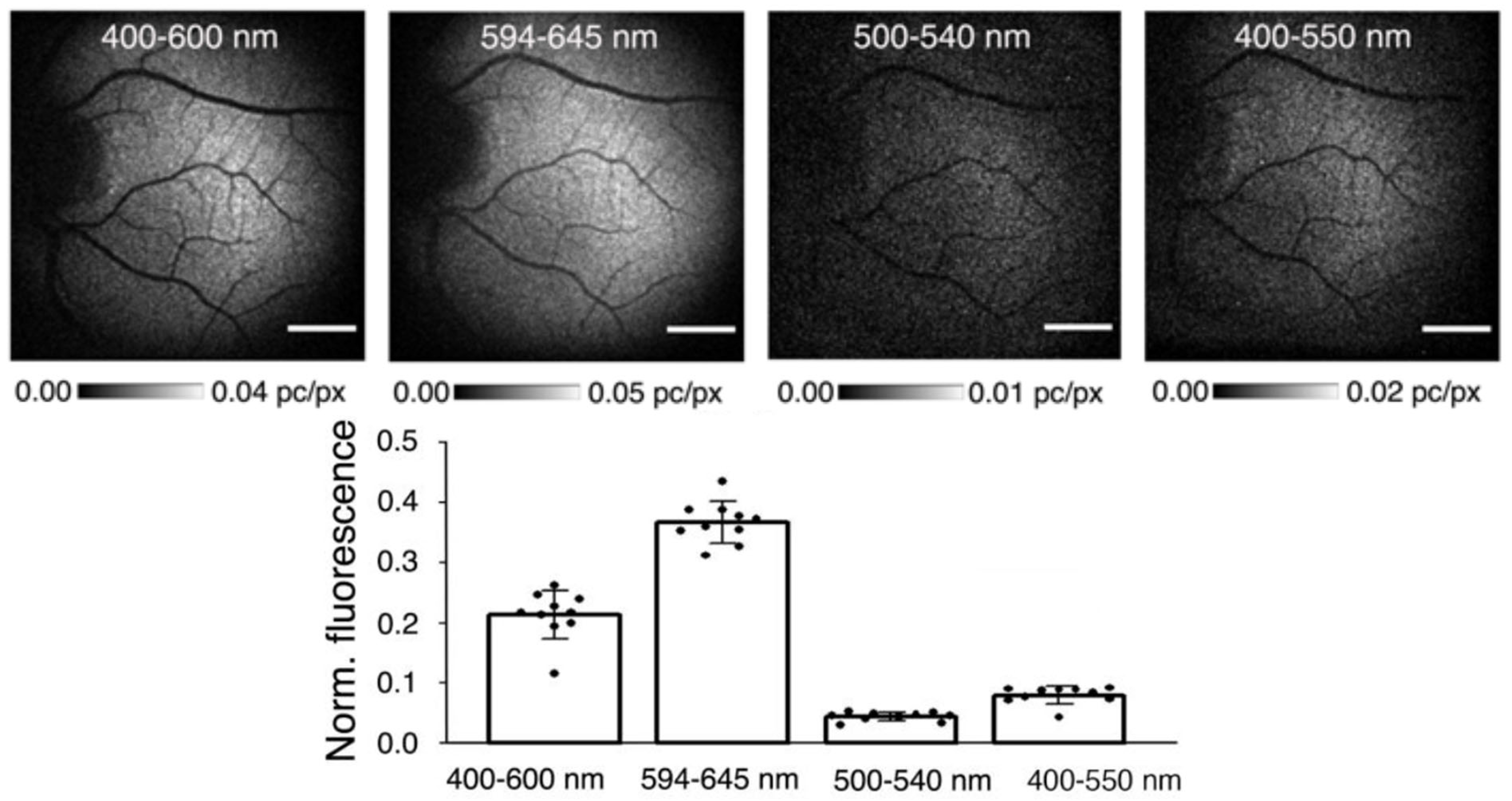
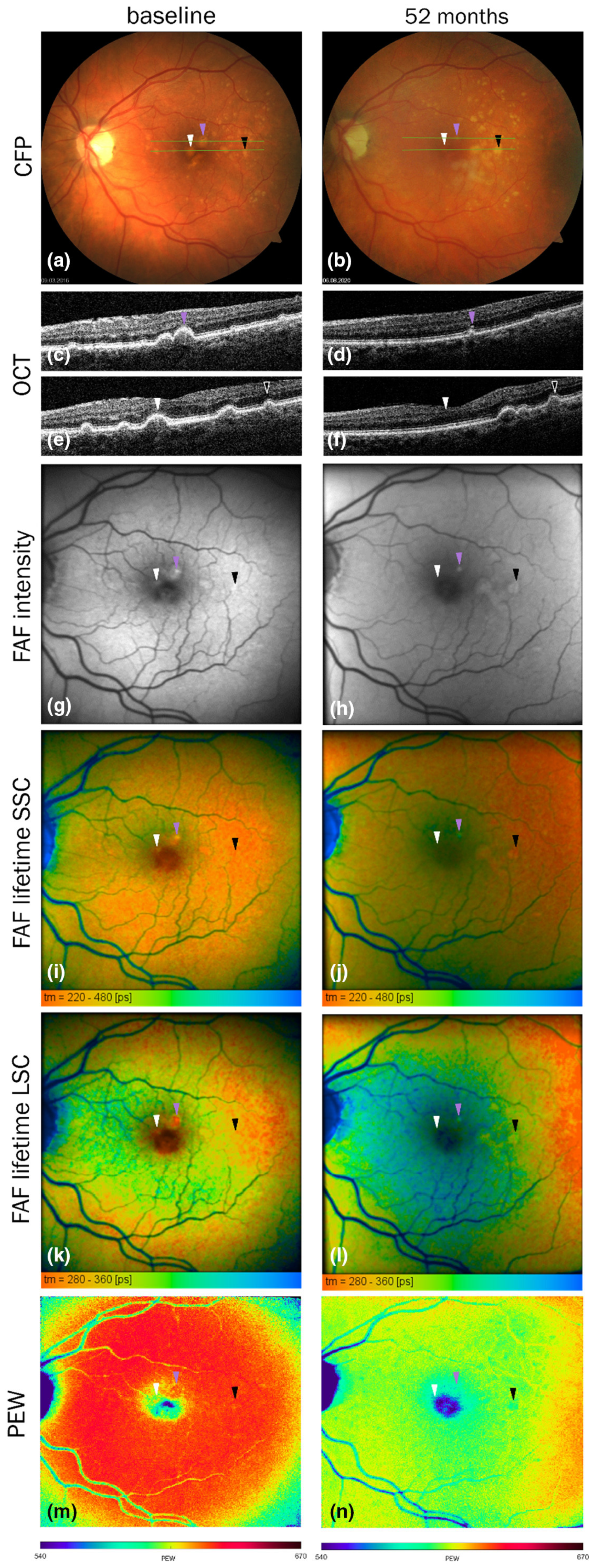
| Fluorophore | The Main Location in the Retina | Excitation | Emission | Effect of (Photo)Oxidation/Degradation |
|---|---|---|---|---|
| NAD(P)H | Photoreceptor inner segment | 300–400 nm (maximum at 340 nm) | 400–650 nm (maxima at 440 and 460 nm in the protein-bound and free state, respectively) | Decrease in fluorescence |
| Flavins | Photoreceptor inner segment | 300–500 nm (maxima at 360 and 445 nm) | 470–650 nm (maximum at 540 nm) | Decrease in fluorescence of the reduced form (due to interaction with superoxide); increase in 400–480 nm fluorescence of the oxidized form |
| Retinyl esters | RPE | 300–360 nm (maximum at 330 nm) | 400–650 nm (maximum at ~490 nm) | Decrease in fluorescence |
| Lipofuscin | RPE | Broad excitation spectrum covering 300–550 nm; 568 nm, 633 nm | Broad emission spectrum dependent on the excitation wavelength covering at least 420–725 nm range | An increase in the short-wavelength fluorescence and a decrease in the long-wavelength fluorescence |
| Melanolipofuscin | RPE | Broad excitation spectrum covering at least 300–500 nm | Broad emission spectrum covering at least 420–700 nm | Not determined |
| Melanosomes | RPE | Broad excitation spectrum covering at least 300–500 nm; 633 nm; 780–795 nm | Broad emission spectrum covering visible range 420–700 nm; near-infrared excitation and emission used in clinical settings | An increase in fluorescence |
| Drusen and other deposits in Bruch’s membrane | Bruch’s membrane | Excitation spectra not reported; excited by 364, 488, 545, 555, 568, 605, 633 nm; two-photon excitation with 960 nm | Broad emission spectrum 500–650 nm dependent on the excitation wavelength; emission with a maximum at 540–550 nm upon excitation with 436, 480, or 488 nm; emission maximum at 560 nm upon two-photon excitation with 960 nm | Not determined |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Różanowska, M.B. Lipofuscin, Its Origin, Properties, and Contribution to Retinal Fluorescence as a Potential Biomarker of Oxidative Damage to the Retina. Antioxidants 2023, 12, 2111. https://doi.org/10.3390/antiox12122111
Różanowska MB. Lipofuscin, Its Origin, Properties, and Contribution to Retinal Fluorescence as a Potential Biomarker of Oxidative Damage to the Retina. Antioxidants. 2023; 12(12):2111. https://doi.org/10.3390/antiox12122111
Chicago/Turabian StyleRóżanowska, Małgorzata B. 2023. "Lipofuscin, Its Origin, Properties, and Contribution to Retinal Fluorescence as a Potential Biomarker of Oxidative Damage to the Retina" Antioxidants 12, no. 12: 2111. https://doi.org/10.3390/antiox12122111
APA StyleRóżanowska, M. B. (2023). Lipofuscin, Its Origin, Properties, and Contribution to Retinal Fluorescence as a Potential Biomarker of Oxidative Damage to the Retina. Antioxidants, 12(12), 2111. https://doi.org/10.3390/antiox12122111






