Food Polyphenols as Preventive Medicine
Abstract
1. Introduction
2. Polyphenols as Reducing Agents and Antioxidants
3. Polyphenols as Pro-Oxidants
4. Polyphenols and the GI Tract
4.1. Mouth
4.2. Stomach
4.3. Intestine
4.4. Colon
5. Cardiovascular System
5.1. Polyphenols Action in the Cardiovascular System
5.2. Proliferation and Angiogenesis
5.3. Protection, Adaptation, and Cell Surviving
6. Polyphenols and Diabetes
6.1. Beta Cells
6.2. Diabetes, Liver and Hepatic Gluconeogenesis
6.3. Diabetes, Cardiovascular, Muscle Cells, Adipocyte, and Kidney
7. Polyphenols Neurons, Brain Function and Ageing
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Pourcel, L.; Routaboul, J.M.; Cheynier, V.; Lepiniec, L.; Debeaujon, I. Flavonoid oxidation in plants: From biochemical properties to physiological functions. Trends Plant Sci. 2007, 12, 29–36. [Google Scholar] [CrossRef]
- Kanner, J. Polyphenols by Generating H2O2, Affect Cell Redox Signaling, Inhibit PTPs and Activate Nrf2 Axis for Adaptation and Cell Surviving: In Vitro, In Vivo and Human Health. Antioxidants 2020, 9, 797. [Google Scholar] [CrossRef]
- Kanner, J.; German, J.B.; Kinsella, J.E. Initiation of lipid peroxidation in biological systems. Crit. Rev. Food Sci. Nutr. 1987, 25, 317–364. [Google Scholar] [CrossRef]
- Wright, J.S.; Johnson, E.R.; DiLabio, G.A. Predicting the activity of phenolic antioxidants: Theoretical method, analysis of substituent effects, and application to major families of antioxidants. J. Am. Chem. Soc. 2001, 123, 1173–1183. [Google Scholar] [CrossRef]
- Foti, M.C. Antioxidant properties of phenols. J. Pharm. Pharmacol. 2007, 59, 1673–1685. [Google Scholar] [CrossRef]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as Antioxidants-Determination of Radical-Scavenging Efficiencies. Methods Enzymol. 1990, 186, 343–355. [Google Scholar]
- Lapidot, T.; Granit, R.; Kanner, J. Lipid hydroperoxidase activity of myoglobin and phenolic antioxidants in simulated gastric fluid. J. Agric. Food Chem. 2005, 53, 3391–3396. [Google Scholar] [CrossRef]
- Foti, M.; Ingold, K.U.; Lusztyk, J. The Surprisingly High Reactivity of Phenoxyl Radicals. J. Am. Chem. Soc. 1994, 116, 9440–9447. [Google Scholar] [CrossRef]
- Galati, G.; O’Brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef]
- Lapidot, T.; Walker, M.D.; Kanner, J. Antioxidant and prooxidant effects of phenolics on pancreatic beta-cells in vitro. J. Agric. Food Chem. 2002, 50, 7220–7225. [Google Scholar] [CrossRef]
- Lapidot, T.; Walker, M.D.; Kanner, J. Can apple antioxidants inhibit tumor cell proliferation? Generation of H2O2 during interaction of phenolic compounds with cell culture media. J. Agric. Food Chem. 2002, 50, 3156–3160. [Google Scholar] [CrossRef]
- Zhang, K.; Dong, R.; Sun, K.; Wang, X.; Wang, J.; Yang, C.S.; Zhang, J. Synergistic toxicity of epigallocatechin-3-gallate and diethyldithiocarbamate, a lethal encounter involving redox-active copper. Free Radic. Biol. Med. 2017, 113, 143–156. [Google Scholar] [CrossRef]
- Tian, L.; Zhang, S.L.; Yi, J.H.; Zhu, Z.B.; Cui, L.Q.; Decker, E.A.; McClements, D.J. Antioxidant and prooxidant activities of tea polyphenols in oil-in-water emulsions depend on the level used and the location of proteins. Food Chem. 2022, 375, 131672. [Google Scholar] [CrossRef]
- Satoh, T.; McKercher, S.R.; Lipton, S.A. Reprint of: Nrf2/ARE-mediated antioxidant actions of pro-electrophilic drugs. Free Radic. Biol. Med. 2014, 66, 45–57. [Google Scholar] [CrossRef]
- Guo, B.; Chou, F.; Huang, L.; Yin, F.; Fang, J.; Wang, J.B.; Jia, Z. Recent insights into oxidative metabolism of quercetin: Catabolic profiles, degradation pathways, catalyzing metalloenzymes and molecular mechanisms. Crit. Rev. Food Sci. Nutr. 2022, 211, 1–28. [Google Scholar] [CrossRef]
- Xiao, L.; Sun, Y.; Tsao, R. Paradigm Shift in Phytochemicals Research: Evolution from Antioxidant Capacity to Anti-Inflammatory Effect and to Roles in Gut Health and Metabolic Syndrome. J. Agric. Food Chem. 2022, 70, 8551–8568. [Google Scholar] [CrossRef]
- Gorelik, S.; Kohen, R.; Ligumsky, M.; Kanner, J. Saliva plays a dual role in oxidation process in stomach medium. Arch. Biochem. Biophys. 2007, 458, 236–243. [Google Scholar] [CrossRef]
- Ginsburg, I.; Koren, E.; Shalish, M.; Kanner, J.; Kohen, R. Saliva increases the availability of lipophilic polyphenols as antioxidants and enhances their retention in the oral cavity. Arch. Oral Biol. 2012, 57, 1327–1334. [Google Scholar] [CrossRef]
- Bodet, C.; Grenier, D.; Chandad, F.; Ofek, I.; Steinberg, D.; Weiss, E.I. Potential oral health benefits of cranberry. Crit. Rev. Food Sci. Nutr. 2008, 48, 672–680. [Google Scholar] [CrossRef]
- Archer, M.C.; Tannenbaum, S.R.; Fan, T.Y.; Weisman, M. Reaction of nitrite with ascorbate and its relation to nitrosamine formation. J. Natl. Cancer Inst. 1975, 54, 1203–1205. [Google Scholar] [CrossRef]
- Volk, J.; Gorelik, S.; Granit, R.; Kohen, R.; Kanner, J. The dual function of nitrite under stomach conditions is modulated by reducing compounds. Free Radic. Biol. Med. 2009, 47, 496–502. [Google Scholar] [CrossRef]
- Kanner, J.; Lapidot, T. The stomach as a bioreactor: Dietary lipid peroxidation in the gastric fluid and the effects of plant-derived antioxidants. Free Radic. Biol. Med. 2001, 31, 1388–1395. [Google Scholar] [CrossRef]
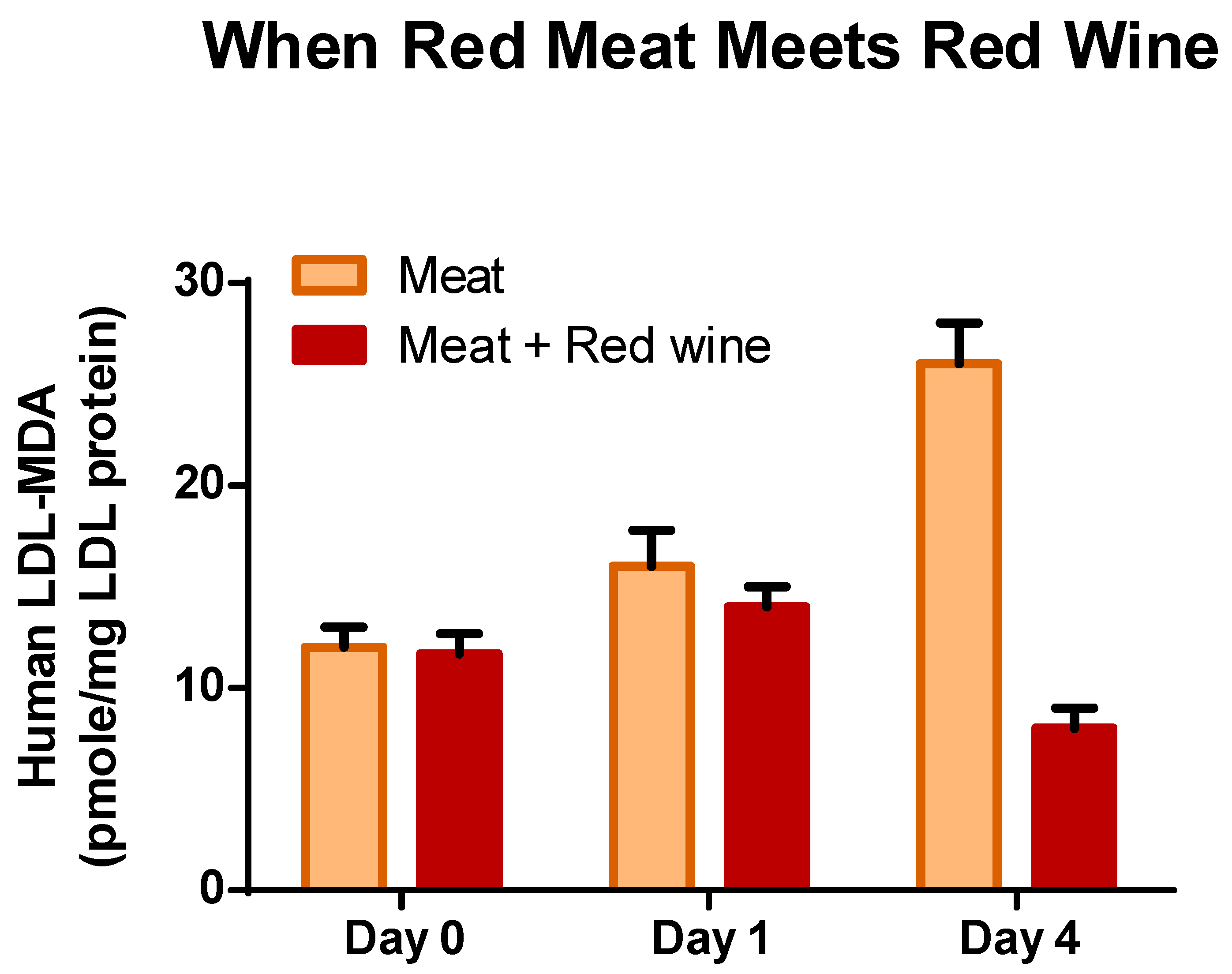
- Gorelik, S.; Ligumsky, M.; Kohen, R.; Kanner, J. The stomach as a “bioreactor”: When red meat meets red wine. J. Agric. Food Chem. 2008, 56, 5002–5007. [Google Scholar] [CrossRef]
- Natella, F.; Macone, A.; Ramberti, A.; Forte, M.; Mattivi, F.; Matarese, R.M.; Scaccini, C. Red wine prevents the postprandial increase in plasma cholesterol oxidation products: A pilot study. Br. J. Nutr. 2011, 105, 1718–1723. [Google Scholar] [CrossRef]
- Gorelik, S.; Lapidot, T.; Shaham, I.; Granit, R.; Ligumsky, M.; Kohen, R.; Kanner, J. Lipid peroxidation and coupled vitamin oxidation in simulated and human gastric fluid inhibited by dietary polyphenols: Health implications. J. Agric. Food Chem. 2005, 53, 3397–3402. [Google Scholar] [CrossRef]
- Kanner, J.; Gorelik, S.; Roman, S.; Kohen, R. Protection by polyphenols of postprandial human plasma and low-density lipoprotein modification: The stomach as a bioreactor. J. Agric. Food Chem. 2012, 60, 8790–8796. [Google Scholar] [CrossRef]
- Kanner, J. Dietary advanced lipid oxidation endproducts are risk factors to human health. Mol. Nutr. Food Res. 2007, 51, 1094–1101. [Google Scholar] [CrossRef]
- Van Hecke, T.; Ho, P.L.; Goethals, S.; De Smet, S. The potential of herbs and spices to reduce lipid oxidation during heating and gastrointestinal digestion of a beef product. Food Res. Int. 2017, 102, 785–792. [Google Scholar] [CrossRef]
- Tirosh, O.; Shpaizer, A.; Kanner, J. Lipid Peroxidation in a Stomach Medium Is Affected by Dietary Oils (Olive/Fish) and Antioxidants: The Mediterranean versus Western Diet. J. Agric. Food Chem. 2015, 63, 7016–7023. [Google Scholar] [CrossRef]
- Duman, E.; Kurban, H. Investigation of in vitro formation of advanced lipoxidation end products and advanced glycation end products precursors in high-fat processed meat products. Food Sci. Technol. 2022, 42, e110921. [Google Scholar] [CrossRef]
- Grootveld, M.; Percival, B.C.; Leenders, J.; Wilson, P.B. Potential Adverse Public Health Effects Afforded by the Ingestion of Dietary Lipid Oxidation Product Toxins: Significance of Fried Food Sources. Nutrients 2020, 12, 974. [Google Scholar] [CrossRef]
- Wang, Z.M.; Wu, Z.Y.; Tu, J.C.; Xu, B.C. Muscle food and human health: A systematic review from the perspective of external and internal oxidation. Trends Food Sci. Technol. 2023, 138, 85–99. [Google Scholar] [CrossRef]
- Draper, H.H.; Polensek, L.; Hadley, M.; McGirr, L.G. Urinary malondialdehyde as an indicator of lipid peroxidation in the diet and in the tissues. Lipids 1984, 19, 836–843. [Google Scholar] [CrossRef]
- Suomela, J.-P.; Ahotupa, M.; Kallio, H. Triacylglycerol oxidation in pig lipoproteins after a diet rich in oxidized sunflower seed oil. Lipids 2005, 40, 437–444. [Google Scholar] [CrossRef]
- Cepas, V.; Collino, M.; Mayo, J.C.; Sainz, R.M. Redox Signaling and Advanced Glycation Endproducts (AGEs) in Diet-Related Diseases. Antioxidants 2020, 9, 142. [Google Scholar] [CrossRef]
- Snelson, M.; Lucut, E.; Coughlan, M.T. The Role of AGE-RAGE Signalling as a Modulator of Gut Permeability in Diabetes. Int. J. Mol. Sci. 2022, 23, 1766. [Google Scholar] [CrossRef]
- Bloomer, R.J.; Kabir, M.M.; Marshall, K.E.; Canale, R.E.; Farney, T.M. Postprandial oxidative stress in response to dextrose and lipid meals of differing size. Lipids Health Dis. 2010, 9, 79. [Google Scholar] [CrossRef]
- Fisher-Wellman, K.H.; Bloomer, R.J. Exacerbated Postprandial Oxidative Stress Induced by the Acute Intake of a Lipid Meal Compared to Isoenergetically Administered Carbohydrate, Protein, and Mixed Meals in Young, Healthy Men. J. Am. Coll. Nutr. 2010, 29, 373–381. [Google Scholar] [CrossRef]
- Garay-Sevilla, M.E.; Rojas, A.; Portero-Otin, M.; Uribarri, J. Dietary AGEs as Exogenous Boosters of Inflammation. Nutrients 2021, 13, 2802. [Google Scholar] [CrossRef]
- Grootveld, M.; Atherton, M.D.; Sheerin, A.N.; Hawkes, J.; Blake, D.R.; Richens, T.E.; Silwood, C.J.; Lynch, E.; Claxson, A.W. In vivo absorption, metabolism, and urinary excretion of alpha, beta-unsaturated aldehydes in experimental animals. Relevance to the development of cardiovascular diseases by the dietary ingestion of thermally stressed polyunsaturate-rich culinary oils. J. Clin. Investig. 1998, 101, 1210–1218. [Google Scholar] [CrossRef]
- Gorelik, S.; Ligumsky, M.; Kohen, R.; Kanner, J. A novel function of red wine polyphenols in humans: Prevention of absorption of cytotoxic lipid peroxidation products. FASEB J. 2008, 22, 41–46. [Google Scholar] [CrossRef]
- Cruz, N.; Flores, M.; Urquiaga, I.; Avila, F. Modulation of 1,2-Dicarbonyl Compounds in Postprandial Responses Mediated by Food Bioactive Components and Mediterranean Diet. Antioxidants 2022, 11, 1513. [Google Scholar] [CrossRef]
- Urquiaga, I.; Avila, F.; Echeverria, G.; Perez, D.; Trejo, S.; Leighton, F. A Chilean Berry Concentrate Protects against Postprandial Oxidative Stress and Increases Plasma Antioxidant Activity in Healthy Humans. Oxid. Med. Cell. Longev. 2017, 2017, 8361493. [Google Scholar] [CrossRef]
- Urquiaga, I.; Troncoso, D.; Mackenna, M.J.; Urzua, C.; Perez, D.; Dicenta, S.; de la Cerda, P.M.; Amigo, L.; Carreno, J.C.; Echeverria, G.; et al. The Consumption of Beef Burgers Prepared with Wine Grape Pomace Flour Improves Fasting Glucose, Plasma Antioxidant Levels, and Oxidative Damage Markers in Humans: A Controlled Trial. Nutrients 2018, 10, 1388. [Google Scholar] [CrossRef]
- Li, Z.; Henning, S.M.; Zhang, Y.; Rahnama, N.; Zerlin, A.; Thames, G.; Tseng, C.H.; Heber, D. Decrease of postprandial endothelial dysfunction by spice mix added to high-fat hamburger meat in men with Type2 diabetes mellitus. Diabet. Med. 2013, 30, 590–595. [Google Scholar] [CrossRef]
- Gobert, M.; Remond, D.; Loonis, M.; Buffiere, C.; Sante-Lhoutellier, V.; Dufour, C. Fruits, vegetables and their polyphenols protect dietary lipids from oxidation during gastric digestion. Food Funct. 2014, 5, 2166–2174. [Google Scholar] [CrossRef]
- Gerasopoulos, K.; Stagos, D.; Petrotos, K.; Kokkas, S.; Kantas, D.; Goulas, P.; Kouretas, D. Feed supplemented with polyphenolic byproduct from olive mill wastewater processing improves the redox status in blood and tissues of piglets. Food Chem. Toxicol. 2015, 86, 319–327. [Google Scholar] [CrossRef]
- Kanner, J.; Selhub, J.; Shpaizer, A.; Rabkin, B.; Shacham, I.; Tirosh, O. Redox homeostasis in stomach medium by foods: The Postprandial Oxidative Stress Index (POSI) for balancing nutrition and human health. Redox Biol. 2017, 12, 929–936. [Google Scholar] [CrossRef]
- Sottero, B.; Rossin, D.; Poli, G.; Biasi, F. Lipid Oxidation Products in the Pathogenesis of Inflammation-related Gut Diseases. Curr. Med. Chem. 2018, 25, 1311–1326. [Google Scholar] [CrossRef]
- Nieva-Echevarria, B.; Goicoechea, E.; Guillen, M.D. Food lipid oxidation under gastrointestinal digestion conditions: A review. Crit. Rev. Food Sci. Nutr. 2018, 60, 461–478. [Google Scholar] [CrossRef]
- Guina, T.; Deiana, M.; Calfapietra, S.; Cabboi, B.; Maina, M.; Tuberoso, C.I.; Leonarduzzi, G.; Gamba, P.; Gargiulo, S.; Testa, G.; et al. The role of p38 MAPK in the induction of intestinal inflammation by dietary oxysterols: Modulation by wine phenolics. Food Funct. 2015, 6, 1218–1228. [Google Scholar] [CrossRef]
- Mascia, C.; Maina, M.; Chiarpotto, E.; Leonarduzzi, G.; Poli, G.; Biasi, F. Proinflammatory effect of cholesterol and its oxidation products on CaCo-2 human enterocyte-like cells effective protection by epigallocatechin-3-gallate. Free Radic. Biol. Med. 2010, 49, 2049–2057. [Google Scholar] [CrossRef]
- Tannenbaum, S.R.; Sinskey, A.J.; Weisman, M.; Bishop, W. Nitrite in Human Saliva-Its Possible Relationship to Nitrosamine Formation. JNCI-J. Natl. Cancer Inst. 1974, 53, 79–84. [Google Scholar] [CrossRef]
- Reddy, D.; Lancaster, J.R., Jr.; Cornforth, D.P. Nitrite inhibition of Clostridium botulinum: Electron spin resonance detection of iron-nitric oxide complexes. Science 1983, 221, 769–770. [Google Scholar] [CrossRef]
- Fox, J.B., Jr.; Thomson, J.S. Formation of bovine nitrosylmyoglobin. I. pH 4.5–6.5. Biochemistry 1963, 2, 465–470. [Google Scholar] [CrossRef]
- Pearson, A.; Love, J.D.; Shorland, F. “Warmed-over” flavor in meat, poultry, and fish. Adv. Food Res. 1977, 23, 1–74. [Google Scholar]
- Kanner, J.; Ben-Gera, I.; Berman, S. Nitric-oxide myoglobin as an inhibitor of lipid oxidation. Lipids 1980, 15, 944. [Google Scholar] [CrossRef]
- Kanner, J.; Harel, S.; Rina, G. Nitric oxide as an antioxidant. Arch. Biochem. Biophys. 1991, 289, 130–136. [Google Scholar] [CrossRef]
- Kanner, J.; Juven, B.J. S-nitrosocysteine as an antioxidant, color-developing, and anticlostridial agent in comminuted turkey meat. J. Food Sci. 1980, 45, 1105–1112. [Google Scholar] [CrossRef]
- IRAC. Monographs Evaluate Consumption of Red Meat and Processed Meat; Press release No. 240; International Agency for Research on Cancer, World Health Organization: Geneva, Switzerland, 2015.
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef]
- Mirvish, S.S.; Shubik, P.; Wallcave, L.; Eagen, M. Ascorbate-Nitrite Reaction-Possible Means of Blocking Formation of Carcinogenic N-Nitroso Compounds. Science 1972, 177, 65–68. [Google Scholar] [CrossRef]
- Tannenbaum, S.R.; Wishnok, J.S.; Leaf, C.D. Inhibition of nitrosamine formation by ascorbic acid. Am. J. Clin. Nutr. 1991, 53, 247S–250S. [Google Scholar] [CrossRef]
- Mirvish, S.S. Blocking the formation of N-nitroso compounds with ascorbic acid in vitro and in vivo. Ann. N. Y. Acad. Sci. 1975, 258, 175–180. [Google Scholar] [CrossRef]
- Leaf, C.D.; Vecchio, A.J.; Roe, D.A.; Hotchkiss, J.H. Influence of Ascorbic-Acid Dose on N-Nitrosoproline Formation in Humans. Carcinogenesis 1987, 8, 791–795. [Google Scholar] [CrossRef]
- Challis, B.C. Rapid Nitrosation of Phenols and Its Implications for Health Hazards from Dietary Nitrites. Nature 1973, 244, 466. [Google Scholar] [CrossRef]
- Tanaka, K.; Hayatsu, T.; Negishi, T.; Hayatsu, H. Inhibition of N-nitrosation of secondary amines in vitro by tea extracts and catechins. Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 1998, 412, 91–98. [Google Scholar] [CrossRef]
- Halliwell, B.; Zhao, K.C.; Whiteman, M. The gastrointestinal tract: A major site of antioxidant action? Free Radic. Res. 2000, 33, 819–830. [Google Scholar] [CrossRef]
- Kuenzig, W.; Chau, J.; Norkus, E.; Holowaschenko, H.; Newmark, H.; Mergens, W.; Conney, A.H. Caffeic and Ferulic Acid as Blockers of Nitrosamine Formation. Carcinogenesis 1984, 5, 309–313. [Google Scholar] [CrossRef]
- Mirvish, S.S.; Cardesa, A.; Wallcave, L.; Shubik, P. Induction of Mouse Lung Adenomas by Amines or Ureas Plus Nitrite and by N-Nitroso Compounds-Effect of Ascorbate, Gallic Acid, Thiocyanate, and Caffeine. J. Natl. Cancer Inst. 1975, 55, 633–636. [Google Scholar] [CrossRef]
- Helser, M.A.; Hotchkiss, J.H.; Roe, D.A. Influence of Fruit and Vegetable Juices on the Endogenous Formation of N-Nitrosoproline and N-Nitrosothiazolidine-4-Carboxylic Acid in Humans on Controlled Diets. Carcinogenesis 1992, 13, 2277–2280. [Google Scholar] [CrossRef]
- Combet, E.; El Mesmari, A.; Preston, T.; Crozier, A.; McColl, K.E.L. Dietary phenolic acids and ascorbic acid: Influence on acid-catalyzed nitrosative chemistry in the presence and absence of lipids. Free Radic. Biol. Med. 2010, 48, 763–771. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Lundberg, J.O. Dietary flavonoids and circulating concentrations of nitrate, nitrite, and S-nitrosothiols. Am. J. Clin. Nutr. 2009, 89, 652. [Google Scholar] [CrossRef][Green Version]
- Curtis, P.J.; van der Velpen, V.; Berends, L.; Jennings, A.; Feelisch, M.; Umpleby, A.M.; Evans, M.; Fernandez, B.O.; Meiss, M.S.; Minnion, M.; et al. Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syndrome-results from a 6-month, double-blind, randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1535–1545. [Google Scholar] [CrossRef]
- McDougall, G.J.; Kulkarni, N.N.; Stewart, D. Current developments on the inhibitory effects of berry polyphenols on digestive enzymes. BioFactors 2008, 34, 73–80. [Google Scholar] [CrossRef]
- Williamson, G. Possible effects of dietary polyphenols on sugar absorption and digestion. Mol. Nutr. Food Res. 2013, 57, 48–57. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.; Ghosh, A.K.; Ghosh, C. Recent developments on polyphenol-protein interactions: Effects on tea and coffee taste, antioxidant properties and the digestive system. Food Funct. 2012, 3, 592–605. [Google Scholar] [CrossRef]
- Velickovic, T.D.C.; Stanic-Vucinic, D.J. The Role of Dietary Phenolic Compounds in Protein Digestion and Processing Technologies to Improve Their Antinutritive Properties. Compr. Rev. Food Sci. Food Saf. 2018, 17, 82–103. [Google Scholar] [CrossRef]
- Gilani, G.S.; Sepehr, E. Protein digestibility and quality in products containing antinutritional factors are adversely affected by old age in rats. J. Nutr. 2003, 133, 220–225. [Google Scholar] [CrossRef]
- Juven, B.; Henis, Y. Studies on Antimicrobial Activity of Olive Phenolic Compounds. J. Appl. Bacteriol. 1970, 33, 721–732. [Google Scholar] [CrossRef]
- Naz, S.; Siddiqi, R.; Dew, T.P.; Williamson, G. Epigallocatechin-3-gallate Inhibits Lactase but Is Alleviated by Salivary Proline-Rich Proteins. J. Agric. Food Chem. 2011, 59, 2734–2738. [Google Scholar] [CrossRef]
- Pyner, A.; Nyambe-Silavwe, H.; Williamson, G. Inhibition of Human and Rat Sucrase and Maltase Activities to Assess Antiglycemic Potential: Optimization of the Assay Using Acarbose and Polyphenols. J. Agric. Food Chem. 2017, 65, 8643–8651. [Google Scholar] [CrossRef]
- Hanhineva, K.; Torronen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkanan, H.; Poutanen, K. Impact of Dietary Polyphenols on Carbohydrate Metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef]
- Juhel, C.; Armand, M.; Pafumi, Y.; Rosier, C.; Vandermander, J.; Lairon, D. Green tea extract (AR25 (R)) inhibits lipolysis of triglycerides in gastric and duodenal medium in vitro. J. Nutr. Biochem. 2000, 11, 45–51. [Google Scholar] [CrossRef]
- Sugiyama, H.; Akazome, Y.; Shoji, T.; Yamaguchi, A.; Yasue, M.; Kanda, T.; Ohtake, Y. Oligomeric procyanidins in apple polyphenol are main active components for inhibition of pancreatic lipase and tri-glyceride absorption. J. Agric. Food Chem. 2007, 55, 4604–4609. [Google Scholar] [CrossRef]
- Wu, X.L.; Feng, Y.; Lu, Y.Q.; Li, Y.; Fan, L.; Liu, L.Z.; Wu, K.M.; Wang, X.R.; Zhang, B.S.; He, Z.D. Effect of phenolic hydroxyl groups on inhibitory activities of phenylpropanoid glycosides against lipase. J. Funct. Foods 2017, 38, 510–518. [Google Scholar] [CrossRef]
- Glisan, S.L.; Grove, K.A.; Yennawar, N.H.; Lambert, J.D. Inhibition of pancreatic lipase by black tea theaflavins: Comparative enzymology and in silico modeling studies. Food Chem. 2017, 216, 296–300. [Google Scholar] [CrossRef]
- Li, S.M.; Zhang, L.; Wan, X.C.; Zhan, J.F.; Ho, C.T. Focusing on the recent progress of tea polyphenol chemistry and perspectives. Food Sci. Hum. Wellness 2022, 11, 437–444. [Google Scholar] [CrossRef]
- Huang, Q.J.; Zhu, Y.D.; Lv, L.S.; Sang, S.M. Translating In Vitro Acrolein-Trapping Capacities of Tea Polyphenol and Soy Genistein to In Vivo Situation is Mediated by the Bioavailability and Biotransformation of Individual Polyphenols. Mol. Nutr. Food Res. 2020, 64, e1900274. [Google Scholar] [CrossRef]
- Bernardi, S.; Del Bo, C.; Marino, M.; Gargari, G.; Cherubini, A.; Andres-Lacueva, C.; Hidalgo-Liberona, N.; Peron, G.; Gonzalez-Dominguez, R.; Kroon, P.; et al. Polyphenols and Intestinal Permeability: Rationale and Future Perspectives. J. Agric. Food Chem. 2020, 68, 1816–1829. [Google Scholar] [CrossRef]
- Lee, S.; Keirsey, K.I.; Kirkland, R.; Grunewald, Z.I.; Fischer, J.G.; de La Serre, C.B. Blueberry Supplementation Influences the Gut Microbiota, Inflammation, and Insulin Resistance in High-Fat-Diet-Fed Rats. J. Nutr. 2018, 148, 209–219. [Google Scholar] [CrossRef]
- Garcia-Mantrana, I.; Selma-Royo, M.; Alcantara, C.; Collado, M.C. Shifts on Gut Microbiota Associated to Mediterranean Diet Adherence and Specific Dietary Intakes on General Adult Population. Front. Microbiol. 2018, 9, 890. [Google Scholar] [CrossRef]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M.; et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun. 2019, 10, 89. [Google Scholar] [CrossRef]
- Yanaka, A. Role of NRF2 in protection of the gastrointestinal tract against oxidative stress. J. Clin. Biochem. Nutr. 2018, 63, 18–25. [Google Scholar] [CrossRef]
- Gil-Cardoso, K.; Gines, I.; Pinent, M.; Ardevol, A.; Blay, M.; Terra, X. Effects of flavonoids on intestinal inflammation, barrier integrity and changes in gut microbiota during diet-induced obesity. Nutr. Res. Rev. 2016, 29, 234–248. [Google Scholar] [CrossRef]
- Shimizu, M. Multifunctions of dietary polyphenols in the regulation of intestinal inflammation. J. Food Drug Anal. 2017, 25, 93–99. [Google Scholar] [CrossRef]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef]
- Kaulmann, A.; Bohn, T. Bioactivity of Polyphenols: Preventive and Adjuvant Strategies toward Reducing Inflammatory Bowel Diseases-Promises, Perspectives, and Pitfalls. Oxid. Med. Cell. Longev. 2016, 2016, 9346470. [Google Scholar] [CrossRef]
- Das, J.; Ramani, R.; Suraju, M.O. Polyphenol compounds and PKC signaling. Biochim. Biophys. Acta 2016, 1860, 2107–2121. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wu, H.; Wang, X.; He, J.; He, S.; Yin, Y. Resveratrol Attenuates Oxidative Stress-Induced Intestinal Barrier Injury through PI3K/Akt-Mediated Nrf2 Signaling Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 7591840. [Google Scholar] [CrossRef]
- Iglesias, D.E.; Cremonini, E.; Fraga, C.G.; Oteiza, P.I. Ellagic acid protects Caco-2 cell monolayers against inflammation -induced permeabilization. Free Radic. Biol. Med. 2020, 152, 776–786. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Jin, S.; Pang, Q.; Shan, A.; Feng, X. Dietary resveratrol alleviated lipopolysaccharide-induced ileitis through Nrf2 and NF-κappaB signalling pathways in ducks (Anas platyrhynchos). J. Anim. Physiol. Anim. Nutr. 2022, 106, 1306–1320. [Google Scholar] [CrossRef]
- Ferrari, D.; Speciale, A.; Cristani, M.; Fratantonio, D.; Molonia, M.S.; Ranaldi, G.; Saija, A.; Cimino, F. Cyanidin-3-O-glucoside inhibits NF-κB signalling in intestinal epithelial cells exposed to TNF-alpha and exerts protective effects via Nrf2 pathway activation. Toxicol. Lett. 2016, 264, 51–58. [Google Scholar] [CrossRef]
- Dangles, O.; Fenger, J.A. The Chemical Reactivity of Anthocyanins and Its Consequences in Food Science and Nutrition. Molecules 2018, 23, 1970. [Google Scholar] [CrossRef]
- Cremonini, E.; Mastaloudis, A.; Hester, S.N.; Verstraeten, S.V.; Anderson, M.; Wood, S.M.; Waterhouse, A.L.; Fraga, C.G.; Oteiza, P.I. Anthocyanins inhibit tumor necrosis alpha-induced loss of Caco-2 cell barrier integrity. Food Funct. 2017, 8, 2915–2923. [Google Scholar] [CrossRef]
- Wu, T.; Guo, Y.; Liu, R.; Wang, K.; Zhang, M. Black tea polyphenols and polysaccharides improve body composition, increase fecal fatty acid, and regulate fat metabolism in high-fat diet-induced obese rats. Food Funct. 2016, 7, 2469–2478. [Google Scholar] [CrossRef]
- Gil-Cardoso, K.; Gines, I.; Pinent, M.; Ardevol, A.; Arola, L.; Blay, M.; Terra, X. Chronic supplementation with dietary proanthocyanidins protects from diet-induced intestinal alterations in obese rats. Mol. Nutr. Food Res. 2017, 61, 1601039. [Google Scholar] [CrossRef]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative Effects of a High-Fat Diet on Intestinal Permeability: A Review. Adv Nutr 2020, 11, 77–91. [Google Scholar] [CrossRef]
- Body-Malapel, M.; Djouina, M.; Waxin, C.; Langlois, A.; Gower-Rousseau, C.; Zerbib, P.; Schmidt, A.M.; Desreumaux, P.; Boulanger, E.; Vignal, C. The RAGE signaling pathway is involved in intestinal inflammation and represents a promising therapeutic target for Inflammatory Bowel Diseases. Mucosal Immunol. 2019, 12, 468–478. [Google Scholar] [CrossRef]
- Saura-Calixto, F. Antioxidant dietary fiber product: A new concept and a potential food ingredient. J. Agric. Food Chem. 1998, 46, 4303–4306. [Google Scholar] [CrossRef]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am. J. Clin. Nutr. 2011, 93, 62–72. [Google Scholar] [CrossRef]
- Henning, S.M.; Yang, J.; Shao, P.; Lee, R.P.; Huang, J.; Ly, A.; Hsu, M.; Lu, Q.Y.; Thames, G.; Heber, D.; et al. Health benefit of vegetable/fruit juice-based diet: Role of microbiome. Sci. Rep. 2017, 7, 2167. [Google Scholar] [CrossRef]
- Lee, H.C.; Jenner, A.M.; Low, C.S.; Lee, Y.K. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res. Microbiol. 2006, 157, 876–884. [Google Scholar] [CrossRef]
- Costabile, A.; Klinder, A.; Fava, F.; Napolitano, A.; Fogliano, V.; Leonard, C.; Gibson, G.R.; Tuohy, K.M. Whole-grain wheat breakfast cereal has a prebiotic effect on the human gut microbiota: A double-blind, placebo-controlled, crossover study. Br. J. Nutr. 2008, 99, 110–120. [Google Scholar] [CrossRef]
- Carvalho-Wells, A.L.; Helmolz, K.; Nodet, C.; Molzer, C.; Leonard, C.; McKevith, B.; Thielecke, F.; Jackson, K.G.; Tuohy, K.M. Determination of the in vivo prebiotic potential of a maize-based whole grain breakfast cereal: A human feeding study. Br. J. Nutr. 2010, 104, 1353–1356. [Google Scholar] [CrossRef]
- Espin, J.C.; Gonzalez-Sarrias, A.; Tomas-Barberan, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly) phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef]
- Hidalgo-Liberona, N.; Gonzalez-Dominguez, R.; Vegas, E.; Riso, P.; Del Bo, C.; Bernardi, S.; Peron, G.; Guglielmetti, S.; Gargari, G.; Kroon, P.A.; et al. Increased Intestinal Permeability in Older Subjects Impacts the Beneficial Effects of Dietary Polyphenols by Modulating Their Bioavailability. J. Agric. Food Chem. 2020, 68, 12476–12484. [Google Scholar] [CrossRef]
- Del Bo, C.; Bernardi, S.; Cherubini, A.; Porrini, M.; Gargari, G.; Hidalgo-Liberona, N.; Gonzalez-Dominguez, R.; Zamora-Ros, R.; Peron, G.; Marino, M.; et al. A polyphenol-rich dietary pattern improves intestinal permeability, evaluated as serum zonulin levels, in older subjects: The MaPLE randomised controlled trial. Clin. Nutr. 2021, 40, 3006–3018. [Google Scholar] [CrossRef]
- Mafra, D.; Borges, N.A.; Lindholm, B.; Shiels, P.G.; Evenepoel, P.; Stenvinkel, P. Food as medicine: Targeting the uraemic phenotype in chronic kidney disease. Nat. Rev. Nephrol. 2021, 17, 153–171. [Google Scholar] [CrossRef]
- Hoek-van den Hil, E.F.; van Schothorst, E.M.; van der Stelt, I.; Swarts, H.J.M.; van Vliet, M.; Amolo, T.; Vervoort, J.J.M.; Venema, D.; Hollman, P.C.H.; Rietjens, I.M.C.M.; et al. Direct comparison of metabolic health effects of the flavonoids quercetin, hesperetin, epicatechin, apigenin and anthocyanins in high-fat-diet-fed mice. Genes Nutr. 2015, 10, 23. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Tav, S.I.; Rothschild, D.; Eijer, M.T.M.; Levy, M.; Moresi, C.; Dohnalova, L.; Braverman, S.; Rozin, S.; Malitsky, S.; et al. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature 2016, 540, 544–551. [Google Scholar] [CrossRef]
- Zou, T.D.; Chen, D.W.; Yang, Q.Y.; Wang, B.; Zhu, M.J.; Nathanielsz, P.W.; Du, M. Resveratrol supplementation of high-fat diet-fed pregnant mice promotes brown and beige adipocyte development and prevents obesity in male offspring. J. Physiol. 2017, 595, 1547–1562. [Google Scholar] [CrossRef]
- Goldwasser, J.; Cohen, P.Y.; Yang, E.; Balaguer, P.; Yarmush, M.L.; Nahmias, Y. Transcriptional Regulation of Human and Rat Hepatic Lipid Metabolism by the Grapefruit Flavonoid Naringenin: Role of PPAR alpha, PPAR gamma and LXR alpha. PLoS ONE 2010, 5, e12399. [Google Scholar] [CrossRef]
- Kudo, N.; Arai, Y.; Suhara, Y.; Ishii, T.; Nakayama, T.; Osakabe, N. A Single Oral Administration of Theaflavins Increases Energy Expenditure and the Expression of Metabolic Genes. PLoS ONE 2015, 10, e0137809. [Google Scholar] [CrossRef]
- Choi, J.H.; Yun, J.W. Chrysin induces brown fat-like phenotype and enhances lipid metabolism in 3T3-L1 adipocytes. Nutrition 2016, 32, 1002–1010. [Google Scholar] [CrossRef]
- Kamble, P.; Litvinov, D.; Narasimhulu, C.A.; Jiang, X.T.; Parthasarathy, S. Aspirin may influence cellular energy status. Eur. J. Pharmacol. 2015, 749, 12–19. [Google Scholar] [CrossRef]
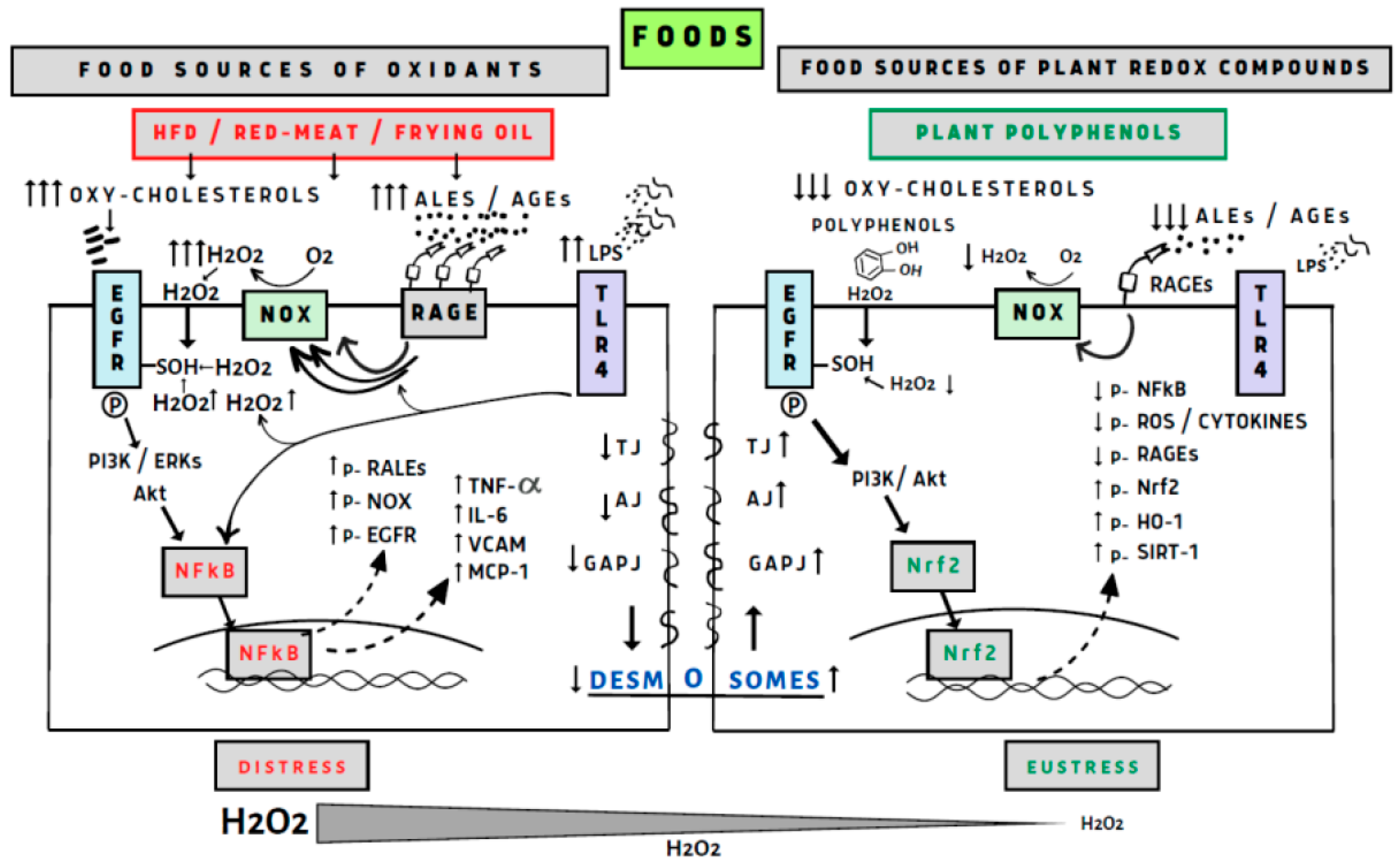
- Perez, S.; Talens-Visconti, R.; Rius-Perez, S.; Finamor, I.; Sastre, J. Redox signaling in the gastrointestinal tract. Free Radic. Biol. Med. 2017, 104, 75–103. [Google Scholar] [CrossRef]
- van der Post, S.; Birchenough, G.M.H.; Held, J.M. NOX1-dependent redox signaling potentiates colonic stem cell proliferation to adapt to the intestinal microbiota by linking EGFR and TLR activation. Cell Rep. 2021, 35, 108949. [Google Scholar] [CrossRef]
- Yanaka, A. Contribution of NRF2 in Gastrointestinal Protection from Oxidative Injury. Curr. Pharm. Des. 2018, 24, 2023–2033. [Google Scholar] [CrossRef]
- Burgueno, J.F.; Fritsch, J.; Gonzalez, E.E.; Landau, K.S.; Santander, A.M.; Fernandez, I.; Hazime, H.; Davies, J.M.; Santaolalla, R.; Phillips, M.C.; et al. Epithelial TLR4 Signaling Activates DUOX2 to Induce Microbiota-Driven Tumorigenesis. Gastroenterology 2021, 160, 797–808.e6. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Frankel, E.N.; Kanner, J.; German, J.B.; Parks, E.; Kinsella, J.E. Inhibition of oxidation of human low-density lipoprotein by phenolic substances in red wine. Lancet 1993, 341, 454–457. [Google Scholar] [CrossRef]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef]
- Kanner, J.; Frankel, E.; Granit, R.; German, B.; Kinsella, J.E. Natural Antioxidants in Grapes and Wines. J. Agric. Food Chem. 1994, 42, 64–69. [Google Scholar] [CrossRef]
- Kinsella, J.E.; Frankel, E.; German, B.; Kanner, J. Possible Mechanisms for the Protective Role of Antioxidants in Wine and Plant Foods. Food Technol. 1993, 47, 85–89. [Google Scholar]
- Halliwell, B. Dietary polyphenols: Good, bad, or indifferent for your health? Cardiovasc. Res. 2007, 73, 341–347. [Google Scholar] [CrossRef]
- Croft, K.D. Dietary polyphenols: Antioxidants or not? Arch. Biochem. Biophys. 2016, 595, 120–124. [Google Scholar] [CrossRef]
- Hollman, P.C.; Cassidy, A.; Comte, B.; Heinonen, M.; Richelle, M.; Richling, E.; Serafini, M.; Scalbert, A.; Sies, H.; Vidry, S. The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established. J. Nutr. 2011, 141, 989S–1009S. [Google Scholar] [CrossRef]
- Gorelik, S.; Kanner, J.; Schurr, D.; Kohen, R. A rational approach to prevent postprandial modification of LDL by dietary polyphenols. J. Funct. Foods 2013, 5, 163–169. [Google Scholar] [CrossRef]
- Uribarri, J.; del Castillo, M.D.; de la Maza, M.P.; Filip, R.; Gugliucci, A.; Luevano-Contreras, C.; Macias-Cervantes, M.H.; Markowicz Bastos, D.H.; Medrano, A.; Menini, T.; et al. Dietary advanced glycation end products and their role in health and disease. Adv. Nutr. 2015, 6, 461–473. [Google Scholar] [CrossRef]
- Del Turco, S.; Basta, G. An update on advanced glycation endproducts and atherosclerosis. BioFactors 2012, 38, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Stinghen, A.E.M.; Massy, Z.A.; Vlassara, H.; Striker, G.E.; Boullier, A. Uremic Toxicity of Advanced Glycation End Products in CKD. J. Am. Soc. Nephrol. 2016, 27, 354–370. [Google Scholar] [CrossRef] [PubMed]
- Tomita, H.; Tanaka, K.; Tanaka, T.; Hara, A. Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget 2016, 7, 11018–11032. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Yin, H. Covalent modification of DNA by α, β-unsaturated aldehydes derived from lipid peroxidation: Recent progress and challenges. Free Radic. Res. 2015, 49, 905–917. [Google Scholar] [CrossRef]
- Uchida, K. Aldehyde adducts generated during lipid peroxidation modification of proteins. Free Radic. Res. 2015, 49, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Thornalley, P.J. Dicarbonyl stress in cell and tissue dysfunction contributing to ageing and disease. Biochem. Biophys. Res. Commun. 2015, 458, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D.; Parthasarathy, S.; Carew, T.E.; Khoo, J.C.; Witztum, J.L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N. Engl. J. Med. 1989, 320, 915–924. [Google Scholar] [CrossRef]
- Amaki, T.; Suzuki, T.; Nakamura, F.; Hayashi, D.; Imai, Y.; Morita, H.; Fukino, K.; Nojiri, T.; Kitano, S.; Hibi, N.; et al. Circulating malondialdehyde modified LDL is a biochemical risk marker for coronary artery disease. Heart 2004, 90, 1211–1213. [Google Scholar] [CrossRef]
- Ito, T.; Fujita, H.; Tani, T.; Ohte, N. Malondialdehyde-modified low-density lipoprotein is a predictor of cardiac events in patients with stable angina on lipid-lowering therapy after percutaneous coronary intervention using drug-eluting stent. Atherosclerosis 2015, 239, 311–317. [Google Scholar] [CrossRef]
- Back, M.; Yurdagul, A.; Tabas, I.; Oorni, K.; Kovanen, P.T. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406. [Google Scholar] [CrossRef]
- Tesoriere, L.; Butera, D.; Gentile, C.; Livrea, M.A. Bioactive components of caper (Capparis spinosa L.) from Sicily and antioxidant effects in a red meat simulated gastric digestion. J. Agric. Food Chem. 2007, 55, 8465–8471. [Google Scholar] [CrossRef] [PubMed]
- Kristinova, V.; Storro, I.; Rustad, T. Influence of human gastric juice on oxidation of marine lipids-in vitro study. Food Chem. 2013, 141, 3859–3871. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remon, A.; Martinez-Gonzalez, M.A.; de la Torre, R.; Corella, D.; Salas-Salvado, J.; Gomez-Gracia, E.; Lapetra, J.; Aros, F.; et al. Inverse association between habitual polyphenol intake and incidence of cardiovascular events in the PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remon, A.; Martinez-Gonzalez, M.A.; Lopez-Sabater, M.C.; Covas, M.I.; Corella, D.; Salas-Salvado, J.; Gomez-Gracia, E.; Lapetra, J.; et al. Polyphenol intake and mortality risk: A re-analysis of the PREDIMED trial. BMC Med. 2014, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A. Berry anthocyanin intake and cardiovascular health. Mol. Asp. Med. 2018, 61, 76–82. [Google Scholar] [CrossRef]
- Rhodin, J.A.G. Ultrastructure of Mammalian Arterioles and Precapillary Sphincters. J. Ultrastruct. Res. 1967, 18, 181–223. [Google Scholar] [CrossRef]
- Lotito, S.; Zhang, W.; Yang, C.; Crozier, A.; Frei, B. Metabolic conversion of dietary flavonoids alters their anti-inflammatory and antioxidant properties. Free Radic. Biol. Med. 2011, 51, 454–463. [Google Scholar] [CrossRef]
- Vong, C.I.; Rathinasabapathy, T.; Moncada, M.; Komarnytsky, S. All Polyphenols Are Not Created Equal: Exploring the Diversity of Phenolic Metabolites. J. Agric. Food Chem. 2022, 70, 2077–2091. [Google Scholar] [CrossRef]
- Erlank, H.; Elmann, A.; Kohen, R.; Kanner, J. Polyphenols activate Nrf2 in astrocytes via H2O2, semiquinones, and quinones. Free Radic. Biol. Med. 2011, 51, 2319–2327. [Google Scholar] [CrossRef]
- Bienert, G.P.; Moller, A.L.B.; Kristiansen, K.A.; Schulz, A.; Moller, I.M.; Schjoerring, J.K.; Jahn, T.P. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 2007, 282, 1183–1192. [Google Scholar] [CrossRef]
- Stone, J.R.; Yang, S.P. Hydrogen peroxide: A signaling messenger. Antioxid. Redox Signal. 2006, 8, 243–270. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G. H2O2, a necessary evil for cell signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef] [PubMed]
- Mu, K.; Yao, Y.; Wang, D.; Kitts, D.D. Prooxidant capacity of phenolic acids defines antioxidant potential. Biochim. Biophys. Acta Gen. Subj. 2023, 1867, 130371. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, M.V.; Lee, C.Y.; Liu, R.H. Nutrition: Antioxidant activity of fresh apples. Nature 2000, 405, 903. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.; Bollag, W.B.; Lewis, J.; Huang, Q.; Singh, B.; Sharawy, M.; Yamamoto, T.; Schuster, G. Green tea polyphenols induce differentiation and proliferation in epidermal keratinocytes. J. Pharmacol. Exp. Ther. 2003, 306, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Zrelli, H.; Matsuoka, M.; Kitazaki, S.; Araki, M.; Kusunoki, M.; Zarrouk, M.; Miyazaki, H. Hydroxytyrosol induces proliferation and cytoprotection against oxidative injury in vascular endothelial cells: Role of Nrf2 activation and HO-1 induction. J. Agric. Food Chem. 2011, 59, 4473–4482. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, T.; Matsumoto, A.; Kihara, T.; Akaike, A.; Sugimoto, H. Protective effect of H2O2 against subsequent H2O2-induced cytotoxicity involves activation of the PI3K-Akt signaling pathway. Cell. Mol. Biol. 2010, 56, OL1447–OL1452. [Google Scholar] [PubMed]
- Angeloni, C.; Motori, E.; Fabbri, D.; Malaguti, M.; Leoncini, E.; Lorenzini, A.; Hrelia, S. H2O2 preconditioning modulates phase II enzymes through p38 MAPK and PI3K/Akt activation. Am. J. Physiol.-Heart Circ. Physiol. 2011, 300, H2196–H2205. [Google Scholar] [CrossRef]
- Mo, L.; Yang, C.T.; Gu, M.F.; Zheng, D.D.; Lin, L.; Wang, X.Y.; Lan, A.P.; Hu, F.; Feng, J.Q. PI3K/Akt signaling pathway-induced heme oxygenase-1 upregulation mediates the adaptive cytoprotection of hydrogen peroxide preconditioning against oxidative injury in PC12 cells. Int. J. Mol. Med. 2012, 30, 314–320. [Google Scholar] [CrossRef][Green Version]
- Woo, A.Y.H.; Cheng, C.H.K.; Waye, M.M.Y. Baicalein protects rat cardiomyocytes from hypoxia/reoxygenation damage via a prooxidant mechanism. Cardiovasc. Res. 2005, 65, 244–253. [Google Scholar] [CrossRef]
- Gao, Z.H.; Huang, K.X.; Xu, H.B. Protective effects of flavonoids in the roots of Scutellaria baicalensis Georgi against hydrogen peroxide-induced oxidative stress in HS-SY5Y cells. Pharmacol. Res. 2001, 43, 173–178. [Google Scholar] [CrossRef]
- Zrelli, H.; Matsuoka, M.; Kitazaki, S.; Zarrouk, M.; Miyazaki, H. Hydroxytyrosol reduces intracellular reactive oxygen species levels in vascular endothelial cells by upregulating catalase expression through the AMPK-FOXO3a pathway. Eur. J. Pharmacol. 2011, 660, 275–282. [Google Scholar] [CrossRef]
- Walter, A.; Etienne-Selloum, N.; Sarr, M.; Kane, M.O.; Beretz, A.; Schini-Kerth, V.B. Angiotensin II induces the vascular expression of VEGF and MMP-2 in vivo: Preventive effect of red wine polyphenols. J. Vasc. Res. 2008, 45, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Tasatargil, A.; Tanriover, G.; Barutcigil, A.; Turkmen, E. Protective effect of resveratrol on methylglyoxal-induced endothelial dysfunction in aged rats. Aging Clin. Exp. Res. 2019, 31, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [PubMed]
- Miller, V.; Mente, A.; Dehghan, M.; Rangarajan, S.; Zhang, X.; Swaminathan, S.; Dagenais, G.; Gupta, R.; Mohan, V.; Lear, S.; et al. Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): A prospective cohort study. Lancet 2017, 390, 2037–2049. [Google Scholar] [CrossRef]
- Shimokawa, H. Hydrogen peroxide as an endothelium-derived hyperpolarizing factor. Pflug. Arch. Eur. J. Physiol. 2010, 459, 915–922. [Google Scholar] [CrossRef]
- Ushio-Fukai, M.; Alexander, R.W.; Akers, M.; Yin, Q.; Fujio, Y.; Walsh, K.; Griendling, K.K. Reactive oxygen species mediate the activation of Akt/protein kinase B by angiotensin II in vascular smooth muscle cells. J. Biol. Chem. 1999, 274, 22699–22704. [Google Scholar] [CrossRef]
- Covas, G.; Marinho, H.S.; Cyrne, L.; Antunes, F. Activation of Nrf2 by H2O2: De novo synthesis versus nuclear translocation. Methods Enzymol. 2013, 528, 157–171. [Google Scholar] [CrossRef]
- Sies, H. Role of metabolic H2O2 generation: Redox signaling and oxidative stress. J. Biol. Chem. 2014, 289, 8735–8741. [Google Scholar] [CrossRef]
- Alhosin, M.; Anselm, E.; Rashid, S.; Kim, J.H.; Madeira, S.V.; Bronner, C.; Schini-Kerth, V.B. Redox-sensitive up-regulation of eNOS by purple grape juice in endothelial cells: Role of PI3-kinase/Akt, p38 MAPK, JNK, FoxO1 and FoxO3a. PLoS ONE 2013, 8, e57883. [Google Scholar] [CrossRef] [PubMed]
- Oak, M.H.; Auger, C.; Belcastro, E.; Park, S.H.; Lee, H.H.; Schini-Kerth, V.B. Potential mechanisms underlying cardiovascular protection by polyphenols: Role of the endothelium. Free Radic. Biol. Med. 2018, 122, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.P.; Harmon, J.S. Diabetes, glucose toxicity, and oxidative stress: A case of double jeopardy for the pancreatic islet beta cell. Free Radic. Biol. Med. 2006, 41, 177–184. [Google Scholar] [CrossRef]
- Robertson, R.P. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J. Biol. Chem. 2004, 279, 42351–42354. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Elksnis, A.; Wikstrom, P.; Walum, E.; Welsh, N.; Carlsson, P.O. The novel NADPH oxidase 4 selective inhibitor GLX7013114 counteracts human islet cell death in vitro. PLoS ONE 2018, 13, e0204271. [Google Scholar] [CrossRef]
- Youl, E.; Bardy, G.; Magous, R.; Cros, G.; Sejalon, F.; Virsolvy, A.; Richard, S.; Quignard, J.F.; Gross, R.; Petit, P.; et al. Quercetin potentiates insulin secretion and protects INS-1 pancreatic beta-cells against oxidative damage via the ERK1/2 pathway. Br. J. Pharmacol. 2010, 161, 799–814. [Google Scholar] [CrossRef]
- Li, J.; Wu, N.H.; Chen, X.; Chen, H.G.; Yang, X.S.; Liu, C. Curcumin protects islet cells from glucolipotoxicity by inhibiting oxidative stress and NADPH oxidase activity both in vitro and in vivo. Islets 2019, 11, 152–164. [Google Scholar] [CrossRef]
- Vanitha, P.; Senthilkumar, S.; Dornadula, S.; Anandhakumar, S.; Rajaguru, P.; Ramkumar, K.M. Morin activates the Nrf2-ARE pathway and reduces oxidative stress-induced DNA damage in pancreatic beta cells. Eur. J. Pharmacol. 2017, 801, 9–18. [Google Scholar] [CrossRef]
- Abebe, T.; Mahadevan, J.; Bogachus, L.; Hahn, S.; Black, M.; Oseid, E.; Urano, F.; Cirulli, V.; Robertson, R.P. Nrf2/antioxidant pathway mediates beta cell self-repair after damage by high-fat diet-induced oxidative stress. JCI Insight 2017, 2, e92854. [Google Scholar] [CrossRef]
- Gao, F.; Fu, Y.; Yi, J.; Gao, A.; Jia, Y.; Cai, S. Effects of Different Dietary Flavonoids on Dipeptidyl Peptidase-IV Activity and Expression: Insights into Structure-Activity Relationship. J. Agric. Food Chem. 2020, 68, 12141–12151. [Google Scholar] [CrossRef]
- Perry, R.J.; Samuel, V.T.; Petersen, K.F.; Shulman, G.I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 2014, 510, 84–91. [Google Scholar] [CrossRef]
- Kikuchi, A.; Takamura, T. Where does liver fat go? A possible molecular link between fatty liver and diabetes. J. Diabetes Investig. 2017, 8, 152–154. [Google Scholar] [CrossRef]
- Sattar, N.; Gill, J.M.R. Type 2 diabetes as a disease of ectopic fat? BMC Med. 2014, 12, 123. [Google Scholar] [CrossRef]
- Cheng, D.M.; Kuhn, P.; Poulev, A.; Rojo, L.E.; Lila, M.A.; Raskin, I. In vivo and in vitro antidiabetic effects of aqueous cinnamon extract and cinnamon polyphenol-enhanced food matrix. Food Chem. 2012, 135, 2994–3002. [Google Scholar] [CrossRef]
- Paoli, P.; Cirri, P.; Caselli, A.; Ranaldi, F.; Bruschi, G.; Santi, A.; Camici, G. The insulin-mimetic effect of Morin: A promising molecule in diabetes treatment. Biochim. Biophys. Acta-Gen. Subj. 2013, 1830, 3102–3111. [Google Scholar] [CrossRef]
- Kang, G.G.; Francis, N.; Hill, R.; Waters, D.; Blanchard, C.; Santhakumar, A.B. Dietary Polyphenols and Gene Expression in Molecular Pathways Associated with Type 2 Diabetes Mellitus: A Review. Int. J. Mol. Sci. 2019, 21, 140. [Google Scholar] [CrossRef]
- Hwang, J.T.; Ha, J.; Park, I.J.; Lee, S.K.; Baik, H.W.; Kim, Y.M.; Park, O.J. Apoptotic effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway. Cancer Lett. 2007, 247, 115–121. [Google Scholar] [CrossRef]
- Collins, Q.F.; Liu, H.Y.; Pi, J.; Liu, Z.; Quon, M.J.; Cao, W. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5’-AMP-activated protein kinase. J. Biol. Chem. 2007, 282, 30143–30149. [Google Scholar] [CrossRef]
- Kim, H.S.; Quon, M.J.; Kim, J.A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef]
- Kane, M.O.; Etienne-Selloum, N.; Madeira, S.V.; Sarr, M.; Walter, A.; Dal-Ros, S.; Schott, C.; Chataigneau, T.; Schini-Kerth, V.B. Endothelium-derived contracting factors mediate the Ang II-induced endothelial dysfunction in the rat aorta: Preventive effect of red wine polyphenols. Pflug. Arch. Eur. J. Physiol. 2010, 459, 671–679. [Google Scholar] [CrossRef]
- Elbatreek, M.H.; Pachado, M.P.; Cuadrado, A.; Jandeleit-Dahm, K.; Schmidt, H. Reactive Oxygen Comes of Age: Mechanism-Based Therapy of Diabetic End-Organ Damage. Trends Endocrinol. Metab. 2019, 30, 312–327. [Google Scholar] [CrossRef]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef]
- Kane, M.O.; Anselm, E.; Rattmann, Y.D.; Auger, C.; Schini-Kerth, V.B. Role of gender and estrogen receptors in the rat aorta endothelium-dependent relaxation to red wine polyphenols. Vascul. Pharmacol. 2009, 51, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, R.D.; Dissard, R.; Jaquet, V.; de Seigneux, S. Potential benefits and harms of NADPH oxidase type 4 in the kidneys and cardiovascular system. Nephrol. Dial. Transplant. 2019, 34, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yang, B.; Tan, J.; Jiang, J.; Li, D. Associations of dietary intakes of anthocyanins and berry fruits with risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective cohort studies. Eur. J. Clin. Nutr. 2016, 70, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Cremonini, E.; Fraga, C.G.; Oteiza, P.I. (-)-Epicatechin in the control of glucose homeostasis: Involvement of redoxregulated mechanisms. Free Radic. Biol. Med. 2019, 130, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Esselman, W.J. Inhibition of PTPs by H2O2 regulates the activation of distinct MAPK pathways. Free Radic. Biol. Med. 2002, 33, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Fratantonio, D.; Speciale, A.; Canali, R.; Natarelli, L.; Ferrari, D.; Saija, A.; Virgili, F.; Cimino, F. Low nanomolar caffeic acid attenuates high glucose-induced endothelial dysfunction in primary human umbilical-vein endothelial cells by affecting NF-κappaB and Nrf2 pathways. BioFactors 2017, 43, 54–62. [Google Scholar] [CrossRef]
- Daveri, E.; Cremonini, E.; Mastaloudis, A.; Hester, S.N.; Wood, S.M.; Waterhouse, A.L.; Anderson, M.; Fraga, C.G.; Oteiza, P.I. Cyanidin and delphinidin modulate inflammation and altered redox signaling improving insulin resistance in high fat-fed mice. Redox Biol. 2018, 18, 16–24. [Google Scholar] [CrossRef]
- Mi, Y.; Zhang, W.; Tian, H.; Li, R.; Huang, S.; Li, X.; Qi, G.; Liu, X. EGCG evokes Nrf2 nuclear translocation and dampens PTP1B expression to ameliorate metabolic misalignment under insulin resistance condition. Food Funct. 2018, 9, 1510–1523. [Google Scholar] [CrossRef]
- Mi, Y.; Qi, G.; Fan, R.; Qiao, Q.; Sun, Y.; Gao, Y.; Liu, X. EGCG ameliorates high-fat- and high-fructose-induced cognitive defects by regulating the IRS/AKT and ERK/CREB/BDNF signaling pathways in the CNS. FASEB J. 2017, 31, 4998–5011. [Google Scholar] [CrossRef]
- Mi, Y.; Qi, G.; Fan, R.; Ji, X.; Liu, Z.; Liu, X. EGCG ameliorates diet-induced metabolic syndrome associating with the circadian clock. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1575–1589. [Google Scholar] [CrossRef]
- Kamble, P.; Selvarajan, K.; Aluganti Narasimhulu, C.; Nandave, M.; Parthasarathy, S. Aspirin may promote mitochondrial biogenesis via the production of hydrogen peroxide and the induction of Sirtuin1/PGC-1alpha genes. Eur. J. Pharmacol. 2013, 699, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G.; Bae, Y.S.; Lee, S.R.; Kwon, J. Hydrogen peroxide: A key messenger that modulates protein phosphorylation through cysteine oxidation. Sci. STKE 2000, 2000, pe1. [Google Scholar] [CrossRef] [PubMed]
- Adair, K.E.; Bowden, R.G. Ameliorating Chronic Kidney Disease Using a Whole Food Plant-Based Diet. Nutrients 2020, 12, 1007. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Tarafdar, A.; Pula, G. The Role of NADPH Oxidases and Oxidative Stress in Neurodegenerative Disorders. Int. J. Mol. Sci. 2018, 19, 3824. [Google Scholar] [CrossRef]
- Sbodio, J.I.; Snyder, S.H.; Paul, B.D. Redox Mechanisms in Neurodegeneration: From Disease Outcomes to Therapeutic Opportunities. Antioxid. Redox Signal. 2019, 30, 1450–1499. [Google Scholar] [CrossRef]
- Casas, A.I.; Geuss, E.; Kleikers, P.W.M.; Mencl, S.; Herrmann, A.M.; Buendia, I.; Egea, J.; Meuth, S.G.; Lopez, M.G.; Kleinschnitz, C.; et al. NOX4-dependent neuronal autotoxicity and BBB breakdown explain the superior sensitivity of the brain to ischemic damage. Proc. Natl. Acad. Sci. USA 2017, 114, 12315–12320. [Google Scholar] [CrossRef]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, J.L.; Tchkonia, T. Cellular Senescence: A Translational Perspective. EBioMedicine 2017, 21, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Biran, A.; Zada, L.; Abou Karam, P.; Vadai, E.; Roitman, L.; Ovadya, Y.; Porat, Z.; Krizhanovsky, V. Quantitative identification of senescent cells in aging and disease. Aging Cell 2017, 16, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Sturmlechner, I.; Durik, M.; Sieben, C.J.; Baker, D.J.; van Deursen, J.M. Cellular senescence in renal ageing and disease. Nat. Rev. Nephrol. 2017, 13, 77–89. [Google Scholar] [CrossRef]
- Palmer, A.K.; Gustafson, B.; Kirkland, J.L.; Smith, U. Cellular senescence: At the nexus between ageing and diabetes. Diabetologia 2019, 62, 1835–1841. [Google Scholar] [CrossRef]
- Tchkonia, T.; Kirkland, J.L. Aging, Cell Senescence, and Chronic Disease: Emerging Therapeutic Strategies. JAMA 2018, 320, 1319–1320. [Google Scholar] [CrossRef]
- Gurau, F.; Baldoni, S.; Prattichizzo, F.; Espinosa, E.; Amenta, F.; Procopio, A.D.; Albertini, M.C.; Bonafe, M.; Olivieri, F. Anti-senescence compounds: A potential nutraceutical approach to healthy aging. Ageing Res. Rev. 2018, 46, 14–31. [Google Scholar] [CrossRef]
- Li, W.; Qin, L.; Feng, R.; Hu, G.; Sun, H.; He, Y.; Zhang, R. Emerging senolytic agents derived from natural products. Mech. Ageing Dev. 2019, 181, 1–6. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Meyer, C.J.; Block, G.A.; Chertow, G.M.; Shiels, P.G. Understanding the role of the cytoprotective transcription factor nuclear factor erythroid 2-related factor 2-lessons from evolution, the animal kingdom and rare progeroid syndromes. Nephrol. Dial. Transplant. 2020, 35, 2036–2045. [Google Scholar] [CrossRef]
- He, J.; Zhang, A.; Song, Z.; Guo, S.; Chen, Y.; Liu, Z.; Zhang, J.; Xu, X.; Liu, J.; Chu, L. The resistant effect of Sirt1 in oxidative stress-induced senescence of rat nucleus pulposus cell is regulated by Akt-FoxO1 pathway. Biosci. Rep. 2019, 39, BSR20190112. [Google Scholar] [CrossRef]
- Man, A.W.C.; Li, H.; Xia, N. The Role of Sirtuin1 in Regulating Endothelial Function, Arterial Remodeling and Vascular Aging. Front. Physiol. 2019, 10, 1173. [Google Scholar] [CrossRef] [PubMed]
- Takano, K.; Tatebe, J.; Washizawa, N.; Morita, T. Curcumin Inhibits Age-Related Vascular Changes in Aged Mice Fed a High-Fat Diet. Nutrients 2018, 10, 1476. [Google Scholar] [CrossRef] [PubMed]
- Senger, D.R.; Li, D.; Jaminet, S.C.; Cao, S. Activation of the Nrf2 Cell Defense Pathway by Ancient Foods: Disease Prevention by Important Molecules and Microbes Lost from the Modern Western Diet. PLoS ONE 2016, 11, e0148042. [Google Scholar] [CrossRef] [PubMed]
- Senger, D.R.; Cao, S. Diabetic Wound Healing and Activation of Nrf2 by Herbal Medicine. J. Nat. Sci. 2016, 2, e247. [Google Scholar]
- Schmidlin, C.J.; Dodson, M.B.; Madhavan, L.; Zhang, D.D. Redox regulation by NRF2 in aging and disease. Free Radic. Biol. Med. 2019, 134, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Bazopoulou, D.; Knoefler, D.; Zheng, Y.; Ulrich, K.; Oleson, B.J.; Xie, L.; Kim, M.; Kaufmann, A.; Lee, Y.T.; Dou, Y.; et al. Developmental ROS individualizes organismal stress resistance and lifespan. Nature 2019, 576, 301–305. [Google Scholar] [CrossRef]
- Siswanto, F.M.; Sakuma, R.; Oguro, A.; Imaoka, S. Chlorogenic Acid Activates Nrf2/SKN-1 and Prolongs the Lifespan of Caenorhabditis elegans via the Akt-FOXO3/DAF16a-DDB1 Pathway and Activation of DAF16f. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1503–1516. [Google Scholar] [CrossRef]
- Golubev, A.; Hanson, A.D.; Gladyshev, V.N. A Tale of Two Concepts: Harmonizing the Free Radical and Antagonistic Pleiotropy Theories of Aging. Antioxid. Redox Signal. 2018, 29, 1003–1017. [Google Scholar] [CrossRef]
- Rendeiro, C.; Rhodes, J.S.; Spencer, J.P. The mechanisms of action of flavonoids in the brain: Direct versus indirect effects. Neurochem. Int. 2015, 89, 126–139. [Google Scholar] [CrossRef]
- Vauzour, D.; Corsini, S.; Muller, M.; Spencer, J.P.E. Inhibition of PP2A by hesperetin may contribute to Akt and ERK1/2 activation status in cortical neurons. Arch. Biochem. Biophys. 2018, 650, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; An, Z. Hesperidin attenuates learning and memory deficits in APP/PS1 mice through activation of Akt/Nrf2 signaling and inhibition of RAGE/NF-κappaB signaling. Arch. Pharm. Res. 2018, 41, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Nouhi, F.; Tusi, S.K.; Abdi, A.; Khodagholi, F. Dietary supplementation with tBHQ, an Nrf2 stabilizer molecule, confers neuroprotection against apoptosis in amyloid beta-injected rat. Neurochem. Res. 2011, 36, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.L.; Yu, S.; Pan, Z.; Ma, J.; Wu, T.Y.; Kong, A.N. tBHQ-induced HO-1 expression is mediated by calcium through regulation of Nrf2 binding to enhancer and polymerase II to promoter region of HO-1. Chem. Res. Toxicol. 2011, 24, 670–676. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Wang, F.; Shi, Y.; Ren, Y.; Liu, Q.; Cao, Y.; Duan, H. Attenuation of glomerular injury in diabetic mice with tert-butylhydroquinone through nuclear factor erythroid 2-related factor 2-dependent antioxidant gene activation. Am. J. Nephrol. 2011, 33, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Shih, A.Y.; Li, P.; Murphy, T.H. A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 10321–10335. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Wang, H.; Fang, J.; Zhu, Y.; Zhou, J.; Wang, X.; Zhou, Y.; Zhou, M. Curcumin provides neuroprotection in model of traumatic brain injury via the Nrf2-ARE signaling pathway. Brain Res. Bull. 2018, 140, 65–71. [Google Scholar] [CrossRef]
- Huang, L.; Chen, C.; Zhang, X.; Li, X.; Chen, Z.; Yang, C.; Liang, X.; Zhu, G.; Xu, Z. Neuroprotective Effect of Curcumin against Cerebral Ischemia-Reperfusion via Mediating Autophagy and Inflammation. J. Mol. Neurosci. 2018, 64, 129–139. [Google Scholar] [CrossRef]
- Allen, E.N.; Potdar, S.; Tapias, V.; Parmar, M.; Mizuno, C.S.; Rimando, A.; Cavanaugh, J.E. Resveratrol and pinostilbene confer neuroprotection against aging-related deficits through an ERK1/2-dependent mechanism. J. Nutr. Biochem. 2018, 54, 77–86. [Google Scholar] [CrossRef]
- Qi, G.; Mi, Y.; Wang, Y.; Li, R.; Huang, S.; Li, X.; Liu, X. Neuroprotective action of tea polyphenols on oxidative stress-induced apoptosis through the activation of the TrkB/CREB/BDNF pathway and Keap1/Nrf2 signaling pathway in SH-SY5Y cells and mice brain. Food Funct. 2017, 8, 4421–4432. [Google Scholar] [CrossRef]
- Gundimeda, U.; McNeill, T.H.; Fan, T.K.; Deng, R.; Rayudu, D.; Chen, Z.; Cadenas, E.; Gopalakrishna, R. Green tea catechins potentiate the neuritogenic action of brain-derived neurotrophic factor: Role of 67-kDa laminin receptor and hydrogen peroxide. Biochem. Biophys. Res. Commun. 2014, 445, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Hervera, A.; De Virgiliis, F.; Palmisano, I.; Zhou, L.; Tantardini, E.; Kong, G.; Hutson, T.; Danzi, M.C.; Perry, R.B.; Santos, C.X.C.; et al. Reactive oxygen species regulate axonal regeneration through the release of exosomal NADPH oxidase 2 complexes into injured axons. Nat. Cell Biol. 2018, 20, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, L.; Chang, C.J. Exosomal NADPH Oxidase: Delivering Redox Signaling for Healing. Biochemistry 2018, 57, 3993–3994. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Understanding mechanisms of antioxidant action in health and disease. Nat. Rev. Mol. Cell Biol. 2023, 12, 1–21. [Google Scholar] [CrossRef]

| Food (var.) | Polyphenols mg/100 g FW | IC100%/g FW a | rPOSI b |
|---|---|---|---|
| Blackberries | 667 ± 4.5 | 16 | 625 |
| Quince (Portugal) | 460 ± 6.3 | 18 | 568 |
| Blueberries | 310 ± 6.7 | 42 | 240 |
| Pomegranate (Wonderful) | 145 ± 3.5 | 72 | 138 |
| Pear (Spadona) | 100 ± 3.8 | 104 | 96 |
| Strawberries (Tamar) | 190 ± 4.7 | 137 | 74 |
| Purple Grapes (Red Globe) | 150 ± 1.5 | 160 | 63 |
| Banana (Ziv) | 114 ± 4.2 | 212 | 47 |
| Peach (White Lady) | 168 ± 6.2 | 288 | 35 |
| Spinach (Winter) | 105 ± 2.0 | 105 | 96 |
| Broccoli (Monaco) | 85 ± 3.5 | 161 | 62 |
| Onion (Yellow) | 80 ± 3.2 | 402 | 25 |
| Red Beet | 196 ± 6.0 | 370 | 27 |
| Eggplant (Black) | 171 ± 2.5 | 460 | 22 |
| Red Wine (Petite Sirah) | 237 ± 5.1 | 168 | 60 |
| Black Coffee (Turkish-ground) | 225 ± 6.3 | 169 | 59 |
| Tea (Green) | 125 ± 2.3 | 416 | 24 |
| Tea (Black) | 113 ± 5.1 | 657 | 15 |
| Coffee (Freeze-dried) | 200 ± 5.3 | 752 | 13 |
| Foods-100 g | Polyphenols (mg)/100 g | IC100%/g FW | rPOSI |
|---|---|---|---|
| Tomato | 29 | 625 | 16 |
| Cucumber | 0 | 0 | 0 |
| Red-Pepper | 47 | 193 | 53 |
| Green-Cabbage | 24 | 240 | 42 |
| Onion (Purple) | 120 | 280 | 36 |
| Olive (Manzanillo 25 g) | 80 | 54 | 45 |
| Total/525 g salad | 300 | 192 | |
| A Salad portion of 274 g | 156 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanner, J. Food Polyphenols as Preventive Medicine. Antioxidants 2023, 12, 2103. https://doi.org/10.3390/antiox12122103
Kanner J. Food Polyphenols as Preventive Medicine. Antioxidants. 2023; 12(12):2103. https://doi.org/10.3390/antiox12122103
Chicago/Turabian StyleKanner, Joseph. 2023. "Food Polyphenols as Preventive Medicine" Antioxidants 12, no. 12: 2103. https://doi.org/10.3390/antiox12122103
APA StyleKanner, J. (2023). Food Polyphenols as Preventive Medicine. Antioxidants, 12(12), 2103. https://doi.org/10.3390/antiox12122103






