Extracts and Scirpusin B from Recycled Seeds and Rinds of Passion Fruits (Passiflora edulis var. Tainung No. 1) Exhibit Improved Functions in Scopolamine-Induced Impaired-Memory ICR Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
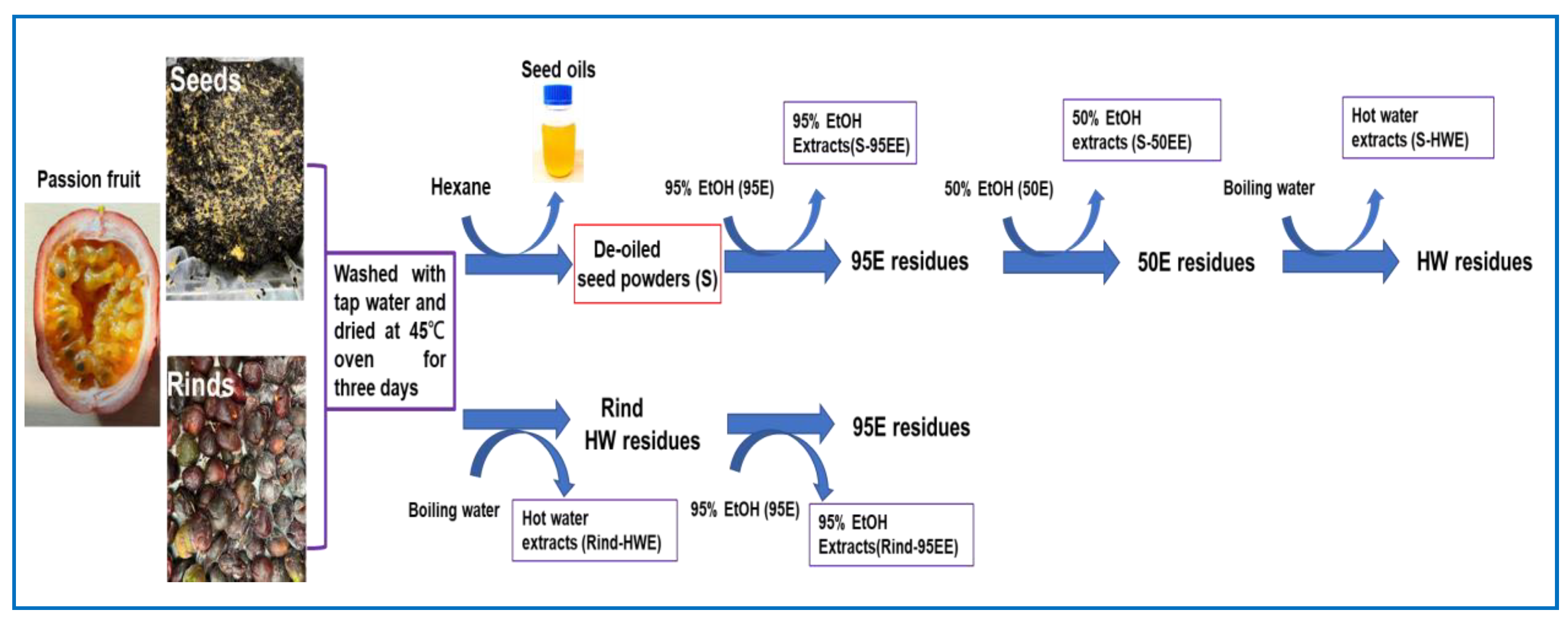
2.2. Extraction of Seeds and Rinds of Passion Fruits
2.3. Isolation and Identification of Piceatannol and Scirpusin B from Seed Extracts of Passion Fruits and HPLC Fingerprinting Analyses
2.4. Biological Activity Assays In Vitro
2.4.1. DPPH Radical-Scavenging Activities
2.4.2. AChE-Inhibitory Activities In Vitro
2.4.3. Inhibition against Aβ Peptide Aggregations In Vitro
2.4.4. Neuroprotection against Hydrogen Peroxide-Induced or Aβ25-35 Peptide-Induced Cell Death in SH-SY5Y Cell Models
2.5. Molecular Docking in Silico
2.6. Effects of Extracts of Seeds and Rinds of Passion Fruits or Scirpuin B Pretreatments on Cognitive Dysfunctions in Scopolamine-Induced Amnesiac ICR Mice
2.6.1. Effects of 7-Day Pretreatments with De-Oiled Seed Powders (S), S-95EE, S-50EE, or Rind-HWE on the Improvement of Learning and Memory Functions
2.6.2. Effects of 18-Day Pretreatments with De-Oiled Seed Powders (S) or Rind-HWE on the Improvement of Learning and Memory Functions
2.6.3. Effects of 7-Day Pretreatments with Scirpusin B or Piceatannol on the Improvement in Learning and Memory Functions
2.7. The Learning Dysfunction of Scopolamine-Induced ICR Mice in Passive Avoidance Tests
2.8. Statistical Analyses
3. Results
3.1. Biological Activities of Extracts of Seeds and Rinds in Vitro
3.2. Biological Activities of Isolated Piceatannol and Scirpusin B
3.3. Molecular Dockings of Scirpusin B with AChE
3.4. Molecular Dockings of Scirpusin B with Aβ1-42 Monomer
3.5. Animal Experiments in Scopolamine-Induced ICR Mice with Cognitive Dysfunctions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Dementia. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 22 August 2023).
- WHO. The Top 10 Causes of Death. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 23 August 2023).
- WHO. Leading Causes of Death and Disability. A Visual Summary of Global and Regional Trends 2000–2019. Available online: https://www.who.int/data/stories/leading-causes-of-death-and-disability-2000-2019-a-visual-summary (accessed on 24 August 2023).
- Alzheimer’s Association. 2023 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2023, 19, 1598–1695. [Google Scholar] [CrossRef] [PubMed]
- National Institute on Aging. What Causes Alzheimer’s Disease? Available online: https://www.nia.nih.gov/health/what-causes-alzheimers-disease (accessed on 6 September 2023).
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef] [PubMed]
- Tarawneh, R.; Holtzman, D.M. The clinical problem of symptomatic Alzheimer disease and mild cognitive impairment. Cold Spring Harb. Perspect. Med. 2012, 2, a006148. [Google Scholar] [CrossRef] [PubMed]
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s disease. N. Eng. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef]
- Craig, L.A.; Hong, N.S.; McDonald, R.J. Revisiting the cholinergic hypothesis in the development of Alzheimer’s disease. Neurosci. Biobehav. Rev. 2011, 35, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. Medications for Memory, Cognition and Dementia-Related Behaviors. Available online: https://www.alz.org/alzheimers-dementia/treatments/medications-for-memory (accessed on 5 September 2023).
- Galimberti, D.; Scarpini, E. Old and new acetylcholinesterase inhibitors for Alzheimer’s disease. Expert Opin. Investig. Drugs 2016, 25, 1181–1187. [Google Scholar] [CrossRef]
- Witt, A.; Macdonald, N.; Kirkpatrick, P. Memantine hydrochloride. Nat. Rev. Drug Discover. 2004, 3, 109–110. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Ames, B.N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science 1983, 221, 1256–1264. [Google Scholar] [CrossRef]
- Beckman, K.B.; Ames, B.N. The free radical theory of aging matures. Physiol. Rev. 1998, 78, 547–581. [Google Scholar] [CrossRef]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M.; Akbar, M.D. The role of reactive oxygen species in the pathogenesis of Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease: A mini review. Oxid. Med. Cell. Longev. 2016, 2016, 8590578. [Google Scholar] [CrossRef]
- Silva, R.F.M.; Pogačnik, L. Polyphenols from food and natural products: Neuroprotection and safety. Antioxidants 2020, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Mazzitelli, S.; Arciello, M.; Capo, C.R.; Rotilio, G. Benefits from dietary polyphenols for brain aging and Alzheimer’s disease. Neurochem. Res. 2008, 33, 2390–2400. [Google Scholar] [CrossRef]
- Hollman, P.C.H.; Cassidy, A.; Comte, B.; Heinonen, M.; Richelle, M.; Richling, E.; Serafini, M.; Scalbert, A.; Sies, H.; Vidry, S. The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established. J. Nutr. 2011, 141, 989S–1009S. [Google Scholar] [CrossRef] [PubMed]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.G.; Lin, S.Y.; Lee, Y.S.; Wang, C.C.; Hou, W.C. Hydrolysable tannins exhibit acetylcholinesterase inhibitory and anti-glycation activities in vitro and learning and memory function improvements in scopolamine-induced amnesiac mice. Biomedicines 2021, 9, 1066. [Google Scholar] [CrossRef]
- Sie, Y.Y.; Chen, L.C.; Li, C.J.; Yuan, Y.H.; Hsiao, S.H.; Lee, M.H.; Wang, C.C.; Hou, W.C. Inhibition of acetylcholinesterase and amyloid-β aggregation by piceatannol and analogs: Assessing in vitro and in vivo impact on a murine model of scopolamine-induced memory impairment. Antioxidants 2023, 12, 1362. [Google Scholar] [CrossRef]
- Chen, L.G.; Wang, C.C.; Lee, Y.S.; Sie, Y.Y.; Chang, C.I.; Hou, W.C. Vitisin A, a resveratrol tetramer, improves scopolamine-induced impaired learning and memory functions in amnesiac ICR mice. Biomedicines 2022, 10, 273. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Passion Fruits. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 4368–4373. [Google Scholar]
- Chen, Y.C.; Chang, C.; Lin, H.L. Topolins and red light improve the micropropagation efficiency of passion fruit (Passiflora edulis Sims) ‘Tainung No. 1’. HortScience 2020, 55, 1337–1344. [Google Scholar] [CrossRef]
- Lo, H.P.; Lou, L.Y.; Huang, T.B. Establishment of integrated propagation system on grafting plantlets of passion fruit (Passiflora edulis). HortScience 2023, 58, 170–177. [Google Scholar] [CrossRef]
- Corrêa, R.C.G.; Peralta, R.M.; Haminiuk, C.W.I.; Maciel, G.M.; Bracht, A.; Ferreira, I.C.F.R. The past decade findings related with nutritional composition, bioactive molecules and biotechnological applications of Passiflora spp. (passion fruit). Trends Food Sci. Technol. 2016, 58, 79–95. [Google Scholar] [CrossRef]
- Rotta, E.M.; Rodrigues, C.A.; Jardim, I.C.S.F.; Maldaner, L.; Visentainer, J.V. Determination of phenolic compounds and antioxidant activity in passion fruit pulp (Passiflora spp.) using a modified QuEChERS method and UHPLC-MS/MS. LWT-Food Sci. Technol. Int. 2019, 100, 397–403. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Stanislas, G.; Douraguia, E.; Gonthier, M.P. Evaluation of nutritional and antioxidant properties of the tropical fruits banana, litchi, mango, papaya, passion fruit and pineapple cultivated in Réunion French Island. Food Chem. 2016, 212, 225–233. [Google Scholar] [CrossRef]
- Fonseca, A.M.A.; Geraldi, M.V.; Junior, M.R.M.; Silvestre, A.J.D.; Rocha, S.M. Purple passion fruit (Passiflora edulis f. edulis): A comprehensive review on the nutritional value, phytochemical profile and associated health effects. Food Res. Int. 2022, 160, 111665. [Google Scholar] [CrossRef]
- de Souza, C.G.; Rodrigues, T.H.S.; Silva, L.M.A.; Ribeiro, P.R.V.; de Brito, E.S. Sequential extraction of flavonoids and pectin from yellow passion fruit rind using pressurized solvent or ultrasound. J. Sci. Food Agric. 2018, 98, 1362–1368. [Google Scholar] [CrossRef]
- Shi, M.; Ali, M.M.; He, Y.; Ma, S.; Rizwan, H.M.; Yang, Q.; Li, B.; Lin, Z.; Chen, F. Flavonoids accumulation in fruit peel and expression profiling of related genes in purple (Passiflora edulis f. edulis) and yellow (Passiflora edulis f. flavicarpa) passion fruits. Plants 2021, 10, 2240. [Google Scholar] [CrossRef]
- Cazarin, C.B.B.; Rodriguez-Nogales, A.; Algieri, F.; Utrilla, M.P.; Rodríguez-Cabezas, M.E.; Garrido-Mesa, J.; Guerra-Hernández, E.; de Campos Braga, P.A.; Reyes, F.G.R.; Maróstica, M.R., Jr.; et al. Intestinal anti-inflammatory effects of Passiflora edulis peel in the dextran sodium sulphate model of mouse colitis. J. Funct. Foods 2016, 26, 565–576. [Google Scholar] [CrossRef]
- Lewis, B.J.; Herrlinger, K.A.; Craig, T.A.; Mehring-Franklin, C.E.; DeFreitas, Z.; Hinojosa-Laborde, C. Antihypertensive effect of passion fruit peel extract and its major bioactive components following acute supplementation in spontaneously hypertensive rats. J. Nutr. Biochem. 2013, 24, 1359–1366. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, G.C.; Batista, Â.G.; Carazin, C.B.B.; Cintra, D.E.; Prado, M.A.; Júnior, M.R.M. Passion fruit peel intake decreases inflammatory response and reverts lipid peroxidation and adiposity in diet-induced obese rats. Nutr. Res. 2020, 76, 106–117. [Google Scholar] [CrossRef]
- Yepes, A.; Ochoa-Bautista, D.; Murillo-Arango, W.; Quintero-Saumeth, J.; Bravo, K.; Osorio, E. Purple passion fruit seeds (Passiflora edulis f. edulis Sims) as a promising source of skin anti-aging agents: Enzymatic, antioxidant and multi-level computational studies. Arab. J. Chem. 2021, 14, 102905. [Google Scholar] [CrossRef]
- Sano, S.; Sugiyama, K.; Ito, T.; Katano, Y.; Ishihata, A. Identification of the strong vasorelaxing substance scirpusin B, a dimer of piceatannol, from passion fruit (Passiflora edulis) seeds. J. Agric. Food Chem. 2011, 59, 6209–6213. [Google Scholar] [CrossRef]
- Matsui, Y.; Sugiyama, K.; Kamei, M.; Takahashi, T.; Suzuki, T.; Katagata, Y.; Ito, T. Extract of passion fruit (Passiflora edulis) seed containing high amounts of piceatannol inhibits melanogenesis and promotes collagen synthesis. J. Agric. Food Chem. 2010, 58, 11112–11118. [Google Scholar] [CrossRef]
- Pan, Z.H.; Ning, D.S.; Fu, Y.X.; Li, D.P.; Zou, Z.Q.; Xie, Y.C.; Yu, L.L.; Li, L.C. Preparative isolation of piceatannol derivatives from passion fruit (Passiflora edulis) seeds by high-speed countercurrent chromatography combined with high-performance liquid chromatography and screening for α-glucosidase inhibitory activities. J. Agric. Food Chem. 2020, 68, 1555–1562. [Google Scholar] [CrossRef]
- Mittas, D.; Mawunu, M.; Magliocca, G.; Lautenschläger, T.; Schwaiger, S.; Stuppner, H.; Marzocco, S. Bioassay-guided isolation of anti-inflammatory constituents of the subaerial parts of Cyperus articulatus (Cyperaceae). Molecules 2022, 27, 5937. [Google Scholar] [CrossRef]
- Lin, Y.S.; Chen, S.H.; Huang, W.J.; Chen, C.H.; Chien, M.Y.; Lin, S.Y.; Hou, W.C. Effects of nicotinic acid derivatives on tyrosinase inhibitory and antioxidant activities. Food Chem. 2012, 132, 2074–2080. [Google Scholar] [CrossRef]
- Liu, Y.H.; Lee, C.J.; Chen, L.C.; Lee, T.L.; Hsieh, Y.Y.; Han, C.H.; Yang, C.H.; Huang, W.J.; Hou, W.C. Acetylcholinesterase inhibitory activity and neuroprotection in vitro, molecular docking, and improved learning and memory functions of demethylcurcumin in scopolamine-induced amnesia ICR mice. Food Funct. 2020, 11, 2328–2338. [Google Scholar] [CrossRef]
- Naiki, H.; Higuchi, K.; Hosokawa, M.; Takeda, T. Fluorometric determination of amyloid fibrils in vitro using the fluorescent dye, thioflavin T1. Anal. Biochem. 1989, 177, 244–249. [Google Scholar] [CrossRef]
- Wang, C.C.; Lin, S.Y.; Cheng, H.C.; Hou, W.C. Pro-oxidant and cytotoxic activities of atractylenolide I in human promyeloleukemic HL-60 cells. Food Chem. Toxicol. 2006, 44, 1308–1315. [Google Scholar] [CrossRef]
- Liu, Y.H.; Lin, Y.S.; Lin, K.L.; Lu, Y.L.; Chen, C.H.; Chien, M.Y.; Shang, H.F.; Lin, S.Y.; Hou, W.C. Effects of hot-water extracts from Ganoderma lucidum residues and solid-state fermentation residues on prebiotic and immune-stimulatory activities in vitro and the powdered residues used as broiler feed additives in vivo. Bot. Stud. 2015, 56, 17. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Enache, T.A.; Chiorcea-Paquim, A.M.; Oliveira-Brett, A.M. Amyloid-β peptides time-dependent structural modifications: AFM and voltammetric characterization. Anal. Chim. Acta 2016, 926, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.C.; Freitas, S.P.; Lorentino, C.M.A.; Fagundes, T.d.S.F.; da Matta, V.M.; dos Santos, A.L.S.; Moreira, D.d.L.; Kunigami, C.N.; Jung, E.P.; Ribeiro, L.d.O. Bioproducts from Passiflora cincinnata seeds: The brazilian caatinga passion fruit. Foods 2023, 12, 2525. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, F.; Zhang, C.; Ji, H.; Hong, P.; Deng, C. Optimization of process parameters for supercritical carbon dioxide extraction of Passiflora seed oil by response surface methodology. J. Supercrit. Fluids 2009, 48, 9–14. [Google Scholar] [CrossRef]
- Sampei, T.; Wu, Y.; Shigemori, H. Amyloid polypeptide disaggregation activity of passion fruit seed-derived polyphenol compounds. Nat. Prod. Commun. 2022, 17, 1–9. [Google Scholar] [CrossRef]
- Sato, A.; Tagai, N.; Ogino, Y.; Uozumi, H.; Kawakami, S.; Yamamoto, T.; Tanuma, S.i.; Maruki-Uchida, H.; Mori, S.; Minoru Morita, M. Passion fruit seed extract protects beta-amyloid-induced neuronal cell death in a differentiated human neuroblastoma SH-SY5Y cell model. Food Sci. Nutr. 2022, 10, 1461–1468. [Google Scholar] [CrossRef]
- Gacar, N.; Mutlu, O.; Utkan, T.; Celikyurt, I.K.; Gocmez, S.S.; Ulak, G. Beneficial effects of resveratrol on scopolamine but not mecamylamine induced memory impairment in the passive avoidance and Morris water maze tests in rats. Pharmacol. Biochem. Behav. 2011, 99, 316–323. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Katano, Y. Cardiovascular protective effects of polyphenols contained in passion fruit seeds namely piceatannol and scirpusin B: A review. Tokai J. Exp. Clin. Med. 2021, 46, 151–161. [Google Scholar]
- Watson, R.R.; Zibadi, S.; Rafatpanah, H.; Jabbari, F.; Ghasemi, R.; Ghafari, J.; Afrasiabi, H.; Foo, L.Y.; Faridhosseini, R. Oral administration of the purple passion fruit peel extract reduces wheeze and cough and improves shortness of breath in adults with asthma. Nutr. Res. 2008, 28, 166–171. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2007, 22, 659–661. [Google Scholar] [CrossRef]
- Xiong, L.; Xiang, D.; Yuan, F.; Tong, H.; Yang, R.; Zhou, L.; Xu, B.; Deng, C.; Li, X. Piceatannol-3′-O-β-D-glucopyranoside atenuates colistin-induced neurotoxicity by suppressing oxidative stress via the NRF2/HO-1 pathway. Biomed. Pharmacother. 2023, 161, 114419. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Khan, H.; Hoi, M.P.M.; Wai San Cheang, W.S. Piceatannol protects brain endothelial cell line (bEnd.3) against lipopolysaccharide-induced inflammation and oxidative stress. Molecules 2022, 27, 1206. [Google Scholar] [CrossRef] [PubMed]
- Han, C.H.; Liu, J.C.; Fang, S.U.; Hou, W.C. Antioxidant activities of the synthesized thiol-contained peptides derived from computer-aided pepsin hydrolysis of yam tuber storage protein, dioscorin. Food Chem. 2013, 138, 923–930. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sie, Y.-Y.; Chen, L.-C.; Li, C.-W.; Wang, C.-C.; Li, C.-J.; Liu, D.-Z.; Lee, M.-H.; Chen, L.-G.; Hou, W.-C. Extracts and Scirpusin B from Recycled Seeds and Rinds of Passion Fruits (Passiflora edulis var. Tainung No. 1) Exhibit Improved Functions in Scopolamine-Induced Impaired-Memory ICR Mice. Antioxidants 2023, 12, 2058. https://doi.org/10.3390/antiox12122058
Sie Y-Y, Chen L-C, Li C-W, Wang C-C, Li C-J, Liu D-Z, Lee M-H, Chen L-G, Hou W-C. Extracts and Scirpusin B from Recycled Seeds and Rinds of Passion Fruits (Passiflora edulis var. Tainung No. 1) Exhibit Improved Functions in Scopolamine-Induced Impaired-Memory ICR Mice. Antioxidants. 2023; 12(12):2058. https://doi.org/10.3390/antiox12122058
Chicago/Turabian StyleSie, Yi-Yan, Liang-Chieh Chen, Cai-Wei Li, Ching-Chiung Wang, Cai-Jhen Li, Der-Zen Liu, Mei-Hsien Lee, Lih-Geeng Chen, and Wen-Chi Hou. 2023. "Extracts and Scirpusin B from Recycled Seeds and Rinds of Passion Fruits (Passiflora edulis var. Tainung No. 1) Exhibit Improved Functions in Scopolamine-Induced Impaired-Memory ICR Mice" Antioxidants 12, no. 12: 2058. https://doi.org/10.3390/antiox12122058
APA StyleSie, Y.-Y., Chen, L.-C., Li, C.-W., Wang, C.-C., Li, C.-J., Liu, D.-Z., Lee, M.-H., Chen, L.-G., & Hou, W.-C. (2023). Extracts and Scirpusin B from Recycled Seeds and Rinds of Passion Fruits (Passiflora edulis var. Tainung No. 1) Exhibit Improved Functions in Scopolamine-Induced Impaired-Memory ICR Mice. Antioxidants, 12(12), 2058. https://doi.org/10.3390/antiox12122058







