Abstract
Filamentous fungi produce several beneficial secondary metabolites, including bioactive compounds, food additives, and biofuels. Trichoderma, which is a teleomorphic Hypocrea that falls under the taxonomic groups Ascomycota and Dikarya, is an extensively studied fungal genus. In an ongoing study that seeks to discover bioactive natural products, we investigated potential bioactive metabolites from the methanolic extract of cultured Trichoderma gamsii. Using liquid chromatography–mass spectrometry (LC–MS), one major compound was isolated and structurally identified as 6-pentyl-α-pyrone (6PP) based on nuclear magnetic resonance data and LC–MS analysis. To determine its antioxidant and anti-inflammatory activity, as well as the underlying mechanisms, we treated lipopolysaccharide (LPS)-stimulated Raw264.7 mouse macrophages with 6PP. We found that 6PP suppresses LPS-induced increase in the levels of nitric oxide, a mediator of oxidative stress and inflammation, and restores LPS-mediated depletion of total glutathione by stabilizing nuclear factor erythroid 2-related factor 2 (Nrf2), an antioxidative factor, and elevating heme oxygenase-1 levels. Furthermore, 6PP inhibited LPS-induced production of proinflammatory cytokines, which are, at least in part, regulated by heme oxygenase-1 (HO-1). 6PP suppressed proinflammatory responses by inhibiting the nuclear localization of nuclear factor kappa B (NF-κB), as well as by dephosphorylating the mitogen-activated protein kinases (MAPKs). These results indicate that 6PP can protect macrophages against oxidative stress and LPS-induced excessive inflammatory responses by activating the Nrf2/HO-1 pathway while inhibiting the proinflammatory, NF-κB, and MAPK pathways.
1. Introduction
Host inflammatory responses that protect from invading pathogens, including bacterial infections, are mainly mediated by innate immune cells, such as neutrophils, macrophages, and dendritic cells [1,2]. Of these, macrophages, which detect invading pathogens via specific receptors, especially toll-like receptor 4, which recognizes lipopolysaccharides (LPS), a constituent of Gram-negative bacterial cell walls, are key inflammation mediators [3]. LPS-interacting macrophages trigger inflammation by expressing proinflammatory mediators, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and nitric oxide (NO) [4,5]. Oxidative stress is also closely associated with the development of chronic inflammatory diseases [6]. Moreover, oxidative stress and inflammatory responses are interdependent because reactive oxygen species induce the release of proinflammatory factors, which, in turn, trigger oxidative stress [7]. To maintain homeostasis in macrophages, the stabilization of nuclear factor erythroid 2-related factor 2 (Nrf2) against oxidative stress is crucial. In response to oxidative stress and LPS, Nrf2 translocates to the nucleus and drives the expression of antioxidant genes, such as heme oxygenase-1 (HO-1) and NAD(P)H dehydrogenase [quinone] 1 [8]. Under excessive oxidative stress and proinflammatory stimuli, the homeostasis of Nrf2 and other anti-inflammatory factors is disrupted, resulting in oxidative stress- and inflammation-mediated diseases, such as asthma, rheumatoid arthritis, hepatitis, and ulcers [9,10,11]. Therefore, the control of macrophage activity is a potential strategy for the development of effective inflammatory disease treatments.
Trichoderma species, which are teleomorphic Hypocrea, fall under the taxonomic groups Ascomycota and Dikarya and are rich in secondary metabolites, including >1000 known compounds with a wide range of structures [12]. Some Trichoderma species, such as Trichoderma reesei, Trichoderma harzianum, Trichoderma atroviride, Trichoderma virens, Trichoderma asperellum, and Trichoderma asperelloides have a high industrial value and have been directly used as biological control agents [13]. Trichoderma species also produce various enzymes with potential biocontrol activity, including cell wall degradation, biotic and abiotic stress tolerance, hyphal growth, and anti-plant pathogen effects. Consequently, cell wall degrading enzymes from Trichoderma species can be used for the development of commercial products [14]. Trichoderma gamsii (T. gamsii) strongly inhibits the growth of wood-damaging fungi by releasing volatile metabolites and is known as a key producer of biological control agents [15]. Previous studies of the secondary metabolites from T. gamsii identified several cytochalasans with distinct structures, such as trichalasins C and D, aspochalasins D, M, and P, and trichoderones A and B [16,17]. Several of these compounds exhibit notable cytotoxicity [16]. Moreover, T. gamsii possesses intriguing metabolites, such as trichoderpyrone (a unique polyketide hybrid with a cyclopentenone-pyrone framework [18]), trichoderamides A and B (a pair of unique stereoisomers), and two novel compounds, trichodenols A and B [19]. Thus, investigation of the secondary metabolites from T. gamsii holds great promise.
In an ongoing study to identify new bioactive compounds from various natural materials, including microbes [20,21,22,23,24], we investigated potential bioactive metabolites from the methanolic extract of cultured T. gamsii. Using column chromatography and high-performance liquid chromatography (HPLC), coupled with liquid chromatography–mass spectrometry (LC–MS), we isolated one major compound, which was identified as 6-pentyl-α-pyrone (6PP).
To determine its antioxidant and anti-inflammatory effects, we treated Raw264.7 macrophages with 6PP at various concentrations in the absence or presence of LPS and then examined its antioxidant and anti-inflammatory effects, as well as the underlying mechanisms. Our findings indicate that 6PP exerts antioxidant and anti-inflammatory effects by activating the Nrf2/HO-1 and the nuclear factor kappa B (NF-κB) signaling pathways while inhibiting the mitogen-activated protein kinase (MAPK) signaling pathway.
2. Materials and Methods
2.1. General Experimental Procedures
Nuclear magnetic resonance (NMR) spectra were acquired using a Bruker Avance III HD 500 NMR spectrometer at 500 MHz (1H), and chemical shifts were determined in ppm (δ). Preparative HPLC was done using a Waters 1525 Binary HPLC pump equipped with a Waters 996 photodiode array detector (Waters Corporation, Milford, CT, USA). LC–MS analysis was done on an Agilent 1200 Series HPLC system (Agilent Technologies, Santa Clara, CA, USA) equipped with a diode array detector and a 6130 Series ESI mass spectrometer on an analytical Kinetex C18 Å column (100 mm × 2.1 mm, 5 μm, Phenomenex) at a flow rate of 0.3 mL/min. Spots were detected on a thin-layer chromatography (TLC) plate under UV light or upon heating after spraying with anisaldehyde–sulfuric acid. Column chromatography was done using a silica gel 60 (230–400 mesh, Merck, Darmstadt, Germany). Merck precoated silica gel F254 plates and RP-18 F254s plates were used for TLC.
2.2. Fungal Material
T. gamsii KUC1747 was purchased from the Korea University Culture Collection (KUC).
2.3. T. gamsii Culture and Isolation of 6PP
T. gamsii was pre-cultured on potato dextrose agar medium ([PDA], BD Difco, Tucker, GA, USA) at 25 °C for five days in the dark. Three inoculums were transferred from the margin of a PDA plate to 5 L PDA plates and incubated at 25 °C for seven days, followed by extraction using 10 L of MeOH to obtain the MeOH extract. The crude methanol (MeOH) extract (3.5 g) was successively partitioned using ethyl acetate (EtOAc), and the resulting EtOAc fraction (1.2 g) was subjected to fractionation using silica gel column chromatography (diameter: 2 × 40 cm) on hexane/EtOAc gradients of 1:0, 1:100, 1:10, 1:1 and 0:1. This generated five EtOAc sub-fractions based on the TLC profiles obtained. The sub-fractions were eluted using hexane/ethyl acetate (1:10) and further separated via chromatography using Sephadex LH-20 (diameter: 2 × 35 cm, CHCl3: MeOH = 1:1) on a C18 column (diameter: 1 × 26 cm) using a 20–100% MeOH gradient and preparative HPLC (YMC J’sphere ODS-H80, 4 μm, 250 × 20 mm i.d., at a flow rate of 4.0 mL/min in 50–70% MeOH, for 50 min), which isolated one major compound (50 mg).
2.4. DPPH (2,2’-Diphenyl-1-picrylhydrazyl radical) Assay
6PP was diluted in 100% MeOH to indicated concentrations, respectively, after which 100 µL of each diluent was transferred to 96-well plates. Afterward, 100 µL of 0.2 mM DPPH solution was added and incubated for 30 min in a 37 °C incubator. After incubation, absorbance was measured at 517 nm using a microplate reader (Synergy HTX, BioTek Instruments Inc., Winooski, VT, USA). The radical scavenging activity was then calculated as (Abssample − AbsMeOH)/AbsMeOH and expressed as relative values. Quercetin (Sigma-Aldrich, St. Louis, MO, USA) was used as the positive control.
2.5. Cell Culture
Raw264.7 cells (ATCC, #TIB-71, Fairfield, NJ, USA) were cultured in high glucose Dulbecco’s Modified Eagle’s Medium (DMEM) (#LM0001-05, WELGENE Inc., Gyeongsan, Republic of Korea) supplemented with 10% fetal bovine serum (#16000044, Gibco, Waltham, MA, USA), 100 IU/mL of penicillin, and 100 µg/mL of streptomycin (#30-002-cl, Corning Inc., Corning, NY, USA). The cells (2nd to 3rd passage) were cultured at 37 °C in a humidified CO2 incubator.
2.6. Cell Viability Assay
Raw264.7 cells were seeded in 96-well plates at a density of 2 × 104 cells/well and then cultured in the presence of 6PP at 12.5, 25, 50, 100, and 200 µM for 24 h. Cell viability assays were then performed using an EZ-Cytox cell viability assay kit (#EZ-1000, DoGenBio, Seoul, Republic of Korea). Briefly, the cells were incubated with the EZ-Cytox solution, which contained water-soluble tetrazolium salt, for 30 min, followed by absorbance reading at 450 nm on a microplate reader (Synergy HTX, BioTek Instruments Inc.). Cell viability was calculated as a percentage relative to the untreated group (100%).
2.7. Glutathione (GSH) Assay
GSH levels were assessed using a glutathione assay kit (#703002, Cayman Chemical Company, Ann Arbor, MI, USA) according to manufacturer instructions. Raw264.7 cells were seeded in 12-well plates at a density of 2 × 105 cells/well and then pretreated with 6PP at 12.5, 25, 50, 100, and 200 µM for 2 h. They were then stimulated with LPS (0.5 µg/mL) for 24 h, washed with cold phosphate-buffered saline (PBS), and then sonicated in cold 2-(N-morpholino) ethane sulfonic acid (MES) buffer (50 mM), which was provided in the GSH assay kit. The samples were then centrifuged at 10,000× g for 15 min at 4 °C. The supernatant was then mixed with an equal volume of 10% meta-phosphoric acid (#239275, Sigma–Aldrich). The solution was then reacted with an enzyme cocktail containing nicotinamide adenine dinucleotide phosphate (NADPH), glutathione reductase, and 5,5′-dithio-bis-2-nitrobenzoic acid in a 96-well plate to quantify total GSH and glutathione disulfide (GSSG). For GSSG measurement, the supernatant was incubated with 1M 2-vinyl pyridine (#13229-2, Sigma-Aldrich) at room temperature (RT) for 1 h before the enzymatic reaction. Following 25 min incubation in the dark, absorbance was read at 410 nm on a microplate reader (Synergy HTX, BioTek Instruments).
The levels of GSH and GSSG were calculated using the formula:
Total GSH or GSSG (μM) = [(absorbance of the sample) − (y-intercept)]/(slope) × 2 × sample dilution.
GSH (μM) = (Total GSH) − 2 × (GSSG)
GSH (μM) = (Total GSH) − 2 × (GSSG)
2.8. Measurement of Nitric Oxide (NO) Levels
Raw264.7 cells were seeded in 12-well plates at a density of 2.5 × 105 cells/well and pretreated with 6PP at 12.5, 25, 50, 100, and 200 µM for 2 h. They were then stimulated with LPS (0.5 µg/mL) for 24 h. Next, 100 µL of the cell culture supernatant was mixed with an equal volume of Griess reagent containing 1% sulfanilamide, 0.1% N-1-naphthyl ethylenediamine dihydrochloride, and 2.5% phosphoric acid, and then incubated in the dark for 10 min. Absorbance was then measured at 540 nm using a microplate reader (Synergy HTX, BioTek Instruments).
2.9. Western Blot Analysis
Western blot analysis was done as previously described [25]. Briefly, the cells were pretreated with 6PP at 25, 50, 100, and 200 µM for 2 h and then stimulated with LPS (0.5 µg/mL) for 15 min or 24 h. Total proteins were extracted using a rapid immunoprecipitation assay buffer (#RC2002-050-00, Biosesang, Seongnam, Republic of Korea) supplemented with a protease inhibitor cocktail and phosphatase inhibitor cocktails II and III (#P8340, #P5726, and #P0044, respectively, Sigma–Aldrich) on ice. After protein quantification, total proteins were denatured, resolved using SDS–PAGE, and transferred onto nitrocellulose membranes. The membranes were blocked using 5% nonfat dry milk (#SKI500, LPS Solution, Daejeon, Republic of Korea) in Tris-buffered saline (25 mM Tris-HCl pH 8.0 and 125 mM NaCl) containing 0.1% Tween-20 (TBS-T) at RT, for 1 h. The membranes were then incubated with the indicated primary antibodies at 4 °C overnight. They were then washed thrice using TBS-T and incubated with horseradish peroxidase-conjugated anti-mouse or anti-rabbit secondary antibodies at RT for 1 h. The protein signal was then developed using enhanced chemiluminescence (#BWP0200, Biomax, Seoul, Republic of Korea) and imaged on a chemiluminescence imaging system (Amersham Imager 680, GE Healthcare, Chicago, IL, USA). Protein band densitometric analysis was determined using Amersham software (ImageQuant TL ver 8.2) (GE Healthcare, Chicago, IL, USA). The following antibodies were used for western blot analyses: anti-iNOS (#610332, BD Biosciences, San Diego, CA, USA), anti-HO-1 (#sc-390991), anti-β-actin (#sc-47778), anti-p38 (#sc-7972), and anti-p44/42 (#sc-514302; Santa Cruz Biotechnology Inc., Dallas, TX, USA), and anti-Nrf2 (#12721), anti-SAPK/JNK (#9252), anti-phospho-SAPK/JNK (#9251), anti-phospho-p38 (#9211), anti-phospho-p44/42 (#9101), anti-p-IκB (#9246), anti-IκB (#9242), anti-mouse (#7076), and anti-rabbit (#7074; Cell Signaling Technology, Danvers, MA, USA).
2.10. Enzyme-Linked Immunosorbent Assay (ELISA)
Raw264.7 cells were seeded in 12-well plates at a density of 2.5 × 105 cells/well, pretreated with 6PP at 25, 50, 100, and 200 µM for 2 h, and then stimulated with LPS (0.5 µg/mL) for 24 h. Cytokine levels in the culture media were then measured using an ELISA kit according to the manufacturer’s instructions. Briefly, purified anti-IL-1β (#14-7012-85) and anti-TNF-α (#14-7423-85; Thermo Fisher Scientific Inc., Waltham, MA, USA) and anti-IL-6 (#554400, BD Pharmingen, San Diego, CA, USA), antibodies were used to coat 96-well plates at 4 °C, overnight. The plates were then washed thrice using PBS-T (0.05% Tween-20 in PBS) and blocked for 1 h at RT, using 1% bovine serum albumin (BSA) in PBS. Following incubation of the supernatants for 2 h at RT, the plates were washed thrice using PBS-T. Next, detection antibodies were added, followed by incubation for 1 h at RT. The plates were then washed four times with PBS-T, incubated with streptavidin-conjugated alkaline phosphatase (AKP, #554065, BD Pharmingen) for 30 min at RT, and then washed four times with PBS-T. Finally, the plates were incubated in the dark with a substrate buffer (pH 9.8) containing 10% diethanolamine (#3032-4400), 0.1% MgCl2·6H2O (#5503-44), and 0.2% NaN3 (#7530-4105; Daejung Chemicals & Metals Co., Siheung, Republic of Korea), and 4-nitrophenyl phosphate (#N2765, Sigma-Aldrich). The reaction was terminated using 1 N NaOH, followed by an absorbance reading at 405 nm on a microplate reader (Synergy HTX, BioTek Instruments).
2.11. Immunofluorescence
Raw264.7 cells were seeded onto coverslips in 24-well plates at a density of 1.0×105 cells/well, pretreated with 6PP at 200 μM for 2 h, and then stimulated with LPS (0.5 µg/mL) for 15 min (or 8 h in the case of Nrf2 translocation experiments). Next, the cells were fixed using 4% paraformaldehyde (#PC2031-050-00, Biosesang), permeabilized with 0.1% Triton X-100 (#T8787, Sigma-Aldrich) for 10 min at RT, and then blocked using 1% BSA (in PBS) for 1 h at RT. The cells were then incubated with an anti-NF-κB/p65 or an anti-Nrf2 antibody at 4 °C overnight. The cells were then incubated with fluorescent secondary antibodies for 1 h in the dark. Finally, the coverslips were mounted onto glass slides using a mounting media (#S36936, Thermo Fisher Scientific Inc.). Then, we used a 1000× objective lens to acquire images and digitally zoomed to obtain images at a magnification of 3000× using software (NIS-Elements Advance Research V5.41.01, Nikon Instruments, Tokyo, Japan) under a confocal microscope (Nikon AX R, Nikon Instruments).
2.12. Statistical Analyses
Statistical analyses were done using SPSS Statistics 27 (IBM Corporation, Inc., New York, NY, USA). All data are presented as the mean ± standard error of the mean (SEM) of three independent experiments. Statistical significance was determined using the nonparametric Mann–Whitney U test. p < 0.05 indicates statistically significant differences.
3. Results
3.1. Isolation and Identification of 6PP
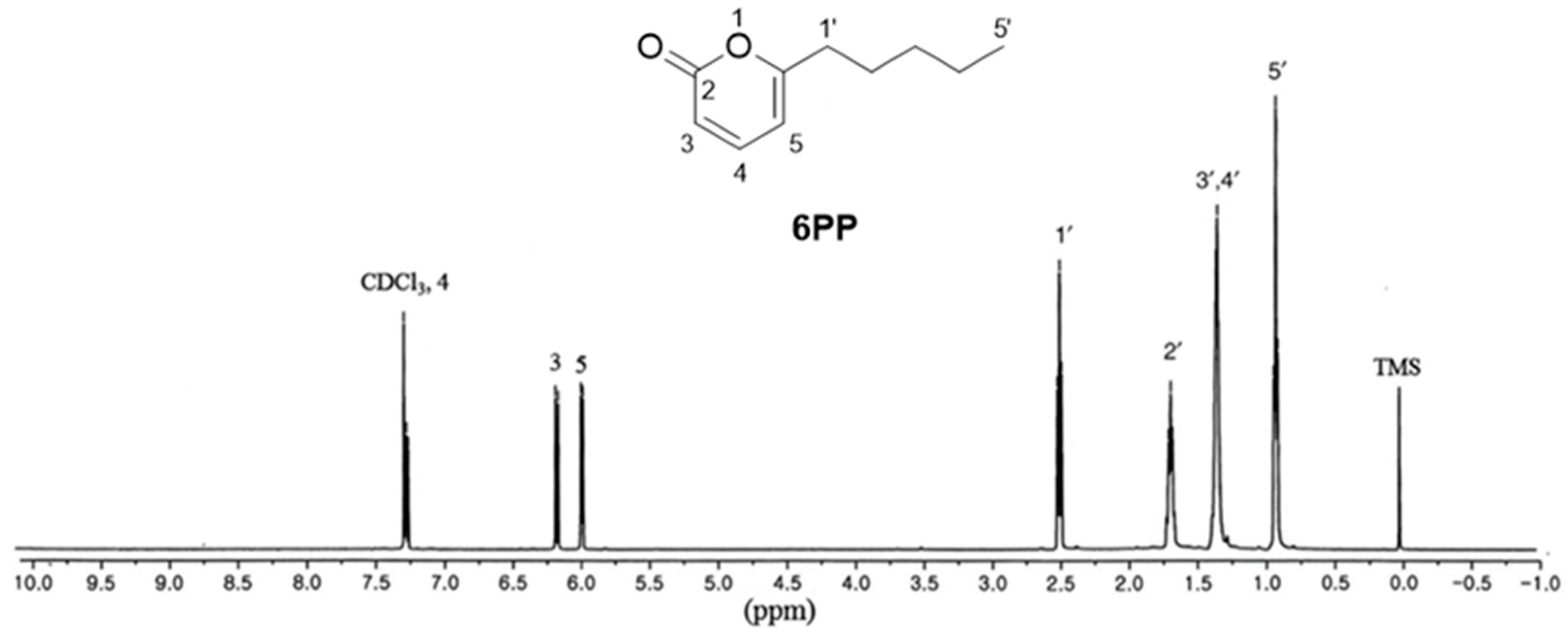
Cultured T. gamsii was subjected to 100% MeOH extraction at RT. The MeOH extract was then successively partitioned using EtOAc, followed by silica gel column chromatography of the resulting EtOAc fraction to obtain sub-fractions. The sub-fractions were then subjected to LC–MS analysis using an in-house ultraviolet (UV) library database to identify one major compound. The sub-fraction containing the major compound was then separated on Sephadex LH-20, using C18 column chromatography, followed by isolation of the major compound using preparative HPLC. The isolated compound was identified as 6PP (Figure 1) by comparing its 1D NMR spectrum [1H-NMR (500 MHz, CDCl3) δ 7.26 (1H, dd, J = 6.5, 9.5 Hz), 6.15 (1H, d, J = 9.5 Hz), 5.97 (1H, d, J = 6.5 Hz), 2.48 (2H, t, J = 7.5 Hz), 1.64–1.70 (2H, m), 1.24–1.36 (4H, m), 0.90 (3H, t, J = 6.5 Hz) ppm] with previously reported data [13], as well as by analyzing the LC-MS data showing the molecular ion peak at m/z 167.1 [M + H]+ (Supplementary Figure S1). The structure was further confirmed through comparison with the standard available in LC-MS analysis.
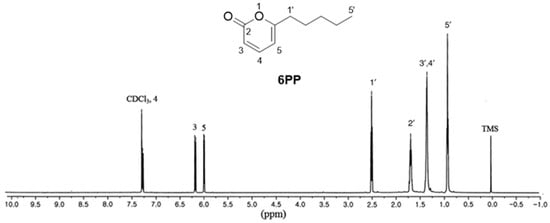
Figure 1.
The chemical structure of 6-pentyl-α-pyrone (6PP) and its 1H-NMR spectrum.
3.2. Non-Cytotoxic 6PP Concentrations Modulate Intracellular GSH Levels
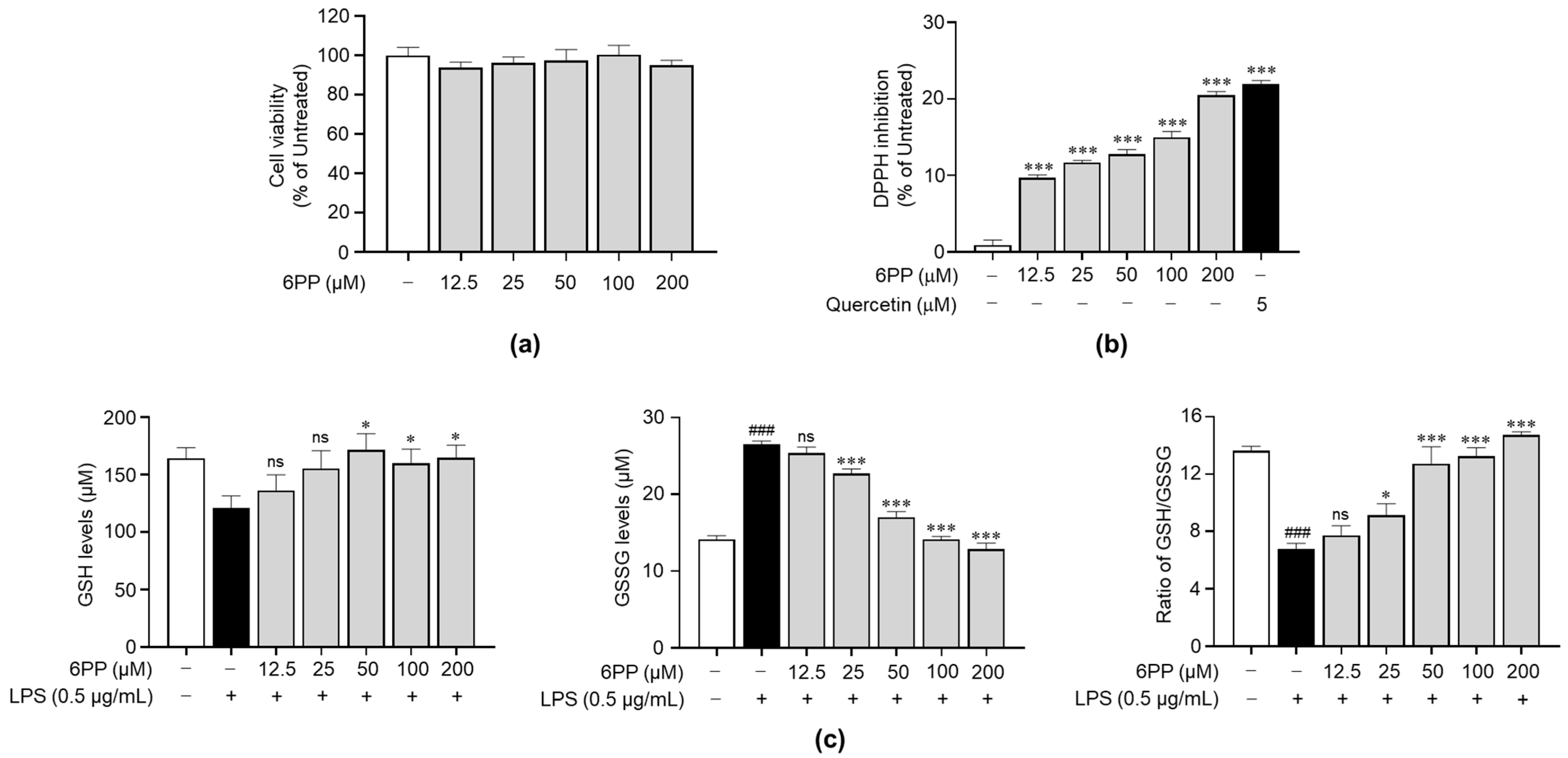
To identify its non-cytotoxic concentrations in Raw264.7 cells, we treated the cells with 6PP at 12.5, 25, 50, 100, and 200 μM for 24 h. This analysis revealed that when compared with the untreated group, 6PP was not significantly cytotoxic at the tested concentrations (Figure 2a). Therefore, subsequent experiments were conducted using 6PP at concentrations of up to 200 μM. Prior to identifying the antioxidant property of 6PP in cells, a DPPH assay was conducted to verify whether 6PP has direct antioxidant activity. As shown in Figure 2b, its radical scavenging activity was not as strong as quercetin, a well-known antioxidant molecule. Next, we investigated the potential antioxidant activity of 6PP in Raw264.7 cells following LPS-induced oxidative stress by assessing the intracellular levels of GSH and GSSG, as well as the GSH/GSSG ratio (Figure 2c). This analysis found that the LPS-treated group exhibited a reduction of GSH levels and GSH/GSSG ratio as well as the induction of GSSH. However, pretreatment with the indicated concentrations of 6PP showed notable increases in intracellular GSH levels and GSH/GSSG ratio. GSSG levels were also reduced by 6PP treatment in a dose-dependent manner.
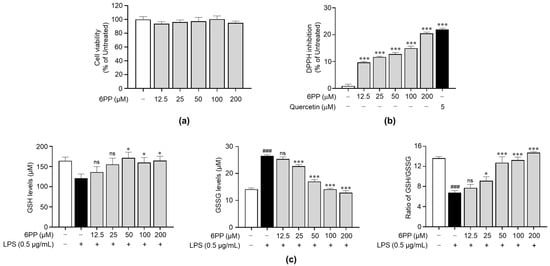
Figure 2.
Reducing effects of 6PP under non-cytotoxic concentration. (a) Raw264.7 cells were treated with 6PP at indicated concentrations for 24 h, followed by cell viability analysis. (b) Inhibition of the free radical DPPH elicited by a range of 6PP concentrations (0–200 µM). Quercetin was used as the positive control. Statistical significance between the groups was determined using the Mann–Whitney U test. *** indicate p < 0.001 vs. untreated groups. (c) The cells were pretreated with 6PP at indicated concentrations for 2 h and then stimulated with LPS (0.5 µg/mL) for 24 h. Total GSH, GSSG, and the GSH/GSSG ratio were determined using a GSH assay kit. The data represent the mean ± standard error of the mean (SEM) of three independent experiments. Statistical significance between the groups was determined using the Mann–Whitney U test. ### indicates p < 0.001 in the untreated group vs. the LPS-treated group. * and *** indicate p < 0.05 and <0.001, respectively, in the LPS- vs. the 6PP-treated groups. ns indicates not significant. 6PP, 6-pentyl-α-pyrone; GSH, glutathione; GSSG, glutathione disulfide.
3.3. 6PP Inhibits LPS-Induced Production of Inflammatory Mediators
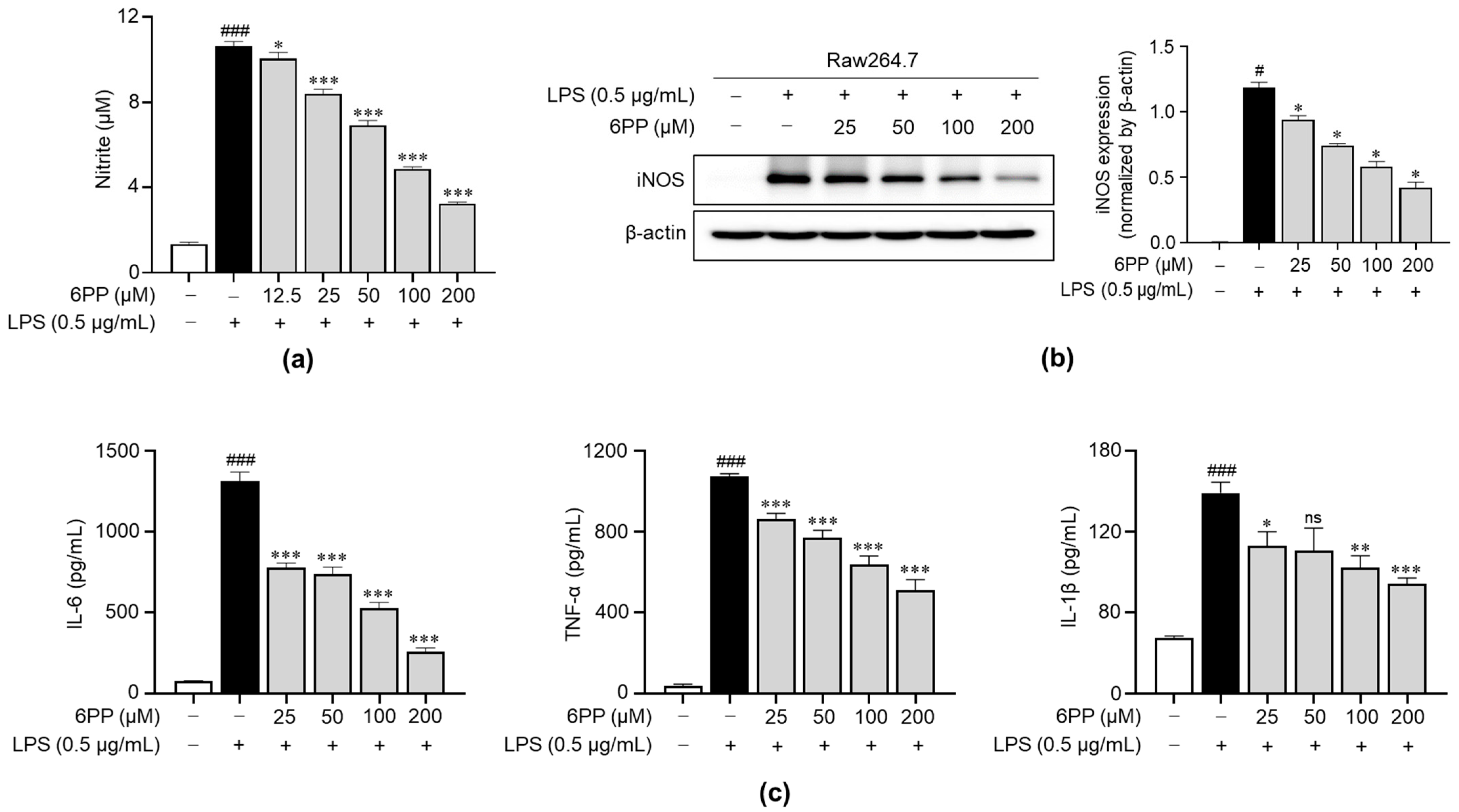
We examined the impact of 6PP on the production of NO upon stimulation with LPS. Data revealed that LPS triggered a significant increase in NO levels and that this effect was dose-dependently inhibited by pretreatment with 6PP (Figure 3a). Moreover, iNOS expression, a responsible enzyme for the production of NO [26,27,28], was reduced by 6PP treatment. To determine the anti-inflammatory potential of 6PP, we examined its ability to inhibit the secretion of proinflammatory cytokines, including IL-6, TNF-α, and IL-1β in Raw264.7 cells. LPS-induced production of proinflammatory cytokines was alleviated by pretreatment of 6PP (Figure 3c).
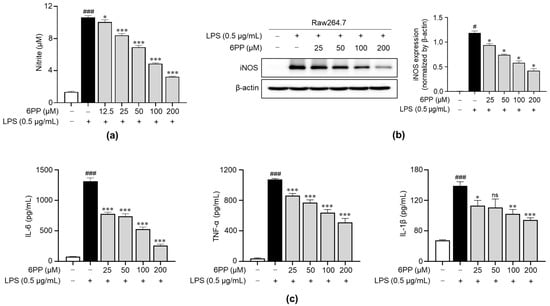
Figure 3.
Inhibitory effects of 6PP on the production of proinflammatory mediators in LPS-stimulated Raw264.7 cells. Raw264.7 cells were pretreated at the indicated 6PP concentrations for 2 h and then stimulated with LPS (0.5 µg/mL) for 24 h. (a) The levels of NO in the cell culture media were quantified using Griess reagent. NO levels were determined by comparing its absorbance to that of a standard curve of nitrite standard solution concentrations. (b) iNOS protein levels were determined using western blotting, with β-actin as the loading control. (c) The levels of IL-6, TNF-α, and IL-1β were determined using ELISA. The data represent the mean ± SEM of three independent experiments. Differences between groups were compared using the Mann–Whitney U test. # and ### represent p < 0.05 and <0.001, respectively, vs. the LPS-untreated group. *, **, and *** indicate p < 0.05, <0.01, and <0.001, respectively, vs. the LPS-treated group. ns indicates not significant. NO, nitric oxide; iNOS, inducible nitric oxide synthase; IL, interleukin; TNF, tumor necrosis factor.
3.4. 6PP Exerts Anti-Inflammatory Effects by Activating HO-1
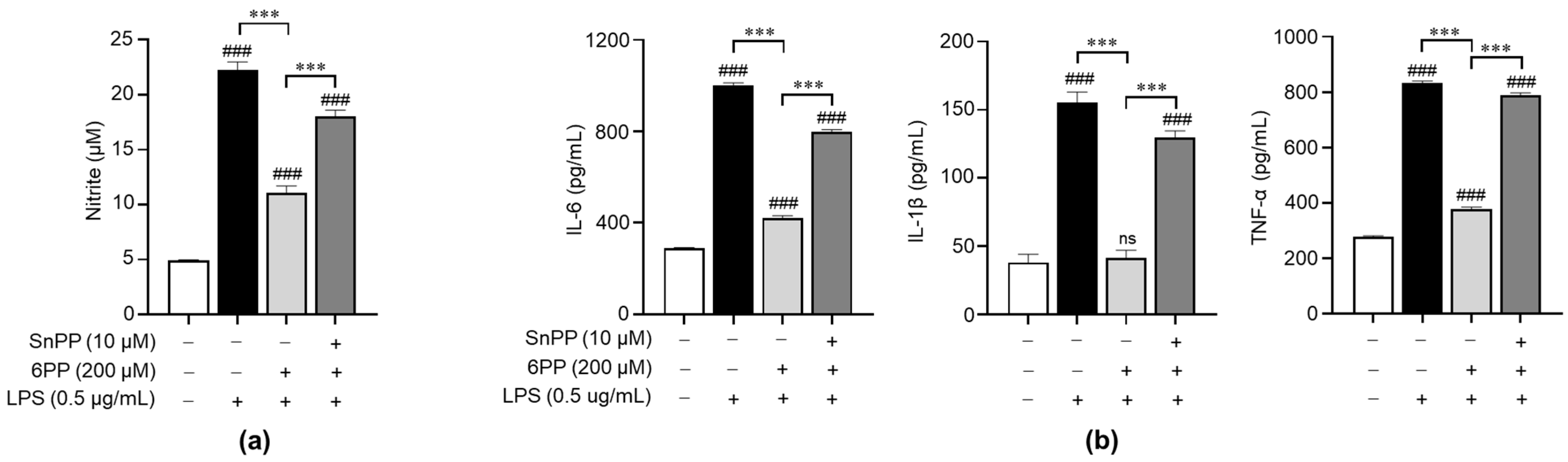
To investigate the relationship between the anti-inflammatory effects of 6PP and the activation of antioxidant signaling molecules, we treated LPS-stimulated macrophages with Sn-protoporphyrin (SnPP), a HO-1 inhibitor [29], and examined its effects on the production of NO and proinflammatory cytokines. SnPP significantly recovered 6PP-mediated inhibition of NO production (Figure 4a), indicating that HO-1 activity is involved in the regulation of 6PP-mediated NO production, at least in part. Furthermore, SnPP showed similar recovery effects for 6PP-mediated alleviation of proinflammatory cytokines’ production (Figure 4b).
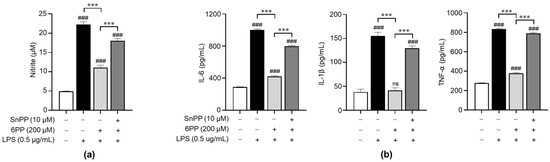
Figure 4.
Involvement of antioxidant HO-1 in the 6PP-mediated regulation of proinflammatory mediators’ production. Raw264.7 cells were pretreated with 6PP (200 µM) for 2 h in the presence or absence of SnPP (10 µM), followed by LPS stimulation (0.5 µg/mL) for 24 h. (a) NO levels in the culture media were determined using the Griess assay and quantified against a standard curve of nitrite standard-solution concentrations. (b) The levels of IL-6, TNF-α, and IL-1β in the culture media were determined using ELISA. The data are presented as mean ± SEM of three independent experiments. Statistical differences between the groups were compared using the Mann-Whitney U test. ### indicates p < 0.001 vs. the LPS-untreated group. *** indicates p < 0.001 between paired groups. ns indicates not significant. HO-1, heme oxygenase-1; SnPP, Sn-protoporphyrin.
3.5. 6PP Mitigates LPS-Induced Oxidative Stress by Activating the Nrf2 Signaling Pathway and Inducing Nrf2 Nuclear Translocation
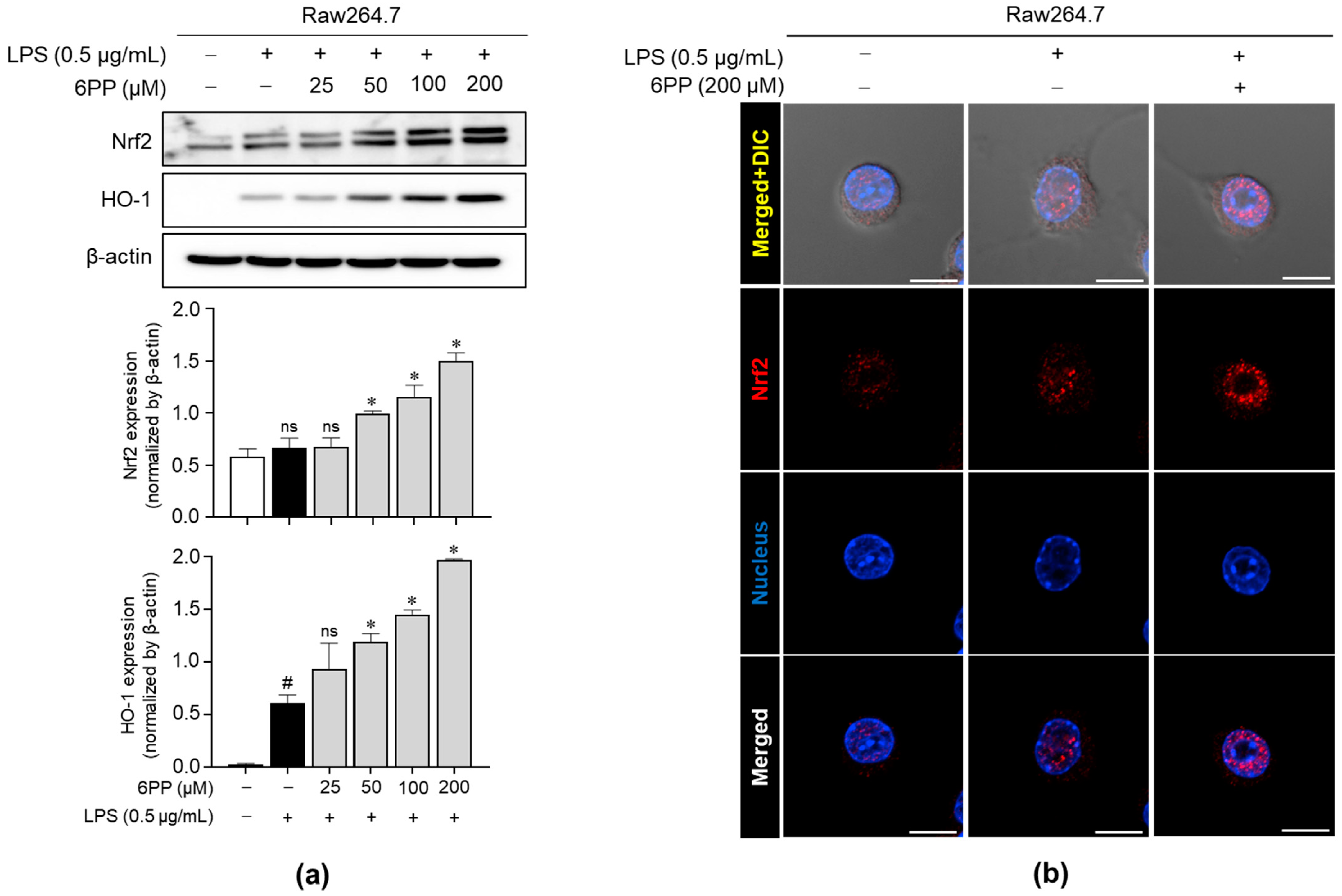
We investigated whether 6PP promotes the upregulation of critical antioxidant proteins, such as Nrf2 and HO-1, in Raw264.7 macrophages. As shown in Figure 5a, 6PP significantly induced the expression levels of Nrf2 and HO-1 in a dose-dependent manner. Moreover, immunofluorescence analysis revealed that 6PP significantly enhanced nuclear expression levels of Nrf2 (Figure 5b and Figure S2).
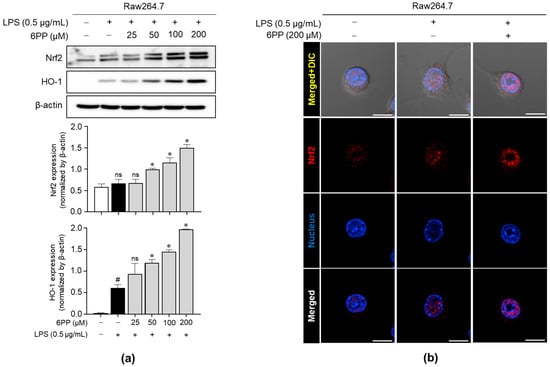
Figure 5.
Enhancing effects of 6PP on the expression of Nrf2 and HO-1. (a) Raw264.7 cells were treated with LPS (0.5 µg/mL) in the presence of 6PP at the indicated concentrations for 24 h. iNOS protein levels were assessed using western blot analysis, with β-actin as the loading control. (b) Nrf2 (red) nuclear translocation was examined using confocal microscopy. Nuclei were counterstained with DAPI (blue). The data represent the mean ± SEM of three independent experiments. Statistical differences between the groups were compared using the Mann–Whitney U test. # indicates p < 0.05 vs. the LPS-untreated group. * indicates p < 0.05 vs. the LPS-treated group. ns indicates not significant. Scale bar: 10 µm. Nrf2, nuclear factor erythroid 2-related factor 2; DAPI, 4′,6-diamidino-2-phenylindole.
3.6. 6PP Inhibits IκB Phosphorylation and Degradation and NF-κB/p65 Nuclear Translocation
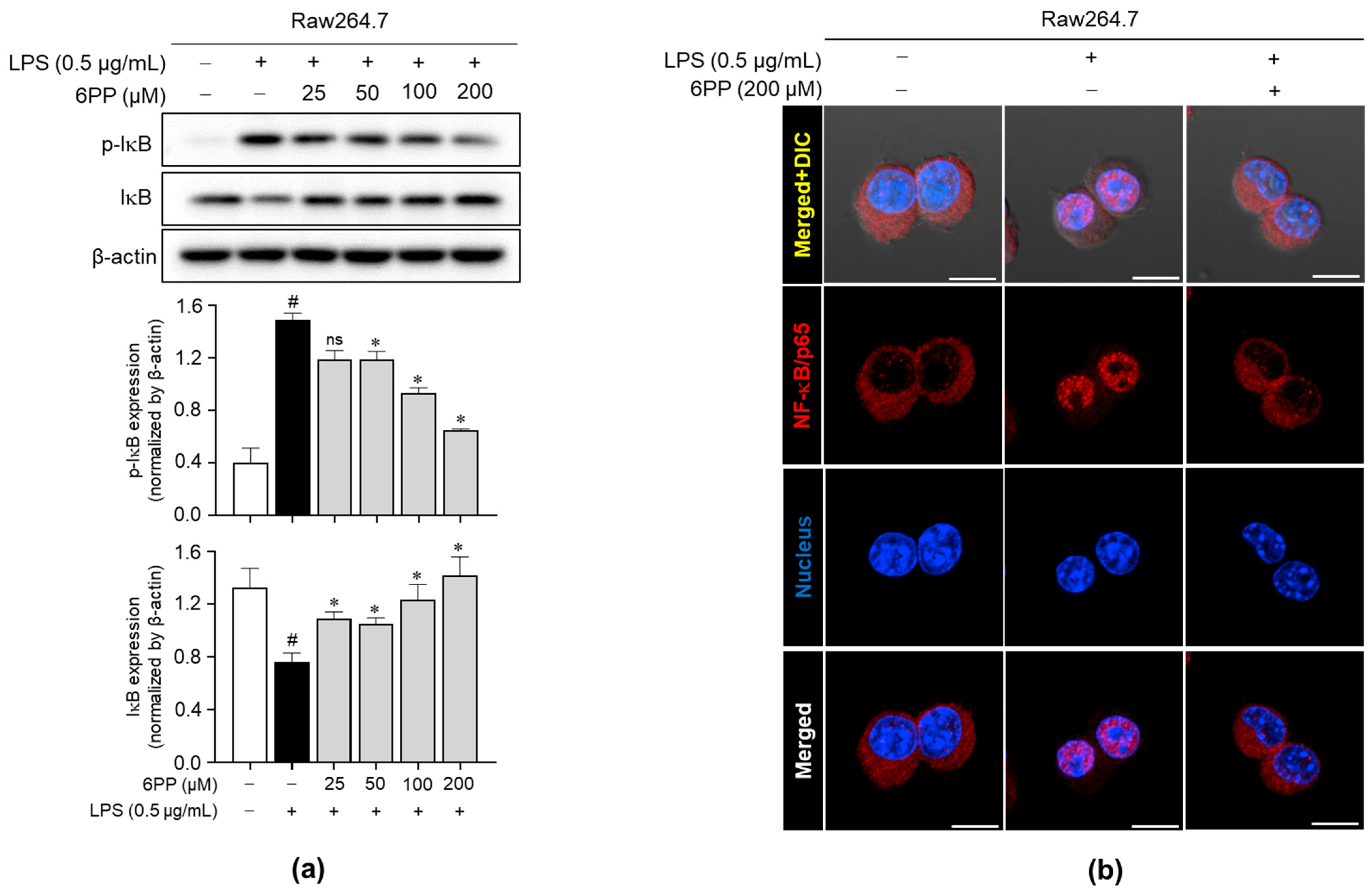
To determine the involvement of the NF-κB signaling pathway in 6PP-mediated anti-inflammatory effects, the effect of 6PP on the IκB phosphorylation and degradation was investigated. Data revealed that 6PP reduces LPS-induced IκB phosphorylation and thereby blocks LPS-induced IκB degradation (Figure 6a). Moreover, LPS-induced NF-κB/p65 nuclear translocation was alleviated by 6PP treatment (Figure 6b and Figure S3).
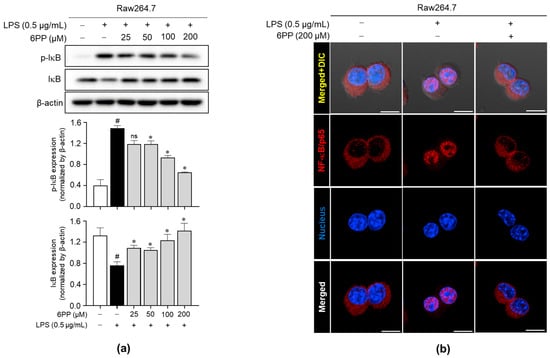
Figure 6.
Inhibitory effects of 6PP on the NF-κB signaling pathway. Raw264.7 cells were pretreated with 6PP (200 µM) for 2 h and then stimulated with LPS (0.5 µg/mL) for 15 min. (a) Western blot analysis of the phosphorylated and total IκB levels, with β-actin as the loading control. (b) NF-κB/p65 (red) nuclear translocation was analyzed using confocal microscopy. Nuclei were counterstained with DAPI (blue). The data represent the mean ± SEM of three independent experiments. Statistical differences between groups were compared using the Mann–Whitney U test. # indicates p < 0.05 vs. the LPS-untreated group; * indicates p < 0.05 vs. the LPS-treated group. ns indicates not significant. Scale bar: 10 µm. IκB, an inhibitor of κB; NF-κB, nuclear factor kappa B.
3.7. 6PP Suppresses MAPK Phosphorylation
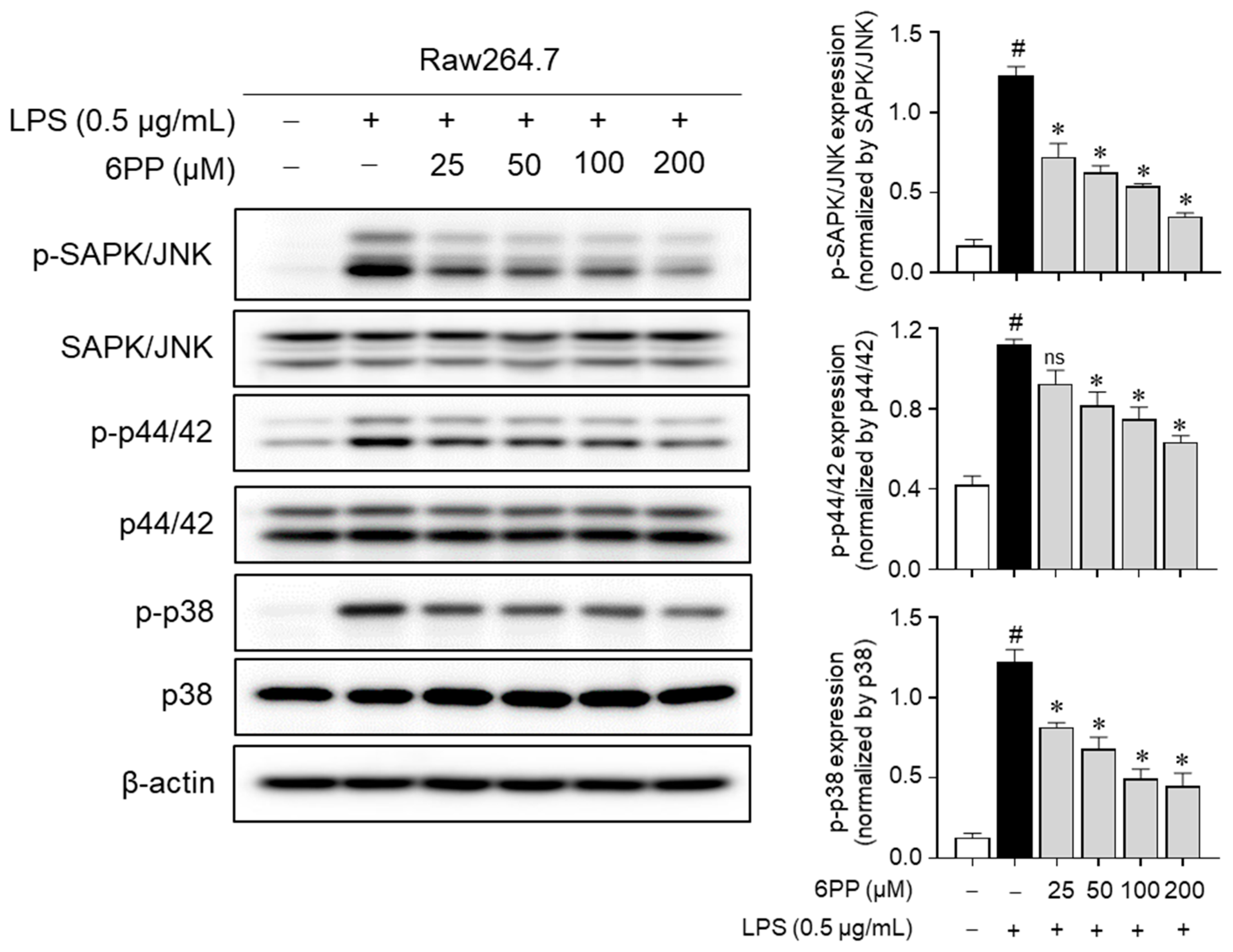
To determine whether 6PP-mediated inhibition of proinflammatory responses is regulated by MAPKs, changes in MAPKs’ phosphorylation level by 6PP were measured in LPS-treated Raw264.7 macrophages. Data showed that LPS-induced phosphorylation of MAPKs was alleviated by 6PP in a dose-dependent manner (Figure 7), implying that 6PP suppresses the production of proinflammatory mediators by inhibiting MAPKs’ activation as well as NF-κB in LPS-stimulated Raw264.7 cells.
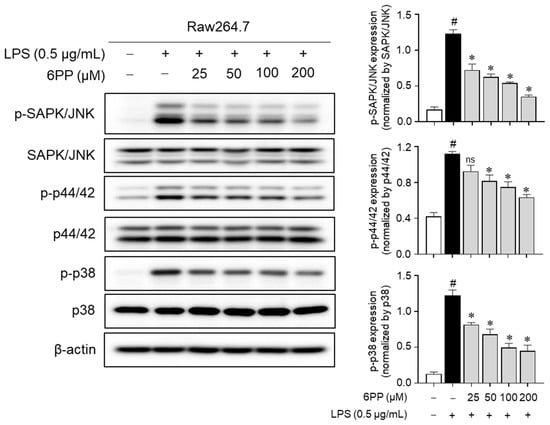
Figure 7.
Inhibitory effects of 6PP on the MAPK phosphorylation. Raw264.7 cells were pretreated with 6PP at indicated concentrations for 2 h and then stimulated with LPS (0.5 µg/mL) for 15 min. The levels of phosphorylated and total MAPK signaling pathway factors (SAPK/JNK, p44/42, and p38) were assessed using western blotting, with β-actin as the loading control. The levels of p-SAPK/JNK, p-p44/42, and p-p38 were normalized to levels of SAPK/JNK, p44/42, and p38, respectively. The data represent the mean ± SEM of three independent experiments. Statistical differences between groups were compared using the Mann–Whitney U test. # indicates p < 0.05 vs. the LPS-untreated group. * indicates p < 0.05 vs. the LPS-treated group. ns indicates not significant. MAPKs, mitogen-activated protein kinase; SAPK/JNK, stress-activated protein kinase/c-Jun N-terminal kinases; p-, phosphorylated.
4. Discussion
6PP is a secondary metabolite produced by fungi and an antifungal natural compound. This unsaturated lactone has a coconut-like smell and has applications as a food aroma enhancer. The pronounced antagonistic effects of 6PP against various fungi have been extensively studied [30,31]. Its ability to inhibit the growth of plant pathogenic fungi, protect from postharvest pathogens, and suppress mycotoxins has gained significant attention. Notably, 6PP has been shown to influence mycotoxins, such as deoxynivalenol and fusaric acid, which are produced by the Fusarium spp. [32].
In this study, we demonstrate that 6PP effectively suppresses LPS-induced inflammation and oxidative stress in Raw264.7 macrophages. Our analyses of the underlying molecular mechanisms indicate that 6PP inhibits the production of NO and proinflammatory cytokines by suppressing the activation of NF-κB and phosphorylation of MAPKs. Furthermore, we found that the Nrf2/HO-1 signaling pathway mediates the antioxidant activity of 6PP. To the best of our knowledge, this is the first study to comprehensively investigate the anti-inflammatory and antioxidant effects of 6PP, as well as their underlying mechanisms.
NO levels, which indicate inflammatory responses in LPS-stimulated macrophages, are primarily regulated by iNOS [33]. Our findings demonstrate that even at a low concentration (25 μM), 6PP potently inhibits NO production. Moreover, 6PP significantly suppressed iNOS protein levels in a concentration-dependent manner (Figure 3a,b). These findings suggest that 6PP attenuates NO production during LPS-induced inflammation by suppressing NOS. Inflammatory cytokines, which play a crucial role in systemic inflammation, are key inflammatory response mediators [34,35,36]. In this study, we found that 6PP suppressed LPS-induced production of the inflammatory cytokines IL-6, IL-1β, and TNF-α in a concentration-dependent manner (Figure 3c). Together, these findings indicate that 6PP effectively suppresses LPS-induced inflammatory responses.
Previous studies have shown a close association between proinflammatory mediators and NF-κB, a crucial regulator of inflammatory responses [37,38]. Upon stimulation by external factors, such as LPS, IκBα undergoes phosphorylation-dependent degradation [39], which triggers the nuclear translocation of activated NF-κB, which drives the expression of various proinflammatory mediators [40]. Thus, the modulation of NF-κB signaling has therapeutic potential against various inflammatory diseases. Our analyses of the mechanisms that underlie the anti-inflammatory effects of 6PP show that pretreating Raw264.7 macrophages with 6PP effectively suppresses LPS-induced IκB phosphorylation, thereby preventing the rapid degradation of IκB and inhibiting the nuclear translocation of the p65 subunit of NF-κB (Figure 6). Consequently, NF-κB signaling inhibition by 6PP suppresses the secretion of various proinflammatory mediators, resulting in anti-inflammatory effects. The MAPK signaling cascade also drives the expression of inflammatory mediators upon LPS stimulation. MAPKs, including JNK, ERK, and p38, are reported to upregulate proinflammatory cytokines, such as TNF-α, IL-6, IL-1β, and iNOS, in LPS-stimulated Raw264.7 macrophages [41]. Therefore, targeting this pathway has the potential to control LPS-induced inflammatory diseases. Our analysis of the impact of 6PP on the expression of MAPKs in LPS-stimulated Raw264.7 cells revealed that pretreatment with 6PP significantly suppresses the phosphorylation of JNK, p44/42, and p38. These findings suggest that 6PP exerts its anti-inflammatory effects by inhibiting MAPK phosphorylation.
Oxidative stress is characterized by a marked elevation of intracellular ROS levels [42]. Several studies have shown that oxidative stress is elevated in inflammatory diseases [43,44,45]. Antioxidant enzymes are closely associated with the transcription factor Nrf2, which plays a crucial role in the regulation of oxidative stress [46,47]. In this study, we observed that pretreatment with 6PP reduces GSH levels and the GSH/GSSG ratio (Figure 1). Since GSH is a representative intracellular antioxidant and is converted to GSSG when oxidative stresses occur, 6PP-induced increases in GSH level and GSH/GSSG ratio indicate that 6PP could be a candidate for the alleviation of excess oxidative stresses. Nrf2, a highly conserved transcription factor, is involved in the primary host defense system [48]. In unstimulated conditions, Nrf2 is sequestered in the cytoplasm by its negative regulator protein Keap1 [49]. However, upon extracellular stimulation, Nrf2 dissociates from keap1 and translocates into the nucleus, where it interacts with antioxidant response elements and induces the expression of cytoprotective proteins, such as HO-1, thereby counteracting oxidative stress [49,50,51]. HO-1, a member of the heat shock protein family, is a key antioxidant, anti-inflammatory, and cytoprotective enzyme, and its expression is regulated by Nrf2 [46]. To determine the antioxidant effects of 6PP, we investigated alterations in the Nrf2/HO-1 signaling pathway in LPS-stimulated Raw264.7 macrophages and found that 6PP strongly triggers Nrf2 nuclear translocation, thereby upregulating HO-1 expression and enhancing antioxidant enzyme activity (Figure 5). Furthermore, we found that SnPP, a HO-1 inhibitor, effectively reversed 6PP-mediated inhibition of LPS-induced production of NO and proinflammatory cytokines (Figure 4). These findings indicate that in Raw264.7 macrophages, 6PP activates the Nrf2/HO-1 signaling pathway, thereby mitigating LPS-induced oxidative stress response.
5. Conclusions
This study provides initial evidence that 6PP has anti-inflammatory and antioxidant effects in LPS-stimulated Raw264.7 macrophages. We demonstrate that 6PP modulates the NF-κB, MAPK, and Nrf2 signaling pathways, thereby suppressing the production of inflammatory mediators, such as NO and proinflammatory cytokines, as well as significantly increasing the levels of GSH and the GSH/GSSG ratio. Furthermore, 6PP triggers Nrf2 nuclear translocation, thereby inducing the expression of antioxidant enzymes. These results suggest that 6PP, a fungi-derived secondary metabolite, could be a beneficial candidate for protection against oxidative stresses and inflammatory states.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox12122028/s1, Figure S1: (A) UV chromatogram of LC/MS (detection wavelength was set as 254 nm) and (B) UV and MS data for 6-pentyl-α-pyrone (6PP); Figure S2: Effect of 6PP on the nucleus translocation of Nrf2; Figure S3: Effect of 6PP on the nucleus translocation of NF-κB.
Author Contributions
Conceptualization, J.S.L. and Y.-C.C.; validation, X.L., D.E.L. and J.U.C.; methodology, J.S.L., D.Y.L. and X.L.; formal analysis, J.S.L., J.-H.H., D.Y.L. and J.U.C.; resources, J.-H.H. and D.E.L.; writing—original draft preparation, J.S.L. and J.-H.H.; writing—review and editing, K.Y.L., K.H.K. and Y.-C.C.; supervision, K.Y.L., K.H.K. and Y.-C.C.; project administration, J.S.L. and Y.-C.C.; funding acquisition, J.S.L., Y.-C.C. and K.H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the National Research Foundation of Korea (NRF) grant funded by the ICT (NRF-2020R1C1C1007261) and the Ministry of Education (NRF-2022R1I1A1A01056975). This work was further supported by National Research Foundation of Korea (NRF) grants funded by the Korean government (MSIT; grant numbers 2019R1A5A2027340 and 2021R1A2C2007937).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
Author Joo-Hyun Hong was employed by the company ILDONG Pharmaceutical Co. Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
References
- Daeron, M. The immune system as a system of relations. Front. Immunol. 2022, 13, 984678. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Coleman, J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxidative Med. Cell. Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Fuertes-Agudo, M.; Luque-Tevar, M.; Cucarella, C.; Martin-Sanz, P.; Casado, M. Advances in Understanding the Role of NRF2 in Liver Pathophysiology and Its Relationship with Hepatic-Specific Cyclooxygenase-2 Expression. Antioxidants 2023, 12, 1491. [Google Scholar] [CrossRef]
- He, W.J.; Lv, C.H.; Chen, Z.; Shi, M.; Zeng, C.X.; Hou, D.X.; Qin, S. The Regulatory Effect of Phytochemicals on Chronic Diseases by Targeting Nrf2-ARE Signaling Pathway. Antioxidants 2023, 12, 236. [Google Scholar] [CrossRef]
- Liu, H.; Johnston, L.J.; Wang, F.; Ma, X. Triggers for the Nrf2/ARE Signaling Pathway and Its Nutritional Regulation: Potential Therapeutic Applications of Ulcerative Colitis. Int. J. Mol. Sci. 2021, 22, 11411. [Google Scholar] [CrossRef]
- Amaresan, N.; Sankaranarayanan, A.; Dwivedi, M.K.; Druzhinina, I.S. Advances in Trichoderma Biology for Agricultural Applications, 2022 ed.; Springer International Publishing: Cham, Switzerland, 2022. [Google Scholar]
- Ferreira, F.V.; Musumeci, M.A. Trichoderma as biological control agent: Scope and prospects to improve efficacy. World J. Microbiol. Biotechnol. 2021, 37, 90. [Google Scholar] [CrossRef]
- Schuster, A.; Schmoll, M. Biology and biotechnology of Trichoderma. Appl. Microbiol. Biotechnol. 2010, 87, 787–799. [Google Scholar] [CrossRef]
- Hong, J.-H.; Lee, J.; Min, M.; Ryu, S.-m.; Lee, D.; Kim, G.-H.; Kim, J.-J. 6-Pentyl-α-pyrone as an anti-sapstain compound produced by Trichoderma gamsii KUC1747 inhibits the germination of ophiostomatoid fungi. Holzforschung 2014, 68, 769–774. [Google Scholar] [CrossRef]
- Ding, G.; Chen, L.; Chen, A.; Tian, X.; Chen, X.; Zhang, H.; Chen, H.; Liu, X.Z.; Zhang, Y.; Zou, Z.M. Trichalasins C and D from the plant endophytic fungus Trichoderma gamsii. Fitoterapia 2012, 83, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Wang, H.; Li, L.; Chen, A.J.; Chen, L.; Chen, H.; Zhang, H.; Liu, X.; Zou, Z. Trichoderones A and B: Two Pentacyclic Cytochalasans from the Plant Endophytic Fungus Trichoderma gamsii. Eur. J. Org. Chem. 2012, 2012, 2516–2519. [Google Scholar] [CrossRef]
- Chen, L.; Niu, S.B.; Li, L.; Ding, G.; Yu, M.; Zhang, G.S.; Wang, M.H.; Li, L.Y.; Zhang, T.; Jia, H.M.; et al. Trichoderpyrone, a Unique Polyketide Hybrid with a Cyclopentenone-Pyrone Skeleton from the Plant Endophytic Fungus Trichoderma gamsii. J. Nat. Prod. 2017, 80, 1944–1947. [Google Scholar] [CrossRef]
- Ding, G.; Chen, L.; Zhou, C.; Hong-Mei, J.; Liu, Y.T.; Chang, X.; Song, B.; Liu, X.Z.; Gu, Y.C.; Zou, Z.M. Trichoderamides A and B, a pair of stereoisomers from the plant endophytic fungus Trichoderma gamsii. J. Antibiot. 2015, 68, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.S.; So, H.M.; Kim, S.; Kim, J.K.; Kim, J.C.; Kang, D.M.; Ahn, M.J.; Ko, Y.J.; Kim, K.H. Comparative evaluation of bioactive phytochemicals in Spinacia oleracea cultivated under greenhouse and open field conditions. Arch. Pharmacal Res. 2022, 45, 795–805. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, J.K.; Yu, J.S.; Jeong, S.Y.; Choi, J.H.; Kim, J.C.; Ko, Y.J.; Kim, S.H.; Kim, K.H. Ginkwanghols A and B, osteogenic coumaric acid-aliphatic alcohol hybrids from the leaves of Ginkgo biloba. Arch. Pharmacal Res. 2021, 44, 514–524. [Google Scholar] [CrossRef]
- Lee, S.R.; Lee, B.S.; Yu, J.S.; Kang, H.; Yoo, M.J.; Yi, S.A.; Han, J.W.; Kim, S.; Kim, J.K.; Kim, J.C.; et al. Identification of anti-adipogenic withanolides from the roots of Indian ginseng (Withania somnifera). J. Ginseng Res. 2022, 46, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Jeong, S.Y.; Li, C.; Oh, T.; Kwon, M.; Ahn, J.S.; Ko, S.K.; Ko, Y.J.; Cao, S.; Kim, K.H. New phenalenone derivatives from the Hawaiian volcanic soil-associated fungus Penicillium herquei FT729 and their inhibitory effects on indoleamine 2,3-dioxygenase 1 (IDO1). Arch. Pharmacal Res. 2022, 45, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Hyejin, C.; Ki Hyun, K.; Sin Hee, H.; Hak-Jae, K.; Ik-Hyun, C.; Sanghyun, L. Structure Determination of Heishuixiecaoline A from Valeriana fauriei and Its Content from Different Cultivated Regions by HPLC/PDA Analysis. Nat. Prod. Sci. 2022, 28, 181–186. [Google Scholar] [CrossRef]
- Lim, J.S.; Bae, J.; Lee, S.; Lee, D.Y.; Yao, L.; Cho, N.; Bach, T.T.; Yun, N.; Park, S.J.; Cho, Y.C. In Vitro Anti-Inflammatory Effects of Symplocos sumuntia Buch.-Ham. Ex D. Don Extract via Blockage of the NF-kappaB/JNK Signaling Pathways in LPS-Activated Microglial Cells. Plants 2022, 11, 3095. [Google Scholar] [CrossRef]
- Koppula, S.; Kumar, H.; Kim, I.S.; Choi, D.K. Reactive oxygen species and inhibitors of inflammatory enzymes, NADPH oxidase, and iNOS in experimental models of Parkinson’ disease. Mediat. Inflamm. 2012, 2012, 823902. [Google Scholar] [CrossRef]
- Xue, Q.; Yan, Y.; Zhang, R.; Xiong, H. Regulation of iNOS on Immune Cells and Its Role in Diseases. Int. J. Mol. Sci. 2018, 19, 3805. [Google Scholar] [CrossRef]
- Zamora, R.; Vodovotz, Y.; Billiar, T.R. Inducible nitric oxide synthase and inflammatory diseases. Mol. Med. 2000, 6, 347–373. [Google Scholar] [CrossRef]
- Pibiri, M.; Leoni, V.P.; Atzori, L. Heme oxygenase-1 inhibitor tin-protoporphyrin improves liver regeneration after partial hepatectomy. Life Sci. 2018, 204, 9–14. [Google Scholar] [CrossRef]
- Ismaiel, A.A.; Ali, D.M.I. Antimicrobial properties of 6-pentyl-α-pyrone produced by endophytic strains of Trichoderma koningii and its effect on aflatoxin B1 production. Biologia 2017, 72, 1403–1415. [Google Scholar] [CrossRef]
- Hao, J.; Wuyun, D.; Xi, X.; Dong, B.; Wang, D.; Quan, W.; Zhang, Z.; Zhou, H. Application of 6-Pentyl-α-Pyrone in the Nutrient Solution Used in Tomato Soilless Cultivation to Inhibit Fusarium oxysporum HF-26 Growth and Development. Agronomy 2023, 13, 1210. [Google Scholar] [CrossRef]
- Degani, O.; Khatib, S.; Becher, P.; Gordani, A.; Harris, R. Trichoderma asperellum Secreted 6-Pentyl-α-Pyrone to Control Magnaporthiopsis maydis, the Maize Late Wilt Disease Agent. Biology 2021, 10, 897. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.R. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Bent, R.; Moll, L.; Grabbe, S.; Bros, M. Interleukin-1 Beta-A Friend or Foe in Malignancies? Int. J. Mol. Sci. 2018, 19, 2155. [Google Scholar] [CrossRef] [PubMed]
- Arias-Salvatierra, D.; Silbergeld, E.K.; Acosta-Saavedra, L.C.; Calderon-Aranda, E.S. Role of nitric oxide produced by iNOS through NF-kappaB pathway in migration of cerebellar granule neurons induced by Lipopolysaccharide. Cell. Signal. 2011, 23, 425–435. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Karin, M.; Ben-Neriah, Y. Phosphorylation meets ubiquitination: The control of NF-[kappa]B activity. Annu. Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef]
- Christian, F.; Smith, E.L.; Carmody, R.J. The Regulation of NF-kappaB Subunits by Phosphorylation. Cells 2016, 5, 12. [Google Scholar] [CrossRef]
- Morrison, D.K. MAP kinase pathways. Cold Spring Harb. Perspect. Biol. 2012, 4, a011254. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Saez, A.; Herrero-Fernandez, B.; Gomez-Bris, R.; Sanchez-Martinez, H.; Gonzalez-Granado, J.M. Pathophysiology of Inflammatory Bowel Disease: Innate Immune System. Int. J. Mol. Sci. 2023, 24, 1526. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Shen, P.; Song, Y.; Huang, Y.; Tu, S. Reactive Oxygen Species in Autoimmune Cells: Function, Differentiation, and Metabolism. Front. Immunol. 2021, 12, 635021. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Jaganjac, M.; Milkovic, L.; Sunjic, S.B.; Zarkovic, N. The NRF2, Thioredoxin, and Glutathione System in Tumorigenesis and Anticancer Therapies. Antioxidants 2020, 9, 1151. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Ulasov, A.V.; Rosenkranz, A.A.; Georgiev, G.P.; Sobolev, A.S. Nrf2/Keap1/ARE signaling: Towards specific regulation. Life Sci. 2022, 291, 120111. [Google Scholar] [CrossRef]
- Zgorzynska, E.; Dziedzic, B.; Walczewska, A. An Overview of the Nrf2/ARE Pathway and Its Role in Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 9592. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Duan, H.; Li, R.; Peng, W.; Wu, C. Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J. Adv. Res. 2021, 34, 43–63. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).