Bilosomes and Biloparticles for the Delivery of Lipophilic Drugs: A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Bilosomes
2.3. Preparation of Biloparticles
2.4. Dimensional Analysis
2.5. Encapsulation Efficiency Evaluation
2.6. Equilibrium Dialysis Experiments
2.7. Cell Culture and Treatments
2.7.1. Cytotoxicity Study (MTT Assay)
2.7.2. Immunofluorescence Staining
2.7.3. IL-1β ELISA Assay
2.8. Mathematical and Statistical Analysis
3. Results and Discussion
3.1. Preformulation Studies
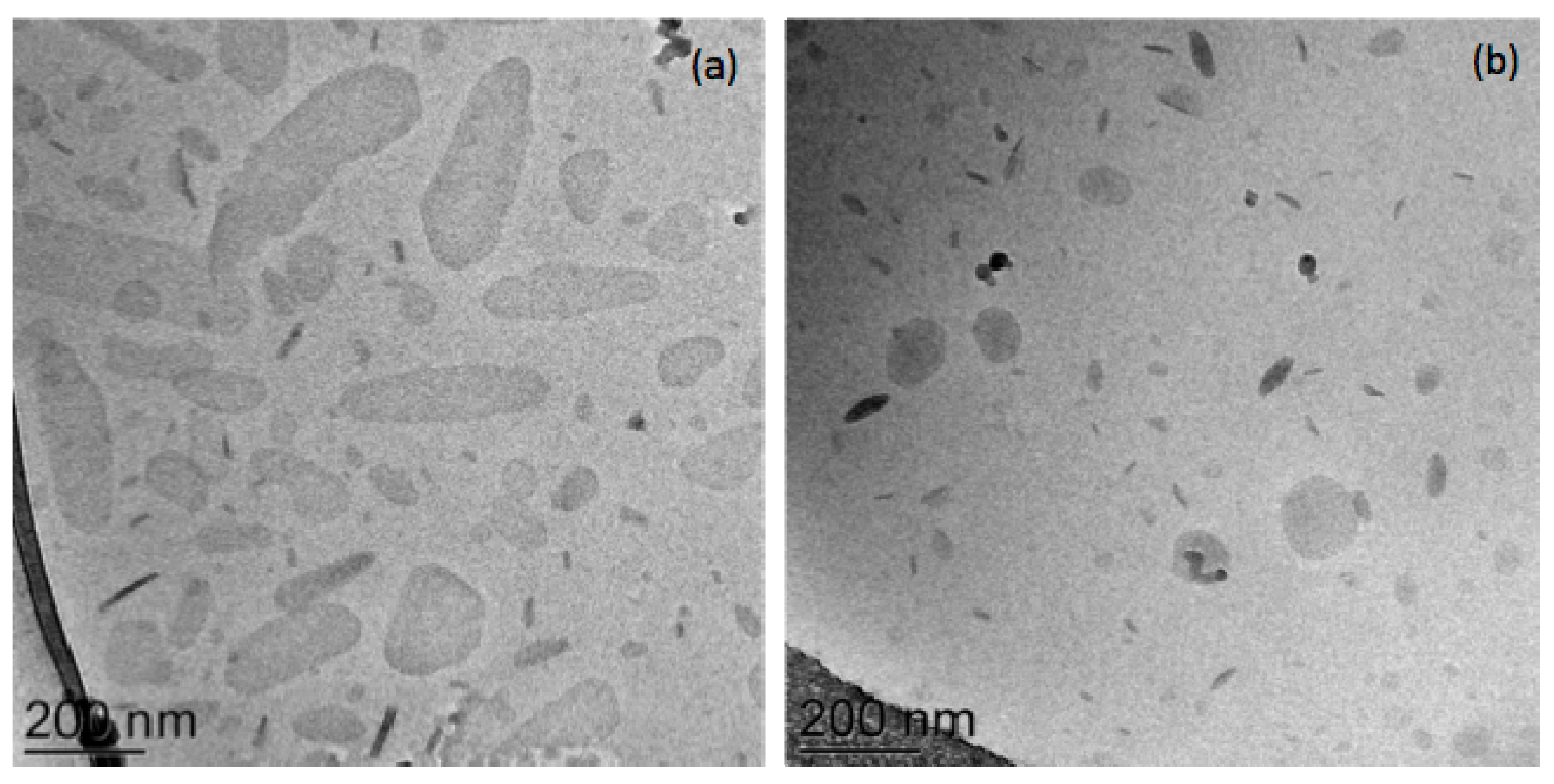
3.1.1. Bilosomes
3.1.2. Biloparticles
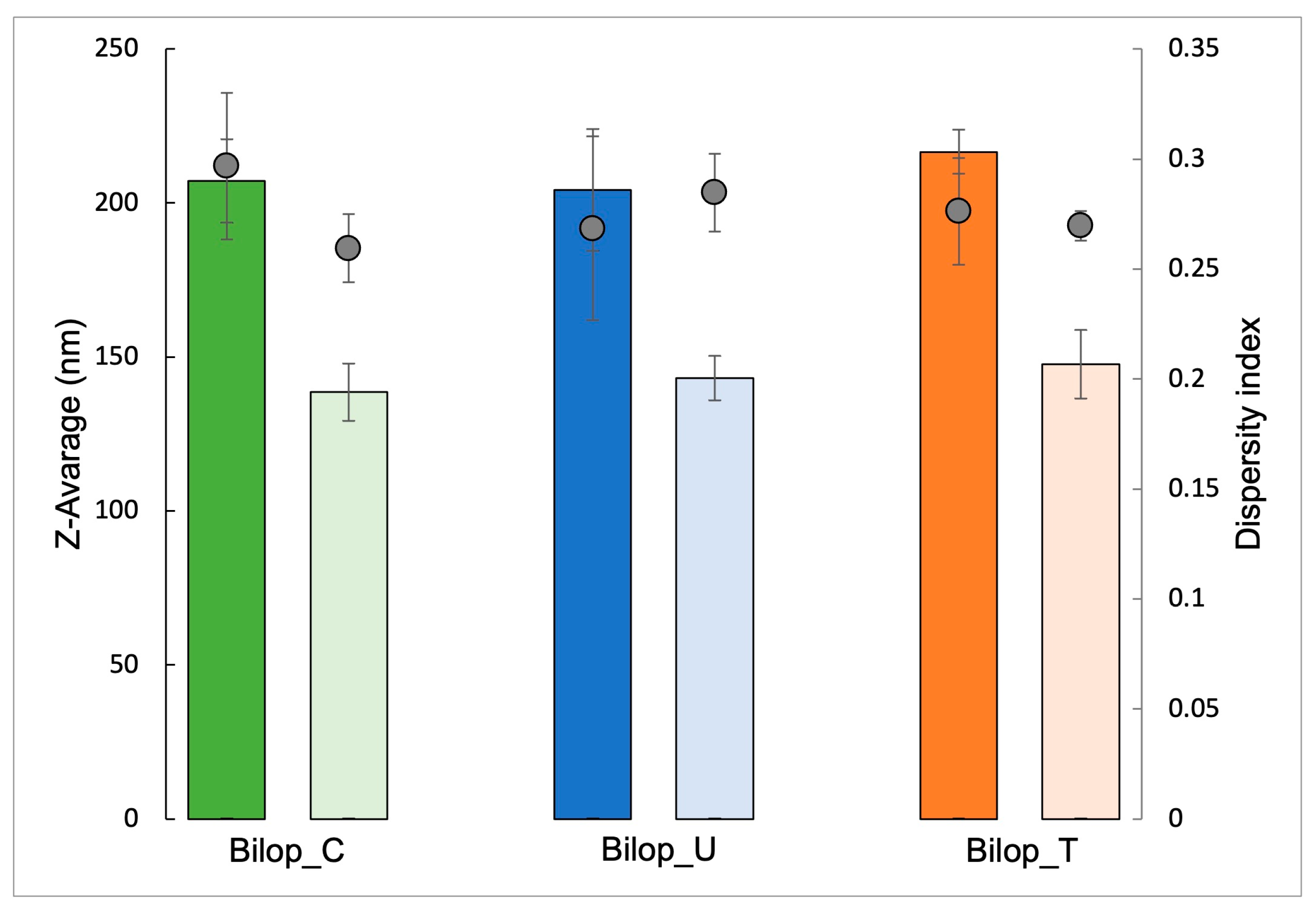
3.2. Production and Characterization of Bilosomes and Biloparticles Containing Budesonide
3.2.1. Bilosomes
3.2.2. Biloparticles
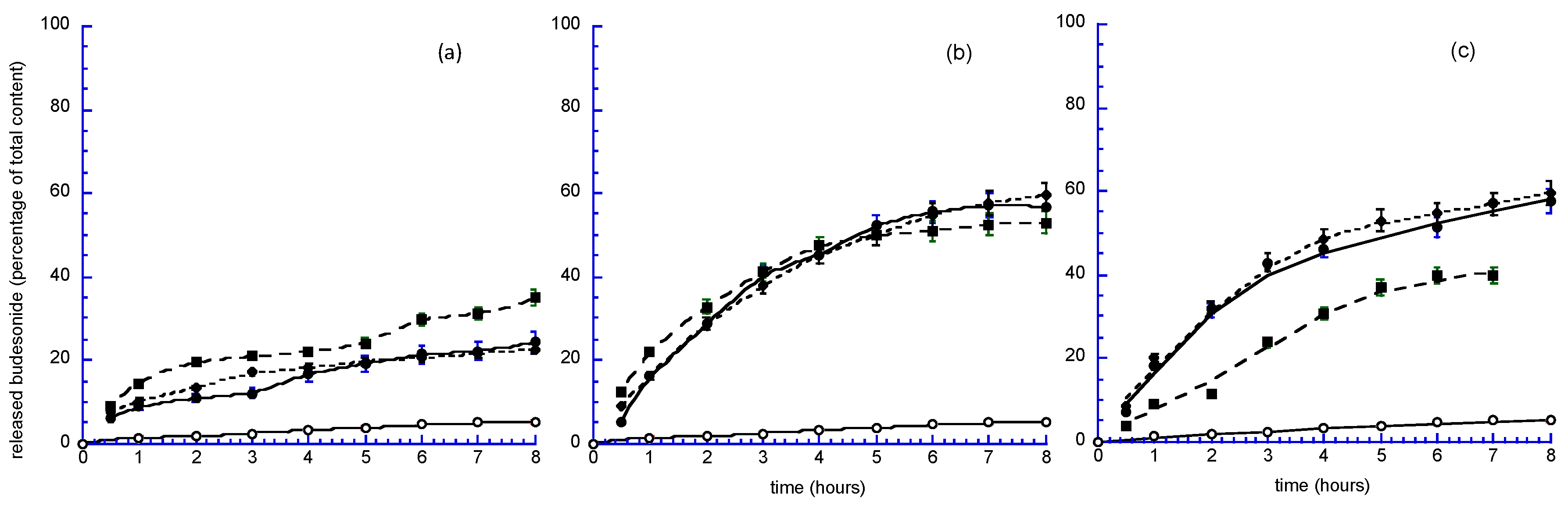
3.3. In Vitro and Ex Vivo Studies
3.3.1. Mathematical Analysis
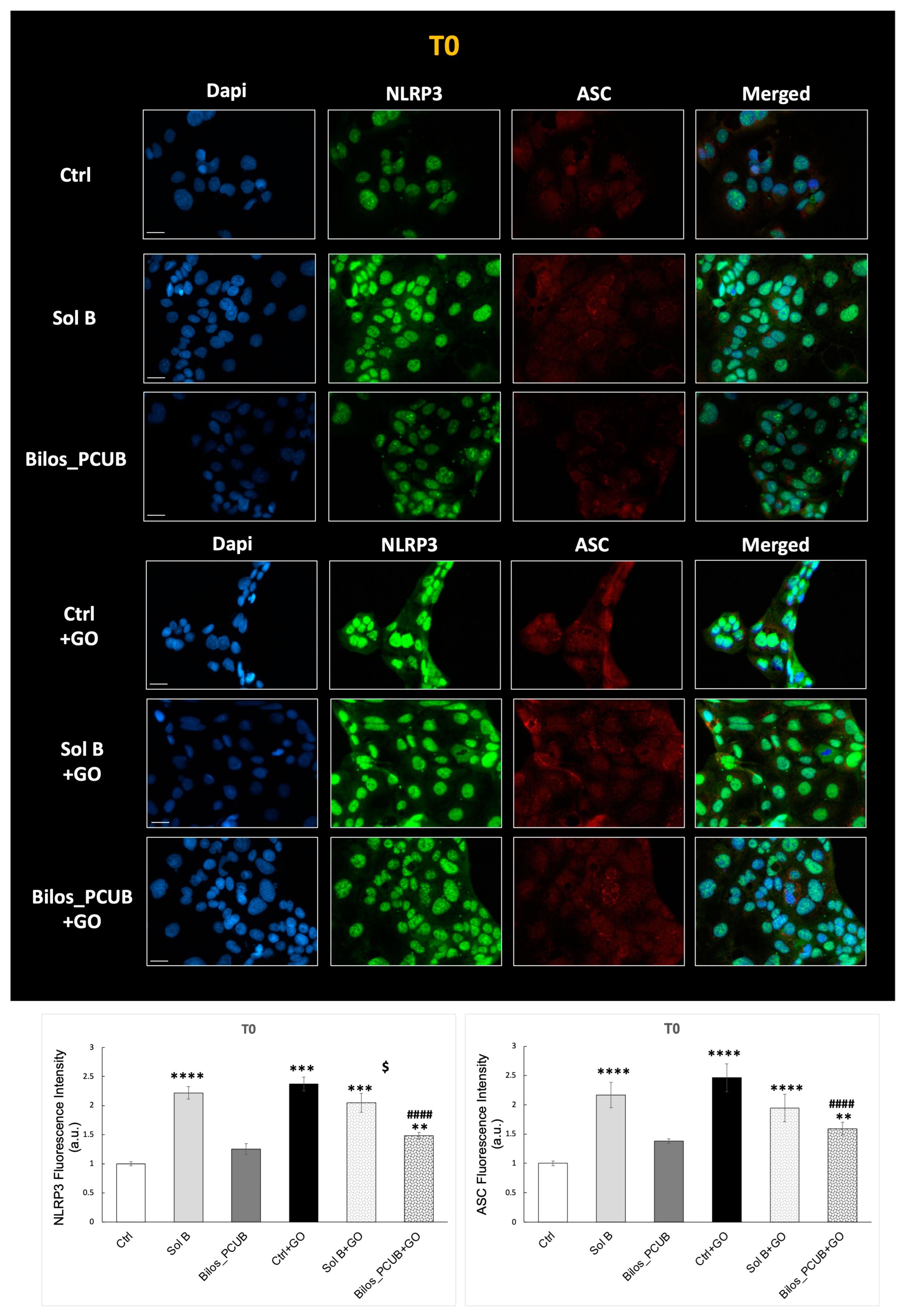
3.3.2. Cell Studies
MTT Assay
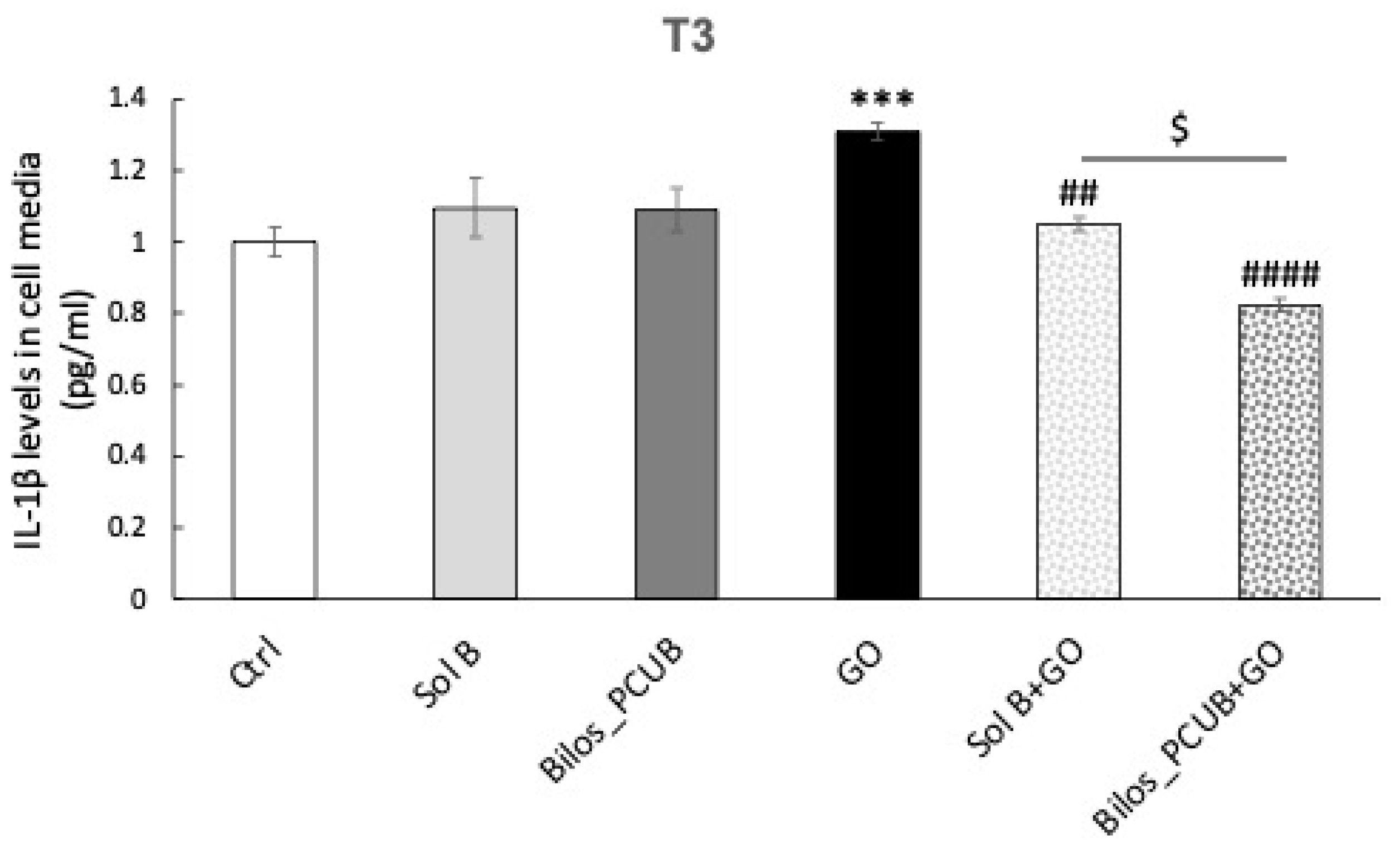
Immunofluorescence NLRP3-ASC and ELISA IL-1β
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Porter, C.J.H.; Pouton, C.W.; Cuine, J.F.; Charman, W.N. Enhancing Intestinal Drug Solubilisation Using Lipid-Based Delivery Systems. Adv. Drug Deliv. Rev. 2008, 60, 673–691. [Google Scholar] [CrossRef]
- Charman, W.N. Lipids, Lipophilic Drugs, and Oral Drug Delivery-Some Emerging Concepts. J. Pharm. Sci. 2000, 89, 967–978. [Google Scholar] [CrossRef]
- Porter, C.J.H.; Trevaskis, N.L.; Charman, W.N. Lipids and Lipid-Based Formulations: Optimizing the Oral Delivery of Lipophilic Drugs. Nat. Rev. Drug Discov. 2007, 6, 231–248. [Google Scholar] [CrossRef]
- Stojančević, M.; Pavlović, N.; Goločorbin-Kon, S.; Mikov, M. Application of Bile Acids in Drug Formulation and Delivery. Front. Life Sci. 2013, 7, 112–122. [Google Scholar] [CrossRef]
- Holm, R.; Müllertz, A.; Mu, H. Bile Salts and Their Importance for Drug Absorption. Int. J. Pharm. 2013, 453, 44–55. [Google Scholar] [CrossRef]
- Zarenezhad, E.; Marzi, M.; Abdulabbas, H.T.; Jasim, S.A.; Kouhpayeh, S.A.; Barbaresi, S.; Ahmadi, S.; Ghasemian, A. Bilosomes as Nanocarriers for the Drug and Vaccine Delivery against Gastrointestinal Infections: Opportunities and Challenges. J. Funct. Biomater. 2023, 14, 453. [Google Scholar] [CrossRef]
- Gupta, D.K.; Ahad, A.; Waheed, A.; Aqil, M.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. Bilosomes: A Novel Platform for Drug Delivery. In Systems of Nanovesicular Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2022; pp. 293–309. ISBN 978-0-323-91864-0. [Google Scholar]
- Ahmad, J.; Singhal, M.; Amin, S.; Rizwanullah, M.; Akhter, S.; Kamal, M.A.; Haider, N.; Midoux, P.; Pichon, C. Bile Salt Stabilized Vesicles (Bilosomes): A Novel Nano-Pharmaceutical Design for Oral Delivery of Proteins and Peptides. Curr. Pharm. Des. 2017, 23, 1575–1588. [Google Scholar] [CrossRef]
- Shukla, A.; Mishra, V.; Kesharwani, P. Bilosomes in the Context of Oral Immunization: Development, Challenges and Opportunities. Drug Discov. Today 2016, 21, 888–899. [Google Scholar] [CrossRef]
- Mohsen, A.M.; Asfour, M.H.; Salama, A.A.A. Improved Hepatoprotective Activity of Silymarin via Encapsulation in the Novel Vesicular Nanosystem Bilosomes. Drug Dev. Ind. Pharm. 2017, 43, 2043–2054. [Google Scholar] [CrossRef]
- Miehlke, S.; Acosta, M.B.; Bouma, G.; Carpio, D.; Magro, F.; Moreels, T.; Probert, C. Oral Budesonide in Gastrointestinal and Liver Disease: A Practical Guide for the Clinician. J. Gastroenterol. Hepatol. 2018, 33, 1574–1581. [Google Scholar] [CrossRef]
- Kozuch, P.L.; Hanauer, S.B. Treatment of Inflammatory Bowel Disease: A Review of Medical Therapy. WJG 2008, 14, 354. [Google Scholar] [CrossRef]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases With Time, Based on Systematic Review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar] [CrossRef]
- Hanauer, S.B. New Steroids for IBD: Progress Report. Gut 2002, 51, 182–183. [Google Scholar] [CrossRef]
- Martinez, M.N.; Amidon, G.L. A Mechanistic Approach to Understanding the Factors Affecting Drug Absorption: A Review of Fundamentals. J. Clin. Pharmacol. 2002, 42, 620–643. [Google Scholar] [CrossRef]
- Stegemann, S.; Leveiller, F.; Franchi, D.; De Jong, H.; Lindén, H. When Poor Solubility Becomes an Issue: From Early Stage to Proof of Concept. Eur. J. Pharm. Sci. 2007, 31, 249–261. [Google Scholar] [CrossRef]
- Liu, L.; Yao, W.; Rao, Y.; Lu, X.; Gao, J. pH-Responsive Carriers for Oral Drug Delivery: Challenges and Opportunities of Current Platforms. Drug Deliv. 2017, 24, 569–581. [Google Scholar] [CrossRef]
- Lautenschläger, C.; Schmidt, C.; Fischer, D.; Stallmach, A. Drug Delivery Strategies in the Therapy of Inflammatory Bowel Disease. Adv. Drug Deliv. Rev. 2014, 71, 58–76. [Google Scholar] [CrossRef]
- Derendorf, H.; Meltzer, E.O. Molecular and Clinical Pharmacology of Intranasal Corticosteroids: Clinical and Therapeutic Implications. Allergy 2008, 63, 1292–1300. [Google Scholar] [CrossRef]
- Vrettos, N.-N.; Roberts, C.J.; Zhu, Z. Gastroretentive Technologies in Tandem with Controlled-Release Strategies: A Potent Answer to Oral Drug Bioavailability and Patient Compliance Implications. Pharmaceutics 2021, 13, 1591. [Google Scholar] [CrossRef]
- Xian, S.; Zhu, J.; Wang, Y.; Song, H.; Wang, H. Oral Liposomal Delivery of an Activatable Budesonide Prodrug Reduces Colitis in Experimental Mice. Drug Deliv. 2023, 30, 2183821. [Google Scholar] [CrossRef]
- Muller, R.H.; Shegokar, R.; Keck, C.M. 20 Years of Lipid Nanoparticles (SLN & NLC): Present State of Development & Industrial Applications. Curr. Drug Discov. Technol. 2011, 8, 207–227. [Google Scholar] [CrossRef]
- Mu, H.; Holm, R. Solid Lipid Nanocarriers in Drug Delivery: Characterization and Design. Expert. Opin. Drug Deliv. 2018, 15, 771–785. [Google Scholar] [CrossRef]
- Talegaonkar, S.; Bhattacharyya, A. Potential of Lipid Nanoparticles (SLNs and NLCs) in Enhancing Oral Bioavailability of Drugs with Poor Intestinal Permeability. AAPS PharmSciTech 2019, 20, 121. [Google Scholar] [CrossRef]
- Maritim, S.; Boulas, P.; Lin, Y. Comprehensive Analysis of Liposome Formulation Parameters and Their Influence on Encapsulation, Stability and Drug Release in Glibenclamide Liposomes. Int. J. Pharm. 2021, 592, 120051. [Google Scholar] [CrossRef]
- Da Silva Santos, V.; Badan Ribeiro, A.P.; Andrade Santana, M.H. Solid Lipid Nanoparticles as Carriers for Lipophilic Compounds for Applications in Foods. Food Res. Int. 2019, 122, 610–626. [Google Scholar] [CrossRef]
- Sguizzato, M.; Pepe, A.; Baldisserotto, A.; Barbari, R.; Montesi, L.; Drechsler, M.; Mariani, P.; Cortesi, R. Niosomes for Topical Application of Antioxidant Molecules: Design and In Vitro Behavior. Gels 2023, 9, 107. [Google Scholar] [CrossRef]
- Sguizzato, M.; Ferrara, F.; Drechsler, M.; Baldisserotto, A.; Montesi, L.; Manfredini, S.; Valacchi, G.; Cortesi, R. Lipid-Based Nanosystems for the Topical Application of Ferulic Acid: A Comparative Study. Pharmaceutics 2023, 15, 1940. [Google Scholar] [CrossRef]
- Esposito, E.; Sguizzato, M.; Drechsler, M.; Mariani, P.; Carducci, F.; Nastruzzi, C.; Cortesi, R. Progesterone Lipid Nanoparticles: Scaling up and in Vivo Human Study. Eur. J. Pharm. Biopharm. 2017, 119, 437–446. [Google Scholar] [CrossRef]
- Pecora, R. Dynamic Light Scattering Measurement of Nanometer Particles in Liquids. J. Nanoparticle Res. 2000, 2, 123–131. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Pozzetti, L.; Ferrara, F.; Marotta, L.; Gemma, S.; Butini, S.; Benedusi, M.; Fusi, F.; Ahmed, A.; Pomponi, S.; Ferrari, S.; et al. Extra Virgin Olive Oil Extracts of Indigenous Southern Tuscany Cultivar Act as Anti-Inflammatory and Vasorelaxant Nutraceuticals. Antioxidants 2022, 11, 437. [Google Scholar] [CrossRef]
- M’Baye, G.; Mély, Y.; Duportail, G.; Klymchenko, A.S. Liquid Ordered and Gel Phases of Lipid Bilayers: Fluorescent Probes Reveal Close Fluidity but Different Hydration. Biophys. J. 2008, 95, 1217–1225. [Google Scholar] [CrossRef]
- Ali Mohammadi, Z.; Foad Aghamiri, S.; Zarrabi, A.; Reza Talaie, M. Liposomal Doxorubicin Delivery Systems: Effects of Formulation and Processing Parameters on Drug Loading and Release Behavior. Curr. Drug Deliv. 2016, 13, 1065–1070. [Google Scholar] [CrossRef]
- Farzaneh, H.; Ebrahimi Nik, M.; Mashreghi, M.; Saberi, Z.; Jaafari, M.R.; Teymouri, M. A Study on the Role of Cholesterol and Phosphatidylcholine in Various Features of Liposomal Doxorubicin: From Liposomal Preparation to Therapy. Int. J. Pharm. 2018, 551, 300–308. [Google Scholar] [CrossRef]
- Liposome Technology: Interactions of Liposomes with the Biological Milieu. Available online: https://www.routledge.com/Liposome-Technology-Interactions-of-Liposomes-with-the-Biological-Milieu/Gregoriadis/p/book/9780367390389 (accessed on 20 October 2023).
- Perrie, Y. Gregory Gregoriadis: Introducing Liposomes to Drug Delivery. J. Drug Target. 2008, 16, 518–519. [Google Scholar] [CrossRef]
- Gregoriadis, G. Drug Entrapment in Liposomes. FEBS Lett. 1973, 36, 292–296. [Google Scholar] [CrossRef]
- Gregoriadis, G. Engineering Liposomes for Drug Delivery: Progress and Problems. Trends Biotechnol. 1995, 13, 527–537. [Google Scholar] [CrossRef]
- Cortesi, R.; Esposito, E.; Drechsler, M.; Pavoni, G.; Cacciatore, I.; Sguizzato, M.; Di Stefano, A. L-Dopa Co-Drugs in Nanostructured Lipid Carriers: A Comparative Study. Mater. Sci. Eng. C 2017, 72, 168–176. [Google Scholar] [CrossRef]
- Sultan, A.A.; Saad, G.A.; El Maghraby, G.M. Permeation Enhancers Loaded Bilosomes for Improved Intestinal Absorption and Cytotoxic Activity of Doxorubicin. Int. J. Pharm. 2023, 630, 122427. [Google Scholar] [CrossRef]
- Aditya, N.P.; Espinosa, Y.G.; Norton, I.T. Encapsulation Systems for the Delivery of Hydrophilic Nutraceuticals: Food Application. Biotechnol. Adv. 2017, 35, 450–457. [Google Scholar] [CrossRef]
- Ahmed, S.; Kassem, M.A.; Sayed, S. Bilosomes as Promising Nanovesicular Carriers for Improved Transdermal Delivery: Construction, in Vitro Optimization, Ex Vivo Permeation and in Vivo Evaluation. Int. J. Nanomed. 2020, 15, 9783–9798. [Google Scholar] [CrossRef]
- Binsuwaidan, R.; Sultan, A.A.; Negm, W.A.; Attallah, N.G.M.; Alqahtani, M.J.; Hussein, I.A.; Shaldam, M.A.; El-Sherbeni, S.A.; Elekhnawy, E. Bilosomes as Nanoplatform for Oral Delivery and Modulated In Vivo Antimicrobial Activity of Lycopene. Pharmaceuticals 2022, 15, 1043. [Google Scholar] [CrossRef]
- Saifi, Z.; Rizwanullah, M.; Mir, S.R.; Amin, S. Bilosomes Nanocarriers for Improved Oral Bioavailability of Acyclovir: A Complete Characterization through in Vitro, Ex-Vivo and in Vivo Assessment. J. Drug Deliv. Sci. Technol. 2020, 57, 101634. [Google Scholar] [CrossRef]
- Elnaggar, Y.S.R.; Omran, S.; Hazzah, H.A.; Abdallah, O.Y. Anionic versus Cationic Bilosomes as Oral Nanocarriers for Enhanced Delivery of the Hydrophilic Drug Risedronate. Int. J. Pharm. 2019, 564, 410–425. [Google Scholar] [CrossRef]
- Dadashzadeh, S.; Haeri, A.; Daeihamed, M.; Arzani, G.; Bakhtiari Kaboutaraki, H. Niosomal Carriers Enhance Oral Bioavailability of Carvedilol: Effects of Bile Salt-Enriched Vesicles and Carrier Surface Charge. Int. J. Nanomed. 2015, 10, 4797. [Google Scholar] [CrossRef]
- Islam, N.; Zahoor, A.F.; Syed, H.K.; Iqbal, M.S.; Khan, I.U.; Abbas, G.; Mushtaq, M.; Rehman, M.U.; Rasul, A.; Ikram, M.; et al. Improvement of Solubility and Dissolution of Ebastine by Fabricating Phosphatidylcholine/ Bile Salt Bilosomes. Pak. J. Pharm. Sci. 2020, 33, 2301–2306. [Google Scholar]
- Elkomy, M.H.; Eid, H.M.; Elmowafy, M.; Shalaby, K.; Zafar, A.; Abdelgawad, M.A.; Rateb, M.E.; Ali, M.R.A.; Alsalahat, I.; Abou-Taleb, H.A. Bilosomes as a Promising Nanoplatform for Oral Delivery of an Alkaloid Nutraceutical: Improved Pharmacokinetic Profile and Snowballed Hypoglycemic Effect in Diabetic Rats. Drug Deliv. 2022, 29, 2694–2704. [Google Scholar] [CrossRef]
- Aburahma, M.H. Bile Salts-Containing Vesicles: Promising Pharmaceutical Carriers for Oral Delivery of Poorly Water-Soluble Drugs and Peptide/Protein-Based Therapeutics or Vaccines. Drug Deliv. 2016, 23, 1847–1867. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Wang, C.; Guo, Y.; Cheng, Y.; Qian, H.; Zhao, Y. Lycopene-Loaded Bilosomes Ameliorate High-Fat Diet-Induced Chronic Nephritis in Mice through the TLR4/MyD88 Inflammatory Pathway. Foods 2022, 11, 3042. [Google Scholar] [CrossRef]
- Wilkhu, J.S.; McNeil, S.E.; Anderson, D.E.; Perrie, Y. Characterization and Optimization of Bilosomes for Oral Vaccine Delivery. J. Drug Target. 2013, 21, 291–299. [Google Scholar] [CrossRef]
- Paes Dutra, J.A.; Gonçalves Carvalho, S.; Soares de Oliveira, A.; Borges Monteiro, J.R.; Rodrigues Pereira de Oliveira Borlot, J.; Tavares Luiz, M.; Bauab, T.M.; Rezende Kitagawa, R.; Chorilli, M. Microparticles and Nanoparticles-Based Approaches to Improve Oral Treatment of Helicobacter Pylori Infection. Crit. Rev. Microbiol. 2023, 1–22. [Google Scholar] [CrossRef]
- Faustino, C.; Serafim, C.; Rijo, P.; Reis, C.P. Bile Acids and Bile Acid Derivatives: Use in Drug Delivery Systems and as Therapeutic Agents. Expert Opin. Drug Deliv. 2016, 13, 1133–1148. [Google Scholar] [CrossRef]
- Yao, W.; Xu, Z.; Sun, J.; Luo, J.; Wei, Y.; Zou, J. Deoxycholic Acid-Functionalised Nanoparticles for Oral Delivery of Rhein. Eur. J. Pharm. Sci. 2021, 159, 105713. [Google Scholar] [CrossRef]
- Mooranian, A.; Raj Wagle, S.; Kovacevic, B.; Takechi, R.; Mamo, J.; Lam, V.; Watts, G.F.; Mikov, M.; Golocorbin-Kon, S.; Stojanovic, G.; et al. Bile Acid Bio-Nanoencapsulation Improved Drug Targeted-Delivery and Pharmacological Effects via Cellular Flux: 6-Months Diabetes Preclinical Study. Sci. Rep. 2020, 10, 106. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Mathematical Modeling of Drug Dissolution. Int. J. Pharm. 2013, 453, 12–24. [Google Scholar] [CrossRef]
- Lao, L.L.; Peppas, N.A.; Boey, F.Y.C.; Venkatraman, S.S. Modeling of Drug Release from Bulk-Degrading Polymers. Int. J. Pharm. 2011, 418, 28–41. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Higuchi Equation: Derivation, Applications, Use and Misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef]
- Higuchi, T. Rate of Release of Medicaments from Ointment Bases Containing Drugs in Suspension. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef]
- Paerewijck, O.; Lamkanfi, M. The Human Inflammasomes. Mol. Asp. Med. 2022, 88, 101100. [Google Scholar] [CrossRef]
- Ferrara, F.; Pecorelli, A.; Valacchi, G. Redox Regulation of Nucleotide-Binding and Oligomerization Domain-Like Receptors Inflammasome. Antioxid. Redox Signal. 2023, 39, 744–770. [Google Scholar] [CrossRef]
- Abbasi-Oshaghi, E.; Mirzaei, F.; Pourjafar, M. NLRP3 Inflammasome, Oxidative Stress, and Apoptosis Induced in the Intestine and Liver of Rats Treated with Titanium Dioxide Nanoparticles: In Vivo and in Vitro Study. Int. J. Nanomed. 2019, 14, 1919–1936. [Google Scholar] [CrossRef]
- Lv, Q.; Xu, D.; Ma, J.; Wang, Y.; Yang, X.; Zhao, P.; Ma, L.; Li, Z.; Yang, W.; Liu, X.; et al. Uric Acid Drives Intestinal Barrier Dysfunction through TSPO-Mediated NLRP3 Inflammasome Activation. Inflamm. Res. 2021, 70, 127–137. [Google Scholar] [CrossRef]
- He, Y.; Li, Z.; Xu, T.; Luo, D.; Chi, Q.; Zhang, Y.; Li, S. Polystyrene Nanoplastics Deteriorate LPS-Modulated Duodenal Permeability and Inflammation in Mice via ROS Drived-NF-κB/NLRP3 Pathway. Chemosphere 2022, 307, 135662. [Google Scholar] [CrossRef]
- Bayiha, J.C.; Evrard, B.; Cataldo, D.; De Tullio, P.; Mingeot-Leclercq, M.-P. The Budesonide-Hydroxypropyl-β-Cyclodextrin Complex Attenuates ROS Generation, IL-8 Release and Cell Death Induced by Oxidant and Inflammatory Stress. Study on A549 and A-THP-1 Cells. Molecules 2020, 25, 4882. [Google Scholar] [CrossRef]
- Valacchi, G.; Sticozzi, C.; Belmonte, G.; Cervellati, F.; Pecorelli, A.; Signorini, C.; Leoncini, S.; Ciccoli, L.; De Felice, C.; Della Ragione, F.; et al. Scavenger Receptor B1 Oxidative Post-Translational Modifications Are Responsible for Its Loss in Rett Syndrome. Free Radic. Biol. Med. 2014, 75, S10–S11. [Google Scholar] [CrossRef]
- Valacchi, G.; Virgili, F.; Cervellati, C.; Pecorelli, A. OxInflammation: From Subclinical Condition to Pathological Biomarker. Front. Physiol. 2018, 9, 858. [Google Scholar] [CrossRef]
- Pérez, S.; Taléns-Visconti, R.; Rius-Pérez, S.; Finamor, I.; Sastre, J. Redox Signaling in the Gastrointestinal Tract. Free Radic. Biol. Med. 2017, 104, 75–103. [Google Scholar] [CrossRef]
- Ahmad, R.; Srivastava, S.; Ghosh, S.; Khare, S.K. Phytochemical Delivery through Nanocarriers: A Review. Colloids Surf. B Biointerfaces 2021, 197, 111389. [Google Scholar] [CrossRef]
- D’Elia, R.V.; Woods, S.; Butcher, W.; McGahon, J.; Khadke, S.; Perrie, Y.; Williamson, E.D.; Roberts, C.W. Exploitation of the Bilosome Platform Technology to Formulate Antibiotics and Enhance Efficacy of Melioidosis Treatments. J. Control. Release 2019, 298, 202–212. [Google Scholar] [CrossRef]
- Mohsen, A.M.; Salama, A.; Kassem, A.A. Development of Acetazolamide Loaded Bilosomes for Improved Ocular Delivery: Preparation, Characterization and in Vivo Evaluation. J. Drug Deliv. Sci. Technol. 2020, 59, 101910. [Google Scholar] [CrossRef]




| Column | Mobile Phase | Flow (mL/min) | Wavelength (nm) | Pressure (bar) | Retention Time (min) |
|---|---|---|---|---|---|
| Kinetex 5 μm C18 100 Å, RP, 150 × 4.6 mm | Acetonitrile and Phosphate buffer (pH 3.2) 60:40 v/v | 0.8 (isocratic mode) | 245 | 80 | 3.4–3.5 |
| Receiving Phase | Component | Concentration |
|---|---|---|
| FaSSGF (pH 1.6) | Sodium cholate Phosphatidylcholine NaCl | 80 μM 20 μM 0.32 mM |
| FaSSIF (pH 6.5) | Sodium taurocholate Phosphatidylcholine porcine lipase | 3.0 mM 0.2 mM 100 U/mL |
| PBS (pH 7.2–7.4) | NaH2PO4 × H2O NaHPO4 × 2 H2O NaCl | 16.08 mM 80 mM 1.5 M |
| Biloparticles | Encapsulation Efficiency * (%) ± s.d. |
|---|---|
| BilopS_CB | 69.25 ± 6.48 |
| BilopS_UB | 59.10 ± 1.96 |
| BilopS_TB | 81.96 ± 2.76 |
| BilopN_CB | 61.87 ± 9.32 |
| BilopN_UB | 57.74 ± 1.66 |
| BilopN_TB | 70.20 ± 5.44 |
| Formulation | Nylon Membrane | Rat Intestinal Membrane | ||||
|---|---|---|---|---|---|---|
| Zero Order | First Order | Second Order | Zero Order | First Order | Second Order | |
| F = K0 t | ln(1 − F) = −K1 t | F = K2 t1/2 | F = K0 t | ln(1 − F) = −K1 t | F = K2 t1/2 | |
| Susp B | 0.8753 | 0.8650 | 0.9878 | 0.9703 | 0.9642 | 0.9624 |
| Bilos_PCUB | 0.9038 | 0.9160 | 0.9614 | 0.9774 | 0.9584 | 0.9768 |
| Bilos_PCCB | 0.9365 | 0.9524 | 0.9850 | 0.9324 | 0.9128 | 0.9256 |
| Bilos_PCTB | 0.9634 | 0.9721 | 0.9763 | 0.9815 | 0.8605 | 0.9170 |
| BilopS_UB | 0.9798 | 0.9922 | 0.9601 | 0.8708 | 0.9343 | 0.9577 |
| BilopS_CB | 0.9717 | 0.9940 | 0.9808 | 0.8327 | 0.8776 | 0.9350 |
| BilopS_TB | 0.9922 | 0.9986 | 0.9589 | 0.9289 | 0.9721 | 0.9878 |
| BilopN_UB | 0.9807 | 0.9935 | 0.9531 | 0.8648 | 0.9305 | 0.9524 |
| BilopN_CB | 0.9461 | 0.9793 | 0.9720 | 0.9460 | 0.9643 | 0.9672 |
| BilopN_TB | 0.9632 | 0.9986 | 0.9572 | 0.8682 | 0.9315 | 0.9574 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sguizzato, M.; Ferrara, F.; Baraldo, N.; Bondi, A.; Guarino, A.; Drechsler, M.; Valacchi, G.; Cortesi, R. Bilosomes and Biloparticles for the Delivery of Lipophilic Drugs: A Preliminary Study. Antioxidants 2023, 12, 2025. https://doi.org/10.3390/antiox12122025
Sguizzato M, Ferrara F, Baraldo N, Bondi A, Guarino A, Drechsler M, Valacchi G, Cortesi R. Bilosomes and Biloparticles for the Delivery of Lipophilic Drugs: A Preliminary Study. Antioxidants. 2023; 12(12):2025. https://doi.org/10.3390/antiox12122025
Chicago/Turabian StyleSguizzato, Maddalena, Francesca Ferrara, Nada Baraldo, Agnese Bondi, Annunziata Guarino, Markus Drechsler, Giuseppe Valacchi, and Rita Cortesi. 2023. "Bilosomes and Biloparticles for the Delivery of Lipophilic Drugs: A Preliminary Study" Antioxidants 12, no. 12: 2025. https://doi.org/10.3390/antiox12122025
APA StyleSguizzato, M., Ferrara, F., Baraldo, N., Bondi, A., Guarino, A., Drechsler, M., Valacchi, G., & Cortesi, R. (2023). Bilosomes and Biloparticles for the Delivery of Lipophilic Drugs: A Preliminary Study. Antioxidants, 12(12), 2025. https://doi.org/10.3390/antiox12122025








