Changes in Oxidative Stress and Intestinal Permeability during Pregnancy in Women with Gestational Diabetes Mellitus Treated with Metformin or Insulin and Healthy Controls: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Protocol
2.3. Oxidative Stress and Intestinal Permeability Analysis
2.4. Statistical Analysis
2.5. Ethics
3. Results
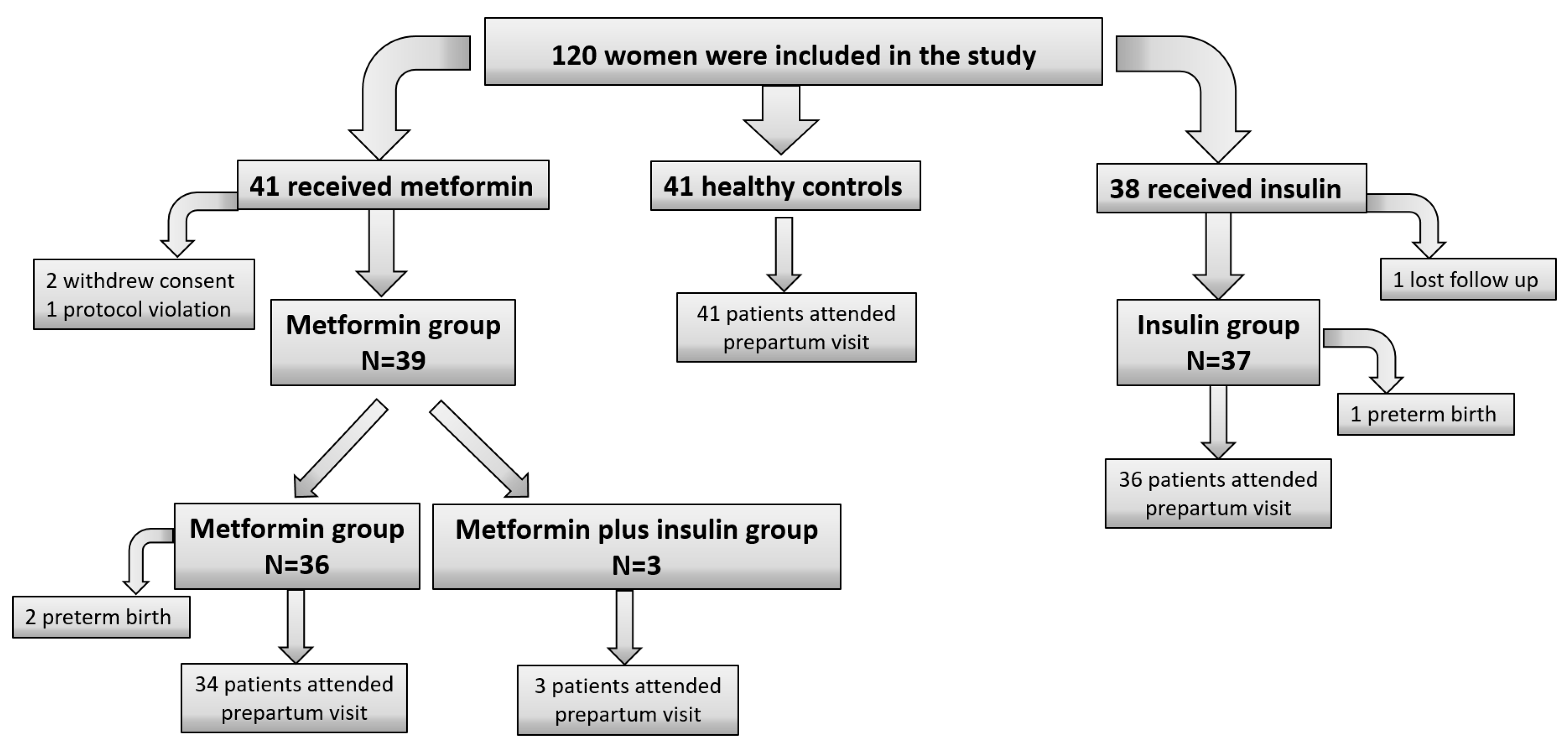
3.1. Study Population
3.2. Characteristics of the Study Population
3.3. Oxidative Stress and Intestinal Permeability Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Metzger, B.E.; Coustan, D.R. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 1998, 21, B161. [Google Scholar] [PubMed]
- Johns, E.C.; Denison, F.C.; Norman, J.E.; Reynolds, R.M. Gestational Diabetes Mellitus: Mechanisms, Treatment, and Complications. Trends Endocrinol. Metab. 2018, 11, 743–754. [Google Scholar] [CrossRef] [PubMed]
- SMFM Statement: Pharmacological treatment of gestational diabetes. Am. J. Obstet. Gynecol. 2018, 218, B2–B4. [CrossRef]
- Elsayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 15. Management of Diabetes in Pregnancy: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S254–S266. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Diabetes in Pregnancy: Management of Diabetes and Its Complications from Preconception to the Postnatal Period. 2015. Available online: www.nice.org.uk/guidance/ng3 (accessed on 28 July 2023).
- Newman, C.; Dunne, F.P. Metformin for pregnancy and beyond: The pros and cons. Diabet. Med. 2022, 39, e14700. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Guo, Q.; Ge, J.; Li, J.; Li, C.; Jing, Z. The efficacy and safety of metformin alone or as an add-on therapy to insulin in pregnancy with GDM or T2DM: A systematic review and meta-analysis of 21 randomized controlled trials. J. Clin. Pharm. Ther. 2022, 47, 168–177. [Google Scholar] [CrossRef]
- Sheng, B.; Ni, J.; Lv, B.; Jiang, G.; Lin, X.; Li, H. Short-term neonatal outcomes in women with gestational diabetes treated using metformin versus insulin: A systematic review and meta-analysis of randomized controlled trials. Acta Diabetol. 2023, 60, 595–608. [Google Scholar] [CrossRef]
- Picón-César, M.J.; Molina-Vega, M.; Suárez-Arana, M.; González-Mesa, E.; Sola-Moyano, A.P.; Roldan-López, R.; Romero-Narbona, F.; Olveira, G.; Tinahones, F.J.; Gónzalez-Romero, S. Metformin for gestational diabetes study: Metformin vs insulin in gestational diabetes: Glycemic control and obstetrical and perinatal outcomes: Randomized prospective trial. Am. J. Obstet. Gynecol. 2021, 225, 517.e1–517.e17. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, H. Metformin—A potentially effective drug for gestational diabetes mellitus: A systematic review and meta-analysis. J. Matern. Fetal Neonatal Med. 2017, 30, 1874–1881. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Sehrawat, A.; Mishra, J.; Sidhu, I.S.; Navik, U.; Khullar, N.; Kumar, S.; Bhatti, G.K.; Reddy, P.H. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free. Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef]
- Banik, S.; Ghosh, A. The association of oxidative stress biomarkers with type 2 diabetes mellitus: A systematic review and meta-analysis. Health Sci. Rep. 2021, 4, e389. [Google Scholar] [CrossRef] [PubMed]
- de Mendonça, E.L.S.S.; Fragoso, M.B.T.; de Oliveira, J.M.; Xavier, J.A.; Goulart, M.O.F.; de Oliveira, A.C.M. Gestational Diabetes Mellitus: The Crosslink among Inflammation, Nitroxidative Stress, Intestinal Microbiota and Alternative Therapies. Antioxidants 2022, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Sudharshana-Murthy, K.A.; Bhandiwada, A.; Chandan, S.L.; Gowda, S.L.; Sindhusree, G. Evaluation of Oxidative Stress and Proinflammatory Cytokines in Gestational Diabetes Mellitus and Their Correlation with Pregnancy Outcome. Indian J. Endocrinol. Metab. 2018, 22, 79–84. [Google Scholar] [CrossRef]
- Rueangdetnarong, H.; Sekararithi, R.; Jaiwongkam, T.; Kumfu, S.; Chattipakorn, N.; Tongsong, T.; Jatavan, P. Comparisons of the oxidative stress biomarkers levels in gestational diabetes mellitus (GDM) and non-GDM among Thai population: Cohort study. Endocr. Connect. 2018, 7, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, M.; Yadegari, A.; Ribaldone, D.G.; Hasanzadeh, M.; Djafarian, K. Zonulin levels in complicated pregnancy: A systematic review and meta-analysis. Obstet. Gynaecol. 2022, 42, 2621–2628. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.B.; Chae, S.U.; Jo, S.J.; Jerng, U.M.; Bae, S.K. The relationship between the gut microbiome and metformin as a key for treating type 2 diabetes mellitus. Int. J. Mol. Sci. 2021, 22, 3566. [Google Scholar] [CrossRef] [PubMed]
- Rad, N.R.; Movahedian, A.; Feizi, A.; Aminorroaya, A.; Aarabi, M.H. Antioxidant effects of astaxanthin and metformin combined therapy in type 2 diabetes mellitus patients: A randomized double-blind controlled clinical trial. Res. Pharm. Sci. 2022, 17, 219–230. [Google Scholar] [CrossRef]
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979, 28, 1039–1057. [Google Scholar] [CrossRef]
- Chiarello, D.I.; Abad, C.; Rojas, D.; Toledo, F.; Vázquez, C.M.; Mate, A.; Sobrevia, L.; Marín, R. Oxidative stress: Normal pregnancy versus preeclampsia. Biochim. Biophys. Acta: Mol. Basis Dis. 2020, 1866, 165354. [Google Scholar] [CrossRef]
- Phoswa, W.N.; Khaliq, O.P. The Role of Oxidative Stress in Hypertensive Disorders of Pregnancy (Preeclampsia, Gestational Hypertension) and Metabolic Disorder of Pregnancy (Gestational Diabetes Mellitus). Oxidative Med. Cell. Longev. 2021, 2021, 5581570. [Google Scholar] [CrossRef] [PubMed]
- de Lucca, L.; Jantsch, L.B.; Vendrame, S.A.; de Paula, H.L.; dos Santos Stein, C.; Gallarreta, F.M.P.; Moresco, R.N.; de Lima Gonçalves, T. Variation of the Oxidative Profile in Pregnant Women With and Without Gestational Complications. Matern. Child Health J. 2022, 26, 2155–2168. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yin, Q.; Li, N.; Ouyang, Z.; Zhong, M. Plasma markers of oxidative stress in patients with gestational diabetes mellitus in the second and third trimester. Obstet. Gynecol. Int. 2016, 2016, 3865454. [Google Scholar] [CrossRef]
- Shang, M.; Zhao, J.; Yang, L.; Lin, L. Oxidative stress and antioxidant status in women with gestational diabetes mellitus diagnosed by IADPSG criteria. Diabetes Res. Clin. Pract. 2015, 109, 404–410. [Google Scholar] [CrossRef]
- Adeshara, K.A.; Bangar, N.S.; Doshi, P.R.; Diwan, A.; Tupe, R.S. Action of metformin therapy against advanced glycation, oxidative stress and inflammation in type 2 diabetes patients: 3 months follow-up study. Diabetol. Metab. Syndr. 2020, 14, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Yarsilikal-Guleroglu, F.; Olgu-Bafali, İ.; Topaktas, M.; Argun-Atalmis, H.; Yavuz-Dogu, S.; Seker-Atas, B.; Ozdemir-Anayurt, E. Muhlise-Okyay, T.; Cetin, A. Comparison of biomarkers of oxidative stress, 8-isoprostane, advanced oxidation protein products, and 8-hydroxy-2′-deoxyguanosine and pro-apoptosis, cytokeratin 18 M30, in women with normal glucose tolerance and gestational diabetes mellitus. Int. J. Diabetes Dev. Ctries. 2022, 42, 621–629. [Google Scholar] [CrossRef]
- Karacay, Ö.; Sepici-Dincel, A.; Karcaaltincaba, D.; Sahin, D.; Yalvaç, S.; Akyol, M.; Kandemir, O.; Altan, N. A quantitative evaluation of total antioxidant status and oxidative stress markers in preeclampsia and gestational diabetic patients in 24–36 weeks of gestation. Diabetes Res. Clin. Pract. 2010, 89, 231–238. [Google Scholar] [CrossRef]
- Parast, V.M.; Paknahad, Z. Antioxidant Status and Risk of Gestational Diabetes Mellitus: A Case-Control Study. Clin. Nutr. Res. 2017, 6, 81. [Google Scholar] [CrossRef]
- Kapustin, R.; Chepanov, S.; Kopteeva, E.; Arzhanova, O. Maternal serum nitrotyrosine, 8-isoprostane and total antioxidant capacity levels in pre-gestational or gestational diabetes mellitus. Gynecol. Endocrinol. 2020, 36, 36–42. [Google Scholar] [CrossRef]
- Zygula, A.; Kosinski, P.; Zwierzchowska, A.; Sochacka, M.; Wroczynski, P.; Makarewicz-Wujec, M.; Pietrzak, B.; Wielgos, M.; Rzentala, M.; Giebultowicz, J. Oxidative stress markers in saliva and plasma differ between diet-controlled and insulin-controlled gestational diabetes mellitus. Diabetes Res. Clin. Pract. 2019, 148, 72–80. [Google Scholar] [CrossRef]
- Zamani-Ahari, U.; Zamani-Ahari, S.; Fardi-Azar, Z.; Falsafi, P.; Ghanizadeh, M. Comparison of Total Antioxidant Capacity of Saliva in Women with Gestational diabetes mellitus and Non-diabetic Pregnant Women. J. Clin. Exp. Dent. 2017, 9, e1282–e1286. [Google Scholar] [CrossRef] [PubMed]
- Surdacka, A.; Ciȩzka, E.; Pioruńska-Stolzmann, M.; Wender-Ozegowska, E.; Korybalska, K.; Kawka, E.; Kaczmarek, E.; Witowski, J. Relation of salivary antioxidant status and cytokine levels to clinical parameters of oral health in pregnant women with diabetes. Arch. Oral Biol. 2011, 56, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, N.; Iannantuoni, F.; Gruevska, A.; Muntane, J.; Rocha, M.; Victor, V.M. Mechanisms of action of metformin in type 2 diabetes: Effects on mitochondria and leukocyte-endothelium interactions. Redox Biol. 2020, 34, 101517. [Google Scholar] [CrossRef]
- Güvey, H.; Çelik, S.; Çalışkan, C.S.; Yılmaz, Z.; Yılmaz, M.; Erten, Ö.; Tinelli, A. How do serum zonulin levels change in gestational diabetes mellitus, pregnancy cholestasis, and the coexistence of both diseases? Int. J. Environ. Res. Public Health 2021, 18, 12555. [Google Scholar] [CrossRef] [PubMed]
- Mokkala, K.; Pussinen, P.; Houttu, N.; Koivuniemi, E.; Vahlberg, T.; Laitinen, K. The impact of probiotics and n-3 long-chain polyunsaturated fatty acids on intestinal permeability in pregnancy: A randomised clinical trial. Benef. Microorg. 2018, 9, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Molina-Vega, M.; Picón-César, M.J.; Gutiérrez-Repiso, C.; Fernández-Valero, A.; Lima-Rubio, F.; González-Romero, S.; Moreno-Indias, I.; Tinahones, F.J. Metformin action over gut microbiota is related to weight and glycemic control in gestational diabetes mellitus: A randomized trial. Biomed. Pharmacother. 2022, 145, 112465. [Google Scholar] [CrossRef] [PubMed]
- Palacios, T.; Vitetta, L.; Coulson, S.; Madigan, C.D.; Lam, Y.Y.; Manuel, R.; Briskey, D.; Hendy, C.; Kim, J.N.; Ishoey, T.; et al. Targeting the intestinal microbiota to prevent type 2 diabetes and enhance the effect of metformin on glycaemia: A randomised controlled pilot study. Nutrients 2020, 12, 2041. [Google Scholar] [CrossRef]
- Lewek, J.; Banach, M. Dyslipidemia Management in Pregnancy: Why Is It not Covered in the Guidelines? Curr. Atheroscler. Rep. 2022, 24, 547–556. [Google Scholar] [CrossRef]
- Hu, J.; Gillies, C.L.; Lin, S.; Stewart, Z.A.; Melford, S.E.; Abrams, K.R.; Baker, P.N.; Khunti, K.; Tan, B.K. Association of maternal lipid profile and gestational diabetes mellitus: A systematic review and meta-analysis of 292 studies and 97,880 women. eClinicalMedicine 2021, 34, 100830. [Google Scholar] [CrossRef]
| Healthy Controls | INS Group | MET Group | p-Value | |
|---|---|---|---|---|
| Baseline | N = 41 | N = 38 | N = 41 | |
| Gestational age | 27.1 ± 1.6 | 23.1 ± 7.1 | 23.4 ± 6.0 | |
| Age (years) | 33.7 ± 4.2 | 34.5 ± 4.9 | 34.9 ± 4.9 | 0.477 |
| Pre-pregnancy BMI (kg/m2) | 26.1 ± 5.2 a | 30.7 ± 4.9 b | 28.9 ± 5.4 b | 0.001 |
| Nulliparous (%) | 55.8 | 18.6 | 25.6 | 0.001 |
| GDM in a previous pregnancy (%) | 5.6 | 44.4 | 50 | 0.005 |
| Previous macrosomia (%) | 16.7 | 44.4 | 38.9 | <0.001 |
| Family history of DM (%) | 23.6 | 40 | 36.4 | 0.055 |
| Fasting glycemia (mg/dL) | 75.6 ± 7.1 a | 85.5 ± 11.4 b | 84.1 ± 12.7 b | <0.001 |
| HbA1c (%) | 5.1 ± 0.3 a | 5.4 ± 0.4 b | 5.3 ± 0.4 b | 0.007 |
| Total cholesterol (mg/dL) | 264.6 ± 42.2 a | 222.6 ± 45.4 b | 220.8 ± 54.5 b | <0.001 |
| HDL cholesterol (mg/dL) | 81.5 ± 17.9 a | 70.9 ± 14.7 b | 67.0 ± 14.5 b | <0.001 |
| LDL cholesterol (mg/dL) | 145.7 ± 36.4 a | 113.5 ± 38.0 b | 118.1 ± 37.4 b | <0.001 |
| Triglycerides (mg/dL) | 192.4 ± 79.2 | 194.1 ± 63.8 | 200.3 ± 78.8 | 0.457 |
| Ferritin (ng/mL) | 18.0 ± 19.2 | 26.2 ± 24.2 | 19.1 ± 17.0 | 0.183 |
| Prepartum Visit | N = 41 | N = 36 | N = 37 | |
| Gestational weight gain (kg) | 11.0 ± 5.1 | 9.3 ± 5.5 | 8.0 ± 5.9 | 0.063 |
| Weight gain from enrollment to prepartum visit (kg) | 4.6 ± 3.0 a | 4.2 ± 4.1 a | 1.7 ± 3.8 b | 0.002 |
| Mean fasting glycemia during follow-up | 92.4 ± 7.8 | 90.3 ± 6.6 | 0.214 | |
| Mean postprandial glycemia during follow-up | 130.3 ± 12.6 | 121.6 ± 8.1 | 0.001 | |
| Mean prepartum fasting glycemia (mg/dL) | 73.7 ± 10.1 | 77.7 ± 11.4 | 76.3 ± 10.2 | 0.250 |
| HbA1c (%) | 5.3 ± 0.3 | 5.5 ± 0.4 | 5.4 ± 0.4 | 0.316 |
| Total cholesterol (mg/dL) | 267.1 ± 41.9 a | 238.4 ± 44.2 b | 239.7 ± 45.3 b | 0.006 |
| HDL cholesterol (mg/dL) | 78.5 ± 21.7 | 72.8 ± 15.4 | 70.6 ± 14.4 | 0.134 |
| LDL cholesterol (mg/dL) | 141.7 ± 35.4 a | 118.9 ± 34.4 b | 116.3 ± 34.3 b | 0.003 |
| Triglycerides (mg/dL) | 248.2 ± 85.3 | 236.1 ± 72.1 | 268.4 ± 91.8 | 0.257 |
| Ferritin (ng/mL) | 18.8 ± 21.0 | 12.9 ± 8.7 | 12.8 ± 7.2 | 0.129 |
| Obstetric and Perinatal Outcomes | ||||
| Induction of labor (%) | 30.8 | 38.5 | 30.8 | 0.331 |
| Type of delivery (%) | 0.088 | |||
| - Non-instrumental vaginal | 47.3 | 36.1 | 68.4 | |
| - Instrumental | 8 | 8.4 | 5.3 | |
| - Cesarean | 44.7 | 55.5 | 26.3 | |
| Birthweight (gr) | 3238.9 ± 418.6 | 3379.9 ± 495.2 | 3300.9 ± 575.8 | 0.502 |
| Birthweight percentile | 46.7 ± 24.9 a | 65.8 ± 32.3 b | 58.0 ± 32.1 a,b | 0.036 |
| Birth length (cm) | 49.9 ± 2.1 | 49.9 ± 2.8 | 50.3 ± 1.8 | 0.635 |
| Head circumference (cm) | 34.5 ± 3.1 | 34.0 ± 1.9 | 34.5 ± 1.9 | 0.539 |
| Healthy Controls | INS Group | MET Group | p-Value | |
|---|---|---|---|---|
| Baseline TAC (µM CRE) | 1710.0 ± 197.9 | 1630.7 ± 268.4 | 1657.4 ± 182.6 | 0.294 |
| Baseline zonulin (ng/mL) | 0.6 ± 0.9 | 0.4 ± 0.3 | 0.4 ± 0.4 | 0.437 |
| Baseline AOPP (µM) | 211.0 ± 151.8 | 146.2 ± 52.0 | 157.4 ± 83.2 | 0.050 |
| Prepartum TAC (µM CRE) | 1814.9 ± 227.5 | 1707.3 ± 281.6 | 1778.9 ± 256.9 | 0.200 |
| Prepartum zonulin (ng/mL) | 1.2 ± 1.7 a | 0.6 ± 0.5 b | 0.5 ± 0.4 b | 0.018 |
| Prepartum AOPP (µM) | 225.7 ± 130.8 | 180.5 ± 88.2 | 220.9 ± 113.5 | 0.166 |
| TAC change (µM CRE) | 104.9 ± 172.5 | 76.6 ± 205.1 | 121.5 ± 187.9 | 0.608 |
| Zonulin change (ng/mL) | 0.6 ± 1.1 a | 0.2 ± 0.5 a,b | 0.1 ± 0.5 b | 0.038 |
| AOPP change (µM) | 45.4 ± 70.3 | 34.2 ± 78.1 | 63.5 ± 89.8 | 0.306 |
| Baseline | Prepartum | p-Value | |
|---|---|---|---|
| Total | |||
| TAC (µM CRE) | 1688.0 ± 218.9 | 1767.0 ± 254.9 | 0.001 |
| Zonulin (ng/mL) | 0.5 ± 0.6 | 0.8 ± 1.1 | <0.001 |
| AOPP (µM) | 173.6 ± 110.3 | 210.1 ± 114.0 | 0.001 |
| Healthy Controls | |||
| TAC (µM CRE) | 1710.0 ± 197.9 | 1814.9 ± 227.5 | 0.389 |
| Zonulin (ng/mL) | 0.6 ± 0.9 | 1.2 ± 1.7 | 0.004 |
| AOPP (µM) | 211.0 ± 151.8 | 225.7 ± 130.8 | 0.551 |
| Ins Group | |||
| TAC (µM CRE) | 1630.7 ± 268.4 | 1707.3 ± 281.6 | 0.037 |
| Zonulin (ng/mL) | 0.4 ± 0.3 | 0.6 ± 0.5 | 0.034 |
| AOPP (µM) | 146.2 ± 52.0 | 180.5 ± 88.2 | 0.015 |
| Met Group | |||
| TAC (µM CRE) | 1657.4 ± 182.6 | 1778.9 ± 256.9 | 0.001 |
| Zonulin (ng/mL) | 0.4 ± 0.4 | 0.5 ± 0.4 | 0.202 |
| AOPP (µM) | 157.4 ± 83.2 | 220.9 ± 113.5 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Valero, A.; Peña-Montero, N.; Lima-Rubio, F.; Gutiérrez-Repiso, C.; Linares-Pineda, T.M.; Picón-César, M.J.; Sancho-Marín, R.; Tinahones, F.J.; Morcillo, S.; Molina-Vega, M. Changes in Oxidative Stress and Intestinal Permeability during Pregnancy in Women with Gestational Diabetes Mellitus Treated with Metformin or Insulin and Healthy Controls: A Randomized Controlled Trial. Antioxidants 2023, 12, 1981. https://doi.org/10.3390/antiox12111981
Fernández-Valero A, Peña-Montero N, Lima-Rubio F, Gutiérrez-Repiso C, Linares-Pineda TM, Picón-César MJ, Sancho-Marín R, Tinahones FJ, Morcillo S, Molina-Vega M. Changes in Oxidative Stress and Intestinal Permeability during Pregnancy in Women with Gestational Diabetes Mellitus Treated with Metformin or Insulin and Healthy Controls: A Randomized Controlled Trial. Antioxidants. 2023; 12(11):1981. https://doi.org/10.3390/antiox12111981
Chicago/Turabian StyleFernández-Valero, Andrea, Nerea Peña-Montero, Fuensanta Lima-Rubio, Carolina Gutiérrez-Repiso, Teresa María Linares-Pineda, María José Picón-César, Raquel Sancho-Marín, Francisco J. Tinahones, Sonsoles Morcillo, and María Molina-Vega. 2023. "Changes in Oxidative Stress and Intestinal Permeability during Pregnancy in Women with Gestational Diabetes Mellitus Treated with Metformin or Insulin and Healthy Controls: A Randomized Controlled Trial" Antioxidants 12, no. 11: 1981. https://doi.org/10.3390/antiox12111981
APA StyleFernández-Valero, A., Peña-Montero, N., Lima-Rubio, F., Gutiérrez-Repiso, C., Linares-Pineda, T. M., Picón-César, M. J., Sancho-Marín, R., Tinahones, F. J., Morcillo, S., & Molina-Vega, M. (2023). Changes in Oxidative Stress and Intestinal Permeability during Pregnancy in Women with Gestational Diabetes Mellitus Treated with Metformin or Insulin and Healthy Controls: A Randomized Controlled Trial. Antioxidants, 12(11), 1981. https://doi.org/10.3390/antiox12111981












