Differential Cellular Interactome in Schizophrenia and Bipolar Disorder—Discriminatory Biomarker Role
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.1.1. Experimental Design
2.1.2. Blood Collection
2.1.3. Clinical Parameters
2.2. Procedures
2.2.1. Oxidative Stress Studies
2.2.2. Inflammation Studies
2.2.3. Western Blotting
2.2.4. Proteasome Activity
2.2.5. ATP Measurement
2.2.6. Apoptosis
2.2.7. Statistical Analysis
3. Results
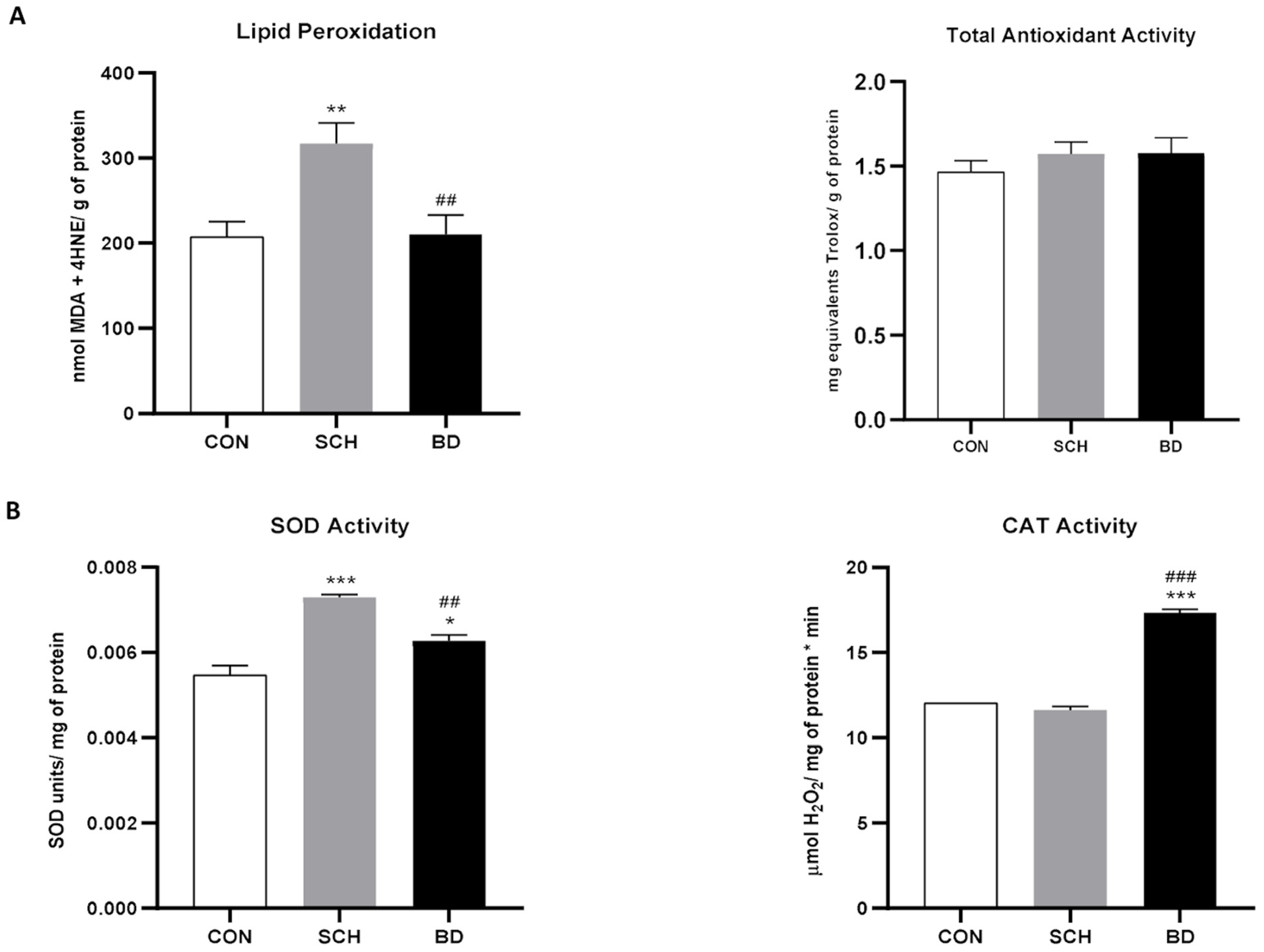
3.1. Widespread Oxidative Stress
3.2. Resulting Inflammation
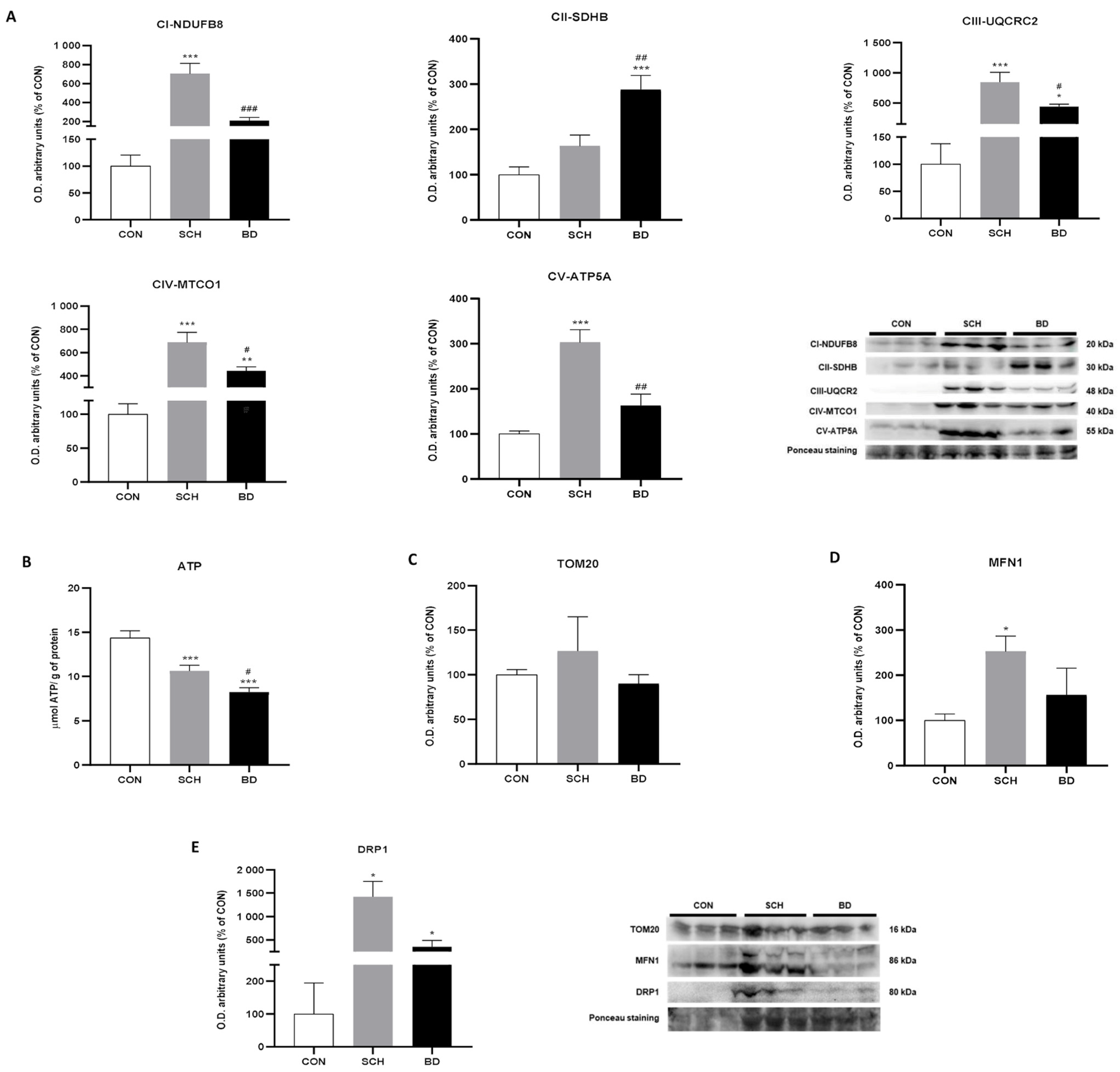
3.3. Mitochondrial Bioenergetics
3.4. Mitochondrial Dynamics
3.5. Endoplasmic Reticulum Stress
3.6. Unfolded Protein Response
3.7. Proteasomal Capacity
3.8. Autophagy Compensation
3.9. Apoptosis through Autophagic Failure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whiteford, H.A.; Degenhardt, L.; Rehm, J.; Baxter, A.J.; Ferrari, A.J.; Erskine, H.E.; Charlson, F.J.; Norman, R.E.; Flaxman, A.D.; Johns, N.; et al. Global Burden of Disease Attributable to Mental and Substance Use Disorders: Findings from the Global Burden of Disease Study 2010. Lancet 2013, 382, 1575–1586. [Google Scholar] [CrossRef] [PubMed]
- WHO. Available online: https://www.who.int/news-room/fact-sheets/detail/mental-disorders (accessed on 14 July 2022).
- Wittchen, H.U.; Jacobi, F.; Rehm, J.; Gustavsson, A.; Svensson, M.; Jönsson, B.; Olesen, J.; Allgulander, C.; Alonso, J.; Faravelli, C.; et al. The Size and Burden of Mental Disorders and Other Disorders of the Brain in Europe 2010. Eur. Neuropsychopharmacol. 2011, 21, 655–679. [Google Scholar] [CrossRef]
- Bipolar Disorder and Schizophrenia Working Group of the Psychiatric Genomics Consortium. Bipolar Disorder and Schizophrenia Working Group of the Psychiatric Genomics Consortium Genomic Dissection of Bipolar Disorder and Schizophrenia, Including 28 Subphenotypes. Cell 2018, 173, 1705–1715.e16. [Google Scholar] [CrossRef]
- Aldinger, F.; Schulze, T.G. Environmental Factors, Life Events, and Trauma in the Course of Bipolar Disorder. Psychiatry Clin. Neurosci. 2017, 71, 6–17. [Google Scholar] [CrossRef]
- Stilo, S.A.; Murray, R.M. Non-Genetic Factors in Schizophrenia. Curr. Psychiatry Rep. 2019, 21, 100. [Google Scholar] [CrossRef] [PubMed]
- Eyckerman, S.; Tavernier, J. Methods to Map Protein Interactions in Mammalian Cells: Different Tools to Address Different Questions. Eur. Cytokine Netw. 2002, 13, 276–284. [Google Scholar] [PubMed]
- Cernea, A.; Fernández-Martínez, J.L.; de Andrés-Galiana, E.J.; Fernández-Muñiz, Z.; Bermejo-Millo, J.C.; González-Blanco, L.; Solano, J.J.; Abizanda, P.; Coto-Montes, A.; Caballero, B. Prognostic Networks for Unraveling the Biological Mechanisms of Sarcopenia. Mech. Ageing Dev. 2019, 182, 111129. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Sawa, A.; Iyo, M. Increased Levels of Glutamate in Brains from Patients with Mood Disorders. Biol. Psychiatry 2007, 62, 1310–1316. [Google Scholar] [CrossRef]
- Li, J.Z.; Vawter, M.P.; Walsh, D.M.; Tomita, H.; Evans, S.J.; Choudary, P.V.; Lopez, J.F.; Avelar, A.; Shokoohi, V.; Chung, T.; et al. Systematic Changes in Gene Expression in Postmortem Human Brains Associated with Tissue pH and Terminal Medical Conditions. Hum. Mol. Genet. 2004, 13, 609–616. [Google Scholar] [CrossRef]
- Liew, C.-C.; Ma, J.; Tang, H.-C.; Zheng, R.; Dempsey, A.A. The Peripheral Blood Transcriptome Dynamically Reflects System Wide Biology: A Potential Diagnostic Tool. J. Lab. Clin. Med. 2006, 147, 126–132. [Google Scholar] [CrossRef]
- Bateman, R.J.; Blennow, K.; Doody, R.; Hendrix, S.; Lovestone, S.; Salloway, S.; Schindler, R.; Weiner, M.; Zetterberg, H.; Aisen, P.; et al. Plasma Biomarkers of AD Emerging as Essential Tools for Drug Development: An EU/US CTAD Task Force Report. J. Prev. Alzheimers Dis. 2019, 6, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.; Wallin, H.; Knudsen, L.E. Oxidative Stress Associated with Exercise, Psychological Stress and Life-Style Factors. Chem.-Biol. Interact. 1996, 102, 17–36. [Google Scholar] [CrossRef]
- Pupić-Bakrač, J.; Pupić-Bakrač, A. Comorbidity of Chronic Tinnitus and Psychological Stress—Which Came First, the Chicken or the Egg? Psychiatr. Danub. 2020, 32, 412–419. [Google Scholar] [PubMed]
- González-Blanco, L.; García-Portilla, M.P.; García-Álvarez, L.; de la Fuente-Tomás, L.; Iglesias García, C.; Sáiz, P.A.; Rodríguez-González, S.; Coto-Montes, A.; Bobes, J. Oxidative Stress Biomarkers and Clinical Dimensions in First 10 Years of Schizophrenia. Rev. Psiquiatr. Salud Ment. 2018, 11, 130–140. [Google Scholar] [CrossRef][Green Version]
- Westover, A.N.; Marangell, L.B. A cross-national relationship between sugar consumption and major depression? Depress. Anxiety 2002, 16, 118–120. [Google Scholar] [CrossRef]
- Cecerska-Heryć, E.; Polikowska, A.; Serwin, N.; Roszak, M.; Grygorcewicz, B.; Heryć, R.; Michalczyk, A.; Dołęgowska, B. Importance of Oxidative Stress in the Pathogenesis, Diagnosis, and Monitoring of Patients with Neuropsychiatric Disorders, a Review. Neurochem. Int. 2022, 153, 105269. [Google Scholar] [CrossRef] [PubMed]
- Rekatsina, M.; Paladini, A.; Piroli, A.; Zis, P.; Pergolizzi, J.V.; Varrassi, G. Pathophysiology and Therapeutic Perspectives of Oxidative Stress and Neurodegenerative Diseases: A Narrative Review. Adv. Ther. 2020, 37, 113–139. [Google Scholar] [CrossRef] [PubMed]
- Amirani, E.; Milajerdi, A.; Mirzaei, H.; Jamilian, H.; Mansournia, M.A.; Hallajzadeh, J.; Ghaderi, A. The Effects of Probiotic Supplementation on Mental Health, Biomarkers of Inflammation and Oxidative Stress in Patients with Psychiatric Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Complement. Ther. Med. 2020, 49, 102361. [Google Scholar] [CrossRef] [PubMed]
- Coto-Montes, A. Editorial (Hot Topic: Mitochondrion: An Organelle More and More Implication in New Processes). Recent Pat. Endocr. Metab. Immune Drug Discov. 2022, 7, 83–85. [Google Scholar] [CrossRef]
- Rubio-González, A.; Reiter, R.J.; de Luxán-Delgado, B.; Potes, Y.; Caballero, B.; Boga, J.A.; Solano, J.J.; Vega-Naredo, I.; Coto-Montes, A. Pleiotropic Role of Melatonin in Brain Mitochondria of Obese Mice. Melatonin Res. 2020, 3, 538–557. [Google Scholar] [CrossRef]
- Kim, P.; Scott, M.R.; Meador-Woodruff, J.H. Dysregulation of the Unfolded Protein Response (UPR) in the Dorsolateral Prefrontal Cortex in Elderly Patients with Schizophrenia. Mol. Psychiatry 2021, 26, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Millo, J.C.; Guimarães, M.R.M.; de Luxán-Delgado, B.; Potes, Y.; Pérez-Martínez, Z.; Díaz-Luis, A.; Caballero, B.; Solano, J.J.; Vega-Naredo, I.; Coto-Montes, A. High-Fructose Consumption Impairs the Redox System and Protein Quality Control in the Brain of Syrian Hamsters: Therapeutic Effects of Melatonin. Mol. Neurobiol. 2018, 55, 7973–7986. [Google Scholar] [CrossRef] [PubMed]
- González-Blanco, L.; Bermúdez, M.; Bermejo-Millo, J.C.; Gutiérrez-Rodríguez, J.; Solano, J.J.; Antuña, E.; Menéndez-Valle, I.; Caballero, B.; Vega-Naredo, I.; Potes, Y.; et al. Cell Interactome in Sarcopenia during Aging. J. Cachexia Sarcopenia Muscle 2022, 13, 919–931. [Google Scholar] [CrossRef]
- Rubio-González, A.; Bermejo-Millo, J.C.; de Luxán-Delgado, B.; Potes, Y.; Pérez-Martínez, Z.; Boga, J.A.; Vega-Naredo, I.; Caballero, B.; Solano, J.J.; Coto-Montes, A.; et al. Melatonin Prevents the Harmful Effects of Obesity on the Brain, Including at the Behavioral Level. Mol. Neurobiol. 2018, 55, 5830–5846. [Google Scholar] [CrossRef] [PubMed]
- Ceccariglia, S.; Sibilia, D.; Parolini, O.; Michetti, F.; Di Sante, G. Altered Expression of Autophagy Biomarkers in Hippocampal Neurons in a Multiple Sclerosis Animal Model. Int. J. Mol. Sci. 2023, 24, 13225. [Google Scholar] [CrossRef]
- Franconi, F.; Capobianco, G.; Diana, G.; Lodde, V.; De Donno, A.; Idda, M.L.; Montella, A.; Campesi, I. Sex Influence on Autophagy Markers and miRNAs in Basal and Angiotensin II-Treated Human Umbilical Vein Endothelial Cells. Int. J. Mol. Sci. 2023, 24, 14929. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, W.; Wang, Y. STING Is an Essential Regulator of Heart Inflammation and Fibrosis in Mice with Pathological Cardiac Hypertrophy via Endoplasmic Reticulum (ER) Stress. Biomed. Pharmacother. 2020, 125, 110022. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Gérard-Monnier, D.; Erdelmeier, I.; Régnard, K.; Moze-Henry, N.; Yadan, J.C.; Chaudière, J. Reactions of 1-Methyl-2-Phenylindole with Malondialdehyde and 4-Hydroxyalkenals. Analytical Applications to a Colorimetric Assay of Lipid Peroxidation. Chem. Res. Toxicol. 1998, 11, 1176–1183. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The Hydrophilic and Lipophilic Contribution to Total Antioxidant Activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Martin, J.P.; Dailey, M.; Sugarman, E. Negative and Positive Assays of Superoxide Dismutase Based on Hematoxylin Autoxidation. Arch. Biochem. Biophys. 1987, 255, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Lubinsky, S.; Bewley, G.C. Genetics of Catalase in DROSOPHILA MELANOGASTER: Rates of Synthesis and Degradation of the Enzyme in Flies Aneuploid and Euploid for the Structural Gene. Genetics 1979, 91, 723–742. [Google Scholar] [CrossRef]
- Fortes, M.A.S.; Marzuca-Nassr, G.N.; Vitzel, K.F.; da Justa Pinheiro, C.H.; Newsholme, P.; Curi, R. Housekeeping Proteins: How Useful Are They in Skeletal Muscle Diabetes Studies and Muscle Hypertrophy Models. Anal. Biochem. 2016, 504, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Li, C.; Hu, S.; Xue, F.; Kang, Y.J.; Zhang, W. An Appropriate Loading Control for Western Blot Analysis in Animal Models of Myocardial Ischemic Infarction. Biochem. Biophys. Rep. 2017, 12, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Sander, H.; Wallace, S.; Plouse, R.; Tiwari, S.; Gomes, A.V. Ponceau S Waste: Ponceau S Staining for Total Protein Normalization. Anal. Biochem. 2019, 575, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Bošković, M.; Vovk, T.; Kores Plesničar, B.; Grabnar, I. Oxidative Stress in Schizophrenia. Curr. Neuropharmacol. 2011, 9, 301–312. [Google Scholar] [CrossRef]
- Fernández-Checa, J.C.; Fernández, A.; Morales, A.; Marí, M.; García-Ruiz, C.; Colell, A. Oxidative Stress and Altered Mitochondrial Function in Neurodegenerative Diseases: Lessons from Mouse Models. CNS Neurol. Disord. Drug Targets 2010, 9, 439–454. [Google Scholar] [CrossRef]
- Hussain, Y.; Singh, J.; Meena, A.; Sinha, R.A.; Luqman, S. Escin-Sorafenib Synergy up-Regulates LC3-II and P62 to Induce Apoptosis in Hepatocellular Carcinoma Cells. Environ. Toxicol. 2023; early view. [Google Scholar] [CrossRef]
- Houessinon, A.; Gicquel, A.; Bochereau, F.; Louandre, C.; Nyga, R.; Godin, C.; Degonville, J.; Fournier, E.; Saidak, Z.; Drullion, C.; et al. Alpha-Fetoprotein Is a Biomarker of Unfolded Protein Response and Altered Proteostasis in Hepatocellular Carcinoma Cells Exposed to Sorafenib. Cancer Lett. 2016, 370, 242–249. [Google Scholar] [CrossRef]
- Cawkwell, P.B.; Bolton, K.W.; Karmacharya, R.; Öngür, D.; Shinn, A.K. Two-Year Diagnostic Stability in a Real-World Sample of Individuals with Early Psychosis. Early Interv. Psychiatry 2020, 14, 751–754. [Google Scholar] [CrossRef]
- Salvatore, S.; Davanzati, G.F.; Potì, S.; Ruggieri, R. Mainstream Economics and Sense-Making. Integr. Psychol. Behav. Sci. 2009, 43, 158–177. [Google Scholar] [CrossRef] [PubMed]
- Dondé, C.; Achim, A.M.; Brunelin, J.; Poulet, E.; Mondino, M.; Haesebaert, F. A Meta-Analysis of Craving Studies in Schizophrenia Spectrum Disorders. Schizophr. Res. 2020, 222, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Thrash-Williams, B.; Karuppagounder, S.S.; Bhattacharya, D.; Ahuja, M.; Suppiramaniam, V.; Dhanasekaran, M. Methamphetamine-Induced Dopaminergic Toxicity Prevented Owing to the Neuroprotective Effects of Salicylic Acid. Life Sci. 2016, 154, 24–29. [Google Scholar] [CrossRef]
- Ciobica, A.; Padurariu, M.; Dobrin, I.; Stefanescu, C.; Dobrin, R. Oxidative Stress in Schizophrenia—Focusing on the Main Markers. Psychiatr. Danub. 2011, 23, 237–245. [Google Scholar]
- Medina-Hernández, V.; Ramos-Loyo, J.; Luquin, S.; Sánchez, L.F.C.; García-Estrada, J.; Navarro-Ruiz, A. Increased Lipid Peroxidation and Neuron Specific Enolase in Treatment Refractory Schizophrenics. J. Psychiatr. Res. 2007, 41, 652–658. [Google Scholar] [CrossRef]
- Wu, J.Q.; Kosten, T.R.; Zhang, X.Y. Free Radicals, Antioxidant Defense Systems, and Schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 46, 200–206. [Google Scholar] [CrossRef]
- Rubio-González, A.; Potes, Y.; Illán-Rodríguez, D.; Vega-Naredo, I.; Sierra, V.; Caballero, B.; Fàbrega, E.; Velarde, A.; Dalmau, A.; Oliván, M.; et al. Effect of Animal Mixing as a Stressor on Biomarkers of Autophagy and Oxidative Stress during Pig Muscle Maturation. Animal 2015, 9, 1188–1194. [Google Scholar] [CrossRef]
- Matthijssens, F.; Back, P.; Braeckman, B.P.; Vanfleteren, J.R. Prooxidant Activity of the Superoxide Dismutase (SOD)-Mimetic EUK-8 in Proliferating and Growth-Arrested Escherichia coli Cells. Free Radic. Biol. Med. 2008, 45, 708–715. [Google Scholar] [CrossRef]
- Schuessel, K.; Schäfer, S.; Bayer, T.A.; Czech, C.; Pradier, L.; Müller-Spahn, F.; Müller, W.E.; Eckert, A. Impaired Cu/Zn-SOD Activity Contributes to Increased Oxidative Damage in APP Transgenic Mice. Neurobiol. Dis. 2005, 18, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Müller, N.; Schwarz, M.J. A Psychoneuroimmunological Perspective to Emil Kraepelins Dichotomy: Schizophrenia and Major Depression as Inflammatory CNS Disorders. Eur. Arch. Psychiatry Clin. Neurosci. 2008, 258 (Suppl. S2), 97–106. [Google Scholar] [CrossRef]
- García-Álvarez, L.; Caso, J.R.; García-Portilla, M.P.; de la Fuente-Tomás, L.; González-Blanco, L.; Sáiz Martínez, P.; Leza, J.C.; Bobes, J. Regulation of Inflammatory Pathways in Schizophrenia: A Comparative Study with Bipolar Disorder and Healthy Controls. Eur. Psychiatry 2018, 47, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Bosmans, E.; Ranjan, R.; Vandoolaeghe, E.; Meltzer, H.Y.; De Ley, M.; Berghmans, R.; Stans, G.; Desnyder, R. Lower Plasma CC16, a Natural Anti-Inflammatory Protein, and Increased Plasma Interleukin-1 Receptor Antagonist in Schizophrenia: Effects of Antipsychotic Drugs. Schizophr. Res. 1996, 21, 39–50. [Google Scholar] [CrossRef]
- Liang, J.; Cai, Y.; Xue, X.; Li, X.; Li, Z.; Xu, C.; Xie, G.; Yu, Y. Does Schizophrenia Itself Cause Obesity? Front. Psychiatry 2022, 13, 934384. [Google Scholar] [CrossRef] [PubMed]
- de Luxán-Delgado, B.; Potes, Y.; Rubio-González, A.; Caballero, B.; Solano, J.J.; Fernández-Fernández, M.; Bermúdez, M.; Rodrigues Moreira Guimarães, M.; Vega-Naredo, I.; Boga, J.A.; et al. Melatonin Reduces Endoplasmic Reticulum Stress and Autophagy in Liver of Leptin-Deficient Mice. J. Pineal Res. 2016, 61, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-J.; Wei, J.; Shang, Y.-H.; Huang, H.-C.; Lao, F.-X. Modulation of AβPP and GSK3β by Endoplasmic Reticulum Stress and Involvement in Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1157–1170. [Google Scholar] [CrossRef]
- Bach, D.; Pich, S.; Soriano, F.X.; Vega, N.; Baumgartner, B.; Oriola, J.; Daugaard, J.R.; Lloberas, J.; Camps, M.; Zierath, J.R.; et al. Mitofusin-2 Determines Mitochondrial Network Architecture and Mitochondrial Metabolism. A Novel Regulatory Mechanism Altered in Obesity. J. Biol. Chem. 2003, 278, 17190–17197. [Google Scholar] [CrossRef]
- Soubannier, V.; McBride, H.M. Positioning Mitochondrial Plasticity within Cellular Signaling Cascades. Biochim. Biophys. Acta 2009, 1793, 154–170. [Google Scholar] [CrossRef]
- Wai, T.; Langer, T. Mitochondrial Dynamics and Metabolic Regulation. Trends Endocrinol. Metab. 2016, 27, 105–117. [Google Scholar] [CrossRef]
- Westermann, B. Bioenergetic Role of Mitochondrial Fusion and Fission. Biochim. Biophys. Acta 2012, 1817, 1833–1838. [Google Scholar] [CrossRef]
- Hui, K.K.; Endo, R.; Sawa, A.; Tanaka, M. A Perspective on the Potential Involvement of Impaired Proteostasis in Neuropsychiatric Disorders. Biol. Psychiatry 2022, 91, 335–345. [Google Scholar] [CrossRef]
- Hagan, I.; Hayles, J.; Nurse, P. Cloning and Sequencing of the Cyclin-Related Cdc13+ Gene and a Cytological Study of Its Role in Fission Yeast Mitosis. J. Cell Sci. 1988, 91 Pt 4, 587–595. [Google Scholar] [CrossRef]
- Soltanmohammadi, E.; Farmaki, E.; Zhang, Y.; Naderi, A.; Kaza, V.; Chatzistamou, I.; Kiaris, H. Coordination in the Unfolded Protein Response during Aging in Outbred Deer Mice. Exp. Gerontol 2021, 144, 111191. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Papa, F.R. The Unfolded Protein Response and Cell Fate Control. Mol. Cell 2018, 69, 169–181. [Google Scholar] [CrossRef]
- Moreno, J.A.; Halliday, M.; Molloy, C.; Radford, H.; Verity, N.; Axten, J.M.; Ortori, C.A.; Willis, A.E.; Fischer, P.M.; Barrett, D.A.; et al. Oral Treatment Targeting the Unfolded Protein Response Prevents Neurodegeneration and Clinical Disease in Prion-Infected Mice. Sci. Transl. Med. 2013, 5, 206ra138. [Google Scholar] [CrossRef] [PubMed]
- Roebuck, M.M.; Jamal, J.; Lane, B.; Wood, A.; Santini, A.; Wong, P.-F.; Bou-Gharios, G.; Frostick, S.P. Cartilage Debris and Osteoarthritis Risk Factors Influence Gene Expression in the Synovium in End Stage Osteoarthritis. Knee 2022, 37, 47–59. [Google Scholar] [CrossRef]
- Ávila-González, D.; Young, L.J.; Camacho, F.; Paredes, R.G.; Díaz, N.F.; Portillo, W. Culture of Neurospheres Derived from the Neurogenic Niches in Adult Prairie Voles. J. Vis. Exp. 2020, 160, e61402. [Google Scholar] [CrossRef]
- Hung, W.-Y.; Chang, J.-H.; Cheng, Y.; Cheng, G.-Z.; Huang, H.-C.; Hsiao, M.; Chung, C.-L.; Lee, W.-J.; Chien, M.-H. Autophagosome Accumulation-Mediated ATP Energy Deprivation Induced by Penfluridol Triggers Nonapoptotic Cell Death of Lung Cancer via Activating Unfolded Protein Response. Cell Death Dis. 2019, 10, 538. [Google Scholar] [CrossRef]
- Vega, I.E. EFhd2, a Protein Linked to Alzheimer’s Disease and Other Neurological Disorders. Front. Neurosci. 2016, 10, 150. [Google Scholar] [CrossRef]
- Zuiki, M.; Chiyonobu, T.; Yoshida, M.; Maeda, H.; Yamashita, S.; Kidowaki, S.; Hasegawa, T.; Gotoh, H.; Nomura, T.; Ono, K.; et al. Luteolin Attenuates Interleukin-6-Mediated Astrogliosis in Human iPSC-Derived Neural Aggregates: A Candidate Preventive Substance for Maternal Immune Activation-Induced Abnormalities. Neurosci. Lett. 2017, 653, 296–301. [Google Scholar] [CrossRef]
- Garza-Lombó, C.; Pappa, A.; Panayiotidis, M.I.; Franco, R. Redox Homeostasis, Oxidative Stress and Mitophagy. Mitochondrion 2020, 51, 105–117. [Google Scholar] [CrossRef]
- Qi, Z.; Chen, L. Endoplasmic Reticulum Stress and Autophagy. In Autophagy: Biology and Diseases; Springer: Singapore, 2019; pp. 167–177. [Google Scholar]
- Kaminskyy, V.O.; Zhivotovsky, B. Free Radicals in Cross Talk between Autophagy and Apoptosis. Antioxid. Redox Signal. 2014, 21, 86–102. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gras, I.; Pérez-Nievas, B.G.; García-Bueno, B.; Madrigal, J.L.M.; Andrés-Esteban, E.; Rodríguez-Jiménez, R.; Hoenicka, J.; Palomo, T.; Rubio, G.; Leza, J.C. The Anti-Inflammatory Prostaglandin 15d-PGJ2 and Its Nuclear Receptor PPARgamma Are Decreased in Schizophrenia. Schizophr. Res. 2011, 128, 15–22. [Google Scholar] [CrossRef]
- Melmed, C.; Karpati, G.; Carpenter, S. Experimental Mitochondrial Myopathy Produced by in Vivo Uncoupling of Oxidative Phosphorylation. J. Neurol. Sci. 1975, 26, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Gaffke, L.; Pierzynowska, K.; Rintz, E.; Cyske, Z.; Giecewicz, I.; Węgrzyn, G. Gene Expression-Related Changes in Morphologies of Organelles and Cellular Component Organization in Mucopolysaccharidoses. Int. J. Mol. Sci. 2021, 22, 2766. [Google Scholar] [CrossRef] [PubMed]




| Controls Mean ± SEM or % | Schizophrenia Mean ± SEM or % | Bipolar Disorder Mean ± SEM or % | |
|---|---|---|---|
| Men; women | 35.7%; 64.3% | 62.5%; 37.5% | 50%; 50% |
| Age (years) | 45.7 ± 4.1 | 46.7 ± 2.7 | 50.0 ± 4.0 |
| BMI (kg/m2) | - | 29.5 ± 1.3 | 29.43 ± 1.5 |
| Years of diagnosis | - | 19.8 ± 2.9 | 14.5 ± 2.7 |
| Age at diagnostic | - | 26.0 ± 1.3 | 38.15 ± 4.6 |
| Disability | |||
| Yes | - | 76.2% | 46.1% |
| No | 100% | 23.8% | 53.8% |
| Antipsychotic | |||
| Aripiprazole oral | - | 5.9% | 13.3% |
| Aripiprazole IM | - | 2.9% | - |
| Clozapine | - | 14.7% | - |
| Levomepromazine * | - | 2.9% | - |
| Olanzapine | - | 23.5% | - |
| Paliperidone oral | - | 11.8% | 6.7% |
| Paliperindone IM | - | 23.5% | 26.7% |
| Quetiapine # | - | 5.9% | 33.3% |
| Risperidone | - | 8.8% | 20.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menéndez-Valle, I.; Cachán-Vega, C.; Boga, J.A.; González-Blanco, L.; Antuña, E.; Potes, Y.; Caballero, B.; Vega-Naredo, I.; Saiz, P.; Bobes, J.; et al. Differential Cellular Interactome in Schizophrenia and Bipolar Disorder—Discriminatory Biomarker Role. Antioxidants 2023, 12, 1948. https://doi.org/10.3390/antiox12111948
Menéndez-Valle I, Cachán-Vega C, Boga JA, González-Blanco L, Antuña E, Potes Y, Caballero B, Vega-Naredo I, Saiz P, Bobes J, et al. Differential Cellular Interactome in Schizophrenia and Bipolar Disorder—Discriminatory Biomarker Role. Antioxidants. 2023; 12(11):1948. https://doi.org/10.3390/antiox12111948
Chicago/Turabian StyleMenéndez-Valle, Iván, Cristina Cachán-Vega, José Antonio Boga, Laura González-Blanco, Eduardo Antuña, Yaiza Potes, Beatriz Caballero, Ignacio Vega-Naredo, Pilar Saiz, Julio Bobes, and et al. 2023. "Differential Cellular Interactome in Schizophrenia and Bipolar Disorder—Discriminatory Biomarker Role" Antioxidants 12, no. 11: 1948. https://doi.org/10.3390/antiox12111948
APA StyleMenéndez-Valle, I., Cachán-Vega, C., Boga, J. A., González-Blanco, L., Antuña, E., Potes, Y., Caballero, B., Vega-Naredo, I., Saiz, P., Bobes, J., García-Portilla, P., & Coto-Montes, A. (2023). Differential Cellular Interactome in Schizophrenia and Bipolar Disorder—Discriminatory Biomarker Role. Antioxidants, 12(11), 1948. https://doi.org/10.3390/antiox12111948








