Effect of Micronutrients and L-Carnitine as Antioxidant on Sperm Parameters, Genome Integrity, and ICSI Outcomes: Randomized, Double-Blind, and Placebo-Controlled Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
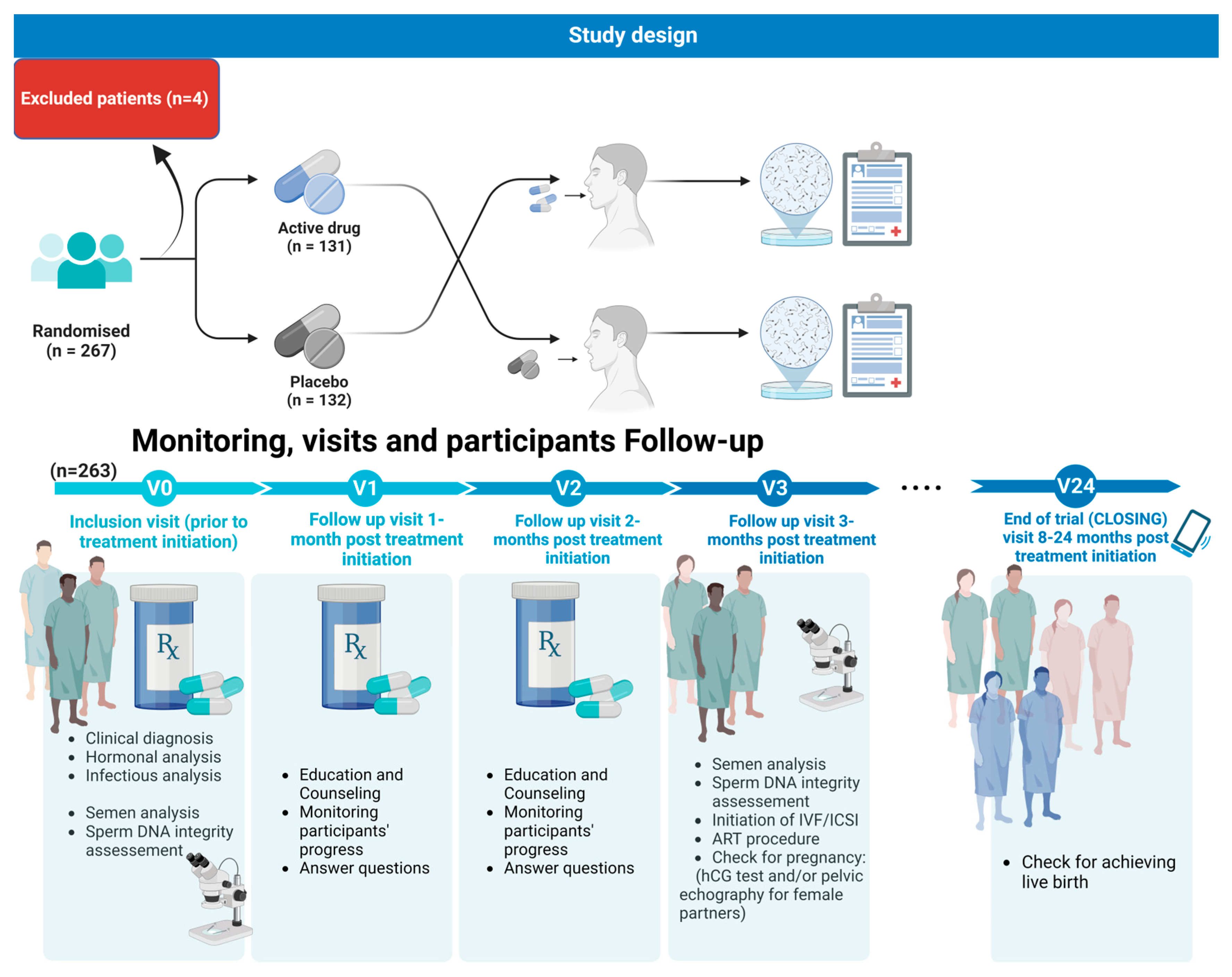
2.1. Study Design and Patients
2.1.1. Questionnaire Design
2.1.2. Ethical Approval
2.1.3. Intervention Details
2.1.4. Monitoring, Visits, and Participants Follow-Up
- -
- Visit two was considered a follow-up visit 1 month post treatment initiation: Male patients were to be given Fertilis homme® treatment or a placebo refill for a period of 1 month;
- -
- Visit three was considered as a follow-up visit 2 months post treatment initiation. Male patients were given Fertilis homme® treatment or a placebo refill for a period of 1 month;
- -
- Visit four was considered as a follow-up visit 3 months post treatment initiation. Patients underwent semen analysis, a DNA fragmentation assay, check for pregnancy: hCG test and/or pelvic echography for the female partner; initiation of IVF/ICSI: assessment of fertilization rate, embryo cleavage rate, and embryo quality;
- -
- Visit five: Female patients underwent a check for pregnancy (hCG test and/or pelvic echography);
- -
- At the end of the trial (CLOSING) visit 24 months post treatment initiation, patients were asked about achieving live birth.
2.2. Semen Collection and Conventional Sperm Parameter Evaluation
2.3. Sperm DNA Fragmentation: TUNEL Assay
2.4. Chromatin Decondensation Test: Toluidine Blue Staining
2.5. Ovarian Stimulation and Oocyte Retrieval
2.6. ART Procedure
2.7. Embryo Culture
2.8. Embryo Transfer
2.9. IVF-ICSI Outcomes
2.10. Statistical Analysis
3. Results
3.1. Conventional Sperm Parameters and Sperm DNA Integrity
3.2. ICSI/IVF Outcome
4. Discussion
5. Conclusions
Limits and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boitrelle, F.; Shah, R.; Saleh, R.; Henkel, R.; Kandil, H.; Chung, E.; Vogiatzi, P.; Zini, A.; Arafa, M.; Agarwal, A. The Sixth Edition of the WHO Manual for Human Semen Analysis: A Critical Review and SWOT Analysis. Life 2021, 11, 1368. [Google Scholar] [CrossRef]
- Agarwal, A.; Parekh, N.; Panner Selvam, M.K.; Henkel, R.; Shah, R.; Homa, S.T.; Ramasamy, R.; Ko, E.; Tremellen, K.; Esteves, S.; et al. Male Oxidative Stress Infertility (MOSI): Proposed Terminology and Clinical Practice Guidelines for Management of Idiopathic Male Infertility. World J. Mens. Health 2019, 37, 296–312. [Google Scholar] [CrossRef] [PubMed]
- Leslie, S.W.; Soon-Sutton, T.L.; Khan, M.A. Male Infertility. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK562258/ (accessed on 5 August 2023).
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Montjean, D.; Godin Pagé, M.-H.; Bélanger, M.-C.; Benkhalifa, M.; Miron, P. An Overview of E-Cigarette Impact on Reproductive Health. Life 2023, 13, 827. [Google Scholar] [CrossRef] [PubMed]
- Montjean, D.; Neyroud, A.-S.; Yefimova, M.G.; Benkhalifa, M.; Cabry, R.; Ravel, C. Impact of Endocrine Disruptors upon Non-Genetic Inheritance. Int. J. Mol. Sci. 2022, 23, 3350. [Google Scholar] [CrossRef] [PubMed]
- Giulioni, C.; Maurizi, V.; Castellani, D.; Scarcella, S.; Skrami, E.; Balercia, G.; Galosi, A.B. The environmental and occupational influence of pesticides on male fertility: A systematic review of human studies. Andrology 2022, 10, 1250–1271. [Google Scholar] [CrossRef] [PubMed]
- Daoud, S.; Sellami, A.; Bouassida, M.; Kebaili, S.; Ammar Keskes, L.; Rebai, T.; Chakroun Feki, N. Routine assessment of occupational exposure and its relation to semen quality in infertile men: A cross-sectional study. Turk. J. Med. Sci. 2017, 47, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J. Male reproductive ageing: A radical road to ruin. Human. Reprod. 2023, 38, 1861–1871. [Google Scholar] [CrossRef] [PubMed]
- Ashapkin, V.; Suvorov, A.; Pilsner, J.R.; Krawetz, S.A.; Sergeyev, O. Age-associated epigenetic changes in mammalian sperm: Implications for offspring health and development. Hum. Reprod. Update 2023, 29, 24–44. [Google Scholar] [CrossRef]
- Lahimer, M.; Capelle, S.; Lefranc, E.; Cabry, R.; Montjean, D.; Bach, V.; Ajina, M.; Ali, H.B.; Benkhalifa, M.; Khorsi-Cauet, H. Effect of pesticide exposure on human sperm characteristics, genome integrity, and methylation profile analysis. Environ. Sci. Pollut. Res. 2023, 30, 77560–77567. [Google Scholar] [CrossRef]
- Lahimer, M.; Montjean, D.; Cabry, R.; Capelle, S.; Lefranc, E.; Bach, V.; Ajina, M.; Ben Ali, H.; Khorsi-Cauet, H.; Benkhalifa, M. Paternal Age Matters: Association with Sperm Criteria’s- Spermatozoa DNA Integrity and Methylation Profile. J. Clin. Med. 2023, 12, 4928. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.; Mann, T.; Sherins, R. Peroxidative breakdown of phospholipids in human spermatozoa, spermicidal properties of fatty acid peroxides, and protective action of seminal plasma. Fertil. Steril. 1979, 31, 531–537. [Google Scholar] [CrossRef]
- Aitken, R.J.; De Iuliis, G.N. On the possible origins of DNA damage in human spermatozoa. Mol. Human. Reprod. 2010, 16, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Kandeel, M.; Metwally, E.; Murtaza, G.; Kalhoro, D.H.; Yin, Y.; Tan, B.; Chughtai, M.I.; Yaseen, A.; Afzal, A.; et al. Unraveling the harmful effect of oxidative stress on male fertility: A mechanistic insight. Front. Endocrinol. 2023, 14, 1070692. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, A.; Argento, F.R.; Fini, E.; Coccia, M.E.; Taddei, N.; Becatti, M.; Fiorillo, C. The Impact of Oxidative Stress in Male Infertility. Front. Mol. Biosci. 2022, 8, 799294. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Virk, G.; Ong, C.; du Plessis, S.S. Effect of Oxidative Stress on Male Reproduction. World J. Mens. Health 2014, 32, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017, 84, 1039–1052. [Google Scholar] [CrossRef]
- Ahmadi, S.; Bashiri, R.; Ghadiri-Anari, A.; Nadjarzadeh, A. Antioxidant supplements and semen parameters: An evidence based review. Int. J. Reprod. Biomed. 2016, 14, 729–736. [Google Scholar] [CrossRef]
- Stenqvist, A.; Oleszczuk, K.; Leijonhufvud, I.; Giwercman, A. Impact of antioxidant treatment on DNA fragmentation index: A double-blind placebo-controlled randomized trial. Andrology 2018, 6, 811–816. [Google Scholar] [CrossRef]
- Agarwal, A.; Leisegang, K.; Majzoub, A.; Henkel, R.; Finelli, R.; Panner Selvam, M.K.; Tadros, N.; Parekh, N.; Ko, E.Y.; Cho, C.-L.; et al. Utility of Antioxidants in the Treatment of Male Infertility: Clinical Guidelines Based on a Systematic Review and Analysis of Evidence. World J. Mens. Health 2021, 39, 233–290. [Google Scholar] [CrossRef]
- Cilio, S.; Rienzo, M.; Villano, G.; Mirto, B.F.; Giampaglia, G.; Capone, F.; Ferretti, G.; Di Zazzo, E.; Crocetto, F. Beneficial Effects of Antioxidants in Male Infertility Management: A Narrative Review. Oxygen 2022, 2, 1–11. [Google Scholar] [CrossRef]
- Li, K.; Yang, X.; Wu, T. The Effect of Antioxidants on Sperm Quality Parameters and Pregnancy Rates for Idiopathic Male Infertility: A Network Meta-Analysis of Randomized Controlled Trials. Front. Endocrinol. 2022, 13, 810242. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, F.; Borgmann, H.; Struck, J.P.; Salem, J.; Kuru, T.H. Antioxidant Supplementation on Male Fertility—A Systematic Review. Antioxidants 2023, 12, 836. [Google Scholar] [CrossRef] [PubMed]
- Osman, R.; Lee, S.; Almubarak, A.; Han, J.-I.; Yu, I.-J.; Jeon, Y. Antioxidant Effects of Myo-Inositol Improve the Function and Fertility of Cryopreserved Boar Semen. Antioxidants 2023, 12, 1673. [Google Scholar] [CrossRef] [PubMed]
- Garolla, A.; Petre, G.C.; Francini-Pesenti, F.; De Toni, L.; Vitagliano, A.; Di Nisio, A.; Grande, G.; Foresta, C. Systematic Review and Critical Analysis on Dietary Supplements for Male Infertility: From a Blend of Ingredients to a Rationale Strategy. Front. Endocrinol. 2022, 12, 824078. [Google Scholar] [CrossRef]
- Scaruffi, P.; Licata, E.; Maccarini, E.; Massarotti, C.; Bovis, F.; Sozzi, F.; Stigliani, S.; Dal Lago, A.; Casciano, I.; Rago, R.; et al. Oral Antioxidant Treatment of Men Significantly Improves the Reproductive Outcome of IVF Cycles. J. Clin. Med. 2021, 10, 3254. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, G.; Cristina Magli, M.; Crippa, A.; Pia Ferraretti, A.; Gianaroli, L. Reduction in sperm aneuploidy levels in severe oligoasthenoteratospermic patients after medical therapy: A preliminary report. Asian J. Androl. 2012, 14, 591–598. [Google Scholar] [CrossRef]
- De Ligny, W.R.; Fleischer, K.; Grens, H.; Braat, D.D.M.; de Bruin, J.P. The lack of evidence behind over-the-counter antioxidant supplements for male fertility patients: A scoping review. Hum. Reprod. Open. 2023, 2023, hoad020. [Google Scholar] [CrossRef]
- Adeoye, O.; Olawumi, J.; Opeyemi, A.; Christiania, O. Review on the role of glutathione on oxidative stress and infertility. JBRA Assist. Reprod. 2018, 22, 61–66. [Google Scholar] [CrossRef]
- Chen, J.Q.; Li, Y.S.; Li, Z.J.; Lu, H.X.; Zhu, P.Q.; Li, C.M. Dietary l-arginine supplementation improves semen quality and libido of boars under high ambient temperature. Animal 2018, 12, 1611–1620. [Google Scholar] [CrossRef]
- Cheng, J.-B.; Zhu, J.; Ni, F.; Jiang, H. L-carnitine combined with coenzyme Q10 for idiopathic oligoasthenozoospermia: A double-blind randomized controlled trial. Zhonghua Nan Ke Xue 2018, 24, 33–38. [Google Scholar] [PubMed]
- Xu, Z.; Liu, M.; Niu, Q.-J.; Huang, Y.-X.; Zhao, L.; Lei, X.G.; Sun, L.-H. Both selenium deficiency and excess impair male reproductive system via inducing oxidative stress-activated PI3K/AKT-mediated apoptosis and cell proliferation signaling in testis of mice. Free. Radic. Biol. Med. 2023, 197, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zeng, Y.; Luo, Y.; He, J.; Luo, B.; Lu, X.; Zhu, L. Effects of folic acid and folic acid plus zinc supplements on the sperm characteristics and pregnancy outcomes of infertile men: A systematic review and meta-analysis. Heliyon 2023, 9, e18224. [Google Scholar] [CrossRef] [PubMed]
- Schisterman, E.F.; Sjaarda, L.A.; Clemons, T.; Carrell, D.T.; Perkins, N.J.; Johnstone, E.; Lamb, D.; Chaney, K.; Van Voorhis, B.J.; Ryan, G.; et al. Effect of Folic Acid and Zinc Supplementation in Men on Semen Quality and Live Birth Among Couples Undergoing Infertility Treatment. JAMA 2020, 323, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Conrad, M.; Moreno, S.G.; Sinowatz, F.; Ursini, F.; Kölle, S.; Roveri, A.; Brielmeier, M.; Wurst, W.; Maiorino, M.; Bornkamm, G.W. The Nuclear Form of Phospholipid Hydroperoxide Glutathione Peroxidase Is a Protein Thiol Peroxidase Contributing to Sperm Chromatin Stability. Mol. Cell. Biol. 2005, 25, 7637–7644. [Google Scholar] [CrossRef] [PubMed]
- Kacem, O.; Harzallah, M.; Zedini, C.; Zidi, I.; Meddeb, S.; Fékih, M.; Saidi, H.; Chaib, A.; Boughizane, S.; Ali, H.B.; et al. Beneficial Effect of an Oral Antioxidant Supplementation (Fertimax2) on IVF-ICSI Outcomes: A Preliminary Clinical Study. Adv. Reprod. Sci. 2014, 2, 47–56. [Google Scholar] [CrossRef]
- Balaban, B.; Brison, D.; Calderon, G.; Catt, J.; Conaghan, J.; Cowan, L.; Ebner, T.; Gardner, D.; Hardarson, T.; Lundin, K.; et al. Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology The Istanbul consensus workshop on embryo assessment: Proceedings of an expert meeting. Hum. Reprod. 2011, 26, 1270–1283. [Google Scholar] [CrossRef]
- Gardner, D.K.; Lane, M.; Stevens, J.; Schlenker, T.; Schoolcraft, W.B. Blastocyst score affects implantation and pregnancy outcome: Towards a single blastocyst transfer. Fertil. Steril. 2000, 73, 1155–1158. [Google Scholar] [CrossRef]
- Mustapha, H.; Lahimer, M.; Makni, M.; Bannour, I.; Kaabia, O.; Derouich, M.; Ferjaoui, M.A.; Arfaoui, R.; Zaouali, M.; Ajina, M. Effect of intrauterine administration of human chorionic gonadotropin one day before fresh blastocyst transfer on clinical outcomes: A quasi-experimental study. Pan Afr. Med. J. 2022, 42, 27. [Google Scholar] [CrossRef]
- Alahmar, A.T. Coenzyme Q10 improves sperm motility and antioxidant status in infertile men with idiopathic oligoasthenospermia. Clin. Exp. Reprod. Med. 2022, 49, 277–284. [Google Scholar] [CrossRef]
- Szymański, M.; Wandtke, T.; Wasilow, K.; Andryszczyk, M.; Janicki, R.; Domaracki, P. Comparison of 3- and 6-Month Outcomes of Combined Oral L-Carnitine Fumarate and Acetyl-L-Carnitine Therapy, Included in an Antioxidant Formulation, in Patients with Idiopathic Infertility. Am. J. Mens. Health 2021, 15, 15579883211036790. [Google Scholar] [CrossRef] [PubMed]
- Nateghian, Z.; Nasr-Esfahani, M.H.; Talaei-Khozani, T.; Tavalaee, M.; Aliabadi, E. L-Carnitine and Pentoxifylline Supplementation Improves Sperm Viability and Motility at Low Temperature. Int. J. Fertil. Steril. 2023, 17, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Micic, S.; Lalic, N.; Djordjevic, D.; Bojanic, N.; Bogavac-Stanojevic, N.; Busetto, G.M.; Virmani, A.; Agarwal, A. Double-blind, randomised, placebo-controlled trial on the effect of L-carnitine and L-acetylcarnitine on sperm parameters in men with idiopathic oligoasthenozoospermia. Andrologia 2019, 51, e13267. [Google Scholar] [CrossRef] [PubMed]
- Jannatifar, R.; Parivar, K.; Roodbari, N.H.; Nasr-Esfahani, M.H. Effects of N-acetyl-cysteine supplementation on sperm quality, chromatin integrity and level of oxidative stress in infertile men. Reprod. Biol. Endocrinol. 2019, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Jannatifar, R.; Asa, E.; Sahraei, S.S.; Verdi, A.; Piroozmanesh, H. N-acetyl-l-cysteine and alpha lipoic acid are protective supplement on human sperm parameters in cryopreservation of asthenoteratozoospermia patients. Andrologia 2022, 54, e14612. [Google Scholar] [CrossRef] [PubMed]
- Tunc, O.; Thompson, J.; Tremellen, K. Improvement in sperm DNA quality using an oral antioxidant therapy. Reprod. BioMedicine Online 2009, 18, 761–768. [Google Scholar] [CrossRef]
- Tremellen, K.; Miari, G.; Froiland, D.; Thompson, J. A randomised control trial examining the effect of an antioxidant (Menevit) on pregnancy outcome during IVF-ICSI treatment. Aust. N. Zeal. J. Obstet. Gynaecol. 2007, 47, 216–221. [Google Scholar] [CrossRef]
- Yaris, M.; Akdogan, N.; Öztürk, M.; Bozkurt, A.; Karabakan, M. The effects of two different antioxidant combinations on sperm parameters. Urologia 2022, 89, 629–635. [Google Scholar] [CrossRef]
- Lafuente, R.; González-Comadrán, M.; Solà, I.; López, G.; Brassesco, M.; Carreras, R.; Checa, M.A. Coenzyme Q10 and male infertility: A meta-analysis. J. Assist. Reprod. Genet. 2013, 30, 1147–1156. [Google Scholar] [CrossRef]
- Cassuto, N.G.; Piquemal, D.; Boitrelle, F.; Larue, L.; Lédée, N.; Hatem, G.; Ruoso, L.; Bouret, D.; Siffroi, J.-P.; Rouen, A.; et al. Molecular Profiling of Spermatozoa Reveals Correlations between Morphology and Gene Expression: A Novel Biomarker Panel for Male Infertility. BioMed Res. Int. 2021, 2021, e1434546. [Google Scholar] [CrossRef]
- Moretti, E.; Signorini, C.; Noto, D.; Corsaro, R.; Collodel, G. The relevance of sperm morphology in male infertility. Front. Reprod. Health 2022, 4, 945351. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Maynou, J.; Novo, S.; Torres, M.; Salas-Huetos, A.; Rovira, S.; Antich, M.; Yeste, M. Sperm DNA integrity does play a crucial role for embryo development after ICSI, notably when good-quality oocytes from young donors are used. Biol. Res. 2022, 55, 41. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.G.; Noonan, E.; von Eckardstein, S.; Auger, J.; Baker, H.W.G.; Behre, H.M.; Haugen, T.B.; Kruger, T.; Wang, C.; Mbizvo, M.T.; et al. World Health Organization reference values for human semen characteristics. Human. Reprod. Update 2010, 16, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2005, 3, 28. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.-L.; Henkel, R.; Vij, S.; Arafa, M.; Panner Selvam, M.K.; Shah, R. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Menezo, Y.; Clement, P.; Amar, E. Evaluation of sperm DNA structure, fragmentation and decondensation: An essential tool in the assessment of male infertility. Transl. Androl. Urol. 2017, 6, S553–S556. [Google Scholar] [CrossRef] [PubMed]
- Delbarba, A.; Arrighi, N.; Facondo, P.; Cappelli, C.; Ferlin, A. Positive effect of nutraceuticals on sperm DNA damage in selected infertile patients with idiopathic high sperm DNA fragmentation. Minerva Endocrinol. 2020, 45, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Marinaro, J.A.; Schlegel, P.N. Sperm DNA Damage and Its Relevance in Fertility Treatment: A Review of Recent Literature and Current Practice Guidelines. Int. J. Mol. Sci. 2023, 24, 1446. [Google Scholar] [CrossRef]
- Robinson, L.; Gallos, I.D.; Conner, S.J.; Rajkhowa, M.; Miller, D.; Lewis, S.; Kirkman-Brown, J.; Coomarasamy, A. The effect of sperm DNA fragmentation on miscarriage rates: A systematic review and meta-analysis. Human. Reprod. 2012, 27, 2908–2917. [Google Scholar] [CrossRef]
- Smith, G.D.; Takayama, S.; Swain, J.E. Rethinking In Vitro Embryo Culture: New Developments in Culture Platforms and Potential to Improve Assisted Reproductive Technologies. Biol. Reprod. 2012, 86, 62. [Google Scholar] [CrossRef]
- Zarbakhsh, S. Effect of antioxidants on preimplantation embryo development in vitro: A review. Zygote 2021, 29, 179–193. [Google Scholar] [CrossRef]
- Łakoma, K.; Kukharuk, O.; Śliż, D. The Influence of Metabolic Factors and Diet on Fertility. Nutrients 2023, 15, 1180. [Google Scholar] [CrossRef]
- Wyck, S.; Herrera, C.; Requena, C.E.; Bittner, L.; Hajkova, P.; Bollwein, H.; Santoro, R. Oxidative stress in sperm affects the epigenetic reprogramming in early embryonic development. Epigenetics Chromatin 2018, 11, 60. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
The trial included patients who meet all of the following criteria:
| Patients with at least one of the following criteria were not included in the trial:
|
| L-Carnitine | 220 mg |
| L-arginine | 125 mg |
| Vitamin E | 60 mg |
| L-glutathione | 40 mg |
| Zinc | 20 mg |
| Coenzyme Q10 | 7.5 mg |
| Folic acid (vitB-9) | 0.4 mg |
| Selenium | 0.03 mg |
| Fertilis (n = 131) | Placebo (n = 132) | p-Value | |||
|---|---|---|---|---|---|
| Sociodemographic characteristics (Male partner), % (n) | BMI male partner kg/m2 | 26.14 ± 4.2 | 26.27 ± 3.9 | ||
| Diabetes (%) | yes | 2.3 (3) | 3.8 (5) | 0.4 | |
| no | 97.7 (128) | 96.2 (127) | |||
| HTA (%) | yes | 3.1 (4) | 1.5 (2) | 0.4 | |
| no | 96.9 (127) | 98.5 (130) | |||
| Smoking (%) | yes | 44.9 (59) | 57.3 (76) | 0.04 | |
| no | 55.1 (72) | 42.7 (56) | |||
| Alcohol (%) | yes | 11.6 (15) | 19.1 (25) | 0.09 | |
| no | 88.4 (116) | 80.9 (107) | |||
| Sociodemographic characteristics (Female partner), % (n) | BMI female partner kg/m2 | 26.18 ± 4.5 | 26.15 ± 4.2 | ||
| Diabetes (%) | yes | 2.3 (3) | 1.5 (2) | 0.1 | |
| no | 97.7 (128) | 98.5 (130) | |||
| HTA (%) | yes | 0 | 0 | - | |
| no | 100 | 100 | |||
| Smoking (%) | yes | 0.8 (1) | 0.8 (1) | 0.9 | |
| no | 99.2 (130) | 99.2 (131) | |||
| Alcohol (%) | yes | 0 | 0 | - | |
| no | 100 | 100 | |||
| Male infertility, % (n) | Male urogenital pathology (%) | 0 | 0 | ||
| Acquired testicular pathology (%) | 0.8 (1) | 0.8 (1) | 0.7 | ||
| Auto-antibody (%) | 6.1 (8) | 9.8 (13) | 0.1 | ||
| Female infertility, % (n) | Right fallopian tube (%): Normal Obstructed/absent/ligated Not defined | 94.7 (124) | 92.4 (122) | 0.7 | |
| 3.8 (5) | 6.1 (8) | 0.7 | |||
| 1.5 (2) | 1.5 (2) | 0.5 | |||
| Left fallopian tube (%): Normal Obstructed/absent/ligated Not defined | 91.6 (120) | 96.2 (127) | 0.3 | ||
| 6.1 (8) | 2.3 (3) | 0.2 | |||
| 1.5 (2) | 1.5 (2) | 0.2 | |||
| Anovulation/dysovulation (%) | 0 | 0 | |||
| Ovarian reserve anomaly (%) | 0.8 (1) | 0.8 (1) | 0.7 | ||
| Endometriosis (%) | 0.8 (1) | 0 | 0.4 | ||
| Cone biopsy (%) | 0 | 0 | |||
| Uterine pathology (%) | 0 | 0.8 (1) | 0.5 | ||
| Cervical pathology (%) | 0.8 (1) | 0 | 0.4 | ||
| Stenosis (%) | 0 | 0 | |||
| Other pathology (%) | 0.8 | 0 | 0.4 | ||
| Infertility type, % (n) | Primary | 71.7 (94) | 82.2 (109) | 0.04 | |
| secondary | 28.3 (37) | 17.8 (23) | |||
| Fertilis (n = 131) | Placebo (n = 132) | p-Value | |
|---|---|---|---|
| Volume (mL) | T0 = 2.4 ± 1.5 TF = 2.8 ± 1.7 p-value = 0.6 | T0 = 2.5 ± 1.5 TF = 2.8 ± 1.7 p-value = 0.5 | 0.7 0.5 |
| Sperm count (106 spz/mL) * | T0 = 44.6 ± 42.2 TF = 45.9 ± 38.2 p-value = 0.9 | T0 = 53.2 ± 44.48 TF = 52.25 ± 45.58 p-value = 0.3 | 0.4 0.9 |
| Sperm motility (%) | T0 = 20.74 ± 13.7 TF = 22.5 ± 15.35 p-value = 0.02 | T0 = 22.9 ± 12.5 TF = 24.6 ± 12.91 p-value = 0.1 | 0.9 0.3 |
| Sperm vitality (%) | T0 = 65.45 ± 16.83 TF = 69.6 ± 15.65 p-value = 0.7 | T0 = 65.93 ± 16.37 TF = 70.25 ± 13.98 p-value = 0.9 | 0.8 0.7 |
| DFI (%) | T0 = 20 TF = 14.5 p-value = 0.01 | T0 = 18 TF = 13 p-value < 0.05 | 0.9 0.2 |
| SDI (%) | T0 = 50 TF = 44 p-value = 0.4 | T0 = 43 TF = 52.5 p-value = 0.3 | 0.2 0.6 |
| Fertilis | Placebo | p-Value | |
|---|---|---|---|
| BMI female partner kg/m2 | 26.18 ± 4.5 | 26.15 ± 4.2 | |
| FSH (UI/I) | 7.5 ± 3.5 | 7.74 ± 3.9 | 0.6 |
| LH (UI/I) | 4.5 ± 3.09 | 5.01 ± 2.3 | 0.3 |
| E2 (pg/mL) | 48.11 ± 36.9 | 49.05 ± 24.83 | 0.8 |
| PRL (ng/mL) | 18.43 ± 25.02 | 20.82 ± 29.27 | 0.5 |
| AMH (ng/mL) | 2.35 ± 3.1 | 2.53 ± 2.4 | 0.6 |
| TSH (mU/I) | 2.36 ± 1.6 | 2.11 ± 1.1 | 0.2 |
| ICSI/IVF Outcome | ICSI (n = 114) | FIV (n = 31) | ||||
|---|---|---|---|---|---|---|
| Fertilis (n = 57) | Placebo (n = 57) | p-Value | Fertilis (n = 18) | Placebo (n = 13) | p-Value | |
| No. of retrieved *COC | 3.7 ± 2.9 | 3.9 ± 3.5 | 0.9 | 3.5 ± 2.8 | 2.4 ± 2.2 | 0.2 |
| No. of mature Oocytes | 2.7 ± 2.2 | 3 ± 2.9 | 0.9 | 2.4 ± 1.9 | 2 ± 2.3 | 0.3 |
| Inseminated oocytes (*IVF) | 0 | 0 | - | 2.6 ± 1.9 | 2.5 ± 2.4 | 0.7 |
| Microinjected oocytes (*ICSI) | 2.7 ± 2.3 | 2.9 ± 2.8 | 0.9 | 0 | 0 | - |
| Zygotes 2 PN | 1.7 ± 1.8 | 1.8 ± 2.2 | 0.9 | 1.8 ± 1.9 | 1.7 ± 2.1 | 0.7 |
| Total embryos | 1.8 ± 1.8 | 1.8 ± 1.9 | 0.9 | 1.6 ± 1.7 | 1.7 ± 1.8 | 0.9 |
| Transferred embryos | 2 ± 0.9 | 2 ± 1 | 0.8 | 2 ± 0.6 | 2 ± 1 | 0.9 |
| Maturation rate (%) | 67.8 ± 38.9 | 57.57 ± 35.8 | 0.2 | 68.8 ± 48.5 | 71.2 ± 39.1 | 0.5 |
| Fertilisation rate (%) | 48.13 ± 45.5 | 38.9 ± 33.7 | 0.4 | 48.5 ± 42.4 | 48.2 ± 41.1 | 0.9 |
| Cleavage rate (%) | 54.1 ± 49.9 | 44.4 ± 49 | 0.3 | 57.1 ± 46.5 | 57.9 ± 46.2 | 0.9 |
| Blastocyst rate (%) | 15.1 ± 63.3 | 10.5 ± 31.5 | 0.6 | 13.1 ± 34 | 11.9 ± 32.7 | 0.7 |
| Fertilis (n = 131) | Placebo (n = 132) | p-Value | |
|---|---|---|---|
| Biochemical pregnancy α (%) | 22.9 (30/131) | 16.7 (22/132) | 0.1 |
| Clinical pregnancy α (%) | 19.8 (26/131) | 11.4 (15/132) | 0.04 |
| Pregnancy rate α (%) | 20.6 (27/131) | 9.8 (13/132) | 0.01 |
| Live birth α (%) | 13 (17/131) | 5.3 (7/132) | 0.031 |
| No. Twins | 2 | 0 |
| Pregnancy | p-Value | ||
|---|---|---|---|
| YES (n = 40) | NO (n = 223) | ||
| No. of retrieved COC | 4.8 ± 3.04 | 3.4 ± 3.07 | 0.08 |
| No. of mature Oocytes | 4.8 ± 2.6 | 2.7 ± 2.5 | 0.04 |
| Zygotes 2 PN | 3.13 ± 1.7 | 1.47 ± 2.07 | 0.004 |
| Total embryos | 2.5 ± 1.06 | 1.29 ± 1.8 | 0.01 |
| Blastocyst | 1.13 ± 1.8 | 0.73 ± 1.9 | 0.4 |
| Maturation rate | 88.44 ± 15.46 | 65.8 ± 40.02 | 0.03 |
| Fertilisation rate | 86.04 ± 21.91 | 42.41 ± 42.76 | <0.0001 |
| Cleavage rate | 89.44 ± 23.24 | 50.24 ± 48.11 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lahimer, M.; Gherissi, O.; Ben Salem, N.; Ben Mustapha, H.; Bach, V.; Khorsi-Cauet, H.; Khairi, H.; Ben Ali, H.; BenKhalifa, M.; Ajina, M. Effect of Micronutrients and L-Carnitine as Antioxidant on Sperm Parameters, Genome Integrity, and ICSI Outcomes: Randomized, Double-Blind, and Placebo-Controlled Clinical Trial. Antioxidants 2023, 12, 1937. https://doi.org/10.3390/antiox12111937
Lahimer M, Gherissi O, Ben Salem N, Ben Mustapha H, Bach V, Khorsi-Cauet H, Khairi H, Ben Ali H, BenKhalifa M, Ajina M. Effect of Micronutrients and L-Carnitine as Antioxidant on Sperm Parameters, Genome Integrity, and ICSI Outcomes: Randomized, Double-Blind, and Placebo-Controlled Clinical Trial. Antioxidants. 2023; 12(11):1937. https://doi.org/10.3390/antiox12111937
Chicago/Turabian StyleLahimer, Marwa, Oumaima Gherissi, Nesrine Ben Salem, Henda Ben Mustapha, Véronique Bach, Hafida Khorsi-Cauet, Hedi Khairi, Habib Ben Ali, Moncef BenKhalifa, and Mounir Ajina. 2023. "Effect of Micronutrients and L-Carnitine as Antioxidant on Sperm Parameters, Genome Integrity, and ICSI Outcomes: Randomized, Double-Blind, and Placebo-Controlled Clinical Trial" Antioxidants 12, no. 11: 1937. https://doi.org/10.3390/antiox12111937
APA StyleLahimer, M., Gherissi, O., Ben Salem, N., Ben Mustapha, H., Bach, V., Khorsi-Cauet, H., Khairi, H., Ben Ali, H., BenKhalifa, M., & Ajina, M. (2023). Effect of Micronutrients and L-Carnitine as Antioxidant on Sperm Parameters, Genome Integrity, and ICSI Outcomes: Randomized, Double-Blind, and Placebo-Controlled Clinical Trial. Antioxidants, 12(11), 1937. https://doi.org/10.3390/antiox12111937






