The Anti-Oxidant Curcumin Solubilized as Oil-in-Water Nanoemulsions or Chitosan Nanocapsules Effectively Reduces Helicobacter pylori Growth, Bacterial Biofilm Formation, Gastric Cell Adhesion and Internalization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Nanocarriers
2.1.2. Scanning Transmission Electron Microscopy (STEM) Images
2.1.3. Cell Studies
2.2. H. pylori Culture
2.3. Cell Culture
2.4. Nanoformulation Preparation Protocols
2.5. Physicochemical Characterization of Nanoformulations
2.6. Antioxidant Capacity Measurement
2.7. Minimal Inhibitory Concentration Assay
2.8. MTS Viability Assay
2.9. Trypan Blue Viability Assay
2.10. Bacterial Internalization Assay
2.11. Biofilm Formation Assay
2.12. Biofilm Formation Assays Determined via Counting Colony-Forming Units
2.13. Statistical Analysis
3. Results
3.1. Nanoformulations Containing Curcumin Are Stable and Show Low Polydispersion Indices
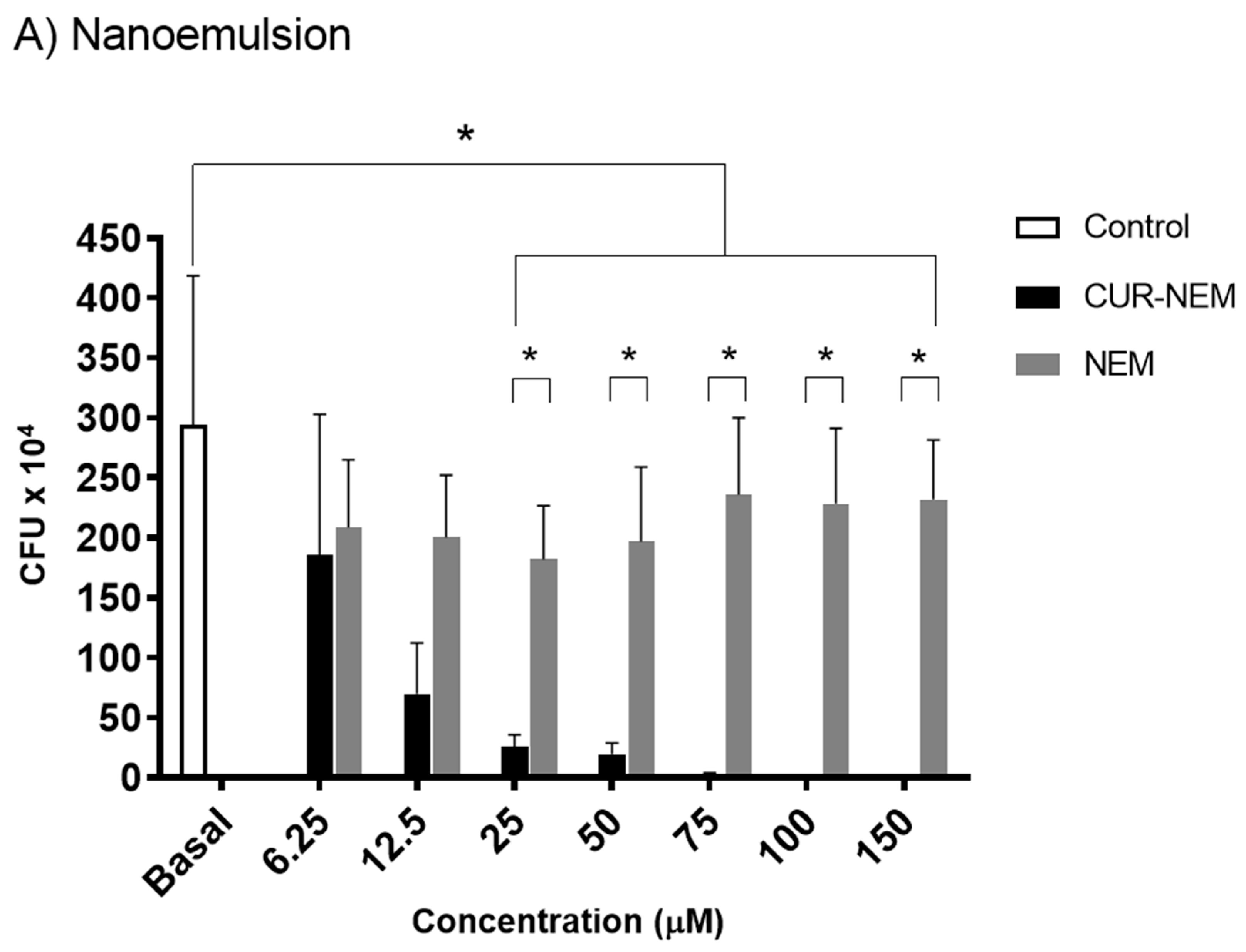
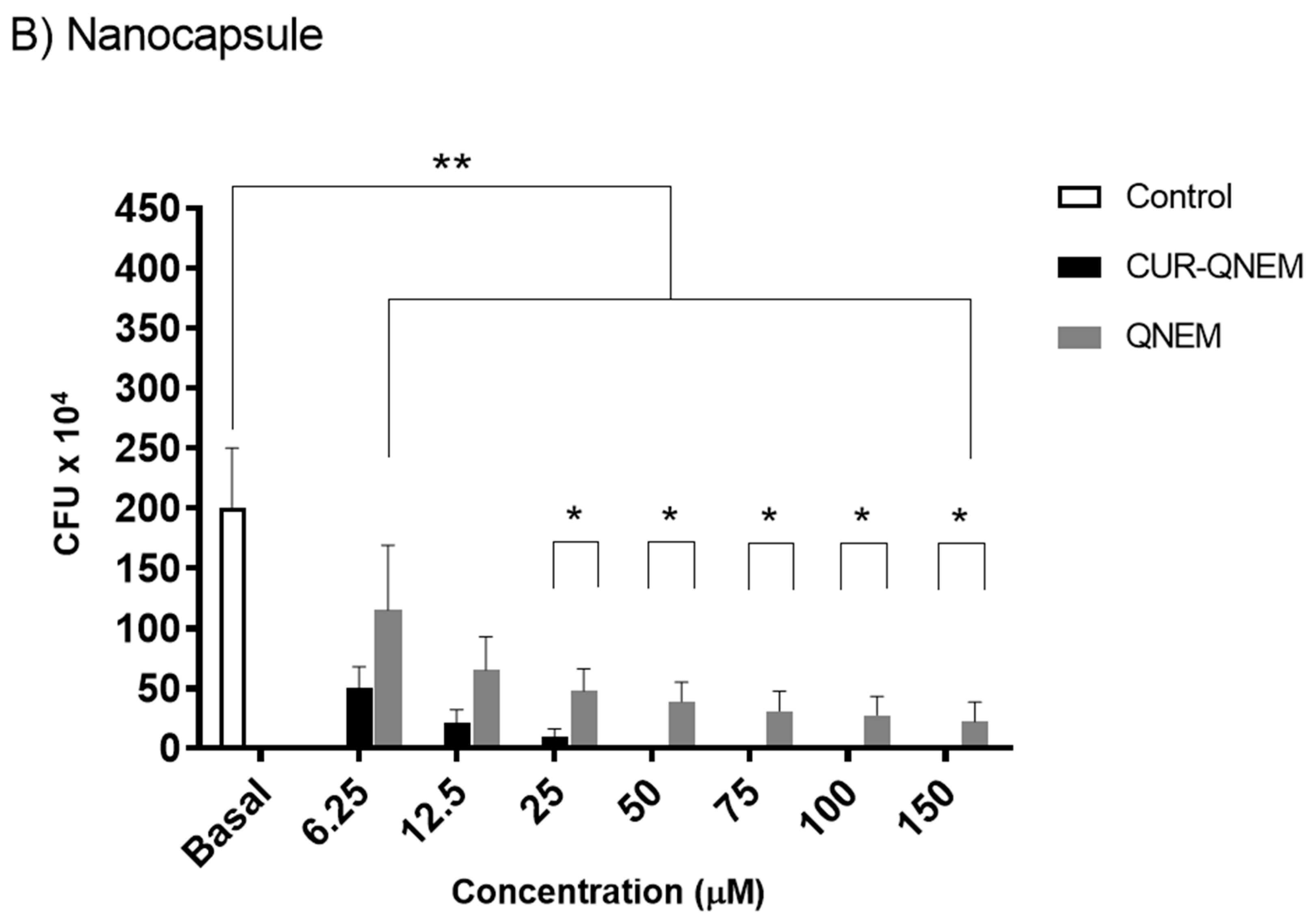
3.2. Nanoemulsions and Nanocapsules Containing Curcumin Inhibit the Growth of H. pylori 26695
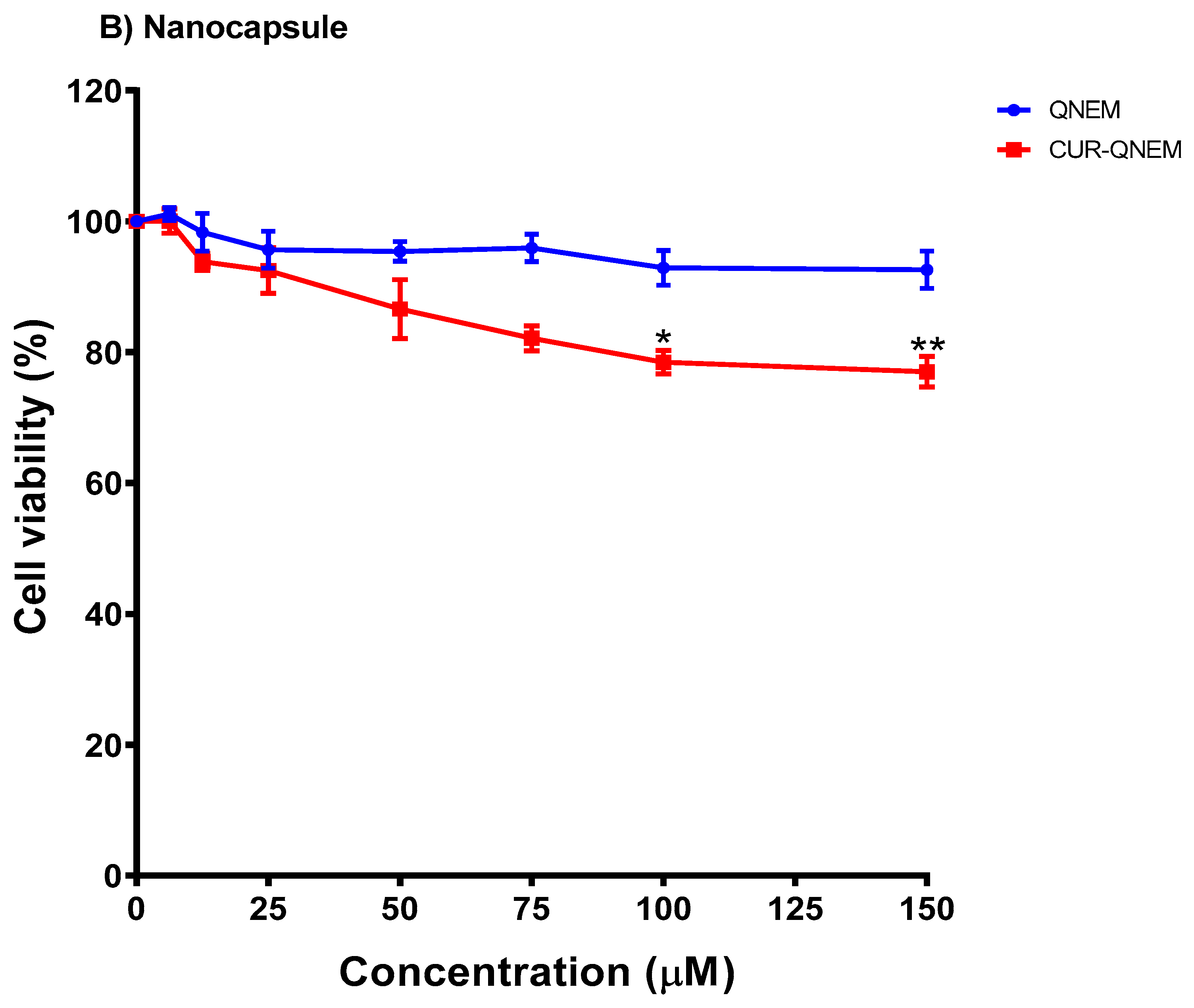
3.2.1. Nanoemulsions but Not Nanocapsules Containing Curcumin Reduce GES-1 Cell Viability in a Dose-Dependent Manner
3.2.2. Nanoformulations Containing Curcumin Diminish the Adherence of H. pylori to GES-1 Cells as Well as Internalization of the Bacteria
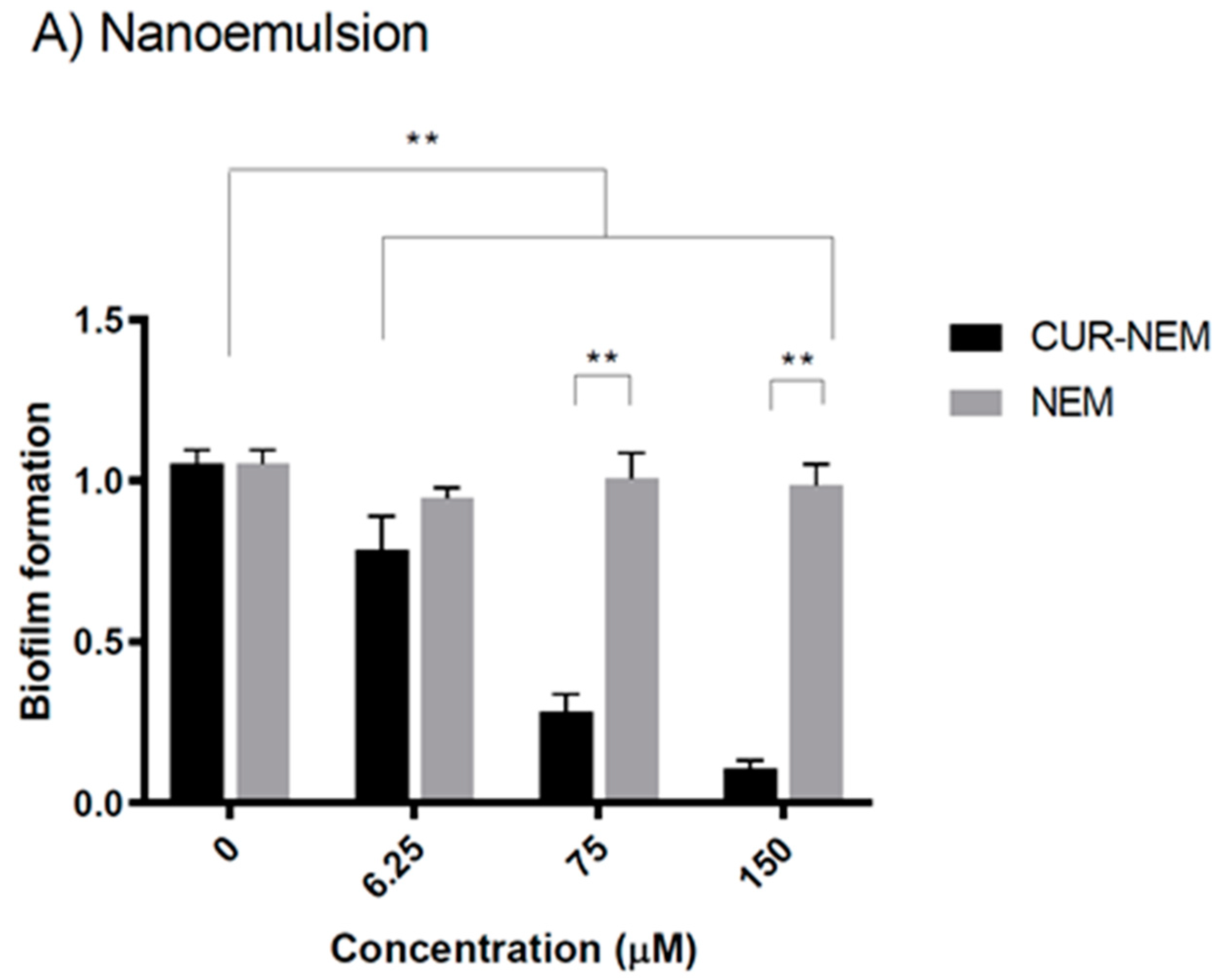
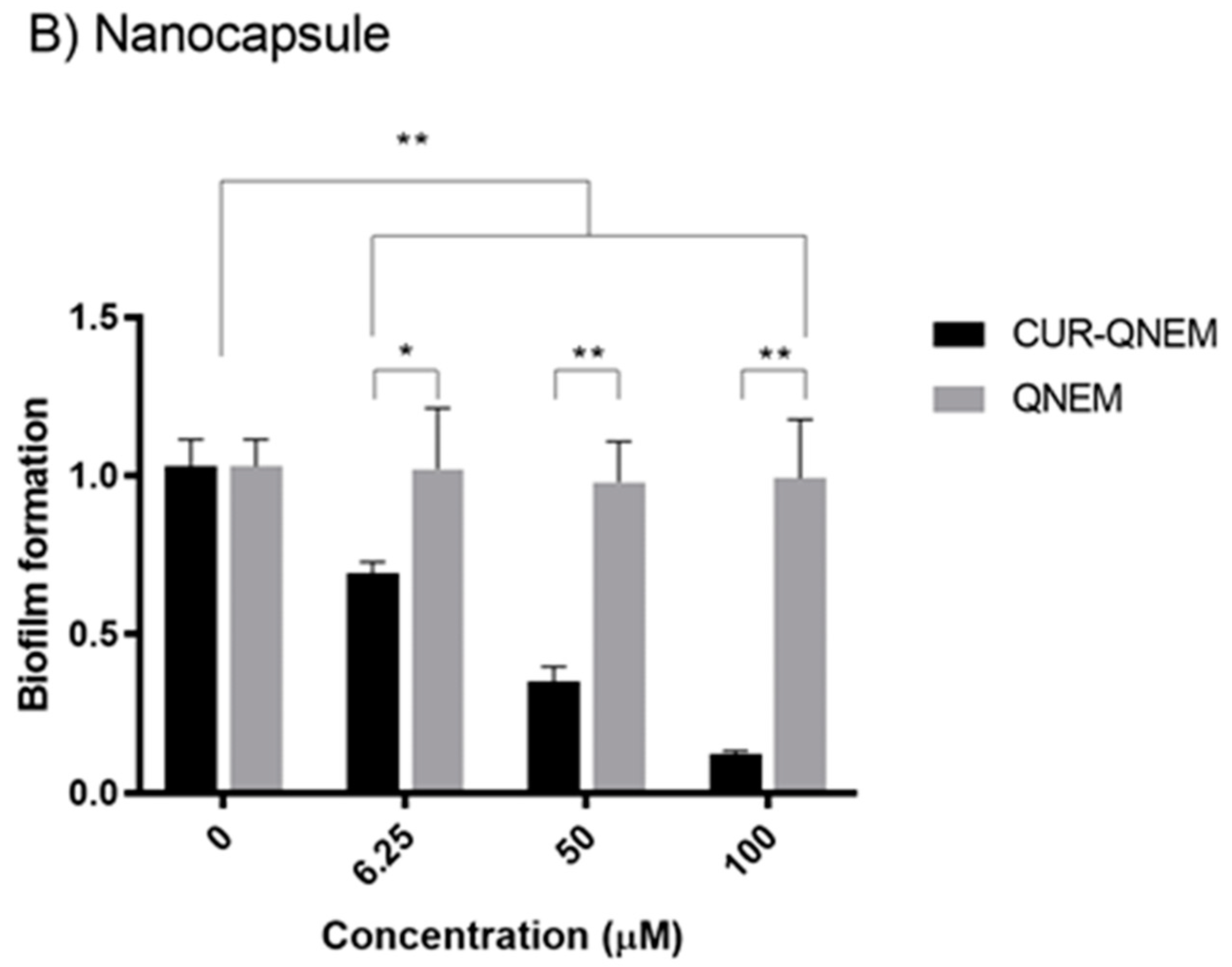
3.2.3. The Nanoformulations Reduce Biofilm Formation by H. pylori
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Collaborators, G.B.D.R.F. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar] [CrossRef]
- Sasazuki, S.; Inoue, M.; Iwasaki, M.; Otani, T.; Yamamoto, S.; Ikeda, S.; Hanaoka, T.; Tsugane, S.; Japan Public Health Center Study, G. Effect of Helicobacter pylori infection combined with CagA and pepsinogen status on gastric cancer development among Japanese men and women: A nested case-control study. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1341–1347. [Google Scholar] [CrossRef]
- Calvet, X.; Ramirez Lazaro, M.J.; Lehours, P.; Megraud, F. Diagnosis and epidemiology of Helicobacter pylori infection. Helicobacter 2013, 18 (Suppl. S1), 5–11. [Google Scholar] [CrossRef]
- Camilo, V.; Sugiyama, T.; Touati, E. Pathogenesis of Helicobacter pylori infection. Helicobacter 2017, 22 (Suppl. S1), e12405. [Google Scholar] [CrossRef] [PubMed]
- Cover, T.L.; Lacy, D.B.; Ohi, M.D. The Helicobacter pylori Cag Type IV Secretion System. Trends Microbiol. 2020, 28, 682–695. [Google Scholar] [CrossRef]
- Sit, W.Y.; Chen, Y.A.; Chen, Y.L.; Lai, C.H.; Wang, W.C. Cellular evasion strategies of Helicobacter pylori in regulating its intracellular fate. Semin. Cell Dev. Biol. 2020, 101, 59–67. [Google Scholar] [CrossRef]
- Zeng, B.; Chen, C.; Yi, Q.; Zhang, X.; Wu, X.; Zheng, S.; Li, N.; She, F. N-terminal region of Helicobacter pylori CagA induces IL-8 production in gastric epithelial cells via the beta1 integrin receptor. J. Med. Microbiol. 2020, 69, 457–464. [Google Scholar] [CrossRef]
- Hathroubi, S.; Servetas, S.L.; Windham, I.; Merrell, D.S.; Ottemann, K.M. Helicobacter pylori Biofilm Formation and Its Potential Role in Pathogenesis. Microbiol. Mol. Biol. Rev. 2018, 82, e00001-18. [Google Scholar] [CrossRef]
- Coticchia, J.M.; Sugawa, C.; Tran, V.R.; Gurrola, J.; Kowalski, E.; Carron, M.A. Presence and density of Helicobacter pylori biofilms in human gastric mucosa in patients with peptic ulcer disease. J. Gastrointest. Surg. 2006, 10, 883–889. [Google Scholar] [CrossRef]
- De Francesco, V.; Ridola, L.; Hassan, C.; Bellesia, A.; Alvaro, D.; Vaira, D.; Zullo, A. Two-week Triple Therapy with either Standard or High-dose Esomeprazole for First-line H. pylori Eradication. J. Gastrointestin Liver Dis. 2016, 25, 147–150. [Google Scholar] [CrossRef]
- Puig, I.; Baylina, M.; Sanchez-Delgado, J.; Lopez-Gongora, S.; Suarez, D.; Garcia-Iglesias, P.; Munoz, N.; Gisbert, J.P.; Dacoll, C.; Cohen, H.; et al. Systematic review and meta-analysis: Triple therapy combining a proton-pump inhibitor, amoxicillin and metronidazole for Helicobacter pylori first-line treatment. J. Antimicrob. Chemother. 2016, 71, 2740–2753. [Google Scholar] [CrossRef]
- Frenck, R.W., Jr.; Clemens, J. Helicobacter in the developing world. Microbes Infect. 2003, 5, 705–713. [Google Scholar] [CrossRef]
- Sjomina, O.; Pavlova, J.; Niv, Y.; Leja, M. Epidemiology of Helicobacter pylori infection. Helicobacter 2018, 23 (Suppl. S1), e12514. [Google Scholar] [CrossRef]
- Dey, S.K.; De, P.K.; De, A.; Ojha, S.; De, R.; Mukhopadhyay, A.K.; Samanta, A. Floating mucoadhesive alginate beads of amoxicillin trihydrate: A facile approach for H. pylori eradication. Int. J. Biol. Macromol. 2016, 89, 622–631. [Google Scholar] [CrossRef]
- Chang, C.H.; Huang, W.Y.; Lai, C.H.; Hsu, Y.M.; Yao, Y.H.; Chen, T.Y.; Wu, J.Y.; Peng, S.F.; Lin, Y.H. Development of novel nanoparticles shelled with heparin for berberine delivery to treat Helicobacter pylori. Acta Biomater. 2011, 7, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Bhawana; Basniwal, R.K.; Buttar, H.S.; Jain, V.K.; Jain, N. Curcumin nanoparticles: Preparation, characterization, and antimicrobial study. J. Agric. Food Chem. 2011, 59, 2056–2061. [Google Scholar] [CrossRef] [PubMed]
- Mahady, G.B.; Pendland, S.L.; Yun, G.; Lu, Z.Z. Turmeric (Curcuma longa) and curcumin inhibit the growth of Helicobacter pylori, a group 1 carcinogen. Anticancer Res. 2002, 22, 4179–4181. [Google Scholar] [PubMed]
- Panchatcharam, M.; Miriyala, S.; Gayathri, V.S.; Suguna, L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol. Cell Biochem. 2006, 290, 87–96. [Google Scholar] [CrossRef]
- Kawamori, T.; Lubet, R.; Steele, V.E.; Kelloff, G.J.; Kaskey, R.B.; Rao, C.V.; Reddy, B.S. Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res. 1999, 59, 597–601. [Google Scholar]
- Aggarwal, B.B.; Surh, Y.-J.; Shishodia, S. The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer: New York, NY, USA, 2007; 21 Chapters; 489p. [Google Scholar]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Hoehle, S.I.; Pfeiffer, E.; Solyom, A.M.; Metzler, M. Metabolism of curcuminoids in tissue slices and subcellular fractions from rat liver. J. Agric. Food Chem. 2006, 54, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Tonnesen, H.H.; Karlsen, J.; van Henegouwen, G.B. Studies on curcumin and curcuminoids. VIII. Photochemical stability of curcumin. Z. Lebensm. Unters. Forsch. 1986, 183, 116–122. [Google Scholar] [CrossRef]
- Tabanelli, R.; Brogi, S.; Calderone, V. Improving Curcumin Bioavailability: Current Strategies and Future Perspectives. Pharmaceutics 2021, 13, 1715. [Google Scholar] [CrossRef]
- de Almeida, M.; da Rocha, B.A.; Francisco, C.R.L.; Miranda, C.G.; Santos, P.D.F.; de Araujo, P.H.H.; Sayer, C.; Leimann, F.V.; Goncalves, O.H.; Bersani-Amado, C.A. Evaluation of the in vivo acute antiinflammatory response of curcumin-loaded nanoparticles. Food Funct. 2018, 9, 440–449. [Google Scholar] [CrossRef]
- Abdel-Mageid, A.D.; Abou-Salem, M.E.S.; Salaam, N.; El-Garhy, H.A.S. The potential effect of garlic extract and curcumin nanoparticles against complication accompanied with experimentally induced diabetes in rats. Phytomedicine 2018, 43, 126–134. [Google Scholar] [CrossRef]
- Chuah, L.H.; Roberts, C.J.; Billa, N.; Abdullah, S.; Rosli, R. Cellular uptake and anticancer effects of mucoadhesive curcumin-containing chitosan nanoparticles. Colloids Surf. B Biointerfaces 2014, 116, 228–236. [Google Scholar] [CrossRef]
- Mirnejad, R.; Jahromi, M.; Ali, M.; Al-Musawi, S.; Pirestani, M.; Fasihi Ramandi, M.; Ahmadi, K.; Rajayi, H.; Mohammad Hassan, Z.; Kamali, M. Curcumin-loaded Chitosan Tripolyphosphate Nanoparticles as a safe, natural and effective antibiotic inhibits the infection of Staphylococcus aureus and Pseudomonas aeruginosa in vivo. Iran. J. Biotechnol. 2014, 12, 1–8. [Google Scholar]
- De, R.; Kundu, P.; Swarnakar, S.; Ramamurthy, T.; Chowdhury, A.; Nair, G.B.; Mukhopadhyay, A.K. Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob. Agents Chemother. 2009, 53, 1592–1597. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vetvickova, J.; Fernandez-Botran, R. Effects of curcumin on Helicobacter pylori infection. Ann. Transl. Med. 2016, 4, 479. [Google Scholar] [CrossRef] [PubMed]
- Pattiyathanee, P.; Vilaichone, R.-K.; Chaichanawongsaroj, N. Effect of curcumin on Helicobacter pylori biofilm formation. Afr. J. Biotechnol. 2009, 8, 5106–5115. [Google Scholar]
- Guerrero, S.; Inostroza-Riquelme, M.; Contreras-Orellana, P.; Diaz-Garcia, V.; Lara, P.; Vivanco-Palma, A.; Cardenas, A.; Miranda, V.; Robert, P.; Leyton, L.; et al. Curcumin-loaded nanoemulsion: A new safe and effective formulation to prevent tumor reincidence and metastasis. Nanoscale 2018, 10, 22612–22622. [Google Scholar] [CrossRef] [PubMed]
- Soto, C.; Rojas, V.; Yanez, L.; Hidalgo, A.; Olivera, M.; Pacheco, M.; Venegas, D.; Salinas, D.; Bravo, D.; Quest, A.F.G. Porphyromonas gingivalis-Helicobacter pylori co-incubation enhances Porphyromonas gingivalis virulence and increases migration of infected human oral keratinocytes. J. Oral Microbiol. 2022, 14, 2107691. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.F.; Burgos-Ravanal, R.; Shao, B.; Heinecke, J.; Valenzuela-Valderrama, M.; Corvalan, A.H.; Quest, A.F.G. Extracellular vesicles from gastric epithelial GES-1 cells infected with Helicobacter pylori promote changes in recipient cells associated with malignancy. Front. Oncol. 2022, 12, 962920. [Google Scholar] [CrossRef]
- Alarcón-Alarcón, C.; Inostroza-Riquelme, M.; Torres-Gallegos, C.; Araya, C.; Miranda, M.; Sánchez-Caamaño, J.C.; Moreno-Villoslada, I.; Oyarzun-Ampuero, F.A. Protection of astaxanthin from photodegradation by its inclusion in hierarchically assembled nano and microstructures with potential as food. Food Hydrocoll. 2018, 83, 36–44. [Google Scholar] [CrossRef]
- Villamizar-Sarmiento, M.G.; Guerrero, J.; Moreno-Villoslada, I.; Oyarzun-Ampuero, F.A. The key role of the drug self-aggregation ability to obtain optimal nanocarriers based on aromatic-aromatic drug-polymer interactions. Eur. J. Pharm. Biopharm. 2021, 166, 19–29. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Bravo, J.; Diaz, P.; Corvalan, A.H.; Quest, A.F.G. A Novel Role for Helicobacter pylori Gamma-Glutamyltranspeptidase in Regulating Autophagy and Bacterial Internalization in Human Gastric Cells. Cancers 2019, 11, 801. [Google Scholar] [CrossRef]
- Oyarzun-Ampuero, F.A.; Rivera-Rodriguez, G.R.; Alonso, M.J.; Torres, D. Hyaluronan nanocapsules as a new vehicle for intracellular drug delivery. Eur. J. Pharm. Sci. 2013, 49, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Rodriguez, G.R.; Lollo, G.; Montier, T.; Benoit, J.P.; Passirani, C.; Alonso, M.J.; Torres, D. In vivo evaluation of poly-l-asparagine nanocapsules as carriers for anti-cancer drug delivery. Int. J. Pharm. 2013, 458, 83–89. [Google Scholar] [CrossRef][Green Version]
- Venkatesan, N.; Perumal, G.; Doble, M. Bacterial resistance in biofilm-associated bacteria. Future Microbiol. 2015, 10, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed. Res. Int. 2014, 2014, 186864. [Google Scholar] [CrossRef]
- Farjam, M.H.; Joukar, M.; Ranjbar, F. Antimicrobial, antifungal and antioxidant activity evaluation of various organic solvent extracts of cleome turkmena Bobrov. Adv. Environ. Biol. 2014, 8, 152–155. [Google Scholar]
- Ungphaiboon, S.; Supavita, T.; Singchangchai, P.; Sungkarak, S.; Rattanasuwan, P.; Itharat, A. Study on antioxidant and antimicrobial activities of turmeric clear liquid soap for wound treatment of HIV patients. Songklanakarin J. Sci. Technol. 2005, 27, 569–578. [Google Scholar]
- Liu, Y.; Ma, M.; Yuan, Y. The potential of curcumin-based co-delivery systems for applications in the food industry: Food preservation, freshness monitoring, and functional food. Food Res. Int. 2023, 171, 113070. [Google Scholar] [CrossRef]
- Choi, I.; Li, N.; Zhong, Q. Co-loading curcumin and quercetin in freeze-dried mushroom microparticles to inhibit lipid oxidation in beef patties. Food Chem. 2022, 374, 131625. [Google Scholar] [CrossRef]
- Feng, F.; Gao, J.; Chen, L.; Xiao, S.; Huang, G. Scalable fabrication of uniform nanoparticles for the efficient co-encapsulation of curcumin and procyanidins driven by multiple interactions. Food Hydrocoll. 2023, 144, 108960. [Google Scholar] [CrossRef]
- Nagy, A.; Harrison, A.; Sabbani, S.; Munson, R.S., Jr.; Dutta, P.K.; Waldman, W.J. Silver nanoparticles embedded in zeolite membranes: Release of silver ions and mechanism of antibacterial action. Int. J. Nanomed. 2011, 6, 1833–1852. [Google Scholar] [CrossRef]
- Divya, K.; Vijayan, S.; George, T.K.; Jisha, M.S. Antimicrobial properties of chitosan nanoparticles: Mode of action and factors affecting activity. Fibers Polym. 2017, 18, 221–230. [Google Scholar] [CrossRef]
- Cuero, R.G.; Osuji, G.; Washington, A. N-carboxymethylchitosan inhibition of aflatoxin production: Role of zinc. Biotechnol. Lett. 1991, 13, 441–444. [Google Scholar] [CrossRef]
- Sintara, K.; Thong-Ngam, D.; Patumraj, S.; Klaikeaw, N.; Chatsuwan, T. Curcumin suppresses gastric NF-kappaB activation and macromolecular leakage in Helicobacter pylori-infected rats. World J. Gastroenterol. 2010, 16, 4039–4046. [Google Scholar] [CrossRef]
- Cole, S.P.; Harwood, J.; Lee, R.; She, R.; Guiney, D.G. Characterization of monospecies biofilm formation by Helicobacter pylori. J. Bacteriol. 2004, 186, 3124–3132. [Google Scholar] [CrossRef]
- Izui, S.; Sekine, S.; Maeda, K.; Kuboniwa, M.; Takada, A.; Amano, A.; Nagata, H. Antibacterial Activity of Curcumin Against Periodontopathic Bacteria. J. Periodontol. 2016, 87, 83–90. [Google Scholar] [CrossRef] [PubMed]
- De Kievit, T.R.; Gillis, R.; Marx, S.; Brown, C.; Iglewski, B.H. Quorum-sensing genes in Pseudomonas aeruginosa biofilms: Their role and expression patterns. Appl. Environ. Microbiol. 2001, 67, 1865–1873. [Google Scholar] [CrossRef] [PubMed]





| Nanoformulation | Diameter (nm) D.E | Polydispersion Index + D.E | Zeta Potential (mV) + D.E |
|---|---|---|---|
| CUR-NEM* | 202 ± 3.0 | 0.16 ± 0.06 | −54.2 ± 1.2 |
| NEM* | 207 ± 0.3 | 0.14 ± 0.03 | −56.3 ± 0.4 |
| CUR-QNEM* | 305 ± 7.0 | 0.29 ± 0.03 | 67.8 ± 0.96 |
| QNEM* | 296 ± 4.1 | 0.29 ± 0.02 | 66.8 ± 0.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidalgo, A.; Bravo, D.; Soto, C.; Maturana, G.; Cordero-Machuca, J.; Zúñiga-López, M.C.; Oyarzun-Ampuero, F.; Quest, A.F.G. The Anti-Oxidant Curcumin Solubilized as Oil-in-Water Nanoemulsions or Chitosan Nanocapsules Effectively Reduces Helicobacter pylori Growth, Bacterial Biofilm Formation, Gastric Cell Adhesion and Internalization. Antioxidants 2023, 12, 1866. https://doi.org/10.3390/antiox12101866
Hidalgo A, Bravo D, Soto C, Maturana G, Cordero-Machuca J, Zúñiga-López MC, Oyarzun-Ampuero F, Quest AFG. The Anti-Oxidant Curcumin Solubilized as Oil-in-Water Nanoemulsions or Chitosan Nanocapsules Effectively Reduces Helicobacter pylori Growth, Bacterial Biofilm Formation, Gastric Cell Adhesion and Internalization. Antioxidants. 2023; 12(10):1866. https://doi.org/10.3390/antiox12101866
Chicago/Turabian StyleHidalgo, Antonio, Denisse Bravo, Cristopher Soto, Gabriela Maturana, Jimena Cordero-Machuca, María Carolina Zúñiga-López, Felipe Oyarzun-Ampuero, and Andrew F. G. Quest. 2023. "The Anti-Oxidant Curcumin Solubilized as Oil-in-Water Nanoemulsions or Chitosan Nanocapsules Effectively Reduces Helicobacter pylori Growth, Bacterial Biofilm Formation, Gastric Cell Adhesion and Internalization" Antioxidants 12, no. 10: 1866. https://doi.org/10.3390/antiox12101866
APA StyleHidalgo, A., Bravo, D., Soto, C., Maturana, G., Cordero-Machuca, J., Zúñiga-López, M. C., Oyarzun-Ampuero, F., & Quest, A. F. G. (2023). The Anti-Oxidant Curcumin Solubilized as Oil-in-Water Nanoemulsions or Chitosan Nanocapsules Effectively Reduces Helicobacter pylori Growth, Bacterial Biofilm Formation, Gastric Cell Adhesion and Internalization. Antioxidants, 12(10), 1866. https://doi.org/10.3390/antiox12101866









