1. Introduction
Natural products have been recognized since the beginning of humanity as an excellent source of bioactive compounds to treat certain diseases or to palliate specific symptoms that may affect human health [
1,
2]. Many of the drugs that are currently available in the market have been obtained either directly from natural products or synthesized from them. This has encouraged a recently growing interest in their properties and promoted research studies on more sophisticated and efficient extraction methods.
Ecuador is one of the countries with the greatest biodiversity in the world, with around 10% of the world’s plant species, and every year new plants are discovered and added to the long list of the species already known [
3]. This makes of Ecuador an invaluable source of potentially interesting new natural products for the pharmaceutical industry.
Prestonia mollis (Malacapa) is an Ecuadorian plant species traditionally used for therapeutic purposes. It belongs to the
Apocinacea family, and its branches are densely tomentose when young and glabrescent at maturity [
4].
Prestonia mollis is considered among the species that dominate the wetland area in the Esmeraldas province on the Ecuadorian coast, and it is also very abundant in the province of Manabí. It is considered a weed in cultivated fields, and it demands laborious removal processes in certain crops such as cassava or corn, which are important sources of income for the population of Manabí. Therefore, this weed could be given an added value after its removal by using it for new purposes. According to a survey where 1593 adults were inquired,
Prestonia mollis was popularly used in therapeutic applications for the treatment of cancer as well as for the disinfection and healing of wounds [
5]. Other authors corroborate the traditional application of
Prestonia mollis extracts to treat skin affections [
3,
6]. Previous research using conventional extraction methods has identified alkaloids, saponins and flavonoids as the main phytochemicals [
6,
7]. However, although a number of projects are being conducted in Ecuador to scientifically study its flora, as far as we know, the
Apocynaceae family, and especially
Prestonia mollis, has not been so far included in these studies. The scientific information available on this plant is scarce, so it is necessary to investigate the biological activity and chemical composition of the extracts.
Extraction is an important step with regard to the isolation of bioactive compounds from vegetable matter. Both the technique and the solvent are key factors to maximize a selective recovery and to avoid the extraction of undesirable substances. Nowadays, great attention is paid to green extraction technologies that allow the use of hazardous substances to be reduced or totally avoided while limiting the toll associated to solvent waste and its disposal [
8]. Among these, supercritical fluid extraction (SFE), enhance solvent extraction (ESE), pressurized liquid extraction (PLE), ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE) and enzyme-assisted extraction (EAE) are all considered as prominent sustainable techniques.
Most industrial extraction processes are based on traditional systems that use organic solvents for the extraction of bioactive compounds. The environmental pollution generated by organic solvents has become a central issue in societal and political decisions. This has led to a growing demand for efficient eco-friendly extraction technologies. In the last two decades, significant advances have been made in the methods used, such as the development of ESE and PLE. The common denominator of newly emerging methods is high efficiency, low solvent consumption and significantly shorter extraction time, resulting in decreased costs, workload and impact to the environment [
9].
Pressurized liquid extraction (PLE)—also known as accelerated solvent extraction (ASE), pressurized solvent extraction (PSE) or pressurized fluid extraction (PFE)— uses liquid solvents under moderately high pressure and temperature conditions that never reach their critical point levels. Under these conditions, the high diffusion coefficients and high solvent strength increase the solubility of the solutes as well as lowering the solvent viscosity and surface tension, all of which favor the extraction process [
10]. Thanks to these favorable conditions, this technique can also guarantee rapid extraction rates, as the dielectric constant of the solvent is also reduced [
11]. Enhanced solvent extraction (ESE), on the other hand, combines the employment of pressurized highly fluid solvents of a low viscosity, such as CO
2, to leverage on the typical benefits of pressurized liquid solvents and the enhanced transport properties of supercritical fluids. Thus, carbon dioxide in combination with a liquid solvent (generally water and/or an alcoholic compound) forms a gas-expanded fluid that easily extracts polar compounds, such as polyphenols [
12]. This process, therefore, requires a smaller amount of solvent to achieve a greater recovery of the compounds in comparison to other traditional methods or even to PLE [
13].
Extraction solvent and temperature are generally the parameters with the greatest impact on these two techniques regarding the size of the extraction yields [
14]. Among the potential green solvents that could be used, water stands out as an excellent option as it poses no harm to human health or the environment, besides its ready availability and low cost [
15]. Ethanol is another solvent that is widely used for ESE and PLE as it is quite affordable and considered non-toxic, even though it is a flammable substance [
16]. Ethanol–water mixtures have also been demonstrated to be, in some cases, more effective than other pure solvents for the extraction of polyphenols from different plant raw materials [
17].
Natural phytochemical compounds are important alternatives for the development of the pharmaceutical industry through phytomedicine [
18]. Polyphenols are compounds with unique chemical and biological properties, notably as bioactive antioxidants. Research has linked many classes of polyphenols to positive health outcomes and potential protection against a host of oxidative degenerative diseases, from cancer to heart disease [
19,
20,
21]. One of the current trends with regard to the use of antioxidant compounds consists in their impregnation into polymeric matrices for biomedical applications. Supercritical impregnation has been reported as an efficient alternative process that can be successfully employed for this purpose [
22,
23,
24]. In this procedure, supercritical CO
2 plays several simultaneous roles as it dissolves the solutes of interest, transfers them to the polymer surface and acts as a diffusion enhancer by temporarily modifying some of the polymer properties [
25]. Carbon dioxide is a gas under regular atmospheric conditions; therefore, it desorbs from the polymer and evaporates by simple depressurization, which results in a solvent-free final product.
Polylactic acid (PLA) is currently used in pharmaceutical formulations, solid dispersions for oral bio availability or drug delivery systems. PLA has good CO
2 sorption, plasticization and swelling properties under high-pressure conditions, as reported by several authors [
26,
27,
28], which facilitates the incorporation of drugs or active extracts. Therefore, it would be interesting to study the extraction of bioactive compounds from the Ecuadorian plant species
Prestonia mollis and their subsequent impregnation in a polymeric matrix used in biomedicine such as PLA.
In this work, solvents generally recognized as safe (GRAS), such as CO2, water and/or ethanol, were used to extract and characterize bioactive compounds from Prestonia mollis leaves. The aim of this study is to evaluate the extraction process by non-conventional techniques, such as ESE and PLE, in terms of total extract yields and phenolic content, as well as to determine the antioxidant capacity of the extracts. The major compounds in the extracts with the highest phenolic content have also been identified by liquid chromatography. Finally, the suitability of a supercritical fluid procedure to incorporate the bioactive extracts obtained into a PLA device has been evaluated.
2. Materials and Methods
2.1. Raw Material and Chemicals
Prestonia mollis sp. (Malacapa) from the Pimpiguasí sector in the Abdón Calderón parish in Portoviejo canton, Manabí province, Ecuador, was the plant variety used for this study. The plants were collected in 2022 from an integral farm located by the lower section of the Chico River basin, at the coordinates −1.012624, −80.365781. The plants had been grown on a sandy loam soil under erratic rainfall conditions, and the temperature records reflected an upward trend since 2013 [
29]. The leaves were dried at room temperature and crushed prior to their use.
For the extraction and impregnation processes, carbon dioxide (99.99%), purchased from Abello-Linde S.A. (Barcelona, Spain), and ethanol (>99%), supplied by Panreac (Barcelona, Spain), were used. The reagents 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) and Folin–Ciocalteu were purchased from Sigma-Aldrich (Steinheim, Germany). The HPLC-grade solvents (acetonitrile and formic acid) were supplied by Panreac (Barcelona, Spain).
For the impregnation process, 1.6 mm diameter PLA filaments were purchased from Mundo Reader S.L. (Madrid, Spain). The polymeric material was 100% PLA with 1.24 g/cm3 density, a fusion temperature of 145–160 °C and a glass transition temperature of 56–64 °C (according to the specifications provided by the manufacturer).
2.2. Extraction under High Pressure
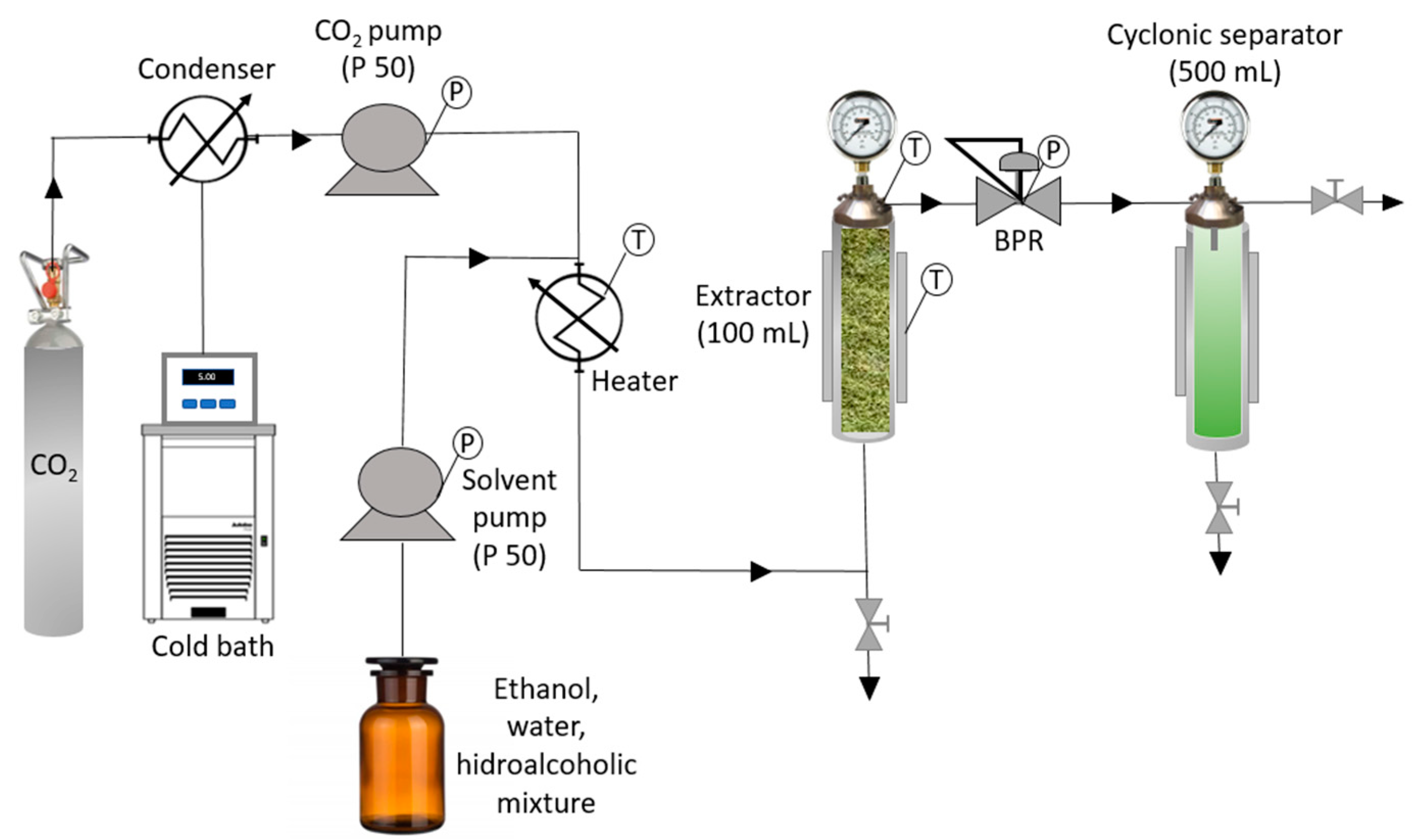
The equipment used for the extractions was an SF100 model supplied by Thar Technologies (Pittsburgh, PA, USA), fitted with an extractor (100 mL capacity) and two pumps, both with a maximum flow rate of 50 g/min, where one was used for the CO
2 and the other for the solvent. The pressure was controlled by means of a back-pressure regulator (BPR) and the temperature by means of a thermostatic jacket.
Figure 1 shows a diagram of the system used for this research work.
The methodology used for the extractions involved the loading of the extraction vessel with approximately 7.4 g of the plant sample, which had been previously homogenized to an apparent density uniform in all the experiments. The extractions were carried out in batch mode for 3 h. Two extraction techniques were evaluated: ESE and PLE; for the ESE process, two pumps were used, one for carbon dioxide and the other for the liquid solvent, while for the PLE, only one pump for liquid solvent was employed. Pressurization was performed by pumping solvents at 10 g min−1 until the set pressure conditions had been achieved, when the extraction time begins. When the extraction time was completed, the extractor pressure was decreased slowly by opening BRP until reaching atmospheric conditions. The extracts were recovered in a cyclonic separator, collected in amber flasks and stored in their respective solvent medium in darkness at 4 °C before testing, in order to prevent degradation of the bioactive compounds. The experiments were carried out in duplicate for each set of conditions to validate any possible discrepancies in the measured values.
The effect on the extract when varying pressure (100–250 bar) and temperature conditions (55–75 °C) was tested. The extraction solvent was selected according to technical requirements, but economic, safety and sustainability aspects were also considered. It is well known that water, ethanol and CO
2 are generally used in biomedical and pharmaceutical applications as they are considered GRAS solvents (generally recognized as safe) with lower toxicity than methanol or other organic solvents.
Table 1 shows the composition of the solvent mixtures used in each extraction method. For the ESE, a higher proportion of CO
2 (50%
v/
v of the total solvent flow) was employed. All conditions were chosen based on the results previously obtained when using other raw materials [
30].
2.3. Characterization of the Extracts
2.3.1. Total Extraction Yields
The total yields obtained were calculated as the ratio of the total dry extract mass divided by the mass of the raw material, and the results were expressed as a percentage according to Equation (1), where
md is the mass of the dry extract, and
ml is the initial mass of the leaves. The extraction yields were determined in triplicate.
2.3.2. Total Phenolic Content
The total phenolic content (TPC) of the extracts was determined through the Folin–Ciocalteu method proposed by Margrat and coworkers [
31], with minor modifications. A 12.5 µL aliquot of each extract was mixed with 12.5 µL of Folin–Ciocalteu reagent and 200 µL of double-distilled water and shaken for 5 min. Then, 25 µL of sodium carbonate solution (20%
w/
v) was added and the mixture was shaken for another 5 min. After 60 min in the absence of light and at room temperature, the absorbance was measured at 725 nm by means of a Synergy HTX Multi-Mode Microplate Reader by BioTek Instruments (Winooski, VT, USA). The absorbance of the same mixture with ethanol instead of the extract or the standard was subtracted from the absorbance of the mixture with the extract. Gallic acid dilutions (25–300 µg/mL) were used as standards to plot a calibration line calculated according to Equation (2), where
A is the absorbance at 725 nm, and
C is the equivalent concentration of gallic acid expressed as µg/mL.
2.3.3. Antioxidant Activity
The antioxidant capacity of the extracts was determined following two free-radical-scavenging methods, 2,2-diphenyl-1-picrylhydrazyl (DPPH) [
32] and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) [
33].
For the DPPH assay, 293 µL of 60 µM DPPH ethanolic solution was added to 7 µL extract aliquots at different concentrations (150–9000 µg/mL) in ethanol. After 2 h of incubation at room temperature in the absence of light, the absorbance was measured at 515 nm. A control experiment was also conducted by replacing the 7 µL extract with ethanol. The percentage of inhibition of the oxidation (%
I) was calculated by comparing the absorbance of the control (
A0) against the absorbance measured after 2 h (
Ai) according to Equation (3).
The EC50 is the efficient concentration that causes 50% DPPH inhibition, and this was calculated graphically using a curve by plotting the %I against the concentration of the extract.
On the other hand, the ABTS solution was prepared by mixing 7.4 μM ABTS diammonium salt and 2.6 μM potassium persulfate (1:1) and allowing them to react in darkness for a minimum period of 16 h. The resulting solution was diluted in ethanol up to an absorbance of 0.7 ± 0.02 at 750 nm.
For the assays, 3 µL of extract at different concentration levels (150–9000 µg/mL) in ethanol) was added to a 300 µL ABTS solution and allowed to react for 60 min in the absence of light; then, the absorbance was measured at 750 nm. The same mixture with ethanol instead of the extract aliquot was tested in the same way to be used as the control sample. The percentage of oxidation inhibition and the IC50 were calculated in the same way as in the DPPH assay.
2.3.4. Identification of Major Phenolic Compounds
The phenolic compounds in the Prestonia mollis extracts were identified and quantified by ultra-high-performance liquid chromatography–electrospray ionization–time of flight–mass spectrometry (UHPLC-ESI-ToF-MS) by means of Xevo G2-S equipment supplied by Waters Corporation (Milford, MA, USA). A UPLC BEH-C18 column of 1.7 μm particle size (2.1 × 100 mm) in a thermostatic oven fixed at 45 °C was employed. A volume of 5 µL of samples was injected and a binary solvent system (A: 0.1% formic acid in water; B: 0.1% formic acid in acetonitrile) was pumped at 0.5 mL/min. The gradient of the mobile phase volumetric composition was 97% of A at the initial time, 90% of A at 3.5 min, 85% of A at 5.0 min, 80% of A at 6.5 min, 75% of A at 8.0 min, 60% of A at 9.5 min, 50% of A at 10.5 min, 25% of A at 11.5 min, 0% of A from 12.5 to 13.5 min and 97% of A at 14.0 min. The electrospray operated in negative ionization mode, with a full-scan analysis (100–1200 Da), 40 V cone voltage, 0.7 kV capillary voltage, 120 °C source temperature and 850 °C desolvation temperature.
The procedure for the identification of the flavonoid compounds was based on their mass spectra according to the bibliography. Their quantification was carried out based on a calibration line for quercetin, at concentrations from 1 to 100 µg/mL (Equation (4)), where
C is the concentration of equivalent quercetin in µg/mL, and
y is the peak area.
2.4. Impregnation at High Pressure
The same high-pressure equipment as described in
Section 2.2 was used for the impregnation process. Firstly, a specific amount of extract (3 mL) was poured into the vessel. Then, five pieces of PLA filament (30 mm long each) were placed in a steel-made supporting basket inside the vessel to prevent any direct contact between the polymer and the extract. The impregnation processes were carried out in batch mode. The CO
2 was first pumped at 10 g/min until the desired pressure level was reached. Then, the CO
2 flow was cut off, and the pressure of the system was maintained throughout the impregnation time. The system was rapidly depressurized (100 bar/min) to facilitate the impregnation of the PLA filaments.
The filaments were subsequently cleaned by means of a wet napkin to remove any extract excess. Each experiment was replicated twice, and the impregnated samples were stored in the absence of light in order to prevent their deterioration prior to use.
The effect of the operating temperature (35–55 °C) and the pressure conditions (100–400 bar) on the impregnation of the hydroethanolic extract CO2/ethanol/water (50:25:25) into the PLA filaments was determined.
Once the best pressure and temperature conditions had been determined, the initial amount of extract was increased up to 6 mL and the impregnation time was increased by 2 h, in order to verify if there was an increment in the amount of antioxidant compounds impregnated.
Loadings of the Impregnated Filaments
The loadings of Prestonia mollis extract impregnated into the PLA filaments were established by spectrophotometric methods. In order to determine the amount of antioxidant compounds that reacted with DPPH, 4 mL of 6 × 10−5 M DPPH solution were kept in contact with a specific amount of impregnated filament (15 mg). After 60 min of incubation at room temperature and in the absence of light, the fall of the absorbance at 515 nm was measured.
The inhibition percentage was determined according to Equation (3), where %I is the percentage of inhibition, A0 is the initial absorbance of the DPPH at 515 nm and Ai is its final absorbance.
2.5. Statistical Analysis
All the analytical determinations were performed in triplicate, and mean and standard deviations were calculated. Multilevel factorial designs were used to determine the effect of the variables on the extraction processes. The conditions for the factorial design were pressures of 100 and 250 bar; temperatures of 55 and 75 °C; and solvent composition expressed as ethanol concentrations of 0, 50 and 100%. On the basis of this design, a total of 12 experiments were carried out with two replicates for each condition and in a random way in order to minimize errors. The response variables were total extraction yield, total phenolic content and antioxidant activities using both DPPH and ABTS methods. The experimental data were processed by means of the software application STATGRAPHICS Plus 4.0 software (Warrenton, VA, USA) to carry out the analysis for the experimental design. A Pareto chart was used to represent the effect of the different factors, where the corresponding sign has been used to indicate a positive or negative effect attributable to each experimental variable.
Using the same software, the effect attributable to the impregnation time and to the amount of extract added was analyzed by ANOVA followed by a multiple-range test in order to obtain homogeneous subgroups.
The normal distribution of the data was determined by the normal probability plot.
4. Discussion
ESE and PLE are solid extraction techniques that allow the selectivity of the extractions to be improved by means of different solvents. In ESE, water and carbon dioxide are widely studied solvents [
40]. It has been reported in the related literature that water in contact with CO
2 becomes acidic because of the formation and dissociation of carbonic acid. This acidification of liquid water contributes to breaking chemical bonds, specifically glycosidic bonds, which are characteristic of phenolic compounds, such as flavonoids, and this, in turn, increases the diffusion coefficient and promotes the release of the phenolic compounds [
41]. Carbon dioxide can be easily removed after extraction by reducing the pressure to ambient temperature and is especially useful in pharmaceutical processing. The presence of water may increase the density of the fluid mixture and cause the swelling of the solutes, which consequently would improve their internal diffusion and the solubility of the compounds of interest. In addition, aqueous-organic mixtures are often used in ESE and PLE procedures to reduce the demand for organic solvents. Ethanol is also a promising solvent that can be used for the extraction of bioactive compounds. As a green solvent, it can contribute to mitigate greenhouse gas emissions, thereby reducing the environmental impact of the process [
42,
43,
44]. Generally, the optimization of these techniques starts by choosing the most adequate extraction solvent, i.e., the one that provides the optimum analyte yield and the targeted biological activity.
On the other hand, as it has already been mentioned, an elevated pressure allows the extraction medium to be maintained in a liquid state, even when the boiling point at atmospheric pressure of the corresponding solvent has been exceeded. This elevated pressure also contributes to increasing the contact area between the matrix and the solvent by inserting the solvent into certain regions of the solid matrix that are not normally accessible to the solvent under normal pressure conditions. On its part, elevated extraction temperatures facilitate the desorption of the analytes in solid matrices and its diffusion into the solvent, and furthermore, it achieves higher values of the partition coefficients [
40,
45]. As a result, greater extraction yields are obtained by ESE or PLE in comparison to other conventional extraction methods.
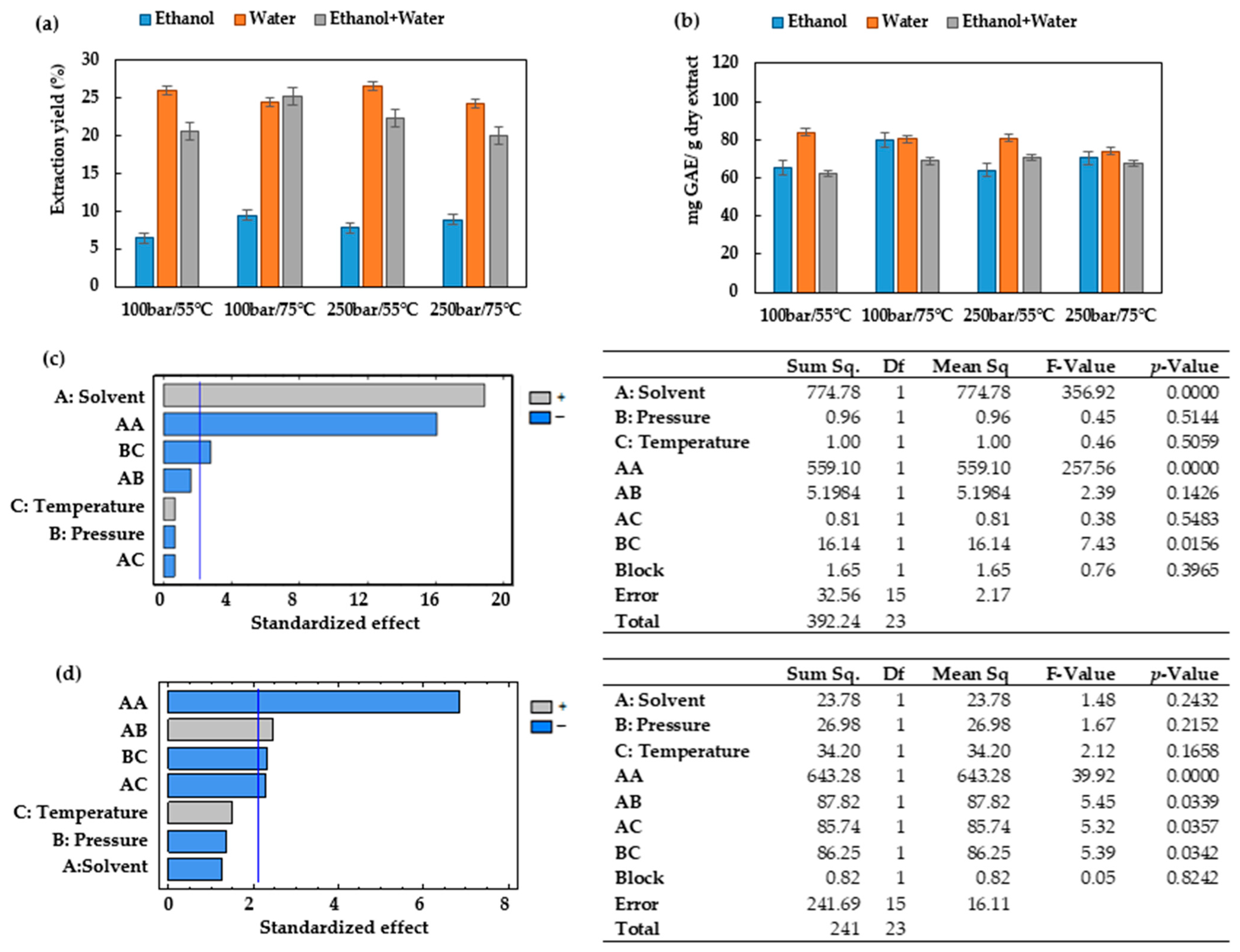
One of the advantages of ESE in comparison to PLE is its low demand for liquid solvents. This allows the time and cost requirements to be moderated for the extract concentration stage, which in many extraction procedures may be rather long and costly. The total extraction yields from
Prestonia mollis leaves through ESE were lower than those obtained by PLE (
Figure 2a and
Figure 3a). This behavior differs from reports concerning other raw materials. Thus, when the extractions were performed on mango leaves at 120 and 200 bar in the range of temperatures between 60 and 100 °C, greater total extraction yields were obtained through ESE [
40]. The same behavior has been reported when using either technique for extractions from elderberry pomace at 40 °C and 209 bar [
12]. Therefore, the composition of the raw material seems to be a critical aspect with regard to the total extraction yields to be expected. On the contrary, even though the total extraction yields obtained from
Prestonia mollis leaves by ESE is discrete, this technique proves to be more selective with regard to the extraction of total polyphenols than PLE (
Figure 2b and
Figure 3b) in the majority of the operating conditions studied. Similarly, the total phenolic content from elderberry pomace [
12] has been reported to present similar results, i.e., higher phenolic content was obtained when using ESE at 40 °C and 209 bar using CO
2/ethanol/water.
Temperature has also been confirmed to have an effect on the total phenolic compounds. Thus, in the range 55–75 °C, higher temperature levels improved the recovery of phenolic compounds as a result of an enhanced mass transfer. Nevertheless, under certain experimental conditions, the use of 75 °C as the extraction temperature resulted in lower or unchanged phenolic yields, probably because of the presence of certain heat-sensitive polyphenols. When the ESE technique was used for the recovery of total phenolic content, temperature had a significant influence, as can be seen from
Figure 2d. Under the conditions studied, the mixtures used for ESE are in a vapor–liquid equilibrium which is more strongly affected by temperature than those solvents used for PLE, all of which are in a liquid state [
46].
The recovery of polyphenols from
Prestonia mollis leaves by PLE using ethanol, water or an ethanol/water mixture (50:50
v/
v) in the temperature range 55–75 °C reveals that this parameter does not have a significant effect on the process. The same occurs with the pressure factor, which in the range 100–250 bar does not have any significant influence either, whereas the pressure–solvent and pressure–temperature interaction do (
Figure 3d). Both results are similar to those achieved during the extraction of the total phenolic compounds from mango leaves [
40].
A previous study evaluated the recovery of total phenolic compounds from
Prestonia mollis leaves by conventional extraction. In this case, the extraction technique used was a dynamic maceration for 4 h at room temperature using methanol as solvent. The total phenolic content obtained was 102.4 ± 2.9 mg GAE/g dry extract [
6]. This result is comparable to those obtained by ESE at 100 bar and at the two temperature conditions studied but higher than the values achieved by PLE. Regarding the antioxidant activity of the extracts obtained by maceration with methanol, an IC
50 = 500 µg extract/mL for ABTS methods and values greater than 1000 µg extract/mL according to the DPPH tests were reported [
6]. The IC
50 values reported in this work, for both extraction techniques ESE and PLE, are lower, and therefore, the extracts have greater antioxidant activity. Nevertheless, it was not possible to confirm that the antioxidant capacity of the extracts was the result of the phenolic compounds only, as the bioactivity can be affected by synergistic effects of phenolic compounds with other non-phenolic compounds, which have been shown to present antioxidant capacity.
Compounds other than phenolics must be extracted using water or the hydro-alcoholic mixture, which probably favors the antioxidant capacity of
Prestonia mollis leaf extracts. Previous studies have suggested that the Maillard reaction inevitably occurs during extraction with hot pressurized water at high temperatures. Some Maillard reaction products, such as 5-hydroxymethylfurfural, have been shown to have a certain antioxidant capacity, and this could therefore contribute to the antioxidant activity of plant extracts [
47].
Ethanolic extracts of the Ecuadorian species
Bidens pilosa L. and
Croton floccosus have also been evaluated in terms of antioxidant activity using the DPPH method. The IC
50 values obtained for
Bidens pilosa L. (239.33 µg/mL) and
Croton floccosus (644.125 µg/mL) show that the extracts of
Prestonia mollis obtained in the present paper exhibit a higher antioxidant activity than those obtained from these species. Malva species are widely used worldwide as traditional remedies.
Malva sylvestris and
Malva pseudolavatera species are the main ones sold in Ecuadorian markets. Antioxidant activity measurements by DPPH and ABTS have shown that the aqueous extract of
M. sylvestris (IC
50DPPH = 78.14 µg/mL; IC
50ABTS = 166.79 µg/mL) was the most antioxidant extract, probably due to the presence of polyols [
48]. These results corroborate how important it is to select the appropriate extraction technique as well as the solvent, as they have a direct influence on the bioactive compounds to be obtained.
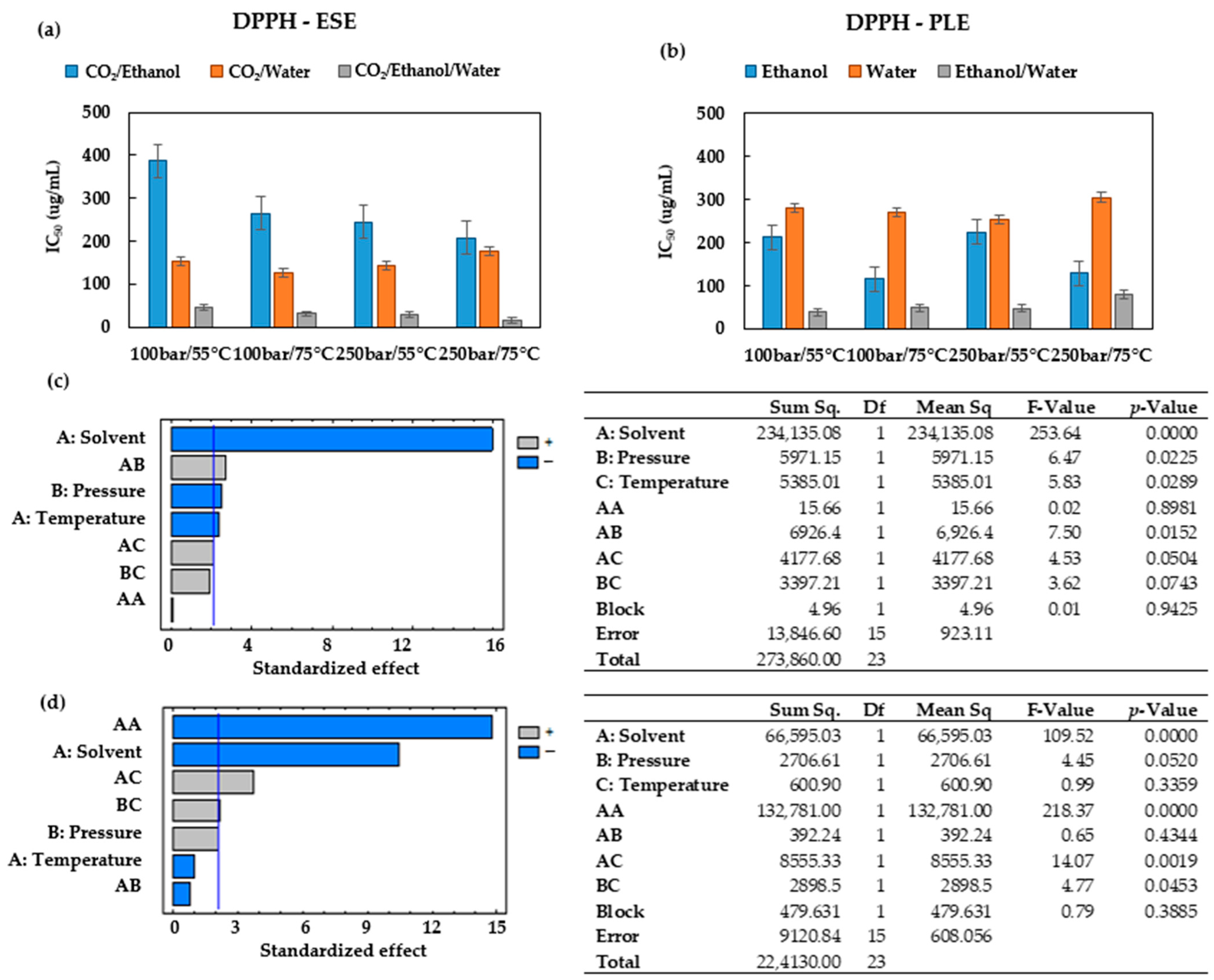
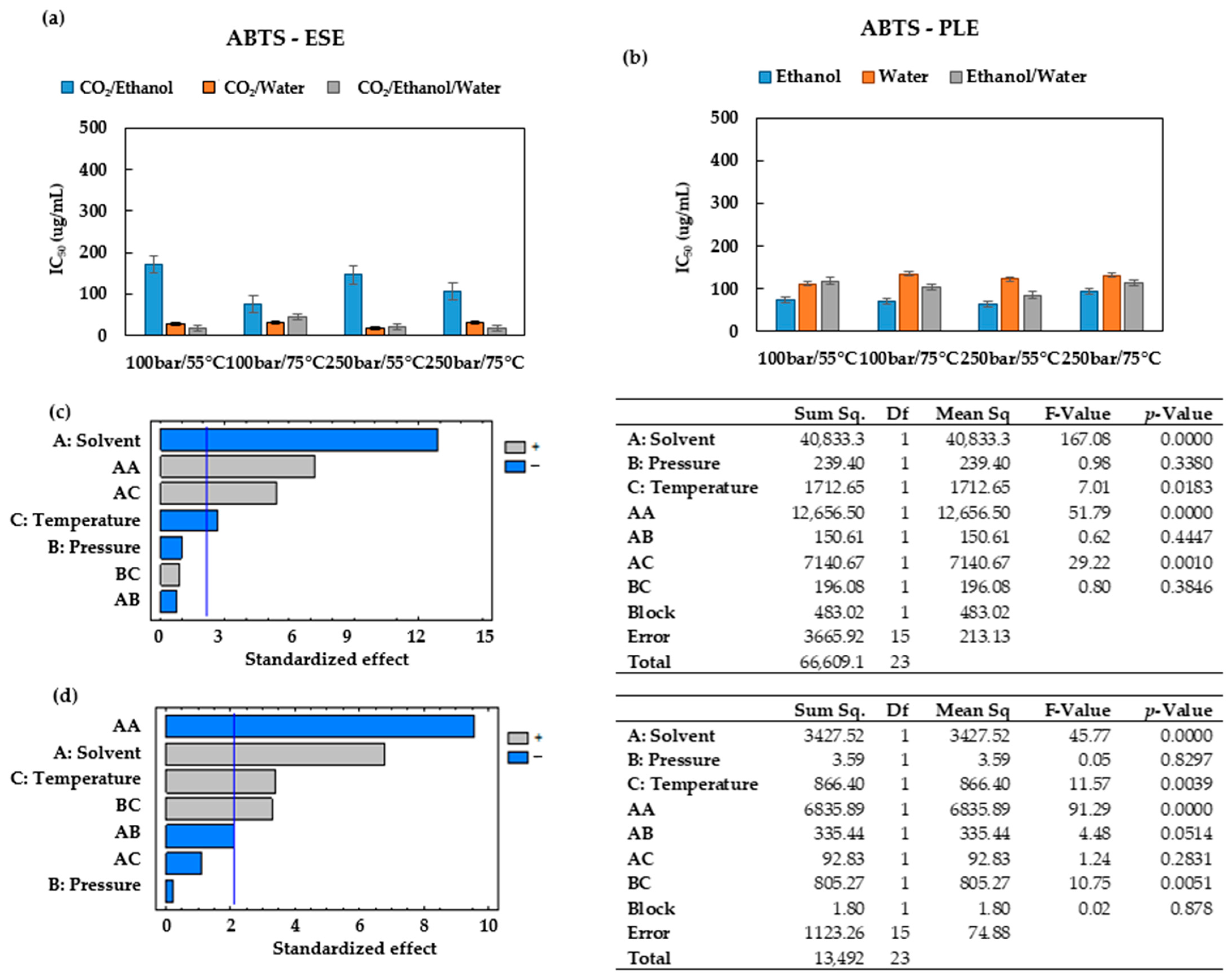
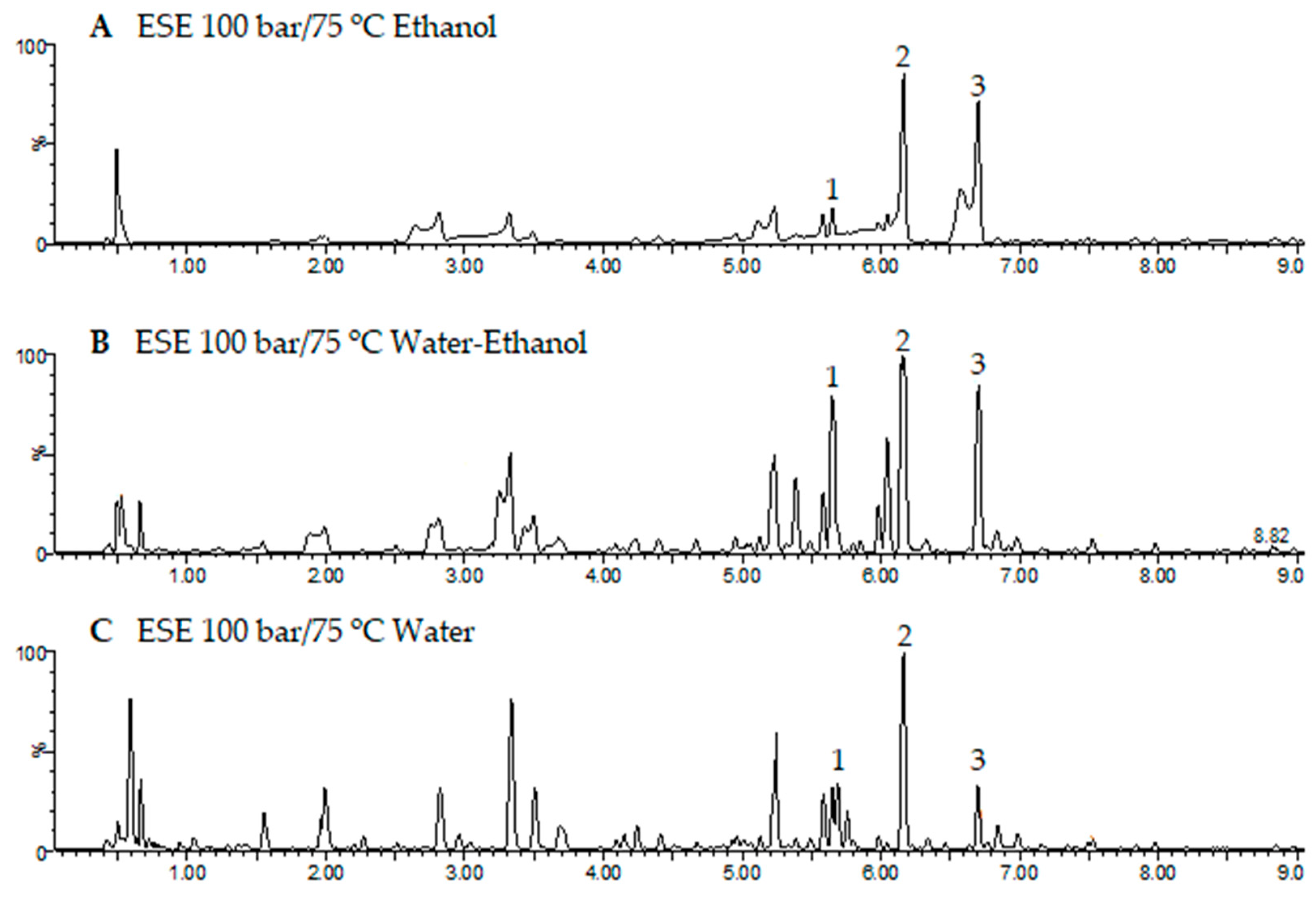
When operating at 75 °C and 100 bar in ESEs, large contents of total phenolic compounds were obtained (
Figure 2b), and the extracts exhibited low IC
50 values (high bioactivity). Therefore, the extracts obtained at these conditions were selected for the UHPLC analysis.
Figure 6 shows the base peak chromatograms of the three solvents studied. Three flavonoids were identified: Quercetin 3-O-xylosyl-rutinoside, Kaempferol 3-(2G-apiosylrobinobioside) and Kaempferol 4′-glucoside 7-rhamnoside, which were found at different concentrations depending on the solvent used for the extraction. Thus, the extracts produced using the CO
2/ethanol/water solvent presented the highest concentration of these three flavonoids and were also the ones with the lowest IC
50 values according to both DPPH and ABTS (
Figure 4a and
Figure 5a). On the other hand, the extract produced by using the CO
2/ethanol mixture presented a higher IC
50 value in comparison to those obtained by the extracts obtained through the other solvents and under similar pressure and temperature conditions. The CO
2/ethanol extract presented the lowest concentration of Quercetin 3-O-xylosyl-rutinoside (538 ± 15 µg/mL).
Extraction studies on
Moringa oleifera Lam. leaves using ESE have identified the presence of some flavanoids among which we should highlight quercetin derivates as quercetin 3-O-glucoside, quercetin malonyl glucoside, quercetin acetyl glucoside, kaempferol 3-O-glucoside and kaempferol malonyl glucoside. Not all the conditions tested extracted all the compounds, quercetin 3-O-glucoside, quercetin malonyl glucoside and kaempferol malonyl glucoside being the most representative ones and those that were recovered under every pressure and temperature configuration tested [
30].
The impregnation of polymers with natural bioactive extracts using supercritical CO2 is a promising technique that is gaining importance with respect to biomedical applications. The extract obtained from Prestonia mollis leaves at 75 °C and 100 bar using CO2/ethanol/water presented large extraction yields of total phenolic compounds and a good antioxidant activity. Therefore, the suitability of this specific extract for the impregnation of biodegradable PLA for biomedical applications was investigated.
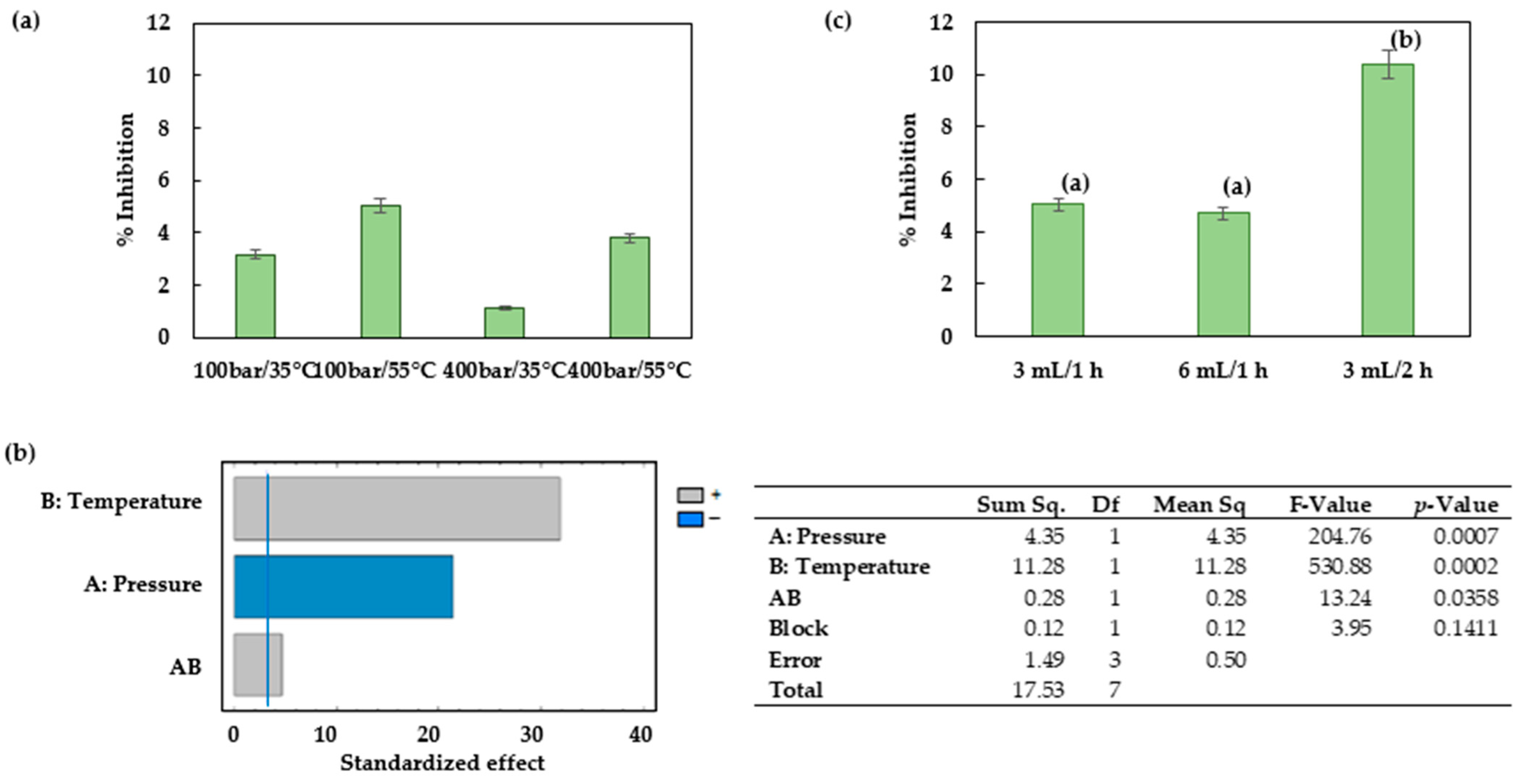
Figure 7 shows a successful impregnation of
Prestonia mollis leaf extract into PLA under all the operating conditions studied. Pressure and temperature were determining factors with regard to the impregnation process with supercritical solvents as it was related to the dissolution and diffusion of the target compounds. However, the selection of pressure and temperature is a complex task, given that the most favorable conditions for the dissolution of the active compounds in the CO
2 phase are not necessarily the best conditions to ensure a good diffusivity of the compounds and their retention within the matrix. In fact, an increase in the solubility of the target compounds (at a certain condition of pressure and temperature) may promote a high affinity of these compounds for the CO
2 phase. Such high solubility favors an easy desorption of the target compounds during the depressurization step, which results in a lower impregnation efficiency. It is therefore necessary to find the balance between the different steps in the process with regard to solubility, diffusivity and retention of the compounds within the matrix in order to determine the partition coefficient that most favors the impregnation of the matrix. The supercritical impregnation of PLA and thermoplastic polyurethane (TPU) with active compounds have been investigated at pressure and temperature ranges from 100 to 400 bar and 35 to 55 °C, respectively. The largest olive leaf extract loadings were achieved at 250 bar and 55 °C for both polymers [
49], with TPU exhibiting nearly fourfold greater OLE loadings and antioxidant activity compared to that of PLA under the optimum conditions. Another study described the impregnation of PLA filaments with mango leaf extract. In this case, the best impregnation conditions were registered at 100 bar and 55 °C [
30]. These results agree with those established in this work. Under these conditions, CO
2 presents a lower density than under the rest of the conditions studied, which results in a lower concentration of the compound in the supercritical phase and a more efficient impregnation of the polymer.
Once the most favorable pressure and temperature conditions for the process had been selected, the effect of the amount of extract was also investigated by increasing it from 3 to 6 mL. According to the results obtained, no significant differences were observed between the two amounts, and therefore, the smallest amount was considered to be adequate. However, a longer impregnation time of 2 h did actually result in significant impregnation differences. Milovanovic et al. impregnated thymol into a mixture of PLA with different amounts of poly(ε-caprolactone) (PCL) using supercritical CO
2 [
27]. They studied the effect of varying impregnation times between 1 and 15 h. According to their results, 1.5 h of impregnation time would be appropriate for the impregnation of the PLA and PCL mixture. In fact, they found out that as the impregnation time was increased up to 5 h, the impregnation loadings were greater, and then they went down as the time was increased up to 15 h. They attributed this behavior to the crystallization of the polymer matrix when saturated with supercritical CO
2, which led to a reduction in the free space within the matrix and subsequently to a poorer impregnation.
5. Conclusions
ESE and PLE are two of the most widely used extraction techniques for the extraction of bioactive compounds from plant materials, as they are versatile, rapid and efficient. Given that they allow to reduce or even operate without organic solvents, they are not only more environmentally friendly but also allow to obtain more efficient extracts in terms of antioxidant activity.
This is the first research work that includes both ESE and PLE where pressure, temperature and extraction solvent are evaluated in terms of total extract yields, as well as phenolic composition and antioxidant capacity of the extracts obtained from Prestonia mollis leaves. A thorough analysis of the extraction results has allowed us to confirm that, even if PLE provided greater extraction yields in comparison to ESE, the total polyphenolic content of these extracts expressed as mg GAE/g dry extract did not present a similar upward trend. In relation to the antioxidant activity as measured by DPPH and ABTS, the extracts obtained by ESE at 100 bar and 75 °C exhibited higher values. The flavonoids identified have been the following: Quercetin 3-O-xylosyl-rutinoside, Kaempferol 3-(2G-apiosylrobinobioside) and Kaempferol 4′-glucoside 7-rhamnoside, all of which appeared at different concentration levels depending on the solvent used for each extraction.
The extract obtained by ESE using CO2/ethanol/water at 75 °C and 100 bar was applied to the supercritical CO2 impregnation process of PLA filaments. All the operating conditions studied achieved good impregnation results as evidenced by the percentages of inhibition achieved. The most successful impregnations were obtained at 100 bar, 55 °C for 2 h.
This study is a first step towards a better insight into the functional properties and potential applications of the extracts obtained from the Ecuadorian plant species Prestonia mollis. However, it would be necessary to further investigate other biological properties of these extracts, as well as other potential applications of the same.