A Comparative Study on UHPLC-HRMS Profiles and Biological Activities of Inula sarana Different Extracts and Its Beta-Cyclodextrin Complex: Effective Insights for Novel Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Sample Preparation
2.4. Total Phenolic and Flavonoids Content
2.5. UHPLC-HRMS Analysis
2.6. Preparation and Characterization of β-CD Complexes
2.7. In Vitro Antioxidant Assays
2.8. Enzyme Inhibitory Activity
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characterization of β-CD Complexes
3.1.1. SEM Analysis
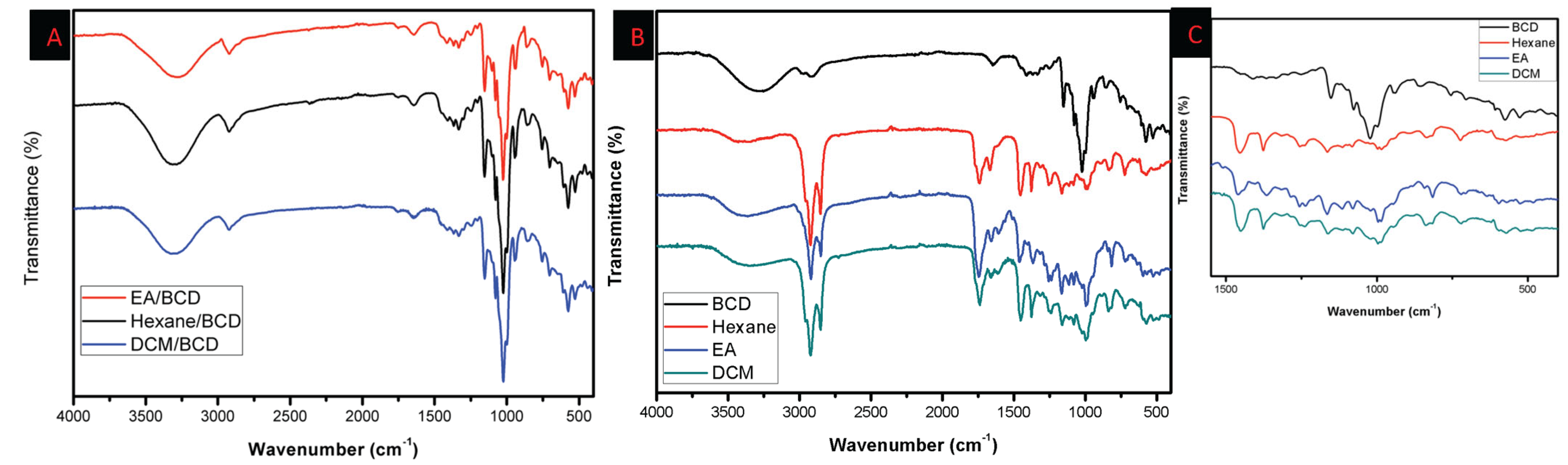
3.1.2. Fourier Transform Infrared Spectroscopic Analysis
3.2. Total Phenolic and Flavonoid Content
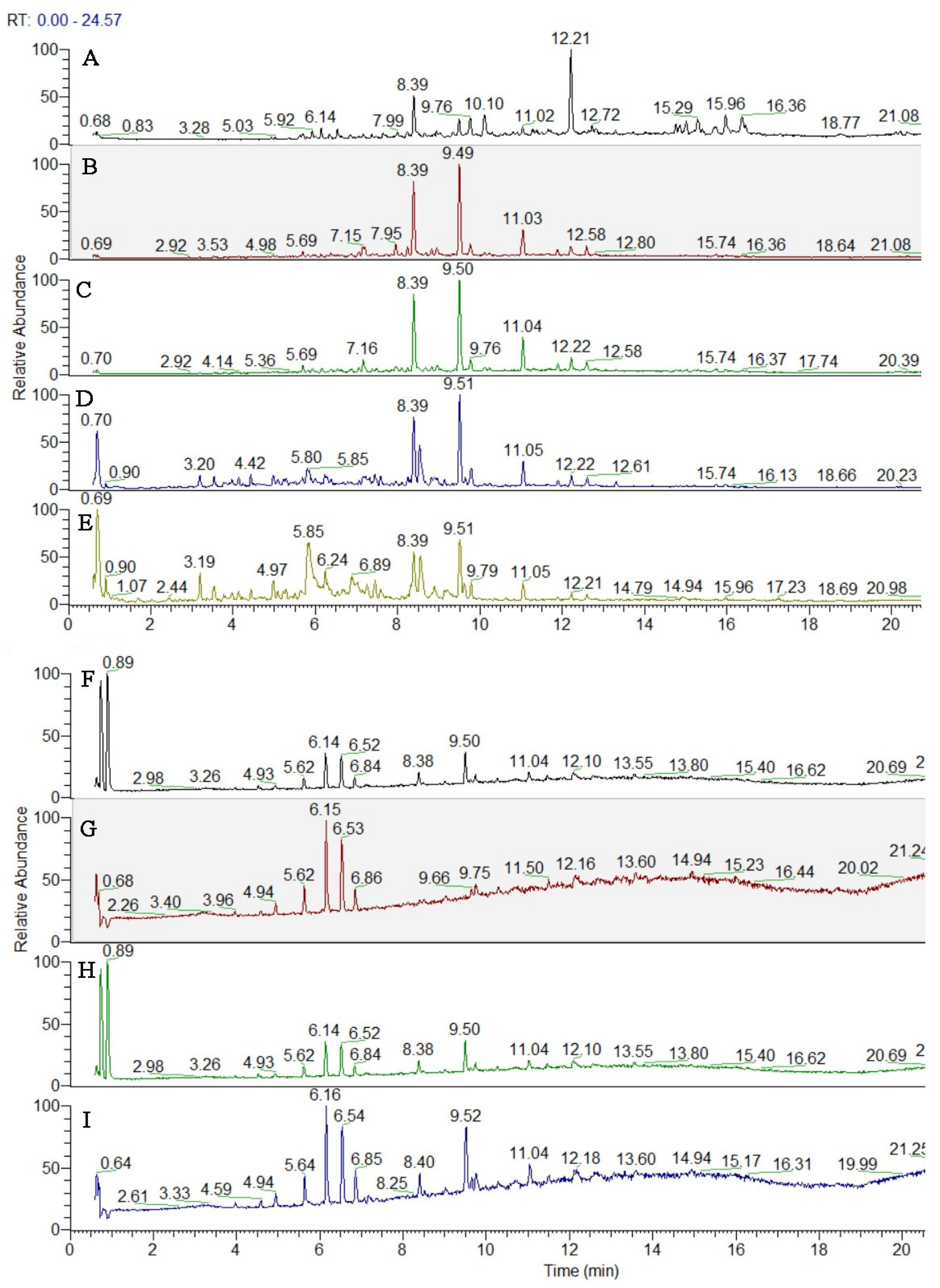
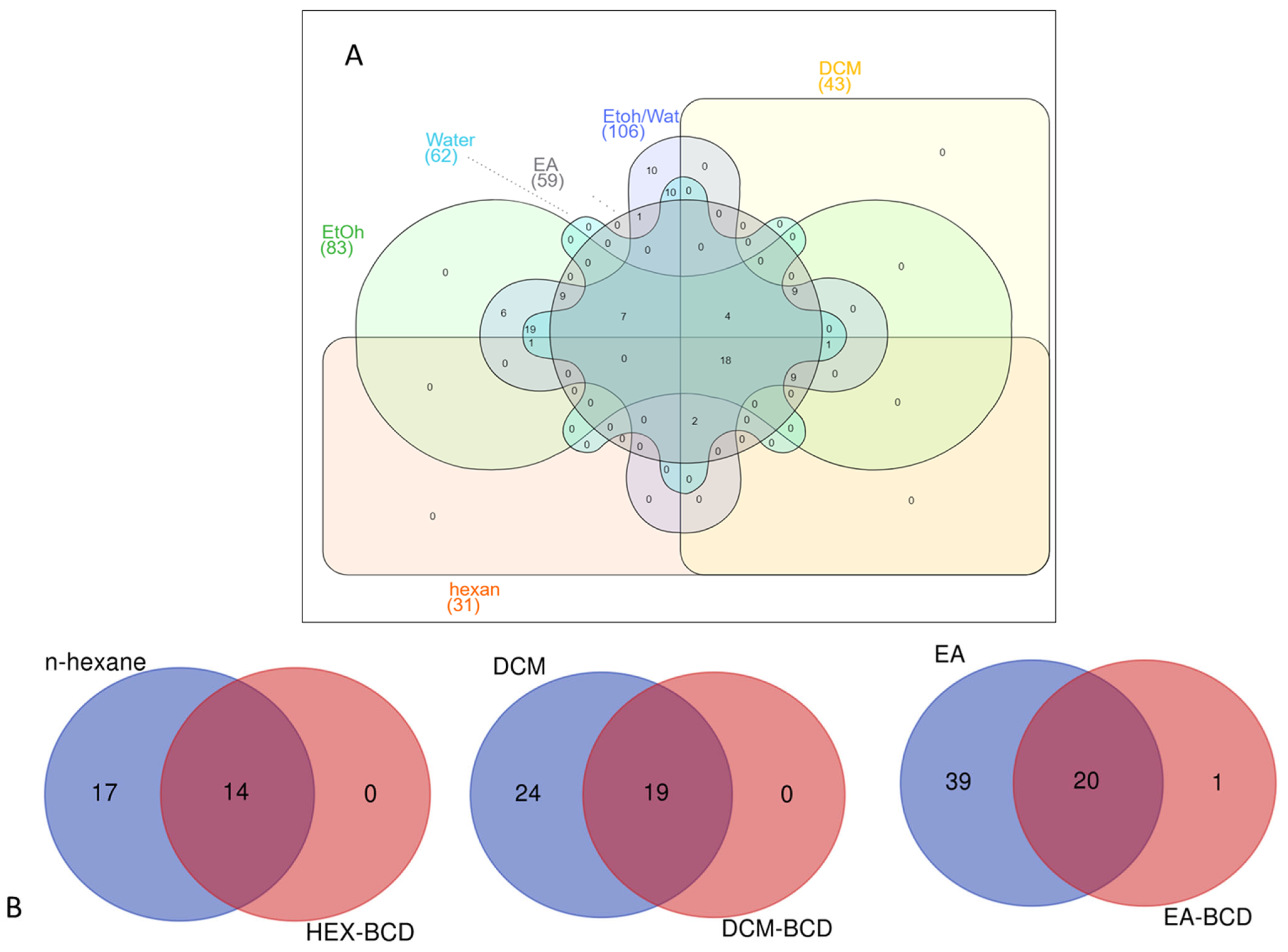
3.3. UHPLC-HRMS Analysis
3.3.1. Hydroxybenzoic and Hydroxycinnamic Acids and Their Derivatives
3.3.2. Acylquinic Acids (AQAs)
3.3.3. Caffeoylhexaric Acids (CHAs)
3.3.4. Flavonoids
3.3.5. Sesquiterpene Lactones and Derivatives
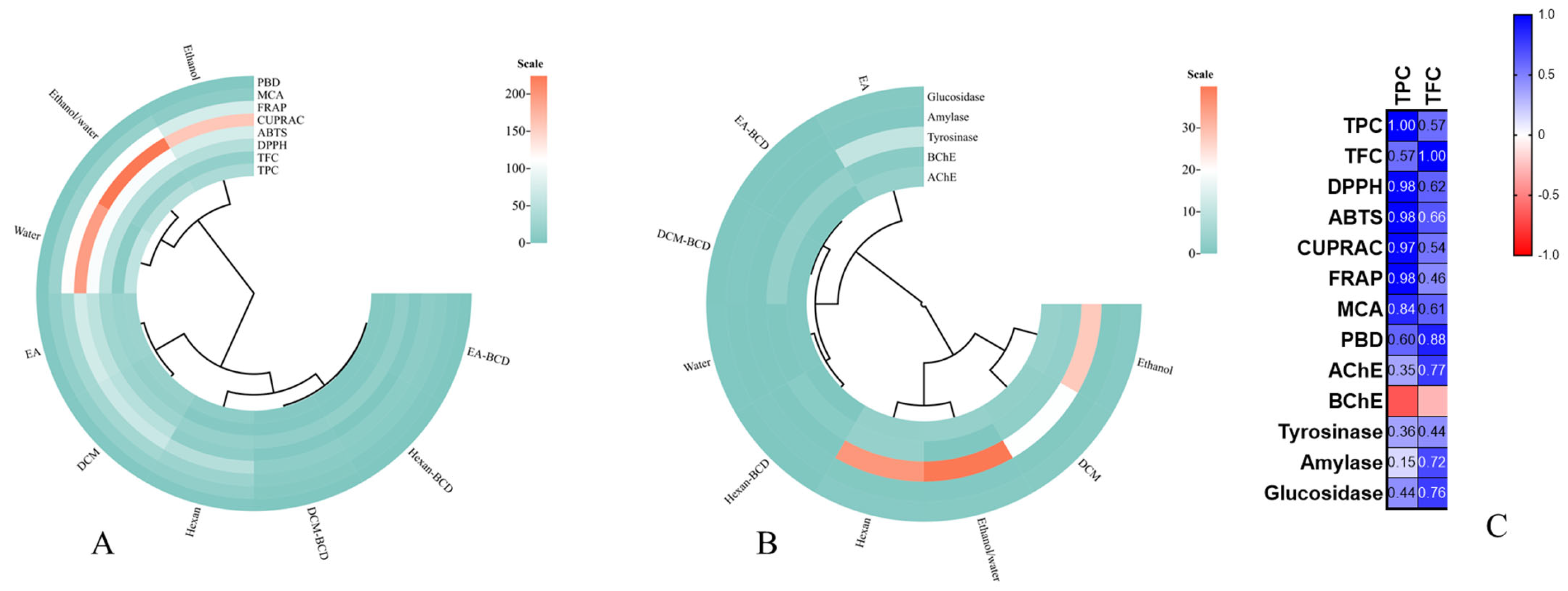
3.4. Antioxidant Properties
3.5. Enzyme Inhibition Effects
3.5.1. α-Amylase and α-Glucosidase
3.5.2. Cholinesterase Inhibitory Activity
3.5.3. Tyrosinase Inhibitory Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Panero, J.; Crozier, B. Macroevolutionary dynamics in the early diversification of Asteraceae. Mol. Phylogeny Evol. 2016, 99, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Zameer, S.; Yaqoob, M. Ethnobotanical, phytochemical and pharmacological properties of Galinsoga parviflora (Asteraceae): A review. Trop. J. Pharm. Res. 2017, 16, 3023–3033. [Google Scholar]
- Rolnik, A.; Olas, B. The plants of the Asteraceae family as agents in the protection of human health. Int. J. Mol. Sci. 2021, 22, 3009. [Google Scholar] [CrossRef] [PubMed]
- Devkota, H.P. An Overview of Medicinal Plants of the Asteraceae Family and Their Role in Human Health. In Medicinal Plants of the Asteraceae Family: Traditional Uses, Phytochemistry and Pharmacological Activities; Springer: Singapore, 2022; pp. 1–15. [Google Scholar] [CrossRef]
- Sevindik, E.; Paksoy, M.Y.; Sevindik, M. Genetic relationship of seven endemic Inula L. (Asteraceae) species grown in Turkey. Turk. J. Agric. Food Sci. Technol. 2022, 10, 678–681. [Google Scholar] [CrossRef]
- Karlıoğlu Kılıç, N.; Yılmaz Dağdeviren, R.; Paksoy, M.Y.; Tuncalı Yaman, T. Pollen morphology of eight endemic Inula L. (Asteraceae) species in Turkey. Palynology 2021, 45, 235–244. [Google Scholar] [CrossRef]
- Englund, M.; Pornpongrungrueng, P.; Gustafsson, M.H.; Anderberg, A.A. Phylogenetic relationships and generic delimitation in Inuleae subtribe Inulinae (Asteraceae) based on ITS and cpDNA sequence data. Cladistics 2009, 25, 319–352. [Google Scholar] [CrossRef]
- Trotta, F.; Zanetti, M.; Cavalli, R. Cyclodextrin-based nanosponges as drug carriers. Beilstein J. Org. Chem. 2012, 8, 2091–2099. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef]
- Mourtzinos, I.; Salta, F.; Yannakopoulou, K.; Chiou, A.; Karathanos, V.T. Encapsulation of olive leaf extract in β-cyclodextrin. J. Agric. Food Chem. 2007, 55, 8088–8094. [Google Scholar] [CrossRef]
- Allahyari, S.; Esmailnezhad, N.; Valizadeh, H.; Ghorbani, M.; Jelvehgari, M.; Ghazi, F.; Zakeri-Milani, P. In-vitro characterization and cytotoxicity study of flutamide loaded cyclodextrin nanosponges. J. Drug Deliv. Sci. Technol. 2021, 61, 102275. [Google Scholar] [CrossRef]
- Mashaqbeh, H.; Obaidat, R.; Al-Shar’i, N. Evaluation and characterization of curcumin-β-cyclodextrin and cyclodextrin-based nanosponge inclusion complexation. Polymers 2021, 13, 4073. [Google Scholar] [CrossRef] [PubMed]
- Obaidat, R.; Al-Shar’i, N.; Tashtoush, B.; Athamneh, T. Enhancement of levodopa stability when complexed with β-cyclodextrin in transdermal patches. Pharm. Dev. Technol. 2018, 23, 986–997. [Google Scholar] [CrossRef] [PubMed]
- Yien, R.M.K.; Matos, A.P.d.S.; Gomes, A.C.C.; Garófalo, D.d.A.; Santos-Oliveira, R.; Simas, N.K.; Ricci-Júnior, E. Nanotechnology promoting the development of products from the biodiversity of the Asteraceae family. Nutrients 2023, 15, 1610. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, G.K.; Caregnato, F.; Moreira, J.C.F.; Teixeira, H.F.; Carvalho, E.L.S. Antioxidant effect of nanoemulsions containing extract of Achyrocline satureioides (Lam) DC—Asteraceae. AAPS PharmSciTech 2016, 17, 844–850. [Google Scholar] [CrossRef]
- Furlan, V.; Bren, U. Helichrysum italicum: From extraction, distillation, and encapsulation techniques to beneficial health effects. Foods 2023, 12, 802. [Google Scholar] [CrossRef] [PubMed]
- Szente, L.; Szejtli, J. Cyclodextrins as food ingredients. Trends Food Sci. Technol. 2004, 15, 137–142. [Google Scholar] [CrossRef]
- Rodis, P.S.; Karathanos, V.T.; Mantzavinou, A. Partitioning of olive oil antioxidants between oil and water phases. J. Agric. Food Chem. 2002, 50, 596–601. [Google Scholar] [CrossRef]
- Acquaviva, A.; Nilofar, N.; Bouyahya, A.; Zengin, G.; Di Simone, S.C.; Recinella, L.; Leone, S.; Brunetti, L.; Uba, A.I.; Cakilcioğlu, U. Chemical characterization of different extracts from Artemisia annua and their antioxidant, enzyme inhibitory and anti-inflammatory properties. Chem. Biodivers. 2023, 20, e202300547. [Google Scholar] [CrossRef]
- Llorent-Martínez, E.J.; Ruiz-Medina, A.; Zengin, G.; Ak, G.; Jugreet, S.; Mahomoodally, M.F.; Emre, G.; Orlando, G.; Libero, M.L.; Nilofar; et al. New Biological and chemical evidences of two Lamiaceae species (Thymbra capitata and Thymus sipyleus subsp. rosulans): In vitro, in silico and ex vivo approaches. Molecules 2022, 27, 9029. [Google Scholar]
- Gevrenova, R.; Zengin, G.; Sinan, K.I.; Zheleva-Dimitrova, D.; Balabanova, V.; Kolmayer, M.; Voynikov, Y.; Joubert, O. An in-Depth study of metabolite profile and biological potential of Tanacetum balsamita L.(Costmary). Plants 2022, 12, 22. [Google Scholar] [CrossRef]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G. A study on in vitro enzyme inhibitory properties of Asphodeline anatolica: New sources of natural inhibitors for public health problems. Ind. Crops Prod. 2016, 83, 39–43. [Google Scholar] [CrossRef]
- Popović, B.M.; Blagojević, B.; Latković, D.; Četojević-Simin, D.; Kucharska, A.Z.; Parisi, F.; Lazzara, G. A one step enhanced extraction and encapsulation system of cornelian cherry (Cornus mas L.) polyphenols and iridoids with β-cyclodextrin. LWT-Food Sci. Technol. 2021, 141, 110884. [Google Scholar] [CrossRef]
- Tong, L. Cyclodextrin Chemistry: Fundamentals and Application; China Science Publishing & Medial Ltd.: Beijing, China, 2001. [Google Scholar]
- Zhu, G.; Zhu, G.; Xiao, Z. A review of the production of slow-release flavor by formation inclusion complex with cyclodextrins and their derivatives. J. Incl. Phenom. Macrocycl. Chem. 2019, 95, 17–33. [Google Scholar] [CrossRef]
- Fan, K. Introduction of Spectroscopy; Higher Education Press: Beijing, China, 2011. [Google Scholar]
- Anwar, F.; Przybylski, R. Effect of solvents extraction on total phenolics and antioxidant activity of extracts from flaxseed (Linum usitatissimum L.). ACTA Sci. Pol. Technol. Aliment. 2012, 11, 293–302. [Google Scholar] [PubMed]
- Hossain, M.A.; Shah, M.D. A study on the total phenols content and antioxidant activity of essential oil and different solvent extracts of endemic plant Merremia borneensis. Arab. J. Chem. 2015, 8, 66–71. [Google Scholar] [CrossRef]
- Ng, Z.X.; Samsuri, S.N.; Yong, P.H. The antioxidant index and chemometric analysis of tannin, flavonoid, and total phenolic extracted from medicinal plant foods with the solvents of different polarities. J. Food Process. Preserv. 2020, 44, e14680. [Google Scholar] [CrossRef]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of cyclodextrins and drug/cyclodextrin complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef]
- Kłeczek, N.; Michalak, B.; Malarz, J.; Kiss, A.K.; Stojakowska, A. Carpesium divaricatum Sieb. & Zucc. revisited: Newly identified constituents from aerial parts of the plant and their possible contribution to the biological activity of the Plant. Molecules 2019, 24, 1614. [Google Scholar]
- Cuyckens, F.; Claeys, M. Mass spectrometry in the structural analysis of flavonoids. J. Mass Spectrom. 2004, 39, 1–15. [Google Scholar] [CrossRef]
- Yao, D.; Li, Z.; Huo, C.; Wang, Y.; Wu, Y.; Zhang, M.; Li, L.; Shi, Q.; Kiyota, H.; Shi, X. Identification of in vitro and in vivo metabolites of alantolactone by UPLC-TOF-MS/MS. J. Chromatogr. B 2016, 1033, 250–260. [Google Scholar] [CrossRef]
- Wang, T.; Guo, S.; Zhang, S.; Yue, W.; Ho, C.-T.; Bai, N. Identification and quantification of seven sesquiterpene lactones in Inula britannica by HPLC-DAD-MS. Anal. Methods 2019, 11, 1822–1833. [Google Scholar] [CrossRef]
- Duan, X.; Subbiah, V.; Xie, C.; Agar, O.T.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A. Evaluation of the antioxidant potential of brown seaweeds extracted by different solvents and characterization of their phenolic compounds by LC–ESI-QTOF–MS/MS. J. Food Sci. 2023, 88, 3737–3757. [Google Scholar] [CrossRef]
- Dulo, B.; Phan, K.; Githaiga, J.; Raes, K.; De Meester, S. Natural quinone dyes: A review on structure, extraction techniques, analysis and application potential. Waste Biomass Valoriz. 2021, 12, 6339–6374. [Google Scholar] [CrossRef]
- Tohma, H.; Altay, A.; Köksal, E.; Gören, A.C.; Gülçin, İ. Measurement of anticancer, antidiabetic and anticholinergic properties of sumac (Rhus coriaria): Analysis of its phenolic compounds by LC–MS/MS. J. Food Meas. Charact. 2019, 13, 1607–1619. [Google Scholar] [CrossRef]
- Jiang, L.; Jia, F.; Han, Y.; Meng, X.; Xiao, Y.; Bai, S. Development and characterization of zein edible films incorporated with catechin/β-cyclodextrin inclusion complex nanoparticles. Carbohydr. Polym. 2021, 261, 117877. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Du, Y.-T.; Xue, W.-Y.; Wang, L.; Li, R.; Jiang, Z.-T.; Tang, S.-H.; Tan, J. Enhanced preservation effects of clove (Syzygium aromaticum) essential oil on the processing of Chinese bacon (preserved meat products) by beta cyclodextrin metal organic frameworks (β-CD-MOFs). Meat Sci. 2023, 195, 108998. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Abellán, C.; Mercader-Ros, M.; Zafrilla, M.; Gabaldón, J.; Núñez-Delicado, E. Comparative study of different methods to measure antioxidant activity of resveratrol in the presence of cyclodextrins. Food Chem. Toxicol. 2011, 49, 1255–1260. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, H.; Yang, B.; Tao, H. Identification of cyclodextrin inclusion complex of chlorogenic acid and its antimicrobial activity. Food Chem. 2010, 120, 1138–1142. [Google Scholar] [CrossRef]
- Kfoury, M.; Landy, D.; Auezova, L.; Greige-Gerges, H.; Fourmentin, S. Effect of cyclodextrin complexation on phenylpropanoids’ solubility and antioxidant activity. Beilstein J. Org. Chem. 2014, 10, 2322–2331. [Google Scholar] [CrossRef]
- Belozertseva, I.V.; Dravolina, O.A.; Tur, M.A.; Semina, M.G.; Zvartau, E.E.; Bespalov, A.Y. Morphine-induced Straub tail reaction in mice treated with serotonergic compounds. Eur. J. Pharmacol. 2016, 791, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Siqueira-Lima, P.S.; Brito, R.G.; Araújo-Filho, H.G.; Santos, P.L.; Lucchesi, A.; Araújo, A.A.; Menezes, P.P.; Scotti, L.; Scotti, M.T.; Menezes, I.R. Anti-hyperalgesic effect of Lippia grata leaf essential oil complexed with β-cyclodextrin in a chronic musculoskeletal pain animal model: Complemented with a molecular docking and antioxidant screening. Biomed. Pharmacother. 2017, 91, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Avello, D.; Avendaño-Godoy, J.; Santos, J.; Lozano-Castellón, J.; Mardones, C.; von Baer, D.; Luengo, J.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A.; Gómez-Gaete, C. Encapsulation of phenolic compounds from a grape cane pilot-plant extract in hydroxypropyl beta-cyclodextrin and maltodextrin by spray drying. Antioxidants 2021, 10, 1130. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Avello, D.; Mardones, C.; Saez, V.; Riquelme, S.; von Baer, D.; Lamuela-Raventos, R.M.; Vallverdu-Queralt, A. Pilot-plant scale extraction of phenolic compounds from grape canes: Comprehensive characterization by LC-ESI-LTQ-Orbitrap-MS. Food Res. Int. 2021, 143, 110265. [Google Scholar] [CrossRef] [PubMed]
- Pinho, E.; Grootveld, M.; Soares, G.; Henriques, M. Cyclodextrins as encapsulation agents for plant bioactive compounds. Carbohydr. Polym. 2014, 101, 121–135. [Google Scholar] [CrossRef]
- Saqib, M.; Iqbal, S.; Mahmood, A.; Akram, R. Theoretical investigation for exploring the antioxidant potential of chlorogenic acid: A density functional theory study. Int. J. Food Prop. 2016, 19, 745–751. [Google Scholar] [CrossRef]
- Choi, S.-S.; Park, H.-R.; Lee, K.-A. A comparative study of rutin and rutin glycoside: Antioxidant activity, anti-inflammatory effect, effect on platelet aggregation and blood coagulation. Antioxidants 2021, 10, 1696. [Google Scholar] [CrossRef]
- Velika, B.; Kron, I. Antioxidant properties of benzoic acid derivatives against superoxide radical. Free. Radic. Antioxid. 2012, 2, 62–67. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Silva, A.M.S.; Pinto, D.C.G.A. Chapter 9—Parthenolide and Parthenolide-Like Sesquiterpene Lactones as Multiple Targets Drugs: Current Knowledge and New Developments. In Studies in Natural Products Chemistry; Attaur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 52, pp. 337–372. [Google Scholar]
- Neeland, I.J.; Patel, K.V. Diabetes: Key markers of injury and prognosis. In Biomarkers in Cardiovascular Disease; Elsevier: Amsterdam, The Netherlands, 2019; pp. 41–51. [Google Scholar]
- Beidokhti, M.N.; Jäger, A.K. Review of antidiabetic fruits, vegetables, beverages, oils and spices commonly consumed in the diet. J. Ethnopharmacol. 2017, 201, 26–41. [Google Scholar] [CrossRef]
- Papoutsis, K.; Zhang, J.; Bowyer, M.C.; Brunton, N.; Gibney, E.R.; Lyng, J. Fruit, vegetables, and mushrooms for the preparation of extracts with α-amylase and α-glucosidase inhibition properties: A review. Food Chem. 2021, 338, 128119. [Google Scholar] [CrossRef]
- Dai, J.; Li, Y.; Zhou, H.; Chen, J.; Chen, M.; Xiao, Z. Genistein promotion of osteogenic differentiation through BMP2/SMAD5/RUNX2 signaling. Int. J. Biol. Sci. 2013, 9, 1089. [Google Scholar] [CrossRef] [PubMed]
- Lordan, S.; Smyth, T.J.; Soler-Vila, A.; Stanton, C.; Ross, R.P. The α-amylase and α-glucosidase inhibitory effects of Irish seaweed extracts. Food Chem. 2013, 141, 2170–2176. [Google Scholar] [CrossRef] [PubMed]
- Shashirekha, M.; Mallikarjuna, S.; Rajarathnam, S. Status of bioactive compounds in foods, with focus on fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2015, 55, 1324–1339. [Google Scholar] [CrossRef] [PubMed]
- Swaraz, A.; Sultana, F.; Bari, M.W.; Ahmed, K.S.; Hasan, M.; Islam, M.M.; Islam, M.A.; Satter, M.A.; Hossain, M.H.; Islam, M.S. Phytochemical profiling of Blumea laciniata (Roxb.) DC. and its phytopharmaceutical potential against diabetic, obesity, and Alzheimer’s. Biomed. Pharmacother. 2021, 141, 111859. [Google Scholar] [CrossRef] [PubMed]
- Tzioras, M.; McGeachan, R.I.; Durrant, C.S.; Spires-Jones, T.L. Synaptic degeneration in Alzheimer disease. Nat. Rev. Neurol. 2023, 19, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.K.L.; Cechinel Filho, V.; de Souza, R.L.R.; Furtado-Alle, L. BChE inhibitors from marine organisms–A review. Chem. Biol. Interact. 2022, 367, 110136. [Google Scholar]
- Aras, A.; Türkan, F.; Yildiko, U.; Atalar, M.N.; Kılıç, Ö.; Alma, M.H.; Bursal, E. Biochemical constituent, enzyme inhibitory activity, and molecular docking analysis of an endemic plant species, Thymus migricus. Chem. Pap. 2021, 75, 1133–1146. [Google Scholar] [CrossRef]
- Imran, M.; Irfan, A.; Ibrahim, M.; Assiri, M.A.; Khalid, N.; Ullah, S.; Al-Sehemi, A.G. Carbonic anhydrase and cholinesterase inhibitory activities of isolated flavonoids from Oxalis corniculata L. and their first-principles investigations. Ind. Crops Prod. 2020, 148, 112285. [Google Scholar] [CrossRef]
- Raza, M.A.; Danish, M.; Mushtaq, M.; Sumrra, S.H.; Saqib, Z.; Rehman, S.U. Phenolic profiling and therapeutic potential of local flora of Azad Kashmir; In vitro enzyme inhibition and antioxidant. Open Chem. 2017, 15, 371–379. [Google Scholar] [CrossRef][Green Version]
- Tamfu, A.N.; Roland, N.; Mfifen, A.M.; Kucukaydin, S.; Gaye, M.; Botezatu, A.V.; Duru, M.E.; Dinica, R.M. Phenolic composition, antioxidant and enzyme inhibitory activities of Parkia biglobosa (Jacq.) Benth., Tithonia diversifolia (Hemsl) A. Gray, and Crossopteryx febrifuga (Afzel.) Benth. Arab. J. Chem. 2022, 15, 103675. [Google Scholar] [CrossRef]
- Zhao, X.-j.; Gong, D.-m.; Jiang, Y.-r.; Guo, D.; Zhu, Y.; Deng, Y.-c. Multipotent AChE and BACE-1 inhibitors for the treatment of Alzheimer’s disease: Design, synthesis and bio-analysis of 7-amino-1, 4-dihydro-2H-isoquilin-3-one derivates. Eur. J. Med. Chem. 2017, 138, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Rasul, A.; Anwar, H.; Aziz, N.; Razzaq, A.; Wei, W.; Ali, M.; Li, J.; Li, X. Role of plant derived alkaloids and their mechanism in neurodegenerative disorders. Int. J. Biol. Sci. 2018, 14, 341. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Saab, A.; Statti, G.; Menichini, F. Composition and α-amylase inhibitory effect of essential oils from Cedrus libani. Fitoterapia 2007, 78, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Olasehinde, T.A.; Ademosun, A.O. Essential oil from lemon peels inhibit key enzymes linked to neurodegenerative conditions and pro-oxidant induced lipid peroxidation. J. Oleo Sci. 2014, 63, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Kinst-Hori, I. Flavonols from saffron flower: Tyrosinase inhibitory activity and inhibition mechanism. J. Agric. Food Chem. 1999, 47, 4121–4125. [Google Scholar] [CrossRef]
- Matsuura, R.; Ukeda, H.; Sawamura, M. Tyrosinase inhibitory activity of citrus essential oils. J. Agric. Food Chem. 2006, 54, 2309–2313. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Peng, X.; Li, S.; Liu, B.; Chu, C. Recent discovery of tyrosinase inhibitors in traditional Chinese medicines and screening methods. J. Ethnopharmacol. 2022, 303, 115951. [Google Scholar] [CrossRef]
- Lee, S.Y.; Baek, N.; Nam, T.-g. Natural, semisynthetic and synthetic tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2016, 31, 1–13. [Google Scholar] [CrossRef]
- Kukula-Koch, W.; Szwajgier, D.; Gaweł-Bęben, K.; Strzępek-Gomółka, M.; Głowniak, K.; Meissner, H.O. Is phytomelatonin complex better than synthetic melatonin? The assessment of the antiradical and anti-Inflammatory properties. Molecules 2021, 26, 6087. [Google Scholar] [CrossRef]
- Nebrigić, V.; Kljakić, A.C.; Zengin, G.; Terzić, M.; Mašković, P.; Radojković, M. Effects of extraction and drying techniques on the chemical composition and biological activities of Helichrysum italicum. Process Biochem. 2023, 130, 96–104. [Google Scholar] [CrossRef]
- Wen, P.; Hu, T.-G.; Linhardt, R.J.; Liao, S.-T.; Wu, H.; Zou, Y.-X. Mulberry: A review of bioactive compounds and advanced processing technology. Trends Food Sci. Technol. 2019, 83, 138–158. [Google Scholar] [CrossRef]
- Eloff, J.N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Medica 1998, 64, 711–713. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Agunloye, O.M.; Akinyemi, A.J.; Ademiluyi, A.O.; Adefegha, S.A. Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to Alzheimer’s disease and some pro-oxidant induced oxidative stress in rats’ brain-in vitro. Neurochem. Res. 2013, 38, 413–419. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, W.; Sun, W.; Chen, S.; Liu, D.; Kong, X.; Tian, J.; Ye, X. Inhibition of porcine pancreatic α-amylase activity by chlorogenic acid. J. Funct. Foods 2020, 64, 103587. [Google Scholar] [CrossRef]
- Dubey, S.; Ganeshpurkar, A.; Ganeshpurkar, A.; Bansal, D.; Dubey, N. Glycolytic enzyme inhibitory and antiglycation potential of rutin. Future J. Pharm. Sci. 2017, 3, 158–162. [Google Scholar] [CrossRef]
- Shen, H.; Wang, J.; Ao, J.; Cai, Y.; Xi, M.; Hou, Y.; Li, M.; Luo, A. Inhibitory kinetics and mechanism of active compounds in green walnut husk against α-glucosidase: Spectroscopy and molecular docking analyses. LWT-Food Sci. Technol. 2022, 172, 114179. [Google Scholar] [CrossRef]
- Budryn, G.; Majak, I.; Grzelczyk, J.; Szwajgier, D.; Rodríguez-Martínez, A.; Pérez-Sánchez, H. Hydroxybenzoic acids as acetylcholinesterase inhibitors: Calorimetric and docking simulation studies. Nutrients 2022, 14, 2476. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Zhou, F.C.; Gao, F.; Bian, J.S.; Shan, F. Comparative evaluation of quercetin, isoquercetin and rutin as inhibitors of α-glucosidase. J. Agric. Food Chem. 2009, 57, 11463–11468. [Google Scholar] [CrossRef] [PubMed]
- Khare, P.; Datusalia, A.K.; Sharma, S.S. Parthenolide, an NF-κB inhibitor ameliorates diabetes-induced behavioural deficit, neurotransmitter imbalance and neuroinflammation in type 2 diabetes rat model. Neuromolecular Med. 2017, 19, 101–112. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]

| Extracts | Total Phenolic Content (mg GAE/g Dry Extract or Complex) | Total Flavonoid Content (mg RE/g Dry Extract or Complex) |
|---|---|---|
| n-Hexane | 12.45 ± 0.44 f | 4.60 ± 0.18 e |
| Ethyl acetate | 23.77 ± 0.15 d | 22.60 ± 0.44 a |
| Dicholoromethane | 20.19 ± 0.78 e | 16.57 ± 0.32 b,c |
| Ethanol | 38.42 ± 0.10 c | 17.23 ± 0.48 b |
| 70% Ethanol | 46.36 ± 0.12 b | 15.93 ± 0.30 c |
| Water | 55.55 ± 0.42 a | 9.11 ± 0.14 d |
| n-Hexane/β-CD | 3.00 ± 0.03 h | 1.96 ± 0.26 f |
| Ethyl acetate/β-CD | 4.17 ± 0.11 g | 3.82 ± 0.41 e |
| DCM/-β-CD | 4.54 ± 0.09 g | 1.81 ± 0.07 f |
| Extracts | DPPH (mg TE/g Dry Extract/Complex) | ABTS (mg TE/g Dry Extract/Complex) | CUPRAC (mg TE/g Dry Extract/Complex) | FRAP (mg TE/g Dry Extract/Complex) | PBD (mmol TE/g Dry Extract/Complex) | MCA (mg EDTAE/g Dry Extract/Complex) |
|---|---|---|---|---|---|---|
| n-Hexane | 17.95 ± 0.24 e | 12.02 ± 0.62 e | 46.56 ± 0.71 e | 24.50 ± 1.87 d | 1.49 ± 0.03 b | 13.85 ± 0.56 c |
| Ethyl acetate | 27.95 ± 0.30 c | 53.24 ± 0.10 c | 71.51 ± 2.43 d | 33.25 ± 0.21 c | 2.07 ± 0.14 a | 15.62 ± 0.97 c |
| Dicholoromethane | 25.90 ± 0.24 d | 48.29 ± 0.37 d | 64.50 ± 2.94 d | 31.39 ± 0.36 c,d | 2.01 ± 0.23 a | 15.44 ± 1.09 c |
| Ethanol | 45.84 ± 0.19 b | 73.61 ± 0.74 b | 159.00 ± 1.30 c | 74.18 ± 0.39 b | 1.97 ± 0.02 a | 10.53 ± 0.41 d |
| 70% Ethanol | 47.97 ± 0.02 a | 106.50 ± 0.09 a | 224.31 ± 0.02 a | 110.40 ± 3.96 a | 1.61 ± 0.06 b | 19.22 ± 0.82 b |
| Water | 47.63 ± 0.04 a | 106.41 ± 0.01 a | 193.85 ± 8.08 b | 113.88 ± 7.43 a | 1.28 ± 0.03 c | 22.99 ± 0.08 a |
| n-Hexane/β-CD | 15.17 ± 0.53 f | na | 9.98 ± 0.22 f | 7.47 ± 0.04 e | 0.12 ± 0.02 e | 0.96 ± 0.25 g |
| Ethyl acetate/β-CD | 13.98 ± 0.36 g | 1.52 ± 0.04 f | 10.67 ± 0.42 f | 8.11 ± 0.18 e | 0.15 ± 0.01 e | 1.87 ± 0.08 f |
| DCM/β-CD | 14.30 ± 0.55 f,g | 2.01 ± 0.36 f | 11.82 ± 0.20 f | 7.97 ± 0.05 e | 0.26 ± 0.02 d | 4.08 ± 0.53 e |
| Extracts | AChE (mg GALAE/g Dry Extract/Complex) | BChE (mg GALAE/g Dry Extract/Complex) | Tyrosinase (mg KAE/g Dry Extract/Complex) | Amylase (mmol ACAE/g Dry Extract/Complex) | Glucosidase (mmol ACAE/g Dry Extract/Complex) |
|---|---|---|---|---|---|
| n-Hexane | 2.99 ± 0.06 c | 3.37 ± 0.31 a | 35.48 ± 2.19 b | 0.47 ± 0.01 a,b | 0.89 ± 0.01 b |
| Ethyl acetate | 3.17 ± 0.08 b,c | 1.43 ± 0.22 c | 10.32 ± 1.38 e | 0.49 ± 0.02 a | 0.80 ± 0.01 c |
| DCM | 2.69 ± 0.06 d | 2.37 ± 0.38 b | 19.57 ± 1.08 d | 0.45 ± 0.01 b,c | 0.90 ± 0.03 a,b |
| Ethanol | 3.42 ± 0.12 a | 2.82 ± 0.18 a,b | 27.87 ± 0.57 c | 0.44 ± 0.01 c | 0.88 ± 0.01 b |
| 70% Ethanol | 3.18 ± 0.05 b | 0.07 ± 0.01 d | 39.88 ± 0.46 a | 0.33 ± 0.01 d | 0.93 ± 0.01 a |
| Water | 0.12 ± 0.06 e | na | na | 0.05 ± 0.01 f | 0.21 ± 0.01 d |
| n-Hexane/β-CD | na | 1.27 ± 0.23 c | na | 0.10 ± 0.01 e | na |
| Ethyl acetate/β-CD | na | 2.97 ± 0.06 a | na | 0.07 ± 0.01 e,f | na |
| DCM/β-CD | 0.16 ± 0.03 e | 2.95 ± 0.09 a,b | na | 0.09 ± 0.01 e | na |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zengin, G.; Nilofar; Yildiztugay, E.; Bouyahya, A.; Cavusoglu, H.; Gevrenova, R.; Zheleva-Dimitrova, D. A Comparative Study on UHPLC-HRMS Profiles and Biological Activities of Inula sarana Different Extracts and Its Beta-Cyclodextrin Complex: Effective Insights for Novel Applications. Antioxidants 2023, 12, 1842. https://doi.org/10.3390/antiox12101842
Zengin G, Nilofar, Yildiztugay E, Bouyahya A, Cavusoglu H, Gevrenova R, Zheleva-Dimitrova D. A Comparative Study on UHPLC-HRMS Profiles and Biological Activities of Inula sarana Different Extracts and Its Beta-Cyclodextrin Complex: Effective Insights for Novel Applications. Antioxidants. 2023; 12(10):1842. https://doi.org/10.3390/antiox12101842
Chicago/Turabian StyleZengin, Gokhan, Nilofar, Evren Yildiztugay, Abdelhakim Bouyahya, Halit Cavusoglu, Reneta Gevrenova, and Dimitrina Zheleva-Dimitrova. 2023. "A Comparative Study on UHPLC-HRMS Profiles and Biological Activities of Inula sarana Different Extracts and Its Beta-Cyclodextrin Complex: Effective Insights for Novel Applications" Antioxidants 12, no. 10: 1842. https://doi.org/10.3390/antiox12101842
APA StyleZengin, G., Nilofar, Yildiztugay, E., Bouyahya, A., Cavusoglu, H., Gevrenova, R., & Zheleva-Dimitrova, D. (2023). A Comparative Study on UHPLC-HRMS Profiles and Biological Activities of Inula sarana Different Extracts and Its Beta-Cyclodextrin Complex: Effective Insights for Novel Applications. Antioxidants, 12(10), 1842. https://doi.org/10.3390/antiox12101842











