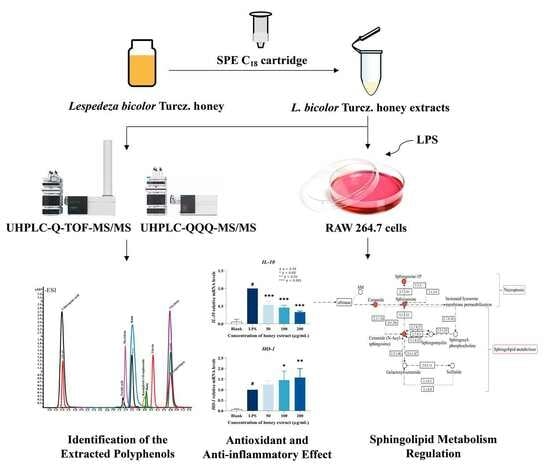
Antioxidant Polyphenols from Lespedeza bicolor Turcz. Honey: Anti-Inflammatory Effects on Lipopolysaccharide-Treated RAW 264.7 Macrophages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Collection and Processing of Honey
2.3. Determination of Polyphenol and Flavonoid Content
2.4. UHPLC-Q-TOF-MS/MS Parameter
2.5. UHPLC-QQQ-MS/MS Analysis
2.6. In Vitro Antioxidant Assays
2.7. Cell Culture
2.7.1. Cell Incubation and Assay
2.7.2. LPS-Induced NO Measurement
2.7.3. RNA Extraction and qRT-PCR
2.7.4. Collection of Metabolites from LPS-Treated Cells
2.7.5. UHPLC-Q-TOF-MS/MS
2.8. Statistics
3. Results and Discussion
3.1. The Polyphenols and Flavonoids Content on L. bicolor Honey
3.2. The Detection of Polyphenols in L. bicolor Honey
3.3. In Vitro Antioxidant Activity of L. bicolor Honey
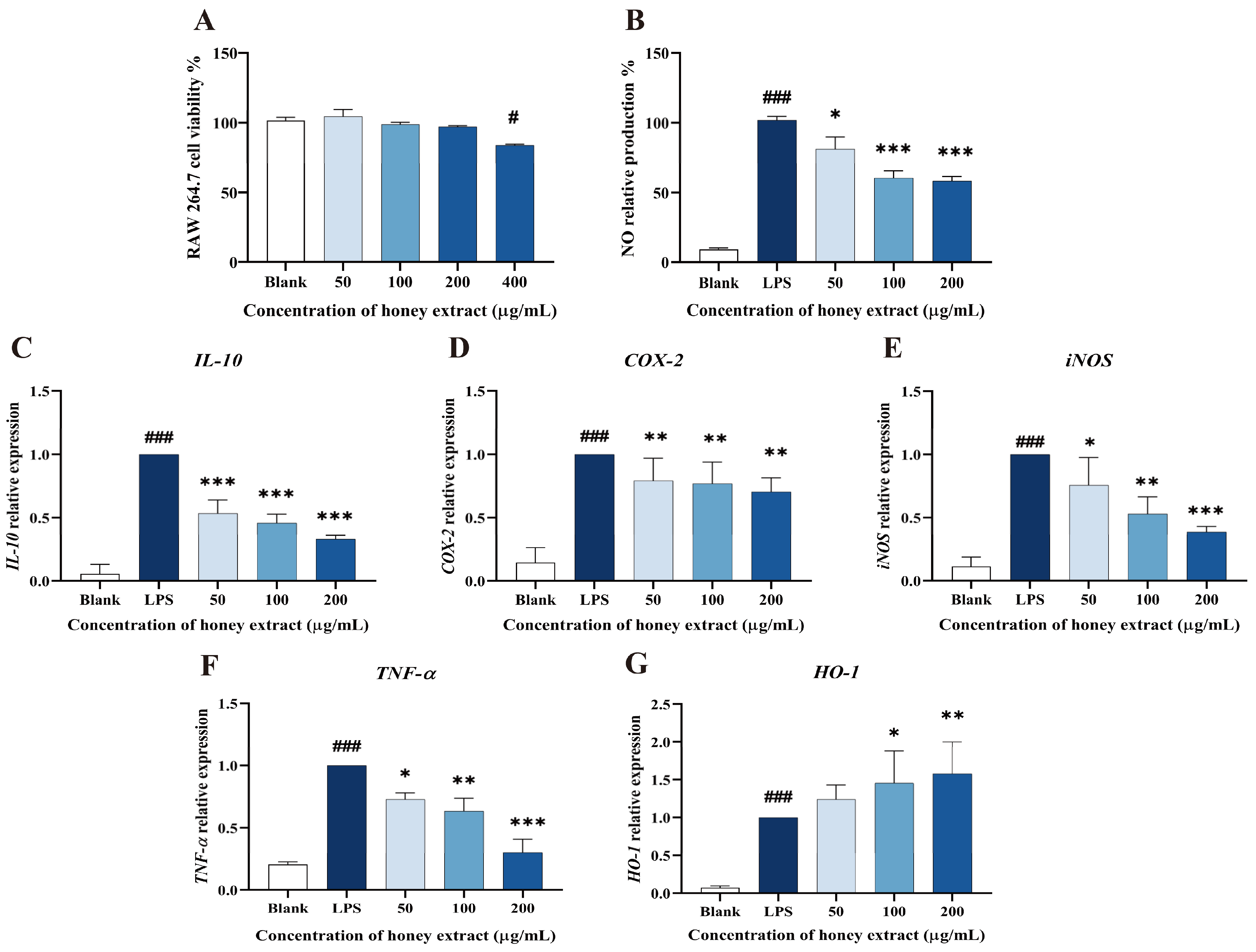
3.4. Effect of L. bicolor Honey Extract on RAW 264.7 Cells Viability
3.5. Influence of NO Production Treating with L. bicolor Honey Extract on RAW 264.7 Cells Inducing by LPS
3.6. L. bicolor Honey Extract Affects the Oxidation- and Inflammation-Response-Related Genes’ Expression
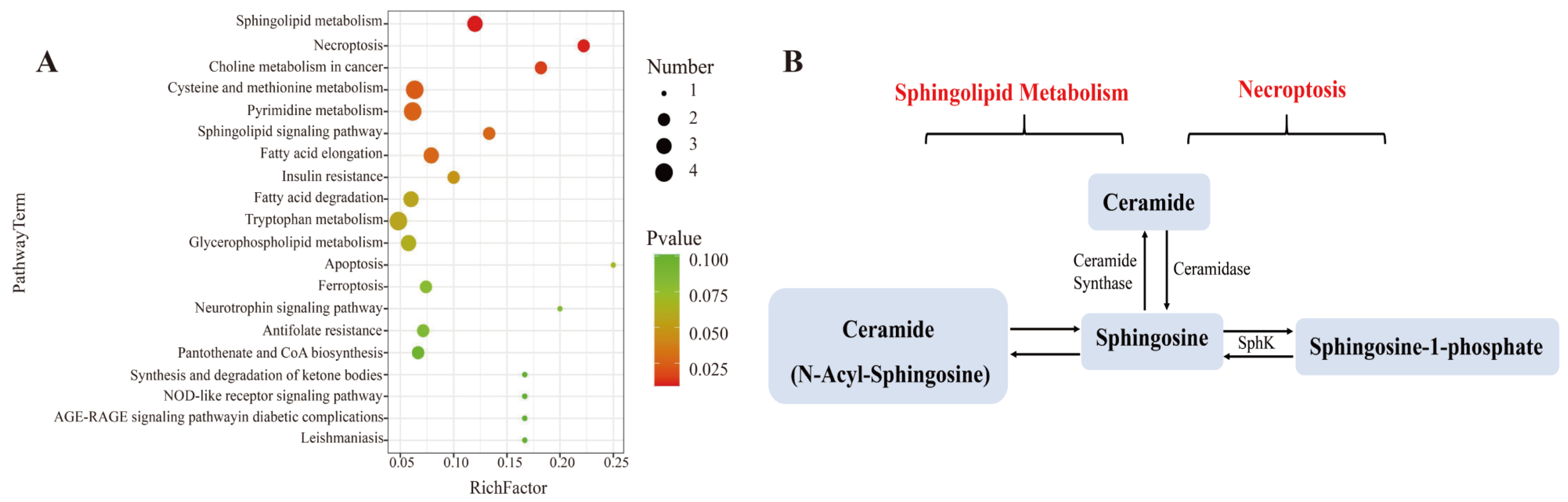
3.7. Effect of L. bicolor Honey Extract on Cell Metabolism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hernández, D.F.; Mojica, L.; Berhow, M.A.; Brownstein, K.; Cervantes, E.L.; de Mejia, E.G. Black and pinto beans (Phaseolus vulgaris L.) unique mexican varieties exhibit antioxidant and anti-inflammatory potential. Food Res. Int. 2023, 169, 112816. [Google Scholar] [CrossRef] [PubMed]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [PubMed]
- Seo, J.; Lee, U.; Seo, S.; Wibowo, A.E.; Pongtuluran, O.B.; Lee, K.; Han, S.B.; Cho, S. Anti-inflammatory and antioxidant activities of methanol extract of Piper betl Linn. (Piper betle L.) leaves and stems by inhibiting NF-κB/MAPK/Nrf2 signaling pathway in RAW 264.7 macrophages. Biomed. Pharmacother. 2022, 155, 113734. [Google Scholar] [CrossRef]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and health: A review of recent clinical research. Pharmacol. Res. 2017, 9, 121–127. [Google Scholar]
- Bouviere, J.; Fortunato, R.S.; Dupuy, C.; Werneck-de-Castro, J.P.; Carvalho, D.P.; Louzada, R.A. Exercise-stimulated ROS sensitive signaling pathways in skeletal muscle. Antioxidants 2021, 10, 537. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.L.; Zhang, L.; Ren, L.C.; Xie, Y.X. Advances in structures required of polyphenols for xanthine oxidase inhibition. Food Front. 2020, 1, 152–167. [Google Scholar] [CrossRef]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–55. [Google Scholar] [CrossRef]
- Deny, M.; Arroba Nuñez, L.A.; Romano, M.; Denis, O.; Casimir, G.; Chamekh, M. Sex difference in innate inflammatory response and macrophage polarization in Streptococcus agalactiae-induced pneumonia and potential role of microRNA-223-3p. Sci. Rep. 2022, 12, 17126. [Google Scholar] [CrossRef] [PubMed]
- Kacem, M.; Simon, G.; Leschiera, R.; Misery, L.; Eifeki, A.; Lebonvallet, N. Antioxidant and anti-inflammatory effects of Ruta chalepensis L. extracts on LPS-stimulated RAW 264.7 cells. In Vitro Cell. Dev. Biol. Anim. 2015, 51, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Souza, N.C.; de Oliveira, J.M.; Morrone, M.D.S.; Albanus, R.D.; Amarante, M.D.S.M.; Camillo, C.D.S.; Langassner, S.M.Z.; Gelain, D.P.; Moreira, J.C.F.; Dalmolin, R.J.S.; et al. Turnera subulata anti-inflammatory properties in lipopolysaccharide-stimulated RAW 264.7 macrophages. J. Med. Food 2016, 19, 922–930. [Google Scholar] [CrossRef]
- Laskin, D.L.; Sunil, V.R.; Gardner, C.R.; Laskin, J.D. Macrophages and tissue injury: Agents of defense or destruction? Annu. Rev. Pharmacol. Toxicol. 2011, 51, 267–288. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Gao, H.; Qi, H.; Chen, Y.; Paul Ross, R.; Stanton, C.; Zhao, J.X.; Zhang, H.; Chen, H.Q.; Chen, W. Linoleate isomerase complex contributes to metabolism and remission of DSS-induced colitis in mice of Lactobacillus plantarum ZS2058. J. Agric. Food Chem. 2021, 69, 8160–8171. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.; Ahmed, M. Evaluation of antioxidant potential of honey drops and honey lozenges. Food Chem. Adv. 2022, 1, 100013. [Google Scholar] [CrossRef]
- Purohit, P.; Roy, D.; Dwivedi, S.; Nebhinani, N.; Sharma, P. Association of miR-155, miR-187 and inflammatory cytokines IL-6, IL-10 and TNF-alpha in chronic opium abusers. Inflammation 2022, 45, 554–566. [Google Scholar] [CrossRef]
- Vyshnevska, L.; Severina, H.I.; Prokopenko, Y.; Shmalko, A. Molecular docking investigation of anti-inflammatory herbal compounds as potential LOX-5 and COX-2 inhibitors. Pharmacia 2022, 69, 733–744. [Google Scholar] [CrossRef]
- Wu, X.; Song, M.Y.; Gao, Z.L.; Sun, Y.; Wang, M.Q.; Li, F.; Zheng, J.K.; Xiao, H. Nobiletin and its colonic metabolites suppress colitis-associated colon carcinogenesis by down-regulating iNOS, inducing antioxidative enzymes and arresting cell cycle progression. J. Nutr. Biochem. 2017, 42, 17–25. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, Z.Q.; Misra, B.R.; Zhang, L.; Cao, Z.X.; Yamamoto, M.; Trush, M.A.; Misra, H.P.; Li, Y.B. Nuclear factor E2-related factor 2-dependent myocardiac cytoprotection against oxidative and electrophilic stress. Cardiovasc. Toxicol. 2008, 8, 71. [Google Scholar] [CrossRef]
- Yang, C.C.; Hsiao, L.D.; Shih, Y.F.; Su, M.H.; Yang, C.M. Sphingosine 1-phosphate-upregulated COX-2/PGE (2) system contributes to human cardiac fibroblast apoptosis: Involvement of MMP-9-dependent transactivation of EGFR cascade. Oxid. Med. Cell. Longev. 2022, 2022, 7664290. [Google Scholar]
- Yan, H.; Zhao, H.; Yi, S.W.; Zhuang, H.; Wang, D.W.; Jiang, J.G.; Shen, G.F. Sphingosine-1-phosphate protects against the development of cardiac remodeling via Sphingosine kinase 2 and the S1PR2/ERK pathway. Curr. Med. Sci. 2022, 42, 702–710. [Google Scholar] [CrossRef]
- Do, M.H.; Lee, J.H.; Cho, K.; Kang, M.C.; Subedi, L.; Parveen, A.; Kim, S.Y. Therapeutic potential of Lespedeza bicolor to prevent methylglyoxal-induced glucotoxicity in familiar diabetic nephropathy. J. Clin. Med. 2019, 8, 1138. [Google Scholar] [CrossRef]
- Guo, N.N.; Zhao, L.W.; Zhao, Y.Z.; Li, Q.Q.; Xue, X.F.; Wu, L.M.; Escalada, M.G.; Wang, K.; Peng, W.J. Comparison of the chemical composition and biological activity of mature and immature honey: An HPLC/QTOF/MS-based metabolomic approach. J. Agri. Food Chem. 2020, 68, 4062–4071. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jhoo, J.W. Antioxidant activity of different parts of Lespedeza bicolor and isolation of antioxidant compound. Korean J. Food Sci. Technol. 2012, 44, 763–771. [Google Scholar] [CrossRef]
- Montaser, M.; Ali, A.T.; Sayed, A.M.; Abdelmohsen, U.R.; Zidan, E.W.; Orfali, R.; Rateb, M.E.; Zaki, M.A.; Hassan, H.M.; Mohammed, R.; et al. 1H-NMR metabolic profiling, antioxidant activity, and docking study of common medicinal plant-derived honey. Antioxidants 2022, 11, 1880. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.Y.; He, Z.Q.; Chen, Y.J.; Li, Z.Y.; Meng, T.M.; Li, Y.F.; Cao, Y. In vitro and in vivo antioxidant activity of eucalyptus leaf polyphenols extract and its effect on chicken meat quality and cecum microbiota. Food Res. Int. 2020, 136, 109302. [Google Scholar] [CrossRef]
- Ren, C.J.; Wang, K.; Luo, T.; Xue, X.F.; Wang, M.; Wu, L.M.; Zhao, L.W. Kaempferol-3-O-galactoside as a marker for authenticating Lespedeza bicolor Turcz. monofloral honey. Food Res. Int. 2022, 160, 111667. [Google Scholar] [CrossRef]
- Vaughn, M.B.; Gretchen, D.J. The r-values of honey: Pollen coefficients. Palynology 2011, 25, 11–28. [Google Scholar]
- Li, Q.Q.; Sun, M.H.; Wan, Z.R.; Liang, J.; Betti, M.; Hrynets, Y.; Xue, X.F.; Wu, L.M.; Wang, K. Bee pollen extracts modulate serum metabolism in lipopolysaccharide-induced acute lung injury mice with anti-inflammatory effects. J. Agric. Food Chem. 2019, 67, 7855–7868. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.T.; Lu, Y.F.; Inbaraj, B.S.; Chen, B.H. Determination of phenolic acids and flavonoids in Rhinacanthus nasutus (L.) kurz. by high-performance-liquid-chromatography with photodiode-array detection and randem mass spectrometry. J. Funct. Foods 2015, 12, 498–508. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Aires, E.; Barreira, J.C.M.; Estevinho, L.M. Antioxidant activity of Portuguese honey samples: Different contributions of the entire honey and phenolic extract. Food Chem. 2009, 114, 1438–1443. [Google Scholar] [CrossRef]
- Silici, S.; Sagdic, O.; Ekici, L. Total phenolic content, antiradical, antioxidant and antimicrobial activities of Rhododendron honeys. Food Chem. 2010, 121, 238–243. [Google Scholar] [CrossRef]
- Kolayli, S.; Can, Z.; Çakir, H.E.; Okan, O.T.; Yildiz, O. An investigation on Trakya region Oak (Quercus spp.) honeys of Turkey: Their physico-chemical, antioxidant and phenolic compounds properties. Turkish J. Biochem. 2018, 43, 362–374. [Google Scholar] [CrossRef]
- Wabaidur, S.M.; Obbed, M.S.; Alothman, Z.A.; Alfaris, N.A.; Badjah-Hadj-Ahmed, A.Y.; Siddiqui, M.R.; Aldayel, T.S. Total phenolic acids and flavonoid contents determination in Yemeni honey of various floral sources: Folin-Ciocalteu and spectrophotometric approach. Food Sci. Technol. Int. 2020, 40, 647–652. [Google Scholar] [CrossRef]
- Hossen, M.S.; Ali, M.Y.; Jahurul, M.H.A.; Abdel-Daim, M.M.; Gan, S.H.; Khalil, M.I. Beneficial roles of honey polyphenols against some human degenerative diseases: A review. Pharmacol. Rep. 2017, 69, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Ramanauskiene, K.; Savickas, A.; Inkeniene, A.; Vitkevicius, K.; Kasparaviciene, G.; Briedis, V.; Amsiejus, A. Analysis of content of phenolic acids in Lithuanian propolis using high-performance liquid chromatography technique. Medicina 2009, 45, 712–717. [Google Scholar] [CrossRef]
- Jasna, B.; Urška, D.; Mojca, J.; Terezija, G. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007, 105, 822–828. [Google Scholar]
- Zhao, T.; Zhao, L.W.; Wang, M.; Qi, S.Z.; Xue, X.F.; Wu, L.M.; Li, Q.Q. Identification of characteristic markers for monofloral honey of Astragalus membranaceus var. mongholicus Hsiao: A combined untargeted and targeted MS-based study. Food Chem. 2023, 404, 134312. [Google Scholar] [CrossRef]
- Rodríguez, J.; Olea-Azar, C.; Cavieres, C.; Norambuena, E.; Delgado-Castro, T.; Soto-Delgado, J.; Araya-Maturana, R. Antioxidant properties and free radical scavenging reactivity of a family of hydroxynaphthalenones and dihydroxyanthracenones. Bioorg. Med. Chem. 2007, 15, 7058–7065. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Antioxidant characterization: Methodology and mechanism. Biochem. Pharmacol. 1995, 49, 1341–1348. [Google Scholar] [CrossRef]
- Zarei, M.; Fazlara, A.; Tulabifard, N. Effect of thermal treatment on physicochemical and antioxidant properties of honey. Heliyon 2019, 5, e01894. [Google Scholar] [CrossRef] [PubMed]
- Burow, P.; Klapperstück, M.; Markwardt, F. Activation of ATP secretion via volume-regulated anion channels by sphingosine-1-phosphate in RAW macrophages. Pflugers Arch. 2015, 467, 1215–1226. [Google Scholar] [CrossRef]
- Xu, L.; Lu, P.; Wang, Y. Sphingosine-1-phosphate receptor modulators for the treatment of inflammatory bowel disease and other immune-mediated diseases. Med. Chem. Res. 2022, 31, 2074–2088. [Google Scholar] [CrossRef]
- Guo, L.; Geng, X.J.; Ma, L.; Luo, C.; Zeng, W.S.; Ou, X.H.; Chen, L.N.; Quan, S.; Li, H. Sphingosine-1-phosphate inhibits ceramide-induced apoptosis during murine preimplantation embryonic development. Theriogenology 2013, 80, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, M.; Young, S.P. Metabolomics—A novel window into inflammatory disease. Swiss Med. Wkly. 2013, 25, w13743. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.X.; Wang, X.; Cao, W.Y.J.; Zheng, S.; Ma, Y.T.; Huang, Q.W. NF-κB and AMPK-Nrf2 pathways support the protective effect of polysaccharides from Polygonatum cyrtonema Hua in lipopolysaccharide-induced acute lung injury. J. Ethnopharmacol. 2022, 291, 115153. [Google Scholar] [CrossRef] [PubMed]
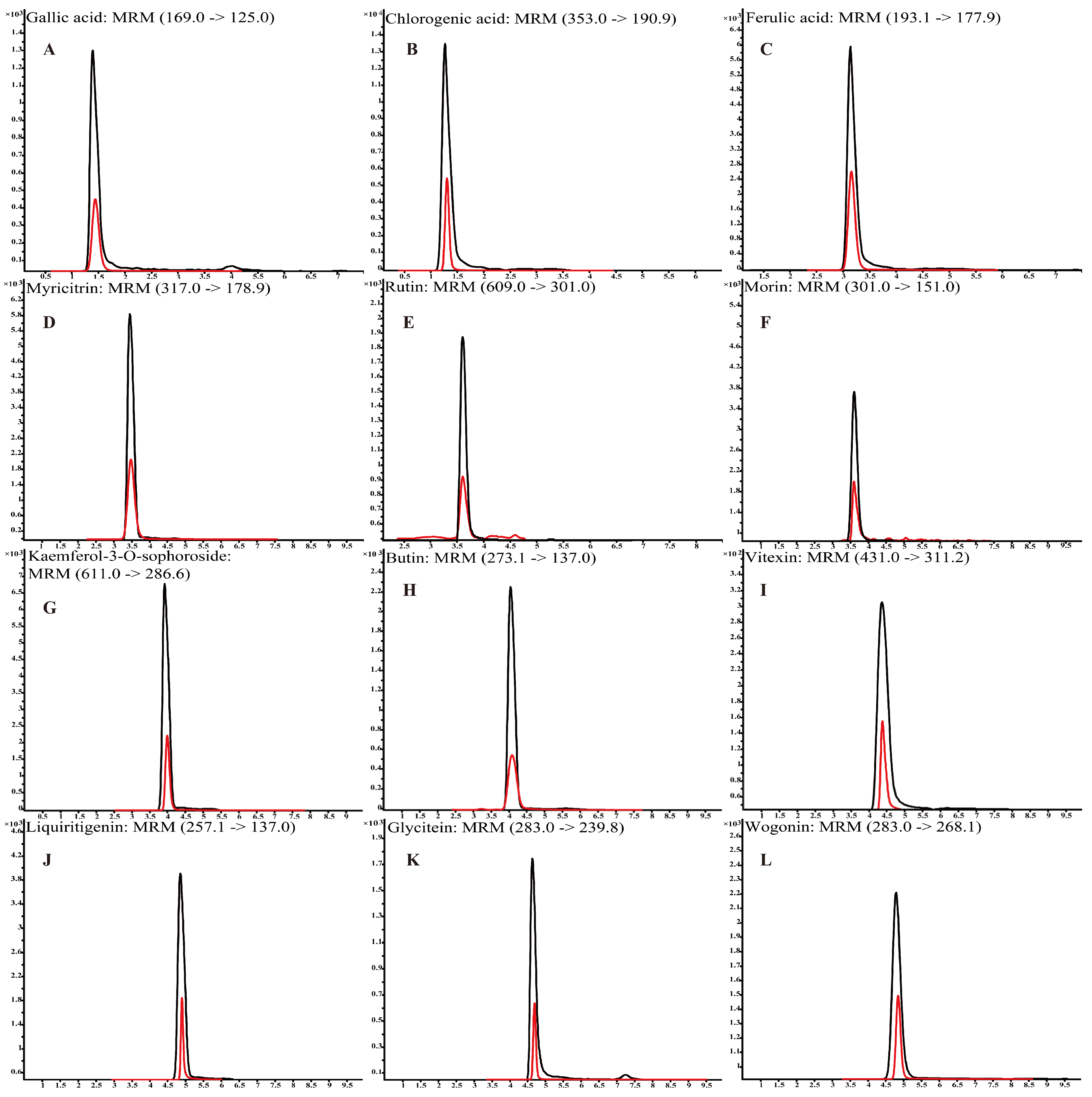

| No. | Compound | Formula | RT/min | Regression Equation | R2 | mg/kg (Mean ± SD) |
|---|---|---|---|---|---|---|
| 1 | Chlorogenic acid | C16H18O9 | 1.4 | y = 21,979x + 3130 | 0.9979 | 16.28 ± 5.68 |
| 2 | Ferulic acid | C10H10O4 | 3.3 | y = 6875x − 4 | 0.9999 | 1.42 ± 0.64 |
| 3 | Vitexin | C21H20O10 | 4.3 | y = 21,424x − 121 | 0.9998 | 9.59 ± 2.79 |
| 4 | Rutin | C27H30O16 | 3.6 | y = 17,807x − 508 | 0.9963 | 7.22 ± 2.90 |
| 5 | Gallic acid | C7H6O5 | 1.4 | y = 2708x − 20 | 0.9924 | 5.04 ± 1.93 |
| 6 | Myricitrin | C15H10O8 | 3.4 | y = 41x − 2 | 0.9949 | 5.43 ± 3.52 |
| 7 | Morin | C15H10O7 | 3.6 | y = 440,451x + 1211 | 0.9963 | 4.39 ± 3.08 |
| 8 | Kaempferol-3-O-sophoroside | C33H40O21 | 4.0 | y = 3201x − 5 | 0.9996 | 7.21 ± 1.94 |
| 9 | Glycitein | C16H12O5 | 4.8 | y = 6452x + 272 | 0.9968 | 17.27 ± 3.37 |
| 10 | Wogonin | C16H12O5 | 4.8 | y = 50,170x + 2956 | 0.9940 | 7.07 ± 4.72 |
| 11 | Butin | C15H12O5 | 4.0 | y = 65,503x − 253 | 0.9972 | 2.77 ± 1.09 |
| 12 | Liquiritigenin | C15H12O4 | 4.8 | y = 126,010x − 67 | 0.9999 | 3.60 ± 1.33 |
| Pathway | Metabolites | LPS Group vs. Blank Group a | Honey Group b vs. LPS Group c | ||||
|---|---|---|---|---|---|---|---|
| p-Value | Log2 (Fold Change) | Trend | p-Value | Log2 (Fold Change) | Trend | ||
| Sphingolipid Metabolism/Necroptosis | Ceramide | 0.0087 | 13.36 | up | 0.0007 | −2.38 | down |
| Sphingosine | 0.0040 | 2.26 | up | 0.0380 | −2.22 | down | |
| Sphingosine-1-phosphate (S1P) | 0.0005 | 13.42 | up | 0.0157 | −4.77 | down | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, C.; Li, Q.; Luo, T.; Betti, M.; Wang, M.; Qi, S.; Wu, L.; Zhao, L. Antioxidant Polyphenols from Lespedeza bicolor Turcz. Honey: Anti-Inflammatory Effects on Lipopolysaccharide-Treated RAW 264.7 Macrophages. Antioxidants 2023, 12, 1809. https://doi.org/10.3390/antiox12101809
Ren C, Li Q, Luo T, Betti M, Wang M, Qi S, Wu L, Zhao L. Antioxidant Polyphenols from Lespedeza bicolor Turcz. Honey: Anti-Inflammatory Effects on Lipopolysaccharide-Treated RAW 264.7 Macrophages. Antioxidants. 2023; 12(10):1809. https://doi.org/10.3390/antiox12101809
Chicago/Turabian StyleRen, Caijun, Qiangqiang Li, Teng Luo, Mirko Betti, Miao Wang, Suzhen Qi, Liming Wu, and Liuwei Zhao. 2023. "Antioxidant Polyphenols from Lespedeza bicolor Turcz. Honey: Anti-Inflammatory Effects on Lipopolysaccharide-Treated RAW 264.7 Macrophages" Antioxidants 12, no. 10: 1809. https://doi.org/10.3390/antiox12101809
APA StyleRen, C., Li, Q., Luo, T., Betti, M., Wang, M., Qi, S., Wu, L., & Zhao, L. (2023). Antioxidant Polyphenols from Lespedeza bicolor Turcz. Honey: Anti-Inflammatory Effects on Lipopolysaccharide-Treated RAW 264.7 Macrophages. Antioxidants, 12(10), 1809. https://doi.org/10.3390/antiox12101809