Caffeic Acid Phenethyl Ester Loaded PEG–PLGA Nanoparticles Enhance Wound Healing in Diabetic Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Preparation of CAPE-NPs
2.4. Characterization of CAPE-NPs’ Size
2.5. Evaluation of CAPE-PEG–PLGA Loading Capacity
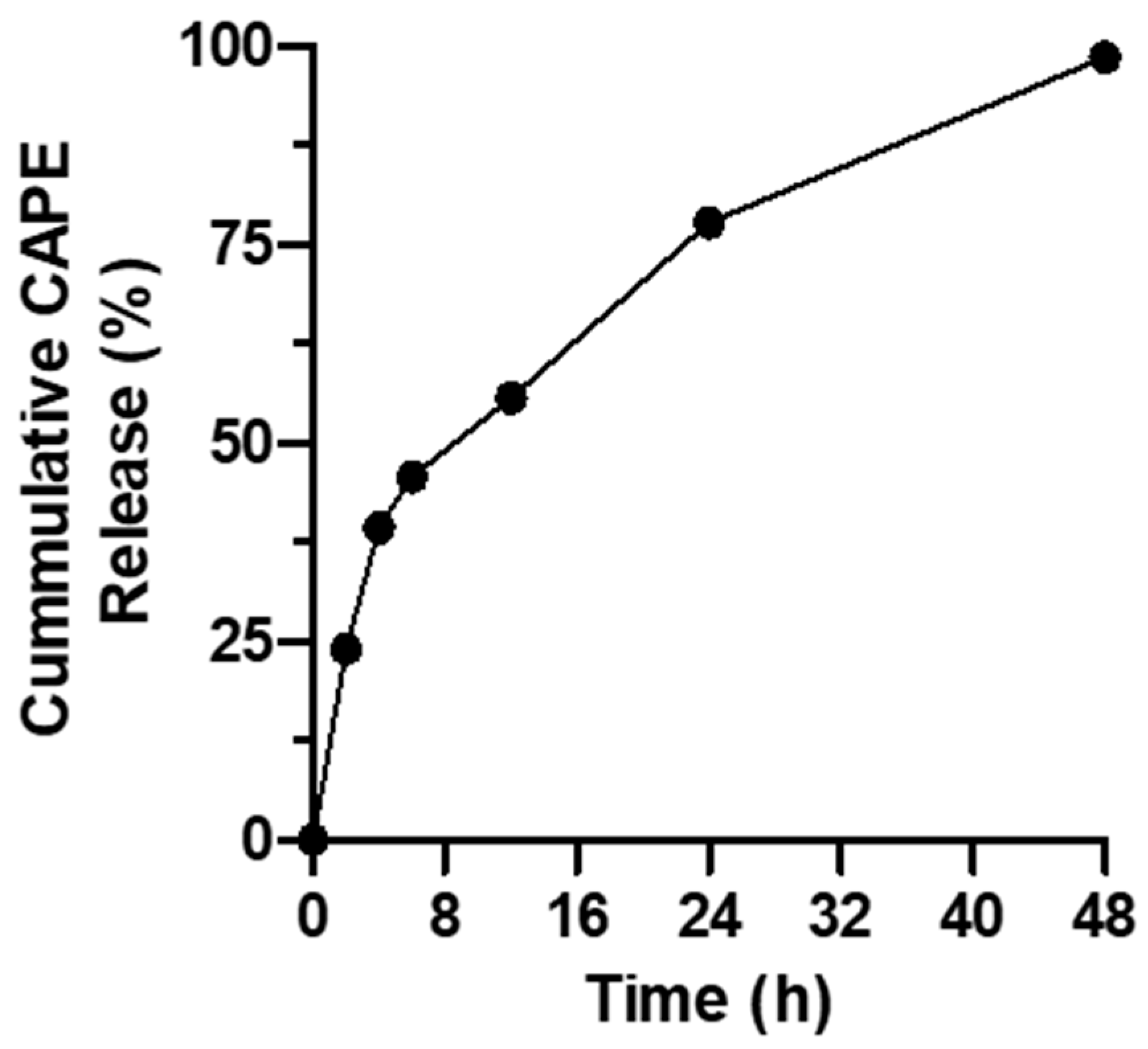
2.6. In Vitro CAPE Release Study
2.7. Experimental Design
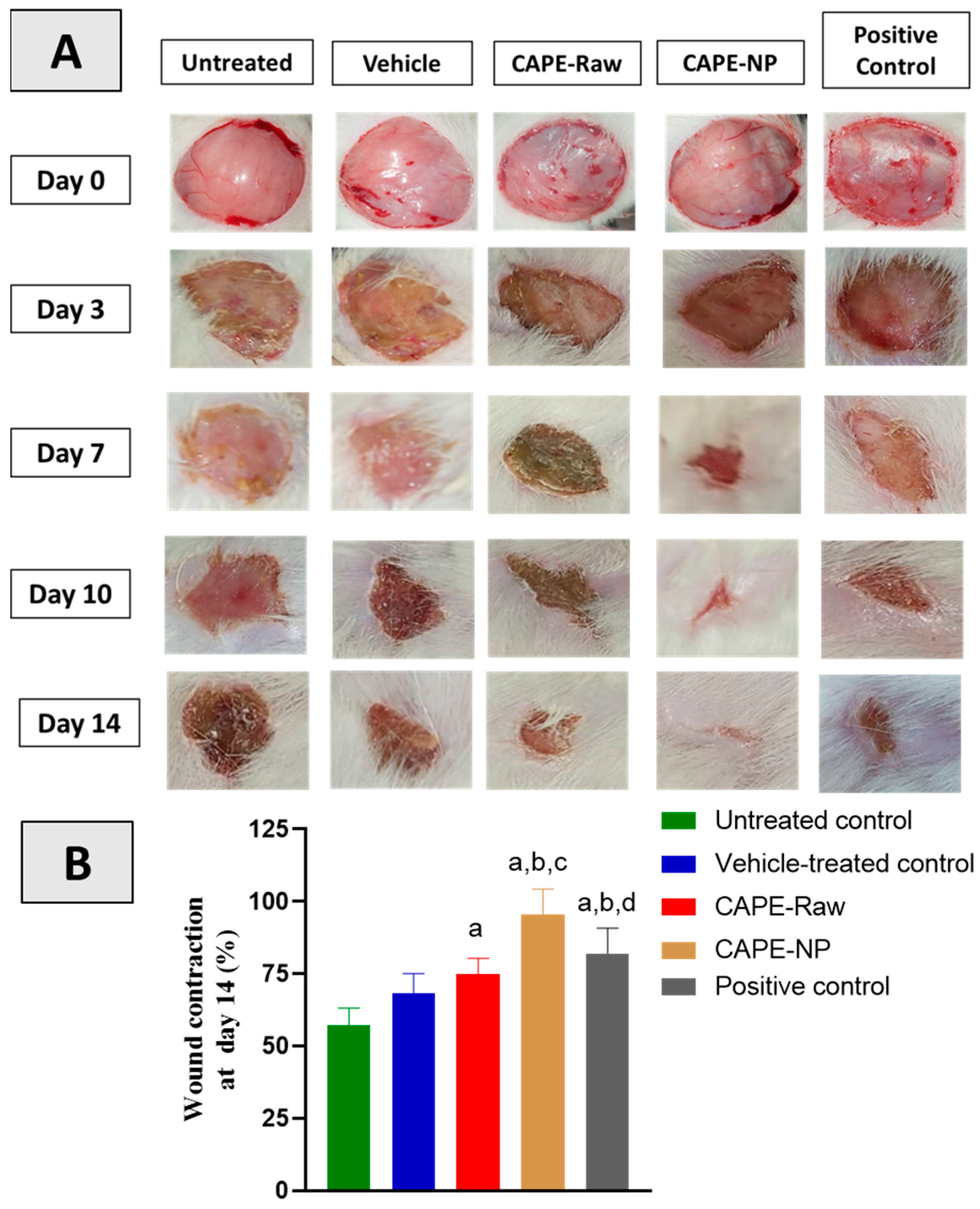
2.8. Assessment of Wound Contraction
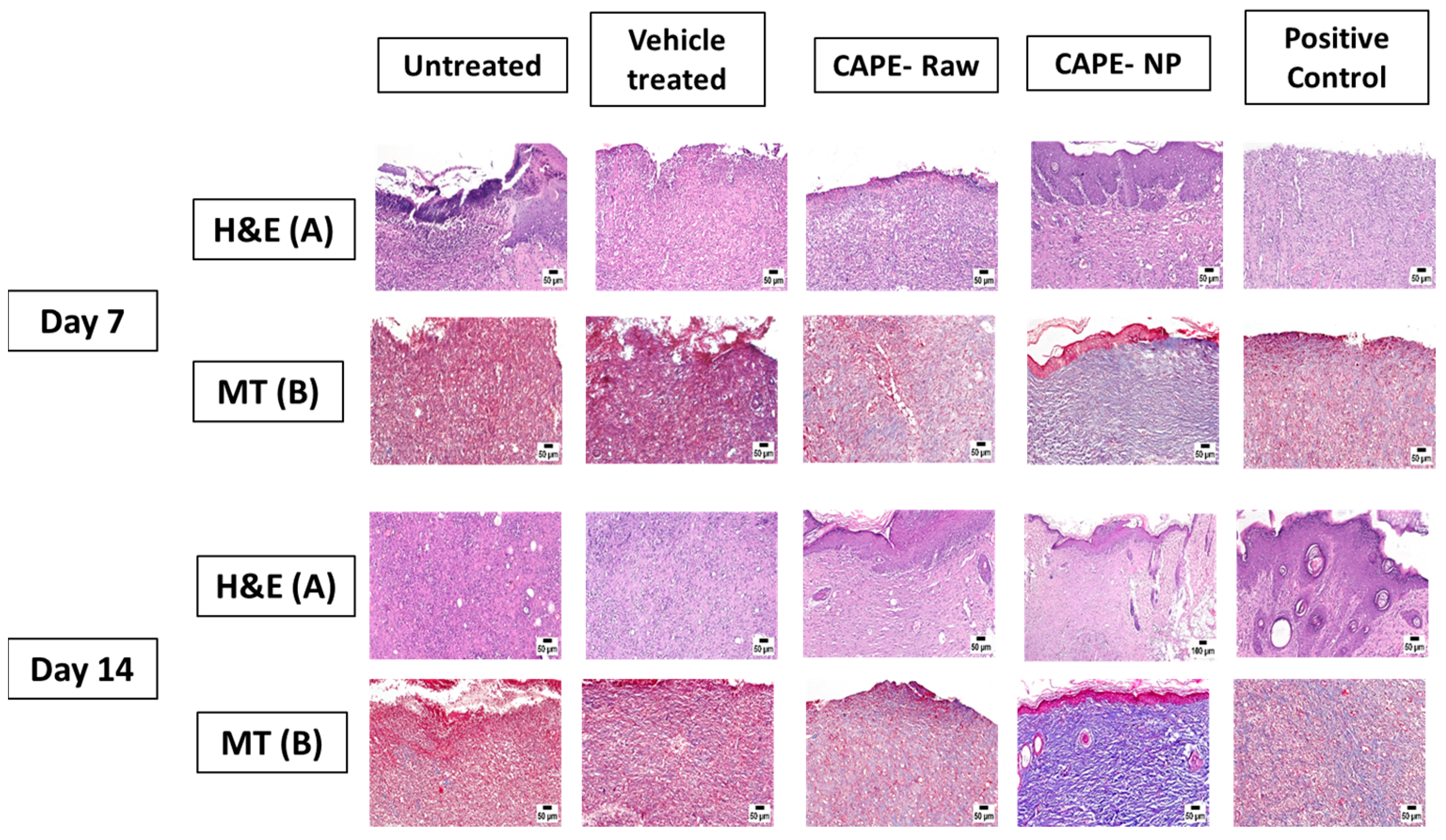
2.9. Histopathology
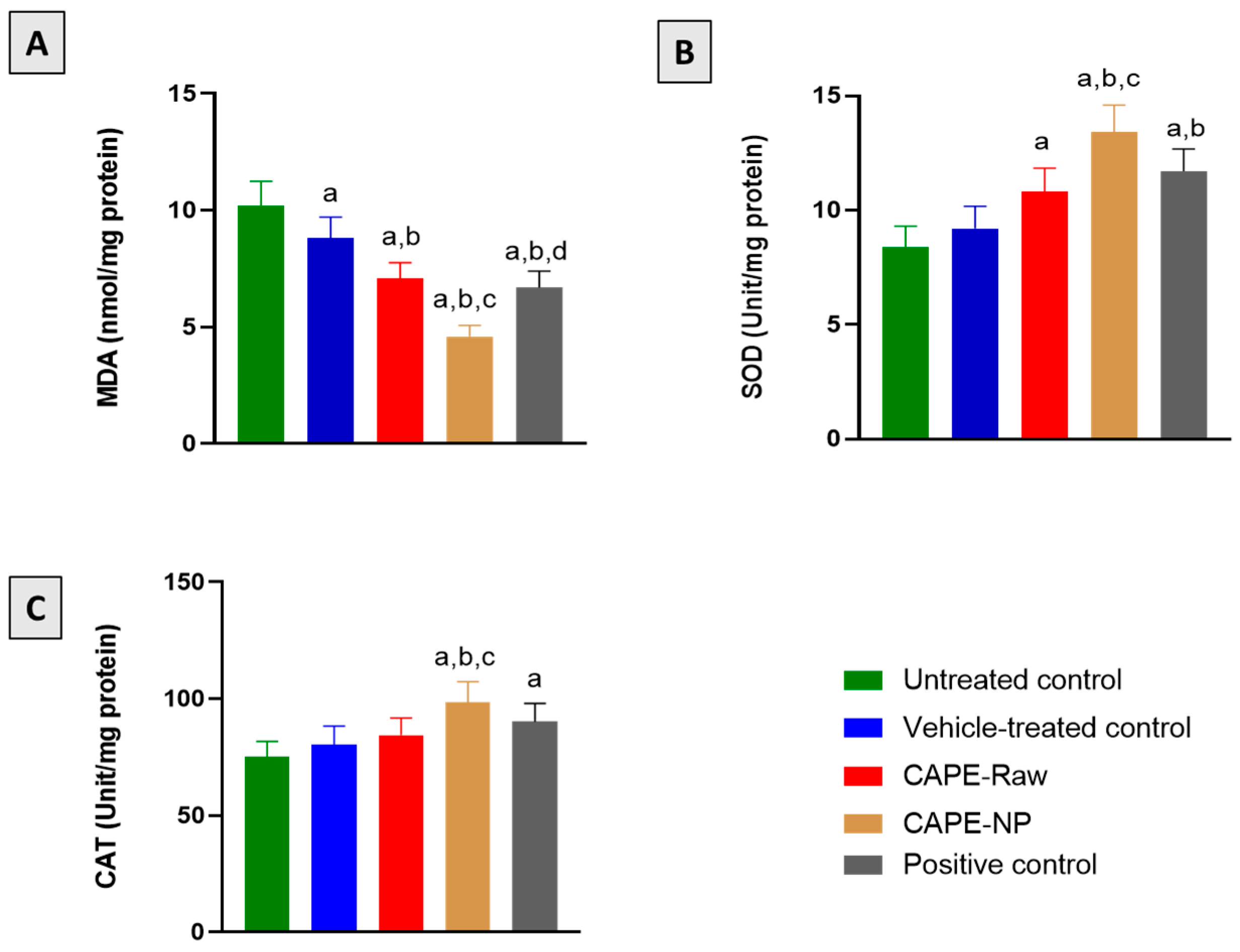
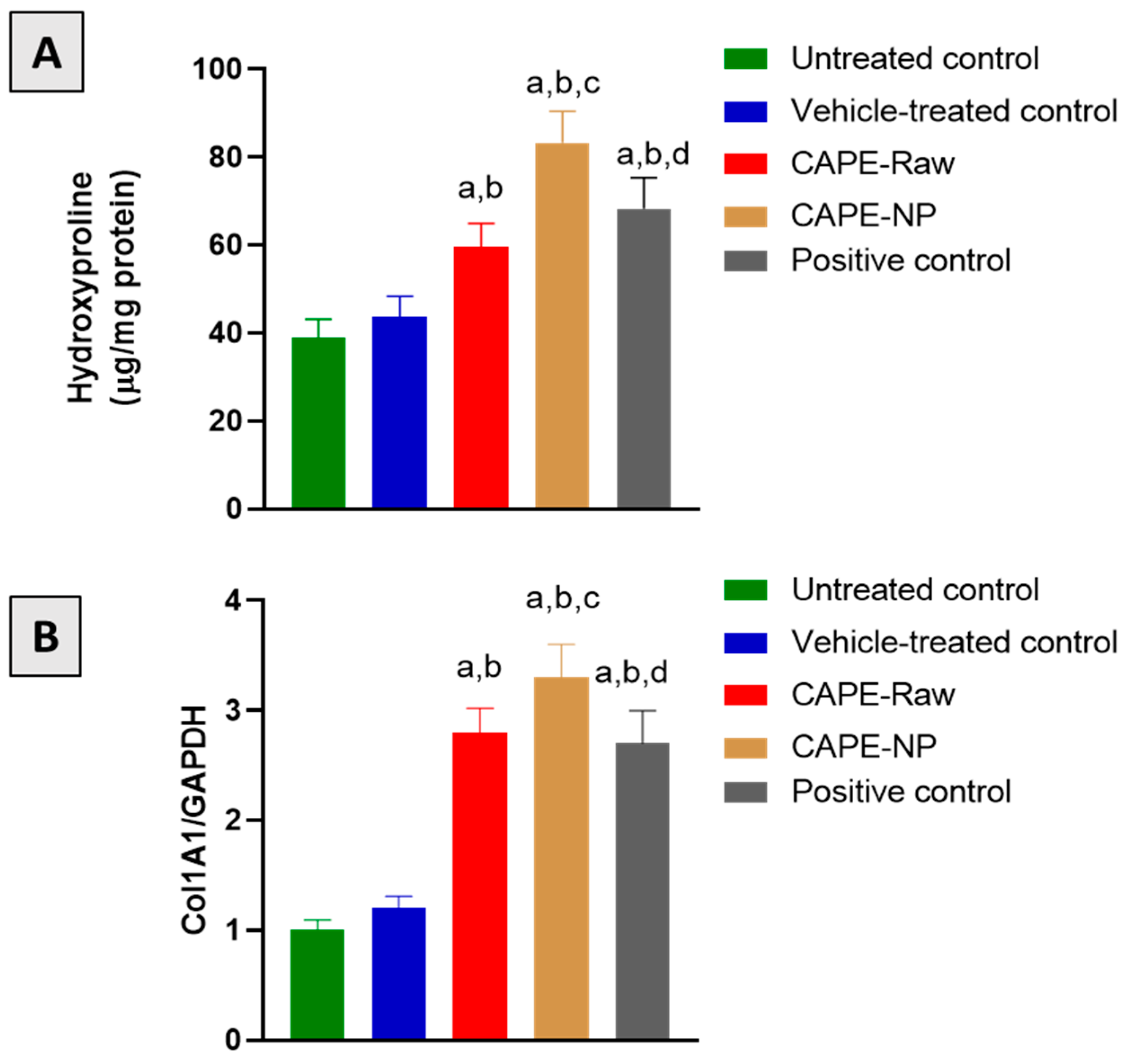
2.10. Biochemical Analyses
2.11. Quantitative Real-Time PCR (qRT-PCR)
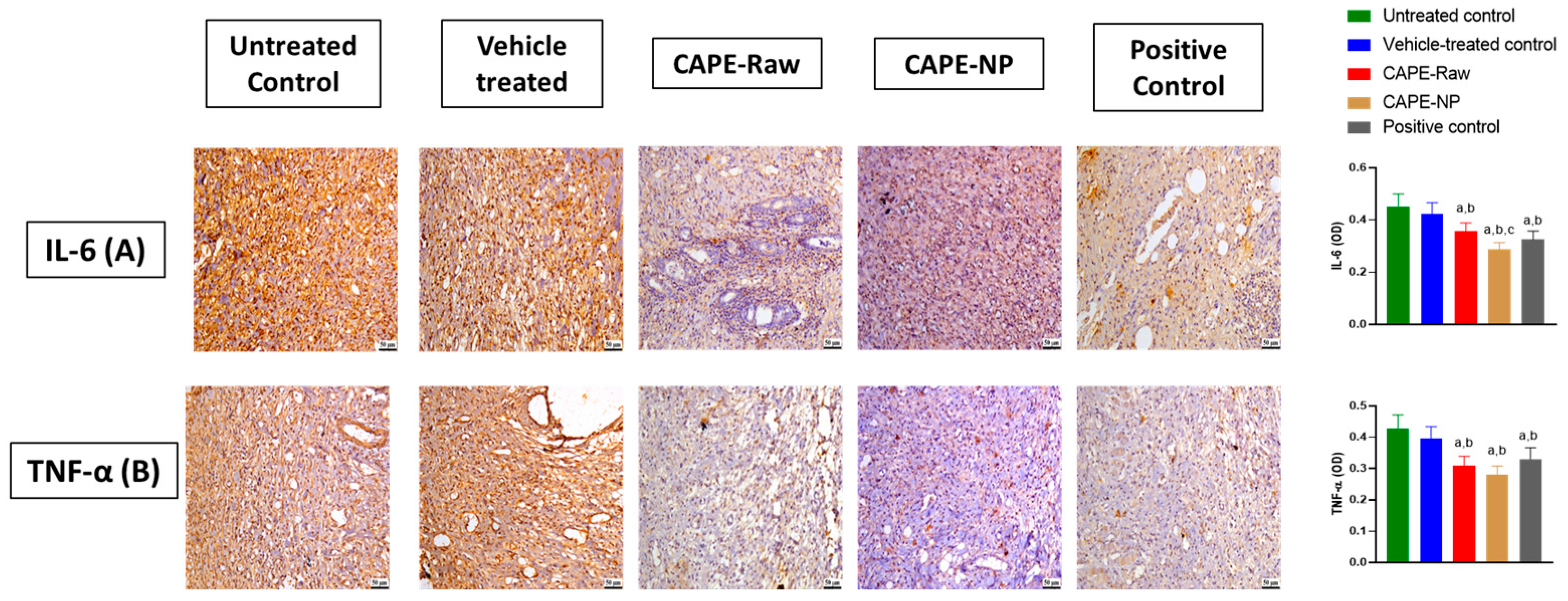
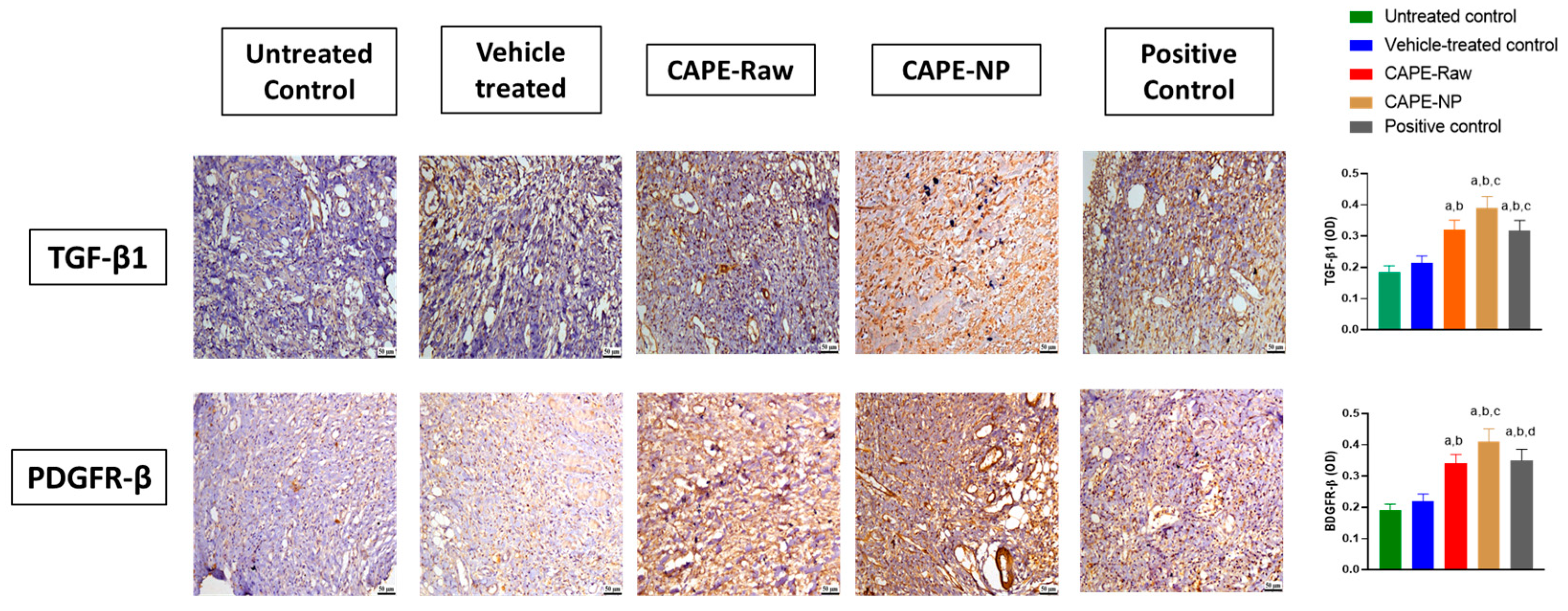
2.12. Assessment of IL-6, TNF-α, TGF-β1, and PDGFRα, Immunohistochemically
2.13. Statistical Analysis
3. Results
3.1. Particle Size, Polydispersity Index, and Loading Capacity
3.2. Assessment of In Vitro CAPE Release
3.3. Effect of CAPE-NPs on Wound Contraction
3.4. Effect of CAPE-NPs on Histological Changes of Wounded Skin (H&E and MT Staining)
3.5. Effect of CAPE-NPs on Antioxidant Status
3.6. Impact of CAPE-NPs on Inflammatory Markers
3.7. Effect of CAPE-NPs on Markers of Collagen Formation
3.8. Impact of CAPE-NPs on Expression of TGF-β1 and PDGF-B
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alam, S.; Hasan, M.K.; Neaz, S.; Hussain, N.; Hossain, M.F.; Rahman, T. Diabetes Mellitus: Insights from Epidemiology, Biochemistry, Risk Factors, Diagnosis, Complications and Comprehensive Management. Diabetology 2021, 2, 36–50. [Google Scholar] [CrossRef]
- Oh, S.H.; Ku, H.; Park, K.S. Prevalence and Socioeconomic Burden of Diabetes Mellitus in South Korean Adults: A Population-Based Study Using Administrative Data. BMC Public Health 2021, 21, 548. [Google Scholar] [CrossRef] [PubMed]
- Naeem, Z. Burden of Diabetes Mellitus in Saudi Arabia. Int. J. Health Sci. 2015, 9, V–VI. [Google Scholar] [CrossRef]
- Dilworth, L.; Facey, A.; Omoruyi, F. Diabetes Mellitus and Its Metabolic Complications: The Role of Adipose Tissues. Int. J. Mol. Sci. 2021, 22, 7644. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J. Limb Loss: The Unspoken Psychological Aspect. J. Vasc. Nurs. 2016, 34, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.C.D.O.; Andrade, Z.D.A.; Costa, T.F.; Medrado, A.R.A.P. Wound Healing—A Literature Review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Wetzler, C.; Kampfer, H.; Stallmeyer, B.; Pfeilschifter, J.; Frank, S. Large and Sustained Induction of Chemokines during Impaired Wound Healing in the Genetically Diabetic Mouse: Prolonged Persistence of Neutrophils and Macrophages during the Late Phase of Repair. J. Investig. Dermatol. 2000, 115, 245–253. [Google Scholar] [CrossRef]
- Chakraborty, R.; Borah, P.; Dutta, P.P.; Sen, S. Evolving Spectrum of Diabetic Wound: Mechanistic Insights and Therapeutic Targets. World J. Diabetes 2022, 13, 696–716. [Google Scholar] [CrossRef]
- Roberts, A.B. Transforming Growth Factor-Beta: Activity and Efficacy in Animal Models of Wound Healing. Wound Repair Regen. 1995, 3, 408–418. [Google Scholar] [CrossRef]
- Caballero, S.; Sengupta, N.; Afzal, A.; Chang, K.H.; Li Calzi, S.; Guberski, D.L.; Kern, T.S.; Grant, M.B. Ischemic Vascular Damage Can Be Repaired by Healthy, but Not Diabetic, Endothelial Progenitor Cells. Diabetes 2007, 56, 960–967. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Everett, E.; Mathioudakis, N. Update on Management of Diabetic Foot Ulcers. Ann. N. Y. Acad. Sci. 2018, 1411, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, S.F.; Caruso, G.I.; De Pasquale, R.; Sortino, M.A.; Merlo, S. The Treatment of Impaired Wound Healing in Diabetes: Looking among Old Drugs. Pharmaceuticals 2020, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Asfour, H.Z.; Alhakamy, N.A.; Ahmed, O.A.A.; Fahmy, U.A.; El-moselhy, M.A.; Rizg, W.Y.; Alghaith, A.F.; Eid, B.G.; Abdel-Naim, A.B. Amitriptyline-Based Biodegradable PEG-PLGA Self-Assembled Nanoparticles Accelerate Cutaneous Wound Healing in Diabetic Rats. Pharmaceutics 2022, 14, 1792. [Google Scholar] [CrossRef] [PubMed]
- Alhakamy, N.A.; Caruso, G.; Privitera, A.; Ahmed, O.A.A.; Fahmy, U.A.; Md, S.; Mohamed, G.A.; Ibrahim, S.R.M.; Eid, B.G.; Abdel-Naim, A.B.; et al. Fluoxetine Ecofriendly Nanoemulsion Enhances Wound Healing in Diabetic Rats: In Vivo Efficacy Assessment. Pharmaceutics 2022, 14, 1133. [Google Scholar] [CrossRef] [PubMed]
- Nikam, A.; Thomas, A.; Giram, P.; Nagore, D.; Chitlange, S. Herbal-Based Dressings in Wound Management. Curr. Diabetes Rev. 2022, 18, e010422202937. [Google Scholar] [CrossRef] [PubMed]
- Olgierd, B.; Kamila, Ż.; Anna, B.; Emilia, M. The Pluripotent Activities of Caffeic Acid Phenethyl Ester. Molecules 2021, 26, 1335. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5281787, Caffeic Acid Phenethyl Ester. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Caffeic_acid_phenethyl_ester (accessed on 20 November 2022).
- Tolba, M.F.; Azab, S.S.; Khalifa, A.E.; Abdel-Rahman, S.Z.; Abdel-Naim, A.B. Caffeic Acid Phenethyl Ester, a Promising Component of Propolis with a Plethora of Biological Activities: A Review on Its Anti-Inflammatory, Neuroprotective, Hepatoprotective, and Cardioprotective Effects. IUBMB Life 2013, 65, 699–709. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, K.; Zheng, S.; Wang, Y.; Ren, Q.; Li, H.; Ding, L.; Li, W.; Zhang, L. Antibacterial Effect of Caffeic Acid Phenethyl Ester on Cariogenic Bacteria and Streptococcus Mutans Biofilms. Antimicrob. Agents Chemother. 2020, 64, e00251-20. [Google Scholar] [CrossRef]
- Arasoglu, T.; Derman, S.; Mansuroglu, B. Comparative Evaluation of Antibacterial Activity of Caffeic Acid Phenethyl Ester and PLGA Nanoparticle Formulation by Different Methods. Nanotechnology 2016, 27, 025103. [Google Scholar] [CrossRef]
- Erdemli, H.; Akyol, S.; Armutcu, F.; Akyol, O. Antiviral Properties of Caffeic Acid Phenethyl Ester and Its Potential Application. J. Intercult. Ethnopharmacol. 2015, 4, 344. [Google Scholar] [CrossRef] [PubMed]
- Albukhari, A.A.; Gashlan, H.M.; El-Beshbishy, H.A.; Nagy, A.A.; Abdel-Naim, A.B. Caffeic Acid Phenethyl Ester Protects against Tamoxifen-Induced Hepatotoxicity in Rats. Food Chem. Toxicol. 2009, 47, 1689–1695. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Wang, M.; Chen, A.; Zhang, C.; Lin, L.; Zhang, Z.; Chen, A. Protective Effect of Caffeic Acid Phenethyl Ester against Imidacloprid-Induced Hepatotoxicity by Attenuating Oxidative Stress, Endoplasmic Reticulum Stress, Inflammation and Apoptosis. Pestic. Biochem. Physiol. 2020, 164, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Kobroob, A.; Chattipakorn, N.; Wongmekiat, O. Caffeic Acid Phenethyl Ester Ameliorates Cadmium-Induced Kidney Mitochondrial Injury. Chem. Biol. Interact. 2012, 200, 21–27. [Google Scholar] [CrossRef]
- Aygün, F.Ö.; Akçam, F.Z.; Kaya, O.; Ceyhan, B.M.; Sütçü, R. Caffeic Acid Phenethyl Ester Modulates Gentamicin-Induced Oxidative Nephrotoxicity in Kidney of Rats. Biol. Trace Elem. Res. 2012, 145, 211–216. [Google Scholar] [CrossRef]
- Balaha, M.; De Filippis, B.; Cataldi, A.; Di Giacomo, V. CAPE and Neuroprotection: A Review. Biomolecules 2021, 11, 176. [Google Scholar] [CrossRef]
- Bıçakçı, N.; Karaboğa; Dökmeci, A.H.; Güzel, S.; Fidanol Erboğa, Z. Cardioprotective Effect of Caffeic Acid Phenethyl Ester on Cardiac Contusion Following Blunt Chest Trauma in Rats. Biotech. Histochem. 2019, 94, 442–448. [Google Scholar] [CrossRef]
- Romana-Souza, B.; dos Santos, J.S.; Monte-Alto-Costa, A. Caffeic Acid Phenethyl Ester Promotes Wound Healing of Mice Pressure Ulcers Affecting NF-ΚB, NOS2 and NRF2 Expression. Life Sci. 2018, 207, 158–165. [Google Scholar] [CrossRef]
- Kinis, V.; Ozbay, M.; Akdag, M.; Alabalik, U.; Gul, A.; Yilmaz, B.; Ozkan, H.; Topcu, I. Effects of Caffeic Acid Phenethyl Ester on Wound Healing of Nasal Mucosa in the Rat: An Experimental Study. Am. J. Otolaryngol. 2014, 35, 482–486. [Google Scholar] [CrossRef]
- Kazancioglu, H.O.; Bereket, M.C.; Ezirganli, S.; Aydin, M.S.; Aksakalli, S. Effects of Caffeic Acid Phenethyl Ester on Wound Healing in Calvarial Defects. Acta Odontol. Scand. 2015, 73, 21–27. [Google Scholar] [CrossRef]
- Serarslan, G.; Altuǧ, E.; Kontas, T.; Atik, E.; Avci, G. Caffeic Acid Phenethyl Ester Accelerates Cutaneous Wound Healing in a Rat Model and Decreases Oxidative Stress. Clin. Exp. Dermatol. 2007, 32, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Celli, N.; Dragani, L.K.; Murzilli, S.; Pagliani, T.; Poggi, A. In Vitro and in Vivo Stability of Caffeic Acid Phenethyl Ester, a Bioactive Compound of Propolis. J. Agric. Food Chem. 2007, 55, 3398–3407. [Google Scholar] [CrossRef] [PubMed]
- Derman, S. Caffeic Acid Phenethyl Ester Loaded PLGA Nanoparticles: Effect of Various Process Parameters on Reaction Yield, Encapsulation Efficiency, and Particle Size. J. Nanomater. 2015, 2015, 341848. [Google Scholar] [CrossRef]
- Tambuwala, M.M.; Khan, M.N.; Thompson, P.; McCarron, P.A. Albumin Nano-Encapsulation of Caffeic Acid Phenethyl Ester and Piceatannol Potentiated Its Ability to Modulate HIF and NF-KB Pathways and Improves Therapeutic Outcome in Experimental Colitis. Drug Deliv. Transl. Res. 2019, 9, 14–24. [Google Scholar] [CrossRef]
- Shahin, N.N.; Shamma, R.N.; Ahmed, I.S. A Nano-Liposomal Formulation of Caffeic Acid Phenethyl Ester Modulates Nrf2 and NF-Κβ Signaling and Alleviates Experimentally Induced Acute Pancreatitis in a Rat Model. Antioxidants 2022, 11, 1536. [Google Scholar] [CrossRef]
- Kaya, S.; Yilmaz, D.E.; Akmayan, I.; Egri, O.; Arasoglu, T.; Derman, S. Caffeic Acid Phenethyl Ester Loaded Electrospun Nanofibers for Wound Dressing Application. J. Pharm. Sci. 2022, 111, 734–742. [Google Scholar] [CrossRef]
- Chen, S.; Cheng, S.X.; Zhuo, R.X. Self-Assembly Strategy for the Preparation of Polymer-Based Nanoparticles for Drug and Gene Delivery. Macromol. Biosci. 2011, 11, 576–589. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Ahmed, O.A.A.; Balata, G.F.; Almalki, W.H. Self-Assembled Biodegradable Polymeric Micelles to Improve Dapoxetine Delivery across the Blood-Brain Barrier. Int. J. Nanomed. 2018, 13, 3679–3687. [Google Scholar] [CrossRef]
- Ashjari, M.; Khoee, S.; Mahdavian, A.R.; Rahmatolahzadeh, R. Self-Assembled Nanomicelles Using PLGA-PEG Amphiphilic Block Copolymer for Insulin Delivery: A Physicochemical Investigation and Determination of CMC Values. J. Mater. Sci. Mater. Med. 2012, 23, 943–953. [Google Scholar] [CrossRef]
- Wang, X.; Bowman, P.D.; Kerwin, S.M.; Stavchansky, S. Stability of Caffeic Acid Phenethyl Ester and Its Fluorinated Derivative in Rat Plasma. Biomed. Chromatogr. 2007, 21, 343–350. [Google Scholar] [CrossRef]
- King, A.J.F. The Use of Animal Models in Diabetes Research. Br. J. Pharmacol. 2012, 166, 877–894. [Google Scholar] [CrossRef]
- Struck, M.B.; Andrutis, K.A.; Ramirez, H.E.; Battles, A.H. Effect of a Short-Term Fast on Ketamine-Xylazine Anesthesia in Rats. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 344–348. [Google Scholar] [PubMed]
- Koshak, A.E.; Algandaby, M.M.; Mujallid, M.I.; Abdel-Naim, A.B.; Alhakamy, N.A.; Fahmy, U.A.; Alfarsi, A.; Badr-Eldin, S.M.; Neamatallah, T.; Nasrullah, M.Z.; et al. Wound Healing Activity of Opuntia Ficus-Indica Fixed Oil Formulated in a Self-Nanoemulsifying Formulation. Int. J. Nanomed. 2021, 16, 3889–3905. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques, 6th ed.; Churchill Livingstone, Elsevier: Beijing, China, 2008. [Google Scholar]
- Buchwalow, I.B.; Böcker, W. Immuno-Histochemistry Basics and Methods; Springer: Heidelberg, Germany, 2010; ISBN 978-3-642-04608-7. [Google Scholar]
- da Fonseca Cerqueira, M.M.B.; Maia, H.M.S.F.; das Mercês, M.C.; Santos da Natividade, M.; dos Santos Almeida, O.; Santana, E.F.; Oliveira, B.A.M.; Araújo, M. Complications Related to Diabetic Foot Ulcer and Associated Social Vulnerability Factors at a Referral Centre in Brazil. J. Wound Care 2022, 31, 946–960. [Google Scholar] [CrossRef] [PubMed]
- Tindong, M.; Palle, J.N.; Nebongo, D.; Aminde, L.N.; Mboue-Djieka, Y.; Mbarga, N.T.F.; Dehayem, M.Y.; Choukem, S.P. Prevalence, Clinical Presentation, and Factors Associated With Diabetic Foot Ulcer in Two Regional Hospitals in Cameroon. Int. J. Low. Extrem. Wounds 2018, 17, 42–47. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J.A. Drug Delivery and Nanoparticles:Applications and Hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Zhang, K.; Tang, X.; Zhang, J.; Lu, W.; Lin, X.; Zhang, Y.; Tian, B.; Yang, H.; He, H. PEG-PLGA Copolymers: Their Structure and Structure-Influenced Drug Delivery Applications. J. Control. Release 2014, 183, 77–86. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Ahmed, O.A.A.; Fahmy, U.A.; Md, S. Development and In Vitro Evaluation of 2-Methoxyestradiol Loaded Polymeric Micelles for Enhancing Anticancer Activities in Prostate Cancer. Polymers 2021, 13, 884. [Google Scholar] [CrossRef]
- Jackson, J.E.; Kopecki, Z.; Cowin, A.J. Nanotechnological Advances in Cutaneous Medicine. J. Nanomater. 2013, 2013, 808234. [Google Scholar] [CrossRef]
- Goyal, R.; Macri, L.K.; Kaplan, H.M.; Kohn, J. Nanoparticles and Nanofibers for Topical Drug Delivery. J. Control. Release 2016, 240, 77–92. [Google Scholar] [CrossRef]
- Andreu, V.; Mendoza, G.; Arruebo, M.; Irusta, S. Smart Dressings Based on Nanostructured Fibers Containing Natural Origin Antimicrobial, Anti-Inflammatory, and Regenerative Compounds. Materials 2015, 8, 5154. [Google Scholar] [CrossRef] [PubMed]
- Korrapati, P.S.; Karthikeyan, K.; Satish, A.; Krishnaswamy, V.R.; Venugopal, J.R.; Ramakrishna, S. Recent Advancements in Nanotechnological Strategies in Selection, Design and Delivery of Biomolecules for Skin Regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 67, 747–765. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.C.; Lancel, S.; Boulanger, E.; Neviere, R. Targeting Oxidative Stress and Mitochondrial Dysfunction in the Treatment of Impaired Wound Healing: A Systematic Review. Antioxidants 2018, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Ramazan Yilmaz, H.; Uz, E.; Yucel, N.; Altuntas, I.; Ozcelik, N. Protective Effect of Caffeic Acid Phenethyl Ester (CAPE) on Lipid Peroxidation and Antioxidant Enzymes in Diabetic Rat Liver. J. Biochem. Mol. Toxicol. 2004, 18, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Tolba, M.F.; Omar, H.A.; Azab, S.S.; Khalifa, A.E.; Abdel-Naim, A.B.; Abdel-Rahman, S.Z. Caffeic Acid Phenethyl Ester: A Review of Its Antioxidant Activity, Protective Effects against Ischemia-Reperfusion Injury and Drug Adverse Reactions. Crit. Rev. Food Sci. Nutr. 2016, 56, 2183–2190. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Cui, H.; Ma, Z.; Liu, X.; Yang, L. Recent Progresses in the Pharmacological Activities of Caffeic Acid Phenethyl Ester. Naunyn. Schmiedebergs. Arch. Pharmacol. 2021, 394, 1327–1339. [Google Scholar] [CrossRef] [PubMed]
- Göçer, H.; Gülçin, I. Caffeic Acid Phenethyl Ester (CAPE): Correlation of Structure and Antioxidant Properties. Int. J. Food Sci. Nutr. 2011, 62, 821–825. [Google Scholar] [CrossRef]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxid. Med. Cell. Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, L.; Xiong, Y.; Panayi, A.C.; Abududilibaier, A.; Hu, Y.; Yu, C.; Zhou, W.; Sun, Y.; Liu, M.; et al. Antioxidant Therapy and Antioxidant-Related Bionanomaterials in Diabetic Wound Healing. Front. Bioeng. Biotechnol. 2021, 9, 554. [Google Scholar] [CrossRef]
- Salazar, J.J.; Ennis, W.J.; Koh, T.J. Diabetes Medications: Impact on Inflammation and Wound Healing. J. Diabetes Complicat. 2016, 30, 746–752. [Google Scholar] [CrossRef]
- Leal, E.C.; Carvalho, E. Heme Oxygenase-1 as Therapeutic Target for Diabetic Foot Ulcers. Int. J. Mol. Sci. 2022, 23, 12043. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, O.; Torres, F.M.; Shireman, P.K. Chemokines and Diabetic Wound Healing. Vascular 2007, 15, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Al-Hariri, M.; Alsunni, A.; Shaikh, M.H.; Eldin, T.G.; Ghamdi, K.A.; Alharbi, A.F.; Alhawaj, H.; Chathoth, S. Caffeic Acid Phenethyl Ester Reduces Pro Inflammatory Cytokines in Moderate Swimming Test in Growing Rats Model. J. Inflamm. Res. 2021, 14, 5653–5657. [Google Scholar] [CrossRef]
- Semis, H.S.; Gur, C.; Ileriturk, M.; Kaynar, O.; Kandemir, F.M. Investigation of the Anti-Inflammatory Effects of Caffeic Acid Phenethyl Ester in a Model of λ-Carrageenan-Induced Paw Edema in Rats. Hum. Exp. Toxicol. 2021, 40, S721–S738. [Google Scholar] [CrossRef] [PubMed]
- Pandurangan, A.K.; Mohebali, N.; Hasanpourghadi, M.; Esa, N.M. Caffeic Acid Phenethyl Ester Attenuates Dextran Sulfate Sodium-Induced Ulcerative Colitis Through Modulation of NF-ΚB and Cell Adhesion Molecules. Appl. Biochem. Biotechnol. 2022, 194, 1091–1104. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.S.; Monte-Alto-Costa, A. Caffeic Acid Phenethyl Ester Improves Burn Healing in Rats through Anti-Inflammatory and Antioxidant Effects. J. Burn Care Res. 2013, 34, 682–688. [Google Scholar] [CrossRef]
- Black, E.; Vibe-Petersen, J.; Jorgensen, L.N.; Madsen, S.M.; Ågren, M.S.; Holstein, P.E.; Perrild, H.; Gottrup, F. Decrease of Collagen Deposition in Wound Repair in Type 1 Diabetes Independent of Glycemic Control. Arch. Surg. 2003, 138, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.; Braunagel, S.; Rosenblum, B.I. Growth Factors in Wound Healing: The Present and the Future? Clin. Podiatr. Med. Surg. 2015, 32, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Mustoe, T.A.; Pierce, G.F.; Thomason, A.; Gramates, P.; Sporn, M.B.; Deuel, T.F. Accelerated Healing of Incisional Wounds in Rats Induced by Transforming Growth Factor-Beta. Science 1987, 237, 1333–1336. [Google Scholar] [CrossRef]
- den Dekker, A.; Davis, F.M.; Kunkel, S.L.; Gallagher, K.A. Targeting Epigenetic Mechanisms in Diabetic Wound Healing. Transl. Res. 2019, 204, 39–50. [Google Scholar] [CrossRef]
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic Strategies for Enhancing Angiogenesis in Wound Healing. Adv. Drug Deliv. Rev. 2019, 146, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Pierce, G.F.; Mustoe, T.A.; Senior, R.M.; Reed, J.; Griffin, G.L.; Thomason, A.; Deuel, T.F. In Vivo Incisional Wound Healing Augmented by Platelet-Derived Growth Factor and Recombinant c-Sis Gene Homodimeric Proteins. J. Exp. Med. 1988, 167, 974–987. [Google Scholar] [CrossRef] [PubMed]
- Steed, D.L.; the Diabetic Ulcer Study Group. Clinical Evaluation of Recombinant Human Platelet-Derived Growth Factor for the Treatment of Lower Extremity Diabetic Ulcers. J. Vasc. Surg. 1995, 21, 71–81. [Google Scholar] [CrossRef] [PubMed]

| Forward | Reverse | Accession Number | |
|---|---|---|---|
| Col1A1 | ATCAGCCCAAACCCCAAGGAGA | CGCAGGAAGGTCAGCTGGATAG | NM_053304.1 |
| GAPDH | CCATTCTTCCACCTTTGATGCT | TGTTGCTGTAGCCATATTCATTGT | NM_017008.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasrullah, M.Z. Caffeic Acid Phenethyl Ester Loaded PEG–PLGA Nanoparticles Enhance Wound Healing in Diabetic Rats. Antioxidants 2023, 12, 60. https://doi.org/10.3390/antiox12010060
Nasrullah MZ. Caffeic Acid Phenethyl Ester Loaded PEG–PLGA Nanoparticles Enhance Wound Healing in Diabetic Rats. Antioxidants. 2023; 12(1):60. https://doi.org/10.3390/antiox12010060
Chicago/Turabian StyleNasrullah, Mohammed Z. 2023. "Caffeic Acid Phenethyl Ester Loaded PEG–PLGA Nanoparticles Enhance Wound Healing in Diabetic Rats" Antioxidants 12, no. 1: 60. https://doi.org/10.3390/antiox12010060
APA StyleNasrullah, M. Z. (2023). Caffeic Acid Phenethyl Ester Loaded PEG–PLGA Nanoparticles Enhance Wound Healing in Diabetic Rats. Antioxidants, 12(1), 60. https://doi.org/10.3390/antiox12010060






