Synthesis and Antioxidant Properties of Novel 1,2,3-Triazole-Containing Nitrones
Abstract
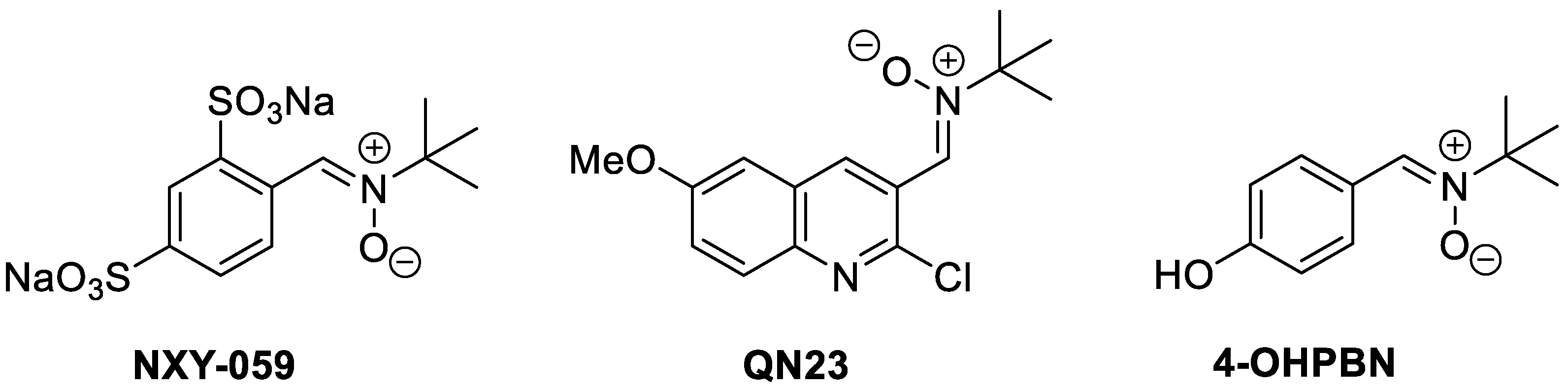
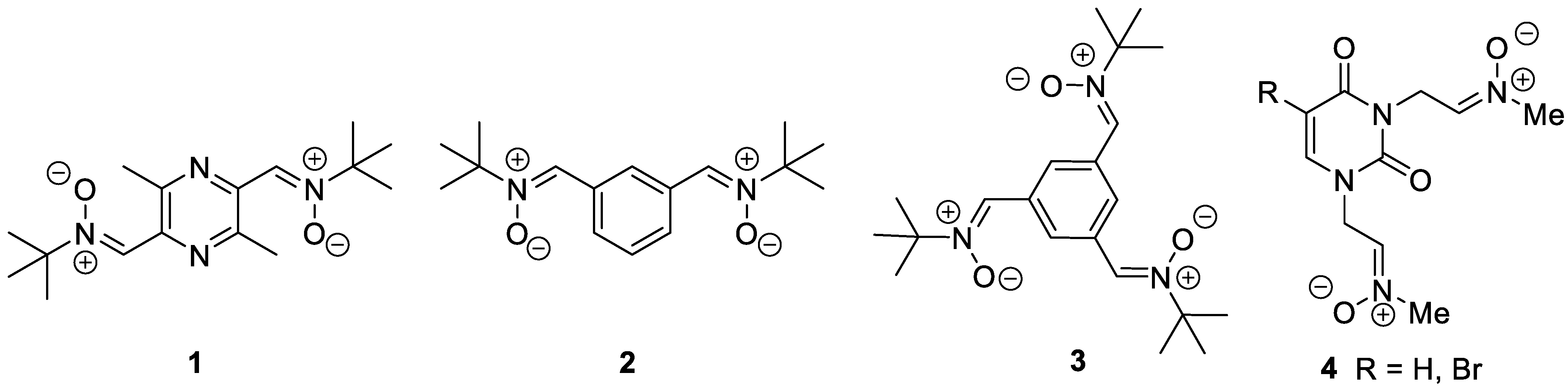
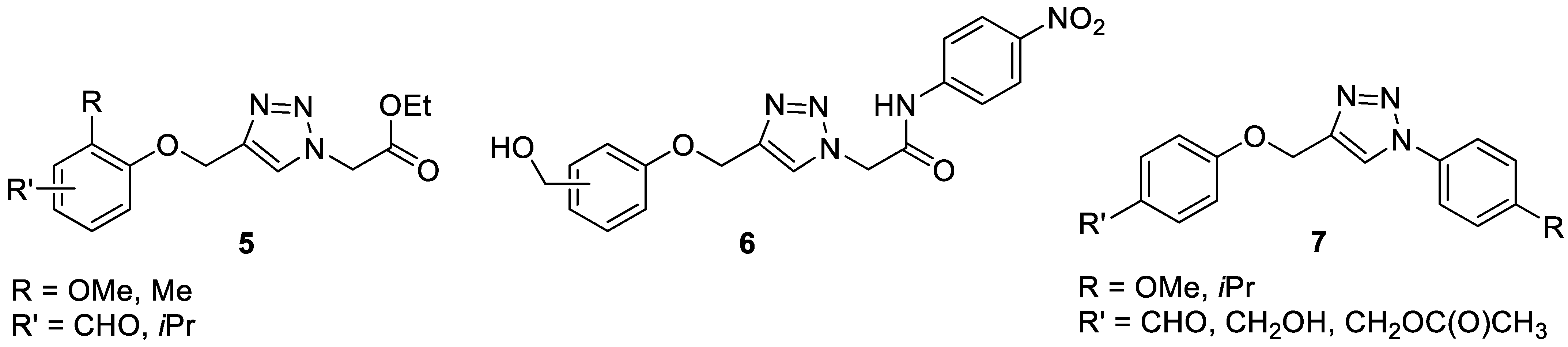
1. Introduction
2. Materials and Methods
2.1. Chemistry
2.1.1. General Information
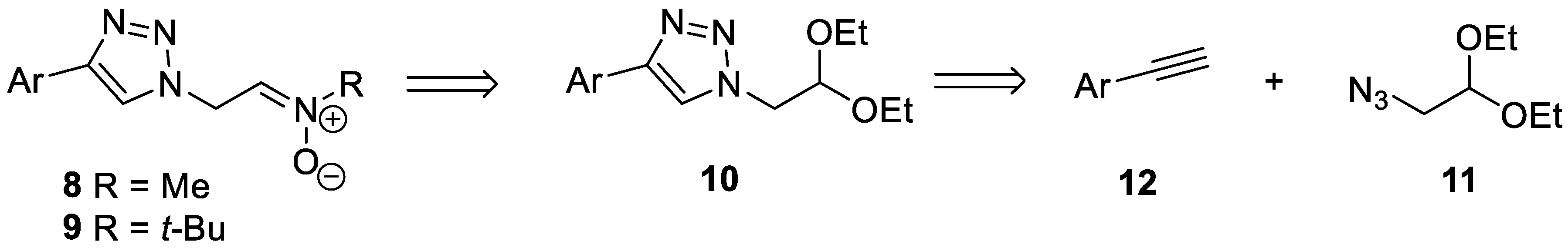
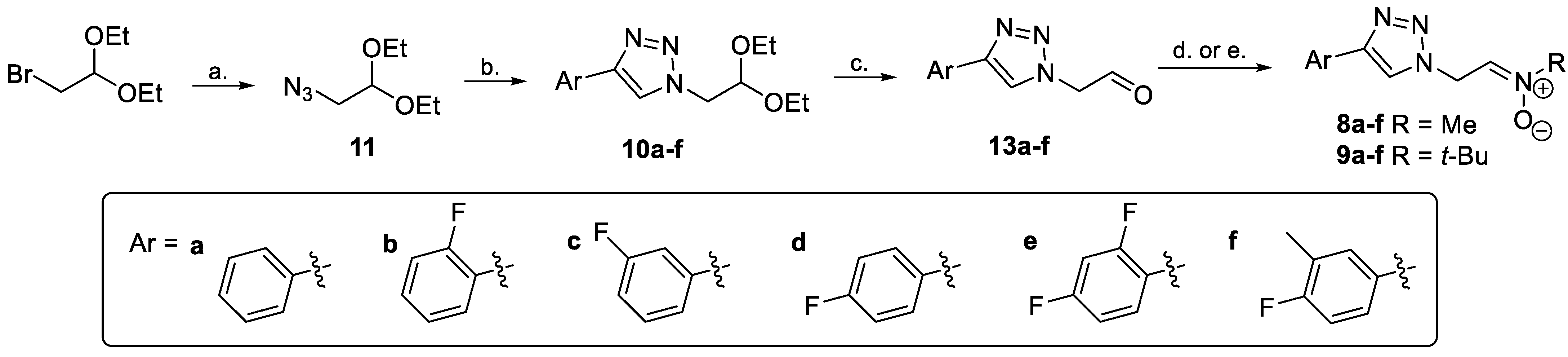
2.1.2. Synthesis of 2-Azidoacetaldehyde Diethyl Acetal 11
2.1.3. General Procedure for the Synthesis of (1,2,3-Triazole)Acetaldehyde Diethyl Acetals 10a–f
1-(2,2-Diethoxyethyl)-4-Phenyl-1H-1,2,3-Triazole (10a)
1-(2,2-Diethoxyethyl)-4-(2-Fluorophenyl)-1H-1,2,3-Triazole (10b)
1-(2,2-Diethoxyethyl)-4-(3-Fluorophenyl)-1H-1,2,3-Triazole (10c)
1-(2,2-Diethoxyethyl)-4-(4-Fluorophenyl)-1H-1,2,3-Triazole (10d)
1-(2,2-Diethoxyethyl)-4-(2,4-Difluorophenyl)-1H-1,2,3-Triazole (10e)
1-(2,2-Diethoxyethyl)-4-(4-Fluoro-3-Methylphenyl)-1H-1,2,3-Triazole (10f)
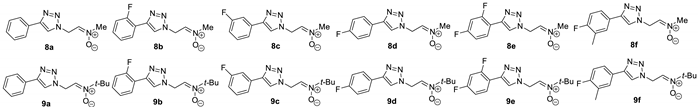
2.1.4. General Procedure for the Synthesis of Nitrones 8a–f and 9a–f
N-(2-(4-Phenyl-1H-1,2,3-Triazol-1-yl)Ethylidene)Methanamine Oxide (8a)
N-(2-(4-(2-Fluorophenyl)-1H-1,2,3-Triazol-1-yl)Ethylidene)Methanamine Oxide (8b)
N-(2-(4-(3-Fluorophenyl)-1H-1,2,3-Triazol-1-yl)Ethylidene)Methanamine Oxide (8c)
N-(2-(4-(4-Fluorophenyl)-1H-1,2,3-Triazol-1-yl)Ethylidene)Methanamine Oxide (8d)
N-(2-(4-(2,4-Difluorophenyl)-1H-1,2,3-Triazol-1-yl)Ethylidene)Methanamine Oxide (8e)
N-(2-(4-(4-Fluoro-3-Methylphenyl)-1H-1,2,3-Triazol-1-yl)Ethylidene)Methanamine Oxide (8f)
2-Methyl-N-(2-(4-Phenyl-1H-1,2,3-Triazol-1-yl)Ethylidene)Propan-2-Amine Oxide (9a)
N-(2-(4-(2-Fluorophenyl)-1H-1,2,3-Triazol-1-yl)Ethylidene)-2-Methylpropan-2-Amine Oxide (9b)
N-(2-(4-(3-Fluorophenyl)-1H-1,2,3-Triazol-1-yl)Ethylidene)-2-Methylpropan-2-Amine Oxide (9c)
N-(2-(4-(4-Fluorophenyl)-1H-1,2,3-Triazol-1-yl)Ethylidene)-2-Methylpropan-2-Amine Oxide (9d)
N-(2-(4-(2,4-Difluorophenyl)-1H-1,2,3-Triazol-1-yl)Ethylidene)-2-Methylpropan-2-Amine Oxide (9e)
N-(2-(4-(4-Fluoro-3-Methylphenyl)-1H-1,2,3-Triazol-1-yl)Ethylidene)-2-Methylpropan-2-Amine Oxide (9f)
2.2. Estimation of Lipophilicity as Clog P
2.3. Antioxidant Assays
2.3.1. Materials and Methods
2.3.2. Inhibition of Linoleic Acid Peroxidation
2.3.3. In Vitro Inhibition of Soybean Lipoxygenase (LOX)
2.3.4. Competition of Nitrones 8 and 9 with DMSO for Hydroxyl Radicals
2.3.5. ABTS∙+–Decolorization Assay in Ethanolic Solution for Antioxidant Activity
3. Results and Discussion
3.1. Chemistry
3.2. Antioxidant Activity of Nitrones 8a–f and 9a–f
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Lutskii, M.A.; Zemskov, A.M.; Razuvaeva, V.V.; Lushnikova, Y.P.; Karpova, O.Y. Oxidative stress as an indicator of metabolic impairments in the pathogenesis of cerebral stroke. Neurosci. Behav. Physiol. 2018, 48, 64–68. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015; ISBN 9780198717478. [Google Scholar]
- Floyd, R.A.; Kopke, R.D.; Choi, C.H.; Foster, S.B.; Doblas, S.; Towner, R.A. Nitrones as therapeutics. Free Radical Biol. Med. 2008, 45, 1361–1374. [Google Scholar] [CrossRef] [PubMed]
- Edenius, C.; Strid, S.; Borgå, O.; Breitholtz-Emanuelsson, A.; Vallén, K.L.; Fransson, B. Pharmacokinetics of NXY-059, a nitrone-based free radical trapping agent, in healthy young and elderly subjects. J. Stroke Cerebrovasc. Dis. 2002, 11, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Chioua, M.; Gonzalo-Gobernado, R.; Ayuso, M.I.; Escobar-Peso, A.; Infantes, L.; Hadjipavlou-Litina, D.; Montoya, J.J.; Montaner, J.; Alcázar, A.; Marco-Contelles, J. New quinolylnitrones for stroke therapy: Antioxidant and neuroprotective (Z)-N-tert-butyl-1-(2-chloro-6-methoxyquinolin-3-yl)methanimine oxide (as a new lead-compound for ischemic stroke treatment. J. Med. Chem. 2019, 62, 2184–2201. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Choi, C.H. Noise-induced neural degeneration and therapeutic effect of antioxidant drugs. J. Audiol. Otol. 2015, 19, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, G.; Zhang, Z.; Yu, P.; Zhong, H.; Du, J.; Wang, Y. Novel multi-functional nitrones for treatment of ischemic stroke. Bioorg. Med. Chem. 2012, 20, 3939–3945. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, B.; Diez-Iriepa, D.; Merás-Sáiz, B.; Chioua, M.; García-Vieira, D.; Iriepa, I.; Hadjipavlou-Litina, D.; López-Muñoz, F.; Martínez-Murillo, R.; González-Nieto, D.; et al. Synthesis, antioxidant properties and neuroprotection of alpha-phenyl-tertbutylnitrone derived homobisnitrones in in vitro and in vivo ischemia models. Sci. Rep. 2020, 10, 14150. [Google Scholar] [CrossRef] [PubMed]
- Diez-Iriepa, D.; Chamorro, B.; Talaván, M.; Chioua, M.; Iriepa, I.; Hadjipavlou-Litina, D.; López-Muñoz, F.; Marco-Contelles, J.; Oset-Gasque, M.J. Homo-tris-nitrones derived from α-phenyl-N-tert-butylnitrone: Synthesis, neuroprotection and antioxidant properties. Int. J. Mol. Sci. 2020, 21, 7949. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, B.; Głowacka, I.E.; Gotkowska, J.; Gulej, R.; Hadjipavlou-Litina, D.; López-Muñoz, F.; Marco-Contelles, J.; Piotrowska, D.G.; Oset-Gasque, M.J. Nucleobase-Derived Nitrones: Synthesis and Antioxidant and Neuroprotective Activities in an In Vitro Model of Ischemia–Reperfusion. Int. J. Mol. Sci. 2022, 23, 3411. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha Lima, J.A.; De Farias Silva, J.; Santos, C.S.; Caiana, R.R.A.; De Moraes, M.M.; Da Camara, C.A.G.; Freitas, J.C.R. Synthesis of new 1,4-disubstituted 1,2,3-triazoles using the CuAAC reaction and determination of their antioxidant activities. An. Acad. Bras. Cienc. 2021, 93, e20201672. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, J.H.; Kadhim, H.Y.; Al_Gahaith Makki, K.; Ali, B.A. Review on Antioxidant evaluation of 1,2,3-triazole derivatives synthesized by click chemistry. Ann. Rom. Soc. Cell Biol. 2021, 25, 2765–2796. [Google Scholar]
- Sahin, I.; Özgeri¸, F.B.; Köse, M.; Bakan, E.; Tümer, F. Synthesis, Characterization, and antioxidant and anticancer activity of 1,4-disubstituted 1,2,3-triazoles. J. Mol. Struct. 2021, 1232, 130042. [Google Scholar] [CrossRef]
- Bellur, E.; Langer, P. Synthesis of functionalized pyrroles and 6,7-dihydro-1H-indol-4(5H)-ones by reaction of 1,3-dicarbonyl compounds with 2-azido-1,1-diethoxyethane. Tetrahedron Lett. 2006, 47, 2151–2154. [Google Scholar] [CrossRef]
- Soriano, E.; Hadjipavlou-Litina, D.; Alcázar, A.; Ayuso, L.I.; Oset-Gasque, M.J.; González, M.P.; Monjas, L.; Rodríguez-Franco, M.I.; Marco-Contelles, J.; Samadi, A. α-Aryl-N-alkyl nitrones, as potential agents for stroke treatment: Synthesis, theoretical calculations, antioxidant, anti-inflammatory, neuroprotective and brain-blood barrier permeability properties. J. Med. Chem. 2012, 55, 153–168. [Google Scholar]
- Liegois, C.; Lermusieau, G.; Colin, S. Measuring antioxidant efficiency of wort, malt, and hops against the 2,2‘-azobis(2-amidinopropane) dihydrochloride-induced oxidation of an aqueous dispersion of linoleic acid. J. Agric. Food Chem. 2000, 48, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
 | |||||
|---|---|---|---|---|---|
| Nitrones/ Standards | Clog P b | LOX Inhibition (% a or IC50 [µM]) | ILP (%) a | Scavenging Activity for HO• (%) a | ABTS+∙ (%) a |
| 8a | 0.21 | 37.5 μM | 48.4 | 93 | 14 |
| 8b | 0.39 | 24% | 2 | 61 | 13 |
| 8c | 0.39 | 40% | 65 | 59 | no |
| 8d | 0.39 | no | 54 | 80 | 5.3 |
| 8e | 0.54 | 87.5 μM | 87 | 76 | no |
| 8f | 0.59 | 44 % | 41 | 91.5 | 34.3 |
| 9a | 1.44 | 44% | 74.5 | 76 | 4 |
| 9b | 1.62 | 40 μM | 73 | 76 | no |
| 9c | 1.44 | no | 21 | 89 | no |
| 9d | 1.62 | 100 μM | 29 | 99 | no |
| 9e | 1.42 | 100 μM | 100 | 100 | no |
| 9f | 2.12 | 27 μM | 87 | 99.9 | 16 |
| NDGA | 87 (0.45 μM) | ||||
| Trolox | 93 | 88 | 91 | ||
| PBN | 23% | 11 | no | 5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadjipavlou-Litina, D.; Głowacka, I.E.; Marco-Contelles, J.; Piotrowska, D.G. Synthesis and Antioxidant Properties of Novel 1,2,3-Triazole-Containing Nitrones. Antioxidants 2023, 12, 36. https://doi.org/10.3390/antiox12010036
Hadjipavlou-Litina D, Głowacka IE, Marco-Contelles J, Piotrowska DG. Synthesis and Antioxidant Properties of Novel 1,2,3-Triazole-Containing Nitrones. Antioxidants. 2023; 12(1):36. https://doi.org/10.3390/antiox12010036
Chicago/Turabian StyleHadjipavlou-Litina, Dimitra, Iwona E. Głowacka, José Marco-Contelles, and Dorota G. Piotrowska. 2023. "Synthesis and Antioxidant Properties of Novel 1,2,3-Triazole-Containing Nitrones" Antioxidants 12, no. 1: 36. https://doi.org/10.3390/antiox12010036
APA StyleHadjipavlou-Litina, D., Głowacka, I. E., Marco-Contelles, J., & Piotrowska, D. G. (2023). Synthesis and Antioxidant Properties of Novel 1,2,3-Triazole-Containing Nitrones. Antioxidants, 12(1), 36. https://doi.org/10.3390/antiox12010036








