Higher Reduced State of Fe/S-Signals, with the Suppressed Oxidation of P700, Causes PSI Inactivation in Arabidopsis thaliana
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Simultaneous Measurements of Chlorophyll (Chl) Fluorescence, P700, and Fe/S-Signals with Gas-Exchange
2.3. Constant High-Intensity Light Treatment
2.4. Statistical Analysis
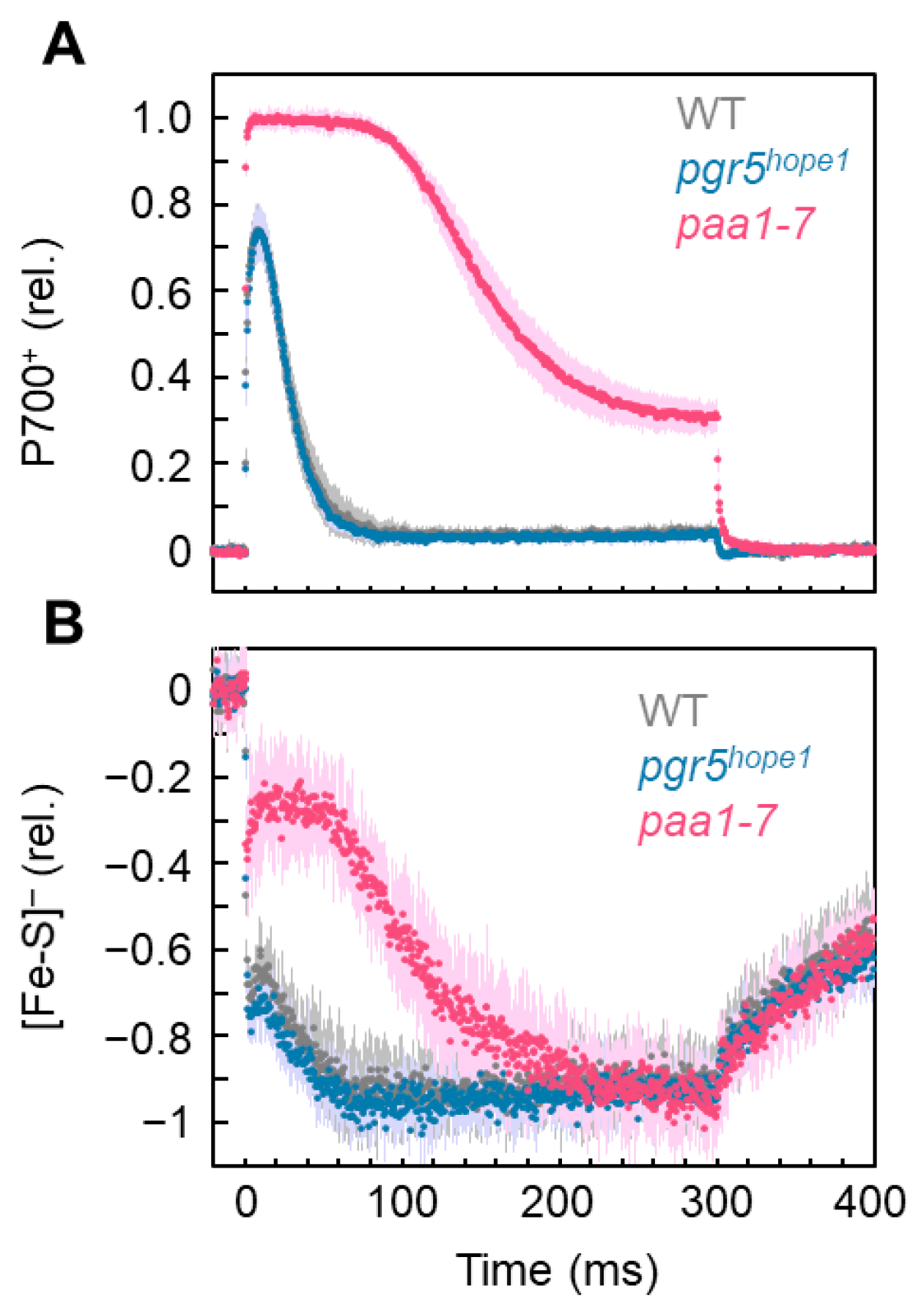
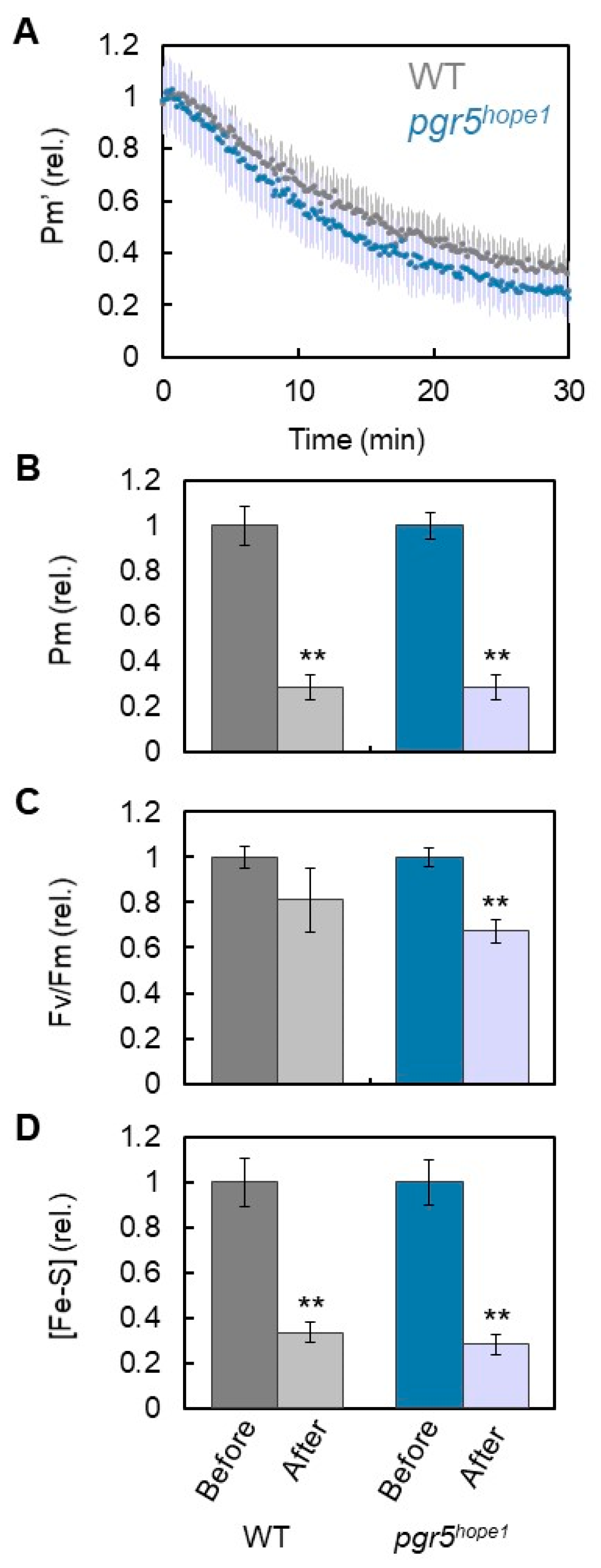
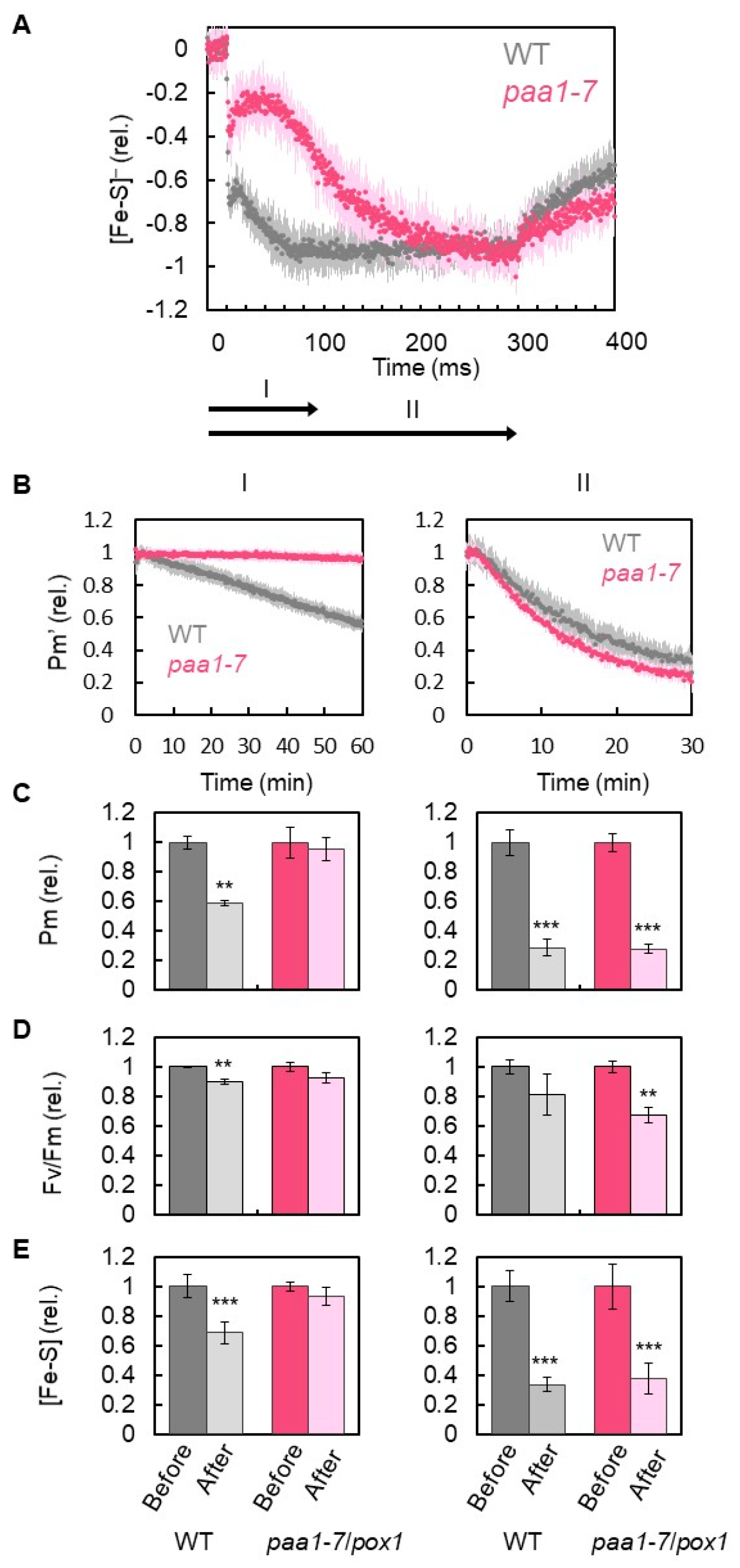
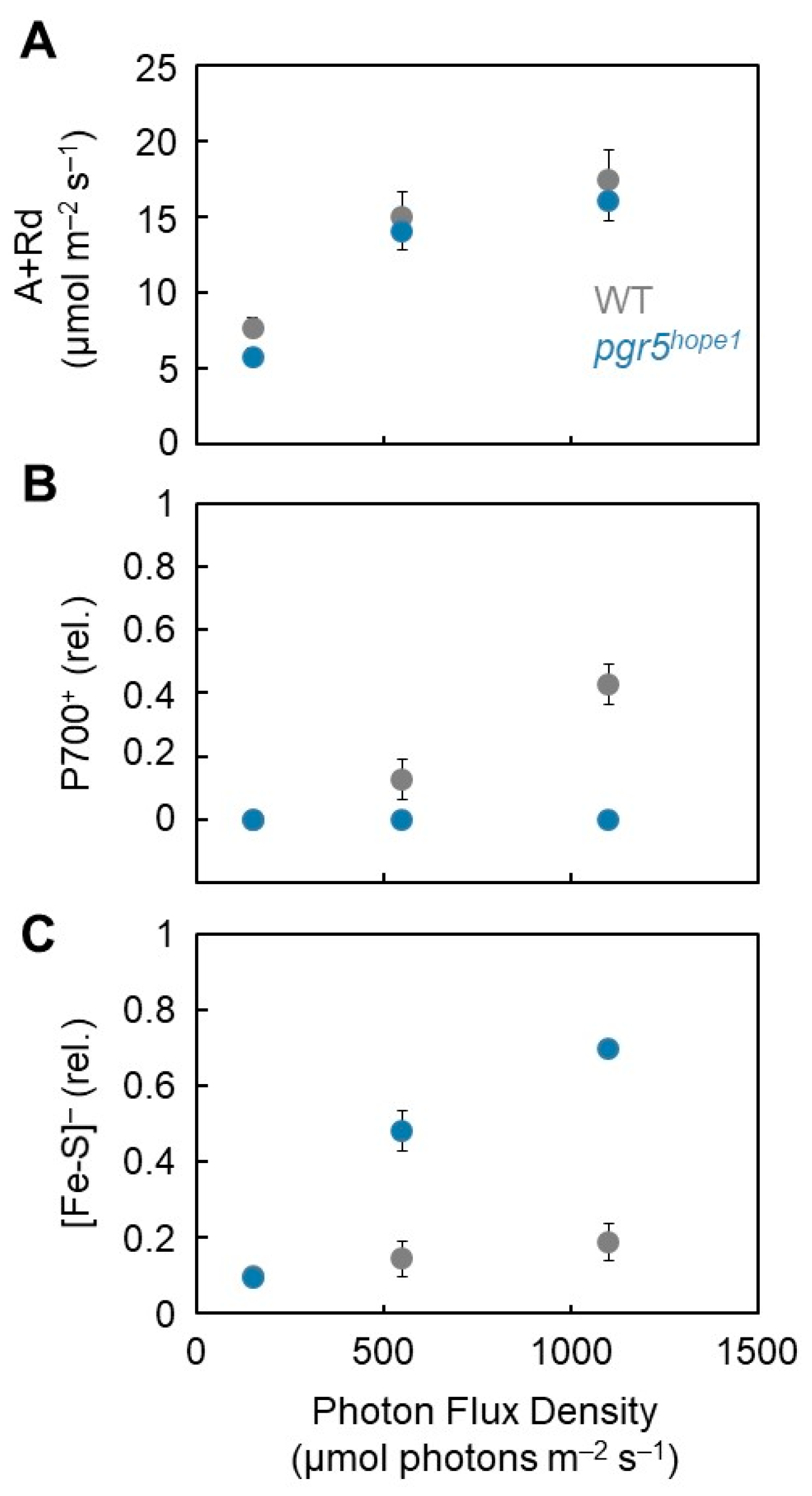
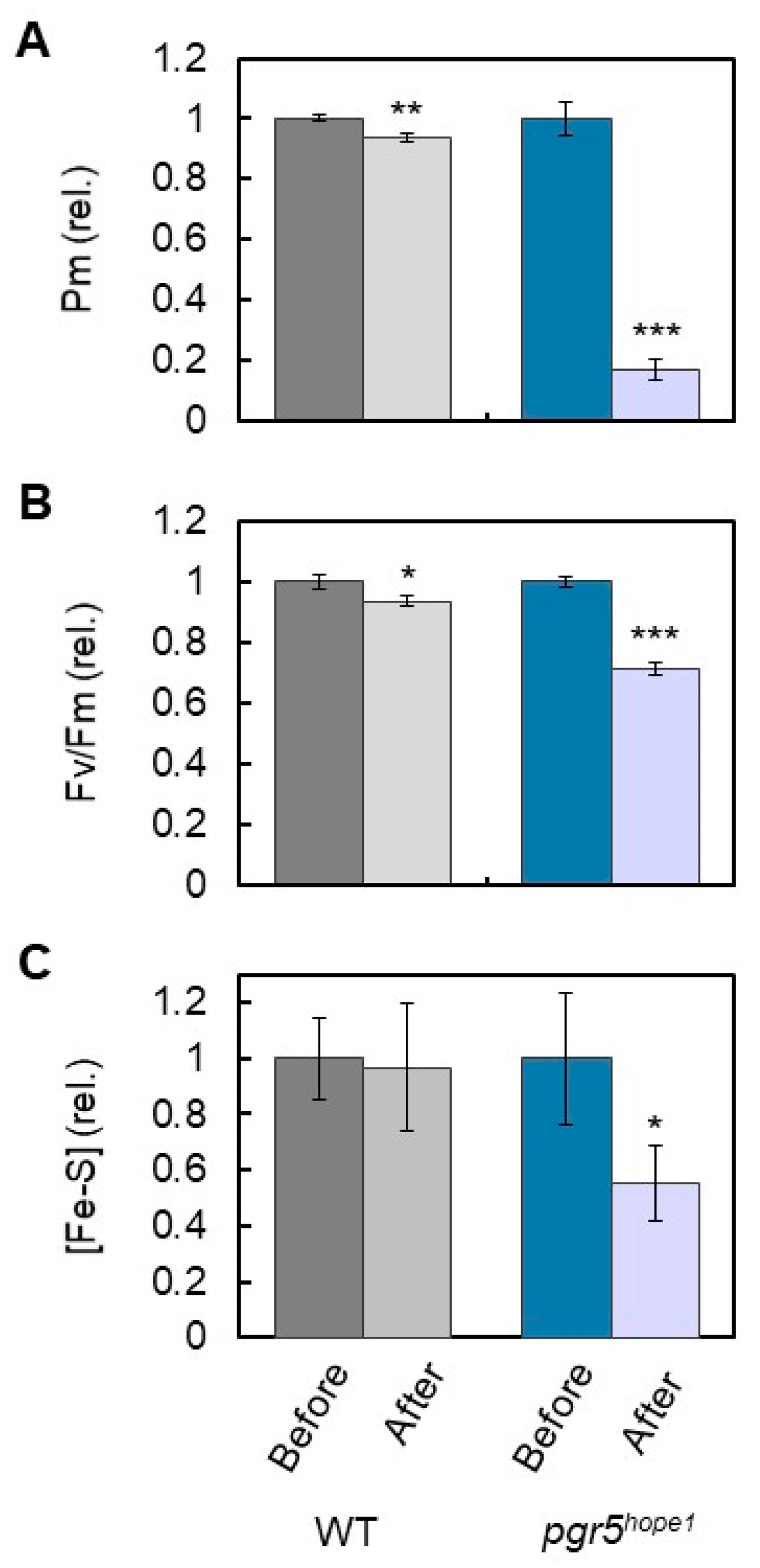
3. Results
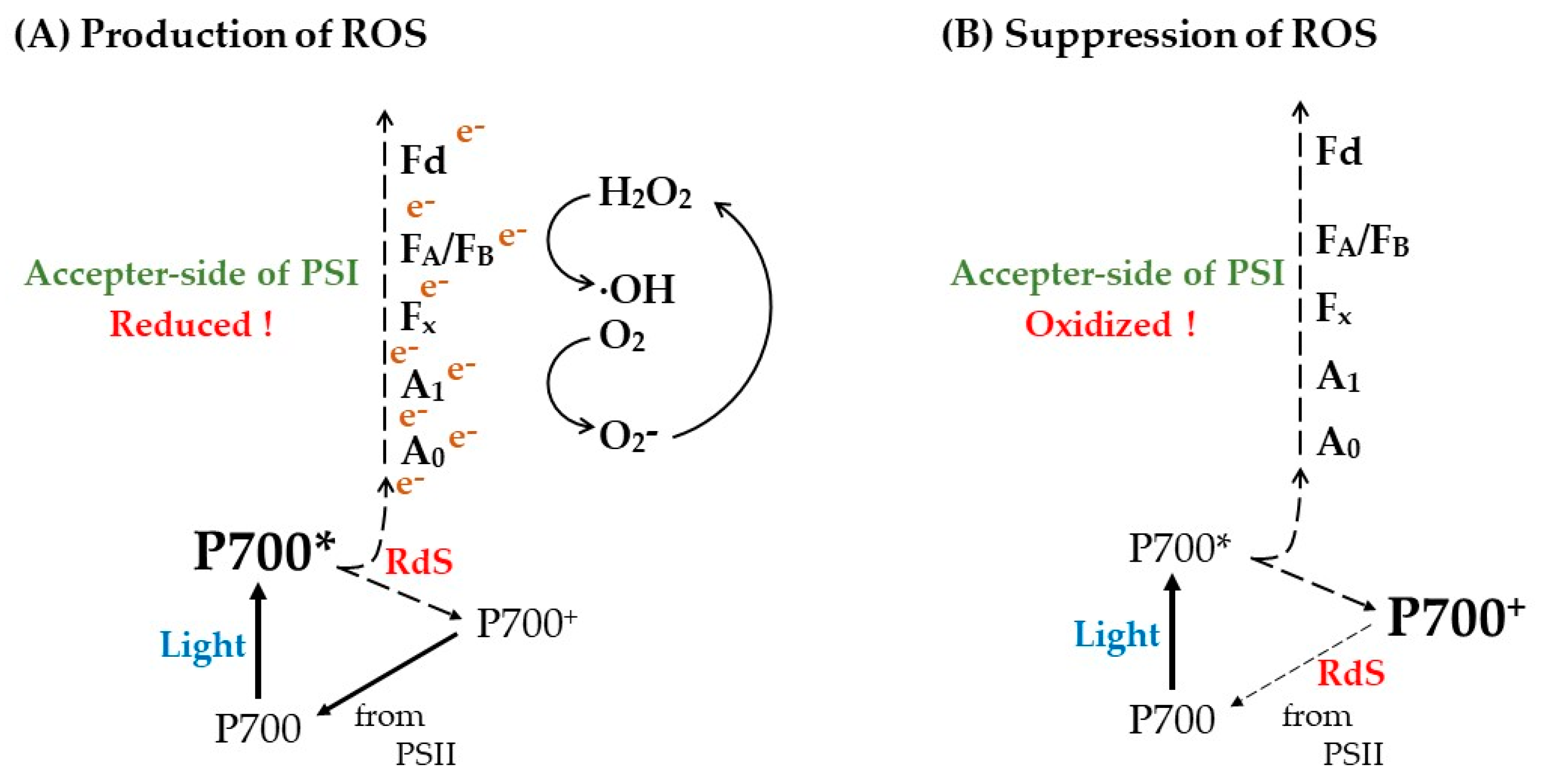
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asada, K. The water–water cycle as alternative photon and electron sinks. Philos. Trans. R. Soc. B Biol. Sci. 2000, 355, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, T.L. The trouble with oxygen: The ecophysiology of extant phototrophs and implications for the evolution of oxygenic photosynthesis. Free Radic. Biol. Med. 2019, 140, 233–249. [Google Scholar] [CrossRef] [PubMed]
- Mattila, H.; Khorobrykh, S.; Havurinne, V.; Tyystjärvi, E. Reactive oxygen species: Reactions and detection from photosynthetic tissues. J. Photochem. Photobiol. B Biol. 2015, 152, 176–214. [Google Scholar] [CrossRef]
- Khorobrykh, S.; Havurinne, V.; Mattila, H.; Tyystjärvi, E. Oxygen and ROS in photosynthesis. Plants 2020, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Cherepanov, D.A.; Milanovsky, G.E.; Petrova, A.A.; Tikhonov, A.N.; Semenov, A.Y. Electron transfer through the acceptor side of photosystem I: Interaction with exogenous acceptors and molecular oxygen. Biochemistry 2017, 82, 1249–1268. [Google Scholar] [CrossRef]
- Mehler, A.H. Studies on reactions of illuminated chloroplasts. I. Mechanism of the reduction of oxygen and other Hill reagents. Arch. Biochem. Biophys. 1951, 33, 65–77. [Google Scholar] [CrossRef]
- Mehler, A.H. Studies on reactions of illuminated chloroplasts. II. Stimulation and inhibition of the reaction with molecular oxygen. Arch. Biochem. Biophys. 1951, 34, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Mehler, A.H.; Brown, A.H. Studies on reactions of illuminated chloroplasts. III. Simultaneous photoproduction and consumption of oxygen studied with oxygen isotopes. Arch. Biochem. Biophys. 1952, 38, 365–370. [Google Scholar] [CrossRef]
- Asada, K.; Kiso, K. The photo-oxidation of epinephrine by spinach chloroplasts and its inhibition by superoxide dismutase: Evidence for the formation of superoxide radicals in chloroplasts. Agri. Biol. Chem. 1973, 37, 453–454. [Google Scholar] [CrossRef]
- Asada, K.; Kiso, K.; Yoshikawa, K. Univalent reduction of molecular oxygen by spinach chloroplasts on illumination. J. Biol. Chem. 1974, 249, 2175–2181. [Google Scholar] [CrossRef]
- Takahashi, M.; Asada, K. Superoxide production in aprotic interior of chloroplast thylakoids. Arch. Biochem. Biophys. 1988, 267, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Kozuleva, M.; Petrova, A.; Milrad, Y.; Semenov, A.; Ivanov, B.; Redding, K.E.; Yacoby, I. Phylloquinone is the principal Mehler reaction site within photosystem I in high light. Plant Physiol. 2021, 186, 1848–1858. [Google Scholar] [CrossRef] [PubMed]
- Kozuleva, M.A.; Petrova, A.A.; Mamedov, M.D.; Semenov, A.Y.; Ivanov, B.N. O2 reduction by photosystem I involves phylloquinone under steady-state illumination. FEBS Lett. 2014, 588, 4364–4368. [Google Scholar] [CrossRef] [PubMed]
- Kruk, J.; Jemioła-Rzemińska, M.; Burda, K.; Schmid, G.H.; Strzałka, K. Scavenging of superoxide generated in photosystem I by plastoquinol and other prenyllipids in thylakoid membranes. Biochemistry 2003, 42, 8501–8505. [Google Scholar] [CrossRef]
- Sejima, T.; Takagi, D.; Fukayama, H.; Makino, A.; Miyake, C. Repetitive short-pulse light mainly inactivates photosystem I in sunflower leaves. Plant Cell Physiol. 2014, 55, 1184–1193. [Google Scholar] [CrossRef]
- Zivcak, M.; Brestic, M.; Kunderlikova, K.; Olsovska, K.; Allakhverdiev, S.I. Effect of photosystem I inactivation on chlorophyll a fluorescence induction in wheat leaves: Does activity of photosystem I play any role in OJIP rise? J. Photochem. Photobiol. B Biol. 2015, 152, 318–324. [Google Scholar] [CrossRef]
- Takahashi, M.; Asada, K. Dependence of oxygen affinity for Mehler reaction on photochemical activity of chloroplast thylakoids. Plant Cell Physiol. 1982, 23, 1457–1461. [Google Scholar]
- Takahashi, M.; Asada, K. Superoxide anion permeability of phospholipid membranes and chloroplast thylakoids. Arch. Biochem. Biophys. 1983, 226, 558–566. [Google Scholar] [CrossRef]
- Munekage, Y.; Hojo, M.; Meurer, J.; Endo, T.; Tasaka, M.; Shikanai, T. PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 2002, 110, 361–371. [Google Scholar] [CrossRef]
- Wada, S.; Amako, K.; Miyake, C. Identification of a novel mutation exacerbated the PSI photoinhibition in pgr5/pgrl1 mutants; Caution for overestimation of the phenotypes in Arabidopsis pgr5-1 mutant. Cells 2021, 10, 2884. [Google Scholar] [CrossRef]
- Furutani, R.; Ohnishi, M.; Mori, Y.; Wada, S.; Miyake, C. The difficulty of estimating the electron transport rate at photosystem I. J. Plant Res. 2021, 135, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Klughammer, C.; Schreiber, U. Deconvolution of ferredoxin, plastocyanin, and P700 transmittance changes in intact leaves with a new type of kinetic LED array spectrophotometer. Photosynth. Res. 2016, 128, 195–214. [Google Scholar] [CrossRef] [PubMed]
- Sétif, P.; Boussac, A.; Krieger-Liszkay, A. Near-infrared in vitro measurements of photosystem I cofactors and electron-transfer partners with a recently developed spectrophotometer. Photosynth. Res. 2019, 142, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.R.; Harbinson, J.; Kramer, D. Determining the limitations and regulation of photosynthetic energy transduction in leaves. Plant Cell Environ. 2007, 30, 1107–1125. [Google Scholar] [CrossRef]
- Shimakawa, G.; Miyake, C. Oxidation of P700 ensures robust photosynthesis. Front. Plant Sci. 2018, 9, 1617. [Google Scholar] [CrossRef]
- Takagi, D.; Ishizaki, K.; Hanawa, H.; Mabuchi, T.; Shimakawa, G.; Yamamoto, H.; Miyake, C. Diversity of strategies for escaping reactive oxygen species production within photosystem I among land plants: P700 oxidation system is prerequisite for alleviating photoinhibition in photosystem I. Physiol. Plant. 2017, 161, 56–74. [Google Scholar] [CrossRef]
- Kadota, K.; Furutani, R.; Makino, A.; Suzuki, Y.; Wada, S.; Miyake, C. Oxidation of P700 induces alternative electron flow in Photosystem I in wheat leaves. Plants 2019, 8, 152. [Google Scholar] [CrossRef]
- Sétif, P.; Shimakawa, G.; Krieger-Liszkay, A.; Miyake, C. Identification of the electron donor to flavodiiron proteins in Synechocystis sp. PCC 6803 by in vivo spectroscopy. Biochim. Biophys. Acta BBA Bioenerg. 2020, 1861, 148256. [Google Scholar] [CrossRef]
- Johnson, M.P.; Ruban, A.V. Rethinking the existence of a steady-state Δψ component of the proton motive force across plant thylakoid membranes. Photosynth. Res. 2013, 119, 233–242. [Google Scholar] [CrossRef]
- Shikanai, T.; Müller-Moulé, P.; Munekage, Y.; Niyogi, K.K.; Pilon, M. PAA1, a P-Type ATPase of Arabidopsis, functions in copper transport in chloroplasts. Plant Cell 2003, 15, 1333–1346. [Google Scholar] [CrossRef]
- Tikkanen, M.; Grieco, M.; Nurmi, M.; Rantala, M.; Suorsa, M.; Aro, E.-M. Regulation of the photosynthetic apparatus under fluctuating growth light. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3486–3493. [Google Scholar] [CrossRef] [PubMed]
- Tikkanen, M.; Rantala, S.; Aro, E.-M. Electron flow from PSII to PSI under high light is controlled by PGR5 but not by PSBS. Front. Plant Sci. 2015, 6, 521. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K. Mechanism of photoinactivation in photosynthetic systems II. The occurrence and properties of two different types of photoinactivation. Plant Cell Physiol. 1970, 11, 29–38. [Google Scholar] [CrossRef]
- Satoh, K. Mechanism of photoinactivation in photosynthetic systems. III. The site and mode of photoinactivation in photosystem I. Plant Cell Physiol. 1970, 11, 187–197. [Google Scholar] [CrossRef]
- Inoue, K.; Fujii, T.; Yokoyama, E.; Matsuura, K.; Hiyama, T.; Sakurai, H. The Photoinhibition site of photosystem I in isolated chloroplasts under extremely reducing conditions. Plant Cell Physiol. 1989, 30, 65–71. [Google Scholar] [CrossRef]
- Inoue, K.; Sakurai, H.; Hiyama, T. Photoinactivation sites of photosystem I in isolated chloroplasts. Plant Cell Physiol. 1986, 27, 961–968. [Google Scholar]
- Havaux, M.; Davaud, A. Photoinhibition of photosynthesis in chilled potato leaves is not correlated with a loss of Photosystem-II activity. Photosynth. Res. 1994, 40, 75–92. [Google Scholar] [CrossRef]
- Sonoike, K.; Terashima, I. Mechanism of photosystem-I photoinhibition in leaves of Cucumis sativus L. Planta 1994, 194, 287–293. [Google Scholar] [CrossRef]
- Sonoike, K.; Terashima, I.; Iwaki, M.; Itoh, S. Destruction of photosystem I iron-sulfur centers in leaves of Cucumis sativus L. by weak illumination at chilling temperatures. FEBS Lett. 1995, 362, 235–238. [Google Scholar] [CrossRef]
- Terashima, I.; Funayama, S.; Sonoike, K. The site of photoinhibition in leaves of Cucumis sativus L. at low temperatures is photosystem I, not photosystem II. Planta 1994, 193, 300–306. [Google Scholar] [CrossRef]
- Ivanov, A.G.; Morgan, R.M.; Gray, G.R.; Velitchkova, M.Y.; Huner, N.P. Temperature/light dependent development of selective resistance to photoinhibition of photosystem I. FEBS Lett. 1998, 430, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Che, Y.; Nakano, T.; Miyake, C.; Ifuku, K. The ability of P700 oxidation in photosystem I reflects chilling stress tolerance in cucumber. J. Plant Res. 2022, 135, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Scheller, H.V.; Haldrup, A. Photoinhibition of photosystem I. Planta 2005, 221, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Tjus, S.E.; Møller, B.L.; Scheller, H. Photosystem I is an early target of photoinhibition in barley illuminated at chilling Temperatures. Plant Physiol. 1998, 116, 755–764. [Google Scholar] [CrossRef]
- Zhang, S.; Scheller, H. Photoinhibition of Photosystem I at chilling temperature and Subsequent Recovery in Arabidopsis thaliana. Plant Cell Physiol. 2004, 45, 1595–1602. [Google Scholar] [CrossRef]
- Furutani, R.; Ifuku, K.; Suzuki, Y.; Noguchi, K.; Shimakawa, G.; Wada, S.; Makino, A.; Sohtome, T.; Miyake, C. P700 Oxidation Suppresses the Production of Reactive Oxygen Species in Photosystem I; Hisabori, T., Ed.; Academic Press: Cambridge, MA, USA, 2000; Volume 96, p. 26. [Google Scholar]
- Furutani, R.; Makino, A.; Suzuki, Y.; Wada, S.; Shimakawa, G.; Miyake, C. Intrinsic fluctuations in transpiration induce photorespiration to oxidize P700 in photosystem I. Plants 2020, 9, 1761. [Google Scholar] [CrossRef]
- Miyake, C. Molecular mechanism of oxidation of P700 and suppression of ROS production in photosystem I in response to electron-sink limitations in C3 Plants. Antioxidants 2020, 9, 230. [Google Scholar] [CrossRef]
- Miyake, C.; Schreiber, U.; Hormann, H.; Sano, S.; Asada, K. The FAD-enzyme monodehydroascorbate radical reductase mediates photoproduction of superoxide radicals in spinach thylakoid membranes. Plant Cell Physiol. 1998, 39, 821–829. [Google Scholar] [CrossRef]
- Ruuska, S.A.; Badger, M.R.; Andrews, T.J.; von Caemmerer, S. Photosynthetic electron sinks in transgenic tobacco with reduced amounts of Rubisco: Little evidence for significant Mehler reaction. J. Exp. Bot. 2000, 51, 357–368. [Google Scholar] [CrossRef]
- Driever, S.M.; Baker, N.R. The water-water cycle in leaves is not a major alternative electron sink for dissipation of excess excitation energy when CO2 assimilation is restricted. Plant Cell Environ. 2011, 34, 837–846. [Google Scholar] [CrossRef]
- Sonoike, K. Photoinhibition of photosystem I. Physiol. Plant. 2011, 142, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Suorsa, M.; Järvi, S.; Grieco, M.; Nurmi, M.; Pietrzykowska, M.; Rantala, M.; Kangasjärvi, S.; Paakkarinen, V.; Tikkanen, M.; Jansson, S.; et al. PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell 2012, 24, 2934–2948. [Google Scholar] [CrossRef] [PubMed]
- Hormann, H.; Neubauer, C.; Asada, K.; Schreiber, U. Intact chloroplasts display pH 5 optimum of O2-reduction in the absence of methyl viologen: Indirect evidence for a regulatory role of superoxide protonation. Photosynth. Res. 1993, 37, 69–80. [Google Scholar] [CrossRef]
- Hormann, H.; Neubauer, C.; Schreiber, U. An active Mehler-peroxidase reaction sequence can prevent cyclic PS I electron transport in the presence of dioxygen in intact spinach chloroplasts. Photosynth. Res. 1994, 41, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Flint, D.; Tuminello, J.; Emptage, M. The inactivation of Fe-S cluster containing hydro-lyases by superoxide. J. Biol. Chem. 1993, 268, 22369–22376. [Google Scholar] [CrossRef] [PubMed]
- Holden, H.M.; Jacobson, B.L.; Hurley, J.K.; Tollin, G.; Oh, B.-H.; Skjeldal, L.; Chae, Y.K.; Cheng, H.; Xia, B.; Markley, J.L. Structure-function studies of [2Fe-2S] ferredoxins. J. Bioenerg. Biomembr. 1994, 26, 67–88. [Google Scholar] [CrossRef] [PubMed]
- Golding, A.J.; Johnson, G.N. Down-regulation of linear and activation of cyclic electron transport during drought. Planta 2003, 218, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Harbinson, J.; Genty, B.; Foyer, C.H. Relationship between photosynthetic electron transport and stromal enzyme activity in pea leaves: Toward an understanding of the nature of photosynthetic control. Plant Physiol. 1990, 94, 545–553. [Google Scholar] [CrossRef]
- Harbinson, J.; Foyer, C.H. Relationships between the efficiencies of photosystems I and II and stromal redox state in CO2-free air: Evidence for cyclic electron flow in vivo. Plant Physiol. 1991, 97, 41–49. [Google Scholar] [CrossRef]
- Harbinson, J.; Hedley, C.L. Changes in P-700 oxidation during the early stages of the induction of photosynthesis. Plant Physiol. 1993, 103, 649–660. [Google Scholar] [CrossRef]
- Kono, M.; Noguchi, K.; Terashima, I. Roles of the cyclic electron flow around PSI (CEF-PSI) and O2-dependent alternative pathways in regulation of the photosynthetic electron flow in short-term fluctuating light in Arabidopsis thaliana. Plant Cell Physiol. 2014, 55, 990–1004. [Google Scholar] [CrossRef] [PubMed]
- Miyake, C.; Miyata, M.; Shinzaki, Y.; Tomizawa, K.-I. CO2 response of cyclic electron flow around PSI (CEF-PSI) in Tobacco leaves—Relative electron fluxes through PSI and PSII determine the magnitude of non-photochemical quenching (NPQ) of Chl fluorescence. Plant Cell Physiol. 2005, 46, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Wada, S.; Miyake, C.; Makino, A.; Suzuki, Y. Photorespiration coupled with CO2 assimilation protects photosystem I from photoinhibition under moderate poly(ethylene glycol)-induced osmotic stress in Rice. Front. Plant Sci. 2020, 11, 1121. [Google Scholar] [CrossRef] [PubMed]
- Wada, S.; Takagi, D.; Miyake, C.; Makino, A.; Suzuki, Y. Responses of the photosynthetic electron transport reactions stimulate the oxidation of the reaction center chlorophyll of photosystem I, P700, under drought and high temperatures in Rice. Int. J. Mol. Sci. 2019, 20, 2068. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Wang, X.; Zeng, Z.; Huang, W. Photoinhibition of photosystem I induced by different intensities of fluctuating light is determined by the kinetics of ∆pH formation rather than linear electron flow. Antioxidants 2022, 11, 2325. [Google Scholar] [CrossRef]
- Tikhonov, A.N. The cytochrome b6f complex at the crossroad of photosynthetic electron transport pathways. Plant Physiol. Biochem. 2014, 81, 163–183. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furutani, R.; Wada, S.; Ifuku, K.; Maekawa, S.; Miyake, C. Higher Reduced State of Fe/S-Signals, with the Suppressed Oxidation of P700, Causes PSI Inactivation in Arabidopsis thaliana. Antioxidants 2023, 12, 21. https://doi.org/10.3390/antiox12010021
Furutani R, Wada S, Ifuku K, Maekawa S, Miyake C. Higher Reduced State of Fe/S-Signals, with the Suppressed Oxidation of P700, Causes PSI Inactivation in Arabidopsis thaliana. Antioxidants. 2023; 12(1):21. https://doi.org/10.3390/antiox12010021
Chicago/Turabian StyleFurutani, Riu, Shinya Wada, Kentaro Ifuku, Shu Maekawa, and Chikahiro Miyake. 2023. "Higher Reduced State of Fe/S-Signals, with the Suppressed Oxidation of P700, Causes PSI Inactivation in Arabidopsis thaliana" Antioxidants 12, no. 1: 21. https://doi.org/10.3390/antiox12010021
APA StyleFurutani, R., Wada, S., Ifuku, K., Maekawa, S., & Miyake, C. (2023). Higher Reduced State of Fe/S-Signals, with the Suppressed Oxidation of P700, Causes PSI Inactivation in Arabidopsis thaliana. Antioxidants, 12(1), 21. https://doi.org/10.3390/antiox12010021







