Importance of Insoluble-Bound Phenolics to the Antioxidant Potential Is Dictated by Source Material
Abstract
1. Introduction
2. Synthesis and Transport of Phenolics in Plants
3. Classification Based on the Structure
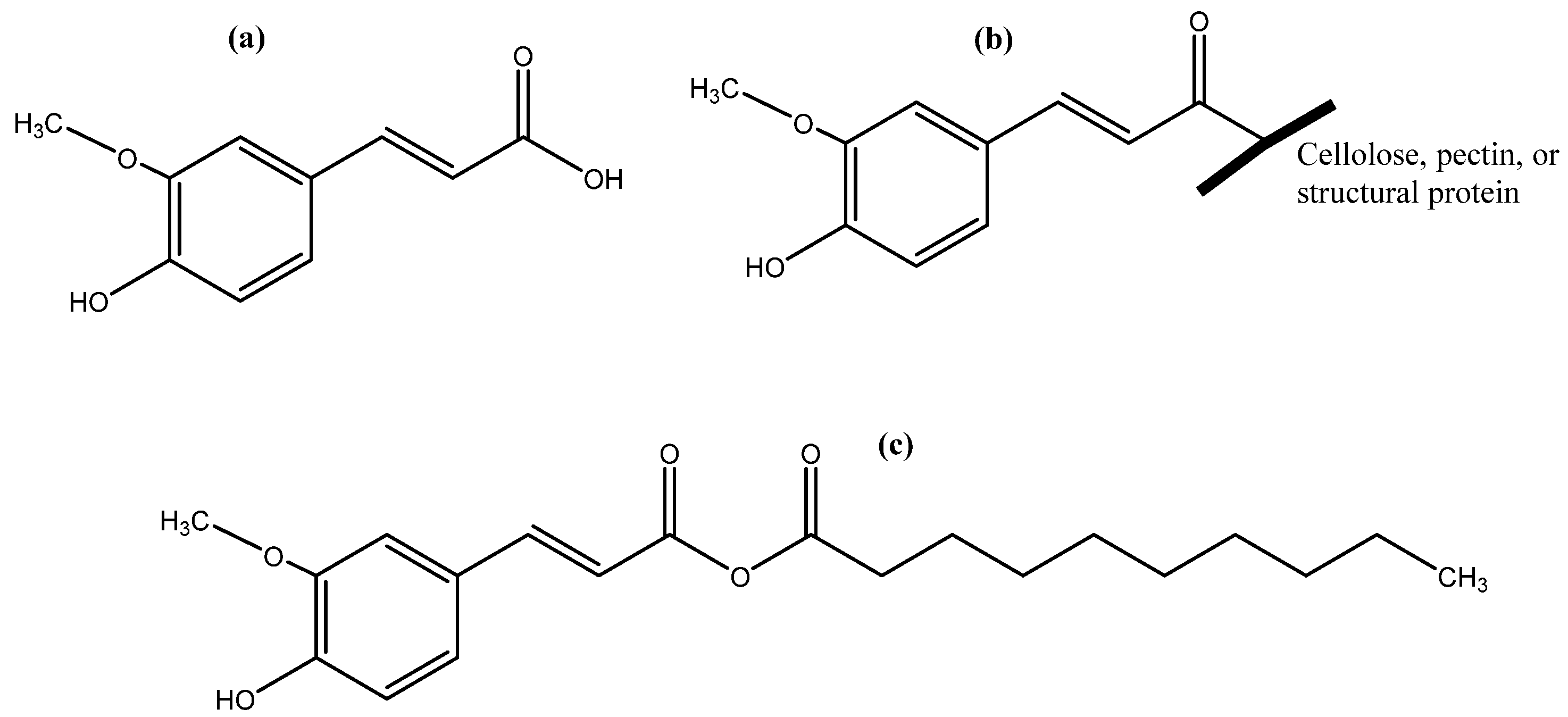
4. Classification and Localization of Phenolics Based on Their Association with Food Matrix
5. Interaction of Phenolics with Other Compounds
6. Effect of Processing on the Release of Insoluble-Bound Phenolics
6.1. Non-Thermal Processing
6.2. Thermal Processing
7. Insoluble-Bound Phenolics in Various Food Matrices
7.1. IBPs in Fruits, Vegetables, Herbs, and Their Different Parts
7.2. IBPs in Cereals, Legumes, Pulses, and Other Seeds
7.3. IBPs in Teas, Coffees, Nuts, Seafoods, and Their By-Products
8. Extraction of IBPs
9. Biological Activities of IBPs
9.1. Antioxidant Properties
9.2. DNA Oxidation Inhibition
9.3. LDL Oxidation Inhibition
9.4. α-Glucosidase, α-Amylase, Pancreatic Lipase, and ACE Inhibitory Activities
9.5. Anticancer Effect
9.6. Other Effects
10. Metabolism of IBPs
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hossain, A.; Dave, D.; Shahidi, F. Antioxidant potential of sea cucumbers and their beneficial effects on human health. Mar. Drugs 2022, 20, 521. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Yeo, J.D. Insoluble-bound phenolics in food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Y.; Li, H.; Deng, Z.; Tsao, R. A review on insoluble-bound phenolics in plant-based food matrix and their contribution to human health with future perspectives. Trends Food Sci. Technol. 2020, 105, 347–362. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Bioactives in spices, and spice oleoresins: Phytochemicals and their beneficial effects in food preservation and health promotion. J. Food Bioact. 2018, 3, 8–75. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J.D. Bioactivities of phenolics by focusing on suppression of chronic diseases: A review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Preservation of aquatic food using edible films and coatings containing essential oils: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 66–105. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Role of lipids in food flavor generation. Molecules 2022, 27, 5014. [Google Scholar] [CrossRef]
- Santos-Zea, L.; Villela-Castrejón, J.; Gutiérrez-Uribe, A.J. Bound Phenolics in Foods. In Bioactive Molecules in Food; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer: Cham, Switzerland, 2019; pp. 973–990. [Google Scholar]
- Wang, Z.; Li, S.; Ge, S.; Lin, S. Review of distribution, extraction methods, and health benefits of bound phenolics in food plants. J. Agric. Food Chem. 2020, 68, 3330–3343. [Google Scholar] [CrossRef]
- Li, M.; Chen, X.; Deng, J.; Ouyang, D.; Wang, D.; Liang, Y.; Chen, Y.; Sun, Y. Effect of thermal processing on free and bound phenolic compounds and antioxidant activities of hawthorn. Food Chem. 2020, 332, 127429. [Google Scholar] [CrossRef]
- Yeo, J.D.; Shahidi, F. Effect of hydrothermal processing on changes of insoluble-bound phenolics of lentils. J. Funct. Foods 2017, 38, 716–722. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Shahidi, F.; Varatharajan, V.; Oh, W.Y.; Peng, H. Phenolic compounds in agri-food by-products, their bioavailability and health effects. J. Food Bioact. 2019, 5, 57–119. [Google Scholar] [CrossRef]
- Rocchetti, G.; Gregorio, R.P.; Lorenzo, J.M.; Barba, F.J.; Oliveira, P.G.; Prieto, M.A.; Simal-Gandara, J.; Mosele, J.I.; Motilva, M.J.; Tomas, M.; et al. Functional implications of bound phenolic compounds and phenolics–food interaction: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 811–842. [Google Scholar] [CrossRef]
- Burlini, I.; Sacchetti, G. Secondary bioactive metabolites from plant-derived food byproducts through ecopharmacognostic approaches: A bound phenolic case study. Plants 2020, 9, 1060. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Singh, V. Antioxidative free and bound phenolic constituents in botanical fractions of Indian specialty maize (Zea mays L.) genotypes. Food Chem. 2016, 201, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.G.; Samac, D.A.; Sarath, G. Modifying crops to increase cell wall digestibility. Plant Sci. 2012, 185, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Hassan, O.; Chang, T.; Hossain, A. Changes in the secondary compounds of persimmon leaves as a defense against circular leaf spot caused by Plurivorosphaerella nawae. PLoS ONE 2020, 15, e0230286. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Renard, C.M.G.C. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar]
- Buitimea-Cantúa, N.E.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Phenolic-protein interactions: Effects on food properties and health benefits. J. Med. Food 2018, 21, 188–198. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Chen, F.; Zhao, G. Effects of Molecular Structure of Polyphenols on Their Noncovalent Interactions with Oat β-glucan. J. Agric. Food Chem. 2013, 61, 4533–4538. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, Y.; Jia, Y.; Pang, M.; Cheng, G.; Cai, S. Phenolic profiles, antioxidant activities and cytoprotective effects of different phenolic fractions from oil palm (Elaeis guineensis Jacq.) fruits treated by ultra-high pressure. Food Chem. 2019, 288, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.D.; Xue, Q.W.; Zhao, T.R.; Khan, A.; Wang, Y.F.; Liu, Y.P.; Cao, J.X.; Cheng, G.G. The effect of ultra-high pretreatment on free, esterified and insoluble-bound phenolics from mango leaves and their antioxidant and cytoprotective activities. Food Chem. 2022, 368, 130864. [Google Scholar] [CrossRef]
- Hossain, A.; Dave, D.; Shahidi, F. Effect of high-pressure processing (HPP) on phenolics of North Atlantic sea cucumber (Cucumaria frondosa). J. Agric. Food Chem. 2022, 70, 3489–3501. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Yeo, J.D.; Dave, D.; Shahidi, F. Phenolic compounds and antioxidant capacity of sea cucumber (Cucumaria frondosa) processing discards as affected by high-pressure processing (HPP). Antioxidants 2022, 11, 337. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Tang, F.; Xu, F.; Wang, Y.; Bao, J. Effects of γ-irradiation on phenolics content, antioxidant activity and physicochemical properties of whole grainrice. Radiat. Phys. Chem. 2013, 85, 227–233. [Google Scholar] [CrossRef]
- Shumoy, H.; Gabaza, M.; Vandevelde, J.; Raes, K. Soluble and bound phenolic contents and antioxidant capacity of tef injera as affected by traditional fermentation. J. Food Compos. Anal. 2017, 58, 52–59. [Google Scholar] [CrossRef]
- Yeo, J.D.; Tsao, R.; Sun, Y.; Shahidi, F. Liberation of insoluble-bound phenolics from lentil hull matrices as affected by Rhizopus oryzae fermentation: Alteration in phenolic profiles and their inhibitory capacities against low-density lipoprotein (LDL) and DNA oxidation. Food Chem. 2021, 363, 130275. [Google Scholar] [CrossRef]
- Rasera, G.B.; Hilkner, M.H.; de Castro, R.J.S. Free and insoluble-bound phenolics: How does the variation of these compounds affect the antioxidant properties of mustard grains during germination? Food Res. Int. 2020, 133, 109115. [Google Scholar] [CrossRef]
- Yeo, J.; Shahidi, F. Critical evaluation of changes in the ratio of insoluble bound to soluble phenolics on antioxidant activity of lentils during germination. J. Agric. Food Chem. 2015, 63, 379–381. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, K.; Guo, M.; Luan, X.; Duan, Z.; Li, X. The effect of thermal pretreatment processing on the distribution of free and bound phenolics in virgin Camellia oleifera seed oil. LWT—Food Sci. Technol. 2022, 161, 113349. [Google Scholar] [CrossRef]
- Peng, G.; Gan, J.; Dong, R.; Chen, Y.; Xie, J.; Huang, Z.; Gu, Y.; Huang, D.; Yu, Q. Combined microwave and enzymatic treatment improve the release of insoluble bound phenolic compounds from the grapefruit peel insoluble dietary fiber. LWT—Food Sci. Technol. 2021, 149, 111905. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, S.; Yao, L.; Wang, L.; Li, C. Free and bound phenolics of buckwheat varieties: HPLC characterization, antioxidant activity, and inhibitory potency towards α-glucosidase with molecular docking analysis. Antioxidants 2019, 8, 606. [Google Scholar] [CrossRef]
- Martín-García, B.; Gómez-Caravaca, A.M.; Marconi, E.; Verardo, V. Distribution of free and bound phenolic compounds, and alkylresorcinols in wheat aleurone enriched fractions. Food Res. Int. 2021, 140, 109816. [Google Scholar] [CrossRef] [PubMed]
- Liyana-Pathirana, C.M.; Shahidi, F. Importance of insoluble-bound phenolics to antioxidant properties of wheat. J. Agric. Food Chem. 2006, 54, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekara, A.; Shahidi, F. Content of insoluble bound phenolics in millets and their contribution to antioxidant capacity. J. Agric. Food Chem. 2010, 58, 6706–6714. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Shahidi, F. Inhibitory activities of soluble and bound millet seed phenolics on free radicals and reactive oxygen species. J. Agric. Food Chem. 2011, 59, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Madhujith, T.; Shahidi, F. Antioxidant potential of barley as affected by alkaline hydrolysis and release of insoluble-bound phenolics. Food Chem. 2009, 117, 615–620. [Google Scholar] [CrossRef]
- Dvořáková, M.; Guido, L.F.; Dostálek, P.; Skulilová, Z.; Moreira, M.M.; Barros, A. Antioxidant properties of free, soluble ester and insoluble-bound phenolic compounds in different barley varieties and corresponding malts. J. Inst. Brew. 2008, 114, 27–33. [Google Scholar] [CrossRef]
- Yang, X.J.; Dang, B.; Fan, M.T. Free and bound phenolic compound content and antioxidant activity of different cultivated blue highland barley varieties from the qinghai-tibet plateau. Molecules 2018, 23, 879. [Google Scholar] [CrossRef] [PubMed]
- de Camargo, A.C.; Alvarez, A.C.; Arias-Santé, M.F.; Oyarzún, J.E.; Andia, M.E.; Uribe, S.; Pizarro, P.N.; Bustos, S.M.; Schwember, A.R.; Shahidi, F.; et al. Soluble free, esterified and insoluble-bound phenolic antioxidants from chickpeas prevent cytotoxicity in human hepatoma HuH-7 cells induced by peroxyl radicals. Antioxidants 2022, 11, 1139. [Google Scholar] [CrossRef] [PubMed]
- Paranavitana, L.; Oh, W.Y.; Yeo, J.D.; Shahidi, F. Determination of soluble and insoluble-bound phenolic compounds in dehulled, whole, and hulls of green and black lentils using electrospray ionization (ESI)-MS/MS and their inhibition in DNA strand scission. Food Chem. 2021, 361, 130083. [Google Scholar] [CrossRef]
- Wang, Y.K.; Zhang, X.; Chen, G.L.; Yu, J.; Yang, L.Q.; Gao, Y.Q. Antioxidant property and their free, soluble conjugate and insoluble-bound phenolic contents in selected beans. J. Funct. Foods 2016, 24, 359–372. [Google Scholar] [CrossRef]
- Rahman, M.J.; Costa de Camargo, A.; Shahidi, F. Phenolic profiles and antioxidant activity of defatted camelina and sophia seeds. Food Chem. 2018, 240, 917–925. [Google Scholar] [CrossRef]
- Rahman, M.J.; de Camargo, A.C.; Shahidi, F. Phenolic and polyphenolic profiles of chia seeds and their in vitro biological activities. J. Funct. Foods 2017, 35, 622–634. [Google Scholar] [CrossRef]
- Yu, M.; Yang, L.; Xue, Q.; Yin, P.; Sun, L.; Liu, Y. Comparison of free, esterified, and insoluble-bound phenolics and their bioactivities in three organs of Ionicera japonica and I. Macranthoides. Molecules 2019, 24, 970. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Xia, C.; Feng, S.; Chen, T.; Zhou, L.; Liu, L.; Kong, Q.; Yang, H.; Ding, C. Assessment of free and bound phenolics in the flowers and floral organs of two Camellia species flower and their antioxidant activities. Food Biosci. 2022, 49, 101905. [Google Scholar] [CrossRef]
- Suwannachot, J.; Reginio, F.C., Jr.; Hamauzu, Y.; Ogawa, Y. Assessment of free, esterified, and insoluble-bound phenolics of green and red perilla leaves and changes during simulated gastrointestinal digestion. Food Chem. Adv. 2022, 1, 100018. [Google Scholar] [CrossRef]
- Chen, G.L.; Zhang, X.; Chen, S.G.; Han, M.D.; Gao, Y.Q. Antioxidant activities and contents of free, esterified and insoluble-bound phenolics in 14 subtropical fruit leaves collected from the south of China. J. Funct. Foods 2017, 30, 290–302. [Google Scholar] [CrossRef]
- Anokwuru, C.; Sigidi, M.; Boukandou, M.; Tshisikhawe, P.; Traore, A.; Potgieter, N. Antioxidant activity and spectroscopic characteristics of extractable and non-extractable phenolics from terminalia sericea burch. ex DC. Molecules 2018, 23, 1303. [Google Scholar] [CrossRef]
- Ayoub, M.; De Camargo, A.C.; Shahidi, F. Antioxidants and bioactivities of free, esterified and insoluble-bound phenolics from berry seed meals. Food Chem. 2016, 197, 221–232. [Google Scholar] [CrossRef]
- Yao, J.; Chen, J.; Yang, J.; Hao, Y.; Fan, Y.; Wang, C.; Li, N. Free, soluble-bound and insoluble-bound phenolics and their bioactivity in raspberry pomace. LWT—Food Sci. Technol. 2021, 135, 109995. [Google Scholar] [CrossRef]
- Albishi, T.; Banoub, J.H.; De Camargo, A.C.; Shahidi, F. Wood extracts as unique sources of soluble and insoluble-bound phenolics: Reducing power, metal chelation and inhibition of oxidation of human LDL-cholesterol and DNA strand scission. J. Food Bioact. 2019, 8, 92–98. [Google Scholar] [CrossRef]
- Prakash, O.; Baskaran, R.; Kudachikar, V.B. Characterization, quantification of free, esterified and bound phenolics in Kainth (Pyrus pashia Buch.-Ham. Ex D.Don) fruit pulp by UPLC-ESI-HRMS/MS and evaluation of their antioxidant activity. Food Chem. 2019, 299, 125114. [Google Scholar] [CrossRef] [PubMed]
- Arruda, H.S.; Pereira, G.A.; de Morais, D.R.; Eberlin, M.N.; Pastore, G.M. Determination of free, esterified, glycosylated and insoluble-bound phenolics composition in the edible part of araticum fruit (Annona crassiflora Mart.) and its by-products by HPLC-ESI-MS/MS. Food Chem. 2018, 245, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, S.; Li, Y.; Xue, X.; Huang, Y.; Luo, H.; Zhang, Y.; Lu, Z. Comparative evaluation of soluble and insoluble-bound phenolics and antioxidant activity of two Chinese mistletoes. Molecules 2018, 23, 359. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Senadheera, R.L.T.; Dave, D.; Shahidi, F. Phenolic profiles of Atlantic sea cucumber (Cucumaria frondosa) tentacles and their biological properties. Food Res. Int. 2022, 163, 112262. [Google Scholar] [CrossRef]
- Bueno-Herrera, M.; Pérez-Magariño, S. Validation of an extraction method for the quantification of soluble free and insoluble bound phenolic compounds in wheat by HPLC-DAD. J. Cereal Sci. 2020, 93, 102984. [Google Scholar] [CrossRef]
- Deng, J.; Xiang, Z.; Lin, C.; Zhu, Y.; Yang, K.; Liu, T.; Xia, C.; Chen, J.; Zhang, W.; Zhang, Y.; et al. Identification and quantification of free, esterified, and insoluble-bound phenolics in grains of hulless barley varieties and their antioxidant activities. LWT—Food Sci. Technol. 2021, 151, 112001. [Google Scholar] [CrossRef]
- Alshikh, N.; de Camargo, A.C.; Shahidi, F. Phenolics of selected lentil cultivars: Antioxidant activities and inhibition of low-density lipoprotein and DNA damage. J. Funct. Foods 2015, 18, 1022–1038. [Google Scholar] [CrossRef]
- Sun, Y.; Deng, Z.; Liu, R.; Zhang, H.; Zhu, H.; Jiang, L.; Tsao, R. A comprehensive profiling of free, conjugated and bound phenolics and lipophilic antioxidants in red and green lentil processing by-products. Food Chem. 2020, 325, 126925. [Google Scholar] [CrossRef]
- Yeo, J.D.; Shahidi, F. Identification and quantification of soluble and insoluble-bound phenolics in lentil hulls using HPLC-ESI-MS/MS and their antioxidant potential. Food Chem. 2020, 315, 126202. [Google Scholar] [CrossRef]
- Lou, X.; Xu, H.; Hanna, M.; Yuan, L. Identification and quantification of free, esterified, glycosylated and insoluble-bound phenolic compounds in hawthorn berry fruit (Crataegus pinnatifida) and antioxidant activity evaluation. LWT—Food Sci. Technol. 2020, 130, 109643. [Google Scholar] [CrossRef]
- Xu, Z.; Xiong, X.; Zeng, Q.; He, S.; Yuan, Y.; Wang, Y.; Wang, Y.; Yang, X.; Su, D. Alterations in structural and functional properties of insoluble dietary fibers-bound phenolic complexes derived from lychee pulp by alkaline hydrolysis treatment. LWT—Food Sci. Technol. 2020, 127, 109335. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; de Camargo, A.C.; Shahidi, F. Phenolic compounds of pomegranate byproducts (outer skin, mesocarp, divider membrane) and their antioxidant activities. J. Agric. Food Chem. 2016, 64, 6584–6604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ma, Y.; Gao, F.; Zhao, Y.; Cai, S.; Pang, M. The free, esterified, and insoluble-bound phenolic profiles of Rhus chinensis Mill. fruits and their pancreatic lipase inhibitory activities with molecular docking analysis. J. Funct. Foods 2018, 40, 729–735. [Google Scholar] [CrossRef]
- Suo, H.; Peng, Z.; Guo, Z.; Wu, C.; Liu, J.; Wang, L.; Xiao, J.; Li, X. Deep eutectic solvent-based ultrasonic-assisted extraction of phenolic compounds from different potato genotypes: Comparison of free and bound phenolic profiles and antioxidant activity. Food Chem. 2022, 388, 133058. [Google Scholar] [CrossRef]
- John, J.A.; Shahidi, F. Phenolic compounds and antioxidant activity of Brazil nut (Bertholletia excelsa). J. Funct. Foods 2010, 2, 196–209. [Google Scholar] [CrossRef]
- Wang, R.; Tian, X.; Li, Q.; Liao, L.; Wu, S.; Tang, F.; Shen, D.; Liu, Y. Walnut pellicle color affects its phenolic composition: Free, esterified and bound phenolic compounds in various colored-pellicle walnuts. J. Food Compos. Anal. 2022, 109, 104470. [Google Scholar] [CrossRef]
- Wu, S.; Shen, D.; Wang, R.; Li, Q.; Mo, R.; Zheng, Y.; Zhou, Y.; Liu, Y. Phenolic profiles and antioxidant activities of free, esterified and bound phenolic compounds in walnut kernel. Food Chem. 2021, 350, 129217. [Google Scholar] [CrossRef] [PubMed]
- Mudenuti, N.V.d.R.; de Camargo, A.C.; de Alencar, S.M.; Danielski, R.; Shahidi, F.; Madeira, T.B.; Hirooka, E.Y.; Spinosa, W.A.; Grossmann, M.V.E. Phenolics and alkaloids of raw cocoa nibs and husk: The role of soluble and insoluble-bound antioxidants. Food Biosci. 2021, 42, 101085. [Google Scholar] [CrossRef]
- Pico, J.; Yan, Y.; Gerbrandt, E.M.; Castellarin, S.D. Determination of free and bound phenolics in northern highbush blueberries by a validated HPLC/QTOF methodology. J. Food Compos. Anal. 2022, 108, 104412. [Google Scholar] [CrossRef]
- Xue, P.; Liao, W.; Chen, Y.; Xie, J.; Chang, X.; Peng, G.; Huang, Q.; Wang, Y.; Sun, N.; Yu, Q. Release characteristic and mechanism of bound polyphenols from insoluble dietary fiber of navel orange peel via mixed solid-state fermentation with Trichoderma reesei and Aspergillus niger. LWT—Food Sci. Technol. 2022, 161, 113387. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, X.; Guo, Z.; Feng, X.; Huang, P.; Du, M.; Zalán, Z.; Kan, J. Distribution and natural variation of free, esterified, glycosylated, and insoluble-bound phenolic compounds in brocade orange (Citrus sinensis L. Osbeck) peel. Food Res. Int. 2022, 153, 110958. [Google Scholar] [CrossRef]
- Gulsunoglu, Z.; Karbancioglu-Guler, F.; Raes, K.; Kilic-Akyilmaz, M. Soluble and insoluble-bound phenolics and antioxidant activity of various industrial plant wastes. Int. J. Food Prop. 2019, 22, 1501–1510. [Google Scholar] [CrossRef]
- Hossain, A.; Moon, H.K.; Kim, J.-K. Antioxidant properties of Korean major persimmon (Diospyros kaki) leaves. Food Sci. Biotechnol. 2018, 27, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Rahman, J. Safety, Nutrition and Functionality of the Traditional Foods. In Traditional Foods-History, Preparation, Processing and Safety; Al-Khusaibi, M., Al-Habsi, N., Rahman, M.S., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 219–238. [Google Scholar]
- Hossain, A.; Moon, H.K.; Kim, J.-K. Effect of pre-treatment and extraction conditions on the antioxidant properties of persimmon (Diospyros kaki) leaves. Biosci. Biotechnol. Biochem. 2017, 81, 2079–2085. [Google Scholar] [CrossRef] [PubMed]
- Kuruburu, M.G.; Bovilla, V.R.; Naaz, R.; Leihang, Z.; Madhunapantula, S.V. Variations in the anticancer activity of free and bound phenolics of finger millet (Eleusine coracana (L) Gaertn; variety KMR-301) seeds. Phytomed. Plus 2022, 2, 100276. [Google Scholar] [CrossRef]
- Feng, Z.; Dong, L.; Zhang, R.; Chi, J.; Liu, L.; Zhang, M.; Jia, X. Structural elucidation, distribution and antioxidant activity of bound phenolics from whole grain brown rice. Food Chem. 2021, 358, 129872. [Google Scholar] [CrossRef]
- Ye, C.; Zhang, R.; Dong, L.; Chi, J.; Huang, F.; Dong, L.; Zhang, M.; Jia, X. α-Glucosidase inhibitors from brown rice bound phenolics extracts (BRBPE): Identification and mechanism. Food Chem. 2022, 372, 131306. [Google Scholar] [CrossRef] [PubMed]
- Takoudjou Miafo, A.P.; Koubala, B.B.; Muralikrishna, G.; Kansci, G.; Fokou, E. Non-starch polysaccharides derived from sorghum grains, bran, spent grain and evaluation of their antioxidant properties with respect to their bound phenolic acids. Bioact. Carbohydr. Diet. Fibre 2022, 28, 100314. [Google Scholar] [CrossRef]
- Xiong, Y.; Ng, K.; Zhang, P.; Warner, R.D.; Shen, S.; Tang, H.Y.; Liang, Z.; Fang, Z. In vitro α-glucosidase and α-amylase inhibitory activities of free and bound phenolic extracts from the bran and kernel fractions of five sorghum grain genotypes. Foods 2020, 9, 1301. [Google Scholar] [CrossRef]
- Mitrović, J.; Nikolić, N.; Karabegović, I.; Lazić, M.; Stojanović, G. Characterization of free and insoluble-bound phenolics of chia (Salvia hispanica L.) seeds. Nat. Prod. Res. 2021, 36, 385–389. [Google Scholar] [CrossRef]
- Naczk, M.; Amarowicz, R.; Pink, D.; Shahidi, F. Insoluble condensed tannins of canola/rapeseed. J. Agric. Food Chem. 2000, 48, 1758–1762. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, Y.; Wang, L.; Tan, Y.; Shi, Y.; Sedjoah, R.C.A.A.; Shao, Y.; Li, L.; Wang, M.; Wan, J.; et al. Ultrasound-assisted extraction of bound phenolic compounds from the residue of Apocynum venetum tea and their antioxidant activities. Food Biosci. 2022, 47, 101646. [Google Scholar] [CrossRef]
- Wang, X.; Contreras, M.d.M.; Xu, D.; Jia, W.; Wang, L.; Yang, D. New insights into free and bound phenolic compounds as antioxidant cluster in tea seed oil: Distribution and contribution. LWT—Food Sci. Technol. 2021, 136, 110315. [Google Scholar] [CrossRef]
- Monente, C.; Ludwig, I.A.; Irigoyen, A.; De Peña, M.P.; Cid, C. Assessment of total (Free and Bound) phenolic compounds in spent coffee extracts. J. Agric. Food Chem. 2015, 63, 4327–4334. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, H.; Wang, Y.; Peng, Z.; Guo, Z.; Ma, Y.; Zhang, R.; Zhang, M.; Wu, Q.; Xiao, J.; et al. Effects of different extraction methods on contents, profiles, and antioxidant abilities of free and bound phenolics of Sargassum polycystum from the South China Sea. J. Food Sci. 2022, 87, 968–981. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Dave, D.; Shahidi, F. Northern sea cucumber (Cucumaria frondosa): A potential candidate for functional food, nutraceutical, and pharmaceutical sector. Mar. Drugs 2020, 18, 274. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.D.; Banerjee, J.; Arora, A. Prebiotic potential of oligosaccharides: A focus on xylan derived oligosaccharides. Bioact. Carbohydr. Diet. Fibre 2015, 5, 19–30. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Dong, L.; Jia, X.; Liu, L.; Ma, Y.; Huang, F.; Zhang, R. Phytochemical profile, bioactivity, and prebiotic potential of bound phenolics released from rice bran dietary fiber during in vitro gastrointestinal digestion and colonic fermentation. J. Agric. Food Chem. 2019, 67, 12796–12805. [Google Scholar] [CrossRef]
- De Camargo, A.C.; Regitano-D’Arce, M.A.B.; Biasoto, A.C.T.; Shahidi, F. Enzyme-assisted extraction of phenolics from winemaking by-products: Antioxidant potential and inhibition of alpha-glucosidase and lipase activities. Food Chem. 2016, 212, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.J.; Ambigaipalan, P.; Shahidi, F. Biological activities of camelina and sophia seeds phenolics: Inhibition of LDL oxidation, DNA damage, and pancreatic lipase and α-glucosidase activities. J. Food Sci. 2018, 83, 237–245. [Google Scholar] [CrossRef] [PubMed]
- da Costa Pinaffi, A.C.; Sampaio, G.R.; Soares, M.J.; Shahidi, F.; de Camargo, A.C.; Torres, E.A.F.S. Insoluble-bound polyphenols released from guarana powder: Inhibition of alpha-glucosidase and proanthocyanidin profile. Molecules 2020, 25, 679. [Google Scholar] [CrossRef]
- Shi, J.; Shan, S.; Li, Z.; Li, H.; Li, X.; Li, Z. Bound polyphenol from foxtail millet bran induces apoptosis in HCT-116 cell through ROS generation. J. Funct. Foods 2015, 17, 958–968. [Google Scholar] [CrossRef]
- Shan, S.; Xie, Y.; Zhao, H.; Niu, J.; Zhang, S.; Zhang, X.; Li, Z. Bound polyphenol extracted from jujube pulp triggers mitochondria-mediated apoptosis and cell cycle arrest of HepG2 cell in vitro and in vivo. J. Funct. Foods 2019, 53, 187–196. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, H.; Xu, F.; Zhang, Y.; Li, Z.; Ju, X.; Wang, L. Insoluble-bound polyphenols of adlay seed ameliorate H2O2-induced oxidative stress in HepG2 cells via Nrf2 signalling. Food Chem. 2020, 325, 126865. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, L.; Jia, X.; Liu, L.; Chi, J.; Huang, F.; Ma, Q.; Zhang, M.; Zhang, R. Bound phenolics ensure the antihyperglycemic effect of rice bran dietary fiber in db/db mice via activating the insulin signaling pathway in skeletal muscle and altering gut microbiota. J. Agric. Food Chem. 2020, 68, 4387–4398. [Google Scholar] [CrossRef]
- Shahidi, F.; Peng, H. Bioaccessibility and bioavailability of phenolic compounds. J. Food Bioact. 2018, 4, 11–68. [Google Scholar] [CrossRef]
- Kroon, A.P.; Faulds, C.B.; Ryden, P.; Robertson, J.A.; Williamson, G. Release of covalently bound ferulic acid from fiber in the human colon. J. Agric. Food Chem. 1997, 45, 661–667. [Google Scholar] [CrossRef]
- Andreasen, F.M.; Kroon, P.A.; Williamson, G.; Garcia-Conesa, M.-T. Esterase activity able to hydrolyze dietary antioxidant hydroxycinnamates is distributed along the intestine of mammals. J. Agric. Food Chem. 2001, 49, 5679–5684. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Fischer, J.; Krewer, G.; Akoh, C.C. Phenolic compounds from blueberries can inhibit colon cancer cell proliferation and induce apoptosis. J. Agric. Food Chem. 2005, 53, 7320–7329. [Google Scholar] [CrossRef] [PubMed]



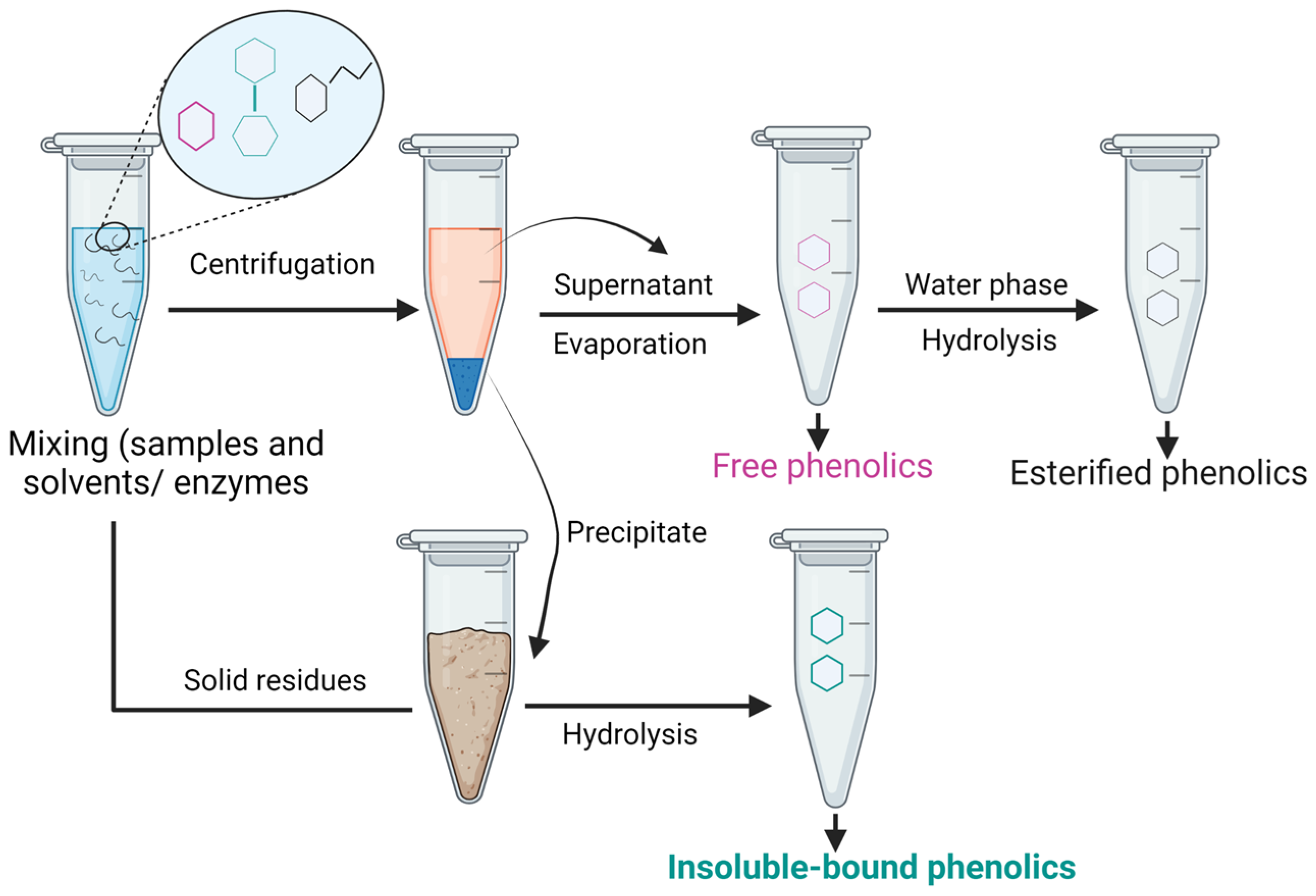
| Sources | Processing Techniques | Content of IBPs | Antioxidant Activity of IBPs | Phenolic Profiles in IBPs | References |
|---|---|---|---|---|---|
| Oil palm (Elaeis guineensis Jacq.) fruits | Ultra-high pressure (UHP, 500 MPa for 10 min) | Increased TPC and TFC by around 2 times upon UHP | Increased DPPH and ABTS radical cation scavenging activities, FRAP values, and ROS inhibitory activity | Increased the content and number of individual phenolics | [24] |
| Mango leaves | UHP (500 MPa for 10 min) | Increased TPC and TFC significantly upon UHP | Increased DPPH and ABTS radical cation scavenging activities and FRAP values | Increased the content and number of individual phenolics | [25] |
| Sea cucumber (C. frondosa) body wall | High-pressure processing (HPP, 200, 400, and 600 MPa for 5, 10, and 15 min) | Increased IBPs in TPC and TFC by about 27 and 35%, respectively | Increased DPPH radical scavenging and metal chelation activities | Increased the content (~28%) and number of individual phenolics | [26] |
| Sea cucumber (C. frondosa) waste | HPP (600 MPa for 10 min) | Increased the overall TPC and TFC | Increased DPPH radical scavenging activity but decreased ABTS radical cation scavenging activity | Increased the content (~26%) and number of individual phenolics | [27] |
| Whole grain rice (black, red, and white) | Gamma (γ)-irradiation (10 kGy) | Bound phenolics increased significantly compared to free phenolic fraction | Increased ABTS radical cation scavenging activity | NA | [28] |
| Fermented pancake (Injera) | Fermentation | Increased TPC and decreased TFC | Increased FRAP values but decreased DPPH and ABTS radical cation scavenging activities | Decreased the content of individual phenolics by 2–100% | [29] |
| Lentil hulls | Fermentation | IBPs decreased significantly | NA | Individual phenolic compounds decreased upon fermentation | [30] |
| Mustard grains (Brassica nigra and Sinapsis alba) | Germination | The TPC and TFC increased or remained the same | Increased DPPH and ABTS radical cation scavenging activities and FRAP and ORAC values for S. alba | Showed an overall positive effect on the phenolic profile | [31] |
| Lentils | Germination | The TPC and TFC increased upon processing | Increased DPPH and ABTS radical cation scavenging activities | NA | [32] |
| Virgin (Camellia oleifera) seed oil | Thermal pre-treatment (0–120 min for 90 and 150 °C) | Fluctuated among different heating conditions | NA | Fluctuated among different heating conditions | [33] |
| Hawthorn fruit | Thermal processing: lightly cooked (80 °C for 20 min and 100 °C for 15 min) and well-cooked (120 °C for 20 min and 150 °C for 15 min) | IBPs increased by 55.84 and 30.35% through being lightly and well-cooked, but overall TPC decreased with cooking | Increased ORAC values but decreased DPPH and ABTS radical cation scavenging activities by both treatments | Decreased the number of individual phenolics and increased the content only by lightly cooking | [11] |
| Lentils | Hydrothermal processing (boiling for 25 min) | Decreased TPC and TFC | Decreased ORAC values, DPPH radical scavenging activity, and reducing power ability | Decreased the overall content and number of phenolics | [12] |
| Grapefruit peels | Microwave and enzymatic treatments | Improved the overall TPC and TFC | Improved DPPH radical scavenging activity and ORAC values | Combined microwave and enzymatic treatment improved the release of phenolic acids | [34] |
| Sources | Free (mg GAE/g) | IBPs (mg GAE/g) | TPC (mg GAE/g) | Ration (IBPs/F) | IBPs/TPC (%) | References |
|---|---|---|---|---|---|---|
| Buckwheat | 5.18–13.74 | 0.63–0.96 | 6.29–14.4 | 0.07–0.12 | 6.67–10.01 | [35] |
| Buckwheat brans | 1242.49 (mg/kg) | 689.89 (mg/kg) | 1932.3 (mg/kg) | 0.56 | 35.7 | [36] |
| Wheat brans (soft and hard) | 0.84–0.98 (mg FAE/g) | 11.3–12.18 (mg FAE/g) | 13.51–14.59 (mg FAE/g) | 13.45–12.42 | 83.48–83.64 | [37] |
| Millets | 0.007–0.032 (mmol FAE/g) | 0.002–0.081 (mmol FAE/g) | 0.009–0.11 (mmol FAE/g) | 0.28–2.53 | 22.23–73.63 | [38] |
| Millet Seeds | 0.004–0.025 (mmol FAE/g) | 0.001–0.062 (mmol FAE/g) | 0.005–0.087 (mmol FAE/g) | 0.25–2.24 | 20–71.26 | [39] |
| Barley varieties | 0.18–0.42 (mg FAE/g) | 2.03–3.36 (mg FAE/g) | 2.63–4.51 (mg FAE/g) | 8–11.27 | 74.50–77.18 | [40] |
| Barley varieties | 0.037–0.16 | 0.21–0.30 | 0.28–0.52 | 1.87–5.67 | 57.69–75 | [41] |
| Barley varieties | 1.66–2.37 | 1.70–2.40 | 3.36–4.53 | 1.01–1.02 | 49.40–52.31 | [42] |
| Corn varieties (pericarp) | 0.013–-0.021 (mmol FAE/g) | 0.27–0.43 (mmol FAE/g) | 0.28–0.45 (mmol FAE/g) | 20.47–20.76 | 95.56–96.42 | [17] |
| Chickpeas | 0.073–3.28 | 0.13–17.98 | 0.17–20.49 | 1.78–5.48 | 76.47–87.75 | [43] |
| Lentil hulls (green and black) | 31.49–40.26 | 40.96–53.88 | 81.22–85.37 | 1.3–1.33 | 50.43–63.11 | [44] |
| Lentil hulls (raw) | 3.22–4.03 | 3–3.64 | 6.22–7.68 | 0.86–0.93 | 47.39–48.23 | [12] |
| Lentils | 3.13–4.25 | 4.78–6.45 | 8.13–10.69 | 1.39–1.66 | 58.79–60.33 | [32] |
| Beans | 0.14–0.52 | 0.14–0.81 | 0.34–1.54 | 1–1.55 | 41.17–52.59 | [45] |
| Camelina (Camelina sativa) | 4.07 | 0.82 | 11.69 | 0.2 | 7.01 | [46] |
| Sophia (Descurainia sophia) | 4.14 | 2.5 | 22.4 | 0.6 | 11.16 | [46] |
| Chia (Salvia hispanica) seeds | 8.69 | 4.59 | 14.22 | 0.52 | 32.27 | [47] |
| Flowers (Lonicera japonica and L. macranthoides) | 0.15 mmol GAE/g | 0.006 mmol GAE/g | 0.19 mmol GAE/g | 0.04 | 3.15 | [48] |
| Flowers (Camellia oleifera and C. polyodonta) | 102.68–137.9 | 1.19–2.04 | 104.72–138.96 | 0.01 | 1.13–1.46 | [49] |
| Leaves (Lonicera japonica and L. macranthoides) | 0.098 mmol GAE/g | 0.029 mmol GAE/g | 0.15 mmol GAE/g | 0.29 | 19.92 | [48] |
| Leaves (green perilla) | 34.18 | 5.08 | 45.03 | 0.14 | 11.28 | [50] |
| Leaves (red perilla) | 12.38 | 17.8 | 35.44 | 0.5 | 49.04 | [50] |
| Fruit leaves (Averrhoa carambola) | 6.27 | 16.11 | 29.96 | 2.53 | 53.77 | [51] |
| Fruit leaves (Artocarpus heterophyllus) | 2.76 | 20.81 | 28.67 | 7.53 | 72.58 | [51] |
| Stem and root (Terminalia sericea) | 15.12 | 10.38–11.62 | 25.5–26.74 | 0.68–0.76 | 40.70–43.44 | [52] |
| Berry seeds (blackberry) | 2.23 | 7.93 | 13.6 | 3.55 | 58.3 | [53] |
| Berry seeds (black raspberry) | 0.8 | 4.6 | 7.3 | 5.75 | 63.01 | [53] |
| Berry seeds (raspberry) | 8.84 | 7.31 | 25.4 | 0.82 | 28.77 | [54] |
| Pomace (raspberry) | 8.66 | 6.39 | 24.14 | 0.73 | 26.47 | [54] |
| Wood (seedling date palm) | 80.03 | 21.05 | 101.08 | 0.26 | 20.82 | [55] |
| Fruit (Pyrus pashia Buch) pulp (Kainth) | 1.78 | 7.07 | 10.36 | 3.97 | 68.24 | [56] |
| Fruit (Annona crassiflora) peel (araticum) | 1.79 | 6.31 | 31.65 | 3.52 | 19.93 | [57] |
| Fruit (Annona crassiflora) pulp (araticum) | 1.41 | 9.04 | 20.49 | 6.41 | 44.11 | [57] |
| Mistletoes (Viscum articulatum and V. liquidambaricolum) | 0.008–0.009 mmol FAE/g | 0.003–0.004 mmol FAE/g | 0.012–0.014 mmol FAE/g | 0.37–9.44 | 25–28 | [58] |
| Dried hawthorn (Crataegus pinnatifida) | 29.34 | 0.47 | 29.81 | 0.016 | 1.57 | [11] |
| Sea cucumber (Cucumaria frondosa) body wall | 2.2 | 0.74 | 3.98 | 0.33 | 18.59 | [26] |
| Sea cucumber (C. frondosa) viscera | 2.27 | 0.56 | 3.02 | 0.24 | 18.54 | [27] |
| Sea cucumber (C. frondosa) tentacles | 2.41 | 0.38 | 3.09 | 0.15 | 12.29 | [59] |
| Sources | Total Bound Phenolics (µg/g) | Major Bound Phenolics (µg/g) | References |
|---|---|---|---|
| Buckwheat brans | 689.81 | Catechin (207.74), syringic acid (85.86), epicatechin (59.08), rutin (51.64), swertiamacroside (39.40), and quercitrin (26.64) | [36] |
| Buckwheat | NA | Rutin (85.02–416.83), dihydromyricetin (57.85–299.93), kaempferol-3-O-rutinoside (43.34–230.85), p-hydroxybenzoic acid (61.57–193.72), gallic acid (59.79–71.78), and syringic acid (4.28–66.97) | [35] |
| Purple wheat (Triticum aestivum) fine brans | 390 | trans-Ferulic acid (279), cis-ferulic acid (25.6), trans-p-coumaric acid (9.24), and sinapic acid (7.76) | [60] |
| Millet seeds | NA | Ferulic acid (132.1–1290) and p-coumaric acid (14.9–778.5) | [39] |
| Millets | NA | Ferulic acid (178.82–1685.04-1290) and p-coumaric acid (20.68–1139.06) | [38] |
| Grain hulls | NA | Ferulic acid (266.9–744.2), sinapic acid (1.35–15.72), chrysoeriol-7-O-glucuronide (10.86–64.93), and luteolin (3.01–12.56) | [61] |
| Barley varieties | 1626.19 | Gallic acid (338.29), benzoic acid (285.79), syringic acid (267.47), naringenin (128.83), p-coumaric acid (127.92), and hesperidin (102.05) | [42] |
| Corn (quality protein corn) | 8675 | Ferulic acid (3522), vanillic acid (2317), isoferulic acid (901), syringic acid (897), and p-hydroxybenzoic acid (532) | [17] |
| Chickpeas | NA | Biochanin A (117.9-841.9), 3-hydroxybenzoic acid (143.2–319.1), and taxifolin (22.9-56.6) | [43] |
| Lentils | Procyanidin dimer B (35.7–167), catechin (15–78.4), epicatechin (0.5–7.94), and catechin-3-glucoside (15.1–122) | [62] | |
| Lentils (red and green) | 1446.80–2204.31 | Dimethoxybenzoic acid derivative (630.76–953.95), coumaric acid derivative (103.97–243.96), catechin (11.22–278.77), gallic acid (186.48–230.31), and p-coumaric acid (83.17–173.61) | [63] |
| Lentils | NA | Catechin (320–2170), protocatechuic acid derivative (160–520), and epicatechin (80–290) | [12] |
| Lentil hulls | NA | Syringic acid (7180–21560), protocatechuic acid (5780–19090), quercetin (5040–14940), catechin (6670–9700), and gallocatechin (5170–7310) | [30] |
| Lentil hulls | 6710–10340 | Catechin (3770–9130), protocatechuic acid (1580–1940), quercetin glucoside (590–1100), and epicatechin (270–620) | [64] |
| Lentils (hull, whole, and dehull) | 48.7–2812.1 | Myricetin (2.1–653.4), catechin (3.1–534.1), gallic acid (0.9–489.9), protocatechuic acid (4.6–439.1), quercetin (3.20–320.7), and quercetin glucoside (1–250.5) | [44] |
| Beans (black) | 1388.71 | Isoquercitrin (462.36), protocatechuic acid (253.42), catechin (109.70), p-coumaric acid (108), vanillic acid (100.22), and quercitrin (86.61) | [45] |
| Camelina (Camelina sativa) | 316.12 | trans-Sinapic acid (172.02), quercetin-hexoside (48.49), protocatechuic acid (31.11), p-hydroxybenzoic acid (16.60), and catechin (12.49) | [46] |
| Sophia (Descurainia sophia) | 187.45 | trans-Sinapic acid (70.48), rosmarinic acid (31.03), quercetin-hexoside (21.54), rutin (21.54), and protocatechuic acid (17.24) | [46] |
| Chia seeds | 578.29 | Apigenin (152.51), genistein (91.98), quercetin-hexoside (91.05), trans-caffeic acid (72.02), trans-ferulic acid (69.70), and cis-hydroxycaffeic acid (67.44) | [47] |
| Leaves (UHP-treated mango) | NA | Mangiferin (18201.35), iriflophenone glucoside (11915.92), catechin gallate (7203.58), gallic acid (6127.62), isoquercitrin (5874.87), 4-O-methylgallic acid (3887.52), homomangiferin (3611.83), quercitrin (2850.16), p-coumaric acid (2470.37), and dihydroquercetin (1094.64) | [25] |
| Leaves (Mangifera indica) | 12619.9 | Epicatechin (7697.95), gallic acid (2424.90), rutin (977.63), and isoquercitrin (605.60) | [51] |
| Leaves (Lonicera macranthoides) | 3190 | Caffeic acid (1150), luteoloside (1210), and isoquercitrin (930) | [48] |
| Flowers (Camellia oleifera and C. polyodonta) | NA | Gallic acid (101.23–580.10), p-coumaric acid (274.88–423.32), astragaline (91.26–304.61), kaempferol-3-O-rutinoside (59.43–119.08), and quercitrin (7.03–116.77) | [49] |
| Seed (black raspberry) | NA | Quercetin 3-O-glucoronide (11.49), quercetin (3.26), epicatechin (3.11), p-coumaric acid (2.43), gallic acid (2.42), caffeic acid (1.58), epigallocatechin (1.36), and protocatechuic acid (0.93) | [53] |
| Fruit (Annona crassiflora) peel (araticum) | 1367.6 | Catechin (812.36), epicatechin (327.31), and protocatechuic acid (125.06) | [57] |
| Grapefruit peel (microwave and enzymatic treatment) | NA | Gallic acid (42.50), naringin (21.54), ferulic acid (18.46), and protocatechuic acid (6.16) | [34] |
| Fruit (Crataeguspinnatifida) peel (hawthorn) | 1000.34 | Epicatechin (265.63), caffeic acid (111.02), catechin (387.23), p-coumaric acid (85.82), and protocatechuic acid (36.04) | [65] |
| Fruit (Annona crassiflora) pulp (araticum) | 716.23 | Catechin (405.54), epicatechin (239.32), and protocatechuic acid (62.89) | [57] |
| Fruit (Pyrus pashia Buch) pulp | NA | Catechin (0.44), epicatechin (0.29), procyanidin B2 (0.08), and p-coumaric acid (0.02) | [56] |
| Lychee pulps | NA | Syringate (12.83–67.14), vanillic acid (7.4–66.58), caffeic acid (54.48–66.51), catechin (12.97–19.95), and epicatechin (12.7–18.19) | [66] |
| Raspberry pomace | 1323.96 | Gallic acid (604.65), ellagic acid (452.44), ferulic acid (76.67), p-coumaric acid (56.67), protocatechuic acid (46.76), and catechin (18.67) | [54] |
| Fruit (pomegranate) outer skin | 28.67 | Gallic acid (11.31), kaempferol 3-O-glucoside (9,67), brevifolin carboxylic acid (3.34), trans-p-coumaric acid (1.17), vanillic acid (1.07), and protocatechuic acid (1.06) | [67] |
| Fruit- hawthorn (C. pinnatifida) | 66020 | Procyanidin B2 (36030), rutin (27120), isoquercetin (13870), chlorogenic acid (9920), and hyperoside (6200) | [11] |
| Fruit (Rhus chinensis) | 99560.4 | Quercitrin (36098.16), gallic acid (1400.92), and myricitrin (425.33) | [68] |
| Fruits-oil palm (Elaeis guineensis) | NA | Caffeic acid (11269.66), p-hydroxybenzoic acid (3605.47), catechin (692.87), ferulic acid (628.79), hesperetin (601.93), p-coumaric acid (531.82), epigallocatechin (448.23), protocatechuic acid (365.05), and gallic acid (311.16) | [24] |
| Mistletoes (Viscum articulatum and V. liquidambaricolum) | 822.3–1135.76 | Epigallocatechin (14.63–223.32), p-coumaric acid (14.26–206.97), ferulic acid (97.94–171.18), catechin hydrate (92.21–129.17), trans-cinnamic acid (46.03–124.38), kaemferol (18.15–99.4), myricetin (33.14–75.23), quercetin (41.44–62.30), p-hydroxybenzoic acid (48.02–55.2), vanillic acid (37.4–52.73), and caffeic acid (28.2–49.88) | [58] |
| Potatoes | NA | Rutin (36.77–1995.73), benzoic acid (263–1831.84), caftaric acid (21.55–940.77), and cryptochlorogenic acid (4.53–32.39) | [69] |
| Brazil nut (brown skin) | 7873.04 | Catechin (2874.55), gallic acid (1638.92), protocatechuic acid (1319.95), gallocatechin (1316.32), taxifolin (333.16), and vanillic acid (285.53) | [70] |
| Walnut pellicle | NA | Gallic acid (234–1142), ellagic acid (432–509), catechin (40.3–89.1), protocatechuic acid (23.6–81.6), and p-hydroxybenzoic acid (25.2–78.3) | [71] |
| Walnut kernel | NA | Ellagic acid (46.38–93.27), gallic acid (3.78–4.58), ferulic acid (3.18–3.79), and sinapic acid (1.93–2.57) | [72] |
| Cocoa (nibs and husk) | NA | Protocatechuic acid (5400–12200), catechin (100–1400), epigallocatechin (300–400), and epicatechin (100–200) | [73] |
| Sea cucumber (Cucumaria frondosa) body wall | 175 | Protocatechuic acid (24), catechin (18), p-coumaric acid (17), gallic acid (17), p-hydroxybenzoic acid (15), quercetin (15), and ellagic acid (14) | [26] |
| Sea cucumber (C. frondosa) viscera | 259.2 | Chlorogenic acid (30.6), p-coumaric acid (28.8), hydroxygallic acid (24.2), catechin (23.3), ellagic acid (21.3), and protocatechuic acid (20.5) | [27] |
| Sources | DPPH RSA (µmol TE/g) | ABTS+ RSA (µmol TE/g) | Hydroxyl RSA (µmol TE/g) | Metal Chelation (µmol EDTAE/g) | ORAC (µmol TE/g) | TEAC (µmol TE/g) | FRAP (µmol TE/g) | Reducing Power (µmol TE/g) | References |
|---|---|---|---|---|---|---|---|---|---|
| Buckwheat | 4.3–7.68 | 7.12–11.54 | 13.23–14.54 | NA | NA | NA | NA | NA | [35] |
| Millet Seeds | 2.77–17.38 (µmolFE/g) | NA | 49.82–1110.2 (µmol FE/g) | NA | 44.2–606.88 (µmol FE/g) | NA | NA | NA | [39] |
| Millets | NA | NA | NA | NA | NA | 6.77–86.13 | 2.96–29.33 (µmol AAE/g) | [38] | |
| Grain hulls | 318.53–607.81 (µg TE/g) | 197.3–880.28 (µg TE/g) | NA | NA | NA | NA | NA | NA | [61] |
| Barley varieties | 3.95–5.62 | NA | NA | NA | 22.13–34.67 | 7.44–9.88 | NA | NA | [40] |
| Corn (quality protein corn) pericarp | 2047 | 958 | NA | NA | NA | NA | 43.3 | [17] | |
| Wheat brans (soft and hard) | 634.6–661.5 | NA | NA | NA | 10550–11350 | 28270–32765 | NA | NA | [37] |
| Lentils | 40–420 | NA | 40–300 | NA | NA | 90–930 | NA | 20–270 | [62] |
| Lentils (raw) | 474–551 (µg TE/g) | NA | NA | NA | 1355–2144 (µg TE/g) | NA | NA | 765–872 (µg AAE/g) | [12] |
| Lentil hulls | 263–719 (µg TE/g) | NA | NA | 174–202 (µg CE/g) | NA | NA | NA | 446–5455 (µg AAE/g) | [64] |
| Lentils (hull, whole, and dehull) | 200–5600 (µg TE/g) | 20–1060 (µg TE/g) | 1620–3550 (µg TE/g) | NA | NA | NA | NA | NA | [44] |
| Beans (black) | 2.38 | 5.7 | NA | NA | NA | NA | NA | NA | [45] |
| Camelina (Camelina sativa) | NA | NA | NA | 13.87 | NA | 14.11 | NA | 6.44 | [46] |
| Sophia (Descurainia sophia) | NA | NA | NA | 6.91 | NA | 39.54 | NA | 26.05 | [46] |
| Chia seeds | 9.98 | 17.9 | 2.5 | NA | 58.35 | NA | 37.19 | [47] | |
| Leaves (Mangifera indica) | 149.7 | 365.13 | NA | NA | NA | NA | 213.88 | NA | [51] |
| Leaves (Lonicera macranthoides) | 12.02 | 20.21 | NA | NA | 248.16 | NA | 213.88 | NA | [48] |
| Flowers (Camellia oleifera and C. polyodonta) | 39.6–54.57 (µg TE/g) | 2144.75–4085.57 (µg TE/g) | NA | NA | NA | NA | 7.52–19.50 (µg TE/g) | NA | [49] |
| Seed (blackberry) | NA | NA | 53.8 | 68.6 (µmol TE/g) | 32.8 | NA | NA | 52.2 | [53] |
| Fruit (Annona crassiflora) peel (araticum) | 41.37 | NA | NA | NA | 117.28 | 63.03 | NA | NA | [57] |
| Grapefruit peel (microwave and enzymatic treatment) | 0.23 | 0.31 | NA | NA | 4.3 | NA | NA | NA | [34] |
| Fruit (Annona crassiflora) pulp (araticum) | 55.57 | NA | NA | NA | 119.19 | 99.07 | NA | NA | [57] |
| Fruit (Pyrus pashia Buch) pulp | 13.8 (IC50 μg/mL) | 12.22 (IC50 μg/mL) | NA | NA | NA | 2970 (μg TE/g) | 2159 (μg TE/g) | NA | [56] |
| Fruit (pomegranate) outer skin | 230 | 11.72 | 29.31 (µmol GAE/g) | 0.86 | 48.44 | NA | NA | NA | [67] |
| Hawthorn fruit (C. pinnatifida) | 27.74 (IC50 mg/mL) | 7.31 (IC50 mg/mL) | NA | NA | NA | NA | NA | [11] | |
| Mistletoes (Viscum articulatum and V. liquidambaricolum) | 1.51–1.83 (µmol FAE/g) | NA | NA | NA | NA | 1.4–5.78 | 8.07–10.31 (µmol FAE/g) | NA | [58] |
| Wood (seedling date palm) | NA | NA | NA | 12.95 | NA | NA | NA | 810 | [55] |
| Brazil nut (brown skin) | 29.13 (µmol CE/g) | NA | 101.26 (µmol CE/g) | NA | 168.35 | 59.83 | NA | 39.9 (µmol AAE/g) | [70] |
| Walnut kernel | 35.25–49.97 (IC50 μg/mL) | NA | NA | NA | NA | NA | NA | NA | [72] |
| Sea cucumber (Cucumaria frondosa) body wall | 949 (µg TE/g) | 1187 (µg TE/g) | 2017 (µg TE/g) | 92 (µg TE/g) | NA | NA | NA | NA | [26] |
| Sea cucumber (C. frondosa) viscera | 727.6 (µg TE/g) | 947.6 (µg TE/g) | 2543 (µg TE/g) | 72.7 (µg TE/g) | NA | NA | NA | NA | [27] |
| Sources | DNA Oxidation | LDL Oxidation | α-Glucosidase | Pancreatic Lipase | References | |
|---|---|---|---|---|---|---|
| Peroxyl Radical | Hydroxyl Radical | |||||
| Pomegranate outer skin | IR: 48.80%, IC50: 0.1 mg/mL | IR: 16.11%, IC50: 0.32 mg/mL | NA | IR: 2.50%, IC50: 20.66 mg/mL | IR: 0.81%, IC50: 64.38 mg/mL | [67] |
| Pomegranate mesocarp | IR: 18.44%, IC50: 0.27 mg/mL | IR: 11.17%, IC50: 0.45 mg/mL | NA | IR: 5.08%, IC50: 9.86 mg/mL | IR: 4.34%, IC50: 11.57 mg/mL | |
| Pomegranate divider | IR: 98.42%, IC50: 0.05 mg/mL | IR: 79.09%, IC50: 0.06 mg/mL | NA | IR: 5.95%, IC50: 8.45 mg/mL | IR: 15.16%, IC50: 3.31 mg/mL | |
| Lentil cultivars | IR: 69.64–88.01% | NA | NA | NA | NA | [62] |
| Lentils (black and green) | IR: 7.7–89.8 mg CE/g | IR: 1.62–3.55 mg CE/g | NA | NA | NA | [44] |
| Date palm wood | IR: 86.39% | IR: 38.64% | NA | NA | NA | [55] |
| Winemaking by-products | NA | NA | NA | IR: 90–100% | IR: 40-50% | [95] |
| Berry seed meals | NA | NA | IR: 48.51–59.93% | NA | NA | [53] |
| Chia seeds | IC50: 5.26 mg/mL | IC50: 25.74 mg/mL | IC50: 0.07 mg/mL | IC50: 192.54 mg/mL | IC50: 17.10 mg/mL | [47] |
| Sophia seed meals | IC50: 2.42 mg/mL | IC50: 15.74 mg/mL | IC50: 0.02 mg/mL | IC50: 152.8 mg/mL | IC50: 12.23 mg/mL | [96] |
| Camelina seed meals | IC50: 5.40 mg/mL | IC50: 5.06 mg/mL | IC50: 12.23 mg/mL | IC50: 128.39 mg/mL | IC50: 4.15 mg/mL | |
| Wheat | NA | IR: 920–1740 µg/g | IR: 6502–25600 µg/g | NA | NA | [37] |
| Barley | IR: 82.34–96.66% | NA | IR: 42.92–72.32% | NA | NA | [40] |
| Guarana powder | NA | NA | NA | IC50: 1.62 µg GAE/mL | NA | [97] |
| Sea cucumber viscera | IR: 80.48% | IR: 66.50% | IR: 20.27% | IR: 26.15% | NA | [27] |
| Sea cucumber tentacles | IR: 80.07% | IR: 68.1% | IR: 15.95% | IR: 26.39% | NA | [59] |
| Sea cucumber body wall | IR: 85.80% | IR: 72.81% | IR: 34.82% | IR: 34.83% | NA | [26] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahidi, F.; Hossain, A. Importance of Insoluble-Bound Phenolics to the Antioxidant Potential Is Dictated by Source Material. Antioxidants 2023, 12, 203. https://doi.org/10.3390/antiox12010203
Shahidi F, Hossain A. Importance of Insoluble-Bound Phenolics to the Antioxidant Potential Is Dictated by Source Material. Antioxidants. 2023; 12(1):203. https://doi.org/10.3390/antiox12010203
Chicago/Turabian StyleShahidi, Fereidoon, and Abul Hossain. 2023. "Importance of Insoluble-Bound Phenolics to the Antioxidant Potential Is Dictated by Source Material" Antioxidants 12, no. 1: 203. https://doi.org/10.3390/antiox12010203
APA StyleShahidi, F., & Hossain, A. (2023). Importance of Insoluble-Bound Phenolics to the Antioxidant Potential Is Dictated by Source Material. Antioxidants, 12(1), 203. https://doi.org/10.3390/antiox12010203