Abstract
Olive-derived bioactive compound oleuropein was evaluated against damage induced by hydrogen peroxide in human trophoblast cells in vitro, by examining the changes in several markers implicated in oxidative stress interactions in the placenta. Trophoblast HTR-8/SVneo cells were preincubated with OLE at 10 and 100 µM and exposed to H2O2, as a model of oxidative stress. Protein and lipid peroxidation, as well as antioxidant enzymes’ activity, were determined spectrophotometrically, and DNA damage was evaluated by comet assay. iNOS protein expression was assessed by Western blot, while the mRNA expression of pro- and anti-apoptotic genes BAX and BCL2 and transcription factor NFE2L2, as well as cytokines IL-6 and TNF α were determined by qPCR. Oleuropein demonstrated cytoprotective effects against H2O2 in trophoblast cells by significantly improving the antioxidant status and preventing protein and lipid damage, as well as reducing the iNOS levels. OLE reduced the mRNA expression of IL-6 and TNF α, however, it did not influence the expression of NFE2L2 or the BAX/BCL2 ratio after H2O2 exposure. Oleuropein per se did not lead to any adverse effects in HTR-8/SVneo cells under the described conditions, confirming its safety in vitro. In conclusion, it significantly attenuated oxidative damage and restored antioxidant functioning, confirming its protective role in trophoblast.
1. Introduction
Among the plants used in traditional and modern medicine, Olea europaea L. tree, popularly known as the olive tree, is one of the most widely used. Olive tree products are key components of the Mediterranean diet (MD) and are abundant in secondary compounds such as polyphenols, particularly flavonoids, with known benefits to human health [1]. One of the most important bioactive and functional food ingredients found in olive oil, olive fruits and olive leaves is phenolic secoiridoid oleuropein (OLE). It is best known for its antioxidant and hypotensive effects, but it is also associated with antiproliferative effects against cancer, anti-angiogenic, neuroprotective, anti-inflammatory and senolytic activity [2,3,4]. Recent studies provided insight into the disease-protective mechanism of OLE and explained many of its effects through oxidative stress modulation and antioxidant activity [5]. OLE showed an ability to suppress the production of reactive oxygen and nitrogen species (ROS and NOS, respectively) in in vitro biochemical assays, as well as in human cells, where it prevented excessive ROS generation [6]. It was also shown to attenuate oxidative stress (OS) by the modulation of the ERK/Nrf2 pathway-mediated signaling, with downstream consequences for a variety of cellular processes [7,8]. Although the many reported benefits of OLE provide ample rationale for its continued use, considerations about its possible interactions and side effects in certain vulnerable states, such as pregnancy, should be further examined. Pregnancy is a state of well-balanced OS, where certain amounts of ROS serve as signaling mediators in key placentation processes [9]. During the normal course of pregnancy, controlled redox signaling is pivotal, and a delicate balance between oxidant production and antioxidant protection is necessary at different stages of pregnancy. An imbalanced oxidative environment created by the overproduction of ROS disrupts these fine-tuned processes and could result in reproductive failure, such as fetal loss, intrauterine growth restriction (IUGR) or pregnancy disorders such as gestational diabetes mellitus (GDM) and preeclampsia (PE) [9,10]. Thus, antioxidant supplements have been proposed as a possible approach in the prevention and treatment of such disorders. The results of recent clinical trials with the MD and olive oil consumption during pregnancy, such as the St Carlos Study, ESTEEM trial, etc., showed that adherence to these diets provides health benefits in pregnant women and newborns and contributes to GDM prevention [11,12,13,14,15]. Furthermore, in the latest study by Minhas et al., the data from the Boston Birth Cohort showed that adherence to a MD was associated with a lower risk for PE development [16]. However, there are no human studies exploring the potential of OLE in pregnancy. The first step to examine its safety and possible benefits in pregnancy is to perform molecular and mechanistic studies that reflect OLE’s impact on the cellular networks involved in the regulation of the key pregnancy events. The initial steps and prerequisites for normal pregnancy establishment and maintenance are the successful implantation of the embryo into the maternal endometrium, and the subsequent invasion of extravillous trophoblast cells into the uterine stroma and maternal spiral arteries [17]. In the earliest stages of pregnancy, ROS-derived signals can act to stimulate or restrict the extent of trophoblast invasion and play an important role in proper placentation via modulation of trophoblast-cell-mediated adhesion, invasion and spiral artery remodeling. Increased OS can cause deregulation in these tightly regulated processes, which can culminate in placental dysfunction [18].
The aim of this research is to explore the impact of OLE on levels of OS in human trophoblast cells, by examining the changes in several OS-related markers (such as reduced glutathione, protein carbonyl levels, malondialdehyde, etc.) and molecular pathways that were found to be implicated in significant interactions between ROS and biological components in healthy and pathological pregnancy [19].
2. Materials and Methods
2.1. Cell Line
For the experiments performed in this research, the HTR-8/SVneo immortalized human extravillous trophoblast cell line (kindly provided by Dr Charles H. Graham, Queen’s, Kingston, ON, Canada) was cultured in a humidified incubator with 5% CO2 at 37 °C in complete culture medium (RPMI 1640 (Gibco, Life Technologies Ltd., Paisley, UK), supplemented with 10% fetal calf serum (Pan Biotech, Aidenbach, Germany) and 1% antibiotic/antimycotic solution (Capricorn Scientific GmbH, Ebsdorfergrund, Germany)).
2.2. Oleuropein Treatment Preparations
A stock solution of oleuropein (Cas No. 32619-42-4, Extrasynthese, 100 mM in DMSO) was diluted in complete RPMI medium to reach final concentrations. In a preliminary experiment, increasing concentrations (1, 10, 25, 50, 100, 200 µM) of OLE in complete medium were tested for 24 h for cytotoxic effects on HTR-8/SVneo cells, using the 2,5-diphenyl-2H-tetrazolium bromide (MTT) assay on cell viability, according to the protocol described in Kostic et al. [20].
2.3. Evaluation of Oleuropein Effects against Cytotoxicity Induced by Hydrogen Peroxide in HTR-8/SVneo Cells
For the evaluation of the OLE against cytotoxic effects induced by hydrogen peroxide, HTR-8/SVneo cells were seeded at a density of 2 × 104 cells/well in 96-well plates and allowed to attach overnight. On the following day, treatments with 10 and 100 μM OLE were added to the cells and incubated for 24 h, while control cells were treated with complete medium alone. After the 24 h treatment, cells were rinsed with phosphate-buffered saline (PBS) and 200 μM H2O2 in serum-free (SF) RPMI medium was added for 2 h. The concentration of 200 μM H2O2 was chosen as this concentration of H2O2significantly reduced cell viability compared to untreated cells. Next, the MTT assay was used to evaluate cell viability in HTR-8/SVneo cells, as described before in Kostic et al. [20]. The absorbance was measured at 570 nm using a microplate reader (ELx800, BioTek, Winooski, VT, USA). Average value for the control cells was set to 100%, and the results of treatments were presented as a percentage of control. Three independent experiments were performed in triplicate.
2.4. Investigation of Genotoxic and Antigenotoxic Effects of Oleuropein
The genoprotective effect of OLE was evaluated using the alkaline comet assay, according to the treatment protocol described in Bruić et al. [21] and following the MIRCA guidelines on the comet assay [22]. The HTR-8/SVneo cells were seeded in 96-well plates (2 × 104 cells/well) and, the following day, the treatments with 10 and 100 μM OLE in complete culture medium were added to the cells. After 24 h incubation with OLE, cells were rinsed, and genotoxic damage was induced by adding 30 µM H2O2 for 30 min in SF medium. This concentration of H2O2was selected because it was the lowest concentration that produced a significant increase in DNA damage compared to non-treated controls. After the induction of DNA damage, the cells were collected using 0.25% trypsin–EDTA solution and centrifuged at 300× g for 5 min, and the obtained pellet was used for the comet assay. In brief, the single-cell suspension was mixed with 0.7% low-melting-point agarose (Sigma Aldrich, St. Louis, MO, USA) and spread on previously pre-coated slides (with 1% normal-melting-point agarose, Sigma Aldrich, St. Louis, MO, USA). After the gel solidified, slides were placed in lysing solution, and a lysis step, followed by DNA unwinding and electrophoresis (at 25 V and 300 mA for 30 min), were performed as described in Bruić et al. [21]. Next, slides were neutralized with PBS and the staining of slides was performed with ethidium bromide (20 µg/mL). Comets were scored at a magnification of 40× on the Olympus BX 50 microscope (Olympus Optical Co., GmbH, Hamburg, Germany), equipped with a mercury lamp, HBO (50 W, 516–560 nm, Zeiss). Visual scoring and classification were performed as described previously by Collins and collaborators [23]. Each of the observed comets was given a value of 0, 1, 2, 3 or 4 (from undamaged, 0, to totally damaged, 4), and DNA damage was expressed in arbitrary units, calculated according to the equation (percentage of cells in class 0 × 0) + (percentage of cells in class 1 × 1) + (percentage of cells in class 2 × 2) + (percentage of cells in class 3 × 3) + (percentage of cells in class 4 × 4). Two replicate slides were analyzed for each treatment, and the scoring was performed on 100 randomly selected comets per slide. The entire experiment was repeated in triplicate.
2.5. Oxidative Stress Parameter Determination
To evaluate the OLE effects on OS parameters, HTR-8/SVneo cells were seeded in 6-well plates (5 × 105 cells/well), left to attach overnight in complete medium and treated for 24 h with 10 and 100 µM OLE. The following day, treatments were removed, and the cells were exposed to 200 µM H2O2 for 2 h. Afterwards, the medium was collected and frozen at −20 °C until further analysis. The cells were rinsed 3 times with chilled PBS and cell lysates were prepared in ice-cold RIPA Buffer (Sigma Aldrich, St. Louis, MO, USA). Protein concentration in cell lysates was determined by using the bicinchoninic acid assay kit (BCA kit, Thermo Scientific, Rockford, IL, USA).
2.6. Antioxidant Enzyme Activity
Catalase (CAT) activity was determined by the UV–kinetic method in the presence of H2O2 [24]. Gluthatione peroxidase (GPx) activity was determined spectrophotometrically, according to the method of Günzler et al. [25]. Superoxide dismutase (SOD) activity was measured using the method based on the inhibition of epinephrine autoxidation [26]. The enzymes’ activity was expressed as enzyme activity per milligram of protein (U/mg protein).
2.7. Markers of Lipid Peroxidation and Oxidative Protein Damage
The method by Stocks and Dormandy [27] based on the reaction with thiobarbituric acid (TBA) was used to evaluate the concentration (nmol/mg protein) of MDA as a marker of lipid peroxidation.
The extracellular lactate dehydrogenase (LDH) was determined through the reaction of the conversion of pyruvate to lactate and measured by the decrease in absorbance at 340 nm [28]. The activity of LDH is expressed as U/mL.
Next, the reduced glutathione (GSH) was determined with the use of Ellman’s reagent (5,5′-dithio-bis(2-nitrobenzoic acid) [29]. Results were expressed as μmol of GSH per milligram of protein (μmol/mg protein). The reaction of carbonyl groups (CG) with 2,4-dinitrophenylhydrazine was measured spectrophotometrically at 365 nm, following the method of [30], and the results were expressed as nmol CG per milligram of protein (nmol/mg protein).
All spectrophotometric measurements were performed on a Cecil CE 2021 UV/VIS spectrophotometer (Cecil Instruments Ltd., Milton, UK).
Finally, a colorimetric method with Griess reagent was employed for the quantitative analysis of nitrite (NO2−) concentrations, using the Guevara et al. method [31]. Absorbance was read on an ELISA reader at 540 nm (Plate reader, Mod. A1, Nubenco Enterprises, ICN, Paramus, NJ, USA). The results were expressed in μmol of NO2− per milligram of protein (μmol/mg protein).
2.8. Western Blot Analysis
Treated cells were lysed in RIPA buffer and incubated on ice for 30 min on a shaker to complete cell lysis, followed by centrifugation at 12,000× g for 20 min. Aliquots of lysates were kept at −80 °C until analysis. SDS–polyacrylamide gel electrophoresis was performed, and target protein levels were determined by Western blot analysis using antibodies for iNOS (ab3523, source: rabbit, 1:500, Abcam, Cambridge, UK) and visualized using enhanced chemiluminescence (ECL) (SERVA Electrophoresis GmbH, Heidelberg Germany) according to a method described elsewhere [32]. TL120 software was used to analyze the optical density of the protein bands and the values were normalized to the optical density of GAPDH.
2.9. Quantitative PCR Analysis
Total RNA was isolated from treated HTR-8/SVneo cells using TRI reagent solution (Thermo Fisher Scientific Baltics, Vilnius, Lithuania). First-strand cDNA was synthesized from 1 μg of total RNA, using 0.5 μg of Oligo(dT) 12–18 primers (Thermo Fisher Scientific Baltics, Vilnius, Lithuania), 250 μM of each dNTP and 200U of RevertAid reverse transcriptase (Thermo Fisher Scientific Baltics, Vilnius, Lithuania). Real-time PCR was performed as described in [33] using the 7500 Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA). Expression levels of BCL2, BAX, NFE2L2 (Nrf2), IL-6 and TNFα were normalized to the housekeeping gene GAPDH. Calculations were made by the comparative ΔΔCt method [34].
The sequences of specific primers were as follows: BCL2 F: AGTTCGGTGGGGTCATGTGT; BCL2 R: GGAGAAATCAAACAGAGGC; BAX F: TTGCTTCAGGGTTTCATC; BAX R: CACTCGCTCAGCTTCTTG; NFE2L2 F: AGTGGATCTGCCAACTACTC; NFE2L2 R: CATCTACAAACGGGAATGTCTG; GAPDH F: GAAGGTGAAGGTCGGAGT; GAPDH R: GAAGATGGTGATGGGATTTC; IL6 F: GGTACATCCTCGACGGCATCT; IL6 R: GTGCCTCTTTGCTGCTTTCAC; TNF F: CCCAGGCAGTCAGATCATCTT; TNF R: TCTCAGCTCCACGCCATT.
2.10. Statistical Analysis
One-way analysis of variance (ANOVA) with Tukey post-hoc analysis was used to evaluate the differences (α = 0.05) in treatments vs. control, after the data passed the normality test. All results were expressed as mean + standard error of the mean (mean + SEM). GraphPad Prism 6.0 (GraphPad Software, Inc., San Diego, CA, USA) was used for statistical analysis and p < 0.05 was considered significant.
3. Results
3.1. Cytotoxicity of Oleuropein in HTR-8/SVneo Cells
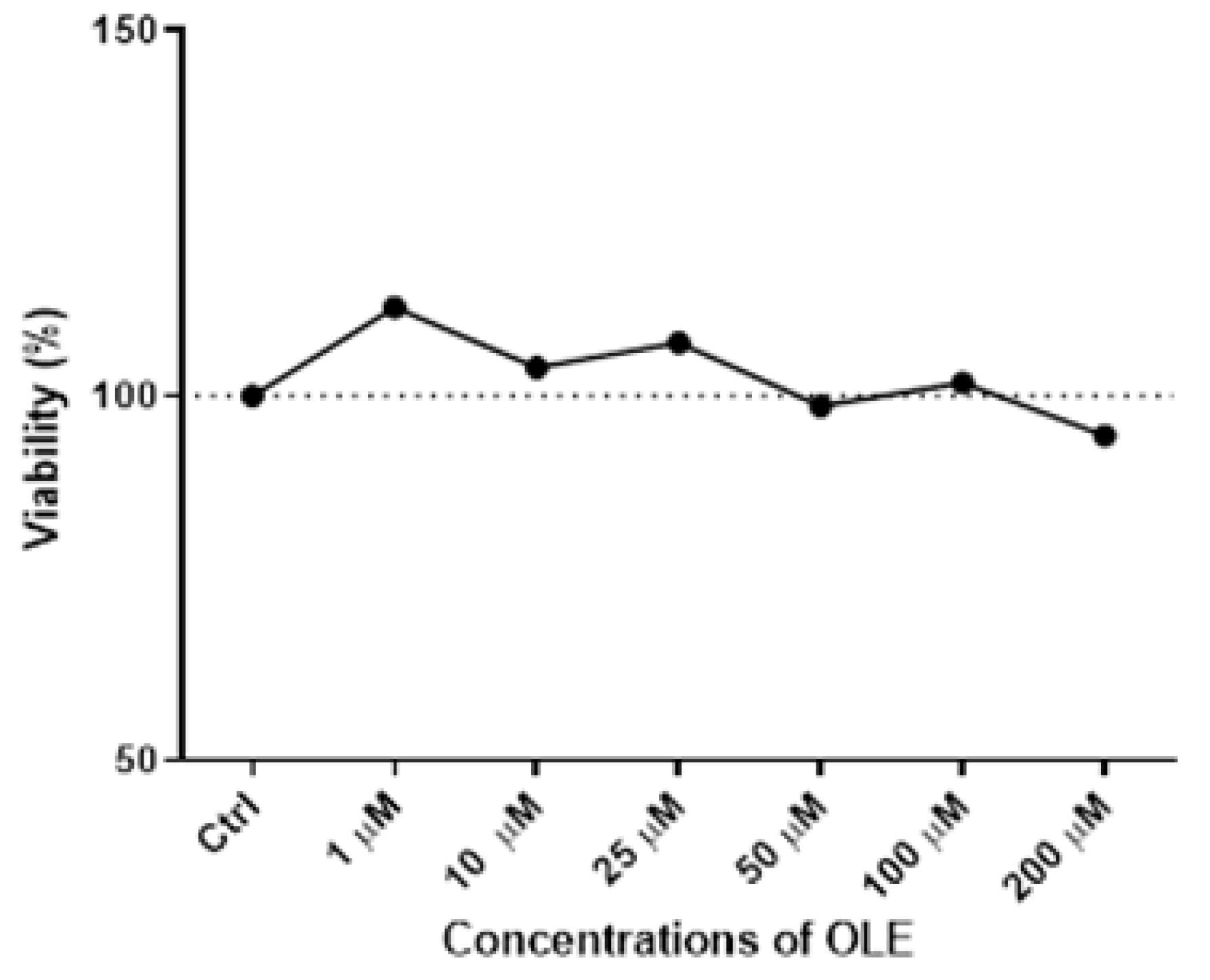
The results of the MTT assay following the 24 h incubation with increasing OLE concentrations ranging from 1 µM to 200 µM in HTR-8/SVneo cells demonstrated a lack of cytotoxicity for the concentrations below 200 µM (Figure 1).
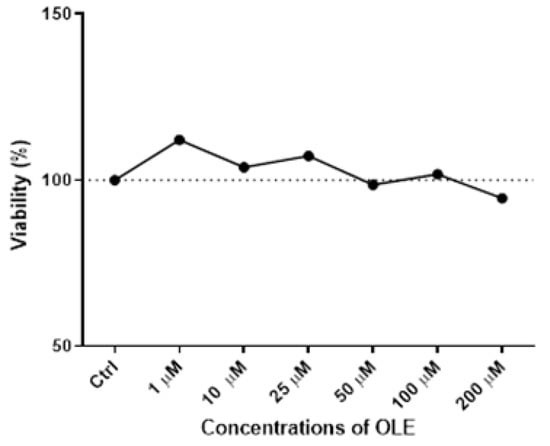
Figure 1.
Cytotoxicity of oleuropein (OLE) in HTR-8/SVneo cells after 24 h treatment with a range of concentrations (1, 10, 25, 50, 100 and 200 µM).
It could be observed that with the increase in OLE concentration, there was discrete cytotoxicity at the highest concentration of 200 µM, but without a statistically significant difference compared to control cells. Based on the obtained results (Figure 1), the concentration of 100 µM of OLE was chosen for testing high-dose OLE effects, as it was the highest concentration of OLE that did not influence cell viability. Accordingly, 10 µM of OLE was chosen for investigating low-concentration OLE effects on HTR-8/SVneo cells in the present study.
3.2. Oleuropein’s Cytoprotective Effect in H2O2-Treated HTR-8/SVneo Cells
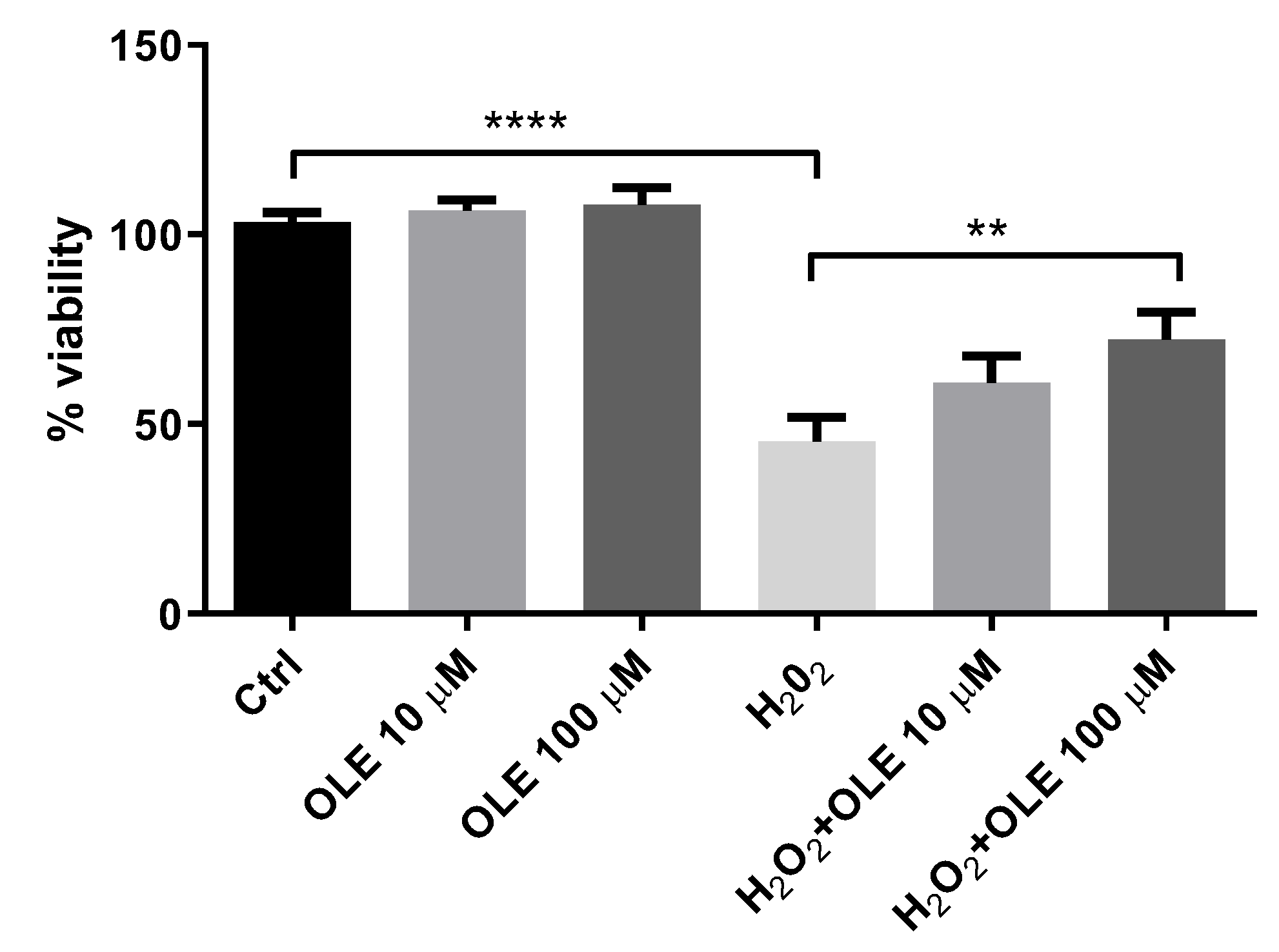
The cell viability of HTR-8/SVneo cells preincubated with 10 and 100 µM OLE was estimated by MTT assay after 2 h exposure to 200 µM H2O2 (Figure 2). It could be observed that 24 h pre-treatment of cells with 10 µM and 100 µM OLE did not influence cell viability compared to control cells. Among HTR-8/SVneo cells exposed to H2O2 alone, the viability was decreased to 45% versus non-treated controls. The pre-treatment of cells with 10 and 100 µM OLE prior to exposure to H2O2 showed a significant cytoprotective effect, i.e., it increased the percentage of viable cells (60.9% and 72.2%, respectively) compared to the cells incubated with H2O2 alone (45%).

Figure 2.
Cytoprotective effect of oleuropein (OLE) on H2O2-induced damage in HTR-8/SVneo cells. The data are expressed as mean + SEM. ** p < 0.01, **** p < 0.0001.
3.3. Genotoxic and Antigenotoxic Properties of Oleuropein in H2O2-Treated HTR-8/SVneo Cells
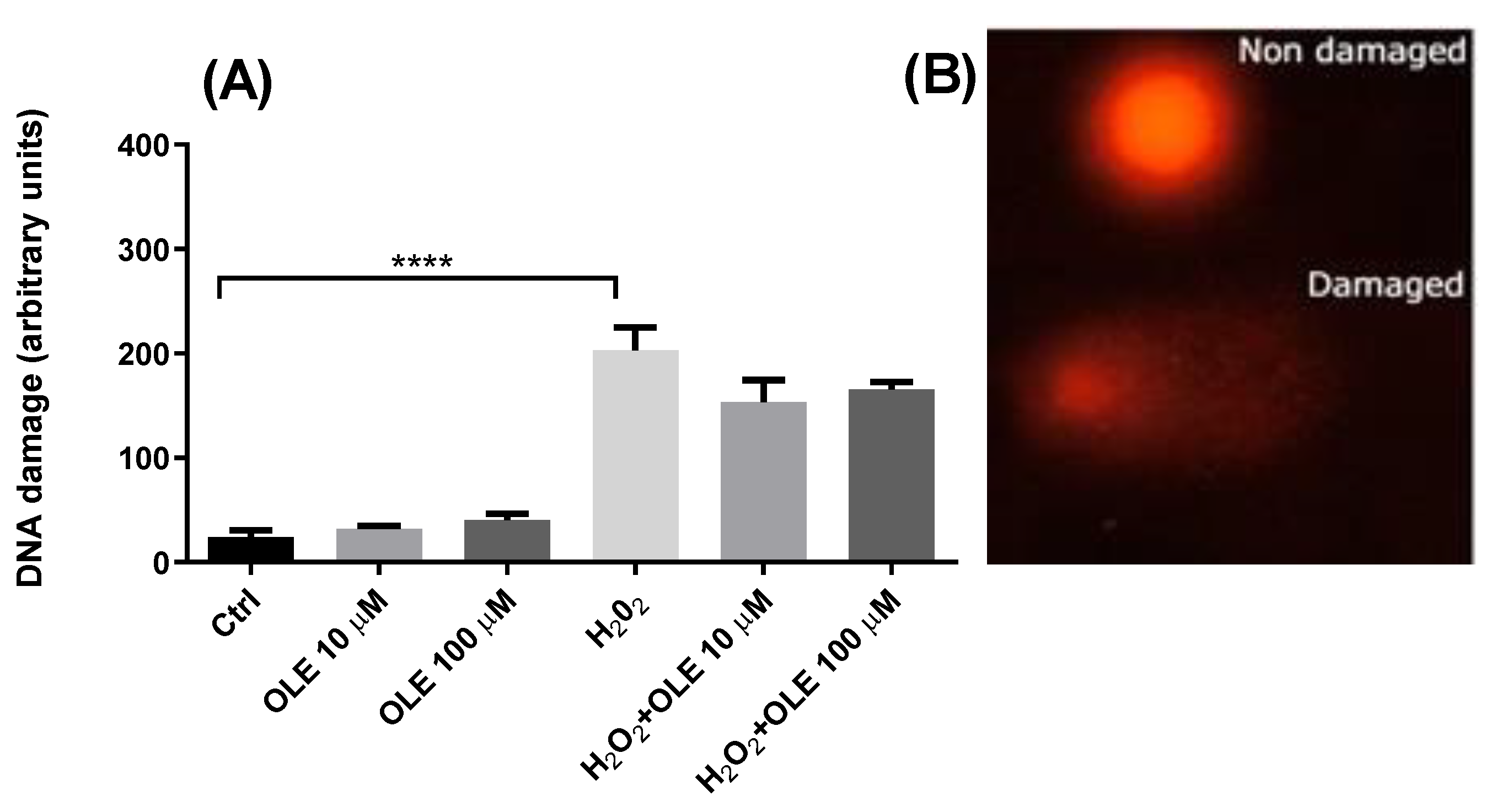
The genotoxic potential of OLE was evaluated by incubating the HTR-8/SVneo cells for 24 h with 10 µM and 100 µM OLE. The results showed that, at the two examined concentrations, OLE did not induce any DNA damage compared to control cells exposed to medium alone (Figure 3). In order to evaluate the genoprotective potential of OLE, a model of OS-induced DNA damage was established by exposing the HTR-8/SVneo cells to 30 µM H2O2 for 30 min in SF RPMI medium. The treatment with H2O2 alone produced a significant increase in DNA damage compared to non-exposed controls. Pre-incubation of cells with OLE at concentrations of 10 µM and 100 μM, prior to H2O2 treatment, reduced the levels of H2O2-induced DNA damage; however, it did not exert statistically significant antigenotoxic effects.

Figure 3.
(A) Genotoxic potential of oleuropein (OLE) and antigenotoxic effects against H2O2-induced damage in HTR-8/SVneo cells. The data are expressed as mean + SEM. **** p < 0.0001. (B) Appearance of comets without DNA damage (Non damaged) and with DNA damage (Damaged).
3.4. The Effects of Oleuropein on Oxidative Stress Parameters in H2O2-Treated HTR-8/SVneo Cells
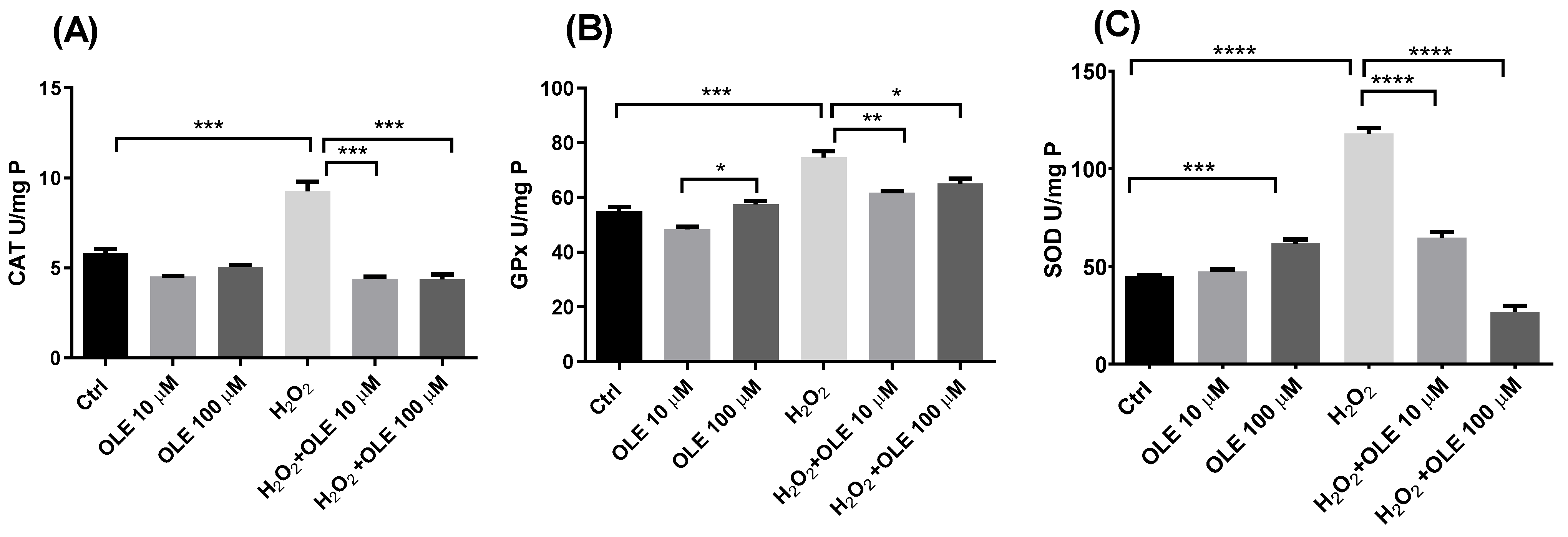
The alterations in the levels of antioxidant enzymes’ activity following the 24 h pre-incubation with OLE and subsequent exposure to H2O2 in HTR-8/SVneo cells are represented in Figure 4. It could be observed that OLE per se at concentrations of 10 µM and 100 µM did not induce changes in the total activity of enzyme CAT compared to non- treated controls (Figure 4A). The treatment of cells with 200 µM H2O2 only produced a significant increase in CAT activity. Further, in cells pre-treated with 10 µM and 100 µM OLE and incubated with H2O2, the activity of CAT was significantly decreased in both treatments in relation to the cells exposed to H2O2 alone. Furthermore, the observed differences were statistically significant for both OLE concentrations. Similar results could be seen for GPx and SOD activity, where OLE at 10 µM alone did not alter enzymes’ activity, while OLE at 100 µM produced an increase in both GPx and SOD activities (Figure 4B,C). Hydrogen peroxide treatment produced a significant rise in GPx and SOD activity compared to non-treated controls. Pre-incubation with 10 µM and 100 µM OLE provided a significant reduction in GPx activity following H2O2 exposure, compared to the treatment with H2O2 alone (Figure 4B). Both concentrations of OLE showed similar efficiency in decreasing the H2O2-induced GPx activity. The results of SOD activity in cells pre-incubated with OLE at 10 and 100 µM and exposed to H2O2 showed a concentration-dependent reduction in SOD activity compared to the high levels observed in the H2O2 treatment alone (Figure 4C).

Figure 4.
The effect of oleuropein (OLE) on activity of antioxidant enzymes in H2O2-exposed HTR-8/SVneo cells. The investigated parameters include catalase (CAT) activity (A), gluthatione peroxidase (GPx) activity (B) and superoxide dismutase (SOD) activity (C). The data are expressed as mean + SEM. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
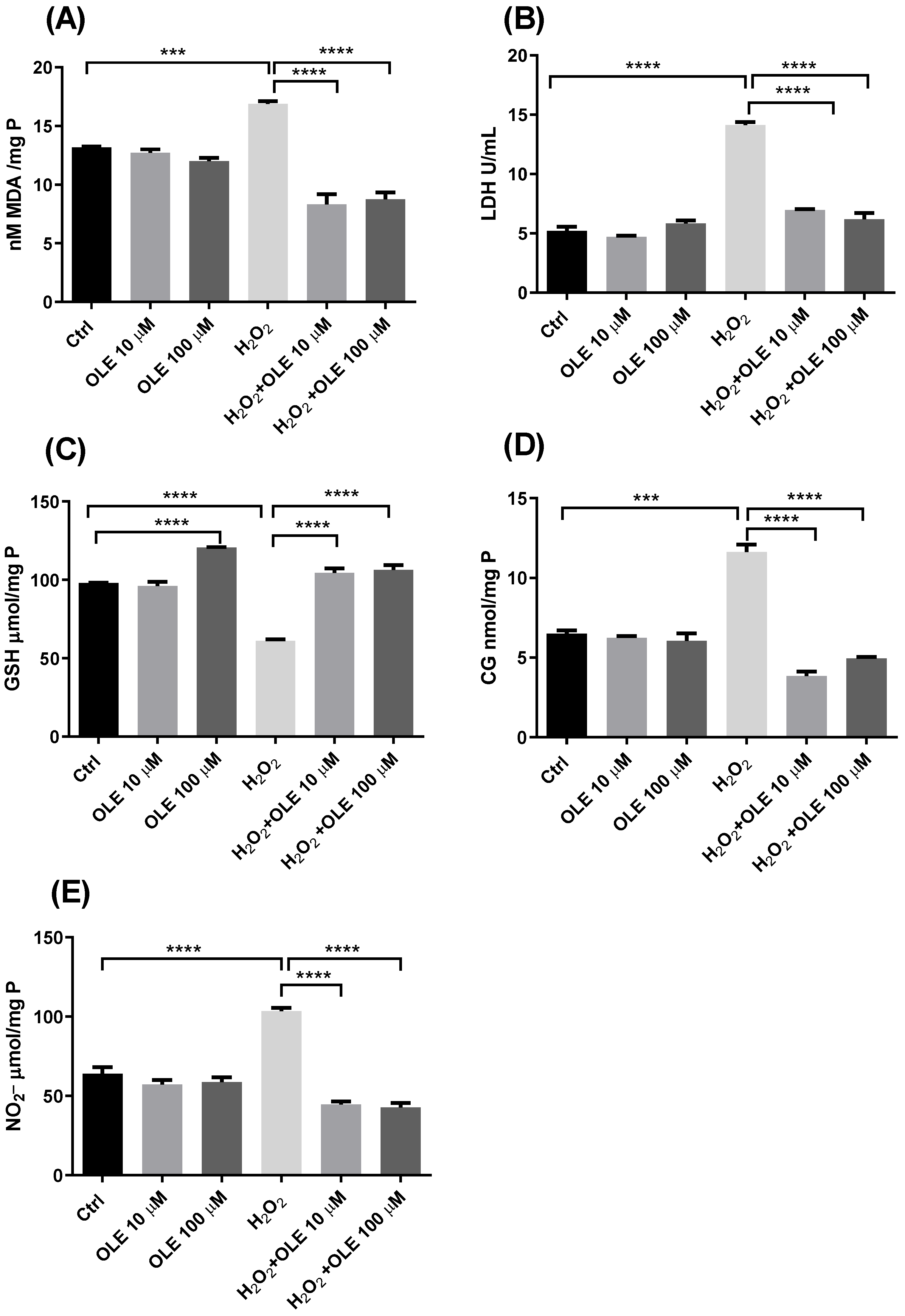
The results of the concentration of MDA as a marker of lipid peroxidation are indicated in Figure 5A. While incubation with the two examined concentrations of OLE did not alter the level of MDA, exposure of cells to H2O2 significantly increased the rate of lipid peroxidation compared to non-treated controls. The cells pre-incubated with OLE and treated with H2O2 showed reduced MDA concentrations compared to the cells exposed to H2O2 alone. Both concentrations of 10 µM and 100 µM OLE provided statistically significant reductions in lipid peroxidation (Figure 5A). The results of extracellular LDH activity presented in Figure 5B showed that the treatment of cells with 10 µM and 100 µM OLE did not lead to membrane damage or the release of LDH extracellularly. In the cells exposed to H2O2 only, there was a significant increase in LDH activity compared to non-exposed controls. The pre-treatment with OLE exhibited protective effects and prevented LDH release after exposure to H2O2. This effect was similar and statistically significant for both OLE concentrations used in this study (Figure 5B).
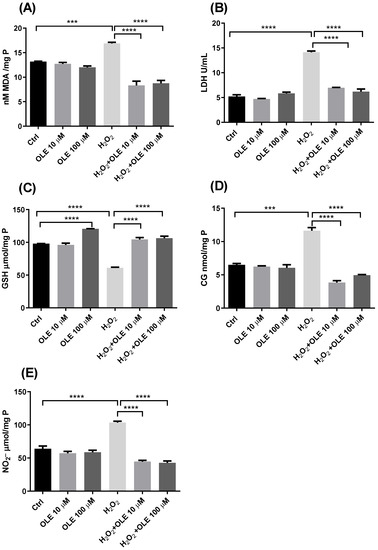
Figure 5.
The effect of oleuropein (OLE) on lipid peroxidation and protein damage in H2O2-exposed HTR-8/SVneo cells. The investigated parameters include malondialdehyde (MDA) concentration (A), extracellular lactate dehydrogenase (LDH) activity (B), reduced glutathione (GSH) concentration (C), protein carbonyl group (CG) concentration (D) and nitrite (NO2−) concentration (E). The data are expressed as mean + SEM., *** p < 0.001, **** p < 0.0001.
The GSH content analysis shown in Figure 5C indicated that the treatment of cells with OLE 100 µM alone can provide a significant rise in GSH concentration, increasing the antioxidant capacity of HTR-8/SVneo cells. On the other hand, when the cells were exposed to H2O2, a significant decrease in GSH was observed compared to the non-treated control cells. Further, after the pre-treatment with OLE and the subsequent H2O2 challenge, an increase in GSH concentration was obtained, whereas both treatments with 10 µM and 100 µM OLE showed the significant recovery of the GSH concentration compared to H2O2 treatment alone (Figure 5C). Measurement of CG concentrations and NO−2, as markers of protein damage, in the cells treated with OLE and/or H2O2 are presented in Figure 5D,E. The results indicated that the 24 h treatment with OLE did not alter the levels of protein carbonylation or concentration of NO2−, whereas H2O2 significantly increased both CG and NO2−. Moreover, following the pre-incubation of cells with OLE and subsequent exposure to H2O2, the CG and NO2− concentrations were significantly reduced and reached the values seen in the non-exposed controls. Both 10 µM and 100µM concentrations of OLE, used in the pre-treatment, showed similar efficiency in preventing CG and NO2− formation after H2O2 exposure, with statistically significant difference compared to the treatment with H2O2 alone.
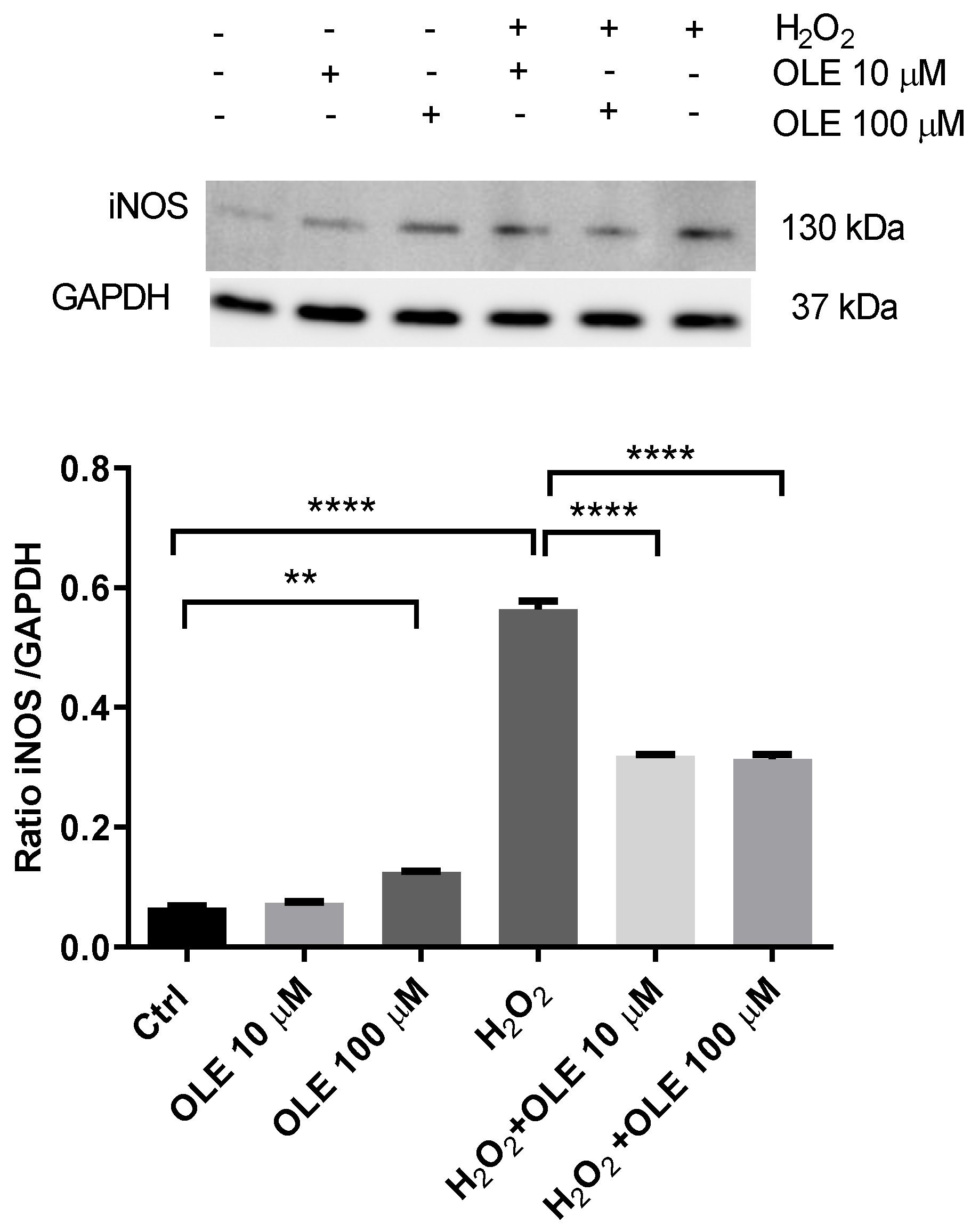
3.5. The Effect of Oleuropein on Inducible Nitric Oxide Synthase (iNOS) in H2O2-Exposed HTR-8/SVneo Cells
The analysis of OLE’s effects on the inducible nitric oxide synthase (iNOS) protein expression in H2O2-exposed HTR-8/SVneo cells is represented in Figure 6. The results show that incubation with 10 µM OLE did not alter the levels of iNOS after 24 h incubation, while 100 µM OLE induced a slight but significant increase in exposed trophoblast cells. When cells were treated with H2O2 only, there was a severe increase in iNOS expression, compared to non-exposed controls. OLE pre-treatment significantly reduced the iNOS protein expression following H2O2 exposure, displaying an equal effect for both OLE concentrations.

Figure 6.
The effect of oleuropein (OLE) on the inducible nitric oxide synthase (iNOS) protein expression in H2O2-exposed HTR-8/SVneo cells. Upper panel, representative Western blot of iNOS; lower panel, densitometric analysis of iNOS expression in HTR-8/SVneo cells. The data are expressed as mean + SEM. ** p < 0.01, **** p < 0.0001.
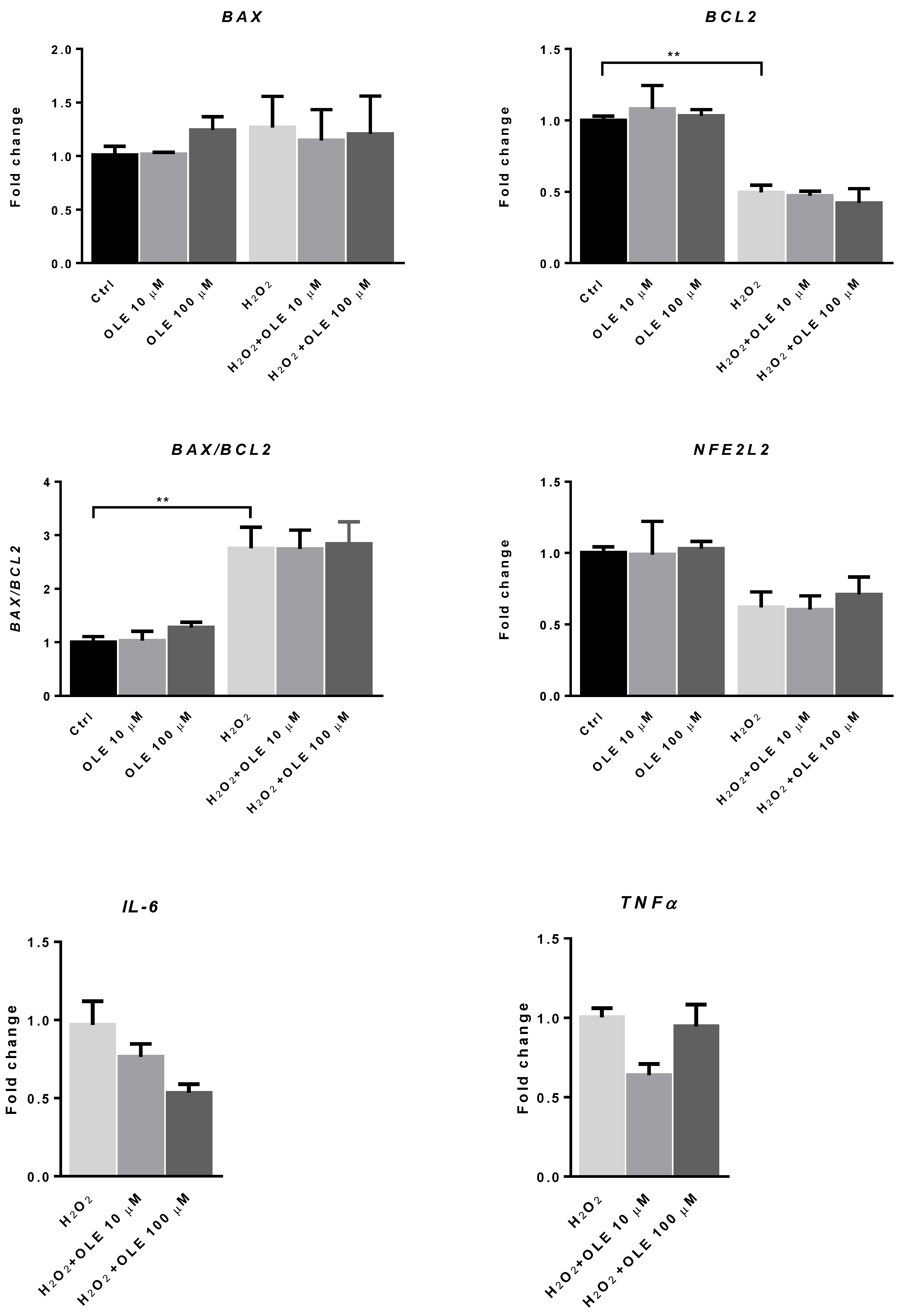
3.6. The Effect of Oleuropein on the mRNA Expression of BAX, BCL2 and NFE2L2 in H2O2-Exposed HTR-8/SVneo Cells
To examine the possible impact of OLE on the mRNA expression of redox-sensitive transcription factor NFE2L2 and the markers of apoptotic signaling BAX and BCL2, trophoblast cells were incubated with OLE at 10 and 100 µM for 24 h. Figure 7 shows that treatment with OLE alone did not change the mRNA expression levels of NFE2L2 or those of BAX and BCL2. To induce the state of increased OS, we treated HTR-8/SVneo cells with H2O2 (200 μM) for 2 h, after 24 h exposure to OLE. When the cells were stimulated with H2O2 alone, the mRNA expression level of the apoptotic marker BAX was increased, while the anti-apoptotic BCL2 mRNA level was significantly decreased compared to non-exposed controls. The BAX/BCL2 mRNA ratio was significantly increased compared to the non-exposed cells, indicating increased pro-apoptotic signaling in H2O2-exposed cells. Although the mRNA level of NFE2L2, a gene encoding for nuclear factor erythroid 2-related factor 2 (Nrf2), which mediates antioxidant gene upregulation, was reduced in the H2O2-exposed cells, this decrease did not reach statistical significance. Moreover, OLE pre-treatment did not significantly influence the expression of the examined pro- and anti-apoptotic genes after H2O2 exposure neither could change the mRNA expression level of NFE2L2 in trophoblast HTR-8/SVneo cells. In terms of the mRNA expression of pro-inflammatory cytokines IL-6 and TNF-α, without exposure to H2O2, the levels were low in control cells and unchanged in OLE-treated samples (data not shown). In the cells exposed to H2O2, the pre-incubation with OLE provided a reduction in the mRNA levels of both cytokines compared to H2O2, and this reduction was observed for both concentrations of OLE. However, the change was not statistically significant.

Figure 7.
The effect of oleuropein (OLE) on the mRNA expression of apoptotic marker BAX, the anti-apoptotic marker BCL2, the mRNA ratio of BAX to BCL2 and the mRNA expression of redox-regulated transcription factor NFE2L2, interleukin 6 (IL6) and tumor necrosis factor α (TNFα), in H2O2-exposed HTR-8/SVneo cells. The data are expressed as mean + SEM. ** p < 0.01.
4. Discussion
Given the importance of ROS during the placentation process and the fact that OS correlates with the severity of some pregnancy-related disorders, such as PE, antioxidant treatment strategies have emerged as an attractive target in clinical trials. However, results derived from clinical trials have been conflicting, and very few antioxidant components showed significant effects in pregnancy disorders [19,35,36,37]. Moreover, most common antioxidant supplements, such as vitamin C and vitamin E, were not only found to be ineffective in reducing the disease risk but have even been associated with increased unexplained stillbirths [38]. These findings suggest that the use of high-dose antioxidants in pregnancy needs to be carefully examined, particularly during the vulnerable early stage of gestation, when interference with oxidative signaling may compromise pregnancy establishment and placental development [39]. Special focus in safety research should be given to the antioxidants that are commonly used in high doses and are readily available throughout various food sources, with the aim to identify the benefits and hazards of their use and evaluate their potential to contribute to the prevention of pregnancy-related disorders [40].
OLE is an olive-derived bioactive compound that harbors many health benefits and is characterized by good absorption, being widely distributed in various organs and tissues, reaching them quickly after ingestion [41]. Studies with olive oil supplementation in the pre-conception period and/or during pregnancy demonstrated that a period of supplementation could improve embryo quality parameters in in vitro human embryo development, positively affect embryonic growth, modify the epigenetic programming in the placenta and induce an anti-inflammatory environment in the placenta and umbilical cord blood [42,43,44]. Furthermore, OLE supplementation was shown to be effective in alleviating symptoms of GDM and improved gestational outcomes in a mouse model [45]. Moreover, studies in pregnant mice showed that supplementation with extra virgin olive oil could induce the inhibition of oxidative stress pathways [46].
It seems that most of the observed health-benefiting effects of olive oil and the derived OLE can be attributed to their antioxidant role and the modulation of OS-related signaling pathways. The results of numerous studies are in support of this assumption. Namely, human trials demonstrated that OLE ingestion could improve the postprandial glycemic profile via hampering NADPH oxidase 2 (Nox2)-derived oxidative stress [47]. It was also shown that OLE exerts its protective role in human liver cells against H2O2-induced apoptosis via an increase in the expression of antioxidant enzymes SOD1, CAT and GPx 1 [48]. In another study, pre-treatment of human glioblastoma cells (U87) with 10 µM OLE significantly prevented the loss of cells and regenerated the total antioxidant capacity and GSH levels after H2O2 exposure [49]. This is in line with our results, which showed that in HTR-8/SVneo trophoblast cells exposed to H2O2, OLE provides the restoration of GSH levels and prevents a decrease in cell viability compromised by H2O2. OLE also restored the H2O2-induced activity of the antioxidant enzymes SOD1, CAT and GPx in trophoblast cells, reaching values found in non-treated controls. These findings indicate that OLE significantly improves trophoblast antioxidant defense and the capacity to withstand OS.
Another significant finding in our study was the decreased levels of MDA and LDH activity following the pre-incubation with OLE and exposure to H2O2, indicating that OLE is able to reduce the rate of lipid peroxidation in trophoblast cells. This effect was also observed in human embryonic kidney (HEK-293) cells, where OLE treatment led to a marked decrease in lipid peroxidation, as evidenced by reduced MDA production, and prevented H2O2-induced apoptosis [50]. This is also in line with the observation of García-Villalba et al. [51], who found that decreased MDA levels in plasma (32%) were associated with the consumption of OLE-rich olive leaf extract. In the current study, we also found that both trophoblast iNOS activity and NO end-product nitrites were significantly reduced in cells pre-treated with OLE and challenged with H2O2. This beneficial effect of OLE is especially important since it is shown that NO plays an important role in regulating trophoblast migration, invasion and apoptosis, which are crucial processes in the establishment of a successful pregnancy [52]. Moreover, increased iNOS activity and NO production are known to directly relate to the severity of PE and are significantly increased in the placentas of patients with this disorder, compared to those with a normal pregnancy [53,54,55].
In addition to oxidative signaling, pro-inflammatory cytokines are also known to influence gestational processes, and tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6) are specified as important regulators of placental function [56,57]. Their physiological levels are required in the placenta for proper regulation of the proliferation and differentiation of various types of cells, including trophoblasts [58]. On the other hand, the increased levels of pro-inflammatory cytokines are associated with pregnancy complications, such as recurrent spontaneous abortions, preterm labor, GDM and PE [56,59,60,61,62]. Therefore, a fine-tuned, balanced inflammatory environment in the placenta is crucial for successful pregnancy [63]. In this work, exposure to the higher levels of H2O2 was used to mimic the state of increased trophoblast OS and induce the expression of the pro-inflammatory cytokines TNF-α and IL-6. The pre-incubation with OLE at 10 µM and 100 µM provided a decrease (although not statistically significant) in TNF-α and IL-6 mRNA expression in H2O2-exposed trophoblasts, suggesting a possible slight, but direct, anti-inflammatory role for OLE in OS, in addition to its antioxidant properties in placental cells.
The ROS–inflammatory axis in normal pregnancy is balanced by anti-inflammatory mediators, endocrine factors and cell signaling pathways mediating OS. If disturbed, alterations in these processes could lead to various pregnancy pathologies [64]. Nuclear factor erythroid-related factor 2 (Nrf2) is one of the main transcription factors that play a key role in the regulation of the cellular redox balance and the protective antioxidant responses against OS [65]. In the state of a balanced (unstressed) redox environment, Nrf2 is localized mainly in the cytoplasm, but upon the induction of oxidative stress, Nrf2 is translocated in the nucleus, where it upregulates the expression of antioxidant genes [66]. It was previously reported that Nrf2 expression levels, as well as the expression of other Nrf2 target genes, were significantly reduced in PE compared to normal pregnancy placentas [67]. Moreover, under hypoxia/reoxygenation conditions in HTR8/SVneo cells, as well as in the placentas of patients with PE, the activity of OS-related enzymes (CAT, GPx, SOD) were significantly lower, possibly related to the altered levels of the Keap1/Nrf2 signaling pathway [68]. It was also shown that the in vitro exposure of the JAR trophoblast cell line to H2O2 reduced Nrf2 expression, as well as the downstream expression of its target cytoprotective genes. This process was shown to be time-dependent, where significant changes in gene expression could be seen after 6 h [66]. As with the mentioned study, our results showed that incubation with H2O2 resulted in decreased gene expression of NFE2L2 and an increased BAX/BCL2 ratio in mRNA in trophoblast cells. Considering that we used higher concentrations and a shorter (2 h) treatment with H2O2, the observed changes for NFE2L2 were most likely not significant due to the short time of exposure. A previous study performed under cyclophosphamide-induced oxidative stress in rats pre-treated with either 100 or 200 mg/kg OLE-rich olive leaf extract showed a marked decrease in Bax gene expression and the Bax/Bcl2 mRNA ratio, and upregulation of the anti-apoptotic Bcl2 and Nfe2l2 genes [69]. The same study showed that a dose of 100 mg/kg olive leaf extract remarkably ameliorated the elevated levels of protein carbonyls and nitrites, as well as MDA, in rats with cyclophosphamide-induced oxidative stress. Further, there was a positive effect of olive leaf extract on the antioxidant defense system via increased GSH content and improved activity of the antioxidant enzymes SOD, CAT and GPx [69]. Similar to the mentioned study, our results demonstrated that OLE pre-treatment in HTR-8/SVneo trophoblast cells increased GSH and restored the normal activity of CAT, GPx and SOD following H2O2 exposure. It also reduced the protein damage and lipid peroxidation induced by OS. However, it could not rescue the decrease in Nrf2 expression and increase in the BAX/BCL2 mRNA ratio, as well as DNA damage, in trophoblast cells under the current conditions, indicating that the observed positive effect on the antioxidant system could be mediated via pathways other than Nrf2. Previous studies demonstrated cytoprotective effects of OLE against OS in mesenchymal stem cells and human embryonic kidney cells, where it reduced H2O2-induced apoptosis through modulating MAPK pathways [50,70]. In synovial fibroblasts, OLE treatment provided anti-inflammatory and antioxidant effects via the downregulation of the MAPK and NF-κB signaling pathways, as well as the induction of Nrf2 [71]. Fröhlich et al. provided an important clue about the underlying molecular mechanisms regulating trophoblast survival under elevated OS, by searching for an answer as to why the number of dead trophoblast cells was not affected by the antioxidant treatment, even when the ROS levels were effectively reduced in these cells. Namely, these authors showed that reduced trophoblast proliferation happens even if the antioxidant markers are increased in these cells, in an ROS-independent manner involving the MAPK pathway—specifically, extracellular signal-regulated protein kinase 1 and 2 (ERK1/2) [72]. The activation of the ERK pathway is a component of the cellular damage response that can be initiated by diverse types of exposure, including OS. It also plays an important functional role in cell cycle arrest in response to DNA damage [73]. ERK activation as a response to DNA damage happens quickly, with the first phase of ERK activation starting already at 1 h post-exposure, and it is known to mediate damage-induced apoptosis [74]. A study by Li et al. showed that treatment of cells with 200 µM H2O2 can simultaneously induce damage by activating the phosphorylation of ERK1/2 and by increasing ROS production and the BAX/BCL2 ratio, with both pathways leading to the induction of apoptosis [75]. In addition, Go et al. demonstrated that H2O2’s effects are mediated both directly, by inducing redox signaling through kinase signaling pathways, and indirectly, by oxidizing the cellular environment [76]. This mechanism is distinct from OS and is regulated by a more isolated and localized redox circuitry [76]. The same study also showed that H2O2 regulates transcription via upstream ERK1/2 and the JNK kinase pathway, separately from the direct regulation of the redox-sensitive transcription factors such as Nrf2. Since our results demonstrated that OLE could not prevent the decrease in NFE2L2 mRNA expression and the significant increase in DNA damage and the BAX/BCL2 mRNA ratio, a surrogate marker of pro-apoptotic signaling after H2O2 exposure [77], it could be assumed that the protective effects of OLE against oxidative damage seen in trophoblast cells in our study may be regulated via the MAPK pathway, which would be interesting to examine in further studies. This assumption is also supported by the fact that the iNOS/NO signaling pathway is triggered by the activation of the ERK signaling pathway in trophoblast cells, as shown by Du and colleagues [78]. Our results showed that OLE treatment effectively reduces iNOS levels following H2O2 exposure; thus, it is plausible that it also influences the upstream ERK pathway. However, the involvement of other mechanisms behind this protective effect of OLE in trophoblast cells cannot be disregarded.
5. Conclusions
Olive-derived bioactive compound OLE displayed cytoprotective effects against H2O2 in HTR-8/SVneo trophoblast cells, by increasing antioxidant cell defense, mirrored in the significantly increased expression of antioxidant markers, and the prevention of protein oxidation and lipid peroxidation, as well as by reducing the iNOS levels and the mRNA expression of IL-6 and TNFα. Despite harboring these benefits, OLE did not influence the expression of the redox-sensitive antioxidant transcription factor NFE2L2, nor did it prevent the generation of DNA damage or increase pro-apoptotic signaling (according to the increased BAX/BCL2 mRNA ratio), following H2O2 exposure. Having in mind the significant attenuation of OS markers and the restoration of antioxidant functioning in HTR-8/SVneo cells pre-treated with OLE, it can be concluded that its protective role could be ascribed to its antioxidant effect, by alleviating the oxidizing cellular environment. However, its impact on the signaling pathways mediating OS in trophoblast cells remains to be examined in further studies. Most importantly, it should be emphasized that OLE per se did not lead to any adverse effects in HTR-8/SVneo trophoblast cells under the described conditions, confirming its safety in vitro.
Author Contributions
Conceptualization, A.P. and D.D.; methodology, A.P., A.V. and S.B.; software, S.B. and M.J.K.; validation, M.N.-A., Ž.B.-T. and D.D.; formal analysis, A.P., A.V. and S.B.; investigation, A.P., A.V. and S.B.; resources, M.J.K. and S.B.; data curation, A.P. and A.V.; writing—original draft preparation, A.P., A.V. and M.N.-A., writing—review and editing, M.J.K., Ž.B.-T., F.G. and D.D.; visualization, A.P. and F.G.; supervision, M.B. and D.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Education, Science and Technological Development, Republic of Serbia, through a Grant Agreement with the University of Belgrade Institute for the Application of Nuclear Energy—INEP, University of Belgrade, Faculty of Veterinary Medicine (Grant No. 451-03-68/2022-14/200019; 451-03-68/2022-14/200143).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rufino-Palomares, E.E.; Pérez-Jiménez, A.; García-Salguero, L.; Mokhtari, K.; Reyes-Zurita, F.J.; Peragón-Sánchez, J.; Lupiáñez, J.A. Nutraceutical Role of Polyphenols and Triterpenes Present in the Extracts of Fruits and Leaves of Olea Europaea as Anti-oxidants, Anti-Infectives and Anticancer Agents on Healthy Growth. Molecules 2022, 27, 2341. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Frost, B.; Liu, J. Oleuropein, Unexpected Benefits! Oncotarget 2017, 8, 17409. [Google Scholar] [CrossRef] [PubMed]
- Lockyer, S.; Corona, G.; Yaqoob, P.; Spencer, J.P.E.; Rowland, I. Secoiridoids Delivered as Olive Leaf Extract Induce Acute Improvements in Human Vascular Function and Reduction of an Inflammatory Cytokine: A Randomised, Double-Blind, Placebo-Controlled, Cross-over Trial. Br. J. Nutr. 2015, 114, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [PubMed]
- Hassen, I.; Casabianca, H.; Hosni, K. Biological Activities of the Natural Anti-oxidant Oleuropein: Exceeding the Expectation—A Mini-Review. J. Funct. Foods 2015, 18, 926–940. [Google Scholar] [CrossRef]
- Czerwińska, M.; Kiss, A.K.; Naruszewicz, M. A Comparison of Anti-oxidant Activities of Oleuropein and Its Dialdehydic Derivative from Olive Oil, Oleacein. Food Chem. 2012, 131, 940–947. [Google Scholar] [CrossRef]
- Sun, W.; Wang, X.; Hou, C.; Yang, L.; Li, H.; Guo, J.; Huo, C.; Wang, M.; Miao, Y.; Liu, J.; et al. Oleuropein Improves Mitochondrial Function to Attenuate Oxidative Stress by Activating the Nrf2 Pathway in the Hypothalamic Paraventricular Nucleus of Spontaneously Hypertensive Rats. Neuropharmacology 2017, 113, 556–566. [Google Scholar] [CrossRef]
- Domitrović, R.; Jakovac, H.; Marchesi, V.V.; Šain, I.; Romić, Ž.; Rahelić, D. Preventive and Therapeutic Effects of Oleuropein against Carbon Tetrachloride-Induced Liver Damage in Mice. Pharmacol. Res. 2012, 65, 451–464. [Google Scholar] [CrossRef]
- Hussain, T.; Murtaza, G.; Metwally, E.; Kalhoro, D.H.; Kalhoro, M.S.; Rahu, B.A.; Sahito, R.G.A.; Yin, Y.; Yang, H.; Chughtai, M.I.; et al. The Role of Oxidative Stress and Antioxidant Balance in Pregnancy. Mediators Inflamm. 2021, 2021, 9962860. [Google Scholar] [CrossRef]
- Schoots, M.H.; Gordijn, S.J.; Scherjon, S.A.; van Goor, H.; Hillebrands, J.-L. Oxidative Stress in Placental Pathology. Placenta 2018, 69, 153–161. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, P.; Zheng, Q.; Deka, A.; Choudhury, R.; Rastogi, S. Does a MediDiet With Additional Extra Virgin Olive Oil and Pistachios Reduce the Incidence of Gestational Diabetes? Endocr. Pract. 2022, 28, 135–141. [Google Scholar] [CrossRef]
- Melero, V.; Assaf-Balut, C.; de la Torre, N.G.; Jiménez, I.; Bordiú, E.; del Valle, L.; Valerio, J.; Familiar, C.; Durán, A.; Runkle, I.; et al. Benefits of Adhering to a Mediterranean Diet Supplemented with Extra Virgin Olive Oil and Pistachios in Pregnancy on the Health of Offspring at 2 Years of Age. Results of the San Carlos Gestational Diabetes Mellitus Prevention Study. J. Clin. Med. 2020, 9, 1454. [Google Scholar] [CrossRef]
- García de la Torre, N.; Assaf-Balut, C.; Jiménez Varas, I.; del Valle, L.; Durán, A.; Fuentes, M.; del Prado, N.; Bordiú, E.; Valerio, J.J.; Herraiz, M.A.; et al. Effectiveness of Following Mediterranean Diet Recommendations in the Real World in the Incidence of Gestational Diabetes Mellitus (GDM) and Adverse Maternal-Foetal Outcomes: A Prospective, Universal, Interventional Study with a Single Group. The St Carlos. Nutrients 2019, 11, 1210. [Google Scholar] [CrossRef]
- Al Wattar, H.B.; Dodds, J.; Placzek, A.; Beresford, L.; Spyreli, E.; Moore, A.; Gonzalez Carreras, F.J.; Austin, F.; Murugesu, N.; Roseboom, T.J.; et al. Mediterranean-Style Diet in Pregnant Women with Metabolic Risk Factors (ESTEEM): A Pragmatic Multicentre Randomised Trial. PLoS Med. 2019, 16, e1002857. [Google Scholar] [CrossRef]
- Martínez-Galiano, J.; Olmedo-Requena, R.; Barrios-Rodríguez, R.; Amezcua-Prieto, C.; Bueno-Cavanillas, A.; Salcedo-Bellido, I.; Jimenez-Moleon, J.; Delgado-Rodríguez, M. Effect of Adherence to a Mediterranean Diet and Olive Oil Intake during Pregnancy on Risk of Small for Gestational Age Infants. Nutrients 2018, 10, 1234. [Google Scholar] [CrossRef]
- Minhas, A.S.; Hong, X.; Wang, G.; Rhee, D.K.; Liu, T.; Zhang, M.; Michos, E.D.; Wang, X.; Mueller, N.T. Mediterranean-Style Diet and Risk of Preeclampsia by Race in the Boston Birth Cohort. J. Am. Heart Assoc. 2022, 11, e022589. [Google Scholar] [CrossRef]
- Fournier, S.B.; D’Errico, J.N.; Stapleton, P.A. Uterine Vascular Control Preconception and During Pregnancy. In Comprehensive Physiology; Wiley: Hoboken, NJ, USA, 2021; pp. 1871–1893. [Google Scholar]
- Mukherjee, I.; Dhar, R.; Singh, S.; Sharma, J.B.; Nag, T.C.; Mridha, A.R.; Jaiswal, P.; Biswas, S.; Karmakar, S. Oxidative Stress-Induced Impairment of Trophoblast Function Causes Preeclampsia through the Unfolded Protein Response Pathway. Sci. Rep. 2021, 11, 18415. [Google Scholar] [CrossRef]
- Di Fabrizio, C.; Giorgione, V.; Khalil, A.; Murdoch, C.E. Anti-oxidants in Pregnancy: Do We Really Need More Trials? Antioxidants 2022, 11, 812. [Google Scholar] [CrossRef]
- Kostić, S.; Vilotić, A.; Pirković, A.; Dekanski, D.; Borozan, S.; Nacka-Aleksić, M.; Vrzić-Petronijević, S.; Jovanović Krivokuća, M. Caffeic Acid Protects Human Trophoblast HTR-8/SVneo Cells from H2O2-Induced Oxidative Stress and Genotoxicity. Food Chem. Toxicol. 2022, 163, 112993. [Google Scholar] [CrossRef]
- Bruić, M.; Pirković, A.; Vilotić, A.; Jovanović-Krivokuća, M.; Spremo-Potparević, B. Cytoprotective and Genoprotective Effects of Taxifolin against Oxidative Damage in HTR-8/SVneo Human Trophoblast Cells. Mutagenesis 2022, geac013. [Google Scholar] [CrossRef]
- Møller, P.; Azqueta, A.; Boutet-Robinet, E.; Koppen, G.; Bonassi, S.; Milić, M.; Gajski, G.; Costa, S.; Teixeira, J.P.; Costa Pereira, C.; et al. Minimum Information for Reporting on the Comet Assay (MIRCA): Recommendations for Describing Comet Assay Procedures and Results. Nat. Protoc. 2020, 15, 3817–3826. [Google Scholar] [CrossRef]
- Collins, A.R.; Dusinská, M.; Gedik, C.M.; Stĕtina, R. Oxidative Damage to DNA: Do We Have a Reliable Biomarker? Environ. Health Perspect. 1996, 104, 465–469. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in Vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; pp. 121–126. [Google Scholar]
- Günzler, W.A.; Kremers, H.; Flohé, L. An Improved Coupled Test Procedure for Glutathione Peroxidase (EC 1.11.1.9.) in Blood. Clin. Chem. Lab. Med. 1974, 12, 444–448. [Google Scholar] [CrossRef]
- Sun, M.; Zigman, S. An Improved Spectrophotometric Assay for Superoxide Dismutase Based on Epinephrine Autoxidation. Anal. Biochem. 1978, 90, 81–89. [Google Scholar] [CrossRef]
- Stocks, J.; Dormandy, T.L. The Autoxidation of Human Red Cell Lipids Induced by Hydrogen Peroxide. Br. J. Haematol. 1971, 20, 95–111. [Google Scholar] [CrossRef]
- Bergmayer, H.U. Methods of Enzymatic Analysis. Verlag Chemie G.m.b.H., Weinheim/Bergstr. (Germany) and Academic Press, New York and London. 1963. Starch—Stärke 1963, 15, 272. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.-G.; Ahn, B.-W.; Shaltiel, S.; Stadtman, E.R. [49] Determination of Carbonyl Content in Oxidatively Modified Proteins. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1990; pp. 464–478. [Google Scholar]
- Guevara, I.; Iwanejko, J.; Dembińska-Kieć, A.; Pankiewicz, J.; Wanat, A.; Anna, P.; Gołąbek, I.; Bartuś, S.; Malczewska-Malec, M.; Szczudlik, A. Determination of Nitrite/Nitrate in Human Biological Material by the Simple Griess Reaction. Clin. Chim. Acta 1998, 274, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Ćupić Miladinović, D.; Prevendar Crnić, A.; Peković, S.; Dacić, S.; Ivanović, S.; Santibanez, J.F.; Ćupić, V.; Borozan, N.; Antonijević Miljaković, E.; Borozan, S. Recovery of Brain Cholinesterases and Effect on Parameters of Oxidative Stres and Apoptosis in Quails (Coturnix Japonica) after Chlorpyrifos and Vitamin B1 Administration. Chem. Biol. Interact. 2021, 333, 109312. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic-Krivokuca, M.; Stefanoska, I.; Rabi, A.; Vilotic, A.; Petronijevic, M.; Vrzic-Petronijevic, S.; Radojcic, L.; Vicovac, L. MIF Is among the Proinflammatory Cytokines Increased by LPS in the Human Trophoblast Line. Arch. Biol. Sci. 2016, 68, 715–722. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tenório, M.B.; Ferreira, R.C.; Moura, F.A.; Bueno, N.B.; Goulart, M.O.F.; Oliveira, A.C.M. Oral Anti-oxidant Therapy for Prevention and Treatment of Preeclampsia: Meta-Analysis of Randomized Controlled Trials. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 865–876. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Qiu, J.; Ni, Y.; Chai, S.; Zhou, L.; Li, J.; Yan, B.; Yang, J.; Liu, Q. The Association between Dietary Vitamin C/E and Gestational Hypertensive Disorder: A Case-Control Study. J. Nutr. Sci. Vitaminol. 2018, 64, 454–465. [Google Scholar] [CrossRef]
- Chappell, L.C.; Seed, P.T.; Kelly, F.J.; Briley, A.; Hunt, B.J.; Charnock-Jones, D.S.; Mallet, A.; Poston, L.; CStat. Vitamin C and E Supplementation in Women at Risk of Preeclampsia Is Associated with Changes in Indices of Oxidative Stress and Placental Function. Am. J. Obstet. Gynecol. 2002, 187, 777–784. [Google Scholar] [CrossRef]
- Poston, L.; Briley, A.; Seed, P.; Kelly, F.; Shennan, A. Vitamin C and Vitamin E in Pregnant Women at Risk for Pre-Eclampsia (VIP Trial): Randomised Placebo-Controlled Trial. Lancet 2006, 367, 1145–1154. [Google Scholar] [CrossRef]
- Poston, L.; Igosheva, N.; Mistry, H.D.; Seed, P.T.; Shennan, A.H.; Rana, S.; Karumanchi, S.A.; Chappell, L.C. Role of Oxidative Stress and Anti-oxidant Supplementation in Pregnancy Disorders. Am. J. Clin. Nutr. 2011, 94, 1980S–1985S. [Google Scholar] [CrossRef]
- Rumbold, A.; Duley, L.; Crowther, C.A.; Haslam, R.R. Anti-oxidants for Preventing Pre-Eclampsia. Cochrane Database Syst. Rev. 2008, 1, 1–86, Art. No.: CD004227. [Google Scholar] [CrossRef]
- Otero, D.M.; Lorini, A.; Oliveira, F.M.; da Fonseca Antunes, B.; Oliveira, R.M.; Zambiazi, R.C. Leaves of Olea europaea L. as a Source of Oleuropein: Characteristics and Biological Aspects. Res. Soc. Dev. 2021, 10, e185101321130. [Google Scholar] [CrossRef]
- Gomez Ribot, D.; Diaz, E.; Fazio, M.V.; Gómez, H.L.; Fornes, D.; Macchi, S.B.; Gresta, C.A.; Capobianco, E.; Jawerbaum, A. An Extra Virgin Olive Oil-enriched Diet Improves Maternal, Placental, and Cord Blood Parameters in GDM Pregnancies. Diabetes. Metab. Res. Rev. 2020, 36, e3349. [Google Scholar] [CrossRef]
- Parisi, F.; Rousian, M.; Huijgen, N.A.; Koning, A.H.J.; Willemsen, S.P.; de Vries, J.H.M.; Cetin, I.; Steegers, E.A.P.; Steegers-Theunissen, R.P.M. Periconceptional Maternal ‘High Fish and Olive Oil, Low Meat’ Dietary Pattern Is Associated with Increased Embryonic Growth: The Rotterdam Periconceptional Cohort (Predict) Study. Ultrasound Obstet. Gynecol. 2017, 50, 709–716. [Google Scholar] [CrossRef]
- Acevedo, N.; Frumento, P.; Harb, H.; Alashkar Alhamwe, B.; Johansson, C.; Eick, L.; Alm, J.; Renz, H.; Scheynius, A.; Potaczek, D. Histone Acetylation of Immune Regulatory Genes in Human Placenta in Association with Maternal Intake of Olive Oil and Fish Consumption. Int. J. Mol. Sci. 2019, 20, 1060. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, H.; Wang, A. Oleuropein Alleviates Gestational Diabetes Mellitus by Activating AMPK Signaling. Endocr. Connect. 2021, 10, 45–53. [Google Scholar] [CrossRef]
- Chiang, Y.-F.; Hung, H.-C.; Chen, H.-Y.; Huang, K.-C.; Lin, P.-H.; Chang, J.-Y.; Huang, T.-C.; Hsia, S.-M. The Inhibitory Effect of Extra Virgin Olive Oil and Its Active Compound Oleocanthal on Prostaglandin-Induced Uterine Hypercontraction and Pain—Ex Vivo and In Vivo Study. Nutrients 2020, 12, 3012. [Google Scholar] [CrossRef]
- Carnevale, R.; Silvestri, R.; Loffredo, L.; Novo, M.; Cammisotto, V.; Castellani, V.; Bartimoccia, S.; Nocella, C.; Violi, F. Oleuropein, a Component of Extra Virgin Olive Oil, Lowers Postprandial Glycaemia in Healthy Subjects. Br. J. Clin. Pharmacol. 2018, 84, 1566–1574. [Google Scholar] [CrossRef]
- Shi, C.; Chen, X.; Liu, Z.; Meng, R.; Zhao, X.; Liu, Z.; Guo, N. Oleuropein Protects L-02 Cells against H2O2-Induced Oxidative Stress by Increasing SOD1, GPx1 and CAT Expression. Biomed. Pharmacother. 2017, 85, 740–748. [Google Scholar] [CrossRef]
- Kucukgul, A.; Isgor, M.M.; Duzguner, V.; Atabay, M.N.; Kucukgul, A. Anti-oxidant Effects of Oleuropein on Hydrogen Peroxide-Induced Neuronal Stress- An In Vitro Study. Antiinflamm. Antiallergy. Agents Med. Chem. 2020, 19, 74–84. [Google Scholar] [CrossRef]
- Maalej, A.; Forte, M.; Bouallagui, Z.; Donato, S.; Mita, L.; Mita, D.G.; Filosa, S.; Crispi, S.; Sayadi, S. Olive Compounds Attenuate Oxidative Damage Induced in HEK-293 Cells via MAPK Signaling Pathway. J. Funct. Foods 2017, 39, 18–27. [Google Scholar] [CrossRef]
- García-Villalba, R.; Larrosa, M.; Possemiers, S.; Tomás-Barberán, F.A.; Espín, J.C. Bioavailability of Phenolics from an Oleuropein-Rich Olive (Olea Europaea) Leaf Extract and Its Acute Effect on Plasma Anti-oxidant Status: Comparison between Pre- and Postmenopausal Women. Eur. J. Nutr. 2014, 53, 1015–1027. [Google Scholar] [CrossRef]
- Harris, L.K.; McCormick, J.; Cartwright, J.E.; Whitley, G.S.; Dash, P.R. S-Nitrosylation of Proteins at the Leading Edge of Migrating Trophoblasts by Inducible Nitric Oxide Synthase Promotes Trophoblast Invasion. Exp. Cell Res. 2008, 314, 1765–1776. [Google Scholar] [CrossRef]
- Shaamash, A.H.; Elsonosy, E.D.; Zakhari, M.M.; Radwan, S.H.; El-Dien, H.M. Placental Nitric Oxide Synthase (NOS) Activity and Nitric Oxide (NO) Production in Normal Pregnancy, Pre-Eclampsia and Eclampsia. Int. J. Gynecol. Obstet. 2001, 72, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Schiessl, B.; Mylonas, I.; Hantschmann, P.; Kuhn, C.; Schulze, S.; Kunze, S.; Friese, K.; Jeschke, U. Expression of Endothelial NO Synthase, Inducible NO Synthase, and Estrogen Receptors Alpha and Beta in Placental Tissue of Normal, Preeclamptic, and Intrauterine Growth-Restricted Pregnancies. J. Histochem. Cytochem. 2005, 53, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, M.; Kim, J.-Y.; Kim, T.; Hwang, J.; Ha, K.-S.; Won, M.-H.; Ryoo, S.; Kwon, Y.-G.; Kim, Y.-M. Circulating MiRNAs Associated with Dysregulated Vascular and Trophoblast Function as Target-Based Diagnostic Biomarkers for Preeclampsia. Cells 2020, 9, 2003. [Google Scholar] [CrossRef] [PubMed]
- Žák, P.; Souček, M. Correlation of Tumor Necrosis Factor Alpha, Interleukin 6 and Interleukin 10 With Blood Pressure, Risk of Preeclampsia and Low Birth Weight in Gestational Diabetes. Physiol. Res. 2019, 68, 395–408. [Google Scholar] [CrossRef]
- Bellos, I.; Karageorgiou, V.; Kapnias, D.; Karamanli, K.-E.; Siristatidis, C. The Role of Interleukins in Preeclampsia: A Comprehensive Review. Am. J. Reprod. Immunol. 2018, 80, e13055. [Google Scholar] [CrossRef]
- Jovanovic, M.; Kovacevic, T.; Stefanoska, I.; Vicovac, L. The Effect of IL-6 on the Trophoblast Cell Line HTR-8/SVneo. Arch. Biol. Sci. 2010, 62, 531–538. [Google Scholar] [CrossRef]
- Haider, S.; Knöfler, M. Human Tumour Necrosis Factor: Physiological and Pathological Roles in Placenta and Endometrium. Placenta 2009, 30, 111–123. [Google Scholar] [CrossRef]
- Fakhr, Y.; Koshti, S.; Habibyan, Y.B.; Webster, K.; Hemmings, D.G. Tumor Necrosis Factor-α Induces a Preeclamptic-like Phenotype in Placental Villi via Sphingosine Kinase 1 Activation. Int. J. Mol. Sci. 2022, 23, 3750. [Google Scholar] [CrossRef]
- Vilotić, A.; Nacka-Aleksić, M.; Pirković, A.; Bojić-Trbojević, Ž.; Dekanski, D.; Jovanović Krivokuća, M. IL-6 and IL-8: An Overview of Their Roles in Healthy and Pathological Pregnancies. Int. J. Mol. Sci. 2022, 23, 14574. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Y.; Alexander, J.S.; Lewis, D.F. Preeclampsia Status Controls Interleukin-6 and Soluble IL-6 Receptor Release from Neutrophils and Endothelial Cells: Relevance to Increased Inflammatory Responses. Pathophysiology 2021, 28, 202–211. [Google Scholar] [CrossRef]
- Romanowska-Próchnicka, K.; Felis-Giemza, A.; Olesińska, M.; Wojdasiewicz, P.; Paradowska-Gorycka, A.; Szukiewicz, D. The Role of TNF-α and Anti-TNF-α Agents during Preconception, Pregnancy, and Breastfeeding. Int. J. Mol. Sci. 2021, 22, 2922. [Google Scholar] [CrossRef]
- Socha, M.W.; Flis, W.; Wartęga, M.; Stankiewicz, M. Impact of Oxidative Stress on Molecular Mechanisms of Cervical Ripening in Pregnant Women. Int. J. Mol. Sci. 2022, 23, 12780. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 System in Development, Oxidative Stress Response and Diseases: An Evolutionarily Conserved Mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Chigusa, Y.; Kawasaki, K.; Kondoh, E.; Mogami, H.; Ujita, M.; Fujita, K.; Tatsumi, K.; Takeda, S.; Konishi, I. Simvastatin Inhibits Oxidative Stress via the Activation of Nuclear Factor Erythroid 2-Related Factor 2 Signaling in Trophoblast Cells. J. Obstet. Gynaecol. Res. 2016, 42, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Chigusa, Y.; Tatsumi, K.; Kondoh, E.; Fujita, K.; Nishimura, F.; Mogami, H.; Konishi, I. Decreased Lectin-Like Oxidized LDL Receptor 1 (LOX-1) and Low Nrf2 Activation in Placenta Are Involved in Preeclampsia. J. Clin. Endocrinol. Metab. 2012, 97, E1862–E1870. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; Wu, J.; Li, M.; Wang, L.; Zhu, X.; Chen, Y. Impaction of Factors Associated with Oxidative Stress on the Pathogenesis of Gestational Hypertension and Preeclampsia. Medicine 2021, 100, e23666. [Google Scholar] [CrossRef]
- ALHaithloul, H.A.S.; Alotaibi, M.F.; Bin-Jumah, M.; Elgebaly, H.; Mahmoud, A.M. Olea Europaea Leaf Extract Up-Regulates Nrf2/ARE/HO-1 Signaling and Attenuates Cyclophosphamide-Induced Oxidative Stress, Inflammation and Apoptosis in Rat Kidney. Biomed. Pharmacother. 2019, 111, 676–685. [Google Scholar] [CrossRef]
- Ji, S.T.; Kim, Y.-J.; Jung, S.Y.; Kim, D.Y.; Kang, S.; Park, J.H.; Jang, W.B.; Ha, J.; Yun, J.; Kwon, S.-M. Oleuropein Attenuates Hydrogen Peroxide-Induced Autophagic Cell Death in Human Adipose-Derived Stem Cells. Biochem. Biophys. Res. Commun. 2018, 499, 675–680. [Google Scholar] [CrossRef]
- Castejón, M.L.; Rosillo, M.Á.; Montoya, T.; González-Benjumea, A.; Fernández-Bolaños, J.M.; Alarcón-de-la-Lastra, C. Oleuropein Down-Regulated IL-1β-Induced Inflammation and Oxidative Stress in Human Synovial Fibroblast Cell Line SW982. Food Funct. 2017, 8, 1890–1898. [Google Scholar] [CrossRef]
- Fröhlich, J.D.; Huppertz, B.; Abuja, P.M.; König, J.; Desoye, G. Oxygen Modulates the Response of First-Trimester Trophoblasts to Hyperglycemia. Am. J. Pathol. 2012, 180, 153–164. [Google Scholar] [CrossRef]
- Park, W. MAPK Inhibitors, Particularly the JNK Inhibitor, Increase Cell Death Effects in H2O2-Treated Lung Cancer Cells via Increased Superoxide Anion and Glutathione Depletion. Oncol. Rep. 2018, 39, 860–870. [Google Scholar] [CrossRef]
- Tang, D.; Wu, D.; Hirao, A.; Lahti, J.M.; Liu, L.; Mazza, B.; Kidd, V.J.; Mak, T.W.; Ingram, A.J. ERK Activation Mediates Cell Cycle Arrest and Apoptosis after DNA Damage Independently of P53. J. Biol. Chem. 2002, 277, 12710–12717. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, Y.; Huang, X.; Xu, C.; Liu, X.; Wang, L.; Yu, M.; Li, D.; Zhu, Y.; Du, M. SCM-198 Protects Endometrial Stromal Cells from Oxidative Damage through Bax/Bcl-2 and ERK Signaling Pathways. Acta Biochim. Biophys. Sin. 2019, 51, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Go, Y.-M.; Gipp, J.J.; Mulcahy, R.T.; Jones, D.P. H2O2-Dependent Activation of GCLC-ARE4 Reporter Occurs by Mitogen-Activated Protein Kinase Pathways without Oxidation of Cellular Glutathione or Thioredoxin-1. J. Biol. Chem. 2004, 279, 5837–5845. [Google Scholar] [CrossRef]
- Du, Y.; Cai, Z.; Zhang, H.; Liang, W.; Wang, H.; Man, Q.; Wang, W. Nitric Oxide Mediates Disruption of Human Placental Trophoblast Invasion Induced by Perfluorobutane Sulfonate. Environ. Pollut. 2021, 283, 117137. [Google Scholar] [CrossRef]
- Salakou, S.; Kardamakis, D.; Tsamandas, A.C.; Zolota, V.; Apostolakis, E.; Tzelepi, V.; Papathanasopoulos, P.; Bonikos, D.S.; Papapetropoulos, T.; Petsas, T.; et al. Increased Bax/Bcl-2 ratio up-regulates caspase-3 and increases apoptosis in the thymus of patients with myasthenia gravis. Vivo 2007, 21, 123–132. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).