Engineered Adipose-Derived Stem Cells Overexpressing RXFP1 via CRISPR Activation Ameliorate Erectile Dysfunction in Diabetic Rats
Abstract
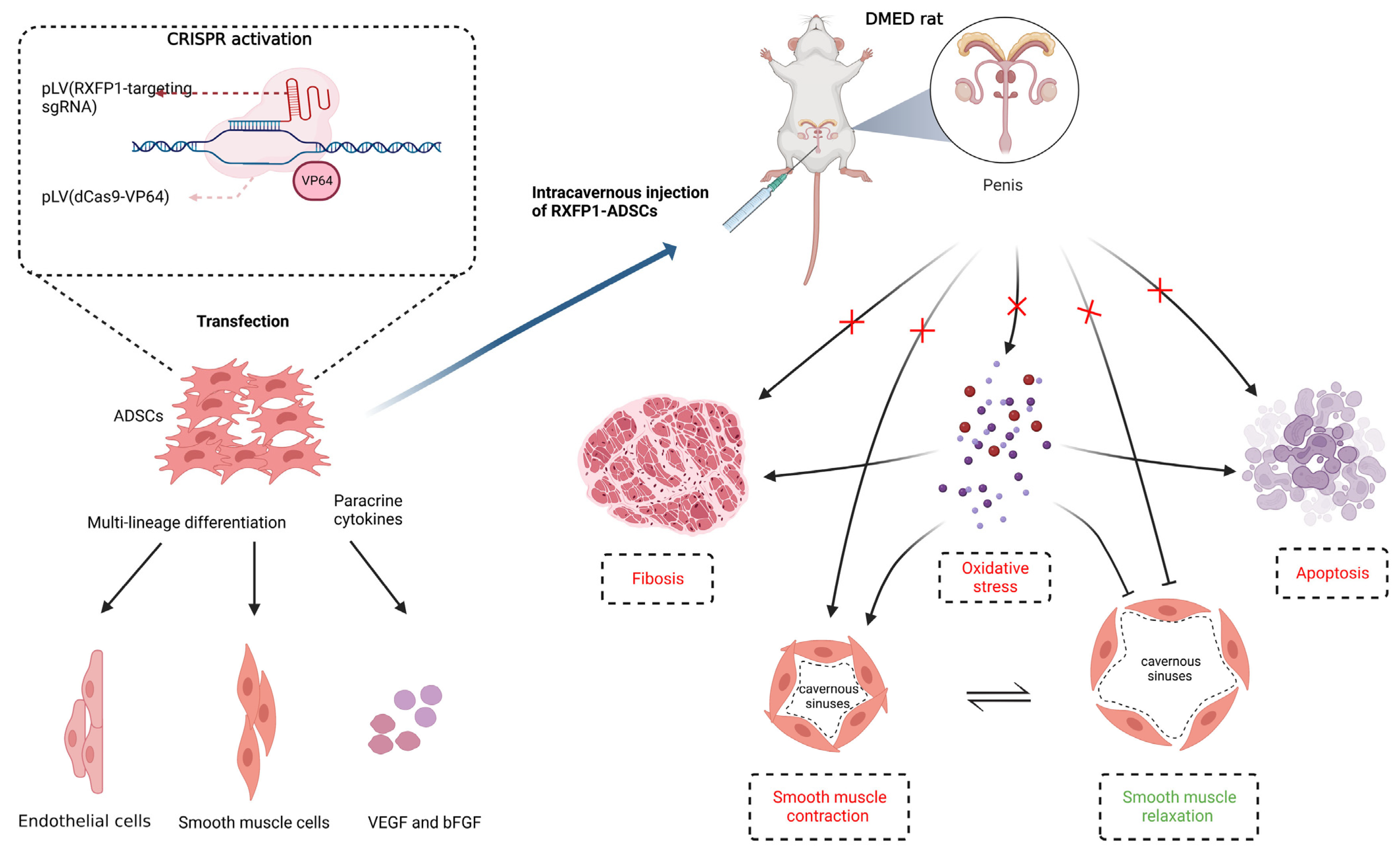
1. Introduction
2. Materials and Methods
2.1. Culture and Identification of Cells
2.2. Transfection of Cells
2.3. Animals
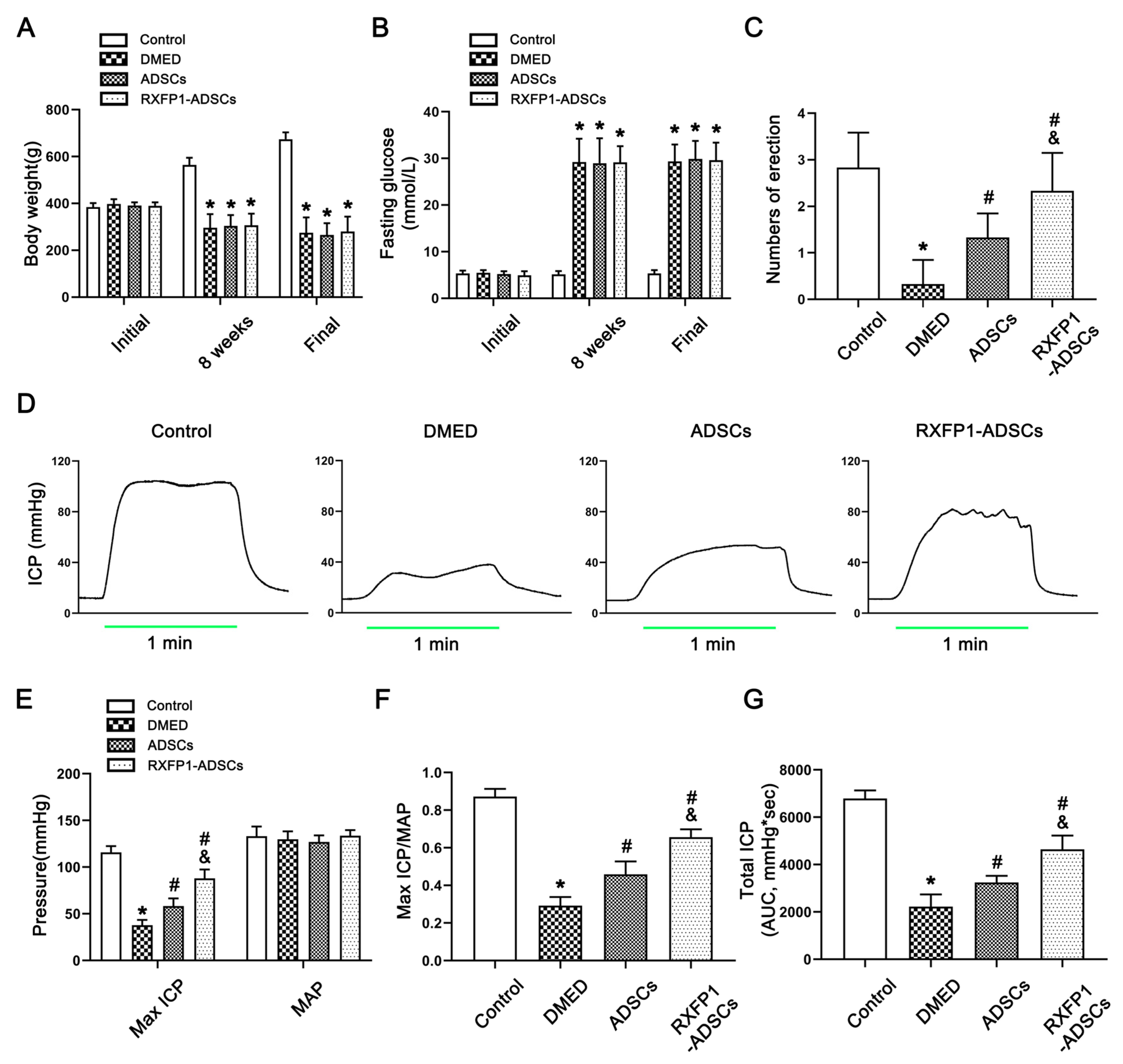
2.4. Evaluation of Erectile Function
2.5. Western Blot
2.6. Quantitative Reverse-Transcription PCR
2.7. Histological Alteration
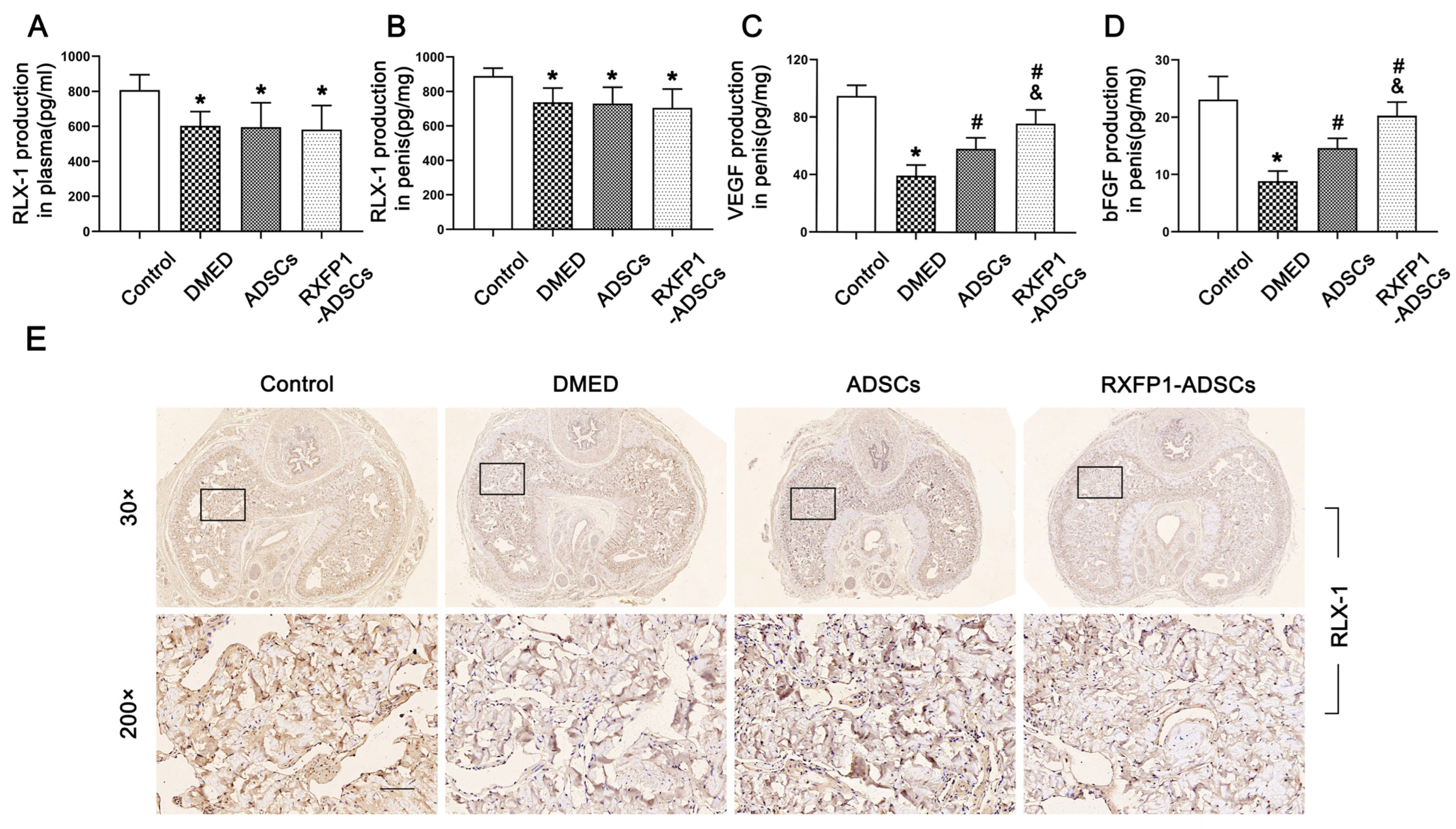
2.8. Detection of Special Substances
2.9. Statistical Analyses
3. Results
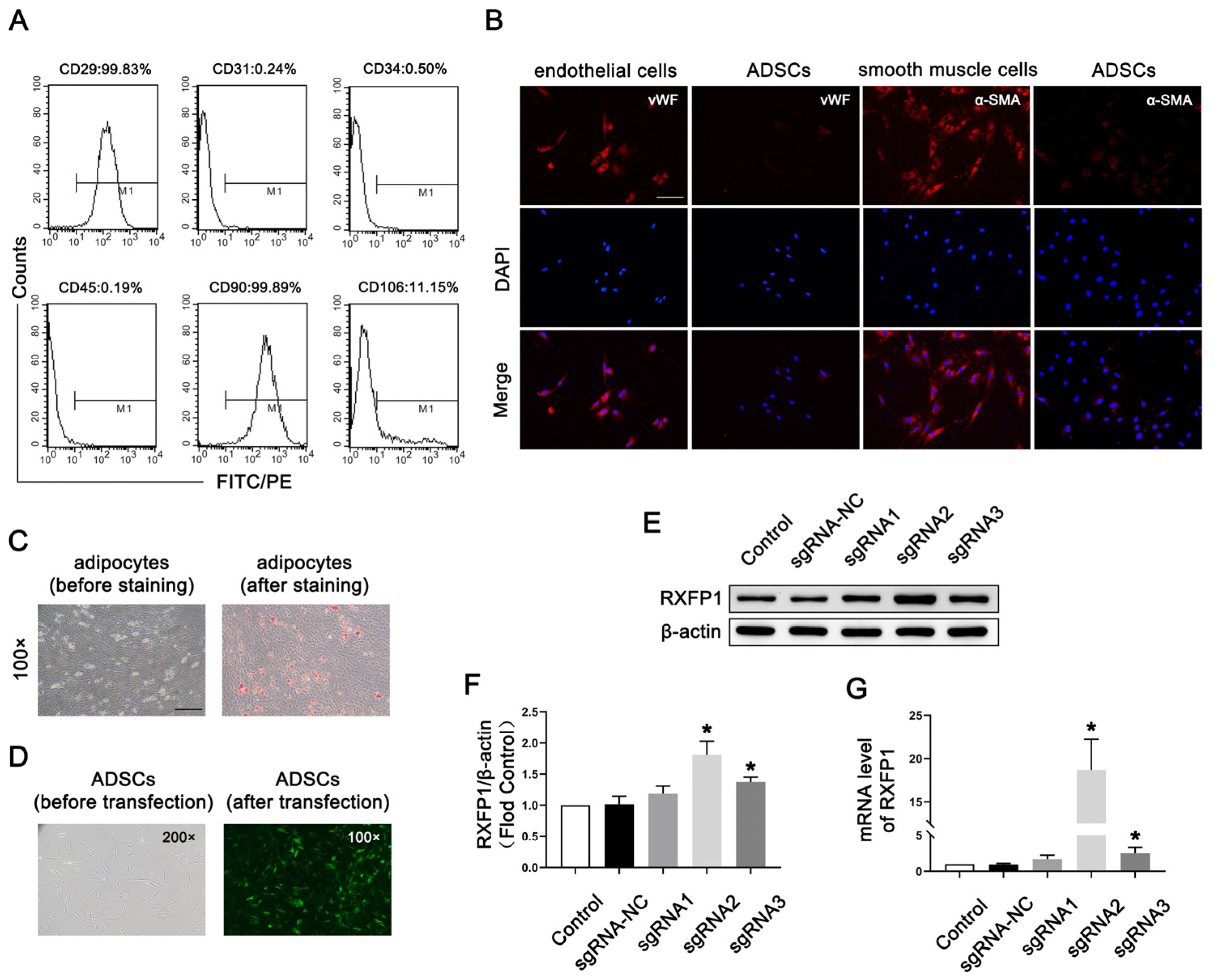
3.1. Preparation and Transfection of ADSCs
3.2. Metabolic and Physiological Parameters
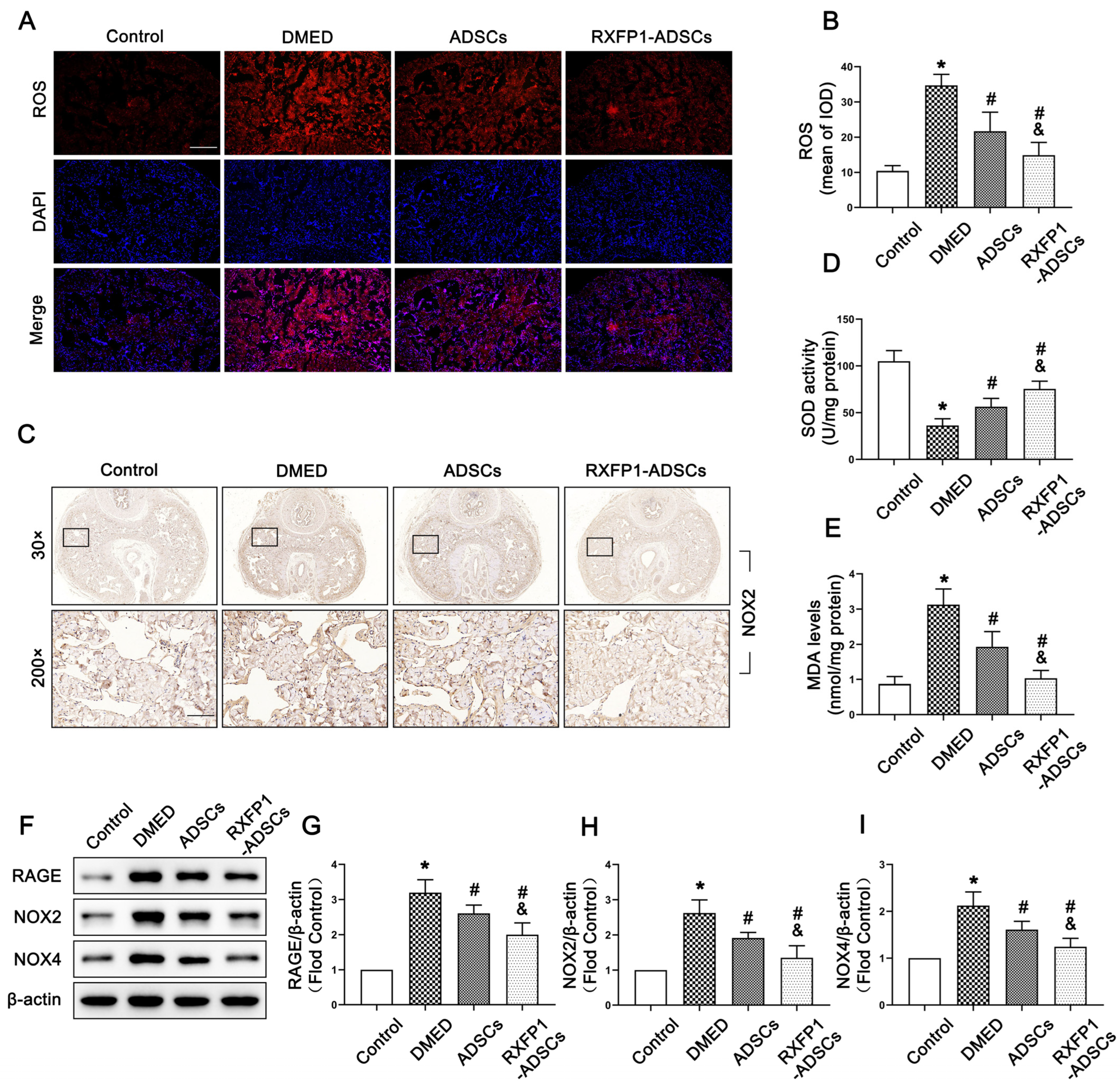
3.3. Transplantation of ADSCs Inhibited Oxidative Stress Damage in Penile Tissue
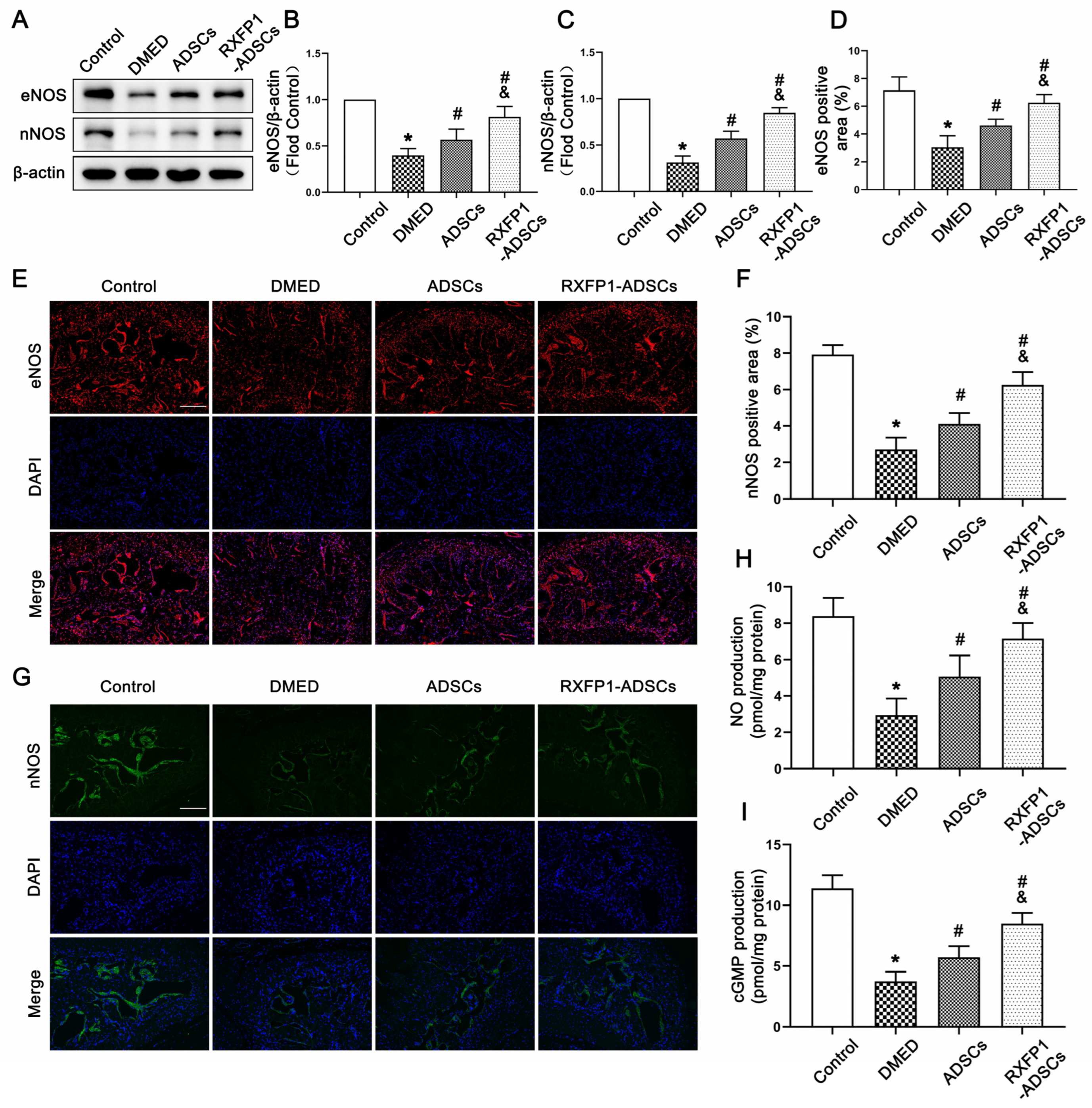
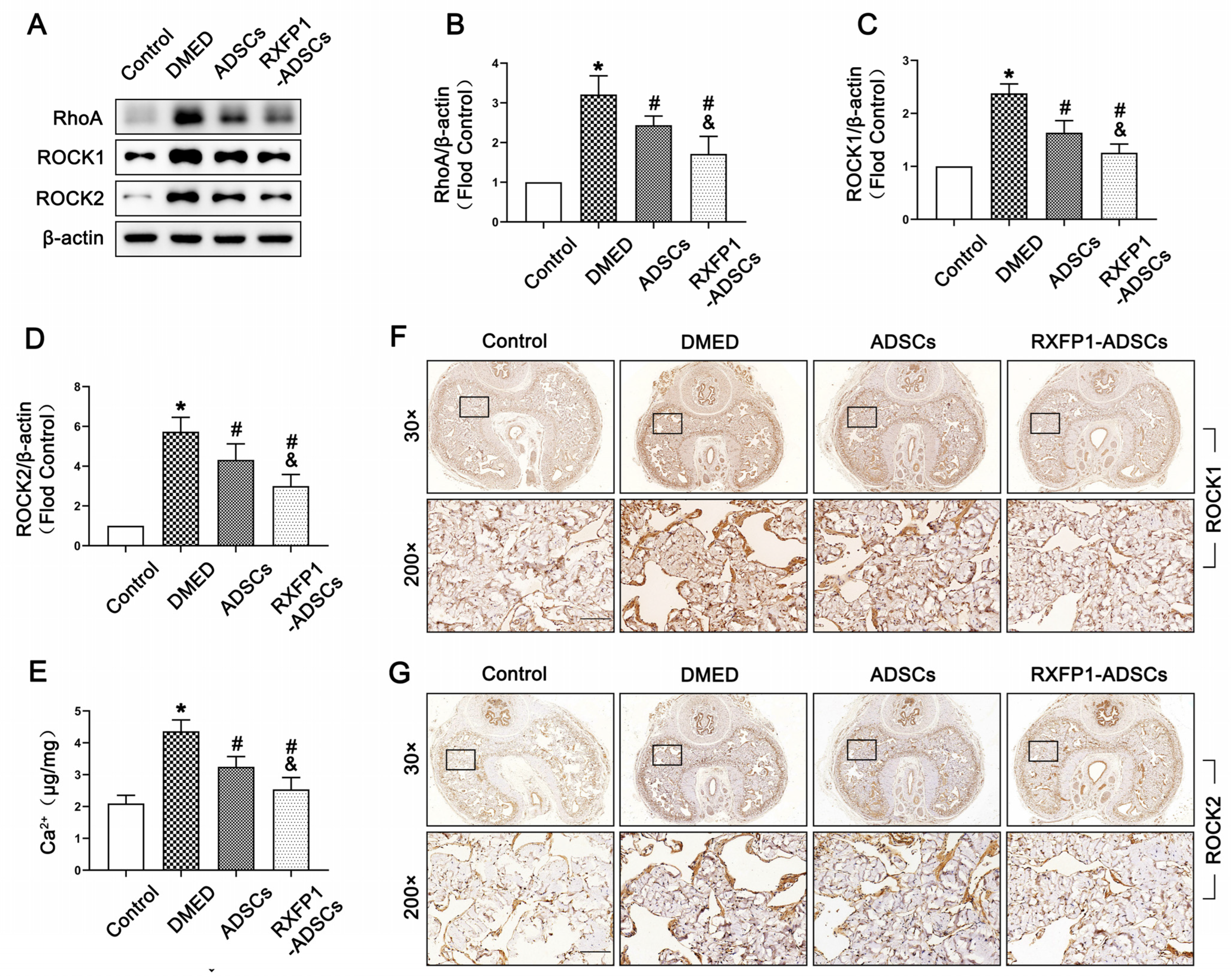
3.4. Transplantation of ADSCs Regulated the NO/cGMP and RhoA/ROCK Pathway in Rats
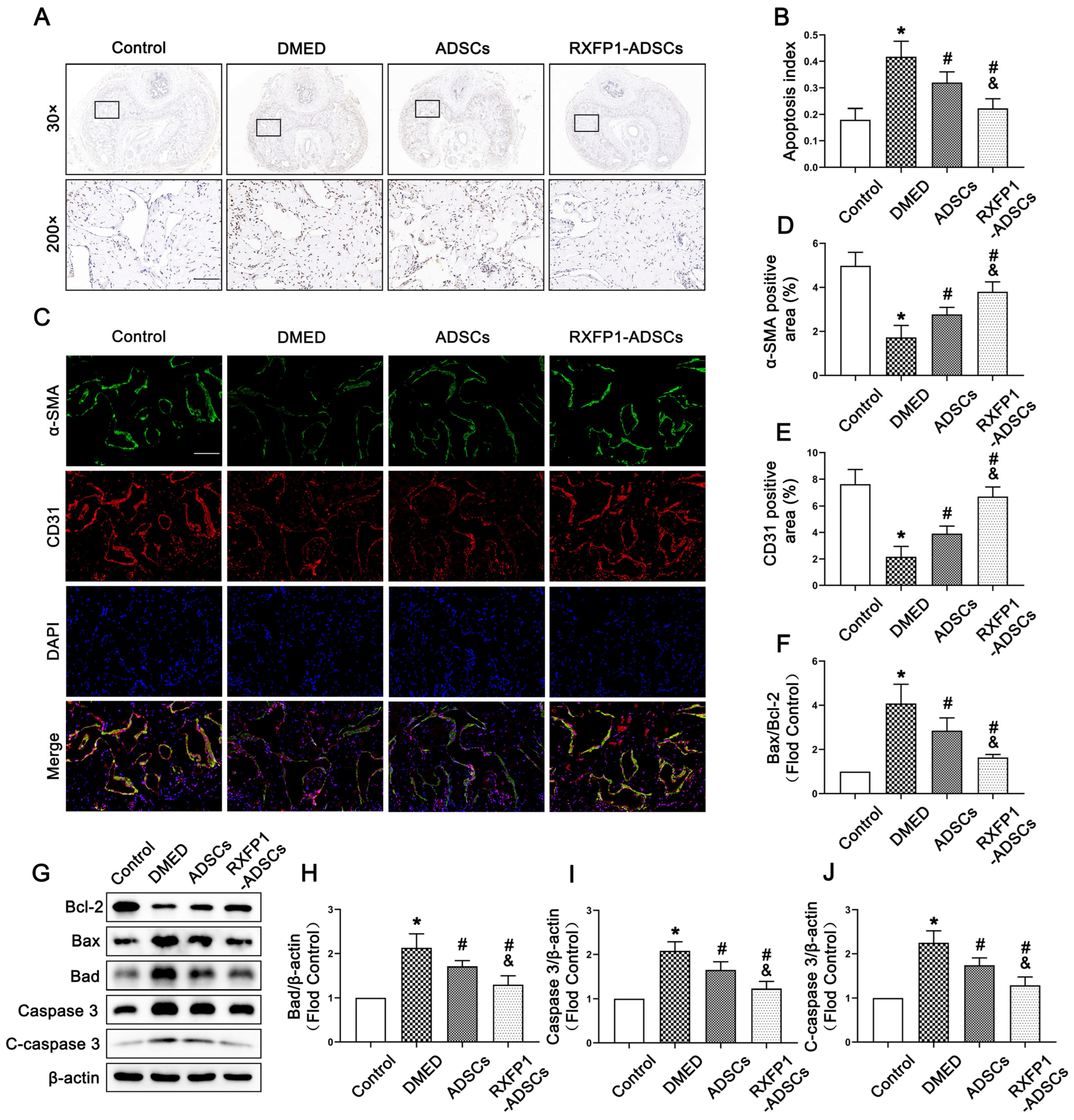
3.5. Transplantation of ADSCs Adjusted Apoptosis In Vivo
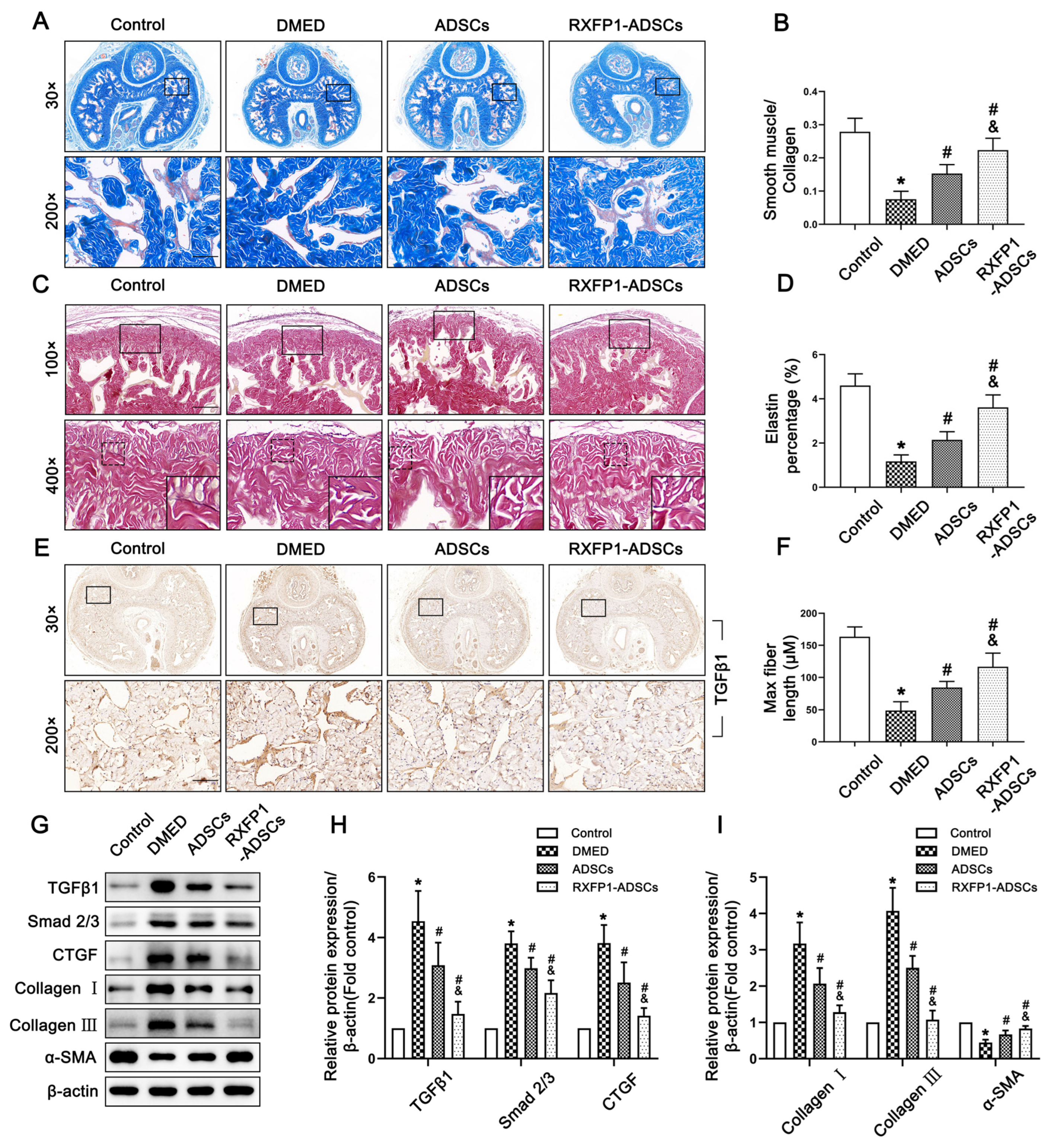
3.6. Transplantation of ADSCs Reduced Fibrosis in the Corpus Cavernosum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shamloul, R.; Ghanem, H. Erectile dysfunction. Lancet 2013, 381, 153–165. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Fink, H.A.; Mac Donald, R.; Rutks, I.R.; Nelson, D.B.; Wilt, T.J. Sildenafil for male erectile dysfunction: A systematic review and meta-analysis. Arch. Intern. Med. 2002, 162, 1349–1360. [Google Scholar] [CrossRef] [PubMed]
- Haahr, M.K.; Jensen, C.H.; Toyserkani, N.M.; Andersen, D.C.; Damkier, P.; Sorensen, J.A.; Lund, L.; Sheikh, S.P. Safety and Potential Effect of a Single Intracavernous Injection of Autologous Adipose-Derived Regenerative Cells in Patients with Erectile Dysfunction Following Radical Prostatectomy: An Open-Label Phase I Clinical Trial. EBioMedicine 2016, 5, 204–210. [Google Scholar] [CrossRef]
- Al Demour, S.; Jafar, H.; Adwan, S.; AlSharif, A.; Alhawari, H.; Alrabadi, A.; Zayed, A.; Jaradat, A.; Awidi, A. Safety and Potential Therapeutic Effect of Two Intracavernous Autologous Bone Marrow Derived Mesenchymal Stem Cells injections in Diabetic Patients with Erectile Dysfunction: An Open Label Phase I Clinical Trial. Urol. Int. 2018, 101, 358–365. [Google Scholar] [CrossRef]
- Ren, M.L.; Peng, W.; Yang, Z.L.; Sun, X.J.; Zhang, S.C.; Wang, Z.G.; Zhang, B. Allogeneic adipose-derived stem cells with low immunogenicity constructing tissue-engineered bone for repairing bone defects in pigs. Cell Transpl. 2012, 21, 2711–2721. [Google Scholar] [CrossRef]
- Wang, X.; Liu, C.; Xu, Y.; Chen, P.; Shen, Y.; Xu, Y.; Zhao, Y.; Chen, W.; Zhang, X.; Ouyang, Y.; et al. Combination of mesenchymal stem cell injection with icariin for the treatment of diabetes-associated erectile dysfunction. PLoS ONE 2017, 12, e0174145. [Google Scholar] [CrossRef]
- Liu, G.Y.; Jiang, X.X.; Zhu, X.; He, W.Y.; Kuang, Y.L.; Ren, K.; Lin, Y.; Gou, X. ROS activates JNK-mediated autophagy to counteract apoptosis in mouse mesenchymal stem cells in vitro. Acta Pharmacol. Sin. 2015, 36, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, D.; Li, P.; Damaser, M.; Zhang, Y. Virus integration and genome influence in approaches to stem cell based therapy for andro-urology. Adv. Drug Deliv. Rev. 2015, 82–83, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, M.; Du, X.J.; Dschietzig, T.B.; Summers, R.J. The actions of relaxin on the human cardiovascular system. Br. J. Pharmacol. 2017, 174, 933–949. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.Y.; Nakabayashi, K.; Nishi, S.; Kumagai, J.; Kudo, M.; Sherwood, O.D.; Hsueh, A.J. Activation of orphan receptors by the hormone relaxin. Science 2002, 295, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Segal, M.S.; Sautina, L.; Li, S.; Diao, Y.; Agoulnik, A.I.; Kielczewski, J.; McGuane, J.T.; Grant, M.B.; Conrad, K.P. Relaxin increases human endothelial progenitor cell NO and migration and vasculogenesis in mice. Blood 2012, 119, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Royce, S.G.; Tominaga, A.M.; Shen, M.; Patel, K.P.; Huuskes, B.M.; Lim, R.; Ricardo, S.D.; Samuel, C.S. Serelaxin improves the therapeutic efficacy of RXFP1-expressing human amnion epithelial cells in experimental allergic airway disease. Clin. Sci. 2016, 130, 2151–2165. [Google Scholar] [CrossRef]
- Moon, J.S.; Kim, S.H.; Oh, S.H.; Jeong, Y.W.; Kang, J.H.; Park, J.C.; Son, H.J.; Bae, S.; Park, B.I.; Kim, M.S.; et al. Relaxin augments BMP-2-induced osteoblast differentiation and bone formation. J. Bone Miner. Res. 2014, 29, 1586–1596. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Sun, T.; Xu, W.; Song, J.; Chen, Y.; Ruan, Y.; Li, H.; Cui, K.; Zhang, Y.; Feng, Y.; et al. Relaxin-2 Prevents Erectile Dysfunction by Cavernous Nerve, Endothelial and Histopathological Protection Effects in Rats with Bilateral Cavernous Nerve Injury. World J. Mens. Health 2022. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; DiCarlo, J.E.; Norville, J.E.; Church, G.M. RNA-guided human genome engineering via Cas9. Science 2013, 339, 823–826. [Google Scholar] [CrossRef]
- Perez-Pinera, P.; Kocak, D.D.; Vockley, C.M.; Adler, A.F.; Kabadi, A.M.; Polstein, L.R.; Thakore, P.I.; Glass, K.A.; Ousterout, D.G.; Leong, K.W.; et al. RNA-guided gene activation by CRISPR-Cas9-based transcription factors. Nat. Methods 2013, 10, 973–976. [Google Scholar] [CrossRef]
- Hsu, M.N.; Chang, Y.H.; Truong, V.A.; Lai, P.L.; Nguyen, T.K.N.; Hu, Y.C. CRISPR technologies for stem cell engineering and regenerative medicine. Biotechnol. Adv. 2019, 37, 107447. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Zhuan, L.; Zang, G.; Wang, T.; Liu, J. Transplantation of adipose-derived stem cells overexpressing inducible nitric oxide synthase ameliorates diabetes mellitus-induced erectile dysfunction in rats. PeerJ 2019, 7, e7507. [Google Scholar] [CrossRef]
- Khazaei, S.; Keshavarz, G.; Bozorgi, A.; Nazari, H.; Khazaei, M. Adipose tissue-derived stem cells: A comparative review on isolation, culture, and differentiation methods. Cell Tissue Bank 2022, 23, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Sun, Z.; Liao, L.; Meng, Y.; Han, Q.; Zhao, R.C. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem. Biophys. Res. Commun. 2005, 332, 370–379. [Google Scholar] [CrossRef]
- Song, J.; Sun, T.; Tang, Z.; Ruan, Y.; Liu, K.; Rao, K.; Lan, R.; Wang, S.; Wang, T.; Liu, J. Exosomes derived from smooth muscle cells ameliorate diabetes-induced erectile dysfunction by inhibiting fibrosis and modulating the NO/cGMP pathway. J. Cell Mol. Med. 2020, 24, 13289–13302. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Zhou, K.; Zhou, M.; Xia, K.; Xu, Y.; Sun, X.; Zhu, Y.; Cui, C.; Deng, C. Influence of Experimental Autoimmune Prostatitis on Sexual Function and the Anti-inflammatory Efficacy of Celecoxib in a Rat Model. Front. Immunol. 2020, 11, 574212. [Google Scholar] [CrossRef]
- Tlachi-Lopez, J.L.; Eguibar, J.R.; Fernandez-Guasti, A.; Lucio, R.A. Copulation and ejaculation in male rats under sexual satiety and the Coolidge effect. Physiol. Behav. 2012, 106, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Heaton, J.P.; Varrin, S.J.; Morales, A. The characterization of a bio-assay of erectile function in a rat model. J. Urol. 1991, 145, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Bathgate, R.A.; Ivell, R.; Sanborn, B.M.; Sherwood, O.D.; Summers, R.J. International Union of Pharmacology LVII: Recommendations for the nomenclature of receptors for relaxin family peptides. Pharmacol. Rev. 2006, 58, 7–31. [Google Scholar] [CrossRef]
- Qiu, X.; Lin, H.; Wang, Y.; Yu, W.; Chen, Y.; Wang, R.; Dai, Y. Intracavernous transplantation of bone marrow-derived mesenchymal stem cells restores erectile function of streptozocin-induced diabetic rats. J. Sex. Med. 2011, 8, 427–436. [Google Scholar] [CrossRef]
- Yang, J.; Yu, Z.; Zhang, Y.; Zang, G.H.; Zhuan, L.; Tang, Z.; Liu, Y.; Wang, T.; Wang, S.G.; Liu, J.H. Preconditioning of adipose-derived stem cells by phosphodiesterase-5 inhibition enhances therapeutic efficacy against diabetes-induced erectile dysfunction. Andrology 2020, 8, 231–240. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Zang, G.; Wang, T.; Yu, Z.; Wang, S.; Tang, Z.; Liu, J. Adipose-derived stem cells improve erectile function partially through the secretion of IGF-1, bFGF, and VEGF in aged rats. Andrology 2018, 6, 498–509. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, H.; Wang, Z.; Ding, W.; Zeng, Q.; Liu, W.; Huang, C.; He, S.; Wei, A. Adipose-Derived Stem Cell-Derived Exosomes Ameliorate Erectile Dysfunction in a Rat Model of Type 2 Diabetes. J. Sex. Med. 2017, 14, 1084–1094. [Google Scholar] [CrossRef]
- Zhu, L.L.; Huang, X.; Yu, W.; Chen, H.; Chen, Y.; Dai, Y.T. Transplantation of adipose tissue-derived stem cell-derived exosomes ameliorates erectile function in diabetic rats. Andrologia 2018, 50, e12871. [Google Scholar] [CrossRef]
- Leo, C.H.; Jelinic, M.; Ng, H.H.; Tare, M.; Parry, L.J. Time-dependent activation of prostacyclin and nitric oxide pathways during continuous i.v. infusion of serelaxin (recombinant human H2 relaxin). Br. J. Pharmacol. 2016, 173, 1005–1017. [Google Scholar] [CrossRef]
- Lin, H.; Yuan, J.; Ruan, K.H.; Yang, W.; Zhang, J.; Dai, Y.; Wang, R. COX-2-10aa-PGIS gene therapy improves erectile function in rats after cavernous nerve injury. J. Sex. Med. 2013, 10, 1476–1487. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.J.; Shen, W.H.; He, P.; Zhou, X.Z.; Zhi, Y.; Dai, Q.; Chen, Z.W.; Zhou, Z.S. Telomerase reverse transcriptase genetically modified adipose tissue derived stem cells improves erectile dysfunction by inhibiting oxidative stress and enhancing proliferation in rat model. Biomed. Pharmacother. 2017, 92, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.H.; Zhu, G.Q.; Bae, W.J.; Choi, S.W.; Jeong, H.C.; Cho, H.J.; Ha, U.S.; Hong, S.H.; Lee, J.Y.; Kwon, E.B.; et al. Engineered Mesenchymal Stem Cells Expressing Stromal Cell-derived Factor-1 Improve Erectile Dysfunction in Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 2018, 19, 3730. [Google Scholar] [CrossRef] [PubMed]
- Sasipong, N.; Schlegel, P.; Wingert, J.; Lederer, C.; Meinhardt, E.; Ziefer, A.; Schmidt, C.; Rapti, K.; Thoni, C.; Frey, N.; et al. Ligand-activated RXFP1 gene therapy ameliorates pressure overload-induced cardiac dysfunction. Mol. Ther. 2021, 29, 2499–2513. [Google Scholar] [CrossRef]
- Becirovic, E. Maybe you can turn me on: CRISPRa-based strategies for therapeutic applications. Cell Mol. Life Sci. 2022, 79, 130. [Google Scholar] [CrossRef]
- Hsu, M.N.; Huang, K.L.; Yu, F.J.; Lai, P.L.; Truong, A.V.; Lin, M.W.; Nguyen, N.T.K.; Shen, C.C.; Hwang, S.M.; Chang, Y.H.; et al. Coactivation of Endogenous Wnt10b and Foxc2 by CRISPR Activation Enhances BMSC Osteogenesis and Promotes Calvarial Bone Regeneration. Mol. Ther. 2020, 28, 441–451. [Google Scholar] [CrossRef]
- Shinkuma, S.; Guo, Z.; Christiano, A.M. Site-specific genome editing for correction of induced pluripotent stem cells derived from dominant dystrophic epidermolysis bullosa. Proc. Natl. Acad. Sci. USA 2016, 113, 5676–5681. [Google Scholar] [CrossRef]
- Neves, D. Advanced glycation end-products: A common pathway in diabetes and age-related erectile dysfunction. Free. Radic. Res. 2013, 47 (Suppl. S1), 49–69. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Yuan, Y.; Chen, P.; Lu, K.; Tang, Z.; Liu, Q.; Xu, W.; Zheng, D.; Xiong, S.; Pei, H. Pathological Roles of Oxidative Stress in Cardiac Microvascular Injury. Curr. Probl. Cardiol. 2022, 48, 101399. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Liu, Q.; Deng, Z.; Li, H.; Zhang, H.; Song, J.; Liu, X.; Liu, J.; Wen, B.; Wang, T. Human umbilical cord mesenchymal stem cells ameliorate erectile dysfunction in rats with diabetes mellitus through the attenuation of ferroptosis. Stem Cell Res. Ther. 2022, 13, 450. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Xu, W.; Wang, J.; Wang, T.; Wang, S.; Liu, K.; Liu, J. Saxagliptin alleviates erectile dysfunction through increasing SDF-1 in diabetes mellitus. Andrology 2022. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, K.; Ruan, Z.; Sun, D.; Zhang, H.; Lin, G.; Hu, L.; Zhao, S.; Fu, Q. Probucol enhances the therapeutic efficiency of mesenchymal stem cells in the treatment of erectile dysfunction in diabetic rats by prolonging their survival time via Nrf2 pathway. Stem. Cell Res. Ther. 2020, 11, 302. [Google Scholar] [CrossRef]
- Yao, X.; Yuan, Y.; Jing, T.; Ye, S.; Wang, S.; Xia, D. Ganoderma lucidum polysaccharide ameliorated diabetes mellitus-induced erectile dysfunction in rats by regulating fibrosis and the NOS/ERK/JNK pathway. Transl. Androl. Urol. 2022, 11, 982–995. [Google Scholar] [CrossRef]
- Luo, C.; Peng, Y.; Zhou, X.; Fan, J.; Chen, W.; Zhang, H.; Wei, A. NLRP3 downregulation enhances engraftment and functionality of adipose-derived stem cells to alleviate erectile dysfunction in diabetic rats. Front. Endocrinol. (Lausanne) 2022, 13, 913296. [Google Scholar] [CrossRef]
- Song, J.; Tang, Z.; Li, H.; Jiang, H.; Sun, T.; Lan, R.; Wang, T.; Wang, S.; Ye, Z.; Liu, J. Role of JAK2 in the Pathogenesis of Diabetic Erectile Dysfunction and an Intervention With Berberine. J. Sex. Med. 2019, 16, 1708–1720. [Google Scholar] [CrossRef]
- Wang, J.; Song, J.; Song, G.; Feng, Y.; Pan, J.; Yang, X.; Xin, Z.; Hu, P.; Sun, T.; Liu, K.; et al. Acetyl-L-carnitine improves erectile function in bilateral cavernous nerve injury rats via promoting cavernous nerve regeneration. Andrology 2022, 10, 984–996. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, T.; Xu, W.; Tu, B.; Wang, T.; Liu, J.; Liu, K.; Luan, Y. Engineered Adipose-Derived Stem Cells Overexpressing RXFP1 via CRISPR Activation Ameliorate Erectile Dysfunction in Diabetic Rats. Antioxidants 2023, 12, 171. https://doi.org/10.3390/antiox12010171
Sun T, Xu W, Tu B, Wang T, Liu J, Liu K, Luan Y. Engineered Adipose-Derived Stem Cells Overexpressing RXFP1 via CRISPR Activation Ameliorate Erectile Dysfunction in Diabetic Rats. Antioxidants. 2023; 12(1):171. https://doi.org/10.3390/antiox12010171
Chicago/Turabian StyleSun, Taotao, Wenchao Xu, Bocheng Tu, Tao Wang, Jihong Liu, Kang Liu, and Yang Luan. 2023. "Engineered Adipose-Derived Stem Cells Overexpressing RXFP1 via CRISPR Activation Ameliorate Erectile Dysfunction in Diabetic Rats" Antioxidants 12, no. 1: 171. https://doi.org/10.3390/antiox12010171
APA StyleSun, T., Xu, W., Tu, B., Wang, T., Liu, J., Liu, K., & Luan, Y. (2023). Engineered Adipose-Derived Stem Cells Overexpressing RXFP1 via CRISPR Activation Ameliorate Erectile Dysfunction in Diabetic Rats. Antioxidants, 12(1), 171. https://doi.org/10.3390/antiox12010171






