Abstract
The fetal systemic inflammatory response associated with intra-amniotic inflammation may play a key role in the pathogenesis of complications of preterm birth. Funisitis is the histologic equivalent of the fetal inflammatory response, whereas chorioamnionitis represents a maternal inflammatory response. We conducted a frequentist and Bayesian model average (BMA) meta-analysis of studies investigating the effects of funisitis on short-term outcomes of prematurity. Thirty-three studies (12,237 infants with gestational age ≤ 34 weeks) were included. Frequentist meta-analysis showed that funisitis was associated with an increased risk of any bronchopulmonary dysplasia (BPD), moderate/severe BPD, retinopathy of prematurity (ROP), intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), any sepsis, early-onset sepsis (EOS), and mortality. However, Bayesian meta-analysis showed that the evidence in favor of the alternative hypothesis (i.e., funisitis is associated with an increased risk of developing the outcome) was strong for any IVH, moderate for severe IVH and EOS, and weak for the other outcomes. When the control group was restricted to infants having chorioamnionitis without funisitis, the only outcome associated with funisitis was any IVH. In conclusion, our data suggest that the presence of funisitis does not add an additional risk to preterm birth when compared to chorioamnionitis in the absence of fetal inflammatory response.
1. Introduction
Preterm birth is a public health problem worldwide, with high rates of infant morbidity and mortality [1,2,3]. Intrauterine infection and/or inflammation are major contributors to the complications of very and extremely preterm birth (i.e., below 32 weeks of gestation) [4,5]. However, discerning between the part of the damage that is due to the infectious insult and the part related to the prenatal infection as a primary inducer of prematurity is a conundrum that continues to challenge perinatologists [4].
Invasion by microorganisms and subsequent intra-amniotic inflammation is generally ascending and progressive [4,6,7]. Histopathological chorioamnionitis is defined as diffuse infiltration of neutrophils into the chorioamniotic membranes and it is referred to as acute villitis if the villous tree is affected [4,6,7]. With progression of inflammation, immune cells infiltrate the umbilical cord (umbilical vessels and Wharton’s jelly), resulting in funisitis [4,6,7,8,9,10]. Therefore, whereas chorioamnionitis represents a maternal inflammatory response, funisitis is widely regarded as a fetal response and is considered the histological equivalent of the fetal inflammatory response syndrome [4,6,7,8,9,10].
The systemic inflammatory response is an initially adaptive mechanism of the fetus to infection, but, when unregulated, it can lead to increased injury in the preterm infant [11,12]. Oxidative stress plays a central role in this damage as the inflammatory process can cause a redox imbalance, increasing the release of free radicals and consuming antioxidant defenses [11,12]. Furthermore, very preterm newborns are particularly susceptible to oxidative stress because their antioxidant defenses are significantly lower compared to term infants [13,14]. In fact, oxidative stress is a major contributor to the pathogenesis of adverse outcomes of prematurity, including retinopathy of prematurity (ROP), necrotizing enterocolitis (NEC), bronchopulmonary dysplasia (BPD), intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), chronic neurodevelopmental and cognitive impairment, and death [13,14]. This has led to the grouping of most of the complications of very preterm birth under the umbrella term oxidative stress disease of prematurity [13,14].
In recent years, numerous observational studies and meta-analyses have investigated the association between chorioamnionitis and complications of prematurity. Some of these studies report data on funisitis and isolated chorioamnionitis, i.e., without funisitis. We hypothesized that the neonatal damage induced by the fetal inflammatory response (funisitis) is more severe than that produced when the inflammatory process is limited to the mother (chorioamnionitis without funisitis). We conducted a systematic review and meta-analysis to test this hypothesis. In addition, we analyzed possible differences in baseline characteristics, such as gestational age (GA), birth weight (BW), sex, or exposure to antenatal corticosteroids, between infants with and without funisitis.
2. Patients and Methods
The methodology of this study is based on that of earlier studies of our group on chorioamnionitis and complications of prematurity [15,16,17,18,19]. The study was performed and reported according to the Guidelines for Meta-Analyses and Systematic Reviews of Observational Studies (MOOSE). Review protocol was registered in the PROSPERO international register of systematic reviews (ID = CRD42020203205). The research question was “Is funisitis a risk factor for developing short-term complications (mortality, BPD, IVH, PVL, PDA, NEC, ROP, sepsis) in preterm infants compared to preterm infants without exposure to funisitis?”.
2.1. Sources and Search Strategy
A comprehensive literature search was undertaken using the PubMed and EMBASE databases. The search terms involved various combinations of the following key words: funisitis, fetal inflammation, fetal inflammatory response, chorioamnionitis, intrauterine infection, intrauterine inflammation, antenatal infection, antenatal inflammation, bronchopulmonary dysplasia, chronic lung disease, retinopathy of prematurity, necrotizing enterocolitis, intraventricular hemorrhage, periventricular leukomalacia, patent ductus arteriosus, sepsis, mortality, risk factors, outcome, cohort, and case-control. No language limit was applied. The literature search was updated up to March 2022. Narrative reviews, systematic reviews, case reports, letters, editorials, and commentaries were excluded, but read to identify potential additional studies. Additional strategies to identify studies included manual review of reference lists from key articles that fulfilled our eligibility criteria, use of “related articles” feature in PubMed, and use of the “cited by” tool in Web of Science and Google scholar.
2.2. Study Selection
The current study included observational studies reporting on the effects of funisitis in very and/or extreme preterm infants. Although the GA cutoff for this category of preterm infants is 32 weeks, studies that had an upper inclusion cutoff of 34 weeks were also included. Subsequently, a sensitivity analysis was performed to evaluate the effect of extending the inclusion limit to 34 weeks GA. Studies that included late preterm infants (GA ≥ 34 weeks) or that combined preterm and term infants were excluded. The primary outcome was short-term complications of prematurity, including mortality during first hospital admission, BPD, IVH, PVL, PDA, NEC, ROP, and sepsis.
2.3. Data Extraction, Definitions, and Risk of Bias Assessment
Data extracted included citation information, location of the research group, time period of study, study objectives, study design, inclusion and exclusion criteria, neonatal and maternal characteristics, data on funisitis, data on chorioamnionitis, and data on the different complications.
The main meta-analysis compared infants with funisitis (Fun+) vs. infants without funisitis (Fun−). In subsequent meta-analyses, the Fun− group was divided into two subgroups: (1) infants without funisitis but with chorioamnionitis (Fun−/CA+); and (2) infants who had neither funisitis nor chorioamnionitis (Fun−/CA−). Outcomes were categorized as follows: any BPD (defined as requiring >21% oxygen at the postnatal age of 28 days), moderate/severe BPD (defined as requiring >21% oxygen at the postmenstrual age of 36 weeks), severe BPD (defined as requiring >30% oxygen or mechanical ventilation at the postmenstrual age of 36 weeks), any ROP, severe ROP (type 1 pre-threshold ROP or ROP requiring treatment), any PDA, PDA requiring medical or surgical treatment, PDA requiring surgery, any PVL, cystic PVL, any NEC, NEC ≥ stage 2, any IVH (grade 1–4), severe IVH (grade ≥ 3), any sepsis, early-onset sepsis (EOS, defined as sepsis within 72 h of life), and late-onset sepsis (LOS, defined as sepsis after 72 h of life).
Risk of bias was assessed using the Newcastle–Ottawa Scale for cohort or case-control studies [20]. This scale assigns a maximum of 9 points (4 for selection, 2 for comparability, and 3 for exposure/outcome). Newcastle–Ottawa Scale scores ≥ 7 were considered as indicative of low risk of bias and scores of 5 to 6 as indicative of moderate risk of bias.
2.4. Statistical Analysis
2.4.1. Frequentist Meta-Analysis
Studies were combined and analyzed using COMPREHENSIVE META-ANALYSIS V3.0 software (Biostat Inc., Englewood, NJ, USA) [20]. Due to anticipated heterogeneity, summary statistics were calculated with a random-effects model. This model accounts for variability between studies as well as within studies [20,21]. Subgroup analyses were conducted according to the mixed-effects model. In this model, a random-effects model is used to combine studies within each subgroup, and a fixed-effect model is used to combine subgroups and yield the overall effect. The study-to-study variance (tau-squared) is not assumed to be the same for all subgroups. This value is computed within subgroups and not pooled across subgroups [20,21].
For dichotomous outcomes, the odds ratio (OR) with 95% confidence interval (CI) was calculated. For continuous outcomes, the mean difference (MD) or Hedges’ g with 95% CI were calculated. When studies reported continuous variables as median and range or interquartile range, we estimated the mean and standard deviation using the method of Wan et al. and the calculator they provided [22]. Statistical heterogeneity was assessed by Cochran’s Q statistic and by the I2 statistic. I2 was interpreted on the basis of Higgins and Thompson criteria, where 25%, 50%, and 75% correspond to low, moderate, and high heterogeneity, respectively [23]. Potential sources of heterogeneity were assessed through subgroup analysis and/or random-effects (method of moments) univariate meta-regression analysis, as previously described [24]. For continuous covariates (examples: difference in mean gestational age between infants exposed and unexposed to funisitis), we used meta-regression analyses to test whether there was a significant relationship between the covariate and effect size, as indicated by a Z-value and an associated p-value. Meta-regression coefficient indicates the change in the log of the OR of the association between mortality and the corresponding exposure for a unit change in the predictor covariate. Subgroups were compared using meta-regression for categorical covariates. For both categorical and continuous covariates, the R2 analog, defined as the total between-study variance explained by the moderator, was calculated based on the meta-regression matrix. We used the Egger’s regression test and funnel plots to assess publication bias. Subgroup analyses, meta-regression, and publication bias assessment were performed only when there were at least ten studies in the meta-analysis. A probability value of less than 0.05 (0.10 for heterogeneity) was considered statistically significant.
2.4.2. Bayesian Model Average Meta-Analysis
The results were further supplemented by a Bayesian model average (BMA) meta-analysis [25,26]. BMA employs Bayes factors (BFs) and Bayesian model averaging to evaluate the likelihood of the data under the combination of models assuming the presence vs. the absence of the meta-analytic effect and heterogeneity [25,26]. The BF10 is the ratio of the probability of the data under the alternative hypothesis (H1) over the probability of the data under the null hypothesis (H0). The BF10 was interpreted using the evidence categories suggested by Lee and Wagenmakers [27]: <1/100 = extreme evidence for H0; from 1/100 to <1/30 = very strong evidence for H0; from 1/30 to <1/10 = strong evidence for H0; from 1/10 to <1/3 = moderate evidence for H0; from 1/3 to <1 weak/inconclusive evidence for H0; from 1 to 3 = weak/inconclusive evidence for H1; from >3 to 10 = moderate evidence for H1; from >10 to 30 = strong evidence for H1; from >30 to 100 = very strong evidence for H1; and >100 = extreme evidence for H1. Consequently, BMA allows us to distinguish the absence of evidence from the evidence of absence [28]. The BFrf is the ratio of the probability of the data under the random-effects model over the probability of the data under the fixed-effects model. The BFrf was interpreted in the following way: <1/100 = extreme evidence for fixed effects; from 1/100 to <1/30 = very strong evidence for fixed effects; from 1/30 to <1/10 = strong evidence for fixed effects; from 1/10 to <1/3 = moderate evidence for fixed effects; from 1/3 to <1 weak/inconclusive evidence for fixed effects; from 1 to 3 = weak/inconclusive evidence for random effects; from >3 to 10 = moderate evidence for random effects; from >10 to 30 = strong evidence for random effects; from >30 to 100 = very strong evidence for random effects; and >100 = extreme evidence for random effects. We used the empirical prior distributions based on the Cochrane Database of Systematic Reviews transformed to logOR; logOR ~ Student-t (µ = 0, σ = 0.78, ν = 5), tau = Inverse-Gamma (k = 1.71, θ = 0.73) [25,26]. We performed the BMA in JASP [29], which utilizes the metaBMA R package [30].
3. Results
3.1. Description of Studies and Quality Assessment
The PRISMA flow diagram of the search process is shown in Supplementary Figure S1. Of 325 potentially relevant studies, 33 were included [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63]. These studies included 12,237 infants. Characteristics of the studies are summarized in Supplementary Table S1. Risk of bias assessment according to the Newcastle–Ottawa Scale is depicted in Supplementary Table S1. All studies received a score of at least six points, indicating a low to moderate risk of bias.
3.2. Main Meta-Analyses
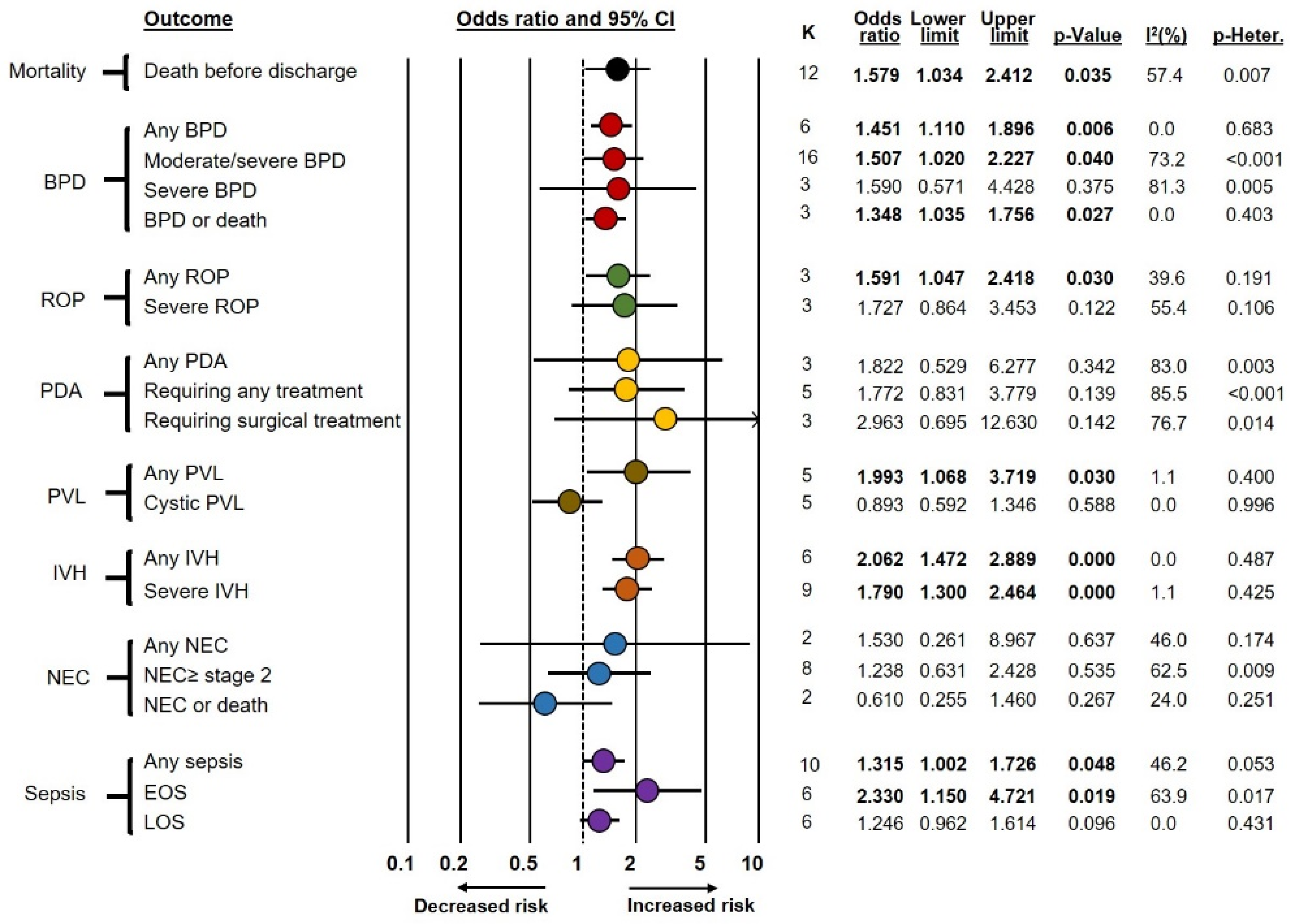
Frequentist meta-analyses on the association between funisitis and short-term complications of prematurity (Fun+ vs. Fun−) are summarized in Figure 1. These meta-analyses showed an association between funisitis and odds of developing any BPD (OR 1.45, CI 1.11–1.90), moderate/severe BPD (OR 1.51, CI 1.02–2.23), BPD or death (OR 1.35, CI 1.04–1.76), any ROP (OR 1.59, CI 1.05–2.42), any PVL (OR 1.99, CI 1.07–3.72), any IVH (OR 2.06, CI 1.47–2.89), severe IVH (OR 1.79, CI 1.30–2.46), any sepsis (OR 1.32, CI 1.00–1.73), EOS (OR 2.33, CI 1.15–4.72), and death before discharge (OR 1.58, CI 1.03–2.41). In contrast, funisitis was not significantly associated with severe BPD, severe ROP, PDA, cystic PVL, NEC, or LOS. As shown in Supplementary Table S2, exclusion of the seven studies that included infants with GA above 32 weeks but below 34 weeks [54,55,57,58,59,60,62] did not significantly affect the results of the meta-analysis.
Figure 1.
Summary of frequentist random-effects meta-analyses on the association between funisitis and short-term outcomes of prematurity. BPD: bronchopulmonary dysplasia; EOS: early-onset sepsis; IVH: intraventricular hemorrhage; K: number of studies included in the meta-analysis; LOS: late-onset sepsis; NEC: necrotizing enterocolitis; PDA: patent ductus arteriosus; PVL: periventricular leukomalacia; ROP: retinopathy of prematurity.
As shown in Table 1, BMA analysis demonstrated that in the Fun+ vs. Fun− comparison, the evidence in favor of the alternative hypothesis was strong (BF10 from >10 to 30) for any IVH; moderate (BF10 from >3 to 10) for severe IVH and EOS; and weak (BF10 from 1 to 3) for mortality, any BPD, moderate/severe BPD, any ROP, severe ROP, PDA requiring treatment, PDA requiring surgery, any PVL, and any sepsis. Conversely, the evidence in favor of the null hypothesis was moderate (BF10 from 1/10 to <1/3) for cystic PVL and weak (BF10 from 1/3 to <1) for severe BPD, BPD or death, any PDA, any NEC, NEC ≥ stage 2, NEC or death, and LOS. Data on heterogeneity of BMA analysis are depicted in Supplementary Table S3.

Table 1.
Bayesian model average (BMA) meta-analysis of the association between funisitis and outcomes of prematurity.
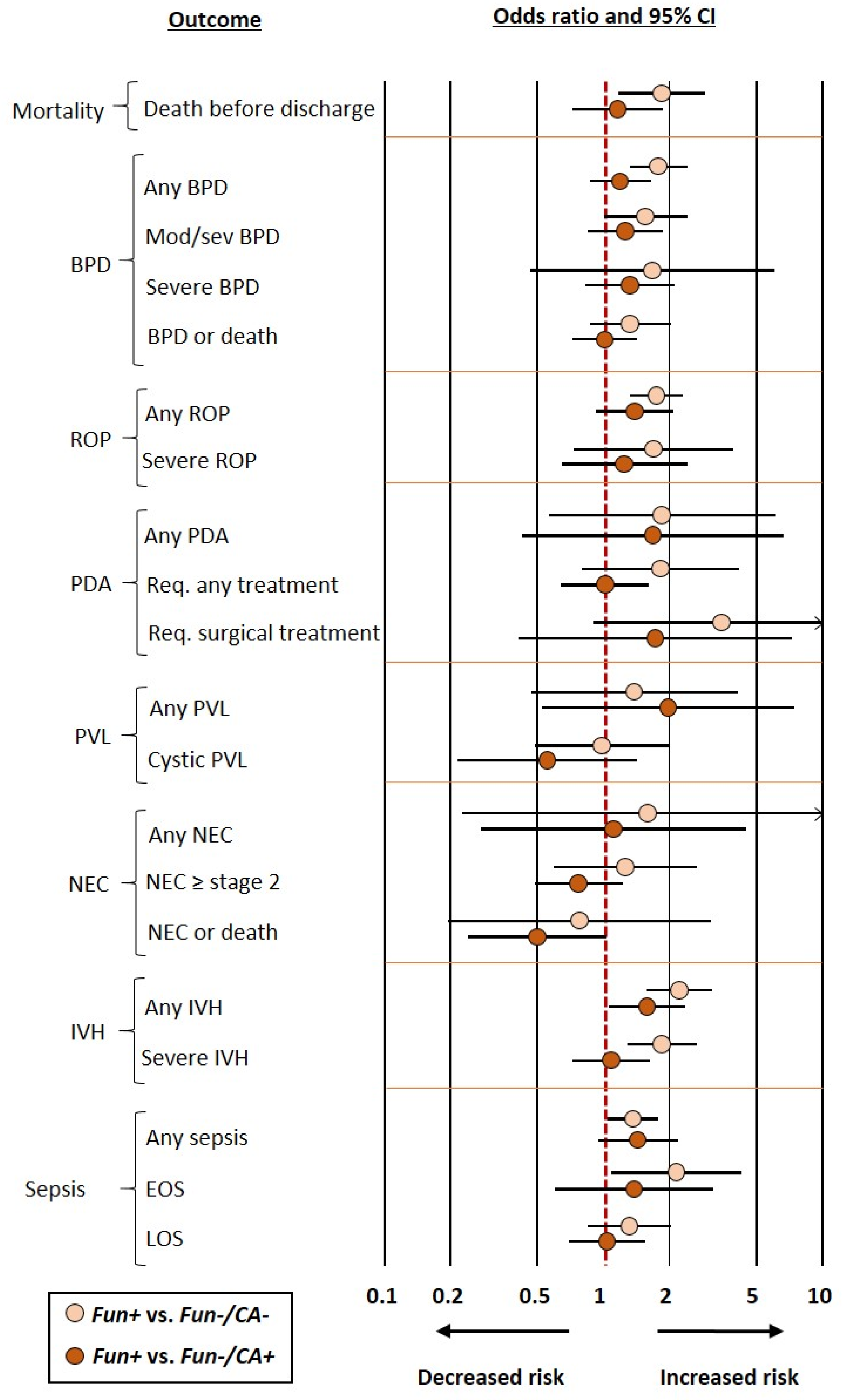
When the control group included infants who had neither funisitis nor chorioamnionitis (Fun−/CA− group), frequentist meta-analysis showed an association between funisitis and odds of developing any BPD, moderate/severe BPD, any ROP, any IVH, severe IVH, any sepsis, EOS, and death before discharge. In contrast, frequentist meta-analysis could not demonstrate a significant association for severe BPD, BPD or death, severe ROP, PDA, NEC, PVL, or LOS (Figure 2 and Table 2). BMA analysis of this comparison (Fun+ vs. Fun−/CA−) showed that the evidence in favor of the alternative hypothesis was strong (BF10 from >10 to 30) for any PBD, any IVH, and severe IVH; moderate (BF10 from >3 to 10) for mortality, any ROP, PDA requiring surgery, and EOS; and weak (BF10 from 1 to 3) for moderate/severe BPD, BPD or death, severe ROP, any PDA, and PDA requiring medical treatment. Conversely, the evidence in favor of the null hypothesis was moderate (BF10 from 1/10 to <1/3) for cystic PVL and weak (BF10 from 1/3 to <1) for severe BPD, any PVL, any NEC, NEC ≥ stage 2, NEC or death, and LOS (Table 1).

Table 2.
Meta-analyses comparing infants with funisitis vs. infants who had neither funisitis nor chorioamnionitis (Fun−/CA−) or infants without funisitis but with chorioamnionitis (Fun−/CA+).
When the control group included infants without funisitis but with chorioamnionitis (Fun−/CA+ group), the only outcome for which the positive association was maintained in the frequentist meta-analysis was any IVH (OR 1.58, CI 1.06–2.36) (Figure 2 and Table 2). BMA analysis showed that the evidence in favor of the alternative hypothesis was weak (BF10 from 1 to 3) for any IVH and NEC or death. Conversely, the evidence in favor of the null hypothesis was moderate (BF10 from 1/10 to <1/3) for mortality, BPD or death, PDA requiring medical treatment, PDA requiring surgery, severe IVH, and LOS; and weak for any BPD, moderate/severe BPD, severe BPD, any ROP, severe ROP, any PDA, any PVL, cystic PVL, any NEC, NEC ≥ stage 2, any sepsis, and EOS (Table 1).
Finally, we also conducted a meta-analysis including the three studies that differentiated grades or severities of funisitis [47,56,61]. As shown in Supplementary Table S4, this meta-analysis did not demonstrate an effect of funisitis grade on any of the outcomes.
Neither visual inspection of funnel plots (Supplementary Figure S2) nor Egger’s test suggested publication or selection bias for any of the meta-analyses that include ten or more studies. Publication bias was not analyzed for meta-analyses including less than ten studies.

Figure 2.
Summary of frequentist random-effects meta-analyses comparing infants with funisitis (Fun+) vs. infants who had neither funisitis nor chorioamnionitis (Fun−/CA−) or infants without funisitis but with chorioamnionitis (Fun−/CA+). BPD: bronchopulmonary dysplasia; EOS: early-onset sepsis; IVH: intraventricular hemorrhage; K: number of studies included in the meta-analysis; LOS: late-onset sepsis; NEC: necrotizing enterocolitis; PDA: patent ductus arteriosus; PVL: periventricular leukomalacia; Req.: requiring, ROP: retinopathy of prematurity.
Figure 2.
Summary of frequentist random-effects meta-analyses comparing infants with funisitis (Fun+) vs. infants who had neither funisitis nor chorioamnionitis (Fun−/CA−) or infants without funisitis but with chorioamnionitis (Fun−/CA+). BPD: bronchopulmonary dysplasia; EOS: early-onset sepsis; IVH: intraventricular hemorrhage; K: number of studies included in the meta-analysis; LOS: late-onset sepsis; NEC: necrotizing enterocolitis; PDA: patent ductus arteriosus; PVL: periventricular leukomalacia; Req.: requiring, ROP: retinopathy of prematurity.
3.3. Additional Meta-Analyses and Meta-Regression
We conducted additional meta-analyses to investigate possible differences in baseline characteristics between infants with and without funisitis. As shown in Table 3, infants in the Fun+ group had significantly lower GA and BW than infants in the Fun− group. In addition, infants in the Fun+ group were more frequently female, were less frequently small for GA or had fetal growth restriction, were less frequently exposed to hypertensive disorders of pregnancy, and were less frequently born by cesarean section than infants in the Fun− group. All these significant differences were maintained when the Fun+ group was compared with the Fun−/CA− group (Table 3). In addition, infants in the Fun−/CA− group showed a lower rate of exposure to antenatal corticosteroids than infants in the Fun+ group (Table 3). In contrast, when the Fun+ group was compared with the Fun−/CA+ group, only the association with sex remained significant. That is, the Fun+ group included significantly more females than the Fun−/CA+ group (Table 3). In addition, three studies reported interleukin (IL)-6 levels in cord blood of preterm infants with funisitis. These levels were markedly elevated in the Fun+ group when compared to the Fun− group, the Fun−/CA+ group, or the Fun−/CA− group (Supplementary Table S5).
As described in our previous meta-analyses on chorioamnionitis [15,16,17,18,19], we conducted a meta-regression analysis to investigate the possible correlation between the difference in GA between the Fun+ group and the Fun− group and the effect size of the association between funisitis and outcome. This meta-regression analysis was only performed for the three outcomes (mortality, moderate/severe BPD, and any sepsis) that were reported in ten or more studies. As shown in Supplementary Table S6, none of these meta-regressions showed statistically significant results. In addition, the meta-regression also showed no significant correlation between the effect size of the association between funisitis–outcome and sex differences or differences in rate of exposure to antenatal corticosteroids (Supplementary Table S6).

Table 3.
Meta-analyses for other covariates.
Table 3.
Meta-analyses for other covariates.
| Control Group | Meta-Analysis | K | Effect Size | 95% CI | p | Heterogeneity | ||
|---|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | I2 (%) | p | |||||
| Absence of funisitis (Fun−) | GA (MD in weeks) | 20 | −1.608 | −2.631 | −0.586 | 0.002 | 99.0 | <0.001 |
| Birth weight (MD in grams) | 20 | −101.6 | −164.6 | −38.6 | 0.002 | 87.8 | <0.001 | |
| Male sex (OR) | 19 | 0.822 | 0.727 | 0.928 | 0.002 | 4.1 | 0.407 | |
| Antenatal corticosteroids (OR) | 17 | 1.190 | 0.905 | 1.563 | 0.213 | 57.7 | 0.002 | |
| Caesarean delivery (OR) | 12 | 0.506 | 0.346 | 0.739 | <0.001 | 81.0 | <0.001 | |
| HDP (OR) | 11 | 0.077 | 0.035 | 0.168 | <0.001 | 76.2 | <0.001 | |
| SGA/IUGR (OR) | 8 | 0.195 | 0.067 | 0.564 | 0.003 | 86.3 | <0.001 | |
| Absence of funisitis and chorioamnionitis (Fun−/CA−) | GA (MD in weeks) | 17 | −1.374 | −1.931 | −0.817 | <0.001 | 95.5 | <0.001 |
| Birth weight (MD in grams) | 15 | −76.2 | −163.6 | 11.2 | 0.087 | 91.1 | <0.001 | |
| Male sex (OR) | 17 | 0.825 | 0.723 | 0.942 | 0.005 | 4.1 | 0.406 | |
| Antenatal corticosteroids (OR) | 14 | 1.420 | 1.098 | 1.837 | 0.008 | 44.2 | 0.038 | |
| Caesarean delivery (OR) | 12 | 0.378 | 0.232 | 0.616 | <0.001 | 87.1 | <0.001 | |
| HDP (OR) | 10 | 0.030 | 0.008 | 0.112 | <0.001 | 89.7 | <0.001 | |
| SGA/IUGR (OR) | 8 | 0.111 | 0.017 | 0.712 | 0.020 | 95.3 | <0.001 | |
| Absence of funisitis but presence of chorioamnionitis (Fun−/CA+) | GA (MD in weeks) | 15 | −0.251 | −0.525 | 0.023 | 0.072 | 50.0 | 0.014 |
| Birth weight (MD in grams) | 15 | −25.4 | −71.4 | 20.7 | 0.281 | 43.1 | 0.039 | |
| Male sex (OR) | 17 | 0.800 | 0.677 | 0.945 | 0.009 | 0.0 | 0.931 | |
| Antenatal corticosteroids (OR) | 15 | 0.955 | 0.703 | 1.298 | 0.768 | 30.2 | 0.129 | |
| Caesarean delivery (OR) | 12 | 0.944 | 0.754 | 1.181 | 0.612 | 8.4 | 0.363 | |
| HDP (OR) | 10 | 0.406 | 0.185 | 0.894 | 0.025 | 50.8 | 0.032 | |
| SGA/IUGR (OR) | 8 | 0.769 | 0.445 | 1.329 | 0.347 | 4.0 | 0.399 | |
Fun−: absence of funisitis regardless of the state of chorioamnionitis; Fun−/CA−: absence of funisitis and chorioamnionitis; Fun−/CA+: absence of funisitis but presence of chorioamnionitis; GA: gestational age; HDP: hypertensive disorders of pregnancy; K: number of studies; MD: mean difference (funisitis minus control group); OR: odds ratio; SGA/IUGR: small-for-gestational age/intrauterine growth restriction.
4. Discussion
To the best of our knowledge, this the most extensive and comprehensive systematic review and meta-analysis that investigated the impact of funisitis, as a proxy of the fetal inflammatory response, on the risk of developing short-term complications of very preterm birth. Frequentist meta-analysis showed that funisitis was associated with an increased risk of any BPD, moderate/severe BPD, ROP, IVH, any PVL, any sepsis, EOS, and death before discharge. However, Bayesian meta-analysis showed that the evidence in favor of the alternative hypothesis (i.e., funisitis is associated with increased risk of developing the outcome) was strong for any IVH, moderate for severe IVH and EOS, and weak for the other outcomes that were significant (p < 0.05) in the frequentist meta-analysis. Interestingly, when the control group consisted of infants exposed to maternal inflammation but without fetal inflammation (i.e., chorioamnionitis without funisitis), the only outcome that remained significantly associated with funisitis in the frequentist meta-analysis was any IVH. Moreover, the Bayesian meta-analysis showed that the evidence in favor of this association was weak (BF10 = 1.9). In addition, the Bayesian evidence in favor of an association between funisitis and complications, such as any BPD, severe IVH, any ROP, PDA requiring surgery, and EOS, becomes moderate-to-strong only when the control group consists of infants with neither fetal (i.e., funisitis) nor maternal (i.e., chorioamnionitis) inflammation. Therefore, our data suggest that the fetal inflammatory response does not add an additional risk to chorioamnionitis for most of the complications of prematurity. We speculate that that a significant part of the pathogenic effects of intrauterine infection/inflammation would be mediated by its role as a trigger of prematurity more than by the alterations induced in perinatal homeostasis.
The main limitation of our meta-analysis is the low number of studies reporting useful data to answer the research question. This is a very common problem in meta-analysis. In fact, a study published in 2011 showed that the median number of trials included in the meta-analyses from the Cochrane Database of Systematic Reviews was three, with an interquartile range from two to six [64]. Bayesian meta-analysis is increasingly being used to address the small-sample challenge [25,26]. In contrast to meta-analysis using frequentist statistics, Bayesian meta-analysis has the ability to quantify evidence in favor or against any hypothesis (including the null hypothesis), and to discriminate, therefore, between absence of evidence and evidence of absence [25,26,65,66]. This is a relevant advantage over the dichotomous interpretation of frequentist inference (significant vs. non-significant), particularly when p-values are close to the conventionally accepted limits (i.e., p < 0.05) [26,66,67]. This is clearly illustrated in Table 1 with the example of “any IVH”. When the control group consisted of infants without funisitis or chorioamnionitis (Fun−/CA−), the association between funisitis and any IVH in the frequentist analysis was “highly significant” (p < 0.001). When the control group consisted of infants without funisitis but with chorioamnionitis (Fun−/CA+), the p-value (=0.026) of the frequentist meta-analysis was higher but still significant. By evaluation of BF10-values, Bayesian meta-analysis allows us to interpret that when the Fun−/CA− group was the control, the data were 65.6 times more likely under the presence-of-effect hypothesis in comparison to the effect-absence hypothesis. In contrast, when the Fun−/CA+ group was the control, the data were only 1.9 times more likely under the presence-of-effect hypothesis in comparison to the effect-absence hypothesis.
Our study did not include a Fun+/CA− group because isolated funisitis (i.e., without chorioamnionitis) is very uncommon (<5%) in preterm placentas [68,69,70]. In contrast, isolated funisitis is described in 17% of term placentas [68,69,70]. When histological examination of a placenta demonstrates both chorioamnionitis and funisitis, it is straightforward that a progressive intrauterine or intra-amniotic infectious process has occurred [70]. However, the clinical significance of isolated funisitis is less clear [70]. Potential explanations for isolated funisitis include a systemic transplacental infection, which affects the fetus but not the membranes. Alternatively, a sampling bias may occur if chorioamnionitis does not involve the entire chorioamniotic membranes [68]. In addition, isolated funisitis in term infants may occur due to damage to the cord resulting from meconium [70]. Interestingly, isolated funisitis in preterm infants is not accompanied by increased levels of cytokines in umbilical cord blood [68].
That the fetal inflammatory response induced by intrauterine infection may influence the outcome of prematurity is a biologically plausible hypothesis. The possible mechanisms linking perinatal inflammation/infection and complications of prematurity include (i) increased cardiopulmonary instability at birth with consequent higher requirement for respiratory and cardiocirculatory support and (ii) the potential pathogenic effects of hypoxia, acidosis, cytokines and other inflammatory mediators, as well as the increased oxidative stress that accompanies infection and/or inflammation [71,72,73,74]. We confirmed these higher cytokine levels associated with funisitis (see Supplementary Table S5). Although this pathophysiological situation could theoretically affect any complication of prematurity, our data suggest that IVH is the outcome most strongly influenced by the presence of a fetal inflammatory response.
IVH generally occurs within the three first days of life and affects infants with higher hemodynamic and respiratory instability, frequently associated with extreme prematurity and/or severe perinatal infections [17,75,76]. Pro-inflammatory cytokines such as IL-6, IL-1β, and tumor necrosis factor (TNF)α can induce hemodynamic disturbances through direct vascular action or by the release of vasoactive mediators, like prostacyclin and nitric oxide [74]. In addition, cytokines can induce a neuro-inflammatory cascade in the fetal brain, which may promote platelet and neutrophil activation and adhesion leading to endothelial cell damage and changes in blood rheology and flow [77,78]. These changes, occurring inside the fragile germinal matrix capillaries or within the vascular connection between germinal matrix and the subependymal venous network, may increase the risk of developing IVH [17,75,76,77,78]. However, it is noteworthy that our findings from the present meta-analysis did not encompass the more severe forms of IVH.
We have attempted to evaluate the effect of funisitis on various complications of prematurity, but it should be noted that all of these complications are multifactorial in their pathogenesis. Oxidative stress is one of the key pathogenic factors, but the causes of oxidative stress are also diverse [79,80,81,82,83]. Low GA is the main risk factor for any complication of prematurity. The development of antioxidant defenses is inversely proportional to GA, but, in addition, the more preterm infants are also the sickest. Therefore, they will have a greater need for aggressive mechanical ventilation and/or oxygen supplementation due to their more severe respiratory insufficiency [79,80,81,82,83]. Moreover, many of these infants will likely experience episodic hypoxia due to their underlying lung disease and clinical instability. They will also be more frequently exposed to postnatal infections, which increase the levels of reactive oxygen species (ROS), and to transfusions of adult erythrocytes. The latter increase the capacity of supplying oxygen to the tissues and augment the risk of iron overload and ROS generation through the Fenton reaction [79,80,81,82,83]. Additional sources of oxidative stress are parenteral nutrition [84] or phototherapy, which reduces the potential antioxidant capacity of bilirubin [85].
As mentioned above, GA is the main prognostic factor in preterm birth and as it decreases, mortality and morbidity increase. However, there is a growing awareness that, beyond GA, the pathophysiological pathway leading to prematurity plays a very relevant role in the outcome of prematurity [86,87,88,89]. The two main pathophysiological pathways, also termed endotypes, leading to very and extremely preterm birth are infection/inflammation and placental dysfunction [86,87,88,89]. Chorioamnionitis is the prototypical example of the infectious/inflammatory endotype. Numerous meta-analyses have demonstrated a robust association between chorioamnionitis and complications of prematurity, including BPD [15,90], ROP [16,91], IVH [17], PVL [92,93], NEC [94], EOS [18], LOS [18], and PDA [19,95]. However, for most of these outcomes, with the exception of IVH [17] and EOS [18], the effect of chorioamnionitis was strongly related to the fact that infants in the control group had significantly higher GAs than those in the chorioamnionitis group [15,16,17,18,19]. It is a well-known fact that the incidence of chorioamnionitis increases as GA decreases, and therefore the majority of extremely preterm newborns belong to the infectious/inflammatory endotype [7].
Our present data further suggest that fetal invasion (i.e., funisitis) occurs more frequently at lower GAs. Therefore, an undetermined component of the pathologic effect of funisitis may be related to this higher degree of prematurity rather than to the inflammatory stress that generates in the fetus. Nevertheless, while in our previous meta-analysis on chorioamnionitis [15,16,17,18,19] meta-regression showed a highly significant correlation between the lower GA of the chorioamnionitis group and the various complications of prematurity, this result could not be reproduced for funisitis (see Supplementary Table S3). However, it should be noted that the number of studies that could be included in the meta-regression was quite limited. The recommendation is to conduct meta-regression studies from a minimum of 10 studies per examined covariate [96,97] and our meta-regressions were just above that limit.
In addition to GA, infants with funisitis differ from those without funisitis in characteristics that may be critically relevant to the prognosis of prematurity, such as sex, exposure to antenatal corticosteroids, mode of birth, or exposure to other pregnancy complications. Some of these findings are not surprising since pregnancies complicated by the infectious/inflammatory endotype are less likely to be associated with fetal growth retardation, hypertensive disorders of pregnancy, or birth by cesarean section than pregnancies complicated by the placental dysfunction endotype [87,88,89,98]. However, an interesting finding of our meta-analysis is that funisitis was negatively associated with male sex. Although some studies had reported such sex differences [58,99], the finding was not consistent and there are cohorts in the literature in which male sex was associated with increased risk of funisitis as compared with female sex [100]. Interestingly, evidence from pre-clinical and clinical studies suggests that the male fetus exists in a relatively more pro-inflammatory environment than the female fetus [101]. As Challis et al., have pointed out, there is a growing need to consider fetal sex in studies of placental function and pathology [101]. In a recent meta-analysis, in which we investigated sex differences in pregnancy complications and outcomes of very preterm birth, we observed no difference in chorioamnionitis risk depending on fetal sex [102]. In contrast, hypertensive disorders of pregnancy were more frequent when the fetus was female [102]. There is a large body of evidence showing that boys are more susceptible than girls to adverse outcomes of prematurity, including BPD, ROP, NEC, IVH, chronic neurodevelopmental and cognitive impairment, and death [102,103,104]. Our group is currently conducting a meta-analysis focused on evaluating the possible effects of fetal sex on the different endotypes of prematurity and how these potential sex differences contribute to the male-female differences in prematurity outcome.
Antenatal corticosteroids in case of anticipated preterm delivery reduce infant mortality and morbidity and are the standard of care in current perinatal practice [105]. Our meta-analysis shows that the rate of use of antenatal corticosteroids is higher in preterm infants with funisitis when compared with infants who had neither funisitis nor chorioamnionitis (Fun−/CA− group). In a previous meta-analysis, we already observed that chorioamnionitis was associated with a higher rate of antenatal corticosteroid use. Paradoxically, clinical chorioamnionitis was considered for a time as a contraindication, at least relative, for the use of antenatal corticosteroids [106,107]. However, there is strong evidence that the positive effect of antenatal corticosteroids also applies to preterm infants with chorioamnionitis [108,109]. We speculate that the more frequent use of antenatal corticosteroids in the infectious/inflammatory endotype may be related to the availability of more time to prepare the fetus for preterm birth compared to the time available, for example, in hypertensive disorders of pregnancy that more often require an emergency cesarean section.
Our meta-analysis has several limitations that should be taken into account. First, as mentioned above, the number of studies for many of the outcomes analyzed was low and some of the meta-analyses had moderate or high heterogeneity. We attempted to overcome these limitations by means of the BMA meta-analysis. In addition, only three studies [47,56,61] reported a gradation of funisitis. Moreover, several studies did not report a clear definition of some of the outcomes, which made classification difficult. On the other hand, the main strength of the present study is the use of rigorous methods, including an extensive and comprehensive search and meta-analysis of baseline and secondary characteristics of the infants included in the studies.
5. Conclusions
In conclusion, our data suggest that funisitis is associated with an increased risk of relevant complications of prematurity, such as BPD, ROP, IVH, PVL, EOS, or death before discharge. However, infants with funisitis are also younger than infants without funisitis and part of the increased risk of complications may be related to this higher degree of prematurity. Moreover, with the exception of IVH, the presence of a fetal inflammatory response does not appear to add additional risk of complications when compared to chorioamniotic membrane inflammation without fetal involvement.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/antiox12020534/s1: Figure S1. PRISMA Diagram; Figure S2. Publication bias; Table S1. Characteristics of the included studies and risk of bias assessment; Table S2. Meta-analysis on association between funisitis and short-term outcomes of prematurity in infants with gestational age up to 32 weeks; Table S3. Data on heterogeneity of the Bayesian model average (BMA) meta-analysis of the association between funisitis and outcomes of prematurity; Table S4. Meta-analysis on association between stages of funisitis and short-term outcomes of prematurity; Table S5. Meta-analysis on association between funisitis and levels of interleukin-6 in umbilical cord blood; Table S6. Meta-regression of the correlation between different covariates and the odds ratio of the association of funisitis with outcome of prematurity.
Author Contributions
E.V. conceived and designed the study, with input from the other authors. T.M.H., M.J.H. and E.V.-M. designed and executed the literature search, screened and reviewed the search results, and abstracted the data. T.M.H. and E.V. checked data extraction for accuracy and completeness. T.M.H. assessed the quality of the included studies. T.M.H. conducted the frequentist analysis, with input from the other authors. F.B. designed and conducted the Bayesian analysis, with input from E.V. and T.M.H. All authors contributed to the interpretation of analyses. T.M.H. and E.V. made the figures and tables. T.M.H. and E.V. drafted the manuscript, with input from the other authors. All authors reviewed the manuscript and provided important intellectual content. T.M.H. and E.V. take responsibility for the article as a whole. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
As this systematic review and meta-analysis did not involve animal subjects or personally identifiable information on human subjects, ethics review board approval was not required.
Informed Consent Statement
As this systematic review and meta-analysis did not involve animal subjects or personally identifiable information on human subjects, patient consent was not required.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as Supplementary Information. Additional data are available upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest. The views expressed in this paper are those of the authors and do not necessarily reflect the policies of Statistics Netherlands.
References
- Blencowe, H.; Cousens, S.; Chou, D.; Oestergaard, M.; Say, L.; Moller, A.B.; Kinney, M.; Lawn, J. Born too soon: The global epidemiology of 15 million preterm births. Reprod. Health 2013, 10, S2. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.S.; Goldenberg, R.L. Global burden of prematurity. Semin. Fetal Neonatal Med. 2016, 21, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Lawn, J.E.; Gravett, M.G.; Nunes, T.M.; Rubens, C.E.; Stanton, C. Global report on preterm birth and stillbirth (1 of 7): Definitions, description of the burden and opportunities to improve data. BMC Pregnancy Childbirth 2010, 10, S1. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.G.; Willis, K.A.; Jobe, A.; Ambalavanan, N. Chorioamnionitis and neonatal outcomes. Pediatr. Res. 2022, 91, 289–296. [Google Scholar] [CrossRef]
- Pietrasanta, C.; Pugni, L.; Merlo, D.; Acaia, B.; Consonni, D.; Ronchi, A.; Ossola, M.W.; Ghirardi, B.; Bottino, I.; Cribiù, F.M. Impact of different stages of intrauterine inflammation on outcome of preterm neonates: Gestational age-dependent and-independent effect. PLoS ONE 2019, 14, e0211484. [Google Scholar] [CrossRef]
- Pacora, P.; Chaiworapongsa, T.; Maymon, E.; Kim, Y.; Gomez, R.; Yoon, B.; Ghezzi, F.; Berry, S.; Qureshi, F.; Jacques, S. Funisitis and chorionic vasculitis: The histological counterpart of the fetal inflammatory response syndrome. J. Matern. Fetal Neonatal Med. 2002, 11, 18–25. [Google Scholar] [CrossRef]
- Kim, C.J.; Romero, R.; Chaemsaithong, P.; Chaiyasit, N.; Yoon, B.H.; Kim, Y.M. Acute chorioamnionitis and funisitis: Definition, pathologic features, and clinical significance. Am. J. Obstet. Gynecol. 2015, 213, S29–S52. [Google Scholar] [CrossRef]
- Boettcher, L.B.; Clark, E.A. Neonatal and childhood outcomes following preterm premature rupture of membranes. Obstet. Gynecol. Clin. 2020, 47, 671–680. [Google Scholar] [CrossRef]
- Lee, S.E.; Romero, R.; Jung, H.; Park, C.-W.; Park, J.S.; Yoon, B.H. The intensity of the fetal inflammatory response in intraamniotic inflammation with and without microbial invasion of the amniotic cavity. Am. J. Obstet. Gynecol. 2007, 197, e291–e296. [Google Scholar] [CrossRef]
- Kamei, M.; Hussein, M.H.; Hattori, A.; Saleh, M.; Kakita, H.; Abdel-Hamid Daoud, G.; Ishiguro, A.; Namba, F.; Yazaki, M.; Goto, H. Oxidative and Inflammatory Markers Are Higher in Full-Term Newborns Suffering Funisitis, and Higher Oxidative Markers Are Associated with Admission. Children 2022, 9, 702. [Google Scholar] [CrossRef]
- Jung, E.; Romero, R.; Yeo, L.; Diaz-Primera, R.; Marin-Concha, J.; Para, R.; Lopez, A.M.; Pacora, P.; Gomez-Lopez, N.; Yoon, B.H. The fetal inflammatory response syndrome: The origins of a concept, pathophysiology, diagnosis, and obstetrical implications. Semin. Fetal Neonatal Med. 2020, 25, 101146. [Google Scholar] [CrossRef]
- Gantert, M.; Been, J.; Gavilanes, A.; Garnier, Y.; Zimmermann, L.; Kramer, B. Chorioamnionitis: A multiorgan disease of the fetus? J. Perinatol. 2010, 30, S21–S30. [Google Scholar] [CrossRef]
- Perez, M.; Robbins, M.E.; Revhaug, C.; Saugstad, O.D. Oxygen radical disease in the newborn, revisited: Oxidative stress and disease in the newborn period. Free Radic. Biol. Med. 2019, 142, 61–72. [Google Scholar] [CrossRef]
- Saugstad, O.D. Oxidative stress in the newborn—A 30-year perspective. Neonatology 2005, 88, 228–236. [Google Scholar] [CrossRef]
- Villamor-Martinez, E.; Alvarez-Fuente, M.; Ghazi, A.M.; Degraeuwe, P.; Zimmermann, L.J.; Kramer, B.W.; Villamor, E. Association of chorioamnionitis with bronchopulmonary dysplasia among preterm infants: A systematic review, meta-analysis, and metaregression. JAMA Netw. Open 2019, 2, e1914611. [Google Scholar] [CrossRef]
- Villamor-Martinez, E.; Cavallaro, G.; Raffaeli, G.; Mohammed Rahim, O.M.; Gulden, S.; Ghazi, A.M.; Mosca, F.; Degraeuwe, P.; Villamor, E. Chorioamnionitis as a risk factor for retinopathy of prematurity: An updated systematic review and meta-analysis. PLoS ONE 2018, 13, e0205838. [Google Scholar] [CrossRef]
- Villamor-Martinez, E.; Fumagalli, M.; Mohammed Rahim, O.; Passera, S.; Cavallaro, G.; Degraeuwe, P.; Mosca, F.; Villamor, E. Chorioamnionitis is a risk factor for intraventricular hemorrhage in preterm infants: A systematic review and meta-analysis. Front. Physiol. 2018, 9, 1253. [Google Scholar] [CrossRef]
- Villamor-Martinez, E.; Lubach, G.A.; Rahim, O.M.; Degraeuwe, P.; Zimmermann, L.J.; Kramer, B.W.; Villamor, E. Association of histological and clinical chorioamnionitis with neonatal sepsis among preterm infants: A systematic review, meta-analysis, and meta-regression. Front. Immunol. 2020, 11, 972. [Google Scholar] [CrossRef]
- Behbodi, E.; Villamor-Martínez, E.; Degraeuwe, P.L.; Villamor, E. Chorioamnionitis appears not to be a risk factor for patent ductus arteriosus in preterm infants: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 37967. [Google Scholar] [CrossRef]
- Borenstein, M. Comprehensive meta-analysis software. In Systematic Reviews in Health Research: Meta-Analysis in Context; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2022; pp. 535–548. [Google Scholar] [CrossRef]
- Borenstein, M.; Higgins, J. Meta-analysis and subgroups. Prev. Sci. 2013, 14, 134–143. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Bartoš, F.; Gronau, Q.F.; Timmers, B.; Otte, W.M.; Ly, A.; Wagenmakers, E.J. Bayesian model-averaged meta-analysis in medicine. Stat. Med. 2021, 40, 6743–6761. [Google Scholar] [CrossRef] [PubMed]
- Gronau, Q.F.; Heck, D.W.; Berkhout, S.W.; Haaf, J.M.; Wagenmakers, E.-J. A primer on Bayesian model-averaged meta-analysis. Adv. Methods Pract. Psychol. Sci. 2021, 4, 25152459211031256. [Google Scholar] [CrossRef]
- Lee, M.; Wagenmakers, E.J. Bayesian Data Analysis for Cognitive Science: A Practical Course; Cambridge University Press: New York, NY, USA, 2013. [Google Scholar]
- Altman, D.G.; Bland, J.M. Statistics notes: Absence of evidence is not evidence of absence. BMJ 1995, 311, 485. [Google Scholar] [CrossRef]
- JASP, version 0.17; JASP Team: Amsterdam, The Netherlands, 2023.
- Heck, D.; Gronau, F.; Wagenmakers, E. metaBMA: Bayesian Model Averaging for Random and Fixed Effects Meta-Analysis, Version 0.6.7; JASP Team: Amsterdam, The Netherlands, 2019.
- Babnik, J.; Stucin-Gantar, I.; Kornhauser-Cerar, L.; Sinkovec, J.; Wraber, B.; Derganc, M. Intrauterine inflammation and the onset of peri-intraventricular hemorrhage in premature infants. Biol. Neonate 2006, 90, 113–121. [Google Scholar] [CrossRef]
- Been, J.V.; Rours, I.G.; Kornelisse, R.F.; De Krijger, R.R.; Kramer, B.W.; Zimmermann, L.J. Chorioamnionitis alters the response to surfactant in preterm infants. Neonatology 2009, 95, 375–376. [Google Scholar] [CrossRef]
- Dessardo, N.S.; Mustac, E.; Dessardo, S.; Banac, S.; Peter, B.; Finderle, A.; Mari, M.; Haller, H. Chorioamnionitis and chronic lung disease of prematurity: A path analysis of causality. Am. J. Perinatol. 2012, 29, 133–140. [Google Scholar] [CrossRef]
- Fujimura, M.; Takeuchi, T.; Kitajima, H.; Nakayama, M. Chorioamnionitis and serum IgM in Wilson-Mikity syndrome. Arch. Dis. Child. 1989, 64, 1379–1383. [Google Scholar] [CrossRef]
- Ikeda, S.; Kihira, K.; Yokoi, A.; Tamakoshi, K.; Miyazaki, K.; Furuhashi, M. The levels of the neutrophil elastase in the amniotic fluid of pregnant women whose infants develop bronchopulmonary dysplasia. J. Matern. Fetal Neonatal Med. 2015, 28, 479–483. [Google Scholar] [CrossRef]
- Jackson, C.M.; Wells, C.B.; Tabangin, M.E.; Meinzen-Derr, J.; Jobe, A.H.; Chougnet, C.A. Pro-inflammatory immune responses in leukocytes of premature infants exposed to maternal chorioamnionitis or funisitis. Pediatr. Res. 2017, 81, 384–390. [Google Scholar] [CrossRef]
- Kelly, M.; Vignes, K.; Cockerham, C.; Su, L.; Stromberg, A.J.; Huang, H.; Schanbacher, B.; Bauer, J.A.; Giannone, P.; O’Brien, J. Cord blood CRP: Preferred biomarker to histologic chorioamnionitis for neonatal outcomes in early preterm infants. Am. J. Obstet. Gynecol. 2022, 226, S94. [Google Scholar] [CrossRef]
- Kent, A.; Dahlstrom, J.E. Chorioamnionitis/funisitis and the development of bronchopulmonary dysplasia. J. Paediatr. Child Health 2004, 40, 356–359. [Google Scholar] [CrossRef]
- Kent, A.; Lomas, F.; Hurrion, E.; Dahlstrom, J.E. Antenatal steroids may reduce adverse neurological outcome following chorioamnionitis: Neurodevelopmental outcome and chorioamnionitis in premature infants. J. Paediatr. Child Health 2005, 41, 186–190. [Google Scholar] [CrossRef]
- Lahra, M.M.; Beeby, P.J.; Jeffery, H.E. Intrauterine inflammation, neonatal sepsis, and chronic lung disease: A 13-year hospital cohort study. Pediatrics 2009, 123, 1314–1319. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, K.H.; Kim, A.; Yang, H.R.; Jung, E.Y.; Cho, S.H. Maternal and Placental Risk Factors for Developing Necrotizing Enterocolitis in Very Preterm Infants. Pediatr. Neonatol. 2017, 58, 57–62. [Google Scholar] [CrossRef]
- Lynch, A.M.; Berning, A.A.; Thevarajah, T.S.; Wagner, B.D.; Post, M.D.; McCourt, E.A.; Cathcart, J.N.; Hodges, J.K.; Mandava, N.; Gibbs, R.S.; et al. The role of the maternal and fetal inflammatory response in retinopathy of prematurity. Am. J. Reprod. Immunol. 2018, 80, e12986. [Google Scholar] [CrossRef]
- Pavcnik-Arnol, M.; Lucovnik, M.; Kornhauser-Cerar, L.; Premru-Srsen, T.; Hojker, S.; Derganc, M. Lipopolysaccharide-binding protein as marker of fetal inflammatory response syndrome after preterm premature rupture of membranes. Neonatology 2014, 105, 121–127. [Google Scholar] [CrossRef]
- Perniciaro, S.; Casarin, J.; Nosetti, L.; Binda, C.; Salvatore, S.; Ghezzi, F.; Agosti, M. Early- and Late-Respiratory Outcome in Very Low Birth Weight with or without Intrauterine Inflammation. Am. J. Perinatol. 2020, 37, S76–S83. [Google Scholar] [CrossRef]
- Plakkal, N.; Soraisham, A.S.; Trevenen, C.; Freiheit, E.A.; Sauve, R. Histological chorioamnionitis and bronchopulmonary dysplasia: A retrospective cohort study. J. Perinatol. 2013, 33, 441–445. [Google Scholar] [CrossRef]
- Puri, K.; Taft, D.H.; Ambalavanan, N.; Schibler, K.R.; Morrow, A.L.; Kallapur, S.G. Association of Chorioamnionitis with Aberrant Neonatal Gut Colonization and Adverse Clinical Outcomes. PLoS ONE 2016, 11, e0162734. [Google Scholar] [CrossRef] [PubMed]
- Salas, A.A.; Faye-Petersen, O.M.; Sims, B.; Peralta-Carcelen, M.; Reilly, S.D.; McGwin, G., Jr.; Carlo, W.A.; Ambalavanan, N. Histological characteristics of the fetal inflammatory response associated with neurodevelopmental impairment and death in extremely preterm infants. J. Pediatr. 2013, 163, e651–e652. [Google Scholar] [CrossRef] [PubMed]
- Smit, A.L.; Been, J.V.; Zimmermann, L.J.; Kornelisse, R.F.; Andriessen, P.; Vanterpool, S.F.; Bischoff, M.P.; Stokroos, R.J.; de Krijger, R.R.; Kremer, B.; et al. Automated auditory brainstem response in preterm newborns with histological chorioamnionitis. J. Matern. Fetal Neonatal Med. 2015, 28, 1864–1869. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.; Seidenspinner, S.; Kawczyńska-Leda, N.; Wirbelauer, J.; Szymankiewicz, M.; Speer, C.P. Soluble receptor for advanced glycation end products (sRAGE) in tracheobronchial aspirate fluid and cord blood of very low birth weight infants with chorioamnionitis and funisitis. Early Hum. Dev. 2010, 86, 593–598. [Google Scholar] [CrossRef]
- Torchin, H.; Lorthe, E.; Goffinet, F.; Kayem, G.; Subtil, D.; Truffert, P.; Devisme, L.; Benhammou, V.; Jarreau, P.H.; Ancel, P.Y. Histologic Chorioamnionitis and Bronchopulmonary Dysplasia in Preterm Infants: The Epidemiologic Study on Low Gestational Ages 2 Cohort. J. Pediatr. 2017, 187, 98–104.e3. [Google Scholar] [CrossRef]
- Trevisanuto, D.; Peruzzetto, C.; Cavallin, F.; Vedovato, S.; Cosmi, E.; Visentin, S.; Chiarelli, S.; Zanardo, V. Fetal placental inflammation is associated with poor neonatal growth of preterm infants: A case-control study. J. Matern. Fetal Neonatal Med. 2013, 26, 1484–1490. [Google Scholar] [CrossRef]
- Woo, S.J.; Jung, H.J.; Kim, S.N.; Choe, G.; Ahn, J.; Park, K.H. Effects of maternal and placental inflammation on retinopathy of prematurity. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 915–923. [Google Scholar] [CrossRef]
- Andersen, C.; Kent, A.; Schmidt, B.; Nahmias, C.; deSa, D.; Bourgeois, J.; Xing, Z.; Kirpalani, H. Pulmonary fluorodeoxyglucose uptake in infants of very low birth weight with and without intrauterine inflammation. J. Pediatr. 2003, 143, 470–476. [Google Scholar] [CrossRef]
- Hong, S.; Jeong, M.; Oh, S.; Oh, J.W.; Park, C.W.; Park, J.S.; Jun, J.K.; Lee, S.M. Funisitis as a Risk Factor for Adverse Neonatal Outcomes in Twin Neonates with Spontaneous Preterm Birth: A Retrospective Cohort Study. Yonsei Med. J. 2021, 62, 822–828. [Google Scholar] [CrossRef]
- Lau, J.; Magee, F.; Qiu, Z.; Houbé, J.; Von Dadelszen, P.; Lee, S.K. Chorioamnionitis with a fetal inflammatory response is associated with higher neonatal mortality, morbidity, and resource use than chorioamnionitis displaying a maternal inflammatory response only. Am. J. Obstet. Gynecol. 2005, 193, 708–713. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, H.J.; Choi, S.J.; Oh, S.Y.; Kim, J.S.; Roh, C.R.; Kim, J.H. Is there a stepwise increase in neonatal morbidities according to histological stage (or grade) of acute chorioamnionitis and funisitis? Effect of gestational age at delivery. J. Perinat. Med. 2015, 43, 259–267. [Google Scholar] [CrossRef]
- Liu, Z.; Tang, Z.; Li, J.; Yang, Y. Effects of placental inflammation on neonatal outcome in preterm infants. Pediatr. Neonatol. 2014, 55, 35–40. [Google Scholar] [CrossRef]
- Richardson, B.S.; Wakim, E.; daSilva, O.; Walton, J. Preterm histologic chorioamnionitis: Impact on cord gas and pH values and neonatal outcome. Am. J. Obstet. Gynecol. 2006, 195, 1357–1365. [Google Scholar] [CrossRef]
- Rocha, G.; Proença, E.; Quintas, C.; Rodrigues, T.; Guimarães, H. Chorioamnionitis and brain damage in the preterm newborn. J. Matern. Fetal Neonatal Med. 2007, 20, 745–749. [Google Scholar] [CrossRef]
- Thorell, A.; Hallingstrom, M.; Hagberg, H.; Fyhr, I.M.; Tsiartas, P.; Olsson, I.; Chaplin, J.E.; Mallard, C.; Jacobsson, B.; Savman, K. Microbial invasion of the amniotic cavity is associated with impaired cognitive and motor function at school age in preterm children. Pediatr. Res. 2020, 87, 924–931. [Google Scholar] [CrossRef]
- Yamada, N.; Sato, Y.; Moriguchi-Goto, S.; Yamashita, A.; Kodama, Y.; Sameshima, H.; Asada, Y. Histological severity of fetal inflammation is useful in predicting neonatal outcome. Placenta 2015, 36, 1490–1493. [Google Scholar] [CrossRef]
- Graham, E.M.; Holcroft, C.J.; Rai, K.K.; Donohue, P.K.; Allen, M.C. Neonatal cerebral white matter injury in preterm infants is associated with culture positive infections and only rarely with metabolic acidosis. Am. J. Obstet. Gynecol. 2004, 191, 1305–1310. [Google Scholar] [CrossRef]
- Wharton, K.N.; Pinar, H.; Stonestreet, B.S.; Tucker, R.; McLean, K.R.; Wallach, M.; Vohr, B.R. Severe umbilical cord inflammation—A predictor of periventricular leukomalacia in very low birth weight infants. Early Hum. Dev. 2004, 77, 77–87. [Google Scholar] [CrossRef]
- Davey, J.; Turner, R.M.; Clarke, M.J.; Higgins, J. Characteristics of meta-analyses and their component studies in the Cochrane Database of Systematic Reviews: A cross-sectional, descriptive analysis. BMC Med. Res. Methodol. 2011, 11, 160. [Google Scholar] [CrossRef]
- Pacheco, R.L.; Fontes, L.E.S.; Martimbianco, A.L.C.; Riera, R. Better understanding of ‘don’t confuse absence of evidence with evidence of absence’. J. Evid. Based Healthc. 2019, 1, 99–102. [Google Scholar] [CrossRef]
- Wagenmakers, E.-J.; Lee, M.; Lodewyckx, T.; Iverson, G.J. Bayesian versus frequentist inference. In Bayesian Evaluation of Informative Hypotheses; Springer: New York, NY, USA, 2008; pp. 181–207. [Google Scholar] [CrossRef]
- Held, L.; Ott, M. On p-values and Bayes factors. Annu. Rev. Stat. Appl. 2018, 5, 393–419. [Google Scholar] [CrossRef]
- Yoon, B.H.; Romero, R.; Park, J.S.; Kim, M.; Oh, S.-Y.; Kim, C.J.; Jun, J.K. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am. J. Obstet. Gynecol. 2000, 183, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Romero, R.; Kim, C.J.; Shim, S.-S.; Yoon, B.H. Funisitis in term pregnancy is associated with microbial invasion of the amniotic cavity and intra-amniotic inflammation. J. Matern. Fetal Neonatal Med. 2006, 19, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Grossman, T.B.; Heller, D.S.; Baergen, R.N. Isolated acute funisitis in the absence of acute chorioamnionitis: What does it mean? Placenta 2019, 75, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Tambor, V.; Kacerovsky, M.; Lenco, J.; Bhat, G.; Menon, R. Proteomics and bioinformatics analysis reveal underlying pathways of infection associated histologic chorioamnionitis in pPROM. Placenta 2013, 34, 155–161. [Google Scholar] [CrossRef]
- Kramer, B.W.; Kallapur, S.G.; Jobe, A.H. Chorioamnionitis and Oxidative Stress: New Ideas from Experimental Models. In Perinatal and Prenatal Disorders; Springer: Berlin/Heidelberg, Germany, 2014; pp. 39–46. [Google Scholar] [CrossRef]
- Ilekis, J.V.; Tsilou, E.; Fisher, S.; Abrahams, V.M.; Soares, M.J.; Cross, J.C.; Zamudio, S.; Illsley, N.P.; Myatt, L.; Colvis, C. Placental origins of adverse pregnancy outcomes: Potential molecular targets: An Executive Workshop Summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Am. J. Obstet. Gynecol. 2016, 215, S1–S46. [Google Scholar] [CrossRef]
- Yanowitz, T.D.; Jordan, J.A.; Gilmour, C.H.; Towbin, R.; Bowen, A.D.; Roberts, J.M.; Brozanski, B.S. Hemodynamic disturbances in premature infants born after chorioamnionitis: Association with cord blood cytokine concentrations. Pediatr. Res. 2002, 51, 310. [Google Scholar] [CrossRef]
- Inder, T.E.; Perlman, J.M.; Volpe, J.J. Preterm Intraventricular Hemorrhage/Posthemorrhagic Hydrocephalus. In Volpe’s Neurology of the Newborn, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 637–698. [Google Scholar]
- Mohamed, M.A.; Aly, H. Transport of premature infants is associated with increased risk for intraventricular haemorrhage. Arch. Dis. Child. Fetal Neonatal Ed. 2010, 95, F403–F407. [Google Scholar] [CrossRef]
- Molina-Holgado, E.; Molina-Holgado, F. Mending the broken brain: Neuroimmune interactions in neurogenesis. J. Neurochem. 2010, 114, 1277–1290. [Google Scholar] [CrossRef]
- Stanimirovic, D.; Satoh, K. Inflammatory mediators of cerebral endothelium: A role in ischemic brain inflammation. Brain Pathol. 2000, 10, 113–126. [Google Scholar] [CrossRef]
- Kimble, A.; Robbins, M.E.; Perez, M. Pathogenesis of Bronchopulmonary Dysplasia: Role of Oxidative Stress from ‘Omics’ Studies. Antioxidants 2022, 11, 2380. [Google Scholar] [CrossRef]
- Lembo, C.; Buonocore, G.; Perrone, S. Oxidative stress in preterm newborns. Antioxidants 2021, 10, 1672. [Google Scholar] [CrossRef]
- Graziosi, A.; Perrotta, M.; Russo, D.; Gasparroni, G.; D’Egidio, C.; Marinelli, B.; Di Marzio, G.; Falconio, G.; Mastropasqua, L.; Li Volti, G. Oxidative stress markers and the retinopathy of prematurity. J. Clin. Med. 2020, 9, 2711. [Google Scholar] [CrossRef]
- Silvestro, S.; Calcaterra, V.; Pelizzo, G.; Bramanti, P.; Mazzon, E. Prenatal hypoxia and placental oxidative stress: Insights from animal models to clinical evidences. Antioxidants 2020, 9, 414. [Google Scholar] [CrossRef]
- Raffaeli, G.; Manzoni, F.; Cortesi, V.; Cavallaro, G.; Mosca, F.; Ghirardello, S. Iron homeostasis disruption and oxidative stress in preterm newborns. Nutrients 2020, 12, 1554. [Google Scholar] [CrossRef]
- Karthigesu, K.; Bertolo, R.F.; Brown, R.J. Parenteral nutrition and oxidant load in neonates. Nutrients 2021, 13, 2631. [Google Scholar] [CrossRef]
- Dani, C.; Poggi, C.; Pratesi, S. Bilirubin and oxidative stress in term and preterm infants. Free Radic. Res. 2019, 53, 2–7. [Google Scholar] [CrossRef]
- Parsons, A.; Netsanet, A.; Seedorf, G.J.; Abman, S.H.; Taglauer, E.S. Understanding the Role of Placental Pathophysiology in the Development of Bronchopulmonary Dysplasia (BPD). Am. J. Physiol. Lung Cell Mol. Physiol. 2022, 323, L651–L658. [Google Scholar] [CrossRef]
- Pierro, M.; Villamor-Martinez, E.; van Westering-Kroon, E.; Alvarez-Fuente, M.; Abman, S.H.; Villamor, E. Association of the dysfunctional placentation endotype of prematurity with bronchopulmonary dysplasia: A systematic review, meta-analysis and meta-regression. Thorax 2022, 77, 268–275. [Google Scholar] [CrossRef]
- Pierro, M.; Van Mechelen, K.; van Westering-Kroon, E.; Villamor-Martínez, E.; Villamor, E. Endotypes of Prematurity and Phenotypes of Bronchopulmonary Dysplasia: Toward Personalized Neonatology. J. Pers. Med. 2022, 12, 687. [Google Scholar] [CrossRef]
- Amelio, G.S.; Provitera, L.; Raffaeli, G.; Tripodi, M.; Amodeo, I.; Gulden, S.; Cortesi, V.; Manzoni, F.; Cervellini, G.; Tomaselli, A. Endothelial dysfunction in preterm infants: The hidden legacy of uteroplacental pathologies. Front. Pediatr. 2022, 10, 1041919. [Google Scholar] [CrossRef] [PubMed]
- Hartling, L.; Liang, Y.; Lacaze-Masmonteil, T. Chorioamnionitis as a risk factor for bronchopulmonary dysplasia: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2012, 97, F8–F17. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Aune, D.; Speer, C.P.; Saugstad, O.D. Chorioamnionitis as a risk factor for retinopathy of prematurity: A systematic review and meta-analysis. Neonatology 2014, 105, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Abiramalatha, T.; Bandyopadhyay, T.; Ramaswamy, V.V.; Shaik, N.B.; Thanigainathan, S.; Pullattayil, A.K.; Amboiram, P. Risk Factors for Periventricular Leukomalacia in Preterm Infants: A Systematic Review, Meta-analysis, and GRADE-Based Assessment of Certainty of Evidence. Pediatr. Neurol. 2021, 124, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.W. Systematic review of chorioamnionitis and cerebral palsy. Ment. Retard. Dev. Disabil. Res. Rev. 2002, 8, 25–29. [Google Scholar] [CrossRef]
- Been, J.V.; Lievense, S.; Zimmermann, L.J.; Kramer, B.W.; Wolfs, T.G. Chorioamnionitis as a risk factor for necrotizing enterocolitis: A systematic review and meta-analysis. J. Pediatr. 2013, 162, 236–242.e2. [Google Scholar] [CrossRef]
- Park, H.W.; Choi, Y.-S.; Kim, K.S.; Kim, S.-N. Chorioamnionitis and patent ductus arteriosus: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0138114. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Geissbühler, M.; Hincapié, C.A.; Aghlmandi, S.; Zwahlen, M.; Jüni, P.; da Costa, B.R. Most published meta-regression analyses based on aggregate data suffer from methodological pitfalls: A meta-epidemiological study. BMC Med. Res. Methodol. 2021, 21, 123. [Google Scholar] [CrossRef]
- Thébaud, B. Preempting Bronchopulmonary Dysplasia: Time to Focus on the Placenta? Am. J. Respir. Cell Mol. 2022, 66, 8–9. [Google Scholar] [CrossRef]
- Dexter, S.C.; Pinar, H.; Malee, M.P.; Hogan, J.; Carpenter, M.W.; Vohr, B.R. Outcome of very low birth weight infants with histopathologic chorioamnionitis. Obstet. Gynecol. 2000, 96, 172–177. [Google Scholar] [CrossRef]
- Liu, Y.; Hoyo, C.; Murphy, S.; Huang, Z.; Overcash, F.; Thompson, J.; Brown, H.; Murtha, A.P. DNA methylation at imprint regulatory regions in preterm birth and infection. Am. J. Obstet. Gynecol. 2013, 208, e391–e397. [Google Scholar] [CrossRef] [PubMed]
- Challis, J.; Newnham, J.; Petraglia, F.; Yeganegi, M.; Bocking, A. Fetal sex and preterm birth. Placenta 2013, 34, 95–99. [Google Scholar] [CrossRef] [PubMed]
- van Westering-Kroon, E.; Huizing, M.J.; Villamor-Martínez, E.; Villamor, E. Male Disadvantage in Oxidative Stress-Associated Complications of Prematurity: A Systematic Review, Meta-Analysis and Meta-Regression. Antioxidants 2021, 10, 1490. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.D.; Dickinson, C.; Kandasamy, Y. Sex difference in mortality for premature and low birth weight neonates: A systematic review. Am. J. Perinatol. 2018, 35, 707–715. [Google Scholar] [CrossRef]
- Zisk, J.L.; Genen, L.H.; Kirkby, S.; Webb, D.; Greenspan, J.; Dysart, K. Do premature female infants really do better than their male counterparts? Am. J. Perinatol. 2011, 28, 241–246. [Google Scholar] [CrossRef]
- McGoldrick, E.; Stewart, F.; Parker, R.; Dalziel, S.R. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 2020. [Google Scholar] [CrossRef]
- Miracle, X.; Di Renzo, G.C.; Stark, A.; Fanaroff, A.; Carbonell-Estrany, X.; Saling, E. Guideline for the use of antenatal corticosteroids for fetal maturation. J. Perinat. Med. 2008, 36, 191–196. [Google Scholar] [CrossRef]
- Gilstrap, L.C.; Christensen, R.; Clewell, W.H.; D’Alton, M.E.; Davidson, E.C.; Escobedo, M.B.; Gjerdingen, D.K.; Goddard-Finegold, J.; Goldenberg, R.L.; Grimes, D.A. Effect of corticosteroids for fetal maturation on perinatal outcomes: NIH consensus development panel on the effect of corticosteroids for fetal maturation on perinatal outcomes. JAMA 1995, 273, 413–418. [Google Scholar] [CrossRef]
- Amiya, R.M.; Mlunde, L.B.; Ota, E.; Swa, T.; Oladapo, O.T.; Mori, R. Antenatal corticosteroids for reducing adverse maternal and child outcomes in special populations of women at risk of imminent preterm birth: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0147604. [Google Scholar] [CrossRef]
- Been, J.V.; Rours, I.G.; Kornelisse, R.F.; Passos, V.L.; Kramer, B.W.; Schneider, T.A.; de Krijger, R.R.; Zimmermann, L.J. Histologic chorioamnionitis, fetal involvement, and antenatal steroids: Effects on neonatal outcome in preterm infants. Am. J. Obstet. Gynecol. 2009, 201, e581–e588. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).