Abstract
Proanthocyanidins (PACs), which are oligomers or polymers of flavan-3ols with potent antioxidative activity, are well known to exert a variety of beneficial health effects. Nonetheless, their bioaccessibility and bioavailability have been poorly assessed. In this review, we focused on the metabolic fate of PACs through the digestive tract. When oligomeric and polymeric PACs are orally ingested, a large portion of the PACs reach the colon, where a small portion is subjected to microbial degradation to phenolic acids and valerolactones, despite the possibility that slight depolymerization of PACs occurs in the stomach and small intestine. Valerolactones, as microbiota-generated catabolites of PACs, may contribute to some of the health benefits of orally ingested PACs. The remaining portion interacts with gut microbiota, resulting in improved microbial diversity and, thereby, contributing to improved health. For instance, an increased amount of beneficial gut bacteria (e.g., Akkermansia muciniphila and butyrate-producing bacteria) could ameliorate host metabolic functions, and a lowered ratio of Firmicutes/Bacteroidetes at the phylum level could mitigate obesity-related metabolic disorders.
1. Introduction
Proanthocyanidins (PACs), also known as condensed tannins, are substances that produce red anthocyanidin pigments when decomposed by acid and are oligomers or polymers of flavan-3-ols, such as epicatechin and catechin. They are widely distributed in fruits, grains, and leaves [1,2,3,4,5], especially in cocoa, black soybeans, cinnamon, apples, and grape seeds [6]. In addition, grape seed PACs have an average degree of polymerization (DP) between 2 and 17 [1].
We previously reported that grape seed PACs have direct antioxidant potential in vitro against di(phenyl)-(2,4,6-trinitrophenyl)iminoazanium (DPPH; a stable radical), superoxide anion radical (O2−•), hydroxyl radical (•OH), singlet oxygen (1O2), and hydrogen peroxide (H2O2) [7]. In oxidative-stress-induced cells, PACs significantly improved antioxidant enzyme activities (e.g., glutathione peroxidase, superoxide dismutase, and catalase), leading to decreased levels of reactive oxygen species and malondialdehyde [8]. In addition, they significantly activate the nuclear factor–erythroid 2-related factor 2 (Nrf2)/antioxidant response element (ARE) pathway, including the increased expression of NAD(P)H:quinone acceptor oxidoreductase 1 and heme oxygenase 1. These characteristic features observed in vitro are thought to contribute to various therapeutic effects, including anti-adipogenesis in adipocytes [9], anti-cancer effects in several cancer cells [10,11,12,13,14], and neuroprotective effects in rat pheochromocytoma cells (PC12 cells) [15,16,17,18].
In in vivo studies, PACs alleviated severe acute pancreatitis in mice via their anti-inflammatory properties [19], exerted anti-obesity and anti-diabetic activity in a type 2 diabetes model of KKAy mice [20] and anti-obesity activity in a mouse model of high-fat-diet-induced obesity [21], and showed neuroprotective activity in zebrafish and rat models of Parkinson’s disease [8,22]. We previously demonstrated that orally administered grape seed PACs prevented bone loss in the lumbar vertebrae and femur in ovariectomized (OVX) mice, and they ameliorated the healing of defects created on the calvaria and osseointegration of a tibial implant in OVX rats, likely by counteracting the accelerated osteoclastogenic activity induced by estrogen deficiency [23]. To attain a better understanding of such health-beneficial activities, pharmacokinetic analysis is imperative. However, there is a paucity of evidence related to the structural complexity of PACs. Thus, in this review, we focused on the bioaccessibility and bioavailability of PACs by exploring their metabolic fate through the digestive tract.
2. Basic Structures of Proanthocyanidins (PACs)
Flavan-3-ols have a basic structure consisting of A, B, and C rings, in which 3, 5, 7, 3′, or 4′ is hydroxylated. For example, the 3-hydroxylated group has two conformations: the 2,3-cis isomer is (−)-epicatechin, and the 2,3-trans isomer is (+)-catechin. Oligomers are formed by C4-C8 or C2-O-C7 bonds between monomers with these basic structures. The isomers are roughly divided into two groups according to their binding modes—those with C4-C8 or C4-C6 bonds are called B-type, and those with additional C2-O-C7 bonds are called A-type (Figure 1). Naturally occurring B-type PACs are predominant in plants such as cocoa, bayberry, and grapes [24,25,26]. Concerning PAC dimers, the A-type dimers and B-type dimers are numbered as A1, A2, B1, and B2; e.g., B1 consists of (−)-epicatechin (C4-C8) (+)-catechin, and B2 consists of (−)-epicatechin (C4-C8) (−)-epicatechin. Apart from the A- and B-type dimers, PAC C1 (epicatechin-(C4-C8)-epicatechin-(C4-C8)-epicatechin) and PAC C2 (catechin-(C4-C8)-catechin-(C4-C8)-catechin) are trimeric and belong to the group of B-type PACs. PACs are also divided into three categories: procyanidins (oligomeric PACs formed from catechin and epicatechin), propelargonidins (from afzelechin and epiafzelechin), and prodelphinidins (from gallocatechin and epigallocatechin) [27]. Based on the DP, PACs with a low DP are called oligomers, and those with a high DP are called polymers. For instance, previous papers defined oligomers and polymers as structures with DP values of four to ten and those with more than ten, respectively [28,29].
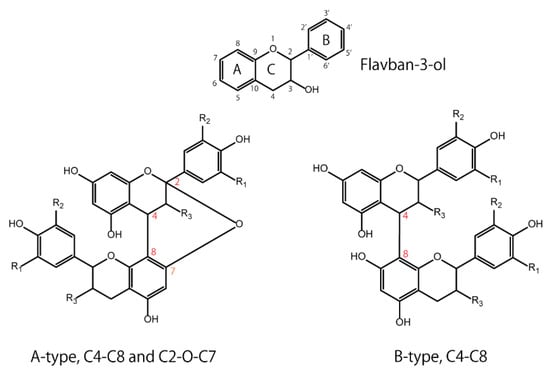
Figure 1.
Basic structures of flavan-3-ol: A-type and B-type proanthocyanidins.
Regarding the DP and stereochemistry of oligomeric PACs, the condensation of monomeric flavan-3-ol units compactly forms a helical PAC structure in an aqueous solution [30,31,32], leading to interactions between saliva proteins, which causes astringency in wine-tasting processes [33]. In addition, the hydrophobicity of PACs, as measured with the octanol–water partition coefficient (logP), significantly decreases with an increase in the degree of polymerization due to the large number of phenolic hydroxyl groups covering them [34,35]. In more detail, PACs with a higher DP have a helical structure comprising a hydrophilic surface covered by a large number of hydroxyl groups; their internal region is hydrophobic, making them likely to interact with biogenic substances, such as proteins, peptides, carbohydrates, lipids, and oligonucleotides [36].
3. Pharmacokinetics of Proanthocyanidins (PACs)
3.1. Oral Stability
Interactions between PACs and biogenic substances in the oral cavity vary. As is the case with biogenic substance–phenolic compound interactions [37,38,39], PACs can interact with carbohydrate polymers via hydrogen bonding, leading to the formation of non-digestible amylose–PAC complexes [40,41,42]. In a previous report, sorghum PACs were extractable after cooking with starches that varied in amylose content [43]. If PACs and carbohydrate polymers interact hydrophobically and/or through hydrogen bonds, the PACs are likely to be extractable. PACs also inhibit α-amylase due to a non-covalent hydrophobic interaction with the enzyme [42,44,45]. Thus, when PACs are orally taken, their bioavailability could be affected depending on the intradigestive environment. In an in vitro oral digestion study where 5 mL of simulated saliva fluid composed of amylase enzyme was applied to Chinese bayberry leaf PACs, the PAC dimers showed no significant differences during in vitro digestion, whereas the trimers were significantly decreased after 2 min of oral digestion [46]. Concomitantly, the flavan-3-ol monomers probably increased due to the degradation of the trimers. However, salivary proteins (proline-rich proteins and histatins) are known to have an affinity to PACs [47,48,49], irrespective of the amylase–PAC interactions, with the salivary protein–PAC complexes being present in the stomach. The protein–PAC complexes that deposit in the stomach then separate due to the acidic environment; for example, PAC trimer–amylase complexes were reported to separate in the gastric environment of the stomach, resulting in an increase in trimer content [46].
3.2. Gastric Stability
To investigate the gastric stability of PACs, several in vitro studies using simulated gastric juice were conducted, but the results were controversial. PAC oligomers (trimer to hexamer) purified from cocoa were hydrolyzed to mixtures of epicatechin monomer and dimer [50], apple dimeric PAC B2 was almost completely degraded into (−)-epicatechin [51], and the PAC content in an extract of Hypericum perfoliatum L. significantly decreased by 25% [52]. On the contrary, other studies reported that PACs with a high DP (mean DP ≥ 6) from grape seeds were remarkably stable in the gastric environment and did not degrade into more readily absorbable monomers [53,54], PACs from Acacia mearnsii remained stable during gastric digestion in vitro [55], and the mean DP of PACs isolated from Choerospondias axillaris peel was not affected [56]. A human in vivo study showed that cocoa beverage PACs were stable during gastric transit, with the pH of gastric contents increasing from 1.9 ± 0.2 to 5.4 ± 0.2 after consumption [57]. Regarding the effects of macronutrients, it was reported that a higher fat content or the presence of carbohydrate-rich food did not greatly affect the in vitro gastric stability of PACs [54,58]. In summary, the gastric stability of PACs depends on their types and on the electrolytes used, the dietary source, the duration of exposure to the gastric environment, and the pH conditions of gastric juice [45,55,57]. The timing of oral intake can be an important factor when considering gastric stability. For instance, in the postprandial state, PACs were present with a mixture of foodstuffs and gastric juice under acidic conditions. However, in the fasting state, there was little gastric juice with slightly higher pH conditions because the acid secretion (a pH of 2 under basal conditions with an empty stomach) was buffered by the food bolus [57]. Collectively, PACs are depolymerized to some extent under gastric conditions and then pass into the small intestine.
3.3. Small-Intestinal Stability and Absorption
The first step after gastric digestion is exposure to pancreatic juice in the duodenum. It was reported that slight depolymerization of PACs could be observed in an in vitro small-intestinal model that used pancreatic enzymes and bile salts [54]. A similar in vitro study showed that the mean DP of PACs was slightly decreased, which was possibly due to interactions with digestive enzymes [56]. Collectively, PACs were rather chemically stable with respect to depolymerization during their passage through the simulated duodenal digestion. Regarding intestinal absorption of PACs, it was reported that the A1, A2, and B2 PAC dimers were slightly absorbed without conjugation or methylation from the small intestine in an in situ perfusion model of the rat small intestine [59]. Similarly, a study on the absorption rate of PACs without digestion, which was measured with the Caco-2 monolayer transport assay, showed that PAC dimers could traverse the Caco-2 monolayer [46], and trimers and tetramers could be transported across Caco-2 cells at low rates [60].
3.4. Colonic Stability and Absorption
When PACs reach the colon, they are likely to be affected by gut microbiota. In in vitro fermentation of grape seed extracts that were rich in B-type PACs, the maximum formation of intermediate metabolites, such as valerolactones, valeric acid, several phenolic acids, and gallic acid, was observed at 5–10 h of incubation with fecal microbiota. Subsequently, the incubations (10–48 h) resulted in the appearance of mono- and non-hydroxylated forms of previous metabolites, which was likely due to dehydroxylation reactions [61,62]. These in vitro results were also consistent with those from a human study. When humans consumed a test drink containing PACs with a DP ranging from 2 to 10, γ-valerolactones were mainly detected in the plasma [63], thus rejecting the notion that PACs are broken down into flavanols prior to their absorption. In another human study that was conducted to comparatively investigate the metabolic fate of (−)-epicatechin, PAC B1 (a dimer) and polymeric PACs, all of which were encapsulated in hard gelatin to minimize interactions with the oral and gastric environments, it was observed that free PAC B1, 4-hydroxy-5-(3′,4′-dihydroxyphenyl)valeric acid, 5-(3′,4′-dihydroxyphenyl)-valerolactone were detected in the plasma after PAC B1 ingestion, but no dimeric or oligomeric PACs were detected in the plasma after the ingestion of polymeric PACs with a mean degree of polymerization of 5.9 [64]. Thus, 5-(3′,4′-dihydroxyphenyl)-valerolactone is a dominant in vivo metabolite of PAC B1 produced by the gut microbiota. Moreover, small portions of PAC B1 were metabolized by the phase II metabolism after entering into circulation. In addition, microbial degradation would be hampered because of the low uptake of compounds by bacteria due to their huge molecular size. These findings were consistent with those of two rat studies that showed that ingested polymeric PACs were present in the colon as the intact parent compounds, and they were responsible for the local beneficial biological actions [65,66].
Phenyl-γ-valerolactones, as microbiota-generated catabolites of PACs, have been shown in preclinical studies to have some potential health-beneficial effects, such as reducing the risk of colorectal cancer [67] and neuroprotection by regulating intracellular proteolysis [68]. In a study in which the cellular antioxidant effect of polyphenol metabolites was examined, 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone showed the highest antioxidant effect among the investigated polyphenol metabolites [69]. It was also shown that 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone had catalase-like activity and promoted the Nrf2/ARE pathway. Collectively, γ-valerolactones produced from PACs by gut microbiota may contribute to some health-beneficial effects following oral ingestion of PACs.
3.5. Effects on Gut Microbiota
Apart from bacterial transformation, PACs could affect the gut microbiota. Although most in vivo studies were conducted to investigate the effects of PACs on altered gut microbiota under certain pathological conditions, a few studies using normal animals have been conducted. It was reported that dietary PACs for 6 days resulted in an ecological shift in the microbiome, dramatically increasing the operational taxonomic units (OTUs) of Lachnospiraceae, unclassified Clostridales, Lactobacillus, and Ruminococcus in crossbred female pigs. Further, intact parent PACs (dimer-pentamer) and major phenolic metabolites (4-hydroxyphenylvaleric acid and 3-hydroxybenzoic acid) were found in feces [70]. It was reported that Lachnospiraceae and Ruminococcus are the major butyrate and propionate producers in human fecal samples [71], and butyrate can modulate oxidative stress in the colonic mucosa of healthy humans [72]. In a review article, butyrate was reported to lead to more specific and efficacious therapeutic strategies for the prevention and treatment of different diseases ranging from genetic/metabolic conditions to neurological degenerative disorders [73]. In particular, in a human study, the transfer of intestinal microbiota from lean donors increased insulin sensitivity in individuals with metabolic syndrome along with levels of butyrate-producing intestinal microbiota, suggesting that intestinal microbiota should be developed as therapeutic agents for increasing insulin sensitivity in humans [74]. If PACs have the ability to increase butyrate producers, they may work not only for colonic health, but also for systemic health. Another study using weaned piglets revealed that dietary grape seed PACs improved the microbial diversity in ileal and colonic digesta, with the most abundant OTUs belonging to two phyla: Firmicutes and Bacteroidetes [75]. The PACs also decreased the abundance of Lactobacillaceae and increased that of Clostridiaceae, accompanied by improved intestinal mucosal barrier function and increased concentration of propionic and butyric acids in the intestinal digesta. In a rat study in which an 8-day oral gavage of grape seed PACs (monomeric (21.3%), dimeric (17.4%), trimeric (16.3%), tetrameric (13.3%), and oligomeric (31.7%)) was administered to normal female rats, the ratio of Firmicutes to Bacteroidetes at the phylum level was lowered with increased plasma glucagon-like-peptide-1 level [76]. More recently, it was reviewed that PACs have a prebiotic and antimicrobial role that favors homeostasis of the intestinal environment, thus reducing the survival of Gram-negative bacteria that produce lipopolysaccharide (LPS) [77]. As LPS triggers the activation of the Toll-like receptor-4 (TLR-4) inflammatory pathway, PACs can minimize endotoxemia.
As for animal studies under pathological conditions, most studies applied high-fat diet (HFD)- or high-fat/high-sucrose diet (HFHSD)-induced metabolic syndrome model animals. PAC-rich grape seed/pomace extract [78,79,80], PAC-rich cranberry extract [81], and apple PACs [82] showed improved symptoms of metabolic syndrome concomitantly with an altered gut microbial environment. Some studies revealed that PACs increase Akkermansia muciniphila [78,80] or Akkermansia at the genus level [82], the former of which is a well-known beneficial gut bacterium that improves host metabolic functions and immune responses [83,84,85,86,87,88,89]. Accounting for 3–5% of the microbial community in healthy individuals, A. muciniphila is a mucinolytic bacterium found in the mucus layer of the human gut [90], and it has the potential to restore mucus thickness and intestinal barrier integrity [91,92]. This bacterium also has the ability to decrease the progression of many diseases, such as obesity and type 2 diabetes mellitus [93,94]. As such, A. muciniphila is considered a promising probiotic candidate [88]. At the phylum level, PACs could decrease the ratio of Firmicutes/Bacteroidetes [79,82]. The dominant gut microbial phyla are Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteria, and Verrucomicrobia, with the first two phyla being the most common in healthy human individuals [95]. Phylum-level analyses of Firmicutes and Bacteroidetes have shown that they are associated with obesity and that an increased population of Bacteroidetes, as well as a reduced population of Firmicutes, could improve obesity [96,97,98,99,100]; this is likely via the depression of the increased capacity for energy harvesting from the diet [99]. In a human study, the relative proportion of Bacteroidetes was decreased in obese people in comparison with that in lean people, and this proportion increased with weight loss with two types of low-calorie diets [101]. These findings indicate that obesity is associated with a microbial component, paving the way for investigations into the potential therapeutic implications of gut microbiota. Aside from HFD- or HFHSD-fed animals, PACs normalized the imbalanced Firmicutes/Bacteroidetes ratio observed in OVX mice in a menopause model and prevented OVX animals from having an increased weight [102].
If the microbial degradation of PACs is hampered because of the low compound uptake by bacteria due to their huge molecular size, how exactly they affect the gut microbiota becomes the primary concern. Some PACs exert antimicrobial activities by preventing bacterial adhesion to human cells [103,104], with PAC-rich cranberry being used clinically as an adjuvant therapy in the prevention of urinary tract infections [105]. It has also been reported that anti-adhesion activity could be challenging in the development of new antimicrobials that are able to withstand the increasing repertoire of bacterial resistance [106]. In dentistry, PACs are known to have specific antibacterial characteristics of attacking periodontopathogenic bacteria (Porphyromonas gingivalis), but not oral commensal bacteria (Streptococcus salivarius) [107,108]. PACs’ antibacterial activity in the oral cavity may be attributed to their biofilm-disrupting properties by interfering with the N-acylhomoserine lactone-mediated quorum sensing of the bacteria, which tightly regulates the expression of multiple virulence factors in opportunistic pathogenic Gram-negative bacteria [109,110]. Thus, PACs could be some of the substances affecting gut microbiota via antibacterial activity. Further studies are needed to clarify the effects of PACs on the gut microbiota.
The aforementioned metabolic fate of PACs through the digestive tract and their health-beneficial effects in association with gut microbiota are summarized in Figure 2 and Figure 3, respectively.
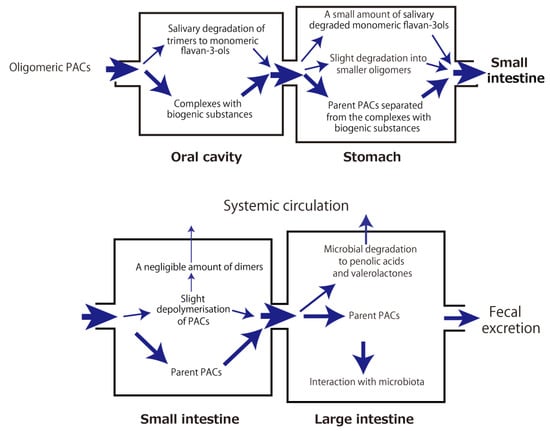
Figure 2.
Intradigestive fate of orally ingested proanthocyanidins (PACs).
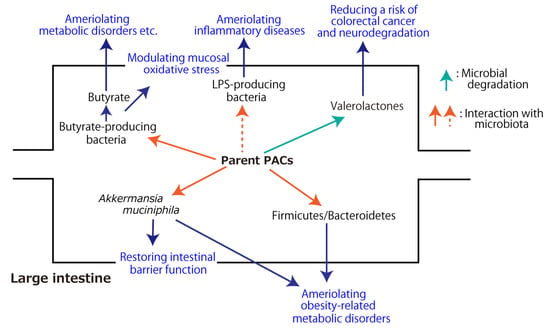
Figure 3.
Health-beneficial effects of proanthocyanidins (PACs) associated with gut microbiota. The dashed arrow indicates the inhibitory effect.
4. Conclusions
A large portion of orally ingested oligomeric and polymeric PACs reach the colon, where a small portion of them are subjected to microbial degradation into phenolic acids and valerolactones. The rest interact with gut microbiota, resulting in improved microbial diversity, which includes an increased amount of beneficial gut bacteria (e.g., Akkermansia muciniphila), which could ameliorate host metabolic functions, and a lowered ratio of Firmicutes/Bacteroidetes at the phylum level, which could mitigate obesity-related metabolic disorders. Further, PACs have the potential to increase butyrate-producing microbiota and decrease LPS-producing bacteria, leading to the prevention and treatment of different diseases ranging from metabolic conditions to neurological degenerative disorders. These microbial changes could confer some of PACs’ health-beneficial effects.
Author Contributions
Conceptualization, Y.N., H.K. and K.N.; methodology, Y.N., M.S. and S.S.; writing—original draft preparation, Y.N., H.K. and K.N.; writing—review and editing, M.S. and S.S.; funding acquisition, Y.N. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partly supported by JSPS KAKENHI, Grant Number: JP20K09996.
Conflicts of Interest
Shirato, Shishido, and Nakamura are members of an academia–industry collaboration laboratory at Tohoku University Graduate School of Dentistry, which receives funding from Luke Co., Ltd. (Sendai, Japan). This academia–industry collaboration has been examined and approved by the Conflict of Interest Management Committee at Tohoku University. Luke Co., Ltd. and the grant funder had no role in the study’s design, the data collection and interpretation, or the decision to submit the work for publication.
References
- Prieur, C.; Rigaud, J.; Cheynier, V.; Moutounet, M. Oligomeric and polymeric procyanidins from grape seeds. Phytochemistry 1994, 36, 781–784. [Google Scholar] [CrossRef]
- Bao, J.; Cai, Y.; Sun, M.; Wang, G.; Corke, H. Anthocyanins, flavonols, and free radical scavenging activity of Chinese bayberry (Myrica rubra) extracts and their color properties and stability. J. Agric. Food Chem. 2005, 53, 2327–2332. [Google Scholar] [CrossRef] [PubMed]
- Spranger, I.; Sun, B.; Mateus, A.M.; Freitas, V.; Ricardo-da-Silva, J.M. Chemical characterization and antioxidant activities of oligomeric and polymeric procyanidin fractions from grape seeds. Food Chem. 2008, 108, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Mkandawire, N.L.; Kaufman, R.C.; Bean, S.R.; Weller, C.L.; Jackson, D.S.; Rose, D.J. Effects of sorghum (Sorghum bicolor (L.) Moench) tannins on α-amylase activity and in vitro digestibility of starch in raw and processed flours. J. Agric Food Chem. 2013, 61, 4448–4454. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Qiao, L.; Cao, Y.; Zhou, X.; Liu, Y.; Ye, X. Structural elucidation and antioxidant activities of proanthocyanidins from Chinese bayberry (Myrica rubra Sieb. et Zucc.) leaves. PLoS ONE 2014, 9, e96162. [Google Scholar] [CrossRef]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, R.L. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [CrossRef]
- Katsuda, Y.; Niwano, Y.; Nakashima, T.; Mokudai, T.; Nakamura, K.; Oizumi, S.; Kanno, T.; Kanetaka, H.; Egusa, H. Cytoprotective effects of grape seed extract on human gingival fibroblasts in relation to its antioxidant potential. PLoS ONE 2015, 10, e0134704. [Google Scholar]
- Chen, J.; Chen, Y.; Zheng, Y.; Zhao, J.; Yu, H.; Zhu, J.; Li, D. Protective effects and mechanisms of procyanidins on parkinson’s disease in vivo and in vitro. Molecules 2021, 26, 5558. [Google Scholar] [CrossRef]
- Choi, Y.E.; Choi, S.I.; Han, X.; Men, X.; Jang, G.W.; Kwon, H.Y.; Kang, S.R.; Han, J.S.; Lee, O.H. Radical scavenging-linked anti-adipogenic activity of Aster scaber ethanolic extract and its bioactive compound. Antioxidants 2020, 9, 1290. [Google Scholar] [CrossRef]
- Upanan, S.; Yodkeeree, S.; Thippraphan, P.; Punfa, W.; Wongpoomchai, R.; Limtrakul Dejkriengkraikul, P. The proanthocyanidin-rich fraction obtained from red rice germ and bran extract induces HepG2 hepatocellular carcinoma cell apoptosis. Molecules 2019, 24, 813. [Google Scholar] [CrossRef]
- Ribas, L.E.; Baravalle, M.E.; Gasser, F.B.; Renna, M.S.; Addona, S.; Ortega, H.H.; Savino, G.H.; van de Velde, F.; Hein, G.J. Extraction of phenolic compounds from the shells of pecan nuts with cytotoxic activity through apoptosis against the colon cancer cell line HT-29. J. Food Sci. 2021, 86, 5409–5423. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, L.; Liu, C.; He, X.; Chen, M.; Chen, J. Lipophilic grape seed proanthocyanidin exerts anti-cervical cancer effects in HeLa cells and a HeLa-derived xenograft zebrafish model. Antioxidants 2022, 11, 422. [Google Scholar] [CrossRef] [PubMed]
- Habib, H.M.; El-Fakharany, E.M.; Kheadr, E.; Ibrahim, W.H. Grape seed proanthocyanidin extract inhibits DNA and protein damage and labile iron, enzyme, and cancer cell activities. Sci. Rep. 2022, 12, 12393. [Google Scholar] [CrossRef] [PubMed]
- Alsharairi, N.A. Insights into the mechanisms of action of proanthocyanidins and anthocyanins in the treatment of nicotine-induced non-small cell lung cancer. Int. J. Mol. Sci. 2022, 23, 7905. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Z.; Ma, Z.J.; Kang, J.H.; Lin, A.X.; Wang, Z.H.; Chen, H.W.; Guo, X.D.; He, X.G.; Kang, X.W. Grape seed proanthocyanidins exert a neuroprotective effect by regulating microglial M1/M2 polarisation in rats with spinal cord injury. Mediat. Inflamm. 2022, 2022, 2579003. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, Y.; Zheng, Y.; Zhao, J.; Yu, H.; Zhu, J.; Li, D. Neuroprotective effects and mechanisms of procyanidins in vitro and in vivo. Molecules 2021, 26, 2963. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, Y.; Zheng, Y.; Zhao, J.; Yu, H.; Zhu, J. Relationship between neuroprotective effects and structure of procyanidins. Molecules 2022, 27, 5007. [Google Scholar] [CrossRef]
- He, X.; Guo, X.; Ma, Z.; Li, Y.; Kang, J.; Zhang, G.; Gao, Y.; Liu, M.; Chen, H.; Kang, X.; et al. Grape seed proanthocyanidins protect PC12 cells from hydrogen peroxide-induced damage via the PI3K/AKT signaling pathway. Neurosci. Lett. 2021, 750, 135793. [Google Scholar] [CrossRef]
- Sheng, L.P.; Han, C.Q.; Ling, X.; Guo, X.W.; Lin, R.; Ding, Z. Proanthocyanidins suppress NLRP3 inflammasome and M1 macrophage polarization to alleviate severe acute pancreatitis in mice. J. Biochem. Mol. Toxicol. 2022, e23242. [Google Scholar] [CrossRef]
- Kashiwada, M.; Nakaishi, S.; Usuda, A.; Miyahara, Y.; Katsumoto, K.; Katsura, K.; Terakado, I.; Jindo, M.; Nakajima, S.; Ogawa, S.; et al. Analysis of anti-obesity and anti-diabetic effects of acacia bark-derived proanthocyanidins in type 2 diabetes model KKAy mice. J. Nat. Med. 2021, 75, 893–906. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, P.; Li, X.; Shen, S.; Li, K. Persimmon proanthocyanidins with different degrees of polymerization possess distinct activities in models of high fat diet induced obesity. Nutrients 2022, 14, 3718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, N.; Chen, M.; Jin, H.; Nie, J.; Shi, J.; Jin, F. Procyanidin protects against 6-hydroxydopamine-induced dopaminergic neuron damage via the regulation of the PI3K/Akt signalling pathway. Biomed. Pharmacother. 2019, 114, 108789. [Google Scholar] [CrossRef] [PubMed]
- Tenkumo, T.; Aobulikasimu, A.; Asou, Y.; Shirato, M.; Shishido, S.; Kanno, T.; Niwano, Y.; Sasaki, K.; Nakamura, K. Proanthocyanidin-rich grape seed extract improves bone loss, bone healing, and implant osseointegration in ovariectomized animals. Sci. Rep. 2020, 10, 8812. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Barberan, F.A.; Cienfuegos-Jovellanos, E.; Marín, A.; Muguerza, B.; Gil-Izquierdo, A.; Cerda, B.; Zafrilla, P.; Morillas, J.; Mulero, J.; Ibarra, A.; et al. A new process to develop a cocoa powder with higher flavonoid monomer content and enhanced bioavailability in healthy humans. J. Agric. Food Chem. 2007, 55, 3926–3935. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ye, X.; Liu, D.; Chen, J.; Zhang, J.; Shen, Y.; Yu, D. Characterization of unusual proanthocyanidins in leaves of bayberry (Myrica rubra Sieb. et Zucc.). J. Agric. Food Chem. 2011, 59, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Fujimaki, T.; Mori, S.; Horikawa, M.; Fukui, Y. Isolation of proanthocyanidins from red wine, and their inhibitory effects on melanin synthesis in vitro. Food Chem. 2018, 248, 61–69. [Google Scholar] [CrossRef]
- Lim, I.; Ha, J. Biosynthetic pathway of proanthocyanidins in major cash crops. Plants 2021, 10, 1792. [Google Scholar] [CrossRef]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Prior, R.L. Screening of foods containing proanthocyanidins and their structural characterization using LC-MS/MS and thiolytic degradation. J. Agric. Food Chem. 2003, 51, 7513–7521. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Li, D.; Ho, C.T.; Li, J.; Wan, X. The absorption, distribution, metabolism and excretion of procyanidins. Food Funct. 2016, 7, 1273–1281. [Google Scholar] [CrossRef]
- Muranaka, A.; Yoshida, K.; Shoji, T.; Moriichi, N.; Masumoto, S.; Kanda, T.; Ohtake, Y.; Kobayashi, N. Chiral recognition of apple procyanidins by complexation with oxotitanium phthalocyanine. Org. Lett. 2006, 8, 2447–2450. [Google Scholar] [CrossRef]
- Tarascou, I.; Barathieu, K.; Simon, C.; Ducasse, M.A.; André, Y.; Fouquet, E.; Dufourc, E.J.; de Freitas, V.; Laguerre, M.; Pianet, I.; et al. A 3D structural and conformational study of procyanidin dimers in water and hydro-alcoholic media as viewed by NMR and molecular modeling. Magn. Reson. Chem. 2006, 44, 868–880. [Google Scholar] [CrossRef] [PubMed]
- Tarascou, I.; Ducasse, M.A.; Dufourc, E.J.; Moskau, D.; Fouquet, E.; Laguerre, M.; Pianet, I. Structural and conformational analysis of two native procyanidin trimers. Magn. Reason. Chem. 2007, 45, 157–166. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, V.; Mateus, N. Structural features of procyanidin interactions with salivary proteins. J. Agric. Food Chem. 2001, 49, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Shibusawa, Y.; Shoji, A.; Yanagida, A.; Shindo, H.; Tagashira, M.; Ikeda, M.; Yoichiro, I. Determination of log Po/w for catechins and their isomers, oligomers, and other organic compounds by stationary phase controlled high speed countercurrent chromatography. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 2819–2837. [Google Scholar] [CrossRef]
- Yanagida, A.; Murao, H.; Ohnishi-Kameyama, M.; Yamakawa, Y.; Shoji, A.; Tagashira, M.; Kanda, T.; Shindo, H.; Shibusawa, Y. Retention behavior of oligomeric proanthocyanidins in hydrophilic interaction chromatography. J. Chromatogr. A 2007, 1143, 153–161. [Google Scholar] [CrossRef]
- Yanagida, A.; Takeshige, S.; Shibusawa, Y. Reversed-phase liquid chromatographic analysis of hydrophobic interaction between proanthocyanidins and a C₈-alkyl compound in aqueous solution. Biosci. Biotechnol. Biochem. 2016, 80, 419–425. [Google Scholar] [CrossRef]
- Rohn, S.; Rawel, H.M.; Rober, M.; Kroli, J. Reactions with phenolic substances can induce changes in some physico-chemical properties and activities of bromelain—The consequences for supplementary food products. Int. J. Food Sci. Technol. 2005, 40, 771–782. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, Z.; Wang, Y.; Fu, L. Dietary protein-phenolic interactions: Characterization, biochemical-physiological consequences, and potential food applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 3589–3615. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Renard, C.M. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef]
- Amoako, D.B.; Awika, J.M. Polymeric tannins significantly alter properties and in vitro digestibility of partially gelatinized intact starch granule. Food Chem. 2016, 208, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Takahama, U.; Hirota, S. Interactions of flavonoids with α-amylase and starch slowing down its digestion. Food Funct. 2018, 9, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Barros, F.; Awika, J.M.; Rooney, L.W. Interaction of tannins and other sorghum phenolic compounds with starch and effects on in vitro starch digestibility. J. Agric. Food Chem. 2012, 60, 11609–11617. [Google Scholar] [CrossRef] [PubMed]
- Czubinski, J.; Dwiecki, K. A review of methods used for investigation of protein-phenolic compound interactions. Int. J. Food Sci. Technol. 2017, 52, 573–585. [Google Scholar] [CrossRef]
- Tao, W.; Zhang, Y.; Shen, X.; Cao, Y.; Shi, J.; Ye, X.; Chen, S. Rethinking the mechanism of the health benefits of proanthocyanidins: Absorption, metabolism, and interaction with gut microbiota. Compr. Rev. Food Sci. Food Saf. 2019, 18, 971–985. [Google Scholar] [CrossRef]
- Tao, W.; Wei, C.; Shen, S.; Wang, M.; Chen, S.; Ye, X.; Cao, Y. Mainly dimers and trimers of Chinese bayberry leaves proanthocyanidins (BLPs) are utilized by gut microbiota: In vitro digestion and fermentation coupled with Caco-2 transportation. Molecules 2020, 25, 184. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Butler, L.G. The specificity of proanthocyanidin-protein interactions. J. Biol. Chem. 1981, 256, 4494–4497. [Google Scholar] [CrossRef]
- Wróblewski, K.; Muhandiram, R.; Chakrabartty, A.; Bennick, A. The molecular interaction of human salivary histatins with polyphenolic compounds. Eur. J. Biochem. 2001, 268, 4384–4397. [Google Scholar] [CrossRef]
- Bennick, A. Interaction of plant polyphenols with salivary proteins. Crit. Rev. Oral Biol. Med. 2002, 13, 184–196. [Google Scholar] [CrossRef]
- Spencer, J.P.; Chaudry, F.; Pannala, A.S.; Srai, S.K.; Debnam, E.; Rice-Evans, C. Decomposition of cocoa procyanidins in the gastric milieu. Biochem. Biophys. Res. Commun. 2000, 272, 236–241. [Google Scholar] [CrossRef]
- Kahle, K.; Kempf, M.; Schreier, P.; Scheppach, W.; Schrenk, D.; Kautenburger, T.; Hecker, D.; Huemmer, W.; Ackermann, M.; Richling, E.; et al. Intestinal transit and systemic metabolism of apple polyphenols. Eur. J. Nutr. 2011, 50, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Celep, E.; İnan, Y.; Akyüz, S.; Yesilada, E. The bioaccessible phenolic profile and antioxidant potential of Hypericum perfoliatum L. after simulated human digestion. Ind. Crops Prod. 2017, 109, 717–723. [Google Scholar] [CrossRef]
- Fernández, K.; Labra, J. Simulated digestion of proanthocyanidins in grape skin and seed extracts and the effects of digestion on the angiotensin I-converting enzyme (ACE) inhibitory activity. Food Chem. 2013, 139, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.; Macià, A.; Romero, M.P.; Valls, J.; Bladé, C.; Arola, L.; Motilva, M.J. Bioavailability of procyanidin dimers and trimers and matrix food effects in in vitro and in vivo models. Br. J. Nutr. 2010, 103, 944–952. [Google Scholar] [CrossRef]
- Chen, X.; Xiong, J.; He, L.; Zhang, Y.; Li, X.; Zhang, L.; Wang, F. Effects of in vitro digestion on the content and biological activity of polyphenols from Acacia mearnsii bark. Molecules 2018, 23, 1824. [Google Scholar] [CrossRef]
- Li, Q.; Chen, J.; Li, T.; Liu, C.; Wang, X.; Dai, T.; McClements, D.J.; Liu, J. Impact of in vitro simulated digestion on the potential health benefits of proanthocyanidins from Choerospondias axillaris peels. Food Res. Int. 2015, 78, 378–387. [Google Scholar] [CrossRef]
- Rios, L.Y.; Bennett, R.N.; Lazarus, S.A.; Rémésy, C.; Scalbert, A.; Williamson, G. Cocoa procyanidins are stable during gastric transit in humans. Am. J. Clin. Nutr. 2002, 76, 1106–1110. [Google Scholar] [CrossRef]
- Gültekin-Özgüven, M.; Berktas, I.; Özçelik, B. Change in stability of procyanidins, antioxidant capacity and in-vitro bioaccessibility during processing of cocoa powder from cocoa beans. Food Sci. Technol. 2016, 72, 559–565. [Google Scholar] [CrossRef]
- Appeldoorn, M.M.; Vincken, J.P.; Gruppen, H.; Hollman, P.C. Procyanidin dimers A1, A2, and B2 are absorbed without conjugation or methylation from the small intestine of rats. J. Nutr. 2009, 139, 1469–1473. [Google Scholar] [CrossRef]
- Ou, K.; Percival, S.S.; Zou, T.; Khoo, C.; Gu, L. Transport of cranberry A-type procyanidin dimers, trimers, and tetramers across monolayers of human intestinal epithelial Caco-2 cells. J. Agric. Food Chem. 2012, 60, 1390–1396. [Google Scholar] [CrossRef]
- Sánchez-Patán, F.; Cueva, C.; Monagas, M.; Walton, C.E.; Gibson, G.R.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V.; Bartolomé, B. Gut microbial catabolism of grape seed flavan-3-ols by human faecal microbiota. Targetted analysis of precursor compounds, intermediate metabolites and end-products. Food Chem. 2012, 131, 337–347. [Google Scholar] [CrossRef]
- Sánchez-Patán, F.; Barroso, E.; van de Wiele, T.; Jiménez-Girón, A.; Martín-Alvarez, P.J.; Moreno-Arribas, M.V.; Martínez-Cuesta, M.C.; Peláez, C.; Requena, T.; Bartolomé, B.; et al. Comparative in vitro fermentations of cranberry and grape seed polyphenols with colonic microbiota. Food Chem. 2015, 183, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, J.I.; Kwik-Uribe, C.; Keen, C.L.; Schroeter, H. Intake of dietary procyanidins does not contribute to the pool of circulating flavanols in humans. Am. J. Clin. Nutr. 2012, 95, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Wiese, S.; Esatbeyoglu, T.; Winterhalter, P.; Kruse, H.P.; Winkler, S.; Bub, A.; Kulling, S.E. Comparative biokinetics and metabolism of pure monomeric, dimeric, and polymeric flavan-3-ols: A randomized cross-over study in humans. Mol. Nutr. Food Res. 2015, 59, 610–621. [Google Scholar] [CrossRef]
- He, J.; Magnuson, B.A.; Giusti, M.M. Analysis of anthocyanins in rat intestinal contents--impact of anthocyanin chemical structure on fecal excretion. J. Agric. Food Chem. 2005, 53, 2859–2866. [Google Scholar] [CrossRef]
- Choy, Y.Y.; Jaggers, G.K.; Oteiza, P.I.; Waterhouse, A.L. Bioavailability of intact proanthocyanidins in the rat colon after ingestion of grape seed extract. J. Agric. Food Chem. 2013, 61, 121–127. [Google Scholar] [CrossRef]
- Rubert, J.; Gatto, P.; Pancher, M.; Sidarovich, V.; Curti, C.; Mena, P.; del Rio, D.; Quattrone, A.; Mattivi, F. A screening of native (poly)phenols and gut-related metabolites on 3D HCT116 spheroids reveals gut health benefits of a flavan-3-ol metabolite. Mol. Nutr. Food Res. 2022, 66, e2101043. [Google Scholar] [CrossRef]
- Cecarini, V.; Cuccioloni, M.; Zheng, Y.; Bonfili, L.; Gong, C.; Angeletti, M.; Mena, P.; del Rio, D.; Eleuteri, A.M. Flavan-3-ol microbial metabolites modulate proteolysis in neuronal cells reducing amyloid-beta (1–42) levels. Mol. Nutr. Food Res. 2021, 65, e2100380. [Google Scholar] [CrossRef]
- Dufour, C.; Villa-Rodriguez, J.A.; Furger, C.; Lessard-Lord, J.; Gironde, C.; Rigal, M.; Badr, A.; Desjardins, Y.; Guyonnet, D. Cellular antioxidant effect of an aronia extract and its polyphenolic fractions enriched in proanthocyanidins, phenolic Acids, and anthocyanins. Antioxidants 2022, 11, 1561. [Google Scholar] [CrossRef]
- Choy, Y.Y.; Quifer-Rada, P.; Holstege, D.M.; Frese, S.A.; Calvert, C.C.; Mills, D.A.; Lamuela-Raventos, R.M.; Waterhouse, A.L. Phenolic metabolites and substantial microbiome changes in pig feces by ingesting grape seed proanthocyanidins. Food Funct. 2014, 5, 2298–2308. [Google Scholar] [CrossRef]
- Reichardt, N.; Duncan, S.H.; Young, P.; Belenguer, A.; McWilliam Leitch, C.; Scott, K.P.; Flint, H.J.; Louis, P. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014, 8, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Jonkers, D.M.; Bast, A.; Vanhoutvin, S.A.; Fischer, M.A.; Kodde, A.; Troost, F.J.; Venema, K.; Brummer, R.J. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin. Nutr. 2009, 28, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Berni Canani, R.; di Costanzo, M.; Leone, L. The epigenetic effects of butyrate: Potential therapeutic implications for clinical practice. Clin. Epigenetics 2012, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012, 143, 913–916. [Google Scholar] [CrossRef]
- Han, M.; Song, P.; Huang, C.; Rezaei, A.; Farrar, S.; Brown, M.A.; Ma, X. Dietary grape seed proanthocyanidins (GSPs) improve weaned intestinal microbiota and mucosal barrier using a piglet model. Oncotarget 2016, 7, 80313–80326. [Google Scholar] [CrossRef]
- Casanova-Martí, À.; Serrano, J.; Portune, K.J.; Sanz, Y.; Blay, M.T.; Terra, X.; Ardévol, A.; Pinent, M. Grape seed proanthocyanidins influence gut microbiota and enteroendocrine secretions in female rats. Food Funct. 2018, 9, 1672–1682. [Google Scholar] [CrossRef]
- Ferreira, Y.A.M.; Jamar, G.; Estadella, D.; Pisani, L.P. Proanthocyanidins in grape seeds and their role in gut microbiota-white adipose tissue axis. Food Chem. 2023, 404, 134405. [Google Scholar] [CrossRef]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary polyphenols promote growth of the gut bacterium Akkermansia muciniphila and attenuate high-fat diet-induced metabolic syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef]
- Griffin, L.E.; Witrick, K.A.; Klotz, C.; Dorenkott, M.R.; Goodrich, K.M.; Fundaro, G.; McMillan, R.P.; Hulver, M.W.; Ponder, M.A.; Neilson, A.P.; et al. Alterations to metabolically active bacteria in the mucosa of the small intestine predict anti-obesity and anti-diabetic activities of grape seed extract in mice. Food Funct. 2017, 8, 3510–3522. [Google Scholar] [CrossRef]
- Zhang, L.; Carmody, R.N.; Kalariya, H.M.; Duran, R.M.; Moskal, K.; Poulev, A.; Kuhn, P.; Tveter, K.M.; Turnbaugh, P.J.; Raskin, I.; et al. Grape proanthocyanidin-induced intestinal bloom of Akkermansia muciniphila is dependent on its baseline abundance and precedes activation of host genes related to metabolic health. J. Nutr. Biochem. 2018, 56, 142–151. [Google Scholar] [CrossRef]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Masumoto, S.; Terao, A.; Yamamoto, Y.; Mukai, T.; Miura, T.; Shoji, T. Non-absorbable apple procyanidins prevent obesity associated with gut microbial and metabolomic changes. Sci. Rep. 2016, 6, 31208. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; de Vos, W.M. Next-generation beneficial microbes: The case of Akkermansia muciniphila. Front. Microbiol. 2017, 8, 1765. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Q.; Cheng, L.; Buch, H.; Zhang, F. Akkermansia muciniphila is a promising probiotic. Microb. Biotechnol. 2019, 12, 1109–1125. [Google Scholar] [CrossRef]
- Zhai, Q.; Feng, S.; Arjan, N.; Chen, W. A next generation probiotic, Akkermansia muciniphila. Crit. Rev. Food Sci. Nutr. 2019, 59, 3227–3236. [Google Scholar] [CrossRef]
- Ansaldo, E.; Slayden, L.C.; Ching, K.L.; Koch, M.A.; Wolf, N.K.; Plichta, D.R.; Brown, E.M.; Graham, D.B.; Xavier, R.J.; Moon, J.J.; et al. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science 2019, 364, 1179–1184. [Google Scholar] [CrossRef]
- Cheng, D.; Xie, M.Z. A review of a potential and promising probiotic candidate-Akkermansia muciniphila. J. Appl. Microbiol. 2021, 130, 1813–1822. [Google Scholar] [CrossRef]
- Bae, M.; Cassilly, C.D.; Liu, X.; Park, S.M.; Tusi, B.K.; Chen, X.; Kwon, J.; Filipčík, P.; Bolze, A.S.; Liu, Z.; et al. Akkermansia muciniphila phospholipid induces homeostatic immune responses. Nature 2022, 608, 168–173. [Google Scholar] [CrossRef]
- Belzer, C.; de Vos, W.M. Microbes inside--from diversity to function: The case of Akkermansia. ISME J. 2012, 6, 1449–1458. [Google Scholar] [CrossRef]
- Ganesh, B.P.; Klopfleisch, R.; Loh, G.; Blaut, M. Commensal Akkermansia muciniphila exacerbates gut inflammation in Salmonella Typhimurium-infected gnotobiotic mice. PLoS ONE 2013, 8, e74963. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Teixeira, C.; Marungruang, N.; Sae-Lim, W.; Tareke, E.; Andersson, R.; Fåk, F.; Nyman, M. Barley malt increases hindgut and portal butyric acid, modulates gene expression of gut tight junction proteins and Toll-like receptors in rats fed high-fat diets, but high advanced glycation end-products partially attenuate the effects. Food Funct. 2015, 6, 3165–3176. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.H.; Krych, L.; Nielsen, D.S.; Vogensen, F.K.; Hansen, L.H.; Sørensen, S.J.; Buschard, K.; Hansen, A.K. Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia 2012, 55, 2285–2294. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Guo, X.; Xia, X.; Tang, R.; Zhou, J.; Zhao, H.; Wang, K. Development of a real-time PCR method for Firmicutes and Bacteroidetes in faeces and its application to quantify intestinal population of obese and lean pigs. Lett. Appl. Microbiol. 2008, 47, 367–373. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Asou, Y.; Ishiyama, K.; Okawa, A.; Kanno, T.; Niwano, Y. Proanthocyanidin-rich grape seed extract modulates intestinal microbiota in ovariectomized mice. J. Food Sci. 2018, 83, 1149–1152. [Google Scholar] [CrossRef] [PubMed]
- Mathison, B.D.; Kimble, L.L.; Kaspar, K.L.; Khoo, C.; Chew, B.P. Consumption of cranberry beverage improved endogenous antioxidant status and protected against bacteria adhesion in healthy humans: A randomized controlled trial. Nutr. Res. 2014, 34, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Maffei, M.E. Metabolomics-based profiling, antioxidant power, and uropathogenic bacterial anti-adhesion activity of SP4™, a formulation with a high content of type-A proanthocyanidins. Antioxidants 2022, 11, 1234. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.Y.; Yang, C.; Xu, D.F.; Xia, H.; Yang, L.G.; Sun, G.J. Consumption of cranberry as adjuvant therapy for urinary tract infections in susceptible populations: A systematic review and meta-analysis with trial sequential analysis. PLoS ONE 2021, 16, e0256992. [Google Scholar] [CrossRef]
- Krachler, A.M.; Orth, K. Targeting the bacteria-host interface: Strategies in anti-adhesion therapy. Virulence 2013, 4, 284–294. [Google Scholar] [CrossRef]
- Savickiene, N.; Jekabsone, A.; Raudone, L.; Abdelgeliel, A.S.; Cochis, A.; Rimondini, L.; Makarova, E.; Grinberga, S.; Pugovics, O.; Dambrova, M.; et al. Efficacy of proanthocyanidins from Pelargonium sidoides root extract in reducing P. gingivalis viability while preserving oral commensal S. salivarius. Materials 2018, 11, 1499. [Google Scholar] [CrossRef]
- Nawrot-Hadzik, I.; Matkowski, A.; Hadzik, J.; Dobrowolska-Czopor, B.; Olchowy, C.; Dominiak, M.; Kubasiewicz-Ross, P. Proanthocyanidins and flavan-3-ols in the prevention and treatment of periodontitis-Antibacterial effects. Nutrients 2021, 13, 165. [Google Scholar] [CrossRef]
- Ulrey, R.K.; Barksdale, S.M.; Zhou, W.; van Hoek, M.L. Cranberry proanthocyanidins have anti-biofilm properties against Pseudomonas aeruginosa. BMC Complement Altern. Med. 2014, 14, 499. [Google Scholar] [CrossRef]
- Maisuria, V.B.; Los Santos, Y.L.; Tufenkji, N.; Déziel, E. Cranberry-derived proanthocyanidins impair virulence and inhibit quorum sensing of Pseudomonas aeruginosa. Sci. Rep. 2016, 6, 30169. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).