Antiamnesic Effects of Feralolide Isolated from Aloe vera Resin Miller against Learning Impairments Induced in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Sample Collection and Identification
2.4. Extraction and Isolation
2.5. Assessment of Feralolide in In Vitro Assays
2.5.1. DPPH Radical Scavenging Assay
2.5.2. ABTS Radical Scavenging Assay
2.5.3. Anti-Cholinesterase Assays
2.6. Assessment of Behavioral Parameters in In Vivo Assays
2.6.1. Assessment of Acute Toxicity Study of Feralolide
2.6.2. Assessment of Antiamnesic Activity
Experimental Design
2.6.3. Novel Object Recognition Test (NORT)
2.6.4. Assessment of Morris Water Maze Test (MWMT)
2.6.5. Assessment in Elevated Plus Maze (EPM)
2.6.6. Assessment in Passive Avoidance Test
2.6.7. Video Recording
2.7. Statistical Analysis
2.8. Molecular Docking
3. Results
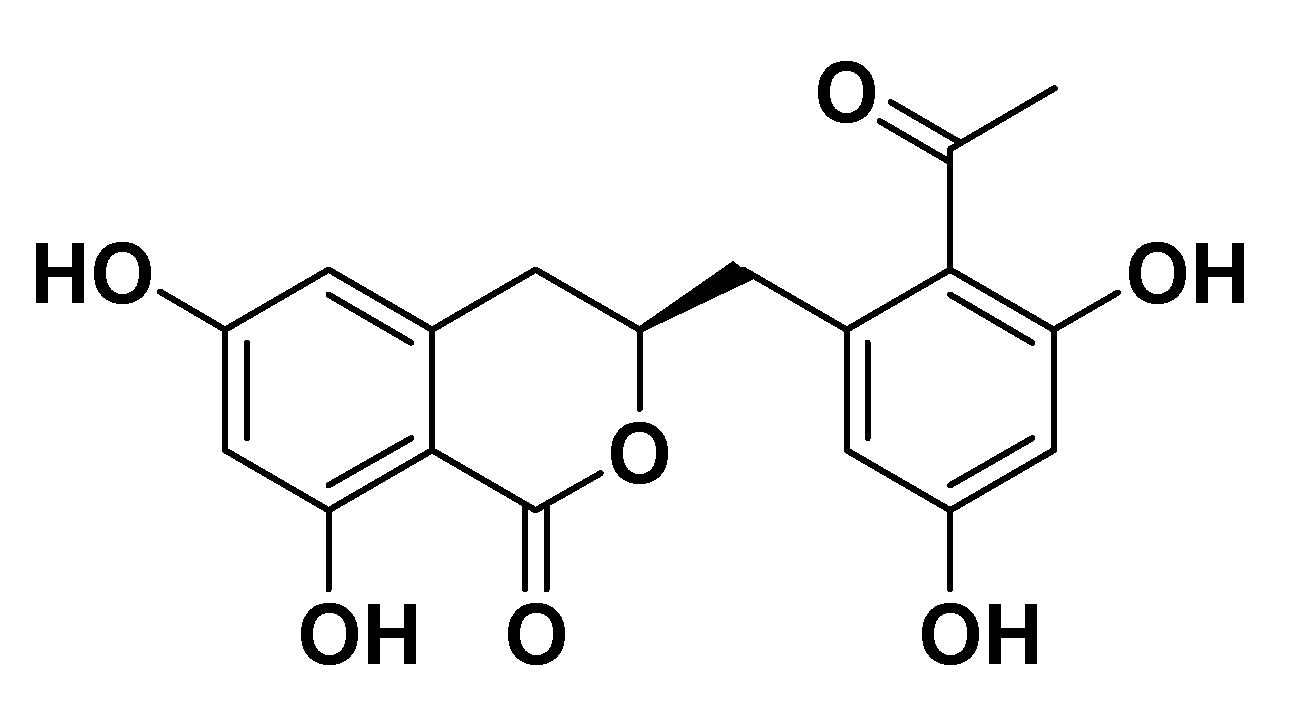
3.1. Chemistry
3.2. Assessment of Free Radical Scavenging Activities
3.2.1. ABTS Free Radical Activity
3.2.2. DPPH Free Radical Activity
3.2.3. Inhibition of AChE Activity
3.2.4. Inhibition of BuChE Activity
3.3. In Vivo Pharmacological Assessment
3.3.1. Effects of Feralolide on the Acute Toxicity of Animals and Their General Behaviors
3.3.2. Effect of Feralolide in Novel Object Recognition Test
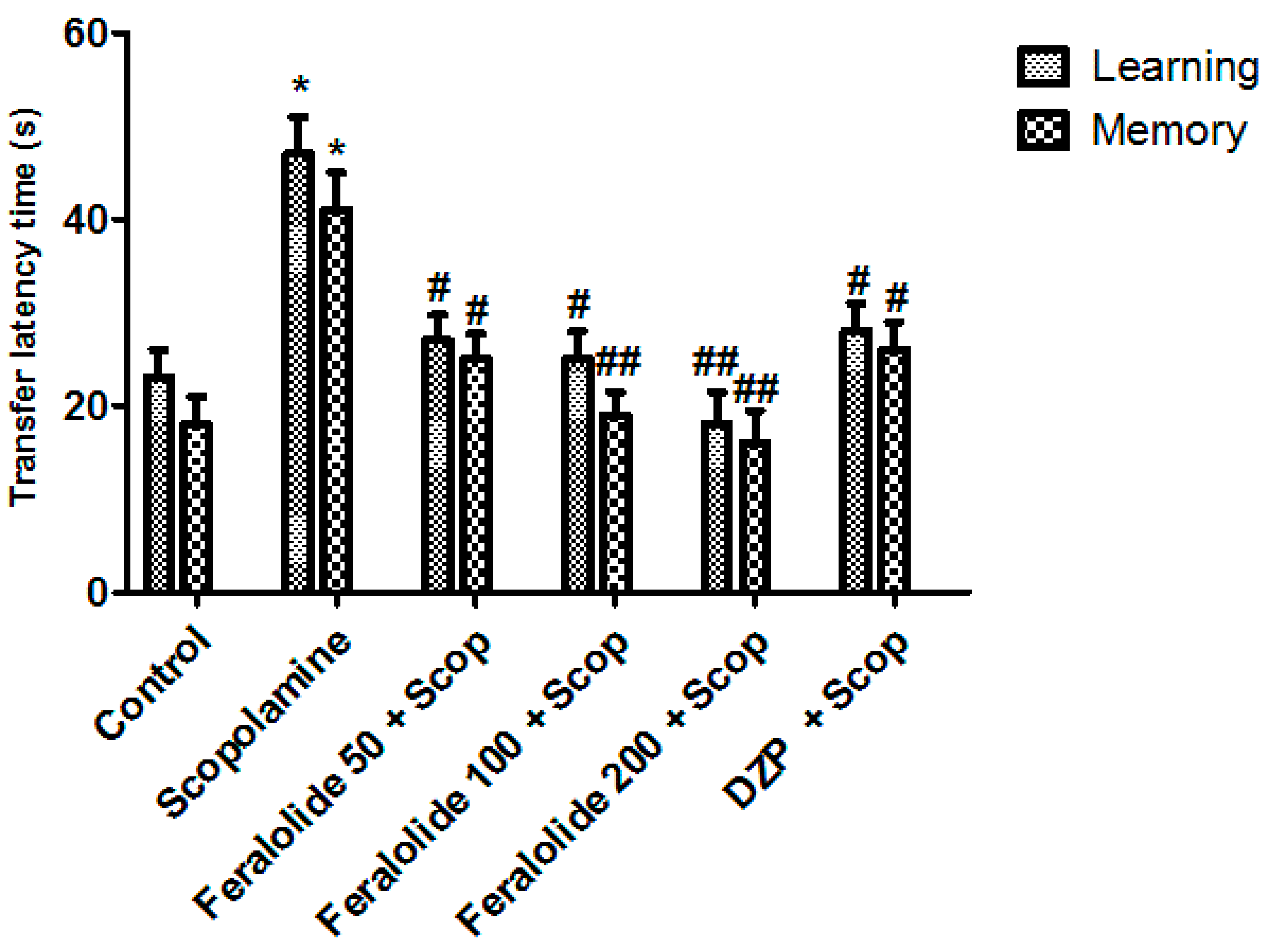
3.3.3. Effect of Feralolide in Morris Water Maze Test (MWMT)
3.3.4. Assessment of Feralolide in Elevated Plus Maze Test (EPM)
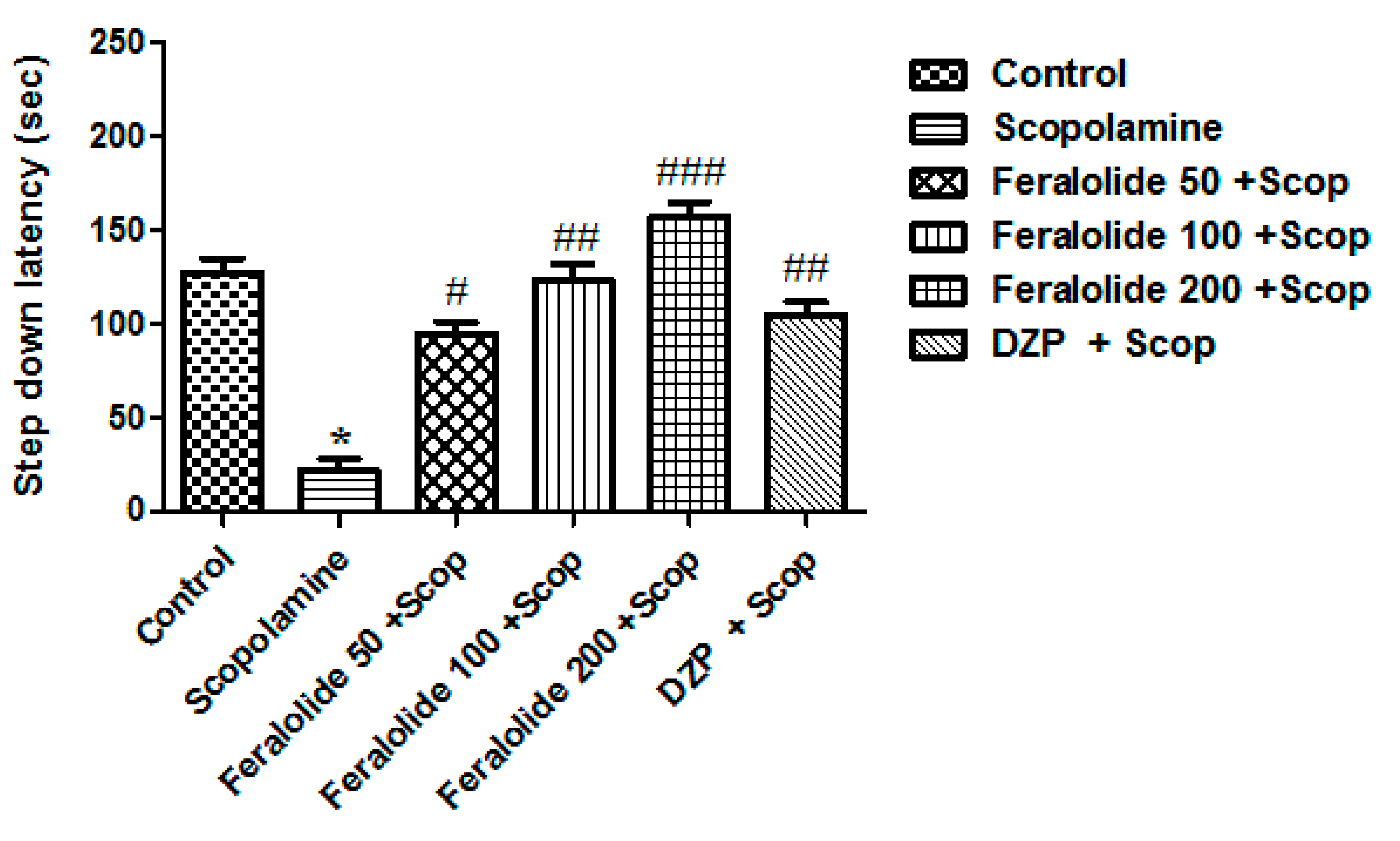
3.3.5. Effect of Feralolide on Passive Avoidance Test (PAT)
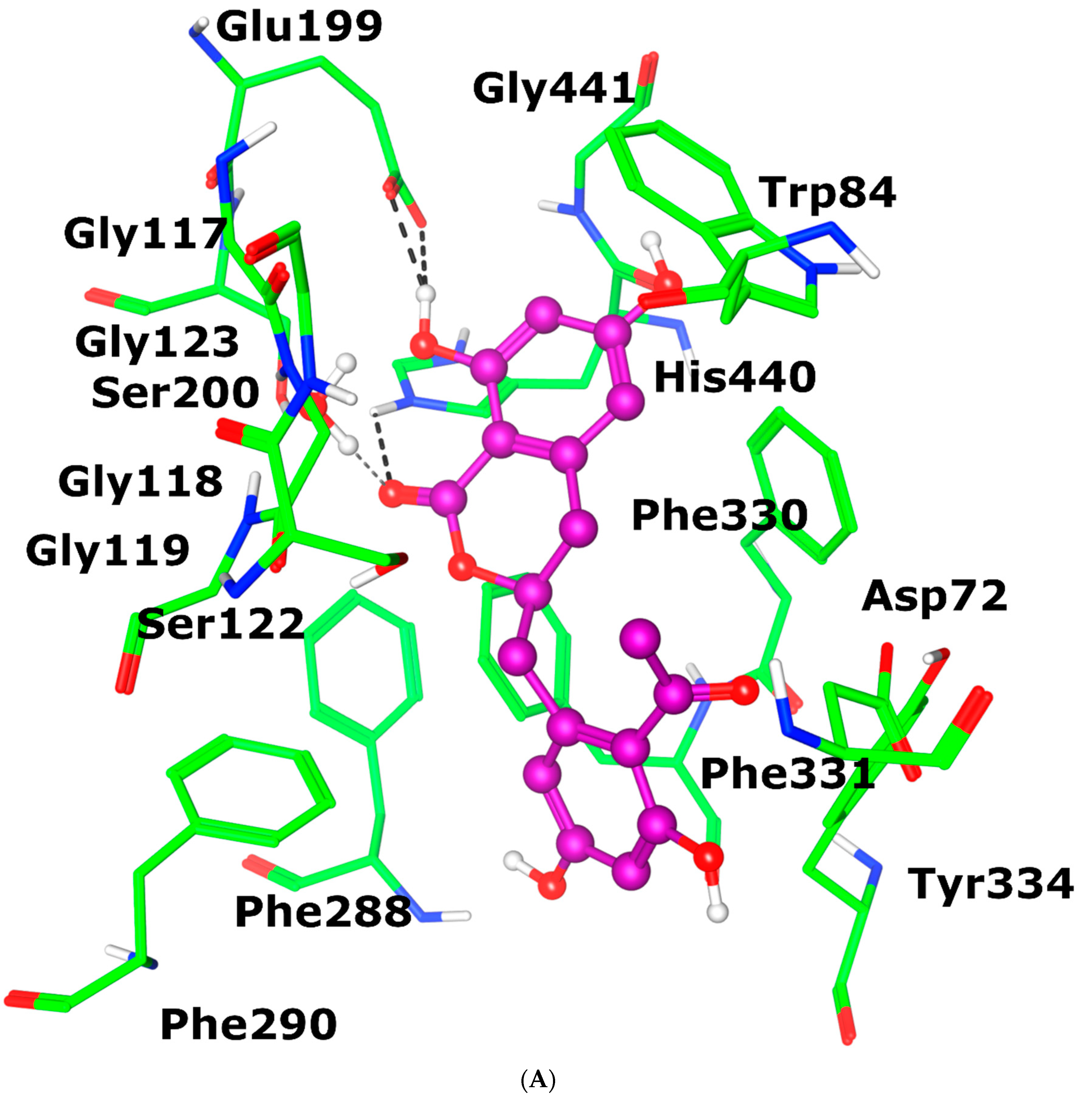
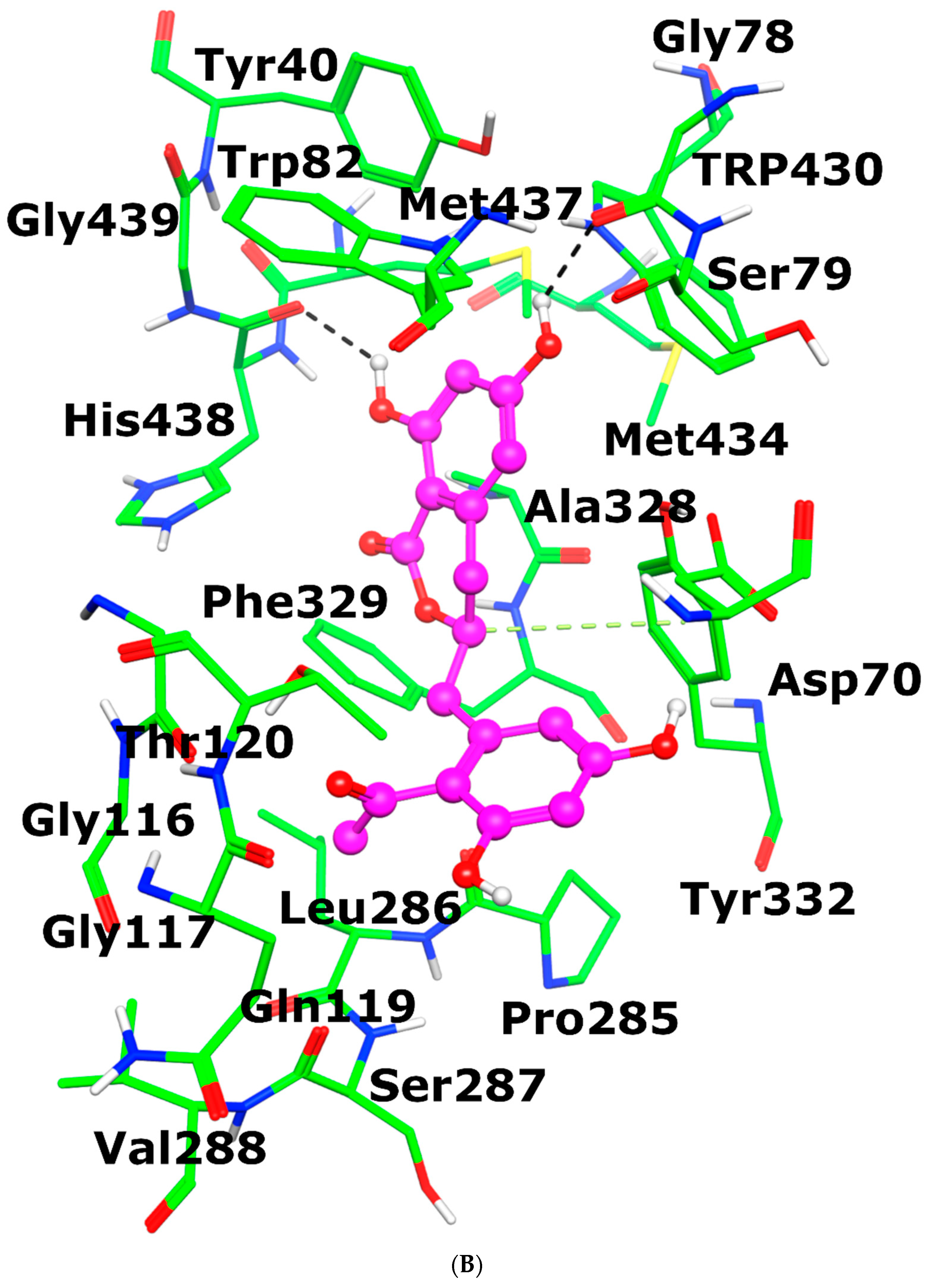
3.3.6. Molecular Docking of Feralolide in AChE and BuChE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yalla Reddy, Y.; Mohana Lakshmi, S.; Saravana, K. Review on effect of natural memory enhancing drugs on dementia. Int. J. Phytopharm. 2010, 1, 1–7. [Google Scholar]
- Faqih, N.T.; Ashoor, A.F.; Alshaikh, S.A.; Maglan, A.F.; Jastaniah, N. Association of Alzheimer’s Disease and Insulin Resistance in King Abdulaziz Medical City, Jeddah. Cureus 2021, 13, e19811. [Google Scholar] [CrossRef]
- Vijay, N.; Tilak, A.V.; Dewda, P.R.; Komma, R.; Tilak, M.A.; More, B. Modulation of working memory by mentat and donepezil using scopolamine induced amnesia in rats. Am. J. Pharmtech Res. 2012, 2, 311–319. [Google Scholar]
- Lazarus, G.G. In Vitro Anti-Platelet Aggregation Activity of the Extracts of Bulbine Natalensis. Ph.D. Thesis, University of Zululand, KwaDlangezwa, South Africa, 2011. [Google Scholar]
- Thompson, S.; Lanctôt, K.L.; Herrmann, N. The benefits and risks associated with cholinesterase inhibitor therapy in Alzheimer’s disease. Expert Opin. Drug Saf. 2004, 3, 425–440. [Google Scholar] [CrossRef]
- Surjushe, A.; Vasani, R.; Saple, D.G. Aloe vera: A short review. Indian J. Dermatol. 2008, 53, 163–166. [Google Scholar] [CrossRef]
- Tao, L.; Xie, J.; Wang, Y.; Wang, S.; Wu, S.; Wang, Q.; Ding, H. Protective effects of aloe-emodin on scopolamine-induced memory impairment in mice and H2O2-induced cytotoxicity in PC12 cells. Bioorganic Med. Chem. Lett. 2014, 24, 5385–5389. [Google Scholar] [CrossRef]
- Rahman, M.S.; Islam, R.; Rana, M.M.; Spitzhorn, L.-S.; Rahman, M.S.; Adjaye, J.; Asaduzzaman, S.M. Characterization of burn wound healing gel prepared from human amniotic membrane and Aloe vera extract. BMC Complement. Altern. Med. 2019, 19, 115. [Google Scholar] [CrossRef]
- Rahman, S.; Carter, P.; Bhattarai, N. Aloe vera for tissue engineering applications. J. Funct. Biomater. 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Abd-Alla, H.I.; Moharram, F.A.; Gaara, A.H.; El-Safty, M.M. Phytoconstituents of Jatropha curcas L. leaves and their immunomodulatory activity on humoral and cell-mediated immune response in chicks. Z. Für. Nat. C 2009, 64, 495–501. [Google Scholar] [CrossRef]
- Karim, N.; Curmi, J.; Gavande, N.; Johnston, G.A.; Hanrahan, J.R.; Tierney, M.L.; Chebib, M. 2′-Methoxy-6-methylflavone: A novel anxiolytic and sedative with subtype selective activating and modulating actions at GABAA receptors. Br. J. Pharmacol. 2012, 165, 880–896. [Google Scholar] [CrossRef]
- Cheng, Z.; Moore, J.; Yu, L. High-throughput relative DPPH radical scavenging capacity assay. J. Agric. Food Chem. 2006, 54, 7429–7436. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Duh, P.D. Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J. Agric. Food Chem. 1994, 42, 629–632. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Khalil, A.; Abass, K. The measurements of the cholinesterase activity of brain and plasma in rabbits by using modified Michel and Ellman assays. Insights Enzym. Res 2017, 1, 9. [Google Scholar] [CrossRef]
- Mugwagwa, A.T.; Gadaga, L.L.; Pote, W.; Tagwireyi, D. Antiamnesic Effects of a Hydroethanolic Extract of Crinum macowanii on Scopolamine-Induced Memory Impairment in Mice. J. Neurodegener. Dis. 2015, 2015, 242505. [Google Scholar] [CrossRef]
- Rodríguez, E.R.; Martín, J.D.; Romero, C.D. Aloe vera as a functional ingredient in foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 305–326. [Google Scholar] [CrossRef]
- Krishna, K.V.; Saha, R.N.; Dubey, S.K. Biophysical, biochemical, and behavioral implications of ApoE3 conjugated donepezil nanomedicine in a Aβ1–42 induced Alzheimer’s disease Rat Model. ACS Chem. Neurosci. 2020, 11, 4139–4151. [Google Scholar] [CrossRef]
- Nazir, S.; Anwar, F.; Saleem, U.; Ahmad, B.; Raza, Z.; Sanawar, M.; Ismail, T. Drotaverine Inhibitor of PDE4: Reverses the Streptozotocin Induced Alzheimer’s Disease in Mice. Neurochem. Res. 2021, 46, 1814–1829. [Google Scholar] [CrossRef]
- Carobrez, A.; Bertoglio, L. Ethological and temporal analyses of anxiety-like behavior: The elevated plus-maze model 20 years on. Neurosci. Biobehav. Rev. 2005, 29, 1193–1205. [Google Scholar] [CrossRef]
- Itoh, J.; Nabeshima, T.; Kameyama, T. Utility of an elevated plus-maze for the evaluation of memory in mice: Effects of nootropics, scopolamine and electroconvulsive shock. Psychopharmacology 1990, 101, 27–33. [Google Scholar] [CrossRef]
- Sachan, A.; Kumar, S.; Singh, H.; Shankar, P.; Kumar, D.; Sachan, A.K.; Dixit, R.K. Potential Anti-Anxiety Effect of Mucuna pruriens in Experimental Model of Swiss Albino Mice. Pharmacol. Toxicol. Biomed. Rep. 2015, 1, 20–23. [Google Scholar] [CrossRef]
- Jafari-Sabet, M.; Mofidi, H.; Attarian-Khosroshahi, M.-S. NMDA receptors in the dorsal hippocampal area are involved in tramadol state-dependent memory of passive avoidance learning in mice. Can. J. Physiol. Pharmacol. 2018, 96, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Halim, S.A.; Uddin, R.; Madura, J.D. Benchmarking docking and scoring protocol for the identification of potential acetylcholinesterase inhibitors. J. Mol. Graph. Model. 2010, 28, 870–882. [Google Scholar]
- Speranza, M.J.; Bagley, A.; Lynch, R. Cells enriched for catalase are sensitized to the toxicities of bleomycin, adriamycin, and paraquat. J. Biol. Chem. 1993, 268, 19039–19043. [Google Scholar] [CrossRef]
- Shaker, M.A. The effect of Aloe Vera extract on Acetylcholinesterase AchE and Monoamine Oxidase MAO enzymes. Al-Mustansiriyah J. Sci. 2019, 29, 88–92. [Google Scholar] [CrossRef]
- Murray, A.P.; Faraoni, M.B.; Castro, M.J.; Alza, N.P.; Cavallaro, V. Natural AChE Inhibitors from Plants and their Contribution to Alzheimer’s Disease Therapy. Curr. Neuropharmacol. 2013, 11, 388–413. [Google Scholar] [CrossRef] [PubMed]
- Youdim, K.A.; Qaiser, M.Z.; Begley, D.J.; Rice-Evans, C.A.; Abbott, N.J. Flavonoid permeability across an in situ model of the blood–brain barrier. Free Radic. Biol. Med. 2004, 36, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Martínez, C.; Medina-Torres, L.; González-Laredo, R.; Calderas, F.; Sánchez-Olivares, G.; Herrera-Valencia, E.; Infante, J.G.; Rocha-Guzman, N.; Rodriguez-Ramirez, J. Study of spray drying of the Aloe vera mucilage (Aloe vera barbadensis Miller) as a function of its rheological properties. LWT-Food Sci. Technol. 2014, 55, 426–435. [Google Scholar] [CrossRef]
- Budson, A.E.; Solomon, P.R. Memory Loss, Alzheimer’s Disease, and Dementia-E-Book: A Practical Guide for Clinicians; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Bajo, R.; Pusil, S.; López, M.; Canuet, L.; Pereda, E.; Osipova, D.; Maestú, F.; Pekkonen, E. Scopolamine effects on functional brain connectivity: A pharmacological model of Alzheimer’s disease. Sci. Rep. 2015, 5, 9748. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Zhou, G.S.; Tan, Y.J.; Tao, H.J.; Chen, J.Q.; Pu, Z.J.; Ma, J.Y.; She, W.; Kang, A.; et al. Studies of the Anti-amnesic Effects and Mechanisms of Single and Combined Use of Donepezil and Ginkgo Ketoester Tablet on Scopolamine-Induced Memory Impairment in Mice. Oxid. Med. Cell Longev. 2019, 2019, 8636835. [Google Scholar] [CrossRef] [PubMed]
- Bhuvanendran, S.; Kumari, Y.; Othman, I.; Shaikh, M.F. Amelioration of Cognitive Deficit by Embelin in a Scopolamine-Induced Alzheimer’s Disease-Like Condition in a Rat Model. Front. Pharmacol. 2018, 9, 665. [Google Scholar] [CrossRef] [PubMed]
- Flood, J.F.; Cherkin, A. Scopolamine effects on memory retention in mice: A model of dementia? Behav. Neural. Biol. 1986, 45, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A.; Kiametis, A.S.; Treptow, W. Donepezil Inhibits Acetylcholinesterase via Multiple Binding Modes at Room Temperature. J. Chem. Inf. Model 2020, 60, 3463–3471. [Google Scholar] [CrossRef] [PubMed]


| Group | Group Category | Test Solution | Route |
|---|---|---|---|
| Group I | Normal control | Vehicle 8 mL/kg | p.o. |
| Group II | Negative control | Scopolamine 1 mg/kg | i.p. |
| Group III | Positive control | Donezepil 2 mg/kg + scopolamine 1 mg/kg | p.o/i.p |
| Group IV | Treatment group-1 | Feralolide 1 mg/kg + scopolamine 1 mg/kg | p.o/i.p |
| Group V | Treatment group-2 | Feralolide 5 mg/kg + scopolamine 1 mg/kg | p.o/i.p |
| Group VI | Treatment group-3 | Feralolide 10 mg/kg + scopolamine 1 mg/kg | p.o/i.p |
| Sample | Concentration (μg/mL) | % ABTS Scavenging Mean ± SEM | IC50 (µg/mL) |
|---|---|---|---|
| Feralolide | 1000 µg/mL | 70.96 ± 0.37 | 170 |
| 500 µg/mL | 67.56 ± 0.58 | ||
| 250 µg/mL | 49.72 ± 0.67 | ||
| 125 µg/mL | 42.12 ± 1.05 | ||
| 62.5 µg/mL | 28.99 ± 0.36 | ||
| 31.25 µg/mL | 11.68 ± 0.56 | ||
| Ascorbic acid | 1000 µg/mL | 83.94 ± 0.47 | 130 |
| 500 µg/mL | 74.68 ± 0.28 | ||
| 250 µg/mL | 63.98 ± 1.00 | ||
| 125 µg/mL | 49.67 ± 0.57 | ||
| 62.5 µg/mL | 45.79 ± 1.09 | ||
| 31.25 µg/mL | 39.73 ± 0.65 |
| Sample | Concentration (μg/mL) | % DPPH Scavenging Mean ± SEM | IC50 (µg/mL) |
|---|---|---|---|
| Feralolide | 1000 µg/mL | 74.98 ± 0.29 | 220 |
| 500 µg/mL | 64.53 ± 0.18 | ||
| 250 µg/mL | 44.9 ± 0.25 | ||
| 125 µg/mL | 39.68 ± 0.19 | ||
| 62.5 µg/mL | 34.03 ± 0.19 | ||
| 31.25 µg/mL | 29.87 ± 0.18 | ||
| Ascorbic acid | 1000 µg/mL | 86.78 ± 0.40 | 63 |
| 500 µg/mL | 74.9 ± 0.35 | ||
| 250 µg/mL | 67.98 ± 0.37 | ||
| 125 µg/mL | 52.87 ± 0.86 | ||
| 62.5 µg/mL | 46.95 ± 0.18 | ||
| 31.25 µg/mL | 30.29 ± 1.10 |
| Sample | Concentration (μg/mL) | % AChEI Scavenging Mean ± SEM | IC50 µg/mL |
|---|---|---|---|
| Feralolide | 1000 µg/mL | 86.91 ± 0.43 | 55 |
| 500 µg/mL | 84.21 ± 0.32 | ||
| 250 µg/mL | 76.98 ± 0.35 | ||
| 125 µg/mL | 61.89 ± 0.66 | ||
| 62.5 µg/mL | 48.88 ± 0.54 | ||
| 31.25 µg/mL | 30.73 ± 0.33 | ||
| Donepezil | 1000 µg/mL | 96.08 ± 0.43 | 60 |
| 500 µg/mL | 94.03 ± 0.32 | ||
| 250 µg/mL | 84.08 ± 0.23 | ||
| 125 µg/mL | 72.09 ± 0.16 | ||
| 62.5 µg/mL | 49.06 ± 0.15 | ||
| 31.25 µg/mL | 19.50 ± 0.23 |
| Sample | Concentration (μg/mL) | % BuChEI Scavenging Mean ± SEM | IC50 µg/mL |
|---|---|---|---|
| Feralolide | 1000 µg/mL | 79.8 ± 0.39 | 52 |
| 500 µg/mL | 68.9 ± 0.30 | ||
| 250 µg/mL | 61.73 ± 0.39 | ||
| 125 µg/mL | 52.68 ± 0.69 | ||
| 62.5 µg/mL | 37.98 ± 0.38 | ||
| 31.25 µg/mL | 24.93 ± 0.29 | ||
| Donepezil | 1000 µg/mL | 93.39 ± 0.48 | 67 |
| 500 µg/mL | 88.95 ± 0.30 | ||
| 250 µg/mL | 79.9 ± 0.29 | ||
| 125 µg/mL | 68.9 ± 0.58 | ||
| 62.5 µg/mL | 41.34 ± 0.48 | ||
| 31.25 µg/mL | 24.93 ± 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, I.; Mohanta, T.K.; Ihsan, N.; Halim, S.A.; Khan, A.; Rehman, N.U.; Khan, F.; Khalid, A.; Abdalla, A.N.; Karim, N.; et al. Antiamnesic Effects of Feralolide Isolated from Aloe vera Resin Miller against Learning Impairments Induced in Mice. Antioxidants 2023, 12, 161. https://doi.org/10.3390/antiox12010161
Khan I, Mohanta TK, Ihsan N, Halim SA, Khan A, Rehman NU, Khan F, Khalid A, Abdalla AN, Karim N, et al. Antiamnesic Effects of Feralolide Isolated from Aloe vera Resin Miller against Learning Impairments Induced in Mice. Antioxidants. 2023; 12(1):161. https://doi.org/10.3390/antiox12010161
Chicago/Turabian StyleKhan, Imran, Tapan Kumar Mohanta, Nuzhat Ihsan, Sobia Ahsan Halim, Ajmal Khan, Najeeb Ur Rehman, Faizullah Khan, Asaad Khalid, Ashraf N. Abdalla, Nasiara Karim, and et al. 2023. "Antiamnesic Effects of Feralolide Isolated from Aloe vera Resin Miller against Learning Impairments Induced in Mice" Antioxidants 12, no. 1: 161. https://doi.org/10.3390/antiox12010161
APA StyleKhan, I., Mohanta, T. K., Ihsan, N., Halim, S. A., Khan, A., Rehman, N. U., Khan, F., Khalid, A., Abdalla, A. N., Karim, N., & Al-Harrasi, A. (2023). Antiamnesic Effects of Feralolide Isolated from Aloe vera Resin Miller against Learning Impairments Induced in Mice. Antioxidants, 12(1), 161. https://doi.org/10.3390/antiox12010161














