Peroxiredoxin 6 Regulates Glutathione Peroxidase 1-Medited Glutamine Synthase Preservation in the Hippocampus of Chronic Epilepsy Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Chemicals
2.2. Generation of Chronic Epilepsy Rats
2.3. Drug Trials
2.4. Tissue Preparation and Immunohistochemistry
2.5. Data Analysis
3. Results
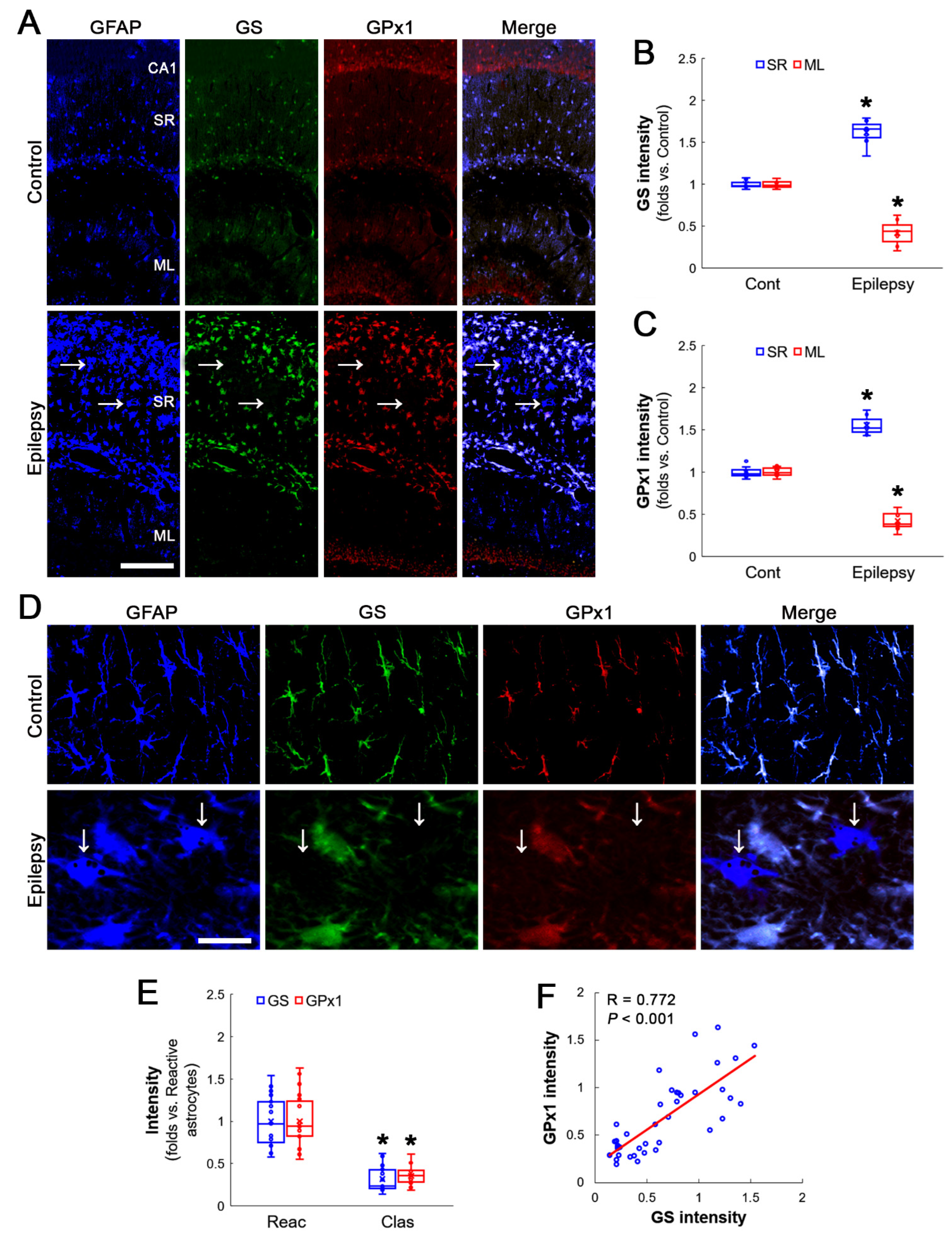
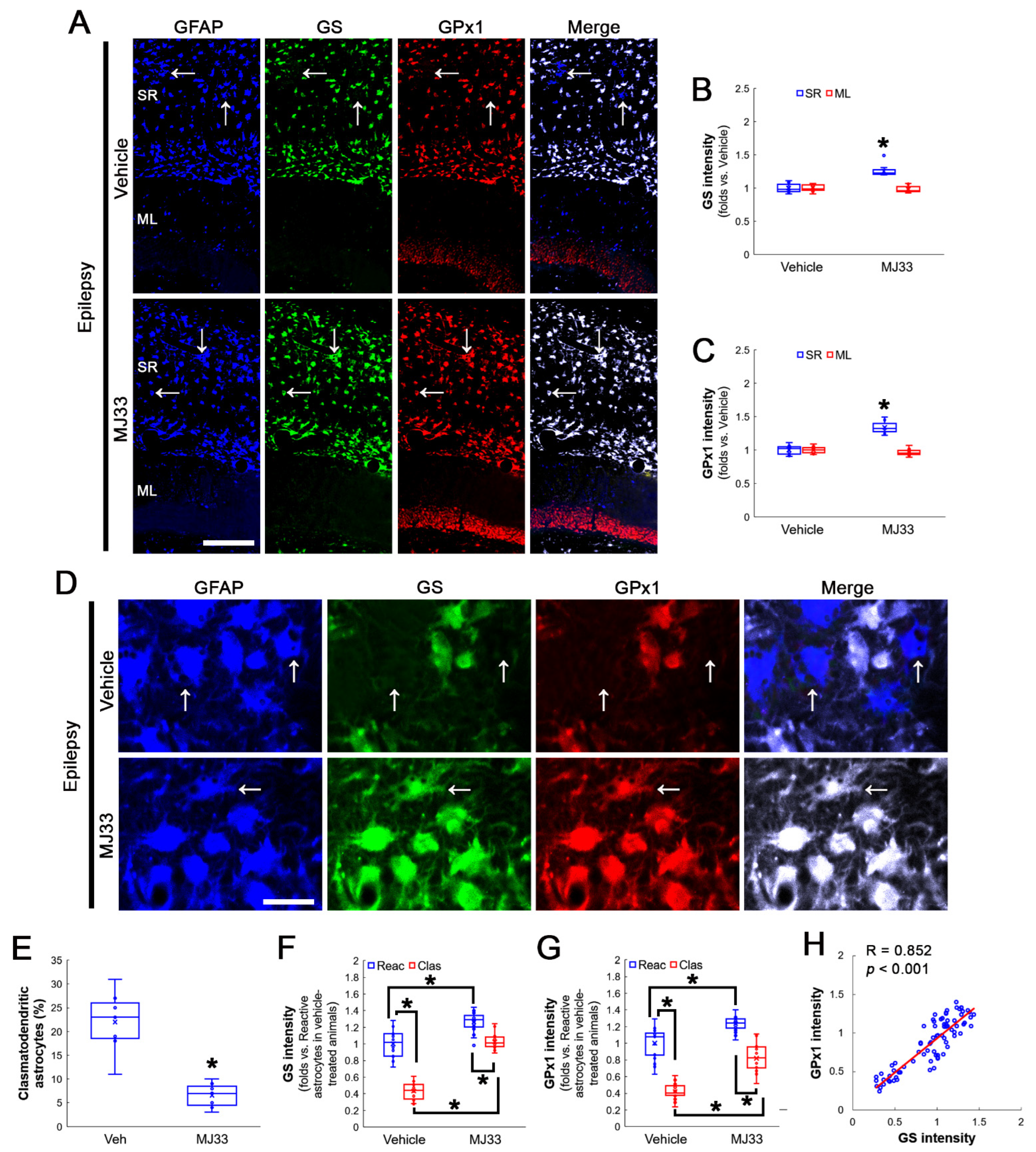
3.1. GS and GPx1 Expressions Are Downregulated in Clasmatodendritic CA1 Astrocytes, But Not Reactive CA1 Astrocytes
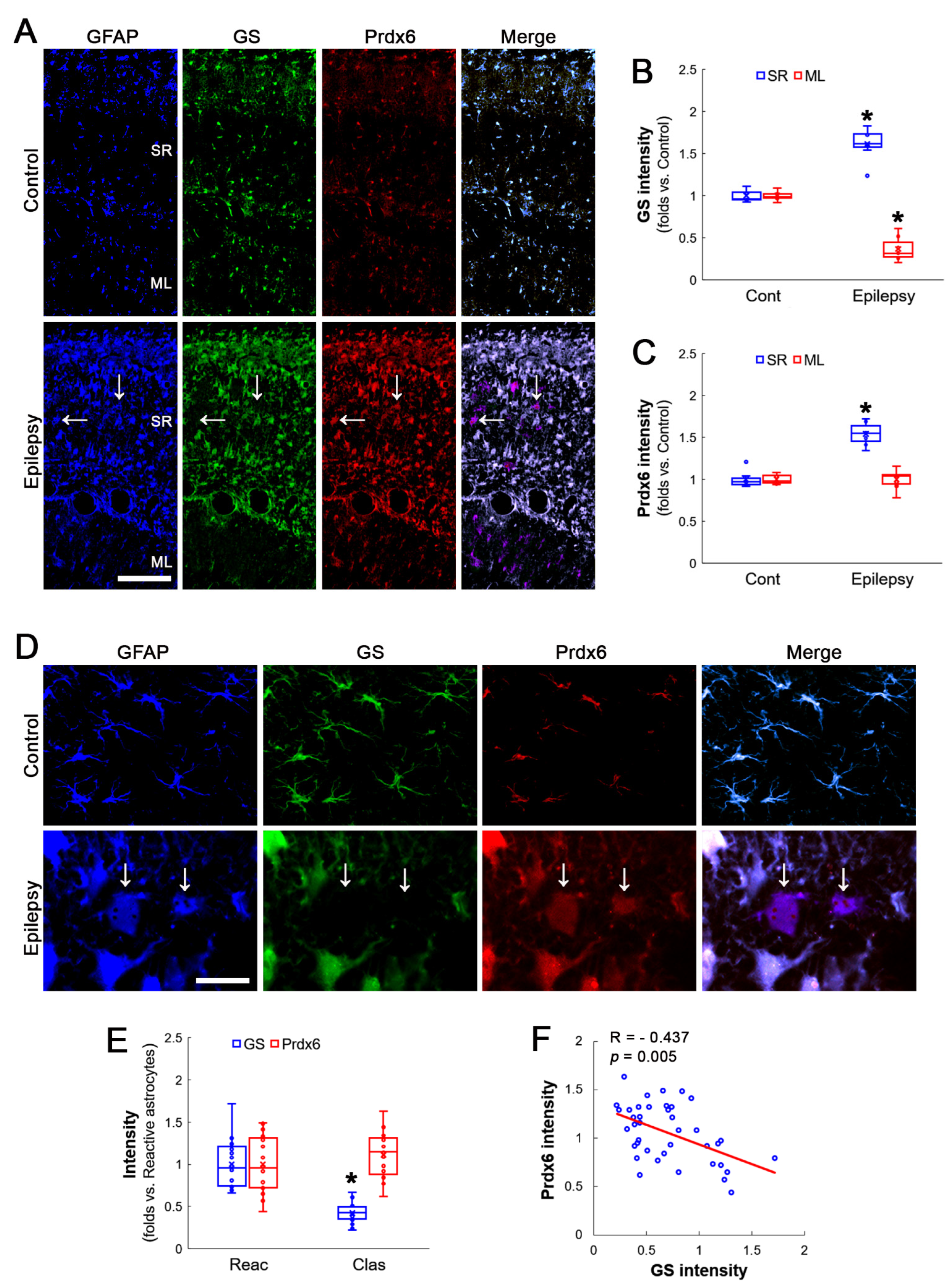
3.2. Prdx6 Expression Is Increased in Both Reactive CA1 Astrocytes and Clasmatodendritic CA1 Astrocytes
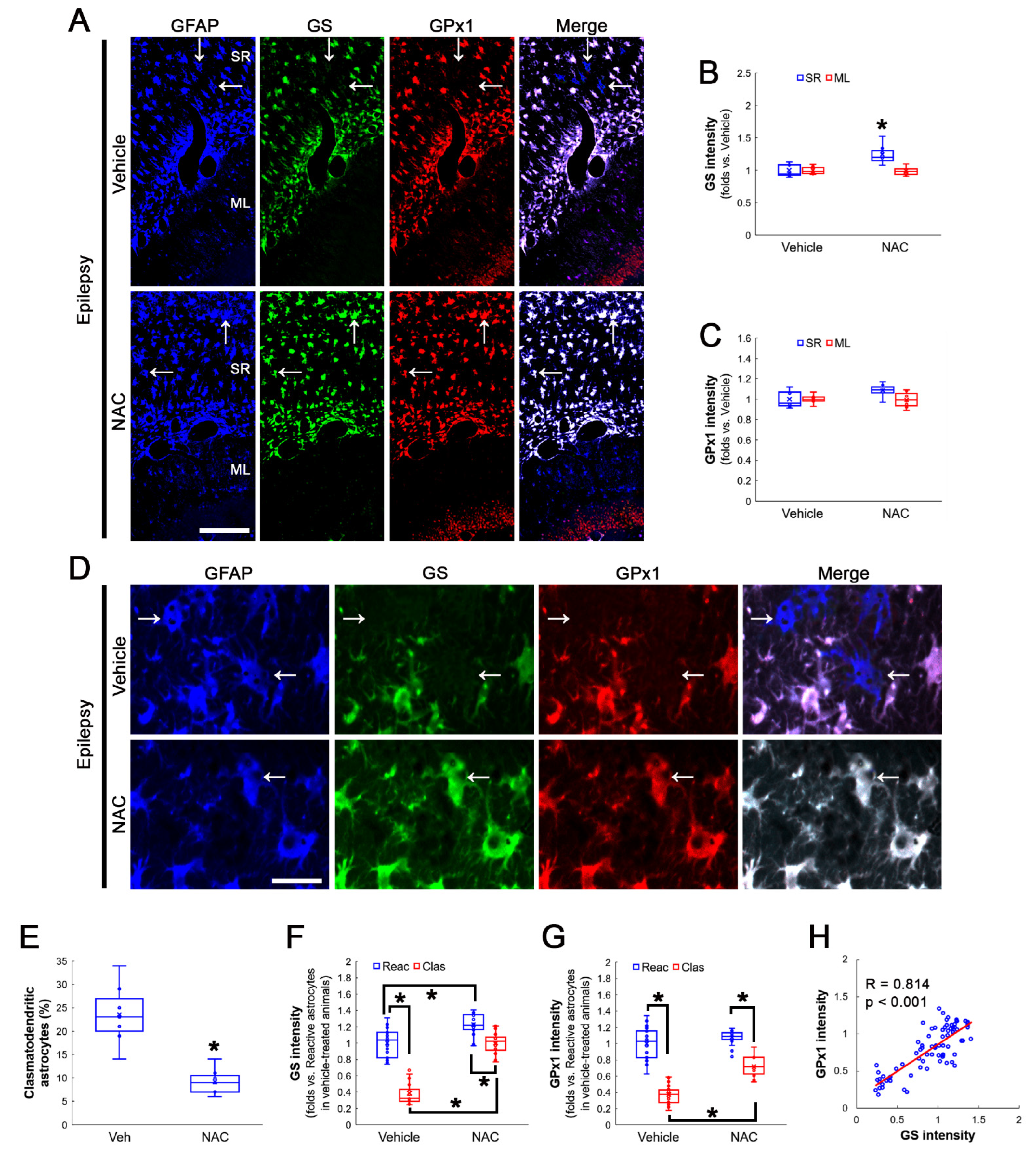
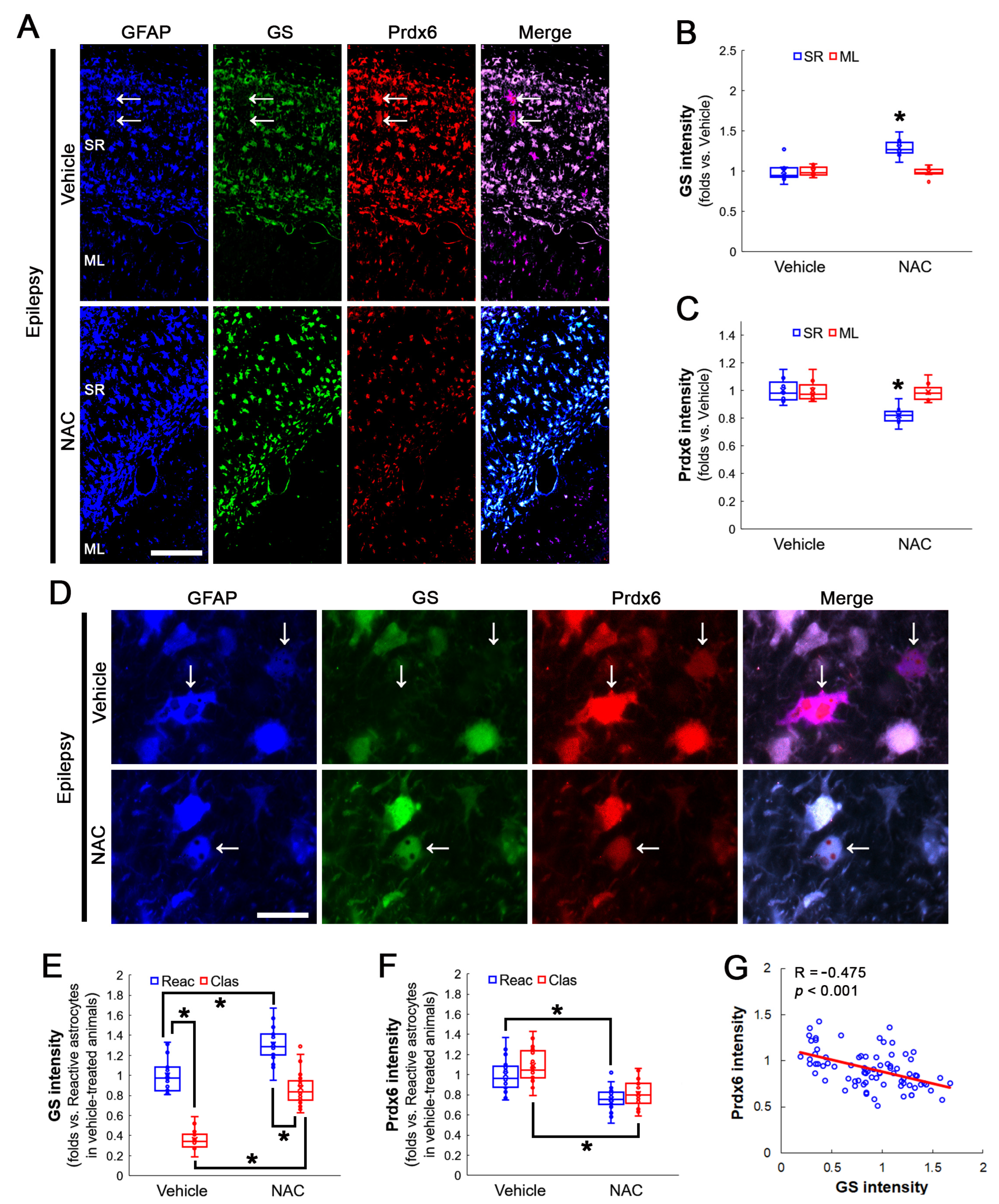
3.3. NAC Enhances GS and GPx1 Expressions, But Reduces Prdx6 Level in Clasmatodendritic CA1 Astrocytes
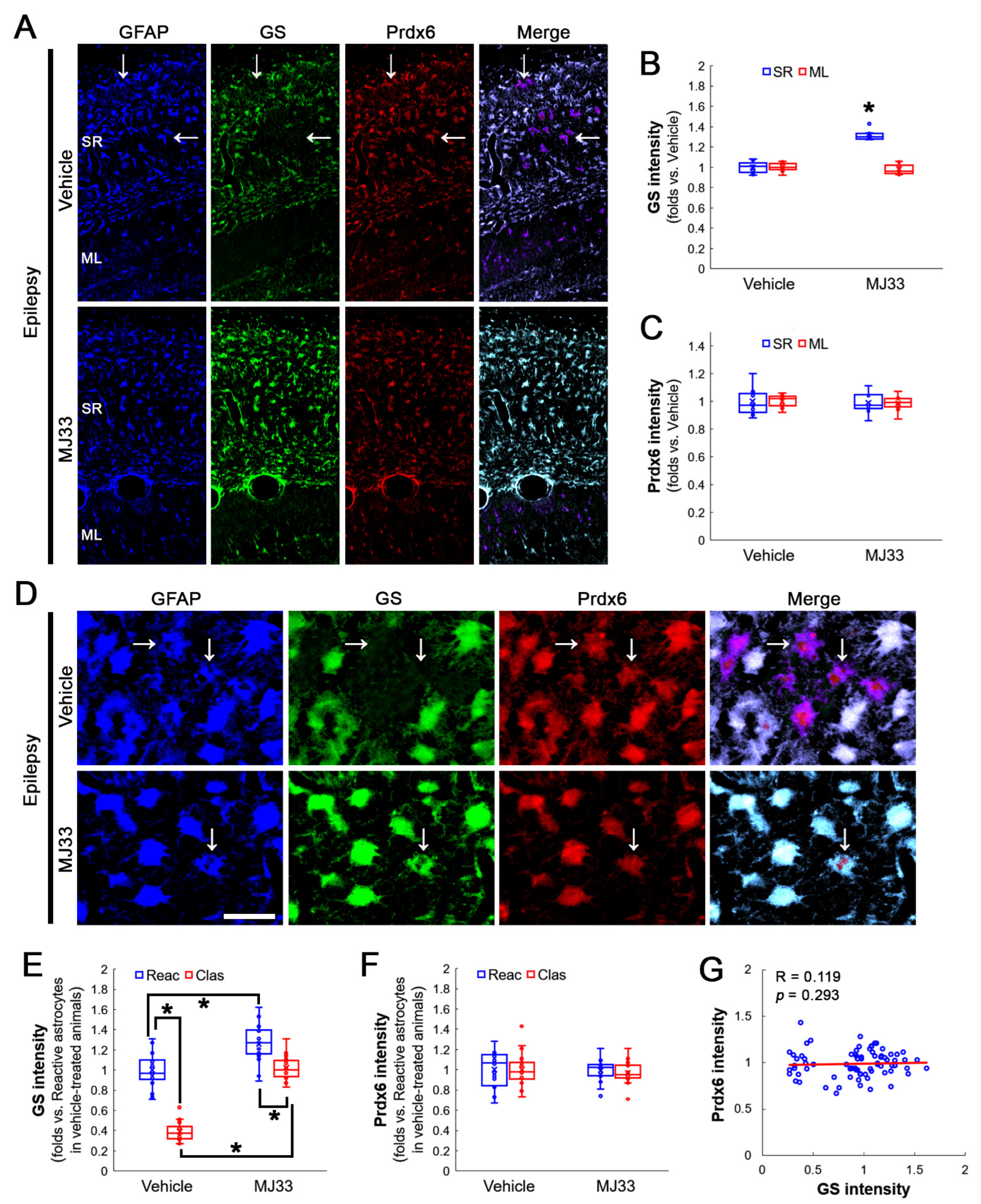
3.4. Inhibition of aiPLA2 Activity of Prdx6 Increases GS and GPx1 Expressions in Clasmatodendritic CA1 Astrocytes without Affecting Prdx6 Levels
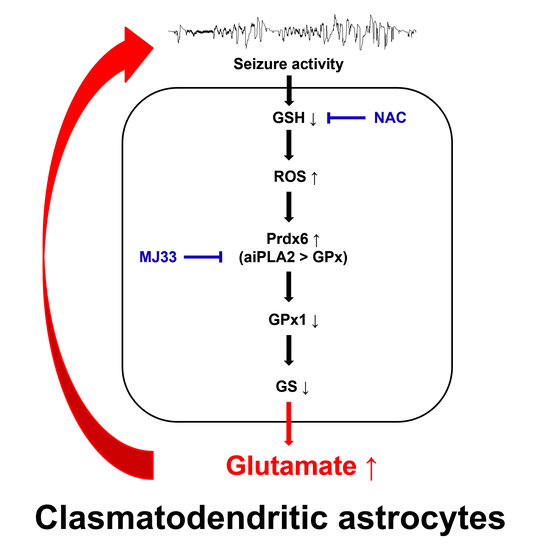
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vinti, V.; Dell’Isola, G.B.; Tascini, G.; Mencaroni, E.; Cara, G.D.; Striano, P.; Verrotti, A. Temporal Lobe Epilepsy and Psychiatric Comorbidity. Front. Neurol. 2021, 12, 775781. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Huang, H.; Chan, C.T.Y.; Li, L. Pathological Targets for Treating Temporal Lobe Epilepsy: Discoveries From Microscale to Macroscale. Front. Neurol. 2022, 12, 779558. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Swanson, R.A. Astrocytes and brain injury. J. Cereb. Blood Flow Metab. 2003, 23, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Penfield, W. Neuroglia and microglia—The interstitial tissue of the central nervous system. Special Cytology, the Form and Function of the Cell in Health and Disease; Cowdry, E.V., Ed.; Hoeber: New York, NY, USA, 1928; pp. 1033–1068. [Google Scholar]
- Duchen, L.W. General pathology of neurons and neuroglia. In Greenfield’s Neuropathology; Adams, J.H., Duchen, L.W., Eds.; Oxford University Press: Oxford, UK, 1992; pp. 1–68. [Google Scholar]
- Ryu, H.J.; Kim, J.E.; Yeo, S.I.; Kim, D.W.; Kwon, O.S.; Choi, S.Y.; Kang, T.C. F-actin depolymerization accelerates clasmatodendrosis via activation of lysosome-derived autophagic astroglial death. Brain Res. Bull. 2011, 85, 368–373. [Google Scholar] [CrossRef]
- Thorburn, A. Apoptosis and autophagy: Regulatory connections between two supposedly different processes. Apoptosis 2008, 13, 1–9. [Google Scholar] [CrossRef]
- Sakai, K.; Fukuda, T.; Iwadate, K. Beading of the astrocytic processes (clasmatodendrosis) following head trauma is associated with protein degradation pathways. Brain Inj. 2013, 27, 1692–1697. [Google Scholar] [CrossRef]
- Bouchat, J.; Gilloteaux, J.; Suain, V.; Van Vlaender, D.; Brion, J.P.; Nicaise, C. Ultrastructural Analysis of Thalamus Damages in a Mouse Model of Osmotic-Induced Demyelination. Neurotox. Res. 2019, 36, 144–162. [Google Scholar] [CrossRef]
- Hulse, R.E.; Winterfield, J.; Kunkler, P.E.; Kraig, R.P. Astrocytic clasmatodendrosis in hippocampal organ culture. Glia 2001, 33, 169–179. [Google Scholar] [CrossRef]
- Kim, J.E.; Hyun, H.W.; Min, S.J.; Kang, T.C. Sustained HSP25 Expression Induces Clasmatodendrosis via ER Stress in the Rat Hippocampus. Front. Cell Neurosci. 2017, 11, 47. [Google Scholar] [CrossRef]
- Kim, J.E.; Ko, A.R.; Hyun, H.W.; Min, S.J.; Kang, T.C. P2RX7-MAPK1/2-SP1 axis inhibits MTOR independent HSPB1-mediated astroglial autophagy. Cell Death Dis. 2018, 9, 546. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R. Tissue-specific functions of individual glutathione peroxidases. Free Radic. Biol. Med. 1999, 27, 951–965. [Google Scholar] [CrossRef]
- Liu, G.; Feinstein, S.I.; Wang, Y.; Dodia, C.; Fisher, D.; Yu, K.; Ho, Y.S.; Fisher, A.B. Comparison of glutathione peroxidase 1 and peroxiredoxin 6 in protection against oxidative stress in the mouse lung. Free Radic. Biol. Med. 2010, 49, 1172–1181. [Google Scholar] [CrossRef] [PubMed]
- Trépanier, G.; Furling, D.; Puymirat, J.; Mirault, M.E. Immunocytochemical localization of seleno-glutathione peroxidase in the adult mouse brain. Neuroscience 1996, 75, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Power, J.H.; Blumbergs, P.C. Cellular glutathione peroxidase in human brain: Cellular distribution, and its potential role in the degradation of Lewy bodies in Parkinson’s disease and dementia with Lewy bodies. Acta Neuropathol. 2009, 117, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Goemaere, J.; Knoops, B. Peroxiredoxin distribution in the mouse brain with emphasis on neuronal populations affected in neurodegenerative disorders. J. Comp. Neurol. 2012, 520, 258–280. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Zhang, Y.; Chen, X.; Zhang, J. The Roles of Peroxiredoxin 6 in Brain Diseases. Mol. Neurobiol. 2021, 58, 4348–4364. [Google Scholar] [CrossRef]
- Dringen, R. Metabolism and functions of glutathione in brain. Prog. Neurobiol. 2000, 62, 649–671. [Google Scholar] [CrossRef]
- Dringen, R.; Brandmann, M.; Hohnholt, M.C.; Blumrich, E.M. Glutathione-dependent detoxification processes in astrocytes. Neurochem. Res. 2015, 40, 2570–2582. [Google Scholar] [CrossRef]
- Anderson, C.M.; Swanson, R.A. Astrocyte glutamate transport: Review of properties, regulation, and physiological functions. Glia 2000, 32, 1–14. [Google Scholar] [CrossRef]
- Merkulova, M.I.; Shuvaeva, T.M.; Radchenko, V.V.; Yanin, B.A.; Bondar, A.A.; Sofin, A.D.; Lipkin, V.M. Recombinant human peroxiredoxin VI: Preparation and protective properties in vitro. Biochemistry 2002, 67, 1235–1239. [Google Scholar]
- Knorpp, T.; Robinson, S.R.; Crack, P.J.; Dringen, R. Glutathione peroxidase-1 contributes to the protection of glutamine synthetase in astrocytes during oxidative stress. J. Neural. Transm. 2006, 113, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.C.; Kim, D.S.; Kwak, S.E.; Kim, J.E.; Won, M.H.; Kim, D.W.; Choi, S.Y.; Kwon, O.S. Epileptogenic roles of astroglial death and regeneration in the dentate gyrus of experimental temporal lobe epilepsy. Glia 2006, 54, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Kim, J.E.; Kwak, S.E.; Choi, K.C.; Kim, D.W.; Kwon, O.S.; Choi, S.Y.; Kang, T.C. Spatiotemporal characteristics of astroglial death in the rat hippocampo-entorhinal complex following pilocarpine-induced status epilepticus. J. Comp. Neurol. 2008, 511, 581–598. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Lee, D.S.; Kim, T.H.; Kang, T.C. Glutathione Regulates GPx1 Expression during CA1 Neuronal Death and Clasmatodendrosis in the Rat Hippocampus following Status Epilepticus. Antioxidants 2022, 11, 756. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, D.S.; Kang, T.C. Sp1-Mediated Prdx6 Upregulation Leads to Clasmatodendrosis by Increasing Its aiPLA2 Activity in the CA1 Astrocytes in Chronic Epilepsy Rats. Antioxidants 2022, 11, 1883. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Kang, T.C. CDDO-Me Attenuates Astroglial Autophagy via Nrf2-, ERK1/2-SP1- and Src-CK2-PTEN-PI3K/AKT-Mediated Signaling Pathways in the Hippocampus of Chronic Epilepsy Rats. Antioxidants 2021, 10, 655. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, D.S.; Park, H.; Kim, T.H.; Kang, T.C. Inhibition of AKT/GSK3β/CREB Pathway Improves the Responsiveness to AMPA Receptor Antagonists by Regulating GRIA1 Surface Expression in Chronic Epilepsy Rats. Biomedicines 2021, 9, 425. [Google Scholar] [CrossRef]
- Ellison, M.A.; Thurman, G.W.; Ambruso, D.R. Phox activity of differentiated PLB-985 cells is enhanced, in an agonist specific manner, by the PLA2 activity of Prdx6-PLA. Eur. J. Immunol. 2012, 42, 1609–1617. [Google Scholar] [CrossRef]
- Krishnaiah, S.Y.; Dodia, C.; Feinstein, S.I.; Fisher, A.B. p67(phox) terminates the phospholipase A(2)-derived signal for activation of NADPH oxidase (NOX2). FASEB J. 2013, 27, 2066–2073. [Google Scholar] [CrossRef]
- Kwon, J.; Wang, A.; Burke, D.J.; Boudreau, H.E.; Lekstrom, K.J.; Korzeniowska, A.; Sugamata, R.; Kim, Y.S.; Yi, L.; Ersoy, I.; et al. Peroxiredoxin 6 (Prdx6) supports NADPH oxidase 1 (Nox1)-based superoxide generation and cell migration. Free Radic. Biol. Med. 2016, 96, 99–115. [Google Scholar] [CrossRef]
- Pauletti, A.; Terrone, G.; Shekh-Ahmad, T.; Salamone, A.; Ravizza, T.; Rizzi, M.; Pastore, A.; Pascente, R.; Liang, L.P.; Villa, B.R.; et al. Targeting oxidative stress improves disease outcomes in a rat model of acquired epilepsy. Brain 2019, 142, e39. [Google Scholar] [CrossRef]
- González-Reyes, S.; Santillán-Cigales, J.J.; Jiménez-Osorio, A.S.; Pedraza-Chaverri, J.; Guevara-Guzmán, R. Glycyrrhizin ameliorates oxidative stress and inflammation in hippocampus and olfactory bulb in lithium/pilocarpine-induced status epilepticus in rats. Epilepsy Res. 2016, 126, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.J.; Kim, J.E.; Yeo, S.I.; Kang, T.C. p65/RelA-Ser529 NF-κB subunit phosphorylation induces autophagic astroglial death (Clasmatodendrosis) following status epilepticus. Cell Mol. Neurobiol. 2011, 31, 1071–1078. [Google Scholar] [CrossRef]
- Zhou, S.; Dodia, C.; Feinstein, S.I.; Harper, S.; Forman, H.J.; Speicher, D.W.; Fisher, A.B. Oxidation of Peroxiredoxin 6 in the Presence of GSH Increases its Phospholipase A₂ Activity at Cytoplasmic pH. Antioxidants 2018, 8, 4. [Google Scholar] [CrossRef]
- Turovsky, E.A.; Varlamova, E.G.; Plotnikov, E.Y. Mechanisms Underlying the Protective Effect of the Peroxiredoxin-6 Are Mediated via the Protection of Astrocytes during Ischemia/Reoxygenation. Int. J. Mol. Sci. 2021, 22, 8805. [Google Scholar] [CrossRef] [PubMed]
- Krapfenbauer, K.; Engidawork, E.; Cairns, N.; Fountoulakis, M.; Lubec, G. Aberrant expression of peroxiredoxin subtypes in neurodegenerative disorders. Brain Res. 2003, 967, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Szeliga, M. Comprehensive analysis of the expression levels and prognostic values of PRDX family genes in glioma. Neurochem. Int. 2022, 153, 105256. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.M.; Park, K.R.; Kim, E.C.; Hong, J.T. PRDX6 controls multiple sclerosis by suppressing inflammation and blood brain barrier disruption. Oncotarget 2015, 6, 20875–20884. [Google Scholar] [CrossRef]
- Garcia-Bonilla, L.; Iadecola, C. Peroxiredoxin sets the brain on fire after stroke. Nat. Med. 2012, 18, 858–859. [Google Scholar] [CrossRef]
- Shichita, T.; Hasegawa, E.; Kimura, A.; Morita, R.; Sakaguchi, R.; Takada, I.; Sekiya, T.; Ooboshi, H.; Kitazono, T.; Yanagawa, T.; et al. Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nat. Med. 2012, 18, 911–917. [Google Scholar] [CrossRef]
- Shanshan, Y.; Beibei, J.; Li, T.; Minna, G.; Shipeng, L.; Li, P.; Yong, Z. Phospholipase A2 of Peroxiredoxin 6 Plays a Critical Role in Cerebral Ischemia/Reperfusion Inflammatory Injury. Front. Cell Neurosci. 2017, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Kraig, R.P.; Chesler, M. Astrocytic acidosis in hyperglycemic and complete ischemia. J. Cereb. Blood Flow Metab. 1990, 10, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Medina, J.P.; Tao, J.Q.; Patel, P.; Bannitz-Fernandes, R.; Dodia, C.; Sorokina, E.M.; Feinstein, S.I.; Chatterjee, S.; Fisher, A.B. Genetic inactivation of the phospholipase A2 activity of peroxiredoxin 6 in mice protects against LPS-induced acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 316, L656–L668. [Google Scholar] [CrossRef] [PubMed]
- Ambruso, D.R. Peroxiredoxin-6 and NADPH oxidase activity. Methods Enzymol. 2013, 527, 145–167. [Google Scholar] [PubMed]
- Gosbell, A.D.; Stefanovic, N.; Scurr, L.L.; Pete, J.; Kola, I.; Favilla, I.; de Haan, J.B. Retinal light damage: Structural and functional effects of the antioxidant glutathione peroxidase-1. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2613–2622. [Google Scholar] [CrossRef]
- Wang, G.; Zhong, W.C.; Bi, Y.H.; Tao, S.Y.; Zhu, H.; Zhu, H.X.; Xu, A.M. The Prognosis Of Peroxiredoxin Family In Breast Cancer. Cancer Manag. Res. 2019, 11, 9685–9699. [Google Scholar] [CrossRef]
- Albright, B.; Dhaher, R.; Wang, H.; Harb, R.; Lee, T.W.; Zaveri, H.; Eid, T. Progressive neuronal activation accompanies epileptogenesis caused by hippocampal glutamine synthetase inhibition. Exp. Neurol. 2017, 288, 122–133. [Google Scholar] [CrossRef]
- Reinehr, R.; Görg, B.; Becker, S.; Qvartskhava, N.; Bidmon, H.J.; Selbach, O.; Haas, H.L.; Schliess, F.; Häussinger, D. Hypoosmotic swelling and ammonia increase oxidative stress by NADPH oxidase in cultured astrocytes and vital brain slices. Glia 2007, 55, 758–771. [Google Scholar] [CrossRef]
- Görg, B.; Karababa, A.; Shafigullina, A.; Bidmon, H.J.; Häussinger, D. Ammonia-induced senescence in cultured rat astrocytes and in human cerebral cortex in hepatic encephalopathy. Glia 2015, 63, 37–50. [Google Scholar] [CrossRef]
- Lu, K.; Zimmermann, M.; Görg, B.; Bidmon, H.J.; Biermann, B.; Klöcker, N.; Häussinger, D.; Reichert, A.S. Hepatic encephalopathy is linked to alterations of autophagic flux in astrocytes. EBioMedicine 2019, 48, 539–553. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, Y.J.; Kim, J.Y.; Kang, T.C. PARP1 activation/expression modulates regional-specific neuronal and glial responses to seizure in a hemodynamic-independent manner. Cell Death Dis. 2014, 5, e1362. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Kwak, S.E.; Choi, S.Y.; Kang, T.C. Region-specific alterations in astroglial TWIK-related acid-sensitive K+-1 channel immunoreactivity in the rat hippocampal complex following pilocarpine-induced status epilepticus. J. Comp. Neurol. 2008, 510, 463–474. [Google Scholar] [CrossRef] [PubMed]
| Antigen | Host | Manufacturer (Catalog Number) | Dilution Used |
|---|---|---|---|
| Prdx6 | Sheep | Biosensis (S-073-100) | 1:1000 |
| GS | Mouse | Millipore (#MAB302) | 1:500 |
| GPx1 | Sheep | Biosensis (S-072-100) | 1:2000 |
| GFAP | Rabbit | Millipore (#AB5804) | 1:500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-E.; Park, H.; Kang, T.-C. Peroxiredoxin 6 Regulates Glutathione Peroxidase 1-Medited Glutamine Synthase Preservation in the Hippocampus of Chronic Epilepsy Rats. Antioxidants 2023, 12, 156. https://doi.org/10.3390/antiox12010156
Kim J-E, Park H, Kang T-C. Peroxiredoxin 6 Regulates Glutathione Peroxidase 1-Medited Glutamine Synthase Preservation in the Hippocampus of Chronic Epilepsy Rats. Antioxidants. 2023; 12(1):156. https://doi.org/10.3390/antiox12010156
Chicago/Turabian StyleKim, Ji-Eun, Hana Park, and Tae-Cheon Kang. 2023. "Peroxiredoxin 6 Regulates Glutathione Peroxidase 1-Medited Glutamine Synthase Preservation in the Hippocampus of Chronic Epilepsy Rats" Antioxidants 12, no. 1: 156. https://doi.org/10.3390/antiox12010156
APA StyleKim, J.-E., Park, H., & Kang, T.-C. (2023). Peroxiredoxin 6 Regulates Glutathione Peroxidase 1-Medited Glutamine Synthase Preservation in the Hippocampus of Chronic Epilepsy Rats. Antioxidants, 12(1), 156. https://doi.org/10.3390/antiox12010156