Influence of the Neuroprotective Properties of Quercetin on Regeneration and Functional Recovery of the Nervous System
Abstract
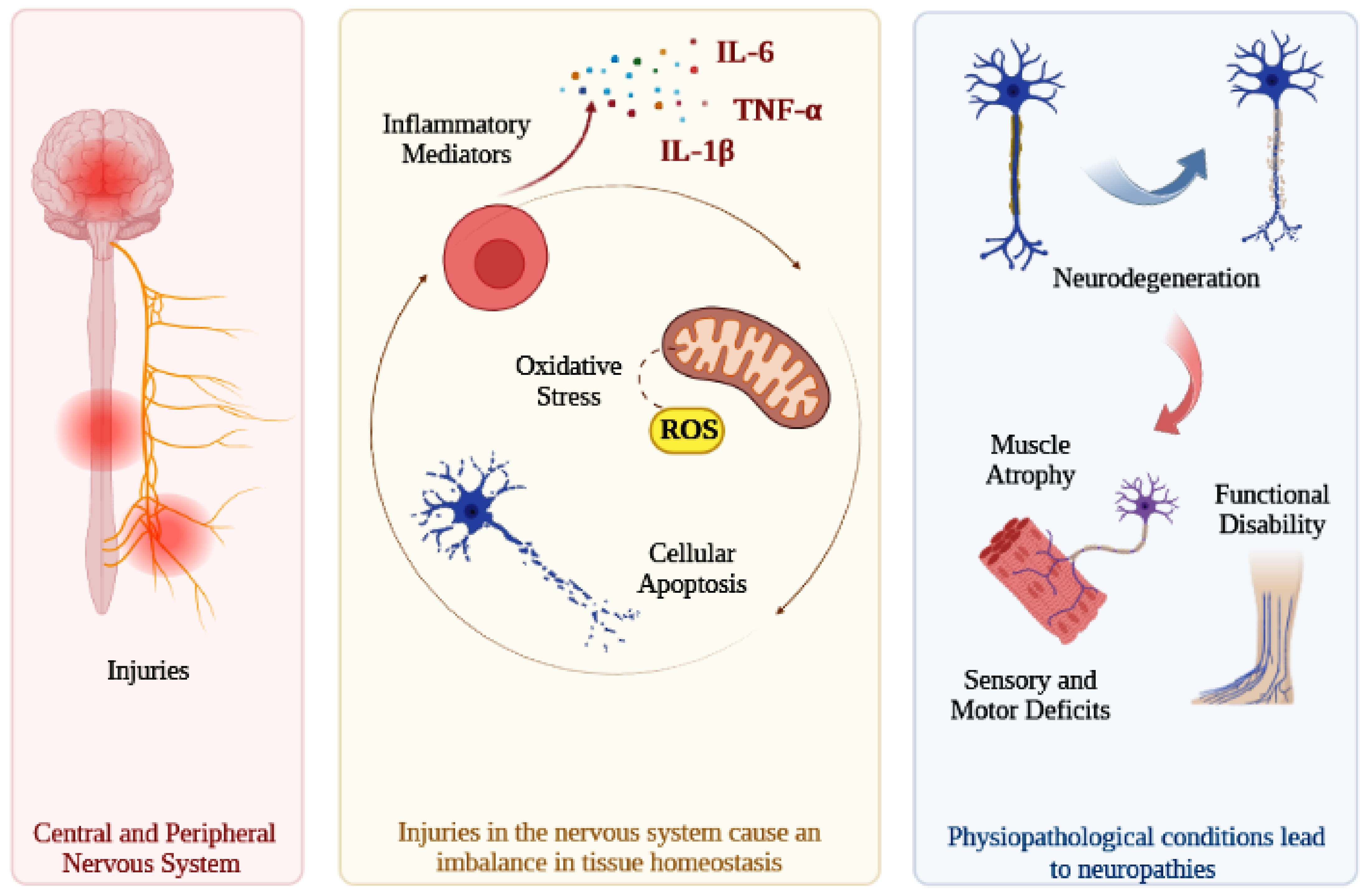
1. Introduction
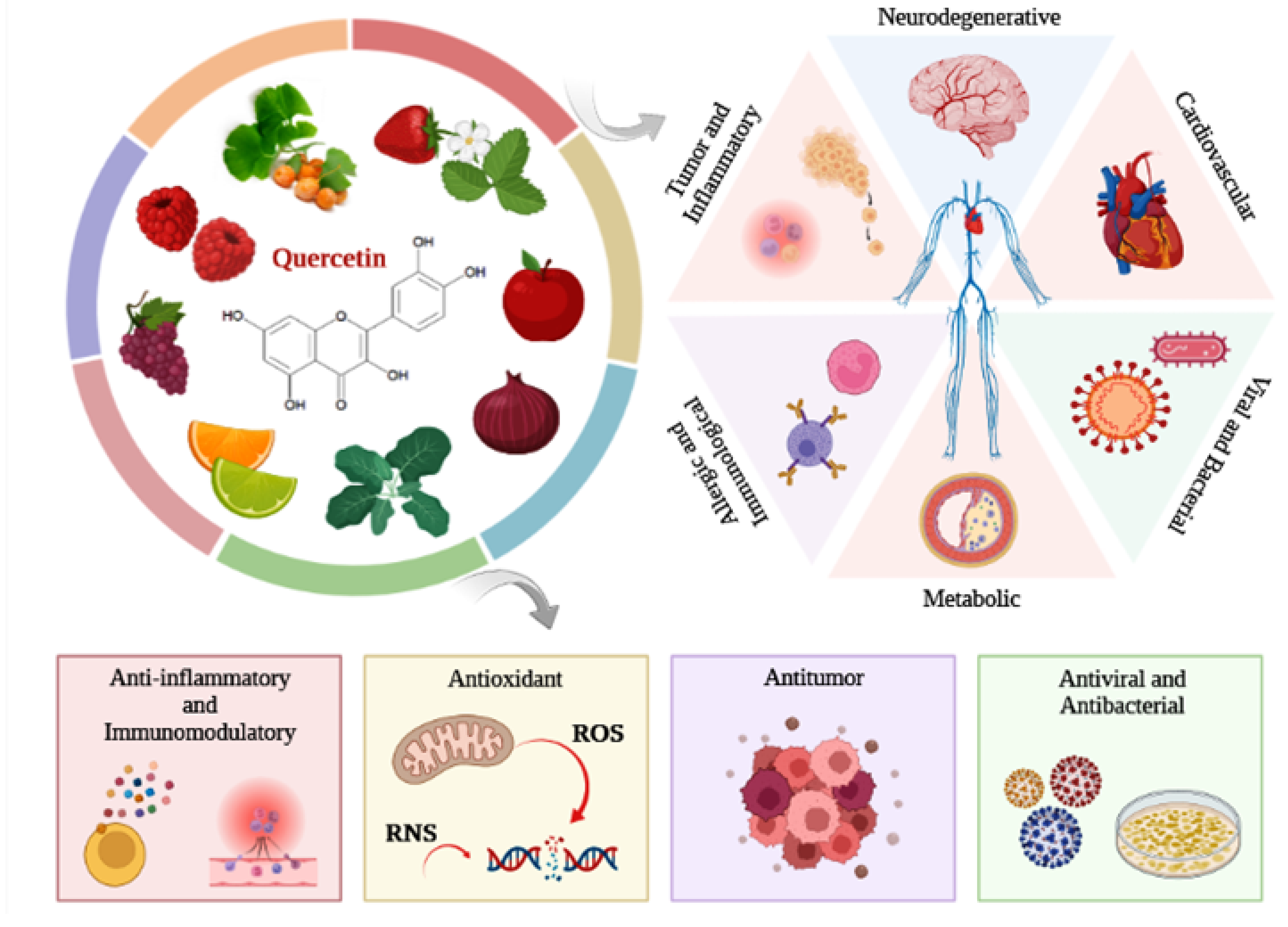
2. Flavonoid Quercetin
Biological Properties
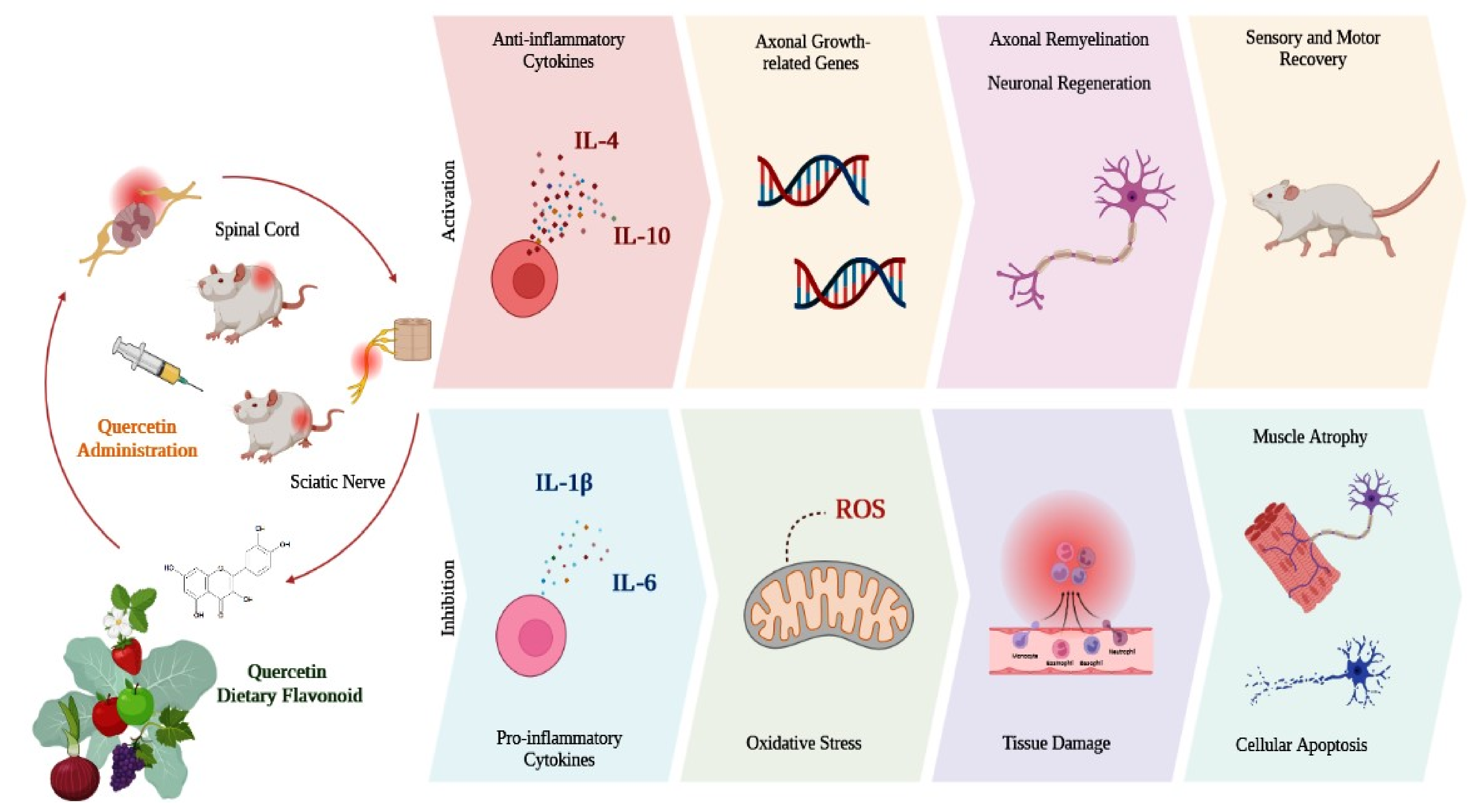
3. Therapeutic Effect of Quercetin
3.1. Animal Models with Central and Peripheral Nervous System Injuries
3.1.1. Spinal Cord Injury (SCI)
3.1.2. Peripheral Nerve Injury
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fan, B.; Wei, Z.; Yao, X.; Shi, G.; Cheng, X.; Zhou, X.; Zhou, H.; Ning, G.; Kong, X.; Feng, S. Microenvironment Imbalance of Spinal Cord Injury. Cell Transplant. 2018, 27, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Radi, E.; Formichi, P.; Battisti, C.; Federico, A. Apoptosis and oxidative stress in neurodegenerative diseases. J. Alzheimers Dis. 2014, 42 (Suppl. S3), S125–S152. [Google Scholar] [CrossRef]
- Modrak, M.; Talukder, M.A.H.; Gurgenashvili, K.; Noble, M.; Elfar, J.C. Peripheral nerve injury and myelination: Potential therapeutic strategies. J. Neurosci. Res. 2020, 98, 780–795. [Google Scholar] [CrossRef] [PubMed]
- Guérout, N. Plasticity of the Injured Spinal Cord. Cells 2021, 10, 1886. [Google Scholar] [CrossRef] [PubMed]
- Brett, B.L.; Gardner, R.C.; Godbout, J.; Dams-O’Connor, K.; Keene, C.D. Traumatic Brain Injury and Risk of Neurodegenerative Disorder. Biol. Psychiatry 2022, 91, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Vella, M.A.; Crandall, M.L.; Patel, M.B. Acute Management of Traumatic Brain Injury. Surg. Clin. N. Am. 2017, 97, 1015–1030. [Google Scholar] [CrossRef] [PubMed]
- Houdek, M.T.; Shin, A.Y. Management and complications of traumatic peripheral nerve injuries. Hand. Clin. 2015, 31, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Rivlin, M.; Graham, J.G.; Beredjiklian, P.K. Peripheral nerve injury, scarring, and recovery. Connect. Tissue Res. 2019, 60, 3–9. [Google Scholar] [CrossRef]
- Grewal, A.K.; Singh, T.G.; Sharma, D.; Sharma, V.; Singh, M.; Rahman, M.H.; Najda, A.; Walasek-Janusz, M.; Kamel, M.; Albadrani, G.M.; et al. Mechanistic insights and perspectives involved in neuroprotective action of quercetin. Biomed. Pharm. 2021, 140, 111729. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; D’Onofrio, G.; Nabavi, S.F.; Daglia, M.; Rastrelli, L.; Nabavi, S.M. Neuroprotective Effects of Quercetin: From Chemistry to Medicine. CNS Neurol. Disord. Drug Targets 2016, 15, 964–975. [Google Scholar] [CrossRef]
- Islam, M.T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Deepika; Maurya, P.K. Health Benefits of Quercetin in Age-Related Diseases. Molecules 2022, 27, 2498. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Minihane, A.M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Birt, D.F.; Jeffery, E. Flavonoids. Adv. Nutr. 2013, 4, 576–577. [Google Scholar] [CrossRef]
- Crasci, L.; Basile, L.; Panico, A.; Puglia, C.; Bonina, F.P.; Basile, P.M.; Rizza, L.; Guccione, S. Correlating In Vitro Target-Oriented Screening and Docking: Inhibition of Matrix Metalloproteinases Activities by Flavonoids. Planta Med. 2017, 83, 901–911. [Google Scholar] [CrossRef]
- Hwang, S.L.; Yen, G.C. Neuroprotective effects of the citrus flavanones against H2O2-induced cytotoxicity in PC12 cells. J. Agric. Food Chem. 2008, 56, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, B.; Xie, H.; He, Y.; Zhong, D.; Chen, D. Antioxidant Structure(-)Activity Relationship Analysis of Five Dihydrochalcones. Molecules 2018, 23, 1162. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Shen, P.; Lin, W.; Deng, X.; Ba, X.; Han, L.; Chen, Z.; Qin, K.; Huang, Y.; Tu, S. Potential Implications of Quercetin in Autoimmune Diseases. Front. Immunol. 2021, 12, 689044. [Google Scholar] [CrossRef]
- Chen, B.H.; Park, J.H.; Ahn, J.H.; Cho, J.H.; Kim, I.H.; Lee, J.C.; Won, M.H.; Lee, C.H.; Hwang, I.K.; Kim, J.D.; et al. Pretreated quercetin protects gerbil hippocampal CA1 pyramidal neurons from transient cerebral ischemic injury by increasing the expression of antioxidant enzymes. Neural Regen. Res. 2017, 12, 220–227. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schäfer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62, 1700447. [Google Scholar] [CrossRef]
- Shabbir, U.; Rubab, M.; Daliri, E.B.; Chelliah, R.; Javed, A.; Oh, D.H. Curcumin, Quercetin, Catechins and Metabolic Diseases: The Role of Gut Microbiota. Nutrients 2021, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Suganthy, N.; Devi, K.P.; Nabavi, S.F.; Braidy, N.; Nabavi, S.M. Bioactive effects of quercetin in the central nervous system: Focusing on the mechanisms of actions. Biomed. Pharm. 2016, 84, 892–908. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Bruno, R.S. Endogenous and exogenous mediators of quercetin bioavailability. J. Nutr. Biochem. 2015, 26, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Mukai, R.; Ishisaka, A. Quercetin and related polyphenols: New insights and implications for their bioactivity and bioavailability. Food Funct. 2015, 6, 1399–1417. [Google Scholar] [CrossRef]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef]
- Ghosh, A.; Sarkar, S.; Mandal, A.K.; Das, N. Neuroprotective role of nanoencapsulated quercetin in combating ischemia-reperfusion induced neuronal damage in young and aged rats. PLoS ONE 2013, 8, e57735. [Google Scholar] [CrossRef]
- Huang, C.; Fu, C.; Qi, Z.P.; Guo, W.L.; You, D.; Li, R.; Zhu, Z. Localised delivery of quercetin by thermo-sensitive PLGA-PEG-PLGA hydrogels for the treatment of brachial plexus avulsion. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1010–1021. [Google Scholar] [CrossRef]
- Sadalage, P.S.; Patil, R.V.; Havaldar, D.V.; Gavade, S.S.; Santos, A.C.; Pawar, K.D. Optimally biosynthesized, PEGylated gold nanoparticles functionalized with quercetin and camptothecin enhance potential anti-inflammatory, anti-cancer and anti-angiogenic activities. J. Nanobiotechnol. 2021, 19, 84. [Google Scholar] [CrossRef]
- Testa, G.; Gamba, P.; Badilli, U.; Gargiulo, S.; Maina, M.; Guina, T.; Calfapietra, S.; Biasi, F.; Cavalli, R.; Poli, G.; et al. Loading into nanoparticles improves quercetin’s efficacy in preventing neuroinflammation induced by oxysterols. PLoS ONE 2014, 9, e96795. [Google Scholar] [CrossRef] [PubMed]
- Thipkaew, C.; Wattanathorn, J.; Muchimapura, S. Electrospun Nanofibers Loaded with Quercetin Promote the Recovery of Focal Entrapment Neuropathy in a Rat Model of Streptozotocin-Induced Diabetes. BioMed Res. Int. 2017, 2017, 2017493. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, S.R.; Ebrahimzadeh, M.A. Quercetin derivatives: Drug design, development, and biological activities, a review. Eur. J. Med. Chem. 2022, 229, 114068. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, E.E.; Burd, R. Quercetin as a systemic chemopreventative agent: Structural and functional mechanisms. Mini Rev. Med. Chem. 2011, 11, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tao, B.; Wan, Y.; Sun, Y.; Wang, L.; Sun, J.; Li, C. Drug delivery based pharmacological enhancement and current insights of quercetin with therapeutic potential against oral diseases. Biomed. Pharm. 2020, 128, 110372. [Google Scholar] [CrossRef]
- Yi, H.; Peng, H.; Wu, X.; Xu, X.; Kuang, T.; Zhang, J.; Du, L.; Fan, G. The Therapeutic Effects and Mechanisms of Quercetin on Metabolic Diseases: Pharmacological Data and Clinical Evidence. Oxi. Med. Cell Longev. 2021, 2021, 6678662. [Google Scholar] [CrossRef]
- Güran, M.; Şanlıtürk, G.; Kerküklü, N.R.; Altundağ, E.M.; Süha Yalçın, A. Combined effects of quercetin and curcumin on anti-inflammatory and antimicrobial parameters in vitro. Eur. J. Pharm. 2019, 859, 172486. [Google Scholar] [CrossRef]
- Han, X.; Xu, T.; Fang, Q.; Zhang, H.; Yue, L.; Hu, G.; Sun, L. Quercetin hinders microglial activation to alleviate neurotoxicity via the interplay between NLRP3 inflammasome and mitophagy. Redox. Biol. 2021, 44, 102010. [Google Scholar] [CrossRef]
- Luo, X.; Bao, X.; Weng, X.; Bai, X.; Feng, Y.; Huang, J.; Liu, S.; Jia, H.; Yu, B. The protective effect of quercetin on macrophage pyroptosis via TLR2/Myd88/NF-κB and ROS/AMPK pathway. Life Sci. 2022, 291, 120064. [Google Scholar] [CrossRef]
- Tang, J.; Diao, P.; Shu, X.; Li, L.; Xiong, L. Quercetin and Quercitrin Attenuates the Inflammatory Response and Oxidative Stress in LPS-Induced RAW264.7 Cells: In Vitro Assessment and a Theoretical Model. BioMed Res. Int. 2019, 2019, 7039802. [Google Scholar] [CrossRef]
- Wang, C.P.; Shi, Y.W.; Tang, M.; Zhang, X.C.; Gu, Y.; Liang, X.M.; Wang, Z.W.; Ding, F. Isoquercetin Ameliorates Cerebral Impairment in Focal Ischemia Through Anti-Oxidative, Anti-Inflammatory, and Anti-Apoptotic Effects in Primary Culture of Rat Hippocampal Neurons and Hippocampal CA1 Region of Rats. Mol. Neurobiol. 2017, 54, 2126–2142. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Du, G.; Wu, H.; Gao, X.; Yang, Z.; Liu, B.; Cui, S. Protective effects of quercetin on traumatic brain injury induced inflammation and oxidative stress in cortex through activating Nrf2/HO-1 pathway. Restor. Neurol. Neurosci. 2021, 39, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Kong, B.; Gu, J.W.; Kuang, Y.Q.; Cheng, L.; Yang, W.T.; Xia, X.; Shu, H.F. Anti-apoptotic and anti-oxidative roles of quercetin after traumatic brain injury. Cell Mol. Neurobiol. 2014, 34, 797–804. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Gao, Y.; Li, L.; Tang, C.; Wen, G.; Yang, Y.; Zhuang, Z.; Zhou, M.; Mao, L.; et al. Quercetin induces mitochondrial biogenesis in experimental traumatic brain injury via the PGC-1α signaling pathway. Am. J. Transl. Res. 2016, 8, 3558–3566. [Google Scholar] [PubMed]
- Li, X.; Wang, H.; Gao, Y.; Li, L.; Tang, C.; Wen, G.; Zhou, Y.; Zhou, M.; Mao, L.; Fan, Y. Protective Effects of Quercetin on Mitochondrial Biogenesis in Experimental Traumatic Brain Injury via the Nrf2 Signaling Pathway. PLoS ONE 2016, 11, e0164237. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Qi, D.; Dong, F.; Wang, B.; Guo, R.; Luo, M.; Yao, R. Quercetin improves hypoxia-ischemia induced cognitive deficits via promoting remyelination in neonatal rat. Brain Res. 2014, 1553, 31–40. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Chang, C.Y.; Lin, S.Y.; Wang, J.D.; Wu, C.C.; Chen, W.Y.; Kuan, Y.H.; Liao, S.L.; Wang, W.Y.; Chen, C.J. Quercetin protects against cerebral ischemia/reperfusion and oxygen glucose deprivation/reoxygenation neurotoxicity. J. Nutr. Biochem. 2020, 83, 108436. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhang, H.T.; Cai, Y.Q.; Han, Y.J.; Yao, F.; Yuan, Z.H.; Wu, B.Y. Anti-inflammatory Effect of Mesenchymal Stromal Cell Transplantation and Quercetin Treatment in a Rat Model of Experimental Cerebral Ischemia. Cell Mol. Neurobiol. 2016, 36, 1023–1034. [Google Scholar] [CrossRef]
- Khan, A.; Ali, T.; Rehman, S.U.; Khan, M.S.; Alam, S.I.; Ikram, M.; Muhammad, T.; Saeed, K.; Badshah, H.; Kim, M.O. Neuroprotective Effect of Quercetin Against the Detrimental Effects of LPS in the Adult Mouse Brain. Front. Pharm. 2018, 9, 1383. [Google Scholar] [CrossRef]
- Josiah, S.S.; Famusiwa, C.D.; Crown, O.O.; Lawal, A.O.; Olaleye, M.T.; Akindahunsi, A.A.; Akinmoladun, A.C. Neuroprotective effects of catechin and quercetin in experimental Parkinsonism through modulation of dopamine metabolism and expression of IL-1β, TNF-α, NF-κB, IκKB, and p53 genes in male Wistar rats. Neurotoxicology 2022, 90, 158–171. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, W.; Zhao, B.; Xie, J.; Sun, Q.; Shi, X.; Yan, B.; Tian, G.; Liang, X. Quercetin Attenuates Diabetic Peripheral Neuropathy by Correcting Mitochondrial Abnormality via Activation of AMPK/PGC-1α Pathway in vivo and in vitro. Front. Neurosci. 2021, 15, 636172. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Juárez, J.V.; Jaramillo-Morales, O.A.; Déciga-Campos, M.; Moreno-Rocha, L.A.; López-Muñoz, F.J. Sigma-1 receptor antagonist (BD-1063) potentiates the antinociceptive effect of quercetin in neuropathic pain induced by chronic constriction injury. Drug. Dev. Res. 2021, 82, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Komirishetty, P.; Areti, A.; Gogoi, R.; Sistla, R.; Kumar, A. Combination strategy of PARP inhibitor with antioxidant prevent bioenergetic deficits and inflammatory changes in CCI-induced neuropathy. Neuropharmacology 2017, 113, 137–147. [Google Scholar] [CrossRef]
- Muto, N.; Matsuoka, Y.; Arakawa, K.; Kurita, M.; Omiya, H.; Taniguchi, A.; Kaku, R.; Morimatsu, H. Quercetin Attenuates Neuropathic Pain in Rats with Spared Nerve Injury. Acta Med. Okayama 2018, 72, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Lin, C.; Zhang, Y.; Ma, Z.; Chen, Y.; Kong, L.; Yuan, L.; Ma, T. Quercetin Alleviates Neuropathic Pain in the Rat CCI Model by Mediating AMPK/MAPK Pathway. J. Pain Res. 2021, 14, 1289–1301. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Tang, H.B.; Shan, L.Q.; Liu, S.C.; Huang, D.G.; Chen, X.; Chen, Z.; Yang, M.; Yin, X.H.; Yang, H.; et al. Quercetin prevents necroptosis of oligodendrocytes by inhibiting macrophages/microglia polarization to M1 phenotype after spinal cord injury in rats. J. Neuroinflam. 2019, 16, 206. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Huang, Y.; Han, N.; He, F.; Li, M.; Bian, Z.; Liu, J.; Sun, T.; Zhu, L. Quercetin suppresses NLRP3 inflammasome activation and attenuates histopathology in a rat model of spinal cord injury. Spinal Cord. 2016, 54, 592–596. [Google Scholar] [CrossRef]
- Qiu, J.; Yang, X.; Wang, L.; Zhang, Q.; Ma, W.; Huang, Z.; Bao, Y.; Zhong, L.; Sun, H.; Ding, F. Isoquercitrin promotes peripheral nerve regeneration through inhibiting oxidative stress following sciatic crush injury in mice. Ann. Transl. Med. 2019, 7, 680. [Google Scholar] [CrossRef]
- Türedi, S.; Yuluğ, E.; Alver, A.; Bodur, A.; İnce, İ. A morphological and biochemical evaluation of the effects of quercetin on experimental sciatic nerve damage in rats. Exp. Med. 2018, 15, 3215–3224. [Google Scholar] [CrossRef]
- Wang, W.; Huang, C.Y.; Tsai, F.J.; Tsai, C.C.; Yao, C.H.; Chen, Y.S. Growth-promoting effects of quercetin on peripheral nerves in rats. Int. J. Artif. Organs 2011, 34, 1095–1105. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Wang, M.; Lin, C.; Li, G.; Zhou, X.; Luo, J.; Jin, D. Quercetin reduces neural tissue damage and promotes astrocyte activation after spinal cord injury in rats. J. Cell Biochem. 2018, 119, 2298–2306. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.Y.; Zhang, L.L.; Li, G.T.; Zhang, H.T. Combinatory effect of mesenchymal stromal cells transplantation and quercetin after spinal cord injury in rat. Eur. Rev. Med. Pharm. Sci. 2018, 22, 2876–2887. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, M.; Wang, M.; Chen, H.; Li, W.; Zhou, X. Quercetin promotes locomotor function recovery and axonal regeneration through induction of autophagy after spinal cord injury. Clin. Exp. Pharm. Physiol. 2021, 48, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.M.; Qin, J.; Chen, S.J.; Yao, L.M.; Zhang, L.Y.; Yin, Z.Q.; Liao, H. Quercetin promotes motor and sensory function recovery following sciatic nerve-crush injury in C57BL/6J mice. J. Nutr. Biochem. 2017, 46, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, J.; Zhang, F.; Zhang, J.; Shi, T.; Zeng, Z. Antioxidant effect of quercetin against acute spinal cord injury in rats and its correlation with the p38MAPK/iNOS signaling pathway. Life Sci. 2013, 92, 1215–1221. [Google Scholar] [CrossRef]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef]
- Huang, T.T.; Zou, Y.; Corniola, R. Oxidative stress and adult neurogenesis--effects of radiation and superoxide dismutase deficiency. Semin. Cell Dev. Biol. 2012, 23, 738–744. [Google Scholar] [CrossRef]
- Pong, K. Oxidative stress in neurodegenerative diseases: Therapeutic implications for superoxide dismutase mimetics. Expert Opin. Biol 2003, 3, 127–139. [Google Scholar] [CrossRef]
- Kleindienst, A.; Hesse, F.; Bullock, M.R.; Buchfelder, M. The neurotrophic protein S100B: Value as a marker of brain damage and possible therapeutic implications. Prog. Brain Res. 2007, 161, 317–325. [Google Scholar] [CrossRef]
- Li, D.; Liu, X.; Liu, T.; Liu, H.; Tong, L.; Jia, S.; Wang, Y.F. Neurochemical regulation of the expression and function of glial fibrillary acidic protein in astrocytes. Glia 2020, 68, 878–897. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, K.K. Glial fibrillary acidic protein: From intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015, 38, 364–374. [Google Scholar] [CrossRef]
- Kovacs, G.G. Cellular reactions of the central nervous system. Handb. Clin. Neurol. 2017, 145, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, S.; Gritti, L.; Crooks, D.; Dombrowski, Y. Oligodendrocytes in Development, Myelin Generation and Beyond. Cells 2019, 8, 1424. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.d.C.; Fideles, S.O.M.; Pomini, K.T.; Bellini, M.Z.; Pereira, E.d.S.B.M.; Reis, C.H.B.; Pilon, J.P.G.; de Marchi, M.Â.; Trazzi, B.F.d.M.; da Silva, W.S.; et al. Potential of Fibrin Glue and Mesenchymal Stem Cells (MSCs) to Regenerate Nerve Injuries: A Systematic Review. Cells 2022, 11, 221. [Google Scholar] [CrossRef] [PubMed]
- Buchaim, R.L.; Andreo, J.C.; Barraviera, B.; Ferreira Junior, R.S.; Buchaim, D.V.; Rosa Junior, G.M.; de Oliveira, A.L.; de Castro Rodrigues, A. Effect of low-level laser therapy (LLLT) on peripheral nerve regeneration using fibrin glue derived from snake venom. Injury 2015, 46, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Rosso, M.P.d.O.; Buchaim, D.V.; Kawano, N.; Furlanette, G.; Pomini, K.T.; Buchaim, R.L. Photobiomodulation Therapy (PBMT) in Peripheral Nerve Regeneration: A Systematic Review. Bioengineering 2018, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Araujo, T.; Andreo, L.; Tobelem, D.D.C.; Silva, T.; Malavazzi, T.C.D.S.; Martinelli, A.; Lemes, B.; Fernandes, K.P.S.; Bussadori, S.K.; Mesquita-Ferrari, R.A. Effects of systemic vascular photobiomodulation using LED or laser on sensory-motor recovery following a peripheral nerve injury in Wistar rats. Photochem. Photobiol. Sci. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
| References | Animals Models | Treatment Groups | Intervention | Main Analysis | Main Outcomes |
|---|---|---|---|---|---|
| Wang et al., (2011) [60] | Sprague Dawley rats. Sciatic Nerve Injury | G1: Saline solution G2: Quercetin 0.1 µg/mL G3: Quercetin 1 µg/mL G4: Quercetin 10 µg/mL | Implantation of silicone rubber nerve chamber filled with the quercetin or saline solutions in the gaps (15 mm) (n = 10). Analyses were performed after 8 weeks of the procedures. | Electrophysiological and histological analysis. | Quercetin-treated groups showed a considerable increase in the number and density of myelinated axons in relation to the control, with satisfactory reinnervation of the gastrocnemius muscle. Quercetin (1 µg/mL) had a considerably larger area of evoked muscle action potential than the control group. |
| Song et al., (2013) [65] | Male Sprague Dawley rats. Spinal Cord Injury (SCI) | G1: Sham surgery G2: SCI G3: SCI + Quercetin 0.2 mg/kg/day G4: SCI + Methylprednisolone (MP) 30 mg/kg/day G5: SCI + specific p38MAPK inhibitor SB20358 (SB) 10 mg/kg/day | Intraperitoneal injections of quercetin, MP, or SB solutions (n = 8). Analyses were performed until the 14th day after the procedures. | Behavioral assessment (BBB: Basso, Beattie and Bresnahan scores), qRT-PCR, Western blot, and immunohistochemical analysis. | Quercetin significantly improved BBB scores, similarly to the positive control (MP). Quercetin suppressed the expression of inducible nitric oxide synthase (iNOS) similarly to SB, showing a neuroprotective effect by inhibiting cellular oxidative stress. |
| Jiang et al., (2016) [57] | Female Sprague Dawley rats. Spinal Cord Injury (SCI) | G1: Sham G2: SCI G3: SCI + Saline solution (vehicle) G4: SCI + Quercetin solution 100 mg/Kg | Intraperitoneal injections with quercetin or vehicle solutions at 12-h intervals for 3 days (n = 5). Analyses were performed until the 14th day after the procedures. | Behavioral assessment (BBB scores), Western blot, histological assays, and biochemical analysis. | Quercetin promoted a significant improvement in functional recovery, reducing histopathological damage, inflammatory cytokines synthesis, and reactive oxygen species production. |
| Chen et al., (2017) [64] | Male C57BL/6J mice. Sciatic Nerve Crush Injury | G1: Sham G2: Saline solution G3: Quercetin 0.2 mg/kg/day G4: Quercetin 2 mg/kg/day G5: Quercetin 20 mg/kg/day G6: mice-derived nerve growth factor (mNGF) 4.86 µg/kg/day | Injection of the solutions into the plantar muscle of the left hind limb once a day (n = 10). Analyses were performed at 7, 14, and 35 days after the procedures. | Behavioral test, qRT-PCR, Western blot, immunofluorescence, transmission electron microscopy, and motor nerve conduction velocity analysis. | Quercetin (mainly at 20 mg/kg/day) and mNGF favored the expression of genes related to intrinsic axon growth and promoted an increase in the number of myelinated fibers. At 20 mg/kg/day, quercetin significantly accelerated sensory and motor function recovery. In addition, quercetin (20 mg/kg/day) and mNGF significantly reduced muscle atrophy. |
| Turedi et al., (2018) [59] | Male Sprague Dawley rats. Sciatic Nerve Crush Injury (T) | G1: Sham (S-7) G2: Sham (S-28) G3: Quercetin (Q-7) 200 mg/kg/day G4: Quercetin (Q-28) 200 mg/kg/day G5: T (T-7) G6: T (T-28) G7: T + Quercetin (T + Q-7) 200 mg/kg/day G8: T + Quercetin (T + Q-28) 200 mg/kg/day | Intragastric administration of quercetin solutions for 7 days (n = 6). Analyses were performed at 7 and 28 days after the procedures. | TUNEL assay, histopathological assay, and biochemical analysis. | Quercetin significantly decreased the index of apoptosis. Nerve fiber regeneration was significantly more expressive in T + Q-28 than in T + Q-7. In addition, T + Q-28 showed significantly more myelinated nerve fibers with thicker myelin sheaths than T + Q-7. |
| Wang et al., (2018) [62] | Female Sprague Dawley rats. Spinal Cord Injury (SCI) | G1: Sham G2: Culture medium G3: Human umbilical cord mesenchymal stromal cells (HUSMCs) G4: Quercetin 50 μmol/kg G5: HUSMCs + Quercetin 50 μmol/kg | Administration of quercetin or saline solutions at 12-h intervals for 3 days. HUSMCs transplantation (2 dosages) into the injured spinal cord. (n = 28). Analyses were performed until the 4th week after the procedures. | Behavioral assessment (BBB scores) and immunohistochemical analysis. | HUMSCs + Quercetin promoted significant improvement in neurological function in relation to the other groups. Similarly, HUMSCs + Quercetin reduced cystic cavities formation, inflammatory cytokines synthesis, and iNOS production, while favoring pro-inflammatory cytokines synthesis. |
| Wang et al., (2018) [61] | Male Sprague Dawley rats. Spinal Cord Injury (SCI) | G1: Sham G2: SCI G3: SCI + Quercetin 20 mg/kg/day | Intraperitoneal injections of quercetin for 7 days (n = 10). Analyses were performed after 7 days of the procedures. | Behavioral assessment (BBB scores), qRT-PCR, Western blot, immunofluorescence, histological assays, and electrophysiological analysis. | Quercetin significantly improved functional capacity and electrophysiological recovery. Quercetin reduced cavity formation, favored axonal regeneration, and promoted astrocyte activation, upregulating the expression of glial fibrillary acidic protein (GFAP) and S100 calcium binding protein B (S100β). |
| Fan et al., (2019) [56] | Male Sprague Dawley rats. Spinal Cord Injury (SCI) | G1: Sham G2: SCI + saline solution (vehicle) G3: SCI + Quercetin 7,5 mg/kg | Intraperitoneal injections of quercetin or vehicle solutions twice daily for 10 days (n = 6). Analyses were performed until the 21st day after the procedures. | Behavioral assessment (BBB scores), qRT-PCR, Western blot, immunohistochemical assays, and electron microscopic analysis. | Quercetin significantly improved functional recovery. Quercetin considerably prevented oligodendrocyte necropsies, in addition to significantly reducing myelin loss and axonal loss after SCI. |
| Qiu et al., (2019) [58] | Male ICR mice. Sciatic Nerve Crush Injury | G1: Sham G2: Saline solution (vehicle) G3: Isoquercitrin (quercetin-3-glucoside) 20 mg/kg/day | Intraperitoneal injections of isoquercitrin or vehicle solutions. Analyses were performed until the 23rd day after the procedures. | Behavioral assessment (sciatic functional index), cell proliferation and migration assays qRT-PCR, Western blot, and electrophysiological analysis. | Isoquercitrin favored peripheral nerve remyelination, improved motor function recovery, reduced muscle atrophy, and inhibited autophagy. In addition, isoquercitrin suppressed cellular oxidative stress, favoring the proliferation and migration of Schwann cells. |
| Wang et al., (2021) [63] | Male Sprague Dawley rats. Spinal Cord Injury (SCI) | G1: Sham surgery + saline solution G2: SCI + saline solution G3: SCI + Quercetin 20 mg/kg/day G4: SCI + Quercetin + 3-methyladenine (3-MA; 400 nmol) | Intraperitoneal injections of solutions for 1, 3, or 7 days (n = 10). Analyses were performed until the 14th days after the procedures. | Behavioral assessment (BBB scores), Western blot and immunohistochemical analysis. | Quercetin favored axonal regeneration and promoted a significant recovery of locomotor capacity, minimizing histological alterations and cavity formation. 3-MA partially abrogated the neuroprotective effects of quercetin. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fideles, S.O.M.; de Cássia Ortiz, A.; Buchaim, D.V.; de Souza Bastos Mazuqueli Pereira, E.; Parreira, M.J.B.M.; de Oliveira Rossi, J.; da Cunha, M.R.; de Souza, A.T.; Soares, W.C.; Buchaim, R.L. Influence of the Neuroprotective Properties of Quercetin on Regeneration and Functional Recovery of the Nervous System. Antioxidants 2023, 12, 149. https://doi.org/10.3390/antiox12010149
Fideles SOM, de Cássia Ortiz A, Buchaim DV, de Souza Bastos Mazuqueli Pereira E, Parreira MJBM, de Oliveira Rossi J, da Cunha MR, de Souza AT, Soares WC, Buchaim RL. Influence of the Neuroprotective Properties of Quercetin on Regeneration and Functional Recovery of the Nervous System. Antioxidants. 2023; 12(1):149. https://doi.org/10.3390/antiox12010149
Chicago/Turabian StyleFideles, Simone Ortiz Moura, Adriana de Cássia Ortiz, Daniela Vieira Buchaim, Eliana de Souza Bastos Mazuqueli Pereira, Maria Júlia Bento Martins Parreira, Jéssica de Oliveira Rossi, Marcelo Rodrigues da Cunha, Alexandre Teixeira de Souza, Wendel Cleber Soares, and Rogerio Leone Buchaim. 2023. "Influence of the Neuroprotective Properties of Quercetin on Regeneration and Functional Recovery of the Nervous System" Antioxidants 12, no. 1: 149. https://doi.org/10.3390/antiox12010149
APA StyleFideles, S. O. M., de Cássia Ortiz, A., Buchaim, D. V., de Souza Bastos Mazuqueli Pereira, E., Parreira, M. J. B. M., de Oliveira Rossi, J., da Cunha, M. R., de Souza, A. T., Soares, W. C., & Buchaim, R. L. (2023). Influence of the Neuroprotective Properties of Quercetin on Regeneration and Functional Recovery of the Nervous System. Antioxidants, 12(1), 149. https://doi.org/10.3390/antiox12010149








