P2X7 Is Involved in the Mouse Retinal Degeneration via the Coordinated Actions in Different Retinal Cell Types
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Scotopic Electroretinogram (ERG) Analysis in Mice
2.3. Spectral-Domain Optical Coherence Tomography (SD-OCT) Imaging in Mice
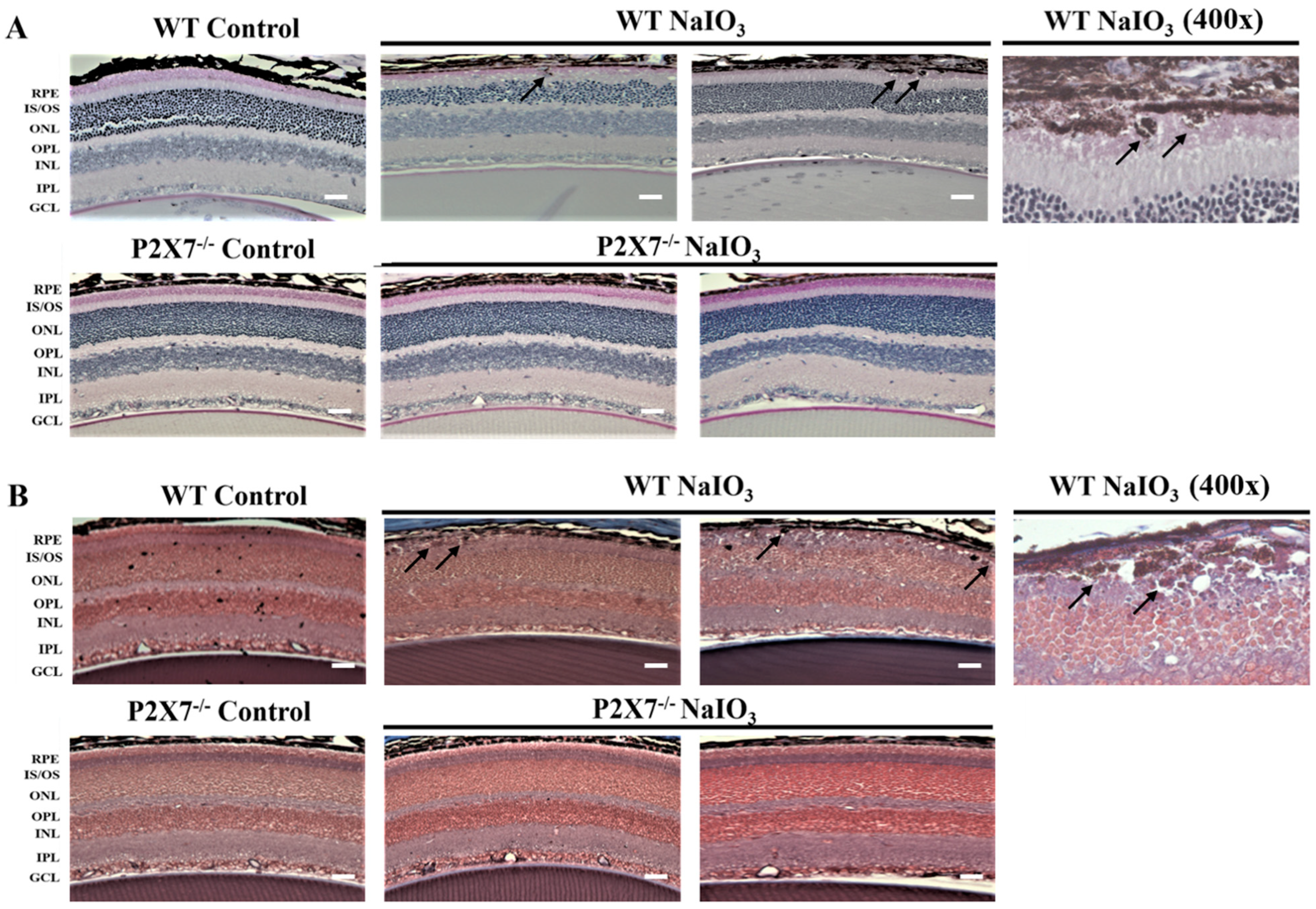
2.4. Histology Analysis
2.5. Measurement of Cytokine Concentrations by Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Cell Culture
2.7. Flow Cytometry Analysis
2.8. Quantitative Real-Time Polymerase Chain Reaction (PCR)
2.9. Immunoblotting
2.10. Determination of Nicotinamide Adenine Dinucleotide Phosphate (NADPH) Level
2.11. Statistical Analysis
3. Results
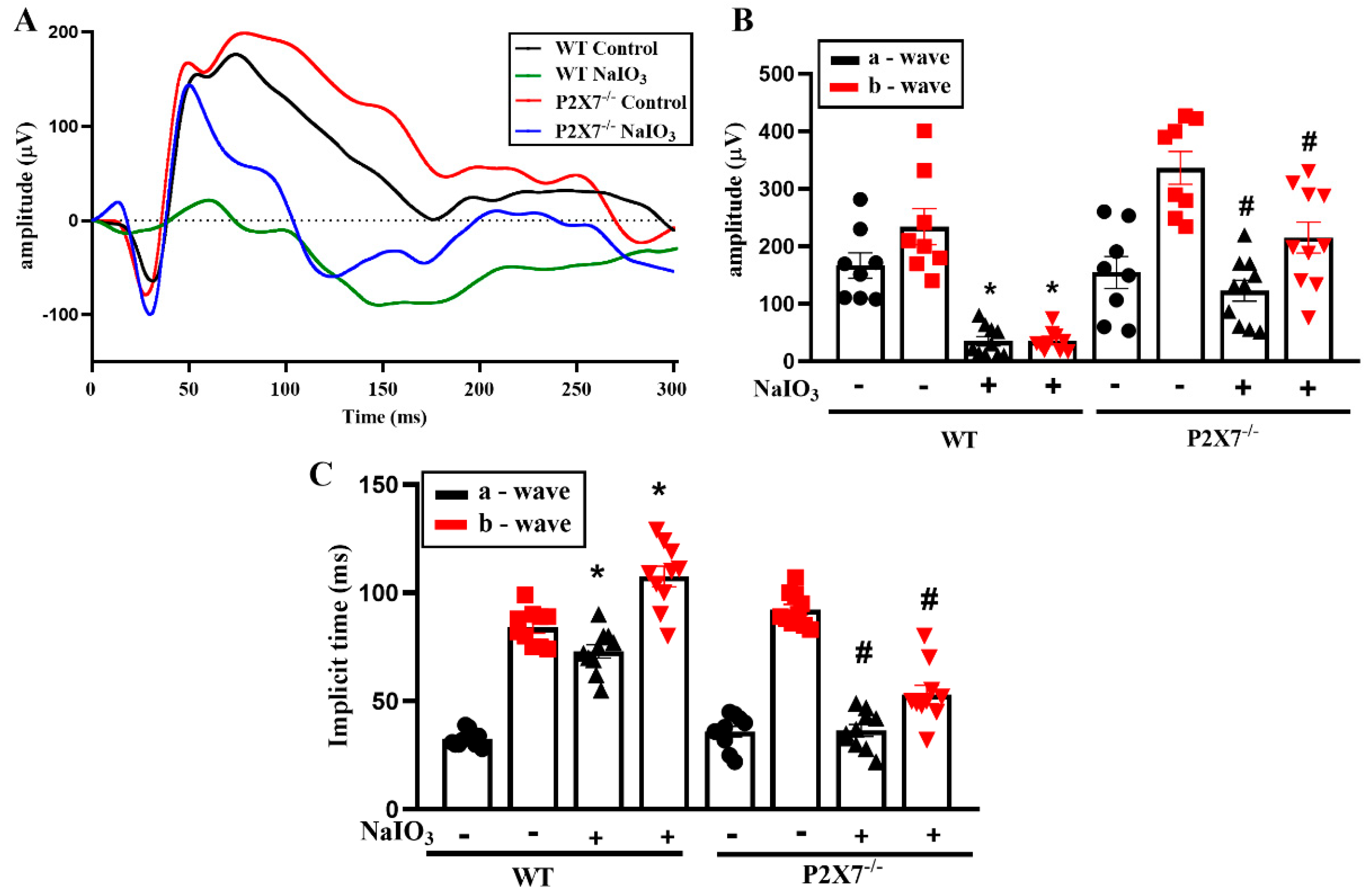
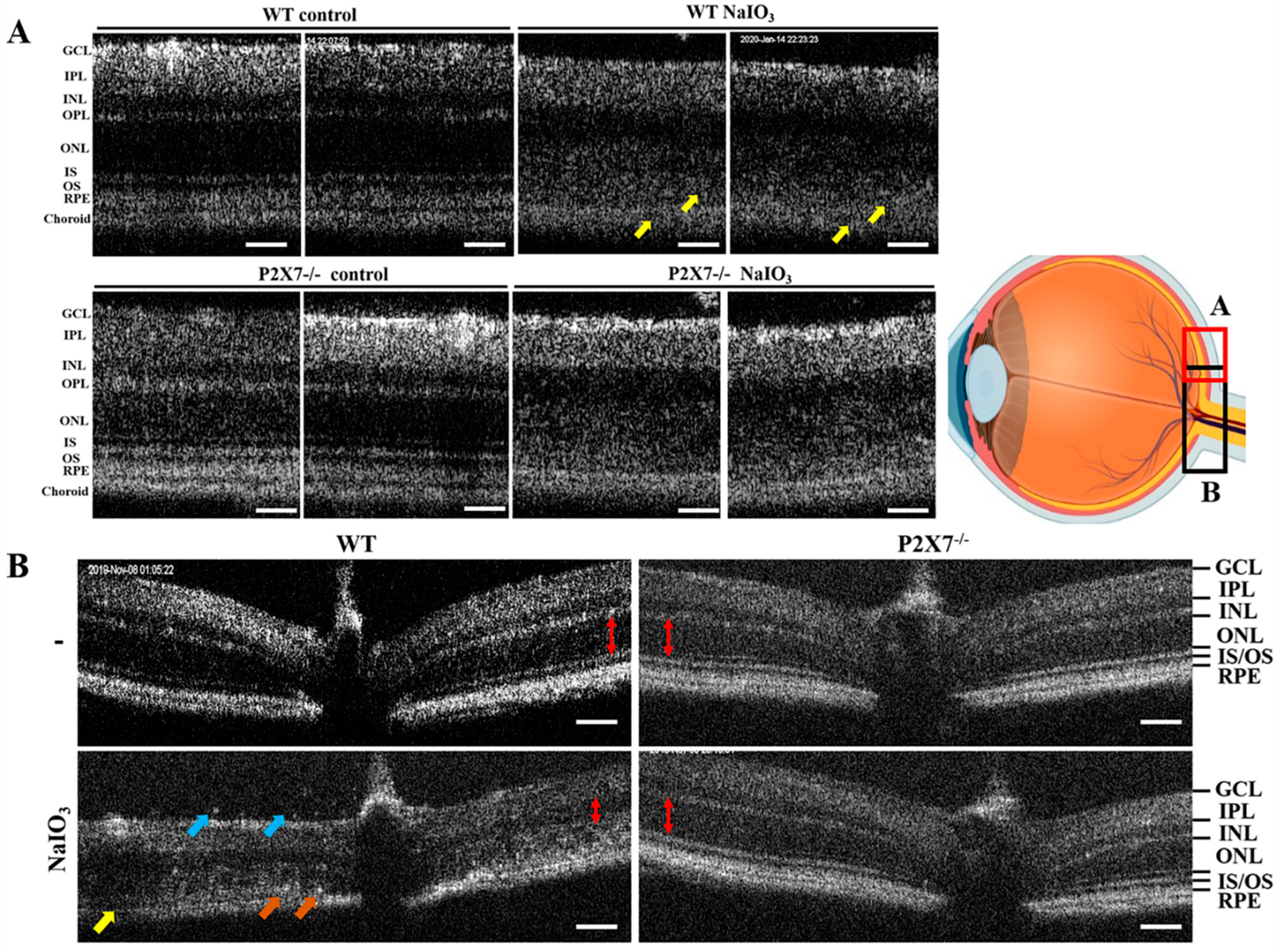
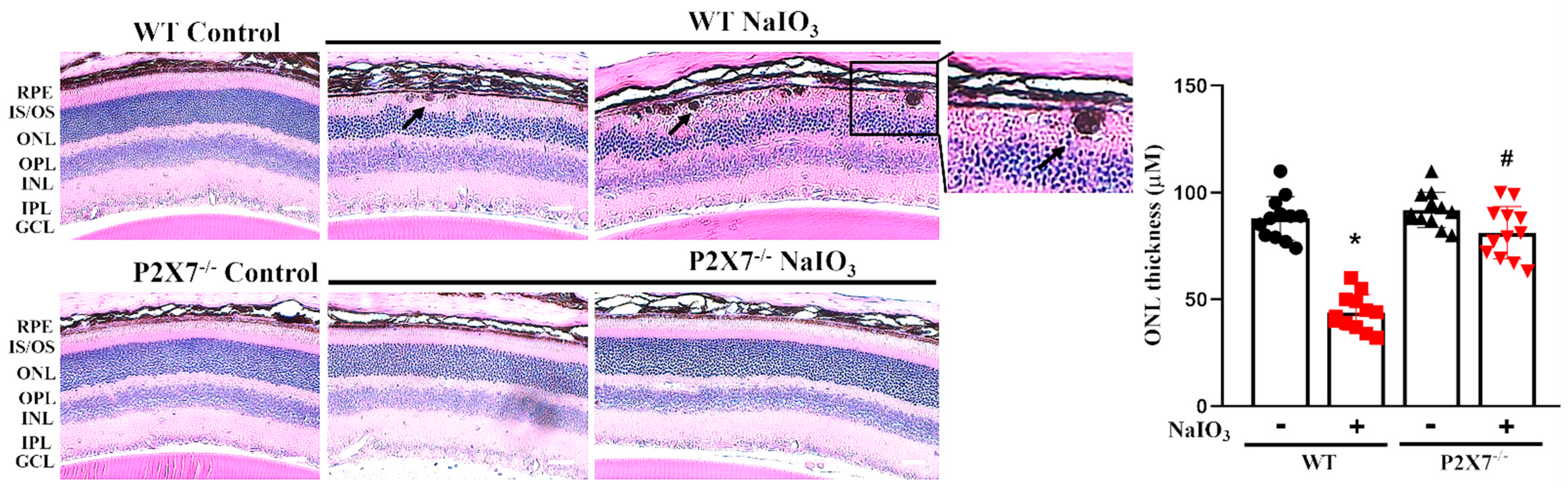
3.1. NaIO3-Induced Retinopathy Is Alleviated in P2X7 Knockout Mice
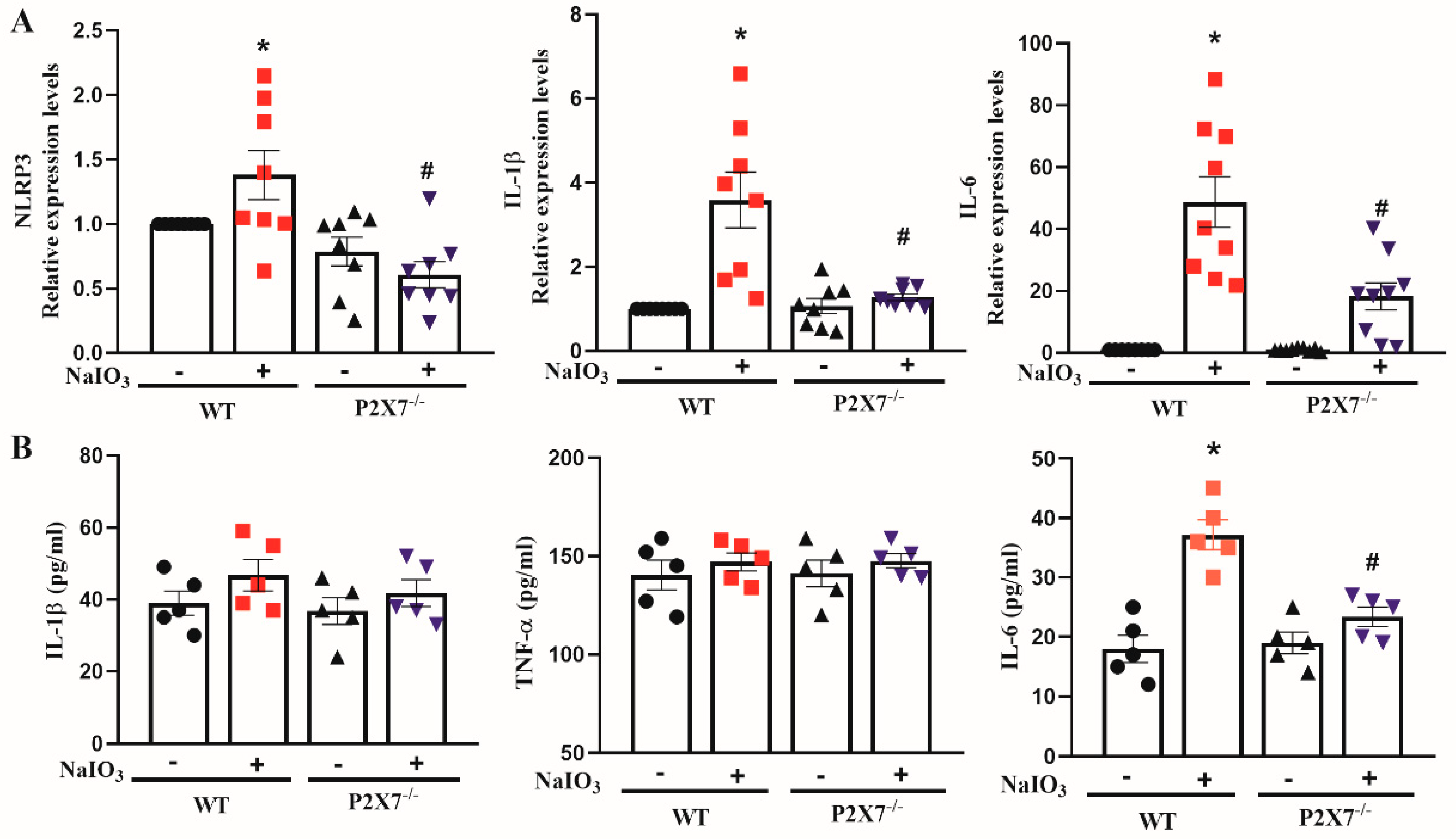
3.2. P2X7 Knockout Reduces NLRP3, IL-1β and IL-6 Gene Expression in Retinal Tissues after NaIO3 Injection
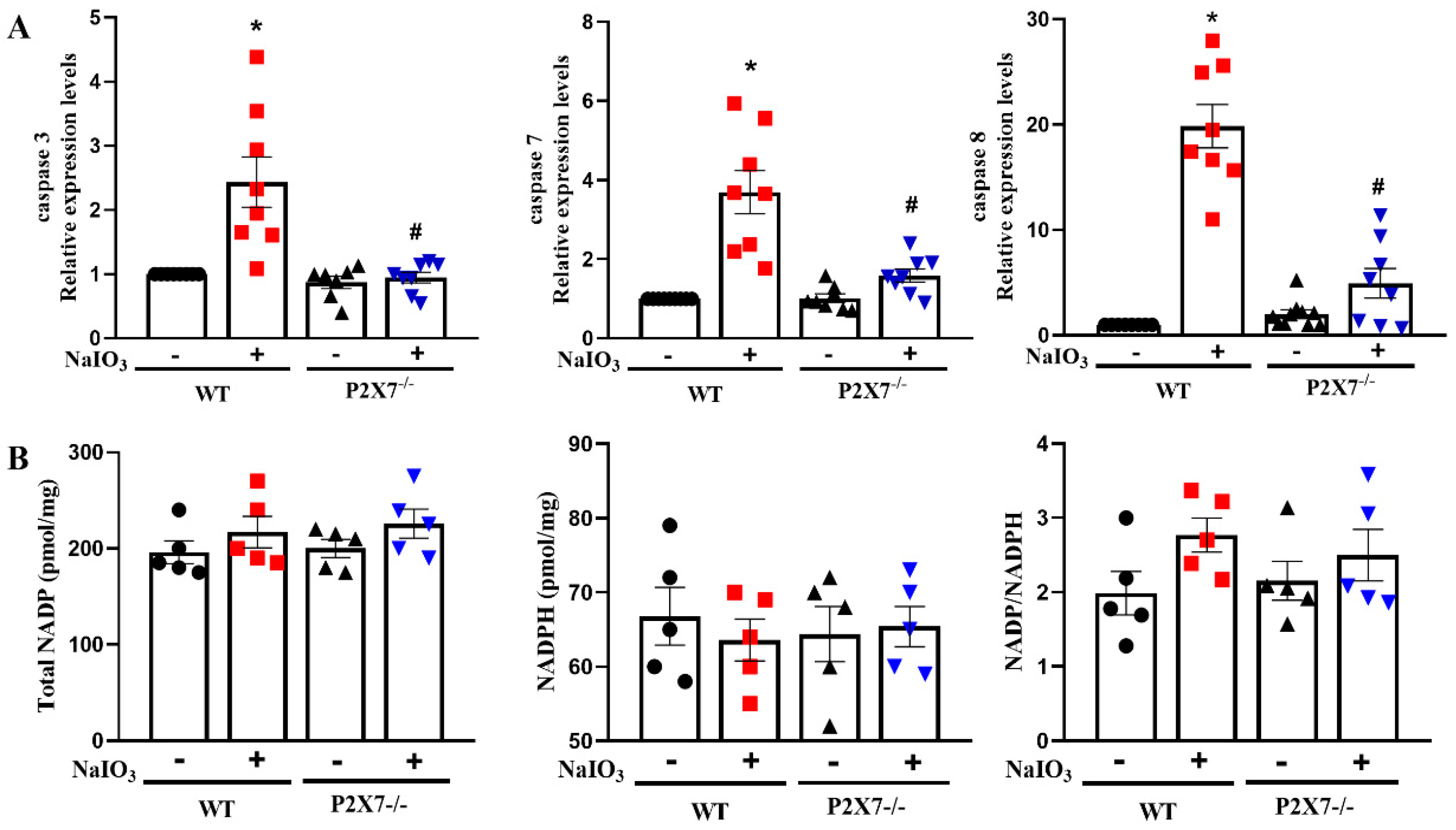
3.3. P2X7 Knockout Reduces Executional Caspases Expression in Retinal Tissues after NaIO3 Injection without Affecting NADP/NADPH
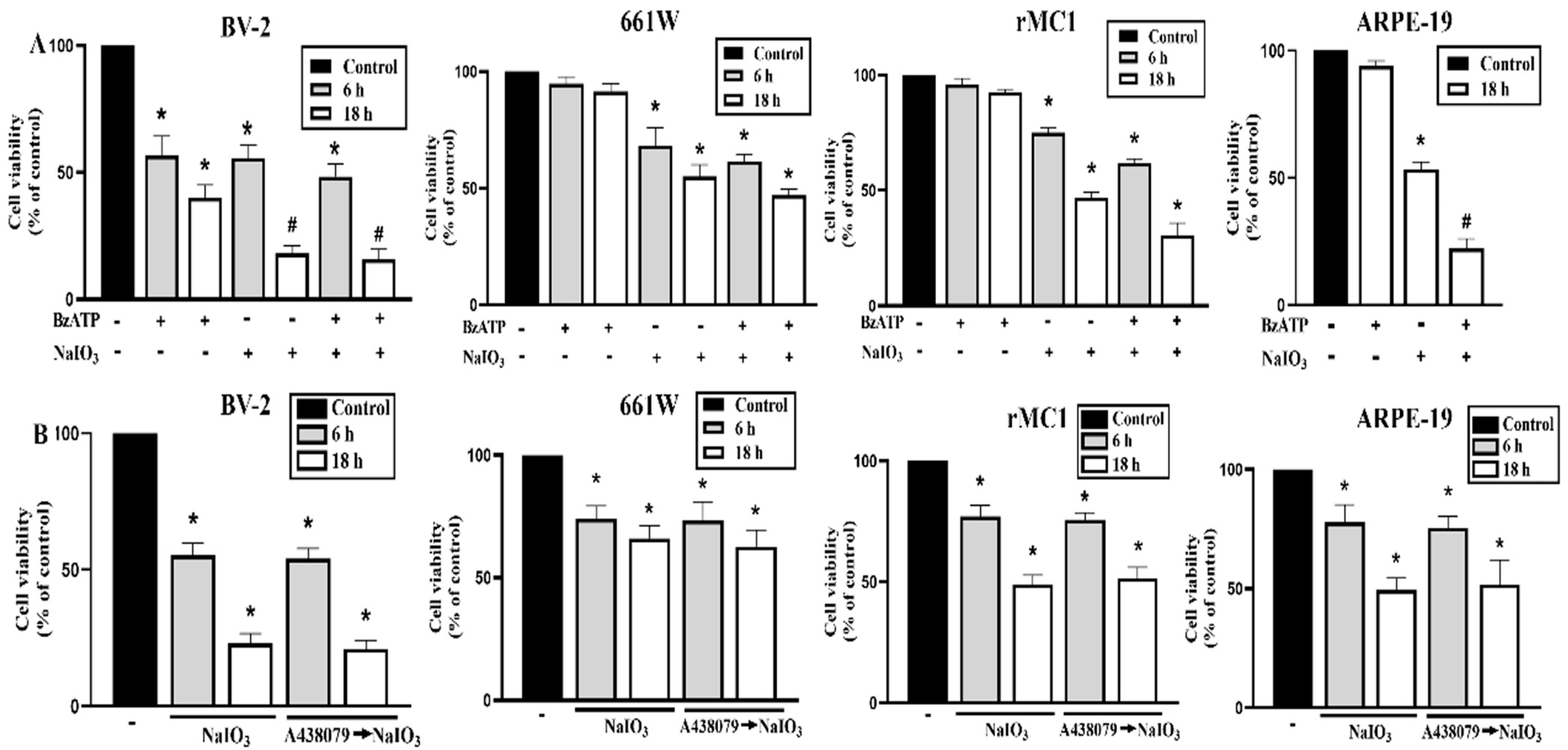
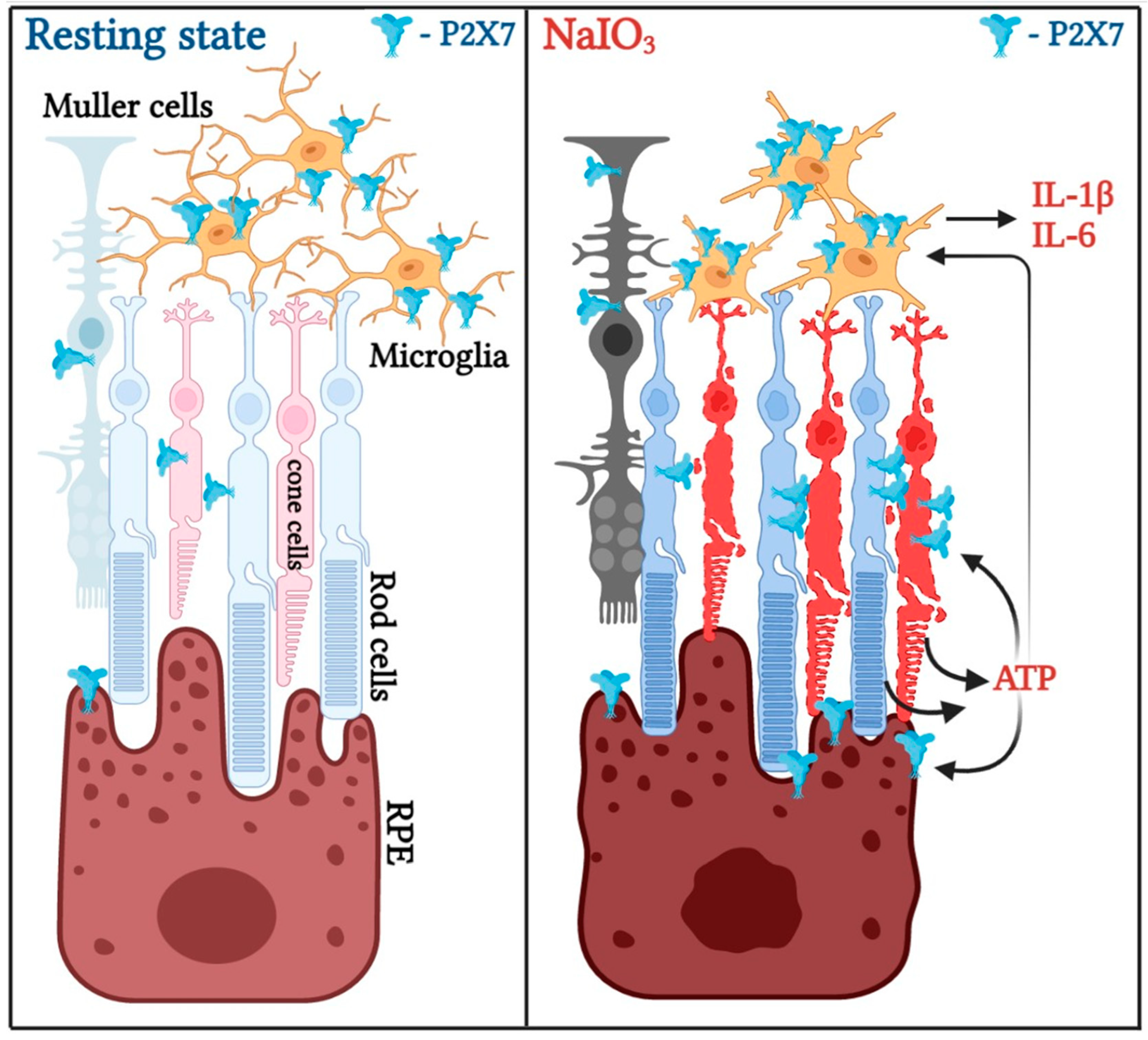
3.4. P2X7 Is Differentially Expressed in Retinal Cells and P2X7 Activation Increases NaIO3-Induced Cytotoxicity
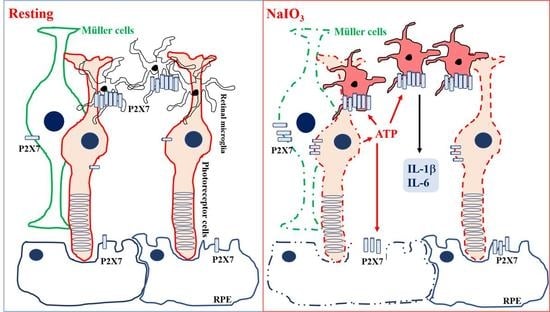
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burnstock, G. Historical review: ATP as a neurotransmitter. Trends Pharmacol. Sci. 2006, 27, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Seeland, S.; Kettiger, H.; Murphy, M.; Treiber, A.; Giller, J.; Kiss, A.; Sube, R.; Krahenbuhl, S.; Hafner, M.; Huwyler, J. ATP-induced cellular stress and mitochondrial toxicity in cells expressing purinergic P2X7 receptor. Pharmacol. Res. Perspect. 2015, 3, e00123. [Google Scholar] [CrossRef] [PubMed]
- Sluyter, R. The P2X7 Receptor. Adv. Exp. Med. Biol. 2017, 1051, 17–53. [Google Scholar] [CrossRef]
- Hwang, S.M.; Koo, N.Y.; Choi, S.Y.; Chun, G.S.; Kim, J.S.; Park, K. P2X7 receptor-mediated membrane blebbing in salivary epithelial cells. Korean J. Physiol. Pharmacol. 2009, 13, 175–179. [Google Scholar] [CrossRef][Green Version]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Sekar, P.; Huang, D.Y.; Hsieh, S.L.; Chang, S.F.; Lin, W.W. AMPK-dependent and independent actions of P2X7 in regulation of mitochondrial and lysosomal functions in microglia. Cell Commun. Signal. 2018, 16, 83. [Google Scholar] [CrossRef] [PubMed]
- Sekar, P.; Huang, D.Y.; Chang, S.F.; Lin, W.W. Coordinate effects of P2X7 and extracellular acidification in microglial cells. Oncotarget 2018, 9, 12718–12731. [Google Scholar] [CrossRef]
- Gupta, M.P.; Herzlich, A.A.; Sauer, T.; Chan, C.C. Retinal anatomy and pathology. Dev. Ophthalmol. 2016, 55, 7–17. [Google Scholar] [CrossRef]
- Bok, D. The retinal pigment epithelium: A versatile partner in vision. J. Cell Sci. Suppl. 1993, 17, 189–195. [Google Scholar] [CrossRef]
- Silverman, S.M.; Wong, W.T. Microglia in the retina: Roles in development, maturity, and disease. Annu. Rev. Vis. Sci. 2018, 4, 45–77. [Google Scholar] [CrossRef]
- Rathnasamy, G.; Foulds, W.S.; Ling, E.A.; Kaur, C. Retinal microglia—A key player in healthy and diseased retina. Prog. Neurobiol. 2019, 173, 18–40. [Google Scholar] [CrossRef] [PubMed]
- Di Pierdomenico, J.; Garcia-Ayuso, D.; Agudo-Barriuso, M.; Vidal-Sanz, M.; Villegas-Perez, M.P. Role of microglial cells in photoreceptor degeneration. Neural Regen. Res. 2019, 14, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Wooff, Y.; Fernando, N.; Wong, J.H.C.; Dietrich, C.; Aggio-Bruce, R.; Chu-Tan, J.A.; Robertson, A.A.B.; Doyle, S.L.; Man, S.M.; Natoli, R. Caspase-1-dependent inflammasomes mediate photoreceptor cell death in photo-oxidative damage-induced retinal degeneration. Sci. Rep. 2020, 10, 2263. [Google Scholar] [CrossRef]
- Ventura, A.L.M.; Dos Santos-Rodrigues, A.; Mitchell, C.H.; Faillace, M.P. Purinergic signaling in the retina: From development to disease. Brain Res. Bull. 2019, 151, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Yang, D. Targeting the P2X7 receptor in age-related macular degeneration. Vision 2017, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, E.L.; Wang, A.Y.; Jobling, A.I.; Rutar, M.V.; Greferath, U.; Gu, B.; Vessey, K.A. Targeting P2X7 receptors as a means for treating retinal disease. Drug Discov. Today 2019, 24, 1598–1605. [Google Scholar] [CrossRef]
- Platania, C.B.M.; Filippo Drago, F.; Bucolo, C. The P2X7 receptor as a new pharmacological target for retinal diseases. Biochem. Pharmacol. 2022, 198, 114942. [Google Scholar] [CrossRef]
- Notomi, S.; Hisatomi, T.; Murakami, Y.; Terasaki, H.; Sonoda, S.; Asato, R.; Takeda, A.; Ikeda, Y.; Enaida, H.; Sakamoto, T.; et al. Dynamic increase in extracellular ATP accelerates photoreceptor cell apoptosis via ligation of P2RX7 in subretinal hemorrhage. PLoS ONE 2013, 8, e53338. [Google Scholar] [CrossRef]
- Pavlou, S.; Augustine, J.; Cunning, R.; Harkin, K.; Stitt, A.W.; Xu, H.; Chen, M. Attenuating diabetic vascular and neuronal defects by targeting P2rx7. Int. J. Mol. Sci. 2019, 20, 2101. [Google Scholar] [CrossRef]
- Kaczmarek-Hajek, K.; Zhang, J.; Kopp, R.; Grosche, A.; Rissiek, B.; Saul, A.; Bruzzone, S.; Engel, T.; Jooss, T.; Krautloher, A.; et al. Re-evaluation of neuronal P2X7 expression using novel mouse models and a P2X7-specific nanobody. elife 2018, 7, e36217. [Google Scholar] [CrossRef] [PubMed]
- Clapp, C.; Diaz-Lezama, N.; Adan-Castro, E.; Ramirez-Hernandez, G.; Moreno-Carranza, B.; Sarti, A.C.; Falzoni, S.; Solini, A.; Di Virgilio, F. Pharmacological blockade of the P2X7 receptor reverses retinal damage in a rat model of type 1 diabetes. Acta Diabetol. 2019, 56, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Perez de Lara, M.J.; Aviles-Trigueros, M.; Guzman-Aranguez, A.; Valiente-Soriano, F.J.; de la Villa, P.; Vidal-Sanz, M.; Pintor, J. Potential role of P2X7 receptor in neurodegenerative processes in a murine model of glaucoma. Brain Res. Bull. 2019, 150, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Morigiwa, K.; Quan, M.; Murakami, M.; Yamashita, M.M.; Fukuda, Y. P2 purinoceptor expression and functional changes of hypoxia-activated cultured rat retinal microglia. Neurosci. Lett. 2000, 282, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Pannicke, T.; Fischer, W.; Biedermann, B.; Schadlich, H.; Grosche, J.; Faude, F.; Wiedemann, P.; Allgaier, C.; Illes, P.; Burnstock, G.; et al. P2X7 receptors in Muller glial cells from the human retina. J. Neurosci. 2000, 20, 5965–5972. [Google Scholar] [CrossRef]
- Wang, J.; Iacovelli, J.; Spencer, C.; Saint-Geniez, M. Direct effect of sodium iodate on neurosensory retina. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1941–1953. [Google Scholar] [CrossRef]
- Hanus, J.; Anderson, C.; Sarraf, D.; Ma, J.; Wang, S. Retinal pigment epithelial cell necroptosis in response to sodium iodate. Cell Death Discov. 2016, 2, 16054. [Google Scholar] [CrossRef]
- Chowers, G.; Cohen, M.; Marks-Ohana, D.; Stika, S.; Eijzenberg, A.; Banin, E.; Obolensky, A. Course of sodium iodate-induced retinal degeneration in albino and pigmented mice. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2239–2249. [Google Scholar] [CrossRef]
- Liu, Y.; Li, R.; Xie, J.; Hu, J.; Huang, X.; Ren, F.; Li, L. Protective effect of hydrogen on sodium iodate-induced age-related macular degeneration in mice. Front. Aging Neurosci. 2018, 10, 389. [Google Scholar] [CrossRef]
- Chan, C.M.; Huang, D.Y.; Sekar, P.; Hsu, S.H.; Lin, W.W. Correction to: Reactive oxygen species-dependent mitochondrial dynamics and autophagy confer protective effects in retinal pigment epithelial cells against sodium iodate-induced cell death. J. Biomed. Sci. 2019, 26, 66. [Google Scholar] [CrossRef]
- Ma, H.; Yang, F.; Ding, X.Q. Inhibition of thyroid hormone signaling protects retinal pigment epithelium and photoreceptors from cell death in a mouse model of age-related macular degeneration. Cell Death Dis. 2020, 11, 24. [Google Scholar] [CrossRef]
- Lin, F.L.; Lin, C.H.; Ho, J.D.; Yen, J.L.; Chang, H.M.; Chiou, G.C.; Cheng, Y.W.; Hsiao, G. The natural retinoprotectant chrysophanol attenuated photoreceptor cell apoptosis in an N-methyl-N-nitrosourea-induced mouse model of retinal degenaration. Sci. Rep. 2017, 7, 41086. [Google Scholar] [CrossRef]
- Balmer, J.; Zulliger, R.; Roberti, S.; Enzmann, V. Retinal cell death caused by sodium iodate involves multiple caspase-dependent and caspase-independent cell death pathways. Int. J. Mol. Sci. 2015, 16, 15086–15103. [Google Scholar] [CrossRef] [PubMed]
- Romano, G.L.; Amato, R.; Lazzara, F.; Porciatti, V.; Chou, T.H.; Drago, F.; Bucolo, C. P2X7 receptor antagonism preserves retinal ganglion cells in glaucomatous mice. Biochem. Pharmacol. 2020, 180, 114199. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.S.; Tang, Y.; Song, J.T. ATP and adenosine in the retina and retinal diseases. Front. Pharmacol. 2021, 12, 654445. [Google Scholar] [CrossRef] [PubMed]
- Vessey, K.A.; Fletcher, E.L. Rod and cone pathway signalling is altered in the P2X7 receptor knock out mouse. PLoS ONE 2012, 7, e29990. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, T.A.; Canning, P.; Tipping, N.; Archer, D.B.; Stitt, A.W. Abnormal glycogen storage by retinal neurons in diabetes. Investig. Ophthalmol. Vis. Sci. 2015, 56, 8008–8018. [Google Scholar] [CrossRef] [PubMed]
- Monif, M.; Burnstock, G.; Williams, D.A. Microglia: Proliferation and activation driven by the P2X7 receptor. Int. J. Biochem. Cell Biol. 2010, 42, 1753–1756. [Google Scholar] [CrossRef]
- Thawkar, B.S.; Kaur, G. Inhibitors of NF-kappaB and P2X7/NLRP3/caspase 1 pathway in microglia: Novel therapeutic opportunities in neuroinflammation induced early-stage Alzheimer’s disease. J. Neuroimmunol. 2019, 326, 62–74. [Google Scholar] [CrossRef]
- Ferrari, D.; Los, M.; Bauer, M.K.; Vandenabeele, P.; Wesselborg, S.; Schulze-Osthoff, K. P2Z purinoreceptor ligation induces activation of caspases with distinct roles in apoptotic and necrotic alterations of cell death. FEBS Lett. 1999, 447, 71–75. [Google Scholar] [CrossRef]
- Kumar, S.; Mishra, A.; Krishnamurthy, S. Purinergic antagonism prevents mitochondrial dysfunction and behavioral deficits associated with dopaminergic toxicity induced by 6-OHDA in rats. Neurochem. Res. 2017, 42, 3414–3430. [Google Scholar] [CrossRef]
- Bringmann, A.; Pannicke, T.; Moll, V.; Milenkovic, I.; Faude, F.; Enzmann, V.; Wolf, S.; Reichenbach, A. Upregulation of P2X(7) receptor currents in Muller glial cells during proliferative vitreoretinopathy. Investig. Ophthalmol. Vis. Sci. 2001, 42, 860–867. [Google Scholar]
- Jabs, R.; Guenther, E.; Marquordt, K.; Wheeler-Schilling, T.H. Evidence for P2X3, P2X4, P2X5 but not for P2X7 containing purinergic receptors in Muller cells of the rat retina. Brain Res. Mol. Brain Res. 2000, 76, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Trueblood, K.E.; Mohr, S.; Dubyak, G.R. Purinergic regulation of high-glucose-induced caspase-1 activation in the rat retinal Muller cell line rMC-1. Am. J. Physiol. Cell Physiol. 2011, 301, C1213–C1223. [Google Scholar] [CrossRef] [PubMed]
- Notomi, S.; Hisatomi, T.; Kanemaru, T.; Takeda, A.; Ikeda, Y.; Enaida, H.; Kroemer, G.; Ishibashi, T. Critical involvement of extracellular ATP acting on P2RX7 purinergic receptors in photoreceptor cell death. Am. J. Pathol. 2011, 179, 2798–2809. [Google Scholar] [CrossRef] [PubMed]
- Corso, L.; Cavallero, A.; Baroni, D.; Garbati, P.; Prestipino, G.; Bisti, S.; Nobile, M.; Picco, C. Saffron reduces ATP-induced retinal cytotoxicity by targeting P2X7 receptors. Purinergic Signal. 2016, 12, 161–174. [Google Scholar] [CrossRef]
- Resta, V.; Novelli, E.; Di Virgilio, F.; Galli-Resta, L. Neuronal death induced by endogenous extracellular ATP in retinal cholinergic neuron density control. Development 2005, 132, 2873–2882. [Google Scholar] [CrossRef]
- Hu, H.; Lu, W.; Zhang, M.; Zhang, X.; Argall, A.J.; Patel, S.; Lee, G.E.; Kim, Y.C.; Jacobson, K.A.; Laties, A.M.; et al. Stimulation of the P2X7 receptor kills rat retinal ganglion cells in vivo. Exp. Eye Res. 2010, 91, 425–432. [Google Scholar] [CrossRef]
- Niyadurupola, N.; Sidaway, P.; Ma, N.; Rhodes, J.D.; Broadway, D.C.; Sanderson, J. P2X7 receptor activation mediates retinal ganglion cell death in a human retina model of ischemic neurodegeneration. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2163–2170. [Google Scholar] [CrossRef]
- Puthussery, T.; Fletcher, E. Extracellular ATP induces retinal photoreceptor apoptosis through activation of purinoceptors in rodents. J. Comp. Neurol. 2009, 513, 430–440. [Google Scholar] [CrossRef]
- Zhang, Q.-L.; Wang, W.; Alatantuya; Dongmei; Lu, Z.-J.; Li, L.-L.; Zhang, T.-Z. Down-regulated miR-187 promotes oxidative stress-induced retinal cell apoptosis through P2X7 receptor. Int. J. Biol. Macromol. 2018, 120, 801–810. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Wang, W.; Jiang, Y.; Li, L.L.; Lu, Z.J.; Chang, H.; Zhang, T.Z. GRGM-13 comprising 13 plant and animal products, inhibited oxidative stress induced apoptosis in retinal ganglion cells by inhibiting P2RX7/p38 MAPK signaling pathway. Biomed. Pharmacother. 2018, 101, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Freitas, H.R.; Isaac, A.R.; Silva, T.M.; Diniz, G.O.F.; Dos Santos Dabdab, Y.; Bockmann, E.C.; Guimaraes, M.Z.P.; da Costa Calaza, K.; de Mello, F.G.; Ventura, A.L.M.; et al. Cannabinoids induce cell death and promote P2X7 receptor signaling in retinal glial progenitors in culture. Mol. Neurobiol. 2019, 56, 6472–6486. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Elner, S.G.; Clark, A.J.; Hughes, B.A.; Petty, H.R.; Elner, V.M. Activation of P2X receptors induces apoptosis in human retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Schmidt, S.; Larsen, P.P.; Meyer, J.H.; Roush, W.R.; Latz, E.; Holz, F.G.; Krohne, T.U. Efficacy of novel selective NLRP3 inhibitors in human and murine retinal pigment epithelial cells. J. Mol. Med. 2019, 97, 523–532. [Google Scholar] [CrossRef]
- Guha, S.; Baltazar, G.C.; Coffey, E.E.; Tu, L.A.; Lim, J.C.; Beckel, J.M.; Patel, S.; Eysteinsson, T.; Lu, W.; O’Brien-Jenkins, A.; et al. Lysosomal alkalinization, lipid oxidation, and reduced phagosome clearance triggered by activation of the P2X7 receptor. FASEB J. 2013, 27, 4500–4509. [Google Scholar] [CrossRef]
- Nie, Q.; Gong, X.; Gong, L.; Zhang, L.; Tang, X.; Wang, L.; Liu, F.; Fu, J.L.; Xiang, J.W.; Xiao, Y.; et al. Sodium iodate-induced mouse model of age-related macular degeneration displayed altered expression patterns of sumoylation enzymes E1, E2 and E3. Curr. Mol. Med. 2018, 18, 550–555. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, H.; Qin, S.; Liu, C.; Chen, Y.; Yang, Y.; Xu, C. The P2X7 receptor involved in gp120-induced cell injury in BV2 microglia. Inflammation 2016, 39, 1814–1826. [Google Scholar] [CrossRef]
- Huang, C.; Yu, W.; Cui, H.; Wang, Y.; Zhang, L.; Han, F.; Huang, T. P2X7 blockade attenuates mouse liver fibrosis. Mol. Med. Rep. 2014, 9, 57–62. [Google Scholar] [CrossRef]
- Xue, B.; Xie, Y.; Xue, Y.; Hu, N.; Zhang, G.; Guan, H.; Ji, M. Involvement of P2X7 receptors in retinal ganglion cell apoptosis induced by activated Muller cells. Exp. Eye Res. 2016, 153, 42–50. [Google Scholar] [CrossRef]
- Portillo, J.C.; Lopez Corcino, Y.; Dubyak, G.R.; Kern, T.S.; Matsuyama, S.; Subauste, C.S. Ligation of CD40 in human Muller cells induces P2X7 receptor-dependent death of retinal endothelial cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6278–6286. [Google Scholar] [CrossRef]
- Kakurai, K.; Sugiyama, T.; Kurimoto, T.; Oku, H.; Ikeda, T. Involvement of P2X7 receptors in retinal ganglion cell death after optic nerve crush injury in rats. Neurosci. Lett. 2013, 534, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Y.; Sun, Q.; Xue, S.; Guan, H.; Ji, M. Activation of P2X7R- NLRP3 pathway in retinal microglia contribute to retinal ganglion cells death in chronic ocular hypertension (COH). Exp. Eye Res. 2019, 188, 107771. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.J.; Calippe, B.; Lavalette, S.; Roubeix, C.; Montassar, F.; Housset, M.; Levy, O.; Delarasse, C.; Paques, M.; Sahel, J.A.; et al. Upregulation of P2RX7 in Cx3cr1-deficient mononuclear phagocytes leads to increased interleukin-1beta secretion and photoreceptor neurodegeneration. J. Neurosci. 2015, 35, 6987–6996. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekar, P.; Hsiao, G.; Chen, Y.-S.; Lin, W.-W.; Chan, C.-M. P2X7 Is Involved in the Mouse Retinal Degeneration via the Coordinated Actions in Different Retinal Cell Types. Antioxidants 2023, 12, 141. https://doi.org/10.3390/antiox12010141
Sekar P, Hsiao G, Chen Y-S, Lin W-W, Chan C-M. P2X7 Is Involved in the Mouse Retinal Degeneration via the Coordinated Actions in Different Retinal Cell Types. Antioxidants. 2023; 12(1):141. https://doi.org/10.3390/antiox12010141
Chicago/Turabian StyleSekar, Ponarulselvam, George Hsiao, Yuan-Shen Chen, Wan-Wan Lin, and Chi-Ming Chan. 2023. "P2X7 Is Involved in the Mouse Retinal Degeneration via the Coordinated Actions in Different Retinal Cell Types" Antioxidants 12, no. 1: 141. https://doi.org/10.3390/antiox12010141
APA StyleSekar, P., Hsiao, G., Chen, Y.-S., Lin, W.-W., & Chan, C.-M. (2023). P2X7 Is Involved in the Mouse Retinal Degeneration via the Coordinated Actions in Different Retinal Cell Types. Antioxidants, 12(1), 141. https://doi.org/10.3390/antiox12010141