The Current State of Knowledge in Biological Properties of Cirsimaritin
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Sources of Cirsimaritin
3.2. Biological and Pharmacological Properties
3.2.1. Antibacterial and Anti-Fungal Activities
3.2.2. Antiviral and Antiparasitic Activities
3.2.3. Antioxidant Activity
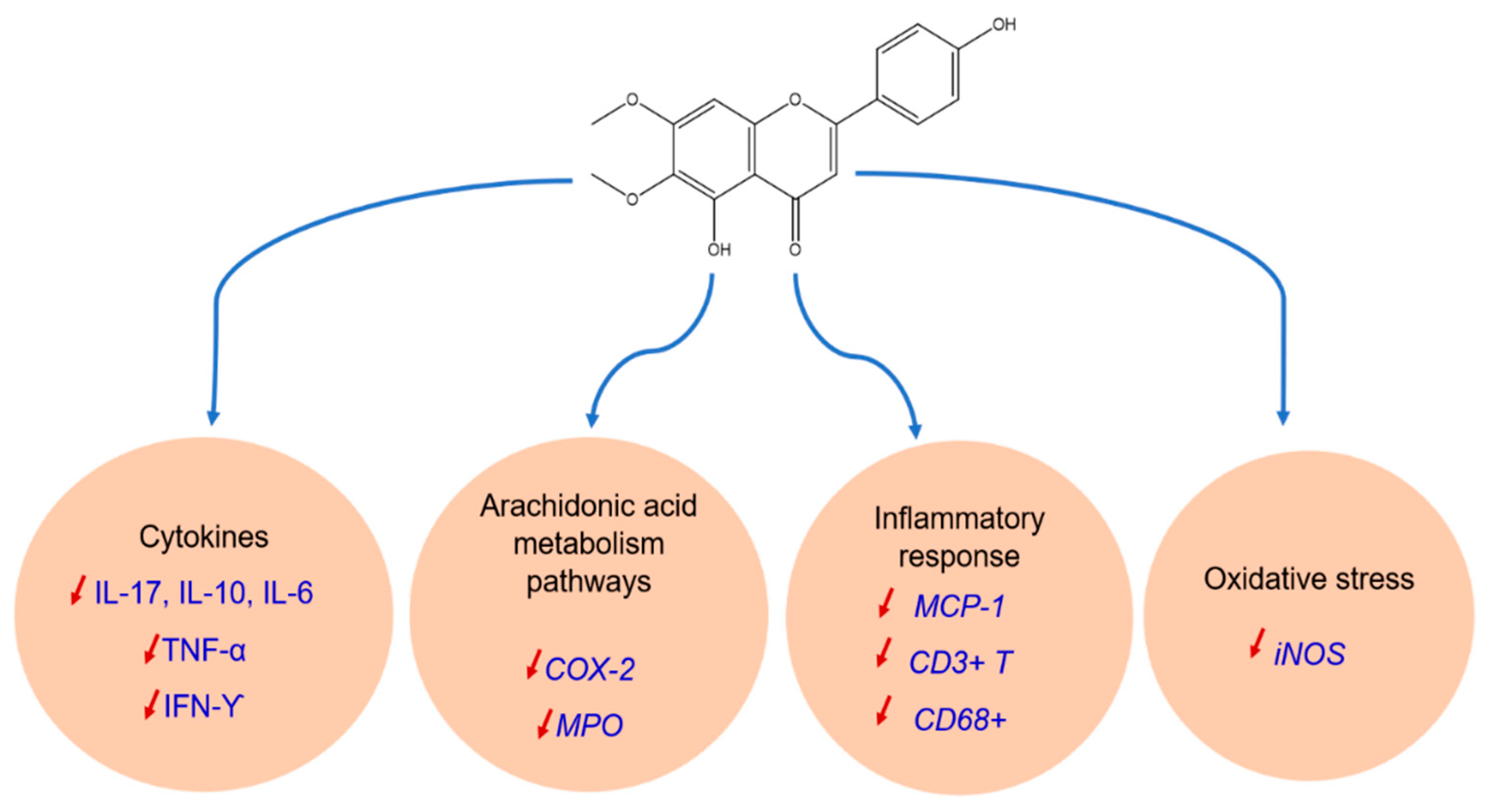
3.2.4. Anti-Inflammatory Activity
3.2.5. Antidiabetic Activity
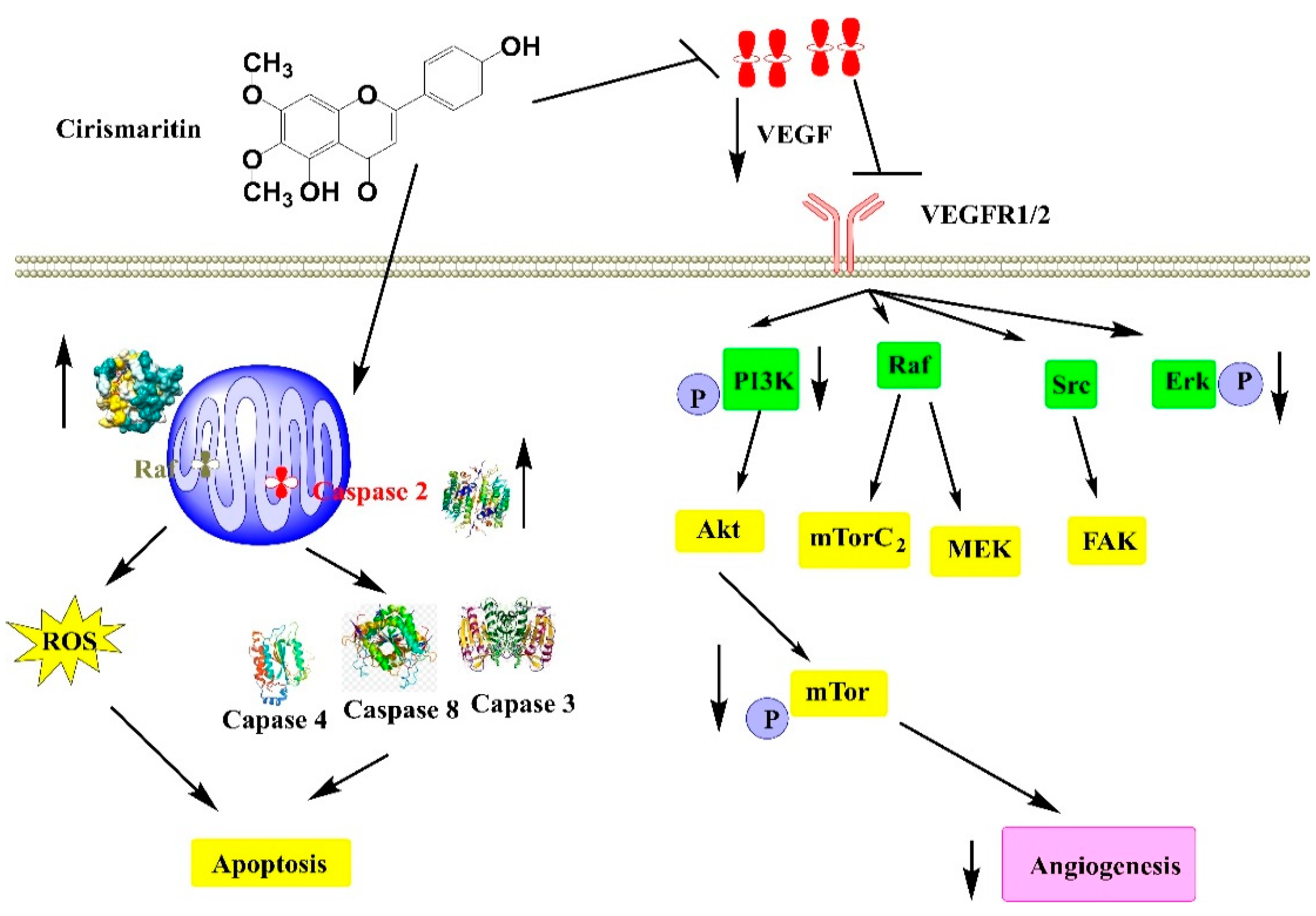
3.2.6. Anti-Cancer Activity
3.2.7. Other Biological Activities
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bouyahya, A.; El Omari, N.; Elmenyiy, N.; Guaouguaou, F.-E.; Balahbib, A.; Belmehdi, O.; Salhi, N.; Imtara, H.; Mrabti, H.N.; El-Shazly, M.; et al. Moroccan Antidiabetic Medicinal Plants: Ethnobotanical Studies, Phytochemical Bioactive Compounds, Preclinical Investigations, Toxicological Validations and Clinical Evidences; Challenges, Guidance and Perspectives for Future Management of Diabetes Worldwide. Trends Food Sci. Technol. 2021, 115, 147–254. [Google Scholar] [CrossRef]
- Bouyahya, A.; El Omari, N.; Hakkur, M.; El Hachlafi, N.; Charfi, S.; Balahbib, A.; Guaouguaou, F.-E.; Rebezov, M.; Maksimiuk, N.; Shariati, M.A.; et al. Sources, Health Benefits, and Biological Properties of Zeaxanthin. Trends Food Sci. Technol. 2021, 118, 519–538. [Google Scholar] [CrossRef]
- Bouyahya, A.; Guaouguaou, F.-E.; El Omari, N.; El Menyiy, N.; Balahbib, A.; El-Shazly, M.; Bakri, Y. Anti-Inflammatory and Analgesic Properties of Moroccan Medicinal Plants: Phytochemistry, in Vitro and in Vivo Investigations, Mechanism Insights, Clinical Evidences and Perspectives. J. Pharm. Anal. 2021, 12, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Chamkhi, I.; Benali, T.; Aanniz, T.; El Menyiy, N.; Guaouguaou, F.-E.; El Omari, N.; El-Shazly, M.; Zengin, G.; Bouyahya, A. Plant-Microbial Interaction: The Mechanism and the Application of Microbial Elicitor Induced Secondary Metabolites Biosynthesis in Medicinal Plants. Plant Physiol. Biochem. 2021, 167, 269–295. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Akram, M.; Semwal, P.; Mujawah, A.A.H.; Muhammad, N.; Riaz, Z.; Munir, N.; Piotrovsky, D.; Vdovina, I.; Bouyahya, A.; et al. Antispasmodic Potential of Medicinal Plants: A Comprehensive Review. Oxid. Med. Cell. Longev. 2021, 2021, e4889719. [Google Scholar] [CrossRef] [PubMed]
- Pina, L.T.; Serafini, M.R.; Oliveira, M.A.; Sampaio, L.A.; Guimarães, J.O.; Guimarães, A.G. Carvone and Its Pharmacological Activities: A Systematic Review. Phytochemistry 2022, 196, 113080. [Google Scholar] [CrossRef]
- Aboulaghras, S.; Sahib, N.; Bakrim, S.; Benali, T.; Charfi, S.; Guaouguaou, F.-E.; Omari, N.E.; Gallo, M.; Montesano, D.; Zengin, G. Health Benefits and Pharmacological Aspects of Chrysoeriol. Pharmaceuticals 2022, 15, 973. [Google Scholar] [CrossRef]
- El Omari, N.; Jaouadi, I.; Lahyaoui, M.; Benali, T.; Taha, D.; Bakrim, S.; El Menyiy, N.; El Kamari, F.; Zengin, G.; Bangar, S.P. Natural Sources, Pharmacological Properties, and Health Benefits of Daucosterol: Versatility of Actions. Appl. Sci. 2022, 12, 5779. [Google Scholar] [CrossRef]
- Bakrim, S.; Machate, H.; Benali, T.; Sahib, N.; Jaouadi, I.; Omari, N.E.; Aboulaghras, S.; Bangar, S.P.; Lorenzo, J.M.; Zengin, G. Natural Sources and Pharmacological Properties of Pinosylvin. Plants 2022, 11, 1541. [Google Scholar] [CrossRef]
- Bouyahya, A.; Chamkhi, I.; Benali, T.; Guaouguaou, F.-E.; Balahbib, A.; El Omari, N.; Taha, D.; Belmehdi, O.; Ghokhan, Z.; El Menyiy, N. Traditional Use, Phytochemistry, Toxicology, and Pharmacology of Origanum majorana L. J. Ethnopharmacol. 2021, 265, 113318. [Google Scholar] [CrossRef]
- Bouyahya, A.; Mechchate, H.; Benali, T.; Ghchime, R.; Charfi, S.; Balahbib, A.; Burkov, P.; Shariati, M.A.; Lorenzo, J.M.; Omari, N.E. Health Benefits and Pharmacological Properties of Carvone. Biomolecules 2021, 11, 1803. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; Chamkhi, I.; Guaouguaou, F.-E.; Benali, T.; Balahbib, A.; El Omari, N.; Taha, D.; El-Shazly, M.; El Menyiy, N. Ethnomedicinal Use, Phytochemistry, Pharmacology, and Food Benefits of Thymus Capitatus. J. Ethnopharmacol. 2020, 259, 112925. [Google Scholar] [CrossRef] [PubMed]
- El Omari, N.; Bakha, M.; Imtara, H.; Guaouguaoua, F.-E.; Balahbib, A.; Zengin, G.; Bouyahya, A. Anticancer Mechanisms of Phytochemical Compounds: Focusing on Epigenetic Targets. Environ. Sci. Pollut. Res. 2021, 28, 47869–47903. [Google Scholar] [CrossRef]
- El Omari, N.; Bakrim, S.; Bakha, M.; Lorenzo, J.M.; Rebezov, M.; Shariati, M.A.; Aboulaghras, S.; Balahbib, A.; Khayrullin, M.; Bouyahya, A. Natural Bioactive Compounds Targeting Epigenetic Pathways in Cancer: A Review on Alkaloids, Terpenoids, Quinones, and Isothiocyanates. Nutrients 2021, 13, 3714. [Google Scholar] [CrossRef] [PubMed]
- El Omari, N.; El Menyiy, N.; Zengin, G.; Goh, B.H.; Gallo, M.; Montesano, D.; Naviglio, D.; Bouyahya, A. Anticancer and Anti-Inflammatory Effects of Tomentosin: Cellular and Molecular Mechanisms. Separations 2021, 8, 207. [Google Scholar] [CrossRef]
- El Omari, N.; Ezzahrae Guaouguaou, F.; El Menyiy, N.; Benali, T.; Aanniz, T.; Chamkhi, I.; Balahbib, A.; Taha, D.; Shariati, M.A.; Zengin, G.; et al. Phytochemical and Biological Activities of Pinus halepensis Mill., and Their Ethnomedicinal Use. J. Ethnopharmacol. 2021, 268, 113661. [Google Scholar] [CrossRef]
- Cheriet, T.; Ben-Bachir, B.; Thamri, O.; Seghiri, R.; Mancini, I. Isolation and Biological Properties of the Natural Flavonoids Pectolinarin and Pectolinarigenin—A Review. Antibiotics 2020, 9, 417. [Google Scholar] [CrossRef]
- Williamson, G.; Barron, D.; Shimoi, K.; Terao, J. In Vitro Biological Properties of Flavonoid Conjugates Found in Vivo. Free Radic. Res. 2005, 39, 457–469. [Google Scholar] [CrossRef]
- Caltagirone, S.; Rossi, C.; Poggi, A.; Ranelletti, F.O.; Natali, P.G.; Brunetti, M.; Aiello, F.B.; Piantelli, M. Flavonoids Apigenin and Quercetin Inhibit Melanoma Growth and Metastatic Potential. Int. J. Cancer 2000, 87, 595–600. [Google Scholar] [CrossRef]
- Xing, N.; Meng, X.; Wang, S. Isobavachalcone: A Comprehensive Review of Its Plant Sources, Pharmacokinetics, Toxicity, Pharmacological Activities and Related Molecular Mechanisms. Phytother. Res. 2022, 36, 3120–3142. [Google Scholar] [CrossRef]
- Ferreira, A.; Pousinho, S.; Fortuna, A.; Falcão, A.; Alves, G. Flavonoid Compounds as Reversal Agents of the P-Glycoprotein-Mediated Multidrug Resistance: Biology, Chemistry and Pharmacology. Phytochem. Rev. 2015, 14, 233–272. [Google Scholar] [CrossRef]
- Dey, D.; Biswas, P.; Paul, P.; Mahmud, S.; Ema, T.I.; Khan, A.A.; Ahmed, S.Z.; Hasan, M.M.; Saikat, A.S.M.; Fatema, B. Natural Flavonoids Effectively Block the CD81 Receptor of Hepatocytes and Inhibit HCV Infection: A Computational Drug Development Approach. Mol. Divers. 2022, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Thapa, A.; Chi, E.Y. Biflavonoids as Potential Small Molecule Therapeutics for Alzheimer’s Disease. In Natural Compounds as Therapeutic Agents for Amyloidogenic Diseases; Springer: Berlin/Heidelberg, Germany, 2015; pp. 55–77. [Google Scholar]
- Bouzid, N.; Moulis, C.; Fouraste, I. Flavones Libres de Artemisia Mesatlantica. Planta Med. 1982, 44, 157–158. [Google Scholar] [CrossRef] [PubMed]
- Saleh, N.A.M.; El-Negoumy, S.I.; Abou-zaid, M.M. Flavonoids of Artemisia Judaica, A. Monosperma and A. Herba-Alba. Phytochemistry 1987, 26, 3059–3064. [Google Scholar] [CrossRef]
- Hasrat, J.A.; De Bruyne, T.; De Backer, J.-P.; Vauquelin, G.; Vlietinck, A.J. (Phytolaccaceae) with Adenosine Antagonistic Properties in Rats: Leads for New Therapeutics in Acute Renal Failure. J. Pharm. Pharmacol. 1997, 49, 1150–1156. [Google Scholar] [CrossRef]
- Kelm, M.A.; Nair, M.G.; Strasburg, G.M.; De Witt, D.L. Antioxidant and Cyclooxygenase Inhibitory Phenolic Compounds from Ocimum Sanctum Linn. Phytomedicine 2000, 7, 7–13. [Google Scholar] [CrossRef]
- Ragasa, C.; Pendon, Z.; Veronica, S.; Rideout, J.A. Antimicrobial flavones from Coleus amboinicus. Philipp. J. Sci. 1999, 128, 347–352. [Google Scholar]
- Rijo, P.; Simões, M.F.; Duarte, A.; Rodríguez, B. Isopimarane Diterpenoids from Aeollanthus Rydingianus and Their Antimicrobial Activity. Phytochemistry 2009, 70, 1161–1165. [Google Scholar] [CrossRef]
- Marino, A.; Zengin, G.; Nostro, A.; Ginestra, G.; Dugo, P.; Cacciola, F.; Miceli, N.; Taviano, M.F.; Filocamo, A.; Bisignano, G.; et al. Antimicrobial Activities, Toxicity and Phenolic Composition of Asphodeline anatolica E. Tuzlaci Leaf Extracts from Turkey. Nat. Prod. Res. 2016, 30, 2620–2623. [Google Scholar] [CrossRef]
- Cazzola, R.; Cestaro, B. Antioxidant Spices and Herbs Used in Diabetes. In Diabetes: Oxidative Stress and Dietary Antioxidants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 89–97. [Google Scholar]
- Shin, M.-S.; Park, J.Y.; Lee, J.; Yoo, H.H.; Hahm, D.-H.; Lee, S.C.; Lee, S.; Hwang, G.S.; Jung, K.; Kang, K.S. Anti-Inflammatory Effects and Corresponding Mechanisms of Cirsimaritin Extracted from Cirsium japonicum var. maackii Maxim. Bioorgan. Med. Chem. Lett. 2017, 27, 3076–3080. [Google Scholar] [CrossRef]
- Bower, A.M.; Real Hernandez, L.M.; Berhow, M.A.; De Mejia, E.G. Bioactive Compounds from Culinary Herbs Inhibit a Molecular Target for Type 2 Diabetes Management, Dipeptidyl Peptidase IV. J. Agric. Food Chem. 2014, 62, 6147–6158. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Wang, H.; Ma, L.; Ma, X.; Yin, J.; Wu, S.; Huang, H.; Li, Y. Cirsimaritin Inhibits Influenza A Virus Replication by Downregulating the NF-ΚB Signal Transduction Pathway. Virol. J. 2018, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Ibañez, E.; Kubátová, A.; Señoráns, F.J.; Cavero, S.; Reglero, U.; Hawthorne, S.B. Subcritical Water Extraction of Antioxidant Compounds from Rosemary Plants. J. Agric. Food Chem. 2003, 51, 375–382. [Google Scholar] [CrossRef]
- Jipa, S.; Zaharescu, T.; Kappel, W.; Dǎneţ, A.F.; Popa, C.V.; Bumbac, M.; Gorghiu, L.M.; Maris, A.M. The Effects of γ-Irradiation on the Antioxidant Activity of Rosemary Extract. Optoelectron. Adv. Mater. Rapid Commun. 2009, 3, 1315–1320. [Google Scholar]
- Tasdemir, D.; Kaiser, M.; Brun, R.; Yardley, V.; Schmidt, T.J.; Tosun, F.; Rüedi, P. Antitrypanosomal and Antileishmanial Activities of Flavonoids and Their Analogues: In Vitro, in Vivo, Structure-Activity Relationship, and Quantitative Structure-Activity Relationship Studies. Antimicrob. Agents Chemother. 2006, 50, 1352–1364. [Google Scholar] [CrossRef]
- Al Ati, H.Y.; Fawzy, G.A.; El Gamal, A.A.; Khalil, A.T.; El Din El Tahir, K.; Abdel-Kader, M.S.; Gilani, A.-H. Phytochemical and Biological Evaluation of Buddleja Polystachya Growing in Saudi Arabia. Pak. J. Pharm. Sci. 2015, 28, 1533–1540. [Google Scholar]
- Morita, N.; Shimizu, M. Naokata Orita and Ineo Shimizu: Of Irsium of the Leaves Iavonoids Components Resources. XXI Runts (Compositae) in Japan. ÝCirsium MAKINO 1963, 83, 615–618. [Google Scholar]
- Shilin, Y.; Roberts, M.F.; Phillipson, J.D. Methoxylated Flavones and Coumarins from Artemisia Annua. Phytochemistry 1989, 28, 1509–1511. [Google Scholar] [CrossRef]
- Namba, T.; Hattori, M.; Takehana, Y.; Tsunezuka, M.; Tomimori, T.; Kizu, H.; Miyaichi, Y.; Medical, T. A flavone Artemisia capillaris. Phytochemistry 1983, 22, 1057–1058. [Google Scholar] [CrossRef]
- Sanz, J.F.; Barbera, O.; Alberto, M.J. Sesquiterpene lactones from Artemisia hispanica. Phytochemistry 1989, 28, 2163–2167. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, D.B.; Li, M.J.; Liu, X.H.; Wang, H.Q. Studies on Flavonoid Constituents from Herbs of Artemisia Ordosica II. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J. Chin. Mater. Medica 2006, 31, 1959–1961. [Google Scholar]
- Chandrasekharan, I.; Khan, H.A.; Ghanim, A. Flavonoids from Artemisia scoparia. Planta Med. 1981, 43, 310–311. [Google Scholar] [CrossRef]
- Chemesova, I.I.; Belenovskaya, L.M.; Markova, L.P. Flavonoids of Artemisia xanthochroa. Chem. Nat. Compd. 1985, 20, 748. [Google Scholar] [CrossRef]
- de Sousa Andrade, L.M.; de Oliveira, A.B.M.; Leal, A.L.A.B.; de Alcântara Oliveira, F.A.; Portela, A.L.; de Sousa Lima Neto, J.; de Siqueira-Júnior, J.P.; Kaatz, G.W.; da Rocha, C.Q.; Barreto, H.M. Antimicrobial Activity and Inhibition of the NorA Efflux Pump of Staphylococcus Aureus by Extract and Isolated Compounds from Arrabidaea brachypoda. Microb. Pathog. 2020, 140, 103935. [Google Scholar] [CrossRef] [PubMed]
- Weimann, C.; Göransson, U.; Pongprayoon-Claeson, U.; Claeson, P.; Bohlin, L.; Rimpler, H.; Heinrich, M. Spasmolytic Effects of Baccharis Conferta and Some of Its Constituents. J. Pharm. Pharmacol. 2001, 54, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Graver, R.E.J.; Veitch, N.C. An 8-hydroxylated external flavone and its 8-O-glucoside from Becium grandiflorum. Phytochemistry 1998, 47, 7–10. [Google Scholar]
- Szoka, L.; Nazaruk, J.; Stocki, M.; Isidorov, V. Santin and Cirsimaritin from Betula Pubescens and Betula Pendula Buds Induce Apoptosis in Human Digestive System Cancer Cells. J. Cell. Mol. Med. 2021, 25, 11085–11096. [Google Scholar] [CrossRef]
- Sen, A.; Turan, S.O.; Bitis, L. Bioactivity-Guided Isolation of Anti-Proliferative Compounds from Endemic Centaurea kilaea. Pharm. Biol. 2016, 55, 541–546. [Google Scholar] [CrossRef]
- Al-Wahaibi, L.H.; Mahmood, A.; Khan, M.; Alkhathlan, H.Z. Phytochemical Analysis and Bioactivity Screening of Three Medicinal Plants of Saudi Arabia. Trop. J. Pharm. Res. 2020, 19, 371–376. [Google Scholar] [CrossRef]
- Diaa, Y.; August, W.F. Constituents of the Aerial Parts of Ruta Montana. Planta Med. 1995, 61, 279. [Google Scholar]
- Lee, D.; Kim, K.H.; Lee, J.; Hwang, G.S.; Lee, H.L.; Hahm, D.H.; Huh, C.K.; Lee, S.C.; Lee, S.; Kang, K.S. Protective Effect of Cirsimaritin against Streptozotocin-Induced Apoptosis in Pancreatic Beta Cells. J. Pharm. Pharmacol. 2017, 69, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Paje, L.A.; Rodriguez, J.P.; Kang, K.S.; Hahm, D.H.; Shim, J.S.; Choi, Y.J.; Lee, S. Validation of an HPLC/UV Analysis Method for Cirsimaritin in Cirsium japonicum var. Maackii. Korean J. Pharmacogn. 2020, 51, 217–221. [Google Scholar] [CrossRef]
- Zhu, M.; Phillipson, J.D.; Greengrass, P.M.; Bowery, N.G. Chemical and Biological Investigation of the Root Bark of Clerodendrum mandarinorum. Planta Med. 1996, 62, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Dawé, A.; Mbiantcha, M.; Yakai, F.; Jabeen, A.; Ali, M.S.; Lateef, M.; Ngadjui, B.T. Flavonoids and Triterpenes from Combretum Fragrans with Anti-Inflammatory, Antioxidant and Antidiabetic Potential. Z. Nat.-Sect. C J. Biosci. 2018, 73, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, M.; Nazeri, V.; Torras-Claveria, L.; Sefidkon, F.; Cusido, R.M.; Zamani, Z.; Palazon, J. Identification and Quantification of Leaf Surface Flavonoids in Wild-Growing Populations of Dracocephalum kotschyi by LC-DAD-ESI-MS. Food Chem. 2013, 141, 139–146. [Google Scholar] [CrossRef]
- Tahtah, Y.; Wubshet, S.G.; Kongstad, K.T.; Heskes, A.M.; Pateraki, I.; Møller, B.L.; Jäger, A.K.; Staerk, D. High-Resolution PTP1B Inhibition Profiling Combined with High-Performance Liquid Chromatography-High-Resolution Mass Spectrometry-Solid-Phase Extraction-Nuclear Magnetic Resonance Spectroscopy: Proof-of-Concept and Antidiabetic Constituents in Crude Extra. Fitoterapia 2016, 110, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ho, D.K.; Cassady, J.M. Isolation of Potential Cancer Chemopreventive Agents. J. Nat. Prod. 1992, 5, 7–13. [Google Scholar]
- Lin, S.; Zhang, Q.W.; Zhang, N.N.; Zhang, Y.X. Determination of Flavonoids in Buds of Herba Artemisiae Scopariae by HPLC. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J. Chin. Mater. Medica 2005, 30, 591–594. [Google Scholar]
- Isobe, T.; Doe, M.; Morimoto, Y.; Nagata, K.; Ohsaki, A. The Anti-Helicobacter Pylori Flavones in a Brazilian Plant, Hyptis fasciculata, and the Activity of Methoxyflavones. Biol. Pharm. Bull. 2006, 29, 1039–1041. [Google Scholar] [CrossRef]
- Yu, Z.; Zhu, H.; Yang, X.; Sun, Q.; Hao, X. Study on Chemical Constituents from Incarvillea Arguta and Their Accelerating PC-12 Cell Differentiation. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J. Chin. Mater. Medica 2005, 30, 1335–1338. [Google Scholar]
- Bai, N.; He, K.; Roller, M.; Lai, C.S.; Shao, X.; Pan, M.H.; Bily, A.; Ho, C.T. Flavonoid Glycosides from Microtea debilis and Their Cytotoxic and Anti-Inflammatory Effects. Fitoterapia 2011, 82, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Berim, A.; Park, J.J.; Gang, D.R. Unexpected Roles for Ancient Proteins: Flavone 8-Hydroxylase in Sweet Basil Trichomes Is a Rieske-Type, PAO-Family Oxygenase. Plant J. 2014, 80, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Vieira, R.F.; Grayer, R.J.; Paton, A.; Simon, J.E. Genetic Diversity of Ocimum gratissimum L. Based on Volatile Oil Constituents, Flavonoids and RAPD Markers. Biochem. Syst. Ecol. 2001, 29, 287–304. [Google Scholar] [CrossRef]
- Bosabalidis, A.; Gabrieli, C.; Niopas, I. Flavone Aglycones in Glandular Hairs of Origanum × intercedens. Phytochemistry 1998, 49, 1549–1553. [Google Scholar] [CrossRef]
- Khaliq, S.; Volk, F.J.; Frahm, A.W. Phytochemical Investigation of Perovskia abrotanoides. Planta Med. 2007, 73, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Huang, C.G.; Yu, Y.J.; Li, Z.Q.; Wang, W.; Huang, X.Z.; Liu, W.X.; Yuan, Y.; Jiang, Z.Y. Chemical Constituents from Perovskia Atriplicifolia. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J. Chin. Mater. Medica 2015, 40, 1108–1113. [Google Scholar] [CrossRef]
- De Azevedo Maia, G.L.; Dos Santos Falcão-Silva, V.; Aquino, P.G.V.; De Araújo-Júnior, J.X.; Tavares, J.F.; Da Silva, M.S.; Rodrigues, L.C.; De Siqueira-Júnior, J.P.; Barbosa-Filho, J.M. Flavonoids from Praxelis clematidea R.M. King and Robinson Modulate Bacterial Drug Resistance. Molecules 2011, 16, 4828–4835. [Google Scholar] [CrossRef] [PubMed]
- Abdelhalim, A.; Karim, N.; Chebib, M.; Aburjai, T.; Khan, I.; Johnston, G.A.R.; Hanrahan, J.R. Antidepressant, Anxiolytic and Antinociceptive Activities of Constituents from Rosmarinus Officinalis. J. Pharm. Pharm. Sci. 2015, 18, 448–459. [Google Scholar] [CrossRef]
- Cavero, S.; Jaime, L.; Martín-Álvarez, P.J.; Señoráns, F.J.; Reglero, G.; Ibañez, E. In Vitro Antioxidant Analysis of Supercritical Fluid Extracts from Rosemary (Rosmarinus officinalis L.). Eur. Food Res. Technol. 2005, 221, 478–486. [Google Scholar] [CrossRef]
- Pérez-Sánchez, A.; Borrás-Linares, I.; Barrajón-Catalán, E.; Arráez-Román, D.; González-Álvarez, I.; Ibáñez, E.; Segura-Carretero, A.; Bermejo, M.; Micol, V. Evaluation of the Intestinal Permeability of Rosemary (Rosmarinus officinalis L.) Extract Polyphenols and Terpenoids in Caco-2 Cell Monolayers. PLoS ONE 2017, 12, e0172063. [Google Scholar] [CrossRef]
- Xu, J.-H.; Lo, Y.M.; Chang, W.-C.; Huang, D.-W.; Wu, J.S.-B.; Jhang, Y.-Y.; Huang, W.-C.; Ko, C.-Y.; Shen, S.-C. Identification of Bioactive Components from Ruellia tuberosa L. on Improving Glucose Uptake in TNF-α-Induced Insulin-Resistant Mouse FL83B Hepatocytes. Evid. Based Complement. Alternat. Med. 2020, 2020, 6644253. [Google Scholar] [CrossRef] [PubMed]
- Srivedavyasasri, R.; Hayes, T.; Ross, S.A. Phytochemical and Biological Evaluation of Salvia apiana. Nat. Prod. Res. 2017, 31, 2058–2061. [Google Scholar] [CrossRef] [PubMed]
- Kanetis, L.; Exarchou, V.; Charalambous, Z.; Goulas, V. Edible Coating Composed of Chitosan and Salvia fruticosa Mill. Extract for the Control of Grey Mould of Table Grapes. J. Sci. Food Agric. 2017, 97, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Exarchou, V.; Kanetis, L.; Charalambous, Z.; Apers, S.; Pieters, L.; Gekas, V.; Goulas, V. HPLC-SPE-NMR Characterization of Major Metabolites in Salvia fruticosa Mill. Extract with Antifungal Potential: Relevance of Carnosic Acid, Carnosol, and Hispidulin. J. Agric. Food Chem. 2015, 63, 457–463. [Google Scholar] [CrossRef]
- Kavvadias, D.; Monschein, V.; Sand, P.; Riederer, P.; Schreier, P. Constituents of Sage (Salvia officinalis) with in Vitro Affinity to Human Brain Benzodiazepine Receptor. Planta Med. 2003, 69, 113–117. [Google Scholar] [CrossRef]
- Miski, M.; Ulubelen, A.; Johansson, C.; Mabry, T.J. Antibacterial Activity Studies of Flavonoids from Salvia palaestina. J. Nat. Prod. 1983, 46, 874–875. [Google Scholar] [CrossRef]
- Cottiglia, F.; Casu, L.; Bonsignore, L.; Casu, M.; Floris, C.; Sosa, S.; Altinier, G.; Della Loggia, R. Topical Anti-Inflammatory Activity of Flavonoids and a New Xanthone from Santolina insularis. Z. Nat.-Sect. C J. Biosci. 2005, 60, 63–66. [Google Scholar] [CrossRef]
- Malmir, M.; Gohari, A.R.; Saeidnia, S.; Silva, O. A New Bioactive Monoterpene-Flavonoid from Satureja khuzistanica. Fitoterapia 2015, 105, 107–112. [Google Scholar] [CrossRef]
- Shafiq, N.; Riaz, N.; Ahmed, S.; Ashraf, M.; Ejaz, S.A.; Ahmed, I.; Saleem, M.; Touseef, M.I.; Tareen, R.B.; Jabbar, A. Bioactive Phenolics from Seriphidium stenocephalum. J. Asian Nat. Prod. Res. 2013, 15, 286–293. [Google Scholar] [CrossRef]
- Beer, M.F.; Frank, F.M.; Germán Elso, O.; Ernesto Bivona, A.; Cerny, N.; Giberti, G.; Luis Malchiodi, E.; Susana Martino, V.; Alonso, M.R.; Patricia Sülsen, V.; et al. Trypanocidal and Leishmanicidal Activities of Flavonoids Isolated from Stevia satureiifolia var. Satureiifolia. Pharm. Biol. 2016, 54, 2188–2195. [Google Scholar] [CrossRef]
- Ren, X.; Bao, Y.; Zhu, Y.; Liu, S.; Peng, Z.; Zhang, Y.; Zhou, G. Isorhamnetin, Hispidulin, and Cirsimaritin Identified in Tamarix Ramosissima Barks from Southern Xinjiang and Their Antioxidant and Antimicrobial Activities. Molecules 2019, 24, 390. [Google Scholar] [CrossRef] [PubMed]
- Polatoğlu, K.; Karakoç, Ö.C.; Demirci, F.; Gökçe, A.; Gören, N. Chemistry and Biological Activities of Tanacetum chiliophyllum var. oligocephalum Extracts. J. AOAC Int. 2013, 96, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Stefkov, G.; Kulevanova, S.; Miova, B.; Dinevska-Kjovkarovska, S.; Mølgaard, P.; Jäger, A.K.; Josefsen, K. Effects of Teucrium polium spp. Capitatum Flavonoids on the Lipid and Carbohydrate Metabolism in Rats. Pharm. Biol. 2011, 49, 885–892. [Google Scholar] [CrossRef]
- Ben Sghaier, M.; Skandrani, I.; Nasr, N.; Franca, M.G.D.; Chekir-Ghedira, L.; Ghedira, K. Flavonoids and Sesquiterpenes from Tecurium ramosissimum Promote Antiproliferation of Human Cancer Cells and Enhance Antioxidant Activity: A Structure-Activity Relationship Study. Environ. Toxicol. Pharmacol. 2011, 32, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.F.; Yang, X.W.; Ma, C.M.; Liu, H.Y.; Shang, M.Y.; Zhang, Q.Y.; Cai, S.Q.; Park, J.H. Trollioside, a New Compound from the Flowers of Trollius chinensis. J. Asian Nat. Prod. Res. 2004, 6, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Nyiligira, E.; Viljoen, A.M.; Van Heerden, F.R.; Van Zyl, R.L.; Van Vuuren, S.F.; Steenkamp, P.A. Phytochemistry and in Vitro Pharmacological Activities of South African Vitex (Verbenaceae) Species. J. Ethnopharmacol. 2008, 119, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Hussain, W.; Amir, A.; Rasool, N. Computer-Aided Study of Selective Flavonoids against Chikungunya Virus Replication Using Molecular Docking and DFT-Based Approach. Struct. Chem. 2020, 31, 1363–1374. [Google Scholar] [CrossRef]
- Kiran, G.; Karthik, L.; Devi, M.S.; Sathiyarajeswaran, P.; Kanakavalli, K.; Kumar, K.M.; Kumar, D.R. In Silico Computational Screening of Kabasura Kudineer-Official Siddha Formulation and JACOM against SARS-CoV-2 Spike Protein. J. Ayurveda Integr. Med. 2020, 13, 100324. [Google Scholar] [CrossRef]
- Tasdemir, D.; Lack, G.; Brun, R.; Rüedi, P.; Scapozza, L.; Perozzo, R. Inhibition of Plasmodium f Alciparum Fatty Acid Biosynthesis: Evaluation of FabG, FabZ, and FabI as Drug Targets for Flavonoids. J. Med. Chem. 2006, 49, 3345–3353. [Google Scholar] [CrossRef]
- Quintanilla-Licea, R.; Vargas-Villarreal, J.; Verde-Star, M.J.; Rivas-Galindo, V.M.; Torres-Hernández, Á.D. Antiprotozoal Activity against Entamoeba Histolytica of Flavonoids Isolated from Lippia Graveolens Kunth. Molecules 2020, 25, 2464. [Google Scholar] [CrossRef]
- Kolak, U.; Hacibekiroǧlu, I.; Öztürk, M.; Özgökçe, F.; Topçu, G.; Ulubelen, A. Antioxidant and Anticholinesterase Constituents of Salvia Poculata. Turk. J. Chem. 2009, 33, 813–823. [Google Scholar] [CrossRef]
- Liu, C.Z.; Murch, S.J.; El-Demerdash, M.; Saxena, P.K. Artemisia Judaica L.: Micropropagation and Antioxidant Activity. J. Biotechnol. 2004, 110, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Burki, S.; Mehjabeen; Burki, Z.G.; Shah, Z.A.; Imran, M.; Khan, M. Phytochemical Screening, Antioxidant, and in Vivo Neuropharmacological Effect of Monotheca buxifolia (Falc.) Barks Extract. Pak. J. Pharm. Sci. 2018, 31, 1519–1528. [Google Scholar] [PubMed]
- Kuo, C.-F.; Su, J.-D.; Chiu, C.-H.; Peng, C.-C.; Chang, C.-H.; Sung, T.-Y.; Huang, S.-H.; Lee, W.-C.; Chyau, C.-C. Anti-Inflammatory Effects of Supercritical Carbon Dioxide Extract and Its Isolated Carnosic Acid from Rosmarinus officinalis Leaves. J. Agric. Food Chem. 2011, 59, 3674–3685. [Google Scholar] [CrossRef]
- Moghaddam, G.; Ebrahimi, S.A.; Rahbar-Roshandel, N.; Foroumadi, A. Antiproliferative Activity of Flavonoids: Influence of the Sequential Methoxylation State of the Flavonoid Structure: Antiproliferative flavonoids from dracocephalum kotschyi. Phytother. Res. 2012, 26, 1023–1028. [Google Scholar] [CrossRef]
- Pathak, G.; Singh, S.; Kumari, P.; Raza, W.; Hussain, Y.; Meena, A. Cirsimaritin, a Lung Squamous Carcinoma Cells (NCIH-520) Proliferation Inhibitor. J. Biomol. Struct. Dyn. 2020, 39, 3312–3323. [Google Scholar] [CrossRef]
- Quan, Z.; Gu, J.; Dong, P.; Lu, J.; Wu, X.; Wu, W.; Fei, X.; Li, S.; Wang, Y.; Wang, J.; et al. Reactive Oxygen Species-Mediated Endoplasmic Reticulum Stress and Mitochondrial Dysfunction Contribute to Cirsimaritin-Induced Apoptosis in Human Gallbladder Carcinoma GBC-SD Cells. Cancer Lett. 2010, 295, 252–259. [Google Scholar] [CrossRef]
- Patel, D.K. Health Beneficial Aspect and Therapeutic Potential of Cirsimaritin in the Medicine for the Treatment of Human Health Complications. Curr. Bioact. Compd. 2022, 18, 27–38. [Google Scholar] [CrossRef]
- Awad, B.M.; Abd-Alhaseeb, M.M.; Habib, E.S.; Ibrahim, A.K.; Ahmed, S.A. Antitumor Activity of Methoxylated Flavonoids Separated from Achillea fragrantissima Extract in Ehrlich’s Ascites Carcinoma Model in Mice. J. Herbmed Pharmacol. 2020, 9, 28–34. [Google Scholar] [CrossRef]
- Kim, H.; Kim, I.; Dong, Y.; Lee, I.-S.; Kim, J.; Kim, J.-S.; Woo, J.-T.; Cha, B.-Y. Melanogenesis-Inducing Effect of Cirsimaritin through Increases in Microphthalmia-Associated Transcription Factor and Tyrosinase Expression. Int. J. Mol. Sci. 2015, 16, 8772–8788. [Google Scholar] [CrossRef]
- Lee, D.; Jung, Y.; Baek, J.Y.; Shin, M.-S.; Lee, S.; Hahm, D.-H.; Lee, S.C.; Shim, J.S.; Kim, S.N.; Kang, K.S. Cirsimaritin Contributes to the Estrogenic Activity of Cirsium Japonicum var. Maackii through the Activation of Estrogen Receptor α: Estrogenic Compounds of Cirsium Japonicum var. Maackii. Bull. Korean Chem. Soc. 2017, 38, 1486–1490. [Google Scholar] [CrossRef]
- Manurung, K.; Sulastri, D.; Zubir, N.; Ilyas, S. In Silico Anticancer Activity and in Vitro Antioxidant of Flavonoids in Plectranthus amboinicus. Pharmacogn. J. 2020, 12, 1573–1577. [Google Scholar] [CrossRef]
- Plochmann, K.; Korte, G.; Koutsilieri, E.; Richling, E.; Riederer, P.; Rethwilm, A.; Schreier, P.; Scheller, C. Structure–Activity Relationships of Flavonoid-Induced Cytotoxicity on Human Leukemia Cells. Arch. Biochem. Biophys. 2007, 460, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yeon Park, J.; Young Kim, H.; Shibamoto, T.; Su Jang, T.; Cheon Lee, S.; Suk Shim, J.; Hahm, D.-H.; Lee, H.-J.; Lee, S.; Sung Kang, K. Beneficial Effects of a Medicinal Herb, Cirsium japonicum var. Maackii, Extract and Its Major Component, Cirsimaritin on Breast Cancer Metastasis in MDA-MB-231 Breast Cancer Cells. Bioorgan. Med. Chem. Lett. 2017, 27, 3968–3973. [Google Scholar] [CrossRef] [PubMed]
- Zehra, B.; Ahmed, A.; Sarwar, R.; Khan, A.; Farooq, U.; Abid Ali, S.; Al-Harrasi, A. Apoptotic and Antimetastatic Activities of Betulin Isolated from Quercus incana against Non-Small Cell Lung Cancer Cells. Cancer Manag. Res. 2019, 11, 1667–1683. [Google Scholar] [CrossRef]
- Abdalla, S.S.; Zarga, M.A. Effects of Cirsimaritin, a Flavone Isolated from Artemisia judaica, on Isolated Guinea-Pig Ileum. Planta Med. 1987, 53, 322–324. [Google Scholar] [CrossRef]
- González-Trujano, M.E.; Hernández-Sánchez, L.Y.; Muñoz Ocotero, V.; Dorazco-González, A.; Guevara Fefer, P.; Aguirre-Hernández, E. Pharmacological Evaluation of the Anxiolytic-like Effects of Lippia graveolens and Bioactive Compounds. Pharm. Biol. 2017, 55, 1569–1576. [Google Scholar] [CrossRef]
- Wang, J.-P.; Chang, L.-C.; Hsu, M.-F.; Chen, S.-C.; Kuo, S.-C. Inhibition of Formyl-Methionyl-Leucyl-Phenylalanine-Stimulated Respiratory Burst by Cirsimaritin Involves Inhibition of Phospholipase D Signaling in Rat Neutrophils. Naunyn. Schmiedebergs Arch. Pharmacol. 2002, 366, 307–314. [Google Scholar] [CrossRef]



| Plants | Part Used | Botanical Families | Type of Extract | Concentration/Fraction | References |
|---|---|---|---|---|---|
| Aeollanthus rydingianus | Aerial parts | Lamiaceae | Me2CO Extract | 58 mg | [29] |
| Artemisia annua | Leaves Stems | Asteraceae | Methanolic extract | 1.45 mg | [40] |
| Artemisia capillaris | Spikes | Asteraceae | Methanolic extract | not specified | [41] |
| Artemisia hispanica | Aerial parts | Asteraceae | Methanolic extract | not specified | [42] |
| Artemisia judaica | Leaves Stems | Asteraceae | Ethanolic extract | not specified | [25] |
| Artemisia meatlanticae | Aerial parts | Asteraceae | Ether extract | not specified | [24] |
| Artemisia monosperma | Leaves Stems | Asteraceae | Ethanolic extract | not specified | [25] |
| Artemisia ordosica II | not specified | Asteraceae | not specified | not specified | [43] |
| Artemisia scoparia | Dried inflorescence | Asteraceae | Chloroform extract | not specified | [44] |
| Artemisia xanthochroa | Epigeal parts | Asteraceae | Ethanolic extract | not specified | [45] |
| Arrabidaea brachypoda | Flowers | Bignoniaceae | Ethanolic extract | not specified | [46] |
| Asphodeline anatolica | Leaves | Liliaceae | Acetone and methanol extract | not specified | [30] |
| Baccharis conferta | Aerial parts | Asteraceae | Ethanolic extract | not specified | [47] |
| Becium grandiflorum | Leaves | Lamiaceae | Methanolic extract | not specified | [48] |
| Betula pendula | Buds | Betulaceae | Carbon dioxide supercritical extraction | 3.79 mg/g | [49] |
| Betula pubescens | Buds | Betulaceae | Carbon dioxide supercritical extraction | 4.21 mg/g | [49] |
| Buddleja polystachya | Aerial parts | Buddlejaceae | Ethanolic extract (cold maceration) | not specified | [38] |
| Centuarea kilaea | Aerial parts | Asteraceae | Chloroform extract | 10.2 mg | [50] |
| Centaurea pseudosinaica | Entire plant (leaves, flowers, stems) | Asteraceae | Ethanolic extract | 0.52 g | [51] |
| Centaurea scoparia | Aerial parts | Asteraceae | Ethanolic extract | 10 mg | [52] |
| Cirsium martimum | Leaves | Asteraceae | Methanolic extract | not specified | [39] |
| Cirsium japonicum | Aerial parts Leaves | Asteraceae | Ethanolic Extract | 6.24 mg/g | [32] |
| 6.24 mg/g | [53] | ||||
| 37.13 mg/g | [54] | ||||
| Clerodendrum mandarinorum | Root bark | Lamiaceae | Ethanolic Extract | 50 mg | [55] |
| Combretum fragrans | Leaves | Combretaceae | Methanolic extract | not specified | [56] |
| Dracocephalum kotschyi | Leaves | Lamiaceae | Diethyl Ether extract | 97.3–637.6 µg/g | [57] |
| Eremophila lucida | Leaves | Myoporaceae | Ethyl acetate extract | not specified | [58] |
| Eriodictyon californicum | Leaves | Hydrophyllaceae | Ethanol extract | 3.85 mg | [59] |
| Herba artemisiae Scopariae | Buds | Compositae | Ethyl acetate extract (ultrasonic) | not specified | [60] |
| Hyptis fasciculata | Aerial parts | Labiatae | Chloroform and methanol extract | 19 mg | [61] |
| Incarvillea arguta | not specified | Bignoniaceae | not specified | not specified | [62] |
| Microtea debilis | Whole plant Aerial parts | Microteaceae | Aqueous & ethanol extractEthanol extract | 0.7 mg/mL 65 mg | [26] [63] |
| Ocimum basilicum | Tricoms | Lamiaceae | Crude protein extract with HCl | not specified | [64] |
| Ocimum gratissimum | Above-ground biomass | Lamiaceae | Clevenger apparatus | 10% of the total flavonoids | [65] |
| Origanum intercedens | Leaves | Lamiaceae | Chloroform extract | not specified | [66] |
| Osimum sanctum | Leaves Stems | Labiatae | Chloroform extract | 1 mg | [27] |
| Perovskia abrotanoies | Aerial parts | Lamiaceae | Methanolic extract | 10 mg | [67] |
| Perovskia atriplicifolia | Leaves | Lamiaceae | Ethanolic Extract | not specified | [68] |
| Praxelis clematidea | Aerial parts | Asteraceae | Ethanolic Extract (exhaustive maceration) | not specified | [69] |
| Rosmarinus officinalis | Leaves | Lamiaceae | Ethyl acetate extract | not specified | [70] |
| Subcritical extraction with water | 1.72% | [35] | |||
| Supercritical fluid extraction | 0.54–17.59% | [71] | |||
| Ethanolic extract | not specified | [36] | |||
| Super critical fluid extraction | not specified | [72] | |||
| Ruellia tuberosa | Leaves & stems | Acanthaceae | Methanolic extract | 805 µg/g | [73] |
| Salvia apiana | Aerial parts | Lamiaceae | Aqueous ethanolic extract | not specified | [74] |
| Salvia fruticosa | Aerial parts | Lamiaceae | Acetonic extract (Soxtec system) | not specified | [75] |
| Ethyl acetate extract (Soxtec extraction) | 10.4 mg/g | [76] | |||
| Salvia officinalis | Leaves | Lamiaceae | Methanolic extraction (ultrasonic bath) | 194 mg | [77] [31] |
| Salvia palaestina | Leaves | Lamiaceae | Benzene extract (Soxhlet) | 30 mg | [78] |
| Santolina insularis | Leaves | Asteraceae | Methanolic extract | 6.9 mg | [79] |
| Satureja khuzistanica | Aerial parts | Lamiaceae | Ethyl acetate extraction | 5 mg | [80] |
| Seriphidium stenocephalum | not specified | Asteraceae | Methanolic extract | 15 mg | [81] |
| Stevia satureiifolia | Aerial parts | Asteraceae | Dichloromethane extract | 1.9% | [82] |
| Tamarix ramosissima | Bark | Tamaricaceae | Ethanolic extract | 13.35 µg/mg | [83] |
| Tanacetum chiliophyllum | Stems | Compositae | Ethyl acetate extract | 36 mg | [84] |
| Teucrium polium | Aerial parts | Lamiaceae | Alcohol extraction | not specified | [85] |
| Teucrium ramosissimum | Leaves | Lamiaceae | Chloroformic extract | not specified | [86] |
| Trollius chinensis | Flowers | Ranunculaceae | Ethanolic extract | 14 mg | [87] |
| Vitex rehmannii | Aerial parts | Verbenaceae | Acetone extract | 5 mg | [88] |
| Methods Used | Strains Tested | Key Results | References |
|---|---|---|---|
| Disk diffusion assay | Escherichia coli | MIC = 31.25 μg/mL, MBC = 125 μg/mL | [78] |
| Klebsiella pneumonia | MIC = 31.25 μg/mL, MBC = 125 μg/mL | ||
| Pseudomonas aeruginosa | MIC = 45 μg/mL, MBC = 90 μg/mL | ||
| Proteus vulgaris | MIC = 31.25 μg/mL, MBC = 125 μg/mL | ||
| Staphylococcus aureus | MIC = 31.25 μg/mL, MBC = 125 μg/mL | ||
| Staphylococcus epidermis | MIC = 62.5 μg/mL, MBC = 125 μg/mL | ||
| Disk diffusion method | Aspergillus niger | Φ = 13 mm at 40 µg | [28] |
| Basilus subtilis | Φ = 0 mm at 40 µg | ||
| Candida albicans | Φ = 12 mm at 40 µg | ||
| Escherichia coli | Φ = 0 mm at 40 µg | ||
| Pseudomonas aeruginosa | Φ = 13 mm at 40 µg | ||
| Staphylococcus aureus | Φ = 11 mm at 40 µg | ||
| Trichophyton mentagrophytes | Φ = 14 mm at 40 µg | ||
| Agar diffusion method | Candida albicans | No activity | [29] |
| Escherichia coli | No activity | ||
| Enterococcus hirae | Growth zone inhibition | ||
| Mycobacterium smegmatis | No activity | ||
| Pseudomonas aeruginosa | No activity | ||
| Staphylococcus aureus | Growth zone inhibition | ||
| Micro-dilution technique | Aspergilus fumigatus | MIC = 1.95 μg/mL | [51] |
| Bacillus subtilis | MIC = 0.03 μg/mL | ||
| Candida albicans | MIC = 1.95 μg/mL | ||
| Escherichia coli | MIC = 11.25 μg/mL | ||
| Geotrichum candidum | MIC = 0.48 μg/mL | ||
| Pseudomonas aeruginosa | MIC = 50.0 μg/mL | ||
| Streptococcus pneumoniae | MIC = 7.81 μg/mL | ||
| Syncephalastrum racemosum | MIC = 12.5 μg/mL | ||
| Micro-dilution method | Bacillus cereus | MIC = 5 mg/mL, MBC = 20 mg/mL | [83] |
| Escherichia coli | MIC = 10 mg/mL, MBC = 25 mg/mL | ||
| Listeria monocytogenes | MIC = 5 mg/mL, MBC = 10 mg/mL | ||
| Pseudomonas aeruginosa | MIC > 10 mg/mL, MBC = NA | ||
| Salmonella typhimurium | MIC > 10 mg/mL, MBC = NA | ||
| Shigella castellani | MIC = 5 mg/mL, MBC = 15 mg/mL | ||
| Staphylococcus aureus | MIC = 5 mg/mL, MBC = 15 mg/mL |
| Used Method | Key Results | References |
|---|---|---|
| DPPH radical scavenging activity | EC50 = 11.3 µg/mL | [35] |
| β-carotene bleaching, superoxide anion radical, and ABTS cation radical scavenging activity assays | No antioxidant activity | [93] |
| ABTS assay | TEAC (µM) = 2.04 | [86] |
| CUPRAC assay | TEAC (µM) = 4.7 | |
| RP (Reducing power) assay | TEAC (µM) = 0.95 | |
| FRAP assay | TEAC (µM) = 0.625 | |
| DPPH Scavenging activity | Inhibition efficiency (%) = 80–100 at a concentration of 100 µg/mL | [53] |
| FRAP assay | AC = 203.39 to 681.27 µmol Fe2+/100 g DW at a concentration of 97.38–637.66 µg/g DW | [57] |
| DPPH assay | Significantly higher capacity to detoxify oxygen radicals | [94] |
| DPPH scavenging | % inhibition (at 500 µg/mL) = 89.55 | [95] |
| Superoxide scavenging | % inhibition (at 500 µg/mL) = 82.10 | |
| Hydrogen peroxide scavenging | % inhibition (at 500 µg/mL) = 80.55 | |
| DPPH assay | IC50 = 55.9 µM | [56] |
| Origin | Biological Model (In Vitro or In Vivo) | Experimental Approach | Results and Mechanism of Action | References |
|---|---|---|---|---|
| Synthetic compound | human cancer cell lines namely COLO-205, MDA-MB-231, HaCaT, K562, A431, A549, MCF-7, PC-3, NCIH- 520, normal cell lines WRL-68, HEK 293 and L132 and in primary macrophages | MTT assay Inhibitory potential and binding interaction with the selected targets were analyzed through in vitro and in silico analysis | Inhibited the growth of NCIH-520 cell-line (IC50 23.29 μM) Induced apoptosis Inhibited the activity of ODC and CATD Exhibited a good binding in silico score with the selected targets and it non-mutagenic | [98] |
| Synthetic compound | gallbladder carcinoma cell lines GBC-SD and GBCSD18H cells, gastric carcinoma cell line BGC-823 cells, and hepatoma cell line SMMC-7721 cells | Cytotoxicity assay Cell apoptosis assay Cell mitochondrial membrane potential assay Subcellular fractionation Western blot Small interference RNA RT)-PCR Detection of intracellular ROS | Inhibited the growth of tumor cells Induced mitochondrial apoptosis in GBC-SD cells Triggered endoplasmic reticulum (ER) stress Down-regulated the phosphorylation of Akt | [99] |
| Centaurea kilaea | one normal cell line (L-929, mouse fibroblast) three human cancer cell lines (Hela, cervix carcinoma; MCF-7, breast carcinoma; PC-3, prostate carcinoma | MTT assay | Inhibited the growth of MCF-7 and PC-3 | [50] |
| Teucrium ramosissimum | Ehrlich’s ascites carcinoma model in mice | (5, 10, 20 mg/kg/d, orally) | Reduced tumor weight compared to EAC-control and cisplatin groups Induced tumor cell necrosis Reduced significantly the level of TNF-α in serum | [101] |
| Teucrium ramosissimum | human chronic myelogenous K562 cells | MTT assay | Exhibited an antiproliferative effect of human cancer cells IC50 = 1.015 × 10−7 mol/mL | [86] |
| Lithocarpus dealbatus | Murine melanoma B16F10 cells (CRL-6415) | Cell Morphology and Cell Viability Measurement Measurement of Cellular Tyrosinase Activity Melanin Content Measurement Western Blotting | Stimulated melanogenesis in B16F10 cells Activated of CREB as well as upregulation of MITF and tyrosinase expression activated by cAMP signaling | [102] |
| Cirsium japonicum var. maackii | human breast cancer (MCF-7) cell-based | Transactivation assay Proliferative activity | Exerted beneficial effects on MCF-7 cells Increased estrogenic activity | [103] |
| Plectranthus amboinicus | Cancer P-Glycoprotein-1, Cyclin Dependent Kinase-2, and Phosphoinositide-3-Kinase receptors | In silico anticancer Test | Exhibited an important strong anti-cancer effect | [104] |
| Dracocephalum kotschyi Boiss. | AGS, HT-29, HL60, SaOs-2, WEHI-164 and HFFF-P16 cells | MTT assay | Exhibited and antiproliferative activity of malignant cells | [97] |
| Isolated | Human T lymphoblasts (Jurkat Clone E6-1) | Cytotoxicity experiments Flow cytometry | Induced cytotoxicity EC50 = 66.8 µM (24 h) EC50 = 44.4 µM (48 h) | [105] |
| Cirsium japonicum | Breast cancer | Cell proliferation assay Tube-formation assay Western blot analysis | Inhibited the viability of HUVECs in a dose-dependent manner Inhibited angiogenesis by downregulation of VEGF, p-Akt and p-ERK in MDA-MB-231 cells | [106] |
| Betula pubescens and Betula pendula | gastric (AGS), colon (DLD-1) and liver (HepG2) cancer cells | Cell viability assay DNA biosynthesis Colony formation assay Apoptosis assay Western immunoblot Immunofluorescence microscopy | Induced apoptosis Activated caspase-3, caspase-7, caspase-8 and caspase-9 expression Upregulated p53 expression | [49] |
| Quercus incana | non-small cell lung carcinoma (NCI-H460) and normal mouse fibroblast (NIH-3T3) cell lines. | mRNA extraction and qRT-PCR Colony formation assay Flow cytometry analysis Cell cycle analysis Western blot analysis | Induced antiproliferative against NIH 3T3 (IC50 = 26.23 ± 0.053 μM) and in NCI-H460 (IC50 = 38.84 ± 0.037 μM) | [107] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benali, T.; Jaouadi, I.; Ghchime, R.; El Omari, N.; Harboul, K.; Hammani, K.; Rebezov, M.; Shariati, M.A.; Mubarak, M.S.; Simal-Gandara, J.; et al. The Current State of Knowledge in Biological Properties of Cirsimaritin. Antioxidants 2022, 11, 1842. https://doi.org/10.3390/antiox11091842
Benali T, Jaouadi I, Ghchime R, El Omari N, Harboul K, Hammani K, Rebezov M, Shariati MA, Mubarak MS, Simal-Gandara J, et al. The Current State of Knowledge in Biological Properties of Cirsimaritin. Antioxidants. 2022; 11(9):1842. https://doi.org/10.3390/antiox11091842
Chicago/Turabian StyleBenali, Taoufiq, Imane Jaouadi, Rokia Ghchime, Nasreddine El Omari, Kaoutar Harboul, Khalil Hammani, Maksim Rebezov, Mohammad Ali Shariati, Mohammad S. Mubarak, Jesus Simal-Gandara, and et al. 2022. "The Current State of Knowledge in Biological Properties of Cirsimaritin" Antioxidants 11, no. 9: 1842. https://doi.org/10.3390/antiox11091842
APA StyleBenali, T., Jaouadi, I., Ghchime, R., El Omari, N., Harboul, K., Hammani, K., Rebezov, M., Shariati, M. A., Mubarak, M. S., Simal-Gandara, J., Zengin, G., Park, M.-N., Kim, B., Mahmud, S., Lee, L.-H., & Bouyahya, A. (2022). The Current State of Knowledge in Biological Properties of Cirsimaritin. Antioxidants, 11(9), 1842. https://doi.org/10.3390/antiox11091842














