NADPH Oxidases in Aortic Aneurysms
Abstract
1. Introduction
2. Nicotinamide Adenine Dinucleotide Phosphate Oxidases (NADPH Oxidases/NOX)
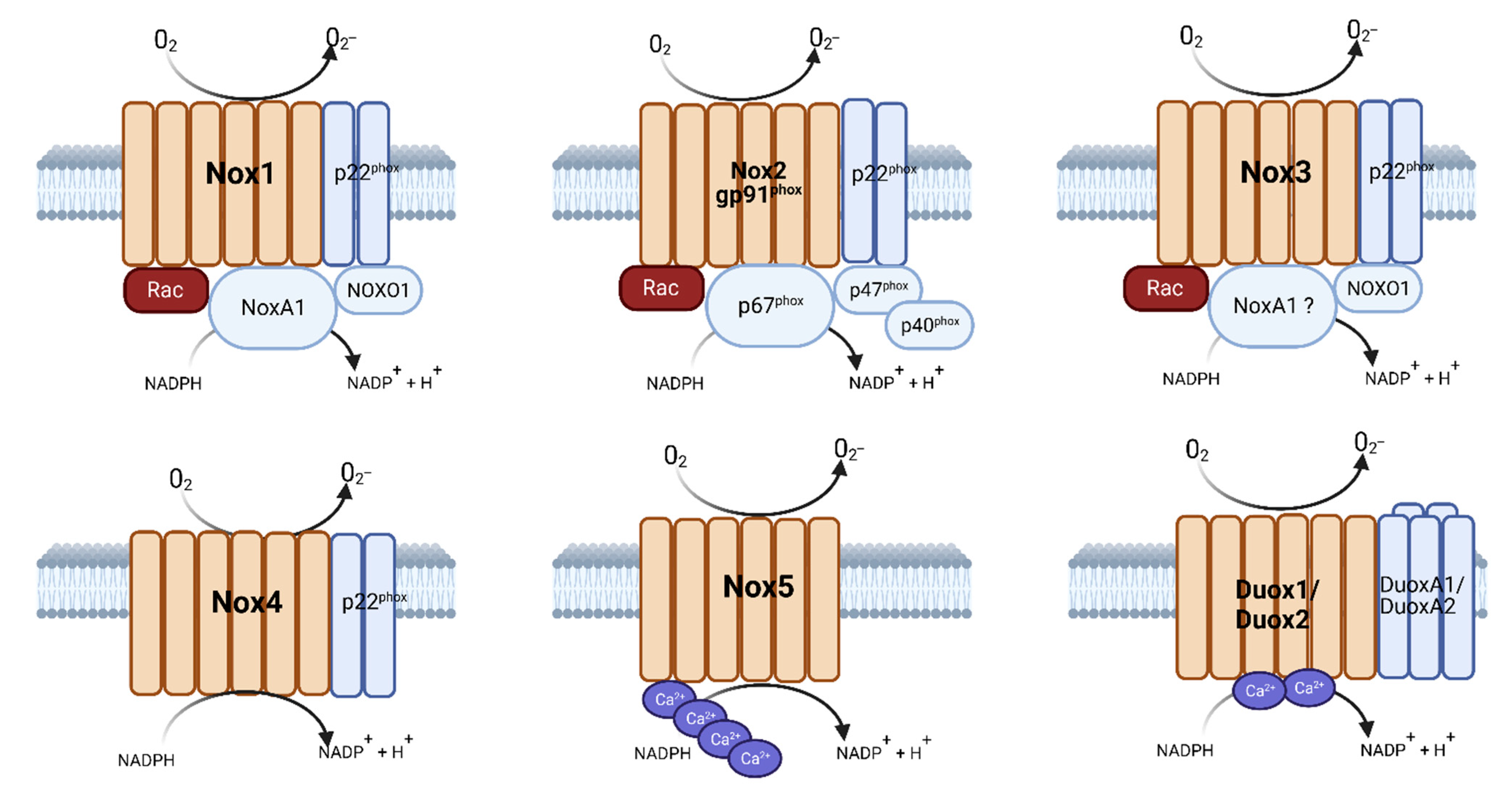
3. Mediators of NADPH Oxidases/NOX in Experimental AAAs
3.1. Reactive Oxygen Species (ROS)
3.2. eNOS
| Mediator | Influence on Experimental AAAs | References |
|---|---|---|
| O2•− | Increased inflammation, iNOS, Nox | [79,80] |
| Increased free radical production | [81] | |
| H2O2 | Decreased catalase production | [84,85] |
| eNOS | Uncoupling increases AAAs | [86,87] |
| Levels decline in aged AAAs | [88] | |
| HMGB1 | Increased and correlated with MMP2 and MMP9 | [93] |
| Decreased aneurysm, decreased elastin breakage | [94] | |
| HIF-1α | Elevated in aneurysms | [95] |
| Elevated at the site of AAA rupture | [96] | |
| Stabilized by DFO, increases MMP2 | [97] | |
| NF-Κβ | Elevated and pharmacologic inhibition decreased AAAs | [98] |
| Endothelial inhibition attenuated AAA formation | [99] |
3.3. HMGB1
3.4. HIF-1α
3.5. NF-Κβ
4. Influence of Genetic NADPH Oxidases/NOX Deficiency Components on Experimental AAAs
5. Influence of Pharmacologic NADPH Oxidases in Experimental AAAs
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAA | Abdominal aortic aneurysm |
| Ang II | Angiotensin II |
| ApoE | Apolipoprotein E |
| ASDR | Age-standardized death rates |
| eNOS | Endothelial nitric oxide synthase |
| HMGB1 | High-mobility group box 1 protein |
| HIF-1α | Hypoxia inducible factor alpha |
| LDLR | Low-density lipoprotein receptor |
| NAPDH Oxidases | Nicotinamide adenine dinucleotide phosphate oxidases |
| PPARα | Peroxisome proliferator-activated receptor alpha |
| ROS | Reactive oxygen species |
| SMCs | Smooth muscle cells |
References
- Eagle, K.A. Rationale and design of the National Registry of Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions (GenTAC). Am. Heart J. 2009, 157, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Lareyre, F.; Raffort, J. Metformin to Limit Abdominal Aortic Aneurysm Expansion: Time for Clinical Trials. Eur. J. Vasc. Endovasc. Surg. 2021, 161, 1030. [Google Scholar] [CrossRef]
- Dalman, R.L.; Wanhainen, A.; Mani, K.; Modarai, B. Top 10 candidate aortic disease trials. J. Intern. Med. 2020, 288, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Baxter, B.T.; Matsumura, J.; Curci, J.A.; McBride, R.; Larson, L.; Blackwelder, W.; Lam, D.; Wijesinha, M.; Terrin, M.; N-TA3CT Investigators. Effect of Doxycycline on Aneurysm Growth Among Patients With Small Infrarenal Abdominal Aortic Aneurysms: A Randomized Clinical Trial. JAMA 2020, 323, 2029–2038. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Kim, K.; Lindsay, M.E.; MacGillivray, T.; Isselbacher, E.M.; Cambria, R.P.; Sundt, T.M. Risk of Rupture or Dissection in Descending Thoracic Aortic Aneurysm. Circulation 2015, 132, 1620–1629. [Google Scholar] [CrossRef]
- Patterson, B.O.; Stenson, K.; Grima, M.; Bruin, J.d.; Al-Subaie, N.; Loftus, I.M.; Thompson, M.M.; Holt, P.J. Postoperative Complications Decrease Long-Term Survival After Thoracic Aneurysm Repair Despite Apparently Successful “Rescue” From Early Mortality. Circulation 2018, 137, 636–638. [Google Scholar] [CrossRef]
- Kent, K.C. Clinical practice. Abdominal aortic aneurysms. N. Engl. J. Med. 2014, 371, 2101–2108. [Google Scholar] [CrossRef]
- Upchurch, G.R., Jr.; Schaub, T.A. Abdominal aortic aneurysm. Am. Fam. Physician 2006, 73, 1198–1204. [Google Scholar]
- Kontopodis, N.; Pantidis, D.; Dedes, A.; Daskalakis, N.; Ioannou, C.V. The-Not So-Solid 5.5 cm Threshold for Abdominal Aortic Aneurysm Repair: Facts, Misinterpretations, and Future Directions. Front. Surg. 2016, 3, 1. [Google Scholar] [CrossRef]
- Brodersen, J.; Hansson, A.; Johansson, M.; Siersma, V.; Langenskiold, M.; Pettersson, M. Consequences of screening in abdominal aortic aneurysm: Development and dimensionality of a questionnaire. J. Patient Rep. Outcomes 2017, 2, 37. [Google Scholar] [CrossRef]
- Absi, T.S.; Sundt, T.M.; Tung, W.S.; Moon, M.; Lee, J.K.; Damiano Jr, R.R.; Thomopson, R.W. Altered patterns of gene expression distinguishing ascending aortic aneurysms from abdominal aortic aneurysms: Complementary DNA expression profiling in the molecular characterization of aortic disease. J. Thorac. Cardiovasc. Surg. 2003, 126, 344–357. [Google Scholar] [CrossRef]
- Castellano, J.M.; Kovacic, J.C.; Sanz, J.; Fuster, V. Are we ignoring the dilated thoracic aorta? Ann. N. Y. Acad. Sci. 2012, 1254, 164–174. [Google Scholar] [CrossRef]
- Chau, K.H.; Elefteriades, J.A. Natural history of thoracic aortic aneurysms: Size matters, plus moving beyond size. Prog. Cardiovasc. Dis. 2013, 56, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Cury, M.; Zeidan, F.; Lobato, A.C. Aortic disease in the young: Genetic aneurysm syndromes, connective tissue disorders, and familial aortic aneurysms and dissections. Int. J. Vasc. Med. 2013, 2013, 267215. [Google Scholar] [CrossRef]
- Elefteriades, J.A.; Farkas, E.A. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J. Am. Coll. Cardiol. 2010, 55, 841–857. [Google Scholar] [CrossRef] [PubMed]
- Gillis, E.; Van Laer, L.; Loeys, B.L. Genetics of thoracic aortic aneurysm: At the crossroad of transforming growth factor-beta signaling and vascular smooth muscle cell contractility. Circ. Res. 2013, 113, 327–340. [Google Scholar] [CrossRef]
- Johansson, G.; Markstrom, U.; Swedenborg, J. Ruptured thoracic aortic aneurysms: A study of incidence and mortality rates. J. Vasc. Surg. 1995, 21, 985–988. [Google Scholar] [CrossRef]
- Milewicz, D.M.; Prakash, S.K.; Ramirez, F. Therapeutics Targeting Drivers of Thoracic Aortic Aneurysms and Acute Aortic Dissections: Insights from Predisposing Genes and Mouse Models. Annu. Rev. Med. 2017, 68, 51–67. [Google Scholar] [CrossRef]
- Olsson, C.; Thelin, S.; Stahle, E.; Ekbom, A.; Granath, F. Thoracic aortic aneurysm and dissection: Increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation 2006, 114, 2611–2618. [Google Scholar] [CrossRef]
- Knops, A.M.; Goossens, A.; Ubbink, D.T.; Balm, R.; Koelemay, M.J.; Vahl, A.C.; de Nie, A.J.; van den Akker, P.J.; Willems, M.C.; Koedam, N.A.; et al. A decision aid regarding treatment options for patients with an asymptomatic abdominal aortic aneurysm: A randomised clinical trial. Eur. J. Vasc. Endovasc. Surg. 2014, 48, 276–283. [Google Scholar] [CrossRef]
- Ashton, H.A.; Buxton, M.J.; Day, N.E.; Kim, L.G.; Marteau, T.M.; Scott, R.A.; Thompson, S.G.; Walker, N.M. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: A randomised controlled trial. Lancet 2002, 360, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, H.; Sonesson, B.J.; Berqvist, D. Incidence and Prevalence of Abdominal Aortic Aneurysms, Estimated by Necropsy Studies and Population Screening by Ultrasounda. Ann. N. Y. Acad. Sci. 1996, 800, 1–24. [Google Scholar] [CrossRef]
- Kent, K.C.; Zwolak, R.M.; Egorova, N.N.; Riles, T.S.; Manganaro, A.; Moskowitz, A.J.; Gelijns, A.C.; Greco, G. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J. Vasc. Surg. 2010, 52, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Lederle, F.A.; Johnson, G.R.; Wilson, S.E.; Chute, E.P.; Hye, R.J.; Makaroun, M.S.; Makhoul, R.G. The aneurysm detection and management study screening program: Validation cohort and final results. Arch. Intern. Med. 2000, 160, 1425–1430. [Google Scholar] [CrossRef] [PubMed]
- Lederle, F.A.; Johnson, G.R.; Wilson, S.E.; Littooy, F.N.; Krupski, W.C.; Bandyk, D.; Makaroun, M.S. YIeld of repeated screening for abdominal aortic aneurysm after a 4-year interval. Arch. Intern. Med. 2000, 160, 1117–1121. [Google Scholar] [CrossRef]
- Lindholt, J.S.; Vammen, S.; Juul, S.; Henneberg, E.W.; Fasting, H. The validity of ultrasonographic scanning as screening method for abdominal aortic aneurysm. Eur. J. Vasc. Endovasc. Surg. 1999, 17, 472–475. [Google Scholar] [CrossRef]
- Scott, R.A.; Wilson, N.M.; Ashton, H.A.; Kay, D.N. Influence of screening on the incidence of ruptured abdominal aortic aneurysm: 5-year results of a randomized controlled study. Br. J. Surg. 1995, 82, 1066–1070. [Google Scholar] [CrossRef]
- Scott, R.A.P.; Bridgewater, S.G.; Ashton, H.A. Randomized clinical trial of screening for abdominal aortic aneurysm in women. Br. J. Surg. 2002, 89, 283–285. [Google Scholar] [CrossRef]
- Thompson, S.G.; Ashton, H.A.; Gao, L.; Buxton, M.J.; Scott, R.A.P.; Multicentre Aneurysm Screening Study (MASS) Group. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br. J. Surg. 2012, 99, 1649–1656. [Google Scholar] [CrossRef]
- Al-Balah, A.; Goodall, R.; Salciccioli, J.D.; Marshall, D.C.; Shalhoub, J. Mortality from abdominal aortic aneurysm: Trends in European Union 15+ countries from 1990 to 2017. Br. J. Surg. 2020, 107, 1459–1467. [Google Scholar] [CrossRef]
- Miller, F.J., Jr.; Sharp, W.J.; Fang, X.; Oberley, L.W.; Oberley, T.D.; Weintraub, N.L. Oxidative stress in human abdominal aortic aneurysms: A potential mediator of aneurysmal remodeling. Arter. Thromb Vasc Biol 2002, 22, 560–565. [Google Scholar] [CrossRef] [PubMed]
- McCormick, M.L.; Gavrila, D.; Weintraub, N.L. Role of Oxidative Stress in the Pathogenesis of Abdominal Aortic Aneurysms. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Selemidis, S.; Sobey, C.G.; Wingler, K.; Schmidt, H.H.; Drummond, G.R. NADPH oxidases in the vasculature: Molecular features, roles in disease and pharmacological inhibition. Pharmacol. Ther. 2008, 120, 254–291. [Google Scholar] [CrossRef] [PubMed]
- Vignais, P.V. The superoxide-generating NADPH oxidase: Structural aspects and activation mechanism. Cell Mol. Life Sci. 2002, 59, 1428–1459. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Yu, L.; Quinn, M.T.; Cross, A.R.; Dinauer, M.C. Gp91(phox) is the heme binding subunit of the superoxide-generating NADPH oxidase. Proc. Natl. Acad. Sci. USA 1998, 95, 7993–7998. [Google Scholar] [CrossRef]
- Sumimoto, H.; Miyano, K.; Takeya, R. Molecular composition and regulation of the Nox family NAD(P)H oxidases. Biochem. Biophys. Res. Commun. 2005, 338, 677–686. [Google Scholar] [CrossRef]
- Carbone, F.; Teixeira, P.C.; Braunersreuther, V.; Mach, F.; Vuilleumier, N.; Montecucco, F. Pathophysiology and Treatments of Oxidative Injury in Ischemic Stroke: Focus on the Phagocytic NADPH Oxidase 2. Antioxid. Redox Signal. 2015, 23, 460–489. [Google Scholar] [CrossRef]
- Lapouge, K.; Smith, S.J.; Groemping, Y.; Rittinger, K. Architecture of the p40-p47-p67phox complex in the resting state of the NADPH oxidase. A central role for p67phox. J. Biol. Chem. 2002, 277, 10121–10128. [Google Scholar] [CrossRef]
- Lambeth, J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004, 4, 181–189. [Google Scholar] [CrossRef]
- Tsunawaki, S.; Yoshikawa, K. Relationships of p40(phox) with p67(phox) in the activation and expression of the human respiratory burst NADPH oxidase. J. Biochem. 2000, 128, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Kleinberg, M.E. Activation of the phagocyte NADPH oxidase protein p47(phox). Phosphorylation controls SH3 domain-dependent binding to p22(phox). J. Biol. Chem. 1999, 274, 19731–19737. [Google Scholar] [CrossRef] [PubMed]
- Leto, T.L.; Adams, A.G.; de Mendez, I. Assembly of the phagocyte NADPH oxidase: Binding of Src homology 3 domains to proline-rich targets. Proc. Natl. Acad. Sci. USA 1994, 91, 10650–10654. [Google Scholar] [CrossRef] [PubMed]
- DeLeo, F.R.; Nauseef, W.M.; Jesaitis, A.J.; Burritt, J.B.; Clark, R.A.; Quinn, M.T. A domain of p47phox that interacts with human neutrophil flavocytochrome b558. J. Biol. Chem. 1995, 270, 26246–26251. [Google Scholar] [CrossRef]
- Inanami, O.; Johnson, J.L.; McAdara, J.K.; Benna, J.E.; Faust, L.R.; Newburger, P.E.; Babior, B.M. Activation of the leukocyte NADPH oxidase by phorbol ester requires the phosphorylation of p47PHOX on serine 303 or 304. J. Biol. Chem. 1998, 273, 9539–9543. [Google Scholar] [CrossRef]
- Ago, T.; Kuribayashi, F.; Hiroaki, H.; Takeya, R.; Ito, T.; Kohda, D.; Sumimoto, H. Phosphorylation of p47phox directs phox homology domain from SH3 domain toward phosphoinositides, leading to phagocyte NADPH oxidase activation. Proc. Natl. Acad. Sci. USA 2003, 100, 4474–4479. [Google Scholar] [CrossRef]
- Cairns, B.; Kim, J.Y.; Tang, X.N.; Yenari, M.A. NOX inhibitors as a therapeutic strategy for stroke and neurodegenerative disease. Curr. Drug. Targets 2012, 13, 199–206. [Google Scholar] [CrossRef]
- Suh, S.W.; Shin, B.S.; Ma, H.; Van Hoecke, M.; Brennan, A.M.; Yenari, M.A.; Swanson, R.A. Glucose and NADPH oxidase drive neuronal superoxide formation in stroke. Ann. Neurol. 2008, 64, 654–663. [Google Scholar] [CrossRef]
- Tang, X.N.; Cairns, B.; Kim, J.Y.; Yenari, M.A. NADPH oxidase in stroke and cerebrovascular disease. Neurol. Res. 2012, 34, 338–345. [Google Scholar] [CrossRef]
- Ma, M.; Wang, J.; Dhandapani, K.; Wang, R.; Brann, D. NADPH Oxidases in Traumatic Brain Injury–Promising Therapeutic Targets? Redox Biol. 2018, 16, 285–293. [Google Scholar] [CrossRef]
- Bánfi, B.; Molnár, G.; Maturana, A.; Steger, K.; Hegedûs, B.; Demaurex, N.; Krause, K.H. A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J. Biol. Chem. 2001, 276, 37594–37601. [Google Scholar] [CrossRef] [PubMed]
- Bánfi, B.; Tirone, F.; Durussel, I.; Knisz, J.; Moskwa, P.; Molnár, G.Z.; Krause, K.H.; Cox, J.A. Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5). J. Biol. Chem. 2004, 279, 18583–18591. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, C.; Ohayon, R.; Valent, A.; Noël-Hudson, M.S.; Dème, D.; Virion, A. Purification of a novel flavoprotein involved in the thyroid NADPH oxidase. Cloning of the porcine and human cdnas. J. Biol. Chem. 1999, 274, 37265–37269. [Google Scholar] [CrossRef]
- De Deken, X.; Wang, D.; Many, M.C.; Costagliola, S.; Libert, F.; Vassart, G.; Dumont, J.E.; Miot, F. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J. Biol. Chem. 2000, 275, 23227–23233. [Google Scholar] [CrossRef] [PubMed]
- Takac, I.; Schröder, K.; Zhang, L.; Lardy, B.; Anilkumar, N.; Lambeth, J.D.; Shah, A.M.; Morel, F.; Brandes, R.P. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J. Biol. Chem. 2011, 286, 13304–13313. [Google Scholar] [CrossRef] [PubMed]
- Madamanchi, N.R.; Runge, M.S. Redox signaling in cardiovascular health and disease. Free Radic. Biol. Med. 2013, 61, 473–501. [Google Scholar] [CrossRef]
- Lassègue, B.; Griendling, K.K. NADPH oxidases: Functions and pathologies in the vasculature. Arter. Thromb. Vasc. Biol. 2010, 30, 653–661. [Google Scholar] [CrossRef]
- Cave, A.C.; Brewer, A.C.; Narayanapanicker, A.; Ray, R.; Grieve, D.J.; Walker, S.; Shah, A.M. NADPH oxidases in cardiovascular health and disease. Antioxid. Redox Signal. 2006, 8, 691–728. [Google Scholar] [CrossRef]
- Sorescu, D.; Weiss, D.; Lassègue, B.; Clempus, R.E.; Szöcs, K.; Sorescu, G.P.; Valppu, L.; Quinn, M.T.; Lambeth, J.D.; Vega, J.D.; et al. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation 2002, 105, 1429–1435. [Google Scholar] [CrossRef]
- Grote, K.; Flach, I.; Luchtefeld, M.; Akin, E.; Holland, S.M.; Drexler, H.; Schieffer, B. Mechanical stretch enhances mRNA expression and proenzyme release of matrix metalloproteinase-2 (MMP-2) via NAD(P)H oxidase-derived reactive oxygen species. Circ. Res. 2003, 92, e80–e86. [Google Scholar] [CrossRef]
- Hwang, J.; Kleinhenz, D.J.; Lassègue, B.; Griendling, K.K.; Dikalov, S.; Hart, C.M. Peroxisome proliferator-activated receptor-gamma ligands regulate endothelial membrane superoxide production. Am. J. Physiol. Cell Physiol. 2005, 288, C899–C905. [Google Scholar] [CrossRef] [PubMed]
- Judkins, C.P.; Diep, H.; Broughton, B.R.; Mast, A.E.; Hooker, E.U.; Miller, A.A.; Selemidis, S.; Dusting, G.J.; Sobey, C.G.; Drummond, G.R. Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE-/- mice. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H24–H32. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.A.; O’Donnell, V.B.; Wood, J.D.; Broughton, J.P.; Hughes, E.J.; Jones, O.T. Expression of phagocyte NADPH oxidase components in human endothelial cells. Am. J. Physiol. 1996, 271, H1626–H1634. [Google Scholar] [CrossRef] [PubMed]
- Görlach, A.; Brandes, R.P.; Nguyen, K.; Amidi, M.; Dehghani, F.; Busse, R. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ. Res. 2000, 87, 26–32. [Google Scholar] [CrossRef]
- Wang, H.D.; Xu, S.; Johns, D.G.; Du, Y.; Quinn, M.T.; Cayatte, A.J.; Cohen, R.A. Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin II in mice. Circ. Res. 2001, 88, 947–953. [Google Scholar] [CrossRef]
- Bendall, J.K.; Cave, A.C.; Heymes, C.; Gall, N.; Shah, A.M. Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation 2002, 105, 293–296. [Google Scholar] [CrossRef]
- Xiao, L.; Pimentel, D.R.; Wang, J.; Singh, K.; Colucci, W.S.; Sawyer, D.B. Role of reactive oxygen species and NAD(P)H oxidase in alpha(1)-adrenoceptor signaling in adult rat cardiac myocytes. Am. J. Physiol. Cell Physiol. 2002, 282, C926–C934. [Google Scholar] [CrossRef]
- Touyz, R.M.; Chen, X.; Tabet, F.; Yao, G.; He, G.; Quinn, M.T.; Pagano, P.J.; Schiffrin, E.L. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: Regulation by angiotensin II. Circ. Res. 2002, 90, 1205–1213. [Google Scholar] [CrossRef]
- Ellmark, S.H.; Dusting, G.J.; Fui, M.N.; Guzzo-Pernell, N.; Drummond, G.R. The contribution of Nox4 to NADPH oxidase activity in mouse vascular smooth muscle. Cardiovasc. Res. 2005, 65, 495–504. [Google Scholar] [CrossRef]
- Clempus, R.E.; Sorescu, D.; Dikalova, A.E.; Pounkova, L.; Jo, P.; Sorescu, G.P.; Schmidt, H.H.; Lassègue, B.; Griendling, K.K. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 42–48. [Google Scholar] [CrossRef]
- Pedruzzi, E.; Guichard, C.; Ollivier, V.; Driss, F.; Fay, M.; Prunet, C.; Marie, J.C.; Pouzet, C.; Samadi, M.; Elbim, C.; et al. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol. Cell Biol. 2004, 24, 10703–10717. [Google Scholar] [CrossRef] [PubMed]
- Colston, J.T.; de la Rosa, S.D.; Strader, J.R.; Anderson, M.A.; Freeman, G.L. H2O2 activates Nox4 through PLA2-dependent arachidonic acid production in adult cardiac fibroblasts. FEBS Lett. 2005, 579, 2533–2540. [Google Scholar] [CrossRef]
- Peng, T.; Lu, X.; Feng, Q. Pivotal role of gp91phox-containing NADH oxidase in lipopolysaccharide-induced tumor necrosis factor-alpha expression and myocardial depression. Circulation 2005, 111, 1637–1644. [Google Scholar] [CrossRef]
- Ago, T.; Kuroda, J.; Pain, J.; Fu, C.; Li, H.; Sadoshima, J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ. Res. 2010, 106, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Jay, D.B.; Papaharalambus, C.A.; Seidel-Rogol, B.; Dikalova, A.E.; Lassègue, B.; Griendling, K.K. Nox5 mediates PDGF-induced proliferation in human aortic smooth muscle cells. Free Radic. Biol. Med. 2008, 45, 329–335. [Google Scholar] [CrossRef] [PubMed]
- BelAiba, R.S.; Djordjevic, T.; Petry, A.; Diemer, K.; Bonello, S.; Banfi, B.; Hess, J.; Pogrebniak, A.; Bickel, C.; Görlach, A. NOX5 variants are functionally active in endothelial cells. Free Radic. Biol. Med. 2007, 42, 446–459. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, N.; Agrotis, A.; Tararak, E.; Antropova, Y.; Kanellakis, P.; Ilyinskaya, O.; Quinn, M.T.; Smirnov, V.; Bobik, A. Cytochrome b558-dependent NAD(P)H oxidase-phox units in smooth muscle and macrophages of atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 2037–2043. [Google Scholar] [CrossRef]
- Stilo, F.; Catanese, V.; Nenna, A.; Montelione, N.; Codispoti, F.A.; Verghi, E.; Gabellini, T.; Jawabra, M.; Chello, M.; Spinelli, F. Biomarkers in EndoVascular Aneurysm Repair (EVAR) and Abdominal Aortic Aneurysm: Pathophysiology and Clinical Implications. Diagn. (Basel) 2022, 12, 183. [Google Scholar] [CrossRef]
- Schramm, A.; Matusik, P.; Osmenda, G.; Guzik, T.J. Targeting NADPH oxidases in vascular pharmacology. Vascul. Pharmacol. 2012, 56, 216–231. [Google Scholar] [CrossRef]
- Guzik, B.; Sagan, A.; Ludew, D.; Mrowiecki, W.; Chwała, M.; Bujak-Gizycka, B.; Filip, G.; Grudzien, G.; Kapelak, B.; Zmudka, K.; et al. Mechanisms of oxidative stress in human aortic aneurysms--association with clinical risk factors for atherosclerosis and disease severity. Int. J. Cardiol. 2013, 168, 2389–2396. [Google Scholar] [CrossRef]
- Xiong, W.; Mactaggart, J.; Knispel, R.; Worth, J.; Zhu, Z.; Li, Y.; Sun, Y.; Baxter, B.T.; Johanning, J. Inhibition of reactive oxygen species attenuates aneurysm formation in a murine model. Atherosclerosis 2009, 202, 128–134. [Google Scholar] [CrossRef]
- Zhang, J.; Schmidt, J.; Ryschich, E.; Mueller-Schilling, M.; Schumacher, H.; Allenberg, J.R. Inducible nitric oxide synthase is present in human abdominal aortic aneurysm and promotes oxidative vascular injury. J. Vasc. Surg. 2003, 38, 360–367. [Google Scholar] [CrossRef]
- Ramos-Mozo, P.; Madrigal-Matute, J.; Martinez-Pinna, R.; Blanco-Colio, L.M.; Lopez, J.A.; Camafeita, E.; Meilhac, O.; Michel, J.B.; Aparicio, C.; Vega de Ceniga, M.; et al. Proteomic analysis of polymorphonuclear neutrophils identifies catalase as a novel biomarker of abdominal aortic aneurysm: Potential implication of oxidative stress in abdominal aortic aneurysm progression. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 3011–3019. [Google Scholar] [CrossRef]
- Grigoryants, V.; Hannawa, K.K.; Pearce, C.G.; Sinha, I.; Roelofs, K.J.; Ailawadi, G.; Deatrick, K.B.; Woodrum, D.T.; Cho, B.S.; Henke, P.K.; et al. Tamoxifen up-regulates catalase production, inhibits vessel wall neutrophil infiltration, and attenuates development of experimental abdominal aortic aneurysms. J. Vasc. Surg. 2005, 41, 108–114. [Google Scholar] [CrossRef]
- Parastatidis, I.; Weiss, D.; Joseph, G.; Taylor, W.R. Overexpression of catalase in vascular smooth muscle cells prevents the formation of abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2389–2396. [Google Scholar] [CrossRef]
- Gao, L.; Chalupsky, K.; Stefani, E.; Cai, H. Mechanistic insights into folic acid-dependent vascular protection: Dihydrofolate reductase (DHFR)-mediated reduction in oxidant stress in endothelial cells and angiotensin II-infused mice: A novel HPLC-based fluorescent assay for DHFR activity. J. Mol. Cell Cardiol. 2009, 47, 752–760. [Google Scholar] [CrossRef]
- Gao, L.; Siu, K.L.; Chalupsky, K.; Nguyen, A.; Chen, P.; Weintraub, N.L.; Galis, Z.; Cai, H. Role of uncoupled endothelial nitric oxide synthase in abdominal aortic aneurysm formation: Treatment with folic acid. Hypertension 2012, 59, 158–166. [Google Scholar] [CrossRef]
- Pimiento, J.M.; Maloney, S.P.; Tang, P.C.; Muto, A.; Westvik, T.S.; Fitzgerald, T.N.; Fancher, T.T.; Tellides, G.; Dardik, A. Endothelial nitric oxide synthase stimulates aneurysm growth in aged mice. J. Vasc. Res. 2008, 45, 251–258. [Google Scholar] [CrossRef]
- Schwartz, E.A.; Reaven, E.; Topper, J.N.; Tsao, P.S. Transforming growth factor-beta receptors localize to caveolae and regulate endothelial nitric oxide synthase in normal human endothelial cells. Biochem. J. 2005, 390, 199–206. [Google Scholar] [CrossRef]
- Habashi, J.P.; Judge, D.P.; Holm, T.M.; Cohn, R.D.; Loeys, B.L.; Cooper, T.K.; Myers, L.; Klein, E.C.; Liu, G.; Calvi, C.; et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 2006, 312, 117–121. [Google Scholar] [CrossRef]
- Daugherty, A.; Manning, M.W.; Cassis, L.A. Antagonism of AT2 receptors augments Angiotensin II-induced abdominal aortic aneurysms and atherosclerosis. Br. J. Pharmacol. 2001, 134, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Gambarin, F.I.; Favalli, V.; Serio, A.; Regazzi, M.; Pasotti, M.; Klersy, C.; Dore, R.; Mannarino, S.; Viganò, M.; Odero, A.; et al. Rationale and design of a trial evaluating the effects of losartan vs. nebivolol vs. the association of both on the progression of aortic root dilation in Marfan syndrome with FBN1 gene mutations. J. Cardiovasc. Med. (Hagerstown) 2009, 10, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Kohno, T.; Anzai, T.; Kaneko, H.; Sugano, Y.; Shimizu, H.; Shimoda, M.; Miyasho, T.; Okamoto, M.; Yokota, H.; Yamada, S.; et al. High-mobility group box 1 protein blockade suppresses development of abdominal aortic aneurysm. J. Cardiol. 2012, 59, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Salmon, M.D.; Lu, G.; Su, G.; Pope, N.H.; Smith, J.R.; Weiss, M.L.; Upchurch, G.R., Jr. Mesenchymal Stem Cells Attenuate NADPH Oxidase-Dependent High Mobility Group Box 1 Production and Inhibit Abdominal Aortic Aneurysms. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 908–918. [Google Scholar] [CrossRef]
- Wang, W.; Xu, B.; Xuan, H.; Ge, Y.; Wang, Y.; Wang, L.; Huang, J.; Fu, W.; Michie, S.A.; Dalman, R.L. Hypoxia-inducible factor 1 in clinical and experimental aortic aneurysm disease. J. Vasc. Surg. 2018, 68, 1538–1550.e1532. [Google Scholar] [CrossRef]
- Choke, E.; Cockerill, G.W.; Dawson, J.; Chung, Y.L.; Griffiths, J.; Wilson, R.W.; Loftus, I.M.; Thompson, M.M. Hypoxia at the site of abdominal aortic aneurysm rupture is not associated with increased lactate. Ann. N. Y. Acad. Sci. 2006, 1085, 306–310. [Google Scholar] [CrossRef]
- Tsai, S.-H.; Huang, P.-H.; Hsu, Y.-J.; Peng, Y.-J.; Lee, C.-H.; Wang, J.-C.; Chen, J.-W.; Lin, S.-J. Inhibition of hypoxia inducible factor-1α attenuates abdominal aortic aneurysm progression through the down-regulation of matrix metalloproteinases. Sci. Rep. 2016, 6, 28612. [Google Scholar] [CrossRef]
- Parodi, F.E.; Mao, D.; Ennis, T.L.; Bartoli, M.A.; Thompson, R.W. Suppression of experimental abdominal aortic aneurysms in mice by treatment with pyrrolidine dithiocarbamate, an antioxidant inhibitor of nuclear factor-kappaB. J. Vasc. Surg. 2005, 41, 479–489. [Google Scholar] [CrossRef]
- Saito, T.; Hasegawa, Y.; Ishigaki, Y.; Yamada, T.; Gao, J.; Imai, J.; Uno, K.; Kaneko, K.; Ogihara, T.; Shimosawa, T.; et al. Importance of endothelial NF-κB signalling in vascular remodelling and aortic aneurysm formation. Cardiovasc. Res. 2013, 97, 106–114. [Google Scholar] [CrossRef]
- Ousaka, D.; Fujii, Y.; Oozawa, S.; Nishibori, M.; Kuroko, Y.; Masuda, Z.; Sano, S. Decreased Serum Levels of High Mobility Group Box 1 (HMGB-1) after Graft Replacement or Stenting of Abdominal Aortic Aneurysm. Ann. Vasc. Surg. 2017, 41, 265–270. [Google Scholar] [CrossRef]
- Hirsch, A.T.; Haskal, Z.J.; Hertzer, N.R.; Bakal, C.W.; Creager, M.A.; Halperin, J.L.; Hiratzka, L.F.; Murphy, W.R.; Olin, J.W.; Puschett, J.B.; et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006, 113, e463–e654. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Nakashima, H.; Aoki, M.; Miyake, T.; Kawasaki, T.; Iwai, M.; Jo, N.; Oishi, M.; Kataoka, K.; Ohgi, S.; Ogihara, T.; et al. Inhibition of Experimental Abdominal Aortic Aneurysm in the Rat by Use of Decoy Oligodeoxynucleotides Suppressing Activity of Nuclear Factor κB and ets Transcription Factors. Circulation 2004, 109, 132–138. [Google Scholar] [CrossRef]
- Gavazzi, G.; Deffert, C.; Trocme, C.; Schäppi, M.; Herrmann, F.R.; Krause, K.-H. NOX1 Deficiency Protects From Aortic Dissection in Response to Angiotensin II. Hypertension 2007, 50, 189–196. [Google Scholar] [CrossRef]
- Siu, K.L.; Li, Q.; Zhang, Y.; Guo, J.; Youn, J.Y.; Du, J.; Cai, H. NOX isoforms in the development of abdominal aortic aneurysm. Redox Biol. 2017, 11, 118–125. [Google Scholar] [CrossRef]
- Hu, X.; Jiang, W.; Wang, Z.; Li, L.; Hu, Z. NOX1 Negatively Modulates Fibulin-5 in Vascular Smooth Muscle Cells to Affect Aortic Dissection. Biol. Pharm. Bull. 2019, 42, 1464–1470. [Google Scholar] [CrossRef]
- Vendrov, A.E.; Sumida, A.; Canugovi, C.; Lozhkin, A.; Hayami, T.; Madamanchi, N.R.; Runge, M.S. NOXA1-dependent NADPH oxidase regulates redox signaling and phenotype of vascular smooth muscle cell during atherogenesis. Redox Biol. 2019, 21, 101063. [Google Scholar] [CrossRef]
- Jiménez-Altayó, F.; Meirelles, T.; Crosas-Molist, E.; Sorolla, M.A.; Del Blanco, D.G.; López-Luque, J.; Mas-Stachurska, A.; Siegert, A.M.; Bonorino, F.; Barberà, L.; et al. Redox stress in Marfan syndrome: Dissecting the role of the NADPH oxidase NOX4 in aortic aneurysm. Free Radic. Biol. Med. 2018, 118, 44–58. [Google Scholar] [CrossRef]
- Ho, F.; Watson, A.M.D.; Elbatreek, M.H.; Kleikers, P.W.M.; Khan, W.; Sourris, K.C.; Dai, A.; Jha, J.; Schmidt, H.H.H.W.; Jandeleit-Dahm, K.A.M. Endothelial reactive oxygen-forming NADPH oxidase 5 is a possible player in diabetic aortic aneurysm but not atherosclerosis. Sci. Rep. 2022, 12, 11570. [Google Scholar] [CrossRef]
- Kigawa, Y.; Miyazaki, T.; Lei, X.-F.; Nakamachi, T.; Oguchi, T.; Kim-Kaneyama, J.-r.; Taniyama, M.; Tsunawaki, S.; Shioda, S.; Miyazaki, A. NADPH Oxidase Deficiency Exacerbates Angiotensin II–Induced Abdominal Aortic Aneurysms in Mice. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2413–2420. [Google Scholar] [CrossRef]
- Ejiri, J.; Inoue, N.; Tsukube, T.; Munezane, T.; Hino, Y.; Kobayashi, S.; Hirata, K.; Kawashima, S.; Imajoh-Ohmi, S.; Hayashi, Y.; et al. Oxidative stress in the pathogenesis of thoracic aortic aneurysm: Protective role of statin and angiotensin II type 1 receptor blocker. Cardiovasc. Res. 2003, 59, 988–996. [Google Scholar] [CrossRef]
- Thomas, M.; Gavrila, D.; McCormick, M.L.; Miller, F.J.; Daugherty, A.; Cassis, L.A.; Dellsperger, K.C.; Weintraub, N.L. Deletion of p47phox Attenuates Angiotensin II–Induced Abdominal Aortic Aneurysm Formation in Apolipoprotein E–Deficient Mice. Circulation 2006, 114, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Rush, C.; Nyara, M.; Moxon, J.V.; Trollope, A.; Cullen, B.; Golledge, J. Whole genome expression analysis within the angiotensin II-apolipoprotein E deficient mouse model of abdominal aortic aneurysm. BMC Genom. 2009, 10, 298. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.; Bendall, J.K.; Channon, K.M. Endothelial-specific Nox2 over-expression increases vascular superoxide production but does not alter atherosclerotic plaque or AngII-dependent aneurysm formation. Atherosclerosis 2010, 213, e12–e13. [Google Scholar] [CrossRef]
- Pepping, J.K.; Vandanmagsar, B.; Fernandez-Kim, S.O.; Zhang, J.; Mynatt, R.L.; Bruce-Keller, A.J. Myeloid-specific deletion of NOX2 prevents the metabolic and neurologic consequences of high fat diet. PLoS ONE 2017, 12, e0181500. [Google Scholar] [CrossRef]
- Carnesecchi, S.; Deffert, C.; Donati, Y.; Basset, O.; Hinz, B.; Preynat-Seauve, O.; Guichard, C.; Arbiser, J.L.; Banfi, B.; Pache, J.C.; et al. A key role for NOX4 in epithelial cell death during development of lung fibrosis. Antioxid. Redox Signal. 2011, 15, 607–619. [Google Scholar] [CrossRef]
- Paffenholz, R.; Bergstrom, R.A.; Pasutto, F.; Wabnitz, P.; Munroe, R.J.; Jagla, W.; Heinzmann, U.; Marquardt, A.; Bareiss, A.; Laufs, J.; et al. Vestibular defects in head-tilt mice result from mutations in Nox3, encoding an NADPH oxidase. Genes Dev. 2004, 18, 486–491. [Google Scholar] [CrossRef]
- Donkó, A.; Ruisanchez, E.; Orient, A.; Enyedi, B.; Kapui, R.; Péterfi, Z.; de Deken, X.; Benyó, Z.; Geiszt, M. Urothelial cells produce hydrogen peroxide through the activation of Duox1. Free Radic. Biol. Med. 2010, 49, 2040–2048. [Google Scholar] [CrossRef]
- Johnson, K.R.; Marden, C.C.; Ward-Bailey, P.; Gagnon, L.H.; Bronson, R.T.; Donahue, L.R. Congenital hypothyroidism, dwarfism, and hearing impairment caused by a missense mutation in the mouse dual oxidase 2 gene, Duox2. Mol. Endocrinol. 2007, 21, 1593–1602. [Google Scholar] [CrossRef]
- Marinkovic, G.; Hibender, S.; Hoogenboezem, M.; van Broekhoven, A.; Girigorie, A.F.; Bleeker, N.; Hamers, A.A.; Stap, J.; van Buul, J.D.; de Vries, C.J.; et al. Immunosuppressive drug azathioprine reduces aneurysm progression through inhibition of Rac1 and c-Jun-terminal-N-kinase in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2380–2388. [Google Scholar] [CrossRef]
- Pincemail, J.; Defraigne, J.O.; Cheramy-Bien, J.P.; Dardenne, N.; Donneau, A.F.; Albert, A.; Labropoulos, N.; Sakalihasan, N. On the potential increase of the oxidative stress status in patients with abdominal aortic aneurysm. Redox Rep. 2012, 17, 139–144. [Google Scholar] [CrossRef]
- Pincemail, J.; Defraigne, J.O.; Courtois, A.; Albert, A.; Cheramy-Bien, J.P.; Sakalihasan, N. Abdominal Aortic Aneurysm (AAA): Is There a Role for the Prevention and Therapy Using Antioxidants? Curr. Drug Targets 2018, 19, 1256–1264. [Google Scholar] [CrossRef]
- Kaneko, H.; Anzai, T.; Morisawa, M.; Kohno, T.; Nagai, T.; Anzai, A.; Takahashi, T.; Shimoda, M.; Sasaki, A.; Maekawa, Y.; et al. Resveratrol prevents the development of abdominal aortic aneurysm through attenuation of inflammation, oxidative stress, and neovascularization. Atherosclerosis 2011, 217, 350–357. [Google Scholar] [CrossRef]
- Wang, L.; Wu, H.; Xiong, L.; Liu, X.; Yang, N.; Luo, L.; Qin, T.; Zhu, X.; Shen, Z.; Jing, H.; et al. Quercetin Downregulates Cyclooxygenase-2 Expression and HIF-1α/VEGF Signaling-Related Angiogenesis in a Mouse Model of Abdominal Aortic Aneurysm. Biomed. Res. Int. 2020, 2020, 9485398. [Google Scholar] [CrossRef]
- Amioka, N.; Miyoshi, T.; Yonezawa, T.; Kondo, M.; Akagi, S.; Yoshida, M.; Saito, Y.; Nakamura, K.; Ito, H. Pemafibrate Prevents Rupture of Angiotensin II-Induced Abdominal Aortic Aneurysms. Front. Cardiovasc. Med. 2022, 9, 904215. [Google Scholar] [CrossRef]
- Dao, V.T.; Elbatreek, M.H.; Altenhöfer, S.; Casas, A.I.; Pachado, M.P.; Neullens, C.T.; Knaus, U.G.; Schmidt, H. Isoform-selective NADPH oxidase inhibitor panel for pharmacological target validation. Free Radic. Biol. Med. 2020, 148, 60–69. [Google Scholar] [CrossRef]
- Gaggini, F.; Laleu, B.; Orchard, M.; Fioraso-Cartier, L.; Cagnon, L.; Houngninou-Molango, S.; Gradia, A.; Duboux, G.; Merlot, C.; Heitz, F.; et al. Design, synthesis and biological activity of original pyrazolo-pyrido-diazepine, -pyrazine and -oxazine dione derivatives as novel dual Nox4/Nox1 inhibitors. Bioorg. Med. Chem. 2011, 19, 6989–6999. [Google Scholar] [CrossRef]
- Gianni, D.; Taulet, N.; Zhang, H.; DerMardirossian, C.; Kister, J.; Martinez, L.; Roush, W.R.; Brown, S.J.; Bokoch, G.M.; Rosen, H. A novel and specific NADPH oxidase-1 (Nox1) small-molecule inhibitor blocks the formation of functional invadopodia in human colon cancer cells. ACS Chem. Biol. 2010, 5, 981–993. [Google Scholar] [CrossRef]
- Gorin, Y.; Cavaglieri, R.C.; Khazim, K.; Lee, D.Y.; Bruno, F.; Thakur, S.; Fanti, P.; Szyndralewiez, C.; Barnes, J.L.; Block, K.; et al. Targeting NADPH oxidase with a novel dual Nox1/Nox4 inhibitor attenuates renal pathology in type 1 diabetes. Am. J. Physiol. Renal. Physiol. 2015, 308, F1276–F1287. [Google Scholar] [CrossRef]
- Hirano, K.; Chen, W.S.; Chueng, A.L.; Dunne, A.A.; Seredenina, T.; Filippova, A.; Ramachandran, S.; Bridges, A.; Chaudry, L.; Pettman, G.; et al. Discovery of GSK2795039, a Novel Small Molecule NADPH Oxidase 2 Inhibitor. Antioxid. Redox Signal. 2015, 23, 358–374. [Google Scholar] [CrossRef]
- Liu, F.C.; Yu, H.P.; Chen, P.J.; Yang, H.W.; Chang, S.H.; Tzeng, C.C.; Cheng, W.J.; Chen, Y.R.; Chen, Y.L.; Hwang, T.L. A novel NOX2 inhibitor attenuates human neutrophil oxidative stress and ameliorates inflammatory arthritis in mice. Redox Biol. 2019, 26, 101273. [Google Scholar] [CrossRef]
- Li, Y.; Cifuentes-Pagano, E.; DeVallance, E.R.; de Jesus, D.S.; Sahoo, S.; Meijles, D.N.; Koes, D.; Camacho, C.J.; Ross, M.; St Croix, C.; et al. NADPH oxidase 2 inhibitors CPP11G and CPP11H attenuate endothelial cell inflammation & vessel dysfunction and restore mouse hind-limb flow. Redox Biol. 2019, 22, 101143. [Google Scholar] [CrossRef]
- Gatto, G.J., Jr.; Ao, Z.; Kearse, M.G.; Zhou, M.; Morales, C.R.; Daniels, E.; Bradley, B.T.; Goserud, M.T.; Goodman, K.B.; Douglas, S.A.; et al. NADPH oxidase-dependent and -independent mechanisms of reported inhibitors of reactive oxygen generation. J. Enzym. Inhib. Med. Chem. 2013, 28, 95–104. [Google Scholar] [CrossRef]
- Schröder, K. NADPH oxidases: Current aspects and tools. Redox Biol. 2020, 34, 101512. [Google Scholar] [CrossRef]
- Wang, X.; Elksnis, A.; Wikström, P.; Walum, E.; Welsh, N.; Carlsson, P.O. The novel NADPH oxidase 4 selective inhibitor GLX7013114 counteracts human islet cell death in vitro. PLoS ONE 2018, 13, e0204271. [Google Scholar] [CrossRef]
- Helfinger, V.; Palfi, K.; Weigert, A.; Schröder, K. The NADPH Oxidase Nox4 Controls Macrophage Polarization in an NFκB-Dependent Manner. Oxid. Med. Cell Longev. 2019, 2019, 3264858. [Google Scholar] [CrossRef]
- Kofler, P.A.; Pircher, H.; von Grafenstein, S.; Diener, T.; Höll, M.; Liedl, K.R.; Siems, K.; Jansen-Dürr, P. Characterisation of Nox4 inhibitors from edible plants. Planta Med. 2013, 79, 244–252. [Google Scholar] [CrossRef]
- Revoltella, S.; Baraldo, G.; Waltenberger, B.; Schwaiger, S.; Kofler, P.; Moesslacher, J.; Huber-Seidel, A.; Pagitz, K.; Kohl, R.; Jansen-Duerr, P.; et al. Identification of the NADPH Oxidase 4 Inhibiting Principle of Lycopus europaeus. Molecules 2018, 23, 653. [Google Scholar] [CrossRef]
- Danyal, K.; de Jong, W.; O’Brien, E.; Bauer, R.A.; Heppner, D.E.; Little, A.C.; Hristova, M.; Habibovic, A.; van der Vliet, A. Acrolein and thiol-reactive electrophiles suppress allergen-induced innate airway epithelial responses by inhibition of DUOX1 and EGFR. Am. J. Physiol. Lung. Cell Mol. Physiol. 2016, 311, L913–L923. [Google Scholar] [CrossRef]
| Gene Knockout | AAA Model | Influence on Experimental AAAs | References |
|---|---|---|---|
| NOX1 | Angiotensin II | Decrease rupture rate, altered TIMP1 expression | [104] |
| Angiotensin II | Decreased rupture, decreased size, decreased eNOS | [105] | |
| NOX2 | Angiotensin II | Decreased rupture, decreased size, decreased eNOS | [105] |
| Elastase | Decreased size, decreased MMP2 and 9, decreased IL-17 and IL-23 production | [94] | |
| NOX3 | n/a | unknown | |
| NOX4 | Angiotensin II | Decreased rupture, decreased size, decreased eNOS | [105] |
| Fbn1C1039G/+ | Decreased aneurysm, decreased elastin breakage | [108] | |
| NOX5 | Diabetic models + Nox5 overexpression | Increased aneurysms | [109] |
| DUOX1 | n/a | Elevated but unknown | [110] |
| DUOX2 | n/a | unknown | |
| p22phox | n/a | Elevated but unknown | [31,80,111] |
| p47phox | Angiotensin II | Decreased rupture, decreased MMP2 | [112] |
| Angiotensin II | Decreased rupture, decreased size, decreased eNOS | [105] | |
| p67phox | n/a | Elevated but unknown | [80,111] |
| Rac1 | n/a | Elevated but unknown | [80,111] |
| Rac2 | n/a | Elevated but unknown | [113] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salmon, M. NADPH Oxidases in Aortic Aneurysms. Antioxidants 2022, 11, 1830. https://doi.org/10.3390/antiox11091830
Salmon M. NADPH Oxidases in Aortic Aneurysms. Antioxidants. 2022; 11(9):1830. https://doi.org/10.3390/antiox11091830
Chicago/Turabian StyleSalmon, Morgan. 2022. "NADPH Oxidases in Aortic Aneurysms" Antioxidants 11, no. 9: 1830. https://doi.org/10.3390/antiox11091830
APA StyleSalmon, M. (2022). NADPH Oxidases in Aortic Aneurysms. Antioxidants, 11(9), 1830. https://doi.org/10.3390/antiox11091830






