Abstract
Plants or plant extracts are widely investigated for preventing/counteracting several chronic disorders. The oral route is the most common route for nutraceutical and drug administration. Currently, it is still unclear as to whether and how the pattern of phenolic compounds (PCs) found in the plants as well as their bioactivity could be modified during the gastrointestinal transit. Recent studies have revealed antioxidant and anti-steatotic properties of Thymbra spicata. Here, we investigated the possible loss of phytochemicals that occurs throughout the sequential steps of a simulated in vitro gastrointestinal (GI) digestion of aqueous and ethanolic extracts of aerial parts of T. spicata. Crude, digested, and dialyzed extracts were characterized in terms of their phenolic profile and biological activities. Total contents of carbohydrates, proteins, PCs, flavonoids, and hydroxycinnamic acids were quantified. The changes in the PC profile and in bioactive compounds upon the simulated GI digestion were monitored by HPLC–MS/MS analysis. The antioxidant activity was measured by different spectrophotometric assays, and the antiproliferative potential was assessed by using three representative human cancer cell lines. We observed that the simulated GI digestion reduced the phytochemical contents in both aqueous and ethanolic T. spicata extracts and modified the PC profile. However, T. spicata extracts improved their antioxidant potential after digestion, while a partial reduction in the antiproliferative activity was observed for the ethanolic extract. Therefore, our results could provide a scientific basis for the employment of T. spicata extract as valuable nutraceutical.
1. Introduction
Dietary phytochemicals are found abundantly in fruits, vegetables, grains, plant-based foods, and beverages [1]. Consumption of phytochemicals plays a main role in healthcare by preventing many chronic diseases including non-alcoholic fatty liver disease (NAFLD) [2], cardiovascular disease [3], neurodegenerative diseases [4], and some types of cancer [5]. For this reason, extracts from plants or plant parts have been largely tested to develop new functional foods for preventing/counteracting many chronic disorders [6]. Phenolic compounds (PCs) are the most abundant phytochemicals in many edible and medicinal plants, and they are the main responsible agents for the beneficial effects, especially the defense against oxidative stress [7]. PCs include numerous varieties of compounds classified into flavonoids and non-flavonoids: flavonoids include flavonols, flavones, flavan-3-ols, flavanones, and anthocyanins; non-flavonoid compounds include phenolic acids, volatile phenols, stilbenes, lignans, and coumarins [8].
Importantly, the bioavailability of several plant extracts as a source of PCs is likely affected by changes occurring during the gastrointestinal (GI) transit. Foods and nutraceuticals introduced by the oral route undergo digestive processes throughout GI compartments and cross physiological barriers that are able to influence their delivery [9]. Indeed, the main challenges for bioactive compounds are the rate and degree of absorption, as well as their solubility, stability, and permeability across the mucosal and intestinal barriers [10]. Moreover, metabolites can show completely different bioavailability compared to the parental phenolic compounds due to the physiological environment and cofactors [11,12]. Experimental approaches using in vitro GI models can overcome difficulties associated with human studies that are often poorly reproducible and comparable, expensive, time-consuming, and might generate ethical issues, depending on the study design and food being tested [13]. In fact, several digestion methods have been proposed in the literature review, often differing in the applied conditions. To give an example, the origin of the used enzymes (porcine, rabbit, or human); the environmental factors (pH, ionic strength, and digestion time); and other parameters such as the presence of phospholipids, digestive emulsifiers vs. their mixtures (e.g., pancreatin and bile salts), and the ratio of food bolus to digestive fluids, which alter enzyme activity, may considerably alter the results. While modifying some of these parameters with a possible and major impact on the matrix release or digestibility of some compounds, we were concerned with applying a standardized and practical simulated in vitro GI digestion method based on physiologically relevant conditions that can be applied for various endpoints and may be amended to accommodate further specific requirements mainly developing a more accurate in vitro human digestion model, taking into consideration the intestinal microbiota presence and conditions.
Lamiaceae is a family of mostly shrubs and herbs with a wide distribution worldwide, especially in the Mediterranean basin [14]. In this family, Thymbra spicata L., locally known as “Za’atar”, is employed in the folk cookery (as salad or tea infusion), but also in traditional medicine, mainly for its antimicrobial and antiseptic properties [6]. Recent studies have revealed several beneficial properties of T. spicata L. leaves such as antioxidant, hypocholesterolemic, and anti-steatotic activities [15], as well as anti-inflammation [16], anti-proliferative, and pro-apoptotic [17] potential. The abundancy of PCs in T. spicata L. leaves including phenolic acids (rosmarinic acid), phenolic monoterpenoids (carvacrol, thymol), and flavonoids (both glycosides and aglycones) stand behind the wide array of its pharmacological activities [18,19]. The beneficial effects of T. spicata L. as herbal medicine or nutraceutical preparation might be modified during the GI transit where the bioconversion is elicited by low gastric pH, digestive enzymes, and the microbiota [11].
In this context, our study aimed to assess if and how two different extracts from T. spicata L. aerial parts were modified after applying a simulated in vitro GI digestion method. The extracts before and after digestion were characterized for their composition in bioactive compounds and their antioxidant potential. The biological effects were assessed by cellular studies focusing on the antiproliferative capacity on different cancer cell lines.
2. Materials and Methods
2.1. Reagents and Enzymes
All reagents otherwise indicated, including enzymes, were purchased from Sigma- Aldrich Corp. (Milan, Italy). All reagents were of analytical purity.
Sources and Activities of Enzymes
- α-Amylase from human saliva (A0521-500 units/mg). α-Amylase catalyzes the hydrolysis of α-1,4 glycosidic linkage in oligosaccharides.
- Pancreatin from porcine pancreas (P3292-100G). Pancreatin contains enzymatic components including trypsin, amylase and lipase, ribonuclease, and protease, produced by the exocrine cells of the porcine pancreas.
- Pepsin from pig gastric mucosa (≈2500 units/mg protein). Pepsin is an aspartic endproteinase used for the unspecific hydrolysis of proteins and peptides in acidic media.
2.2. Plant Collection
Aerial parts of Thymbra spicata L. were collected from flowering plants growing in “Maarakeh”, South Lebanon. Voucher specimen (L1.125/1) was authenticated by Prof. G. Tohme (CNRS, Beirut, Lebanon) and was kept in the Herbarium of the Botanical Department-Lebanese University (Beirut, Lebanon). The in vitro gastrointestinal (GI) digestion of T. spicata aerial parts was performed according to protocol [20] with slight modifications in order to sequentially simulate the mouth, stomach, and small intestine digestion. The composition of buffers is reported in Table 1.

Table 1.
Chemical composition of the buffer employed in the simulated digestion. SSF: salivary fluid; SGF: gastric fluid; SIF: intestinal fluid.
2.3. In Vitro Simulated Digestion
2.3.1. Oral Digestion
To mimic the oral digestion, 25 g of T. spicata dried and powdered aerial parts were mixed with 25 mL SSF and 3 mL (stock 75 U/mL) α-salivary amylase (from human saliva), 0.2 mL CaCl2, and 5.8 mL distilled H2O and then incubated for 2 min at 37 °C on a magnetic stirrer.
2.3.2. Gastric Digestion
To mimic the gastric digestion, 40 mL SGF, 7 mL pepsin (stock 25,000 U/mL), 0.03 mL CaCl2, and 3 mL distilled H2O were added to the oral outcome and the pH was lowered to 3.0 by HCl; the mixture was incubated for 2 h at 37 °C on a magnetic stirrer, and the pH was checked regularly.
2.3.3. Intestinal Digestion
To mimic the intestinal digestion, 50 mL of gastric outcome was mixed with 50 mL of SIF, 20 mL of pancreatin (stock 100 U/mL), 10 mL bile salt (stock 10 mM), 0.024 mL CaCl2, 6 mL distilled H2O, and 0.7 mL of 1 M HCl to neutralize the pH to 7.0.
2.3.4. Extract Preparation
The obtained mixture was incubated for 2 h at 37 °C on a magnetic stirrer. Then, the mixture was heated to 90 °C for 10 min to inactivate the enzymes used in the digestion process. At the end, the samples were centrifuged for 20 min at 7000 rpm. The pellet was incubated with ethanol (96%) at room temperature for 24 h with agitation. The solution was centrifuged for 20 min at 7000 rpm, obtaining a precipitate (TE) that was discharged, and the ethanol in the digested ethanolic extract (TE-dig) was removed using a rotavapor before the lyophilization of the residue. The supernatant obtained from the digestion process was divided into two parts. One part was lyophilized, obtaining the crude digested aqueous extract (TW-dig); the other one was dialyzed with membrane cut-off 3.5 kDa (Spectra/Por molecularporous membrane tubing, Thermo Fisher Scientific, Milan, Italy) against 250 mL of water for 24 h at 4 °C to separate the low molecular weight (mw) fraction (<3.5 kDa) and the high mw fraction (>3.5 kDa). The solutions inside the dialysis tube (>3.5 kDa) and out of the tube (<3.5 kDa) were lyophilized.
To prepare the undigested aqueous and ethanolic extracts (TW and TE, respectively), the same procedure was followed for extraction without the first part of enzymatic digestion. The detailed scheme of in vitro digestion and the obtained extracts and fractions is illustrated in Figure 1.

Figure 1.
A schematic presentation describing the steps of the simulated digestion in the three phases: mouth, stomach, and intestine, in order to obtain the digested ethanolic (TE) and aqueous (TW) extracts. The same procedure was followed for preparation of crude extracts without the use of enzymatic digestion. The TW and TW dig were subjected to a membrane dialysis with a cut-off of 3.5 kDa to obtain low and high mw fractions.
2.4. Total Carbohydrate Content (TCC)
TCC was determined by the phenol-sulfuric acid colorimetric method [21]. Briefly, 0.5 mL of sample (1 mg/mL) was mixed with 0.5 mL 5% aqueous phenol and 2 mL of H2SO4 (96%). After incubation for 30 min at room temperature, the absorbance was read at 320 nm using a UV–VIS microplate reader (FLUOstar Optima, BMG Labtech, Ortenberg, Germany). The results were derived from a glucose calibration curve (0–200 µg/mL). Values are expressed as µg/mg extract.
2.5. Total Protein Content (TPrC)
The protein content was determined by Bradford colorimetric method, using bovine serum albumin (BSA) as standard [22]. Briefly, 0.5 mL from each extract (1 mg/mL) was mixed with 0.5 mL of Bradford reagent; after 30 min incubation, the absorbance was measured at 595 nm using a UV–VIS microplate reader (FLUOstar Optima, BMG Labtech, Ortenberg, Germany). Data are expressed as µg/g.
2.6. Total Phenol Quantification (TPC)
TPC was determined using the Folin–Ciocalteu method [23]. Briefly, 25μL aliquots of sample (1 mg/mL) were incubated with 125 μL of 10% (w/v) Folin–Ciocalteu reagent for 5 min; after adding 125 µL of Na2CO3 (10% w/v), the sample was incubated for 30 min in darkness at room temperature, and the absorbance was read at 320 nm using a UV–VIS microplate reader (FLUOstar Optima, BMG Labtech, Ortenberg, Germany). The results were derived from a gallic acid calibration curve (0–1000 ug/mL) prepared from a stock solution (1 mg/mL in ethanol). Values are expressed as mg of gallic acid equivalents (GAE) per gram of dried weight extract (mg of GAE/g extract).
2.7. Total Flavonoid Quantification (TFC)
TFC was determined using the aluminum chloride colorimetric method [24]. Briefly, a 1 mL aliquot of sample (1 mg/mL) was mixed with 0.2 mL of 10% (w/v) methanolic AlCl3 solution, 0.2 mL (1 M) potassium acetate, and 5.6 mL distilled H2O. After incubation at room temperature in darkness for 30 min, the absorbance was read at 320 nm using a UV–VIS microplate reader. The results were derived from a calibration curve of quercetin (0–200 μg/mL) prepared from a stock solution (5 mg/mL in methanol). Values are expressed as mg of quercetin equivalent (QE) per gram of dried weight extract (mg of QE/g extract).
2.8. Total Hydroxycinnamic Acid Content (THAC)
HCA was determined using the method by Custódio et al. [25]. Briefly, in a 96-well plate, 20 µL of sample (5 mg/mL) was mixed with 20 µL of 95% ethanol containing 0.1% HCl. After the addition of 160 µL of 2% HCl and 10 min incubation, the absorbance was read at 320 nm using a UV–VIS microplate reader. The results were derived from a calibration curve of rosmarinic acid (0–500 µg/mL) prepared from a stock solution (1 mg/mL in ethanol). Values are expressed as mg of rosmarinic acid equivalents (RAE) per gram of dried weight extract (mg of RAE/g extract).
2.9. HPLC–MS Analysis
High-performance liquid chromatography coupled with tandem mass spectrometry (HPLC–MS/MS) was performed using an Agilent 1100 HPLC-MSD Ion Trap XCT system, equipped with an electrospray ion source (HPLC-ESI-MS) (Agilent Technologies, Santa Clara, CA, USA). Separation of extracts was performed on a Jupiter C18 column 1 × 150 mm with 3.5 μm particle size (Phenomenex, Torrance, CA, USA). As eluents, we used water (eluent A) and MeOH (eluent B), both added with 0.1% formic acid. The gradient employed was 15% eluent B for 5 min, linear to 100% eluent B in 35 min, and finally hold at 100% eluent B for another 5 min. The flow rate was set to 50 μL/min with a column temperature of 30 °C. The injection volume was 8 μL. Ions were detected in the positive and negative ion mode, in the m/z 100–800 range, and ion charged control with a target ion value of 100,000 and an accumulation time of 300 ms. A capillary voltage of 3300 V, nebulizer pressure of 20 psi, drying gas of 8 L/min, dry temperature of 325 °C, and 2 rolling averages (averages: 5) were the parameters set for the MS detection. MS/MS analysis was conducted using an amplitude optimized time by time for each compound. From the chromatograms, the percentage of PC for each extract was calculated on the basis of the peak area.
2.10. Radical Scavenging Activity Assays
The radical scavenging activity was measured using the 1,1-diphenyl-2-picrylhydrazyl (DPPH) method [26]. In a 96-multiwell plate, 50 µL aliquot of sample (0–2 mg/mL) or of the standard Trolox (0–100 mg/mL) was added to 200 µL of DPPH solution (0.1 mM in methanol). After incubation in darkness for 30 min at 37 °C, the absorbance was measured at 490 nm using a UV–VIS microplate reader against DPPH solution as a blank. Values are expressed as half maximal inhibitory concentration IC50 (µg/mL) and Trolox equivalent (µg TE/mg dry extract).
The radical cation scavenging activity of each extract was measured using the 2-2′-azino-bis (3-ethylbenzo-thiazoline-6-sulphonate) diammonium salt (ABTS) method [27]. In a 96-multiwell plate, 50 µL aliquot of sample (0–2 mg/mL) was added to 200 µL of ABTS solution (5 mM). ATBS solution was prepared by oxidizing ABTS with MnO2 in distilled water for 30 min in the dark, and then the solution was filtered through filter paper. After 20 min incubation in darkness at room temperature, the absorbance was determined at 734 nm using a UV–VIS microplate reader against ABTS solution as a blank. Values are expressed as half maximal inhibitory concentration IC50 (µg/mL) and Trolox equivalent (µg TE/mg dry extract).
2.11. Ferric Reducing Antioxidant Power (FRAP) Assay
The reducing power was evaluated according to the ferric reducing antioxidant power (FRAP) assay [28]. In a 96-multiwell plate, 25 µL aliquot of sample (0–2 mg/mL) or of standard Trolox (0–100 µg/mL) was added to 175 µL of FRAP working solution containing 300 mmol/L acetate buffer (pH 3.6), 20 mmol/L ferric chloride, and 10 mmol/L TPTZ (2,4,6-tri (2-pyridyl)—S-triazine) made up in 40 mmol/L HCl. The three solutions were mixed at a 10:1:1 ratio (v:v:v). The mixture was incubated in darkness for 30 min at 37 °C and then the absorbance was determined at 593 using a UV–VIS microplate reader against FRAP solution as a blank. Values are expressed as Trolox equivalent (µg TE/mg dry extract).
2.12. Cell Culture
The human cancer cell lines MDA-MB-231 (breast adenocarcinoma), A375 (Melanoma), and HCT116 (colorectal carcinoma) were gently supplied from Prof. Bramucci (Laboratory of Physiology, University of Camerino). The cancer cells were routinely maintained in Dulbecco’s modified Eagle’s minimum essential medium (DMEM) or in RPMI-1640 (Sigma-Aldrich, Beirut, Lebanon) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mM glutamine, and 1% P/S at 37 °C in a humidified incubator containing 5% CO2.
2.13. Cell Proliferation Assay
The cytotoxicity of T. spicata extracts was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method [29]. Stock solution (50 mg/mL) of extracts were prepared in dimethyl sulfoxide (DMSO) or in sterile distilled water. In addition, the pure Carvacrol was used as positive control. Briefly, cells were seeded in a 96-well plate (104 cells per well), and after 24 h, they were treated with increasing concentrations (0, 50, 100, and 200 µg/mL) of each extract for 24 h. At the end, 20 µL of MTT reagent (5.0 mg/mL) was added, and the mixture was incubated for 3 h at 37 °C. After removing the unreacted MTT dye, 100 µL DMSO was added to solubilize purple formazan crystals, and the absorbance was recorded at 570 nm. The IC50 value (concentration that causes 50% growth inhibition) was estimated as that leading to 50% absorbance decrease as compared to the control. Cell viability was expressed in percentage with respect to the control.
2.14. Quantification of ROS Production
2′,7′-Dichlorodihydrofluorescein diacetate (H2DCF-DA; molecular probe) was employed to assess ROS generation [30]. Briefly, cells were seeded on a 96-well plate (105 cells/mL) and incubated overnight. After the treatments, cells were washed twice with PBS and then incubated with 10 µM of H2DCF-DA (in PBS) for 30 min at 37 °C. Then, ROS production level was measured fluorometrically using a microplate reader (lex = 495 nm; lem = 525 nm).
2.15. Quantification of Nitrite/Nitrate Levels
The nitric oxide NOx (nitrites and nitrates) level was measured by spectrophotometric measurement using the Griess reaction [31]. Briefly, 105 cells/mL were seeded on a 96-well plate and incubated overnight. After the treatments, NOx level in the medium was calculated using NaNO2 as a standard curve. Spectrophotometric analyses were performed at 546 nm using a microplate reader.
2.16. Statistical Analysis
All results were expressed as mean ± SD of at least three independent experiments. GraphPad Prism 8.0.1 software was used for statistical evaluation. Comparisons between different conditions were performed using ANOVA with Tukey’s post-test. Difference between percentages was calculated by chi-squared test. The possible correlation between the measured parameters was tested by a two-tailed Pearson’s correlation coefficient analysis. All statistical analysis were performed by GraphPad Software Prism 8.0.1, Inc. (San Diego, CA, USA).
3. Results
3.1. Characterization of T. spicata Extracts before and after Simulated Digestion
We characterized the aqueous and ethanolic extracts from T. spicata aerial parts before (TW and TE) and after (TW-dig and TE-dig) the simulated digestion. For TW, we also assessed the low (<3.5 kDa) and the high (>3.5 kDa) mw fractions obtained by dialysis.
The aqueous and ethanolic crude extracts exhibited a similar content of carbohydrates. The simulated digestion significantly reduced the TCC in the ethanolic extract (from 26.4 in TE to 19.2 µg/mg in TE-dig), without affecting the aqueous extract. However, upon simulated digestion, we observed a different distribution of carbohydrates between the two fractions: TCC was reduced in the high mw fraction (from 40.4 in TW to 17.6 µg/mg in TW-dig) and increased in the low mw fraction (from 25.2 in TW to 33 µg/mg in TW-dig) (Figure 2A). Moreover, the protein content was roughly similar in the crude extracts, and the simulated digestion did not affect it considerably. However, the aqueous extract showed a redistribution of the protein content between the two mw fractions, leading to a TPrC reduction in the high mw fraction (from 10.8 in TW to 2.4 µg/g in TW-dig) and an increase in the low mw fraction (from 22.7 in TW to 33.9 µg/g in TW-dig) (Figure 2B).

Figure 2.
Quantification of total carbohydrate contents (TCC) (A), total protein content (TPrC) (B), total phenol content (TPC) (C), total flavonoid content (TFC) (D), and total hydroxycinnamic acid content (THAC) (E) of TW and TE, and dialyzed fractions before and after simulated in vitro digestion. All the contents were quantified spectrophotometrically and expressed as µg/mg of the dry extract, µg/g of dry extract, mg of gallic acid equivalent per g of dry powder extract (mg GAE/g dry extract), mg of quercetin equivalent per g of dry powder extract (mg QE/g dry extract), and mg of rosmarinic acid equivalents (RAE) per gram of dried weight extract (mg of RAE/g extract), respectively. Samples were measured in triplicate, and significant differences between digested and undigested extracts are denoted by symbols: * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001.
As expected, the ethanolic extract was richer in phenolic compounds compared to the aqueous one (353 vs. 201.4 mg GAE/g). After simulated digestion, the TPC significantly decreased in both the ethanolic (250 mg GAE/g) and the aqueous (138.9 mg GAE/g) extracts. For the aqueous extract, the simulated digestion reduced the TPC in the high mw fraction (from 148.7 to 81.6 mg GAE/g). By contrast, the aqueous extract was richer in flavonoids than the ethanolic extract (172.88 vs. 123.84 mg QE/g).
After simulated digestion, the TFC significantly decreased in both the ethanolic (to 85.18 mg QE/g) and aqueous (to 111.26 mg QE/g) extracts, as well as in both the high (from 117.03 to 25.75 mg QE/g) and low mw fractions (from 278.13 to 236.24 mg QE/g) (Figure 2D). The hydroxycinnamic acid content was higher in the ethanolic than in the aqueous extract (89.2 vs. 35.4 mg RAE/g, respectively). The simulated digestion reduced the THAC in the crude ethanolic extract (76.5 mg RAE/g in TE-dig), while in the aqueous extract, the digestion redistributed the THAC between the two mw fractions (from 101.6 to 95.2 mg RAE/g for low mw fraction, and from 31.4 to 17.8 mg RAE/g in the high mw fraction) (Figure 2E).
3.2. HPLC–MS Characterization of the Phenolic Compounds
Both the extracts were characterized by HPLC–MS/MS analysis before and after digestion (Figure 3). In the ethanolic extract, we detected 14 PCs in both the undigested and digested preparations. The most abundant PCs were monoterpenoic phenols (carvacrol), polyphenolic acids (rosmarinic acid), flavonoids, and their derivatives (rutin, thymusin, and eriodictyol derivative, etc.). The aqueous extract contained less PCs; 19 PCs were detected in both the undigested and digested preparations, which can be classified into three main groups: phenolic acids, phenolic monoterpenoids, and flavonoids. Carvacrol is the most abundant PC in the ethanolic extract (34.8% in TE and 52.9% in TE-dig), rosmarinic acid in TW (57.4%), and salvalonic acid in TW-dig (42.3%).

Figure 3.
HPLC–UV chromatographic profiles for both ethanolic and aqueous extracts of Thymbra spicata before and after digestion wherein their pure polyphenols were recorded at 280 nm: (A) Chromatogram of the ethanolic extract (TE and TE-dig) showing the following peaks: 1: carvacrol; 2: thymusin; 3: rosmarinic acid; 4: eriodictyol. (B) Chromatogram of the aqueous extract (TW and TW-dig) showing the following peaks: 1: salvalonic acid I; 2: rosmarinic acid; 3: carvacrol; 4: vicenin; 5: rutin.
To compare the chromatograms of the two extracts, we normalized them for their TPC. The analysis revealed some differences in the percentages of the major PCs (Table 2). The simulated digestion led to an enrichment in carvacrol abundance in the ethanolic extract (from 34.8 to 52.9%; p ≤ 0.01) and to a reduction in rosmarinic acid abundance in the aqueous extract (from 57.4% to 18.8%; p ≤ 0.01). In TW, the reduction in rosmarinic acid was almost balanced by the increase in salvalonic acid (from 19.5% to 42.3%; p ≤ 0.01). Moreover, a redistribution in phenols, flavonoids, and hydroxycinnamic acids was observed between the two mw fractions, with the low mw fraction being enriched in both TPC (from 1.53 to 2.06) and TFC (from 1.61 to 2.12), balanced by a reduction in the high mw fraction of TFC (from 0.68 to 0.23) and THAC (from 0.89 to 0.54) (Figure 4).

Table 2.
Phenolic compounds identified in TE (A) and TW (B) before and after digestion using HPLC–MS/MS in the negative ionization mode.
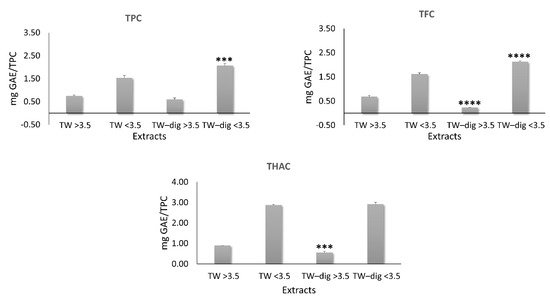
Figure 4.
Normalized total phenol content (TPC), total flavonoid content (TFC), and total hydroxycinnamic acid content (THAC) of high and low mw fractions of the aqueous extract by the TPC of the corresponding extracts. Significant differences between digested and undigested extracts are denoted by symbols: *** p < 0.001 and **** p < 0.0001.
3.3. Effect of Simulated Digestion on Antioxidant Proprieties
The antioxidant potential of each extract before and after the simulated digestion was evaluated by three different spectrophotometric assays. A higher antioxidant activity was observed for the ethanolic extract compared to the aqueous one. Both extracts showed significant changes in the antioxidant potentials upon digestion, as well as between the mw fractions obtained by dialysis before and after digestion (Table 3A).

Table 3.
(A) The radical scavenging activity of the T. spicata extracts before and after digestion. Values are reported as Trolox equivalent (µg TE/mg dry extract). (B) Pearson correlation (two-tailed) between TFC, THAC, TPC, and antioxidant parameters (DPPH, ABTS, and FRAP). All values are mean ± SD from at least three independent experiments. Samples were measured in triplicate for each experiment. Significance is denoted by symbols: * p < 0.05, ** p < 0.01, and *** p < 0.001.
After normalizing the Trolox equivalent values for the TPC in each extract, we appreciated a higher antioxidant activity for the digested extracts than for the crude ones. Briefly, in the ethanolic extract, DPPH increased from 0.25 (TE) to 0.37 (TE-dig), and in the aqueous extract from 0.44 (TW) to 0.60 (TW-dig).
Regarding the FRAP assay, ethanolic extract showed an increase in the reducing capacity after digestion from 0.26 (TE) to 0.35 (TE-dig). Conversely, the ABTS assay of the aqueous extract showed a reduction in the high mw fraction from 3.06 (TW-dig > 3.5 kDa) to 1.68 (TW-dig > 3.5 kDa) (Table 3A).
Finally, the correlation analysis between the phytochemical contents (in terms of TPC, THAC, and TFC) and the three antioxidant activities (evaluated as DPPH, ABTS, and FRAP) for all the extracts showed a significant and strong correlation between DPPH and TPC (r2 = 0.7612). Moreover, a good correlation was calculated between FRAP and the phytochemical contents: FRAP and TFC (r2 = 0.8952), FRAP and THAC (r2 = 0.8913), and FRAP and TPC (r2 = 0.7755) (Table 3B). These results indicate that the phenolic compounds contained in the extracts are the major contributor for the antioxidant capacity.
3.4. In Vivo Effects: Cytotoxic Activity and Oxidative Stress in Cancer Cells
The anti-proliferative effects of the different extracts before and after digestion were assessed using three human cancer cell lines representative of the most common human cancers, i.e., MDA-MB 231, HCT116, and A375 cells (Figure 5). No significant cytotoxic effects were observed for the aqueous extract (data not shown). By contrast, all the ethanolic extracts, both undigested and digested, significantly reduced the cell viability of all cancer cell lines in a concentration-dependent manner. Carvacrol is the most abundant component of the ethanolic extract, and it was employed as a positive control. After 24 h of treatment at the highest concentration (200 µg/mL), both the extracts (TE and TE-dig) and carvacrol dramatically reduced the cell viability in MDA-MB 231 (to 20.1%, 28.8%, and 17.9%; respectively), in HCT116 cells (to 22.3%, 41.1%, and 7.8%, respectively), and in A375 cells (to 38.2%, 26%, and 21.6%, respectively). From the IC50 values listed in Table 4, we can deduce that carvacrol is the most cytotoxic agent for A375 cells at 24h (IC50 of about 19.912 µg/mL), as well as for MDA-MB 231 cells (IC50 about 23.278 µg/mL) and HCT 116 cells (IC50 about 59.625 µg/mL), and other intermediate inhibitory effects have been exhibited on the other cell lines.
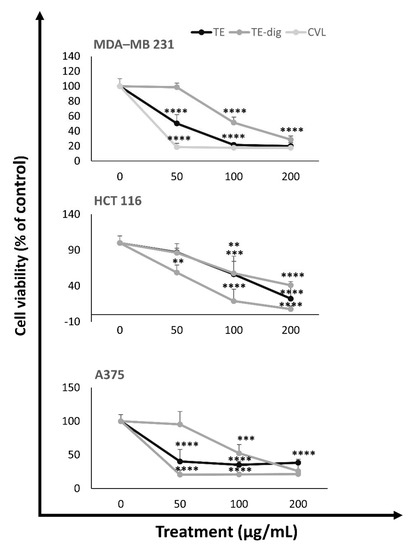
Figure 5.
Antiproliferative activity of TE, TE-dig, and CVL on three representative human cancer cell lines: breast (MDA-MB 231), colon (HCT 116), and melanoma (A375) cells. The cell viability was expressed as a percentage (%) with respect to the control. Data represent the mean of at least five independent experiments. Statistical analysis for cell viability data was performed using two-way ANOVA followed by Tukey’s post-test ** p < 0.01, *** p < 0.001, **** p < 0.0001).

Table 4.
IC50 values (50% cell viability inhibitory concentration) determined for T. spicata ethanolic extracts (TE and TE dig) and Carvacrol (CVL) in the three cell lines over a long-term (24 h) period under analysis.
In attempt to decipher the mechanisms sustaining the cytotoxic effects, we assessed the oxygen and nitrogen radical production. A dose-dependent increase in ROS production was detected in the breast cancer cell line MDA-MD 231 treated with either the crude or digested extracts. At the highest concentration (200 µg/mL), ROS production increased to +158.1% for TE-dig and +154.1% for TE, but carvacrol was more efficient in inducing ROS production (+238.8%). Similar results were recorded for the colon cancer cell line.
On HCT116, carvacrol stimulated ROS production (+253.8%) more than the crude and digested extracts (TE +151.9% and TE-dig +163.6%) at the highest concentration. Moreover, for the melanoma cancer cell A375, carvacrol stimulated ROS production (+293.4%) more than the crude and digested extracts (+191.7% for TE-dig and +142.7% for TE) at the highest concentration (Figure 6A).
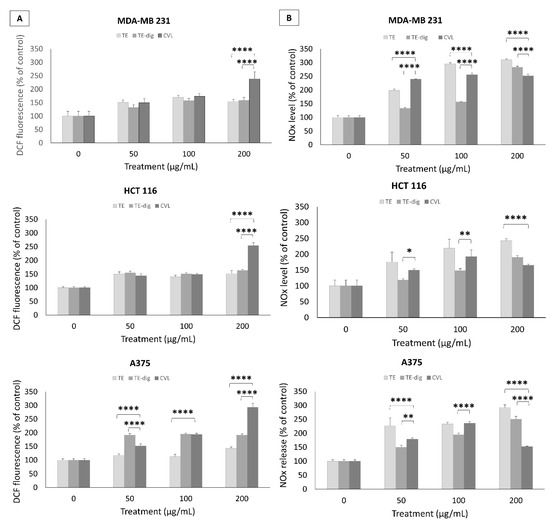
Figure 6.
Pro-apoptotic effects of TE, TE-dig, and CVL on three cell lines: MDA-MB 231, HCT116, and A375, were assessed by measuring the ROS (A) and NO production (B) using spectrophotometric and fluorometric analyses, respectively. Values are expressed as % of control. Data represent the mean of five independent experiments. Statistical analysis for cell viability data was performed using two-way ANOVA followed by Tukey’s post-test (* p < 0.05, ** p < 0.01, **** p < 0.001).
An opposite trend was observed in terms of NO release. In this case, the extracts, at the highest concentration (200 µg/mL), were stronger than carvacrol in triggering the NO release in MDA-MB231 (+283.% for TE-dig, +310.4% for TE, and +251.6% for CVL), as well as in HCT116 and A375 cells, where the release was maximum for TE (+242.7% and +292.6%, respectively) followed by TE-dig (+190.4% and +250.4%, respectively) and by CVL (+165.3% and +151.7%, respectively) in the three cell lines (Figure 6B).
4. Discussion
Although many studies emphasized the uncountable positive effects on human health of the phenolic compounds contained in certain edible or medicinal plants, only a few reports have investigated the possible influence of the gastrointestinal digestion on their efficacy. The main finding of our study using a simulated in vitro GI digestion is that the digestion boosters the antioxidant activity of T. spicata extracts, while it reduces the antiproliferative potential. We may attribute these differences in the biological activity of the extracts to the modifications in the phenolic profile caused by the simulated digestion.
To our knowledge, no studies have previously documented the possible changes in bioactivity of T. spicata extracts or preparations after transit in the gastrointestinal tract. In this context, we assessed the content of the main macromolecules and the phenolome in ethanolic and aqueous extracts from T. spicata leaves, and for the aqueous extract, we also distinguished between the low and high mw fractions.
Regarding the ethanolic extract, the simulated digestion led to a significant decrease in almost all components, while in the aqueous extract, it led to a reduction of only phenols and flavonoids. Although the simulated digestion reduced the carvacrol content in the ethanolic extract in absolute terms, carvacrol became the most abundant PC in relative terms. On the other hand, in the aqueous extract, the simulated digestion significantly increased the content of salvalonic acid, which became the most abundant PC in relative terms (Table 2). The increase in salvalonic acid content likely depends on the reduction in the RA upon digestion, taking into consideration the fact that salvalonic acid derives form condensation of two units of RAs, and this compound appears to be the precursor to many related salvianolic acid derivatives [32]. Therefore, we could hypothesize that the reduction in the rosmarinic acid (RA) upon digestion had been balanced by the increase in the SA.
We wish to emphasize as the biotransformation of parental phenolic compounds during the digestive process could mainly depend on the enzymes and the physiological environment of the GI tract (pH, temperature, and electrolytes) [33]. Indeed, Karas et al. [34] suggested that about 10% of the PCs remain undigested in the plant matrix, with only 90% of them being digested and released. However, the effects of digestion might vary according to the plant materials, and in the literature, we found two different outcomes: one stating the increase in phenolic compounds upon digestion [35], and the other one showing a reduction [36]. Indeed, our data are in accordance with reports showing a reduction such as those showing a reduced PC content in Brassica oleracea [37], as well as in Chilean white strawberry [38] upon digestion.
The idea is that the GI digestion may be unable to release all PCs, leaving a considerable amount of non-extractable polyphenols (NEPs) being trapped by dietary fibers, macromolecules (i.e., proteins), or polysaccharides through hydrophobic, hydrogen, and covalent bonds [39]. Therefore, NEPs reach the colon nearly intact [40]; however, only the phenolic components released from the matrix are absorbable from the GI barriers, and this could explain the enrichment in the secondary metabolites that we observed in the low mw fractions (mainly carbohydrates and proteins). NEPs may be released from the food matrix in the colon by the action of microbiota thus becoming bioavailable, absorbed, and bioactive [41], and this point specifically will inform our upcoming investigations. In conclusion, our findings indicate that the effect of digestion was greater on the ethanolic extract, and this was likely to due to degrees of solubility of different phyto-constituents.
In a previous paper [15], we demonstrated the great antioxidative potential of the T. spicata ethanolic extract, being higher than the aqueous one. Interestingly, the antioxidant potential of both the ethanolic and aqueous extracts was boosted by the simulated digestion. This could be related to the noticeable enrichment of each extract in terms of bioactive compounds, namely, carvacrol in the digested ethanolic extract, and salvalonic acid in the aqueous digested extract according to previous scientific reports [42].
Although polyphenols are generally considered as antioxidant compounds, at very high concentrations, they are known to play a prooxidant effect that might promote apoptosis, especially in highly proliferative cells such as cancer cells [43,44]. A previous study of our group reported remarkable antiproliferative and pro-apoptotic effects on tumor cell lines for the ethanolic extract from T. spicata when tested at a rather high concentration (100 mg/mL) [17]. In the present study, we verified that the in vitro cytotoxic activity of the ethanolic extract on cancer cell lines was maintained after the simulated digestion, but with lower efficacy compared with the crude extract. As widely reported, the antiproliferative activity of a plant is firmly correlated with the PCs [45]. Accordingly, we observed a decreased antiproliferative potency for the digested extract compared to the crude one, and this result parallels well with the reduced PC content upon digestion, in particular the reduction in carvacrol, which is the most potent antiproliferative agent in our study.
We evaluated the free radical production to clarify the mechanisms sustaining the anti-proliferative activity of the ethanolic extract before and after digestion. The results of the present study showed that the pro-oxidant property of the ethanolic extract was not only maintained after the digestion, but it was even bigger in terms of ROS production when compared to TE-dig. On the other hand, the NO release was higher in the crude extract compared to the digested one.
Interestingly, we observed that both the crude and digested ethanolic extracts were less potent in ROS production compared to carvacrol, while in terms of NO release, the extracts were more potent than carvacrol. This can indicate that the antiproliferative potential of T. spicata is exerted by acting as ROS-mediating apoptosis and inducing the release of cytotoxic mediators. Nevertheless, further studies should be performed at this level to have a clear idea about the mechanistic mode of action.
5. Conclusions
In summary, although we observed a reduction in the PCs and modulation in the phenolome of both ethanolic and aqueous T. spicata extracts upon the simulated GI digestion, the antioxidant activity was significantly enhanced. However, the antiproliferative potential of the ethanolic extract was reduced. Accordingly, we can come to an assumption that the digestion process had an impact on the nutritional value of T. spicata, but it kept its biological effectiveness.
As a final word, we can confidently say that T. spicata can represent a good and considerable source of PCs with potent antioxidant and antiproliferative bioactivities. In particular, the detected panel of bioactive compounds in T. spicata makes this edible plant a potent candidate to be used as a dietary supplement for different therapeutic purposes.
Author Contributions
All authors contributed to this work significantly. F.D. contributed to the spectrophotometric analyses, performed the statistical analysis, wrote the original draft, and edited the manuscript. M.K. carried out the cell culture and treatments, as well as the spectrophotometric experiments, and participated in the writing of the manuscript. G.L. contributed to setting and carrying on the in vitro digestion study. H.Z. carried out the digestion experiments. A.S. and G.D. performed HPLC–MS/MS analyses and elaborated data. M.B. participated in the in vitro assays. P.P. participated in conceiving and designing the study. L.V. conceived the focus of the paper, designed the experimental activity, and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the University of Genova (FRA 2020 and 2021).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Datas are contained within the articles.
Acknowledgments
Special thanks to Luana Quassinti, Laboratory of Physiology-School of Pharmacy (University of Camerino) for extract preparation, and to Hala Khalifeh, Department of Biology, Laboratory Rammal Rammal (ATAC, Lebanese University), for providing the plant materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dillard, C.J.; German, J.B. Phytochemicals: Nutraceuticals and Human Health. J. Sci. Food Agric. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- Bagherniya, M.; Nobili, V.; Blesso, C.N.; Sahebkar, A. Medicinal Plants and Bioactive Natural Compounds in the Treatment of Non-Alcoholic Fatty Liver Disease: A Clinical Review. Pharmacol. Res. 2018, 130, 213–240. [Google Scholar] [CrossRef] [PubMed]
- Medina-Remón, A.; Tresserra-Rimbau, A.; Pons, A.; Tur, J.A.; Martorell, M.; Ros, E.; Buil-Cosiales, P.; Sacanella, E.; Covas, M.I.; Corella, D.; et al. Effects of Total Dietary Polyphenols on Plasma Nitric Oxide and Blood Pressure in a High Cardiovascular Risk Cohort. The PREDIMED Randomized Trial. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, Y.; Gao, M.; Bai, X.; Chen, Z. Neuroprotective Effect of Several Phytochemicals and Its Potential Application in the Prevention of Neurodegenerative Diseases. Geriatrics 2016, 1, 29. [Google Scholar] [CrossRef]
- Liu, R.H. Potential Synergy of Phytochemicals in Cancer Prevention: Mechanism of Action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef]
- Khalil, M.; Rita Caponio, G.; Diab, F.; Shanmugam, H.; Di Ciaula, A.; Khalifeh, H.; Vergani, L.; Calasso, M.; De Angelis, M.; Portincasa, P. Unraveling the Beneficial Effects of Herbal Lebanese Mixture “Za’atar”. History, Studies, and Properties of a Potential Healthy Food Ingredient. J. Funct. Foods 2022, 90, 104993. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid Antioxidants: Chemistry, Metabolism and Structure-Activity Relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An Overview of Plant Phenolic Compounds and Their Importance in Human Nutrition and Management of Type 2 Diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef]
- Dupont, D.; Alric, M.; Blanquet-Diot, S.; Bornhorst, G.; Cueva, C.; Deglaire, A.; Denis, S.; Ferrua, M.; Havenaar, R.; Lelieveld, J.; et al. Can Dynamic in Vitro Digestion Systems Mimic the Physiological Reality? Crit. Rev. Food Sci. Nutr. 2019, 59, 1546–1562. [Google Scholar] [CrossRef]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the Encapsulation in Bioavailability of Phenolic Compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical Methods for Determining Bioavailability and Bioaccessibility of Bioactive Compounds from Fruits and Vegetables: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef]
- Stanisavljević, N.; Samardžić, J.; Janković, T.; Šavikin, K.; Mojsin, M.; Topalović, V.; Stevanović, M. Antioxidant and Antiproliferative Activity of Chokeberry Juice Phenolics during in Vitro Simulated Digestion in the Presence of Food Matrix. Food Chem. 2015, 175, 516–522. [Google Scholar] [CrossRef]
- Ge, H.; Chen, Y.; Chen, J.; Tian, J.; Liang, X.; Chen, L. Evaluation of Antioxidant Activities of Ethanol Extract from Ligusticum Subjected to in-Vitro Gastrointestinal Digestion. Food Chem. Toxicol. 2018, 119, 417–424. [Google Scholar] [CrossRef]
- Khoury, M.; Stien, D.; Eparvier, V.; Ouaini, N.; El Beyrouthy, M. Report on the Medicinal Use of Eleven Lamiaceae Species in Lebanon and Rationalization of Their Antimicrobial Potential by Examination of the Chemical Composition and Antimicrobial Activity of Their Essential Oils. Evid. Based Complementary Altern. Med. 2016, 2016, 1–17. [Google Scholar] [CrossRef]
- Khalil, M.; Khalifeh, H.; Baldini, F.; Salis, A.; Damonte, G.; Daher, A.; Voci, A.; Vergani, L. Antisteatotic and Antioxidant Activities of Thymbra Spicata L. Extracts in Hepatic and Endothelial Cells as in Vitro Models of Non-Alcoholic Fatty Liver Disease. J. Ethnopharmacol. 2019, 239, 111919. [Google Scholar] [CrossRef]
- Cam, M.E.; Hazar-Yavuz, A.N.; Yildiz, S.; Ertas, B.; Ayaz Adakul, B.; Taskin, T.; Alan, S.; Kabasakal, L. The Methanolic Extract of Thymus Praecox Subsp. Skorpilii Var. Skorpilii Restores Glucose Homeostasis, Ameliorates Insulin Resistance and Improves Pancreatic β-Cell Function on Streptozotocin/Nicotinamide-Induced Type 2 Diabetic Rats. J. Ethnopharmacol. 2019, 231, 29–38. [Google Scholar] [CrossRef]
- Khalil, M.; Khalifeh, H.; Baldini, F.; Serale, N.; Parodi, A.; Voci, A.; Vergani, L.; Daher, A. Antitumor Activity of Ethanolic Extract from Thymbra Spicata L. Aerial Parts: Effects on Cell Viability and Proliferation, Apoptosis Induction, STAT3, and NF-KB Signaling. Nutr. Cancer 2021, 73, 1193–1206. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Bachmayer, O.; Kosar, M.; Hiltunen, R. Antioxidant Properties of Aqueous Extracts from Selected Lamiaceae Species Grown in Turkey. J. Agric. Food Chem. 2004, 52, 762–770. [Google Scholar] [CrossRef]
- Hanci, S.; Sahin, S.; Yılmaz, L. Isolation of Volatile Oil from Thyme (Thymbra Spicata) by Steam Distillation. Nahrung/Food 2003, 47, 252–255. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static in Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Larsen, B.; Salem, D.M.S.A.; Sallam, M.A.E.; Mishrikey, M.M.; Beltagy, A.I. Characterization of the Alginates from Algae Harvested at the Egyptian Red Sea Coast. Carbohydr. Res. 2003, 338, 2325–2336. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Silva, D.B.; Turatti, I.C.C.; Gouveia, D.R.; Ernst, M.; Teixeira, S.P.; Lopes, N.P. Mass Spectrometry of Flavonoid Vicenin-2, Based Sunlight Barriers in Lychnophora Species. Sci. Rep. 2015, 4, 4309. [Google Scholar] [CrossRef]
- Arvouet-Grand, A.; Vennat, B.; Pourrat, A.; Legret, P. Standardization of Propolis Extract and Identification of Principal Constituents. J. Pharm. Belg. 1994, 49, 462–468. [Google Scholar]
- Custódio, L.; Silvestre, L.; Rocha, M.I.; Rodrigues, M.J.; Vizetto-Duarte, C.; Pereira, H.; Barreira, L.; Varela, J. Methanol Extracts from Cystoseira Tamariscifolia and Cystoseira Nodicaulis Are Able to Inhibit Cholinesterases and Protect a Human Dopaminergic Cell Line from Hydrogen Peroxide-Induced Cytotoxicity. Pharm. Biol. 2016, 54, 1687–1696. [Google Scholar] [CrossRef]
- Srinivasan, R.; Chandrasekar, M.J.N.; Nanjan, M.J.; Suresh, B. Antioxidant Activity of Caesalpinia Digyna Root. J. Ethnopharmacol. 2007, 113, 284–291. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Firuzi, O.; Lacanna, A.; Petrucci, R.; Marrosu, G.; Saso, L. Evaluation of the Antioxidant Activity of Flavonoids by “Ferric Reducing Antioxidant Power” Assay and Cyclic Voltammetry. Biochim. Biophys. Acta-Gen. Subj. 2005, 1721, 174–184. [Google Scholar] [CrossRef]
- Hussain, R.F.; Nouri, A.M.E.; Oliver, R.T.D. A New Approach for Measurement of Cytotoxicity Using Colorimetric Assay. J. Immunol. Methods 1993, 160, 89–96. [Google Scholar] [CrossRef]
- Halliwell, B.; Whiteman, M. Measuring Reactive Species and Oxidative Damage in Vivo and in Cell Culture: How Should You Do It and What Do the Results Mean? Br. J. Pharmacol. 2004, 142, 231–255. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of Nitrate, Nitrite, and [15N]Nitrate in Biological Fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Habtemariam, S. Molecular Pharmacology of Rosmarinic and Salvianolic Acids: Potential Seeds for Alzheimer’s and Vascular Dementia Drugs. Int. J. Mol. Sci. 2018, 19, 458. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Chang, X.; Guo, X.; Brennan, C.S.; Li, T.; Fu, X.; Liu, R.H. Phenolic Compounds, Antioxidant Activity, Antiproliferative Activity and Bioaccessibility of Sea Buckthorn (Hippophaë Rhamnoides L.) Berries as Affected by in Vitro Digestion. Food Funct. 2017, 8, 4229–4240. [Google Scholar] [CrossRef]
- Karaś, M.; Jakubczyk, A.; Szymanowska, U.; Złotek, U.; Zielińska, E. Digestion and Bioavailability of Bioactive Phytochemicals. Int. J. Food Sci. Technol. 2017, 52, 291–305. [Google Scholar] [CrossRef]
- Celep, E.; İnan, Y.; Akyüz, S.; Yesilada, E. The Bioaccessible Phenolic Profile and Antioxidant Potential of Hypericum Perfoliatum L. after Simulated Human Digestion. Ind. Crops Prod. 2017, 109, 717–723. [Google Scholar] [CrossRef]
- İnan, Y.; Kurt-Celep, I.; Akyüz, S.; Barak, T.H.; Celep, E.; Yesilada, E. An Investigation on the Enzyme Inhibitory Activities, Phenolic Profile and Antioxidant Potentials of Salvia Virgata Jacq. S. Afr. J. Bot. 2021, 143, 350–358. [Google Scholar] [CrossRef]
- Scrob, T.; Hosu, A.; Cimpoiu, C. The Influence of in Vitro Gastrointestinal Digestion of Brassica Oleracea Florets on the Antioxidant Activity and Chlorophyll, Carotenoid and Phenolic Content. Antioxidants 2019, 8, 212. [Google Scholar] [CrossRef]
- Thomas-Valdés, S.; Theoduloz, C.; Jiménez-Aspee, F.; Burgos-Edwards, A.; Schmeda-Hirschmann, G. Changes in Polyphenol Composition and Bioactivity of the Native Chilean White Strawberry (Fragaria Chiloensis Spp. Chiloensis f. Chiloensis) after in Vitro Gastrointestinal Digestion. Food Res. Int. 2018, 105, 10–18. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Díaz-Rubio, M.E.; Saura-Calixto, F. Non-Extractable Polyphenols, a Major Dietary Antioxidant: Occurrence, Metabolic Fate and Health Effects. Nutr. Res. Rev. 2013, 26, 118–129. [Google Scholar] [CrossRef]
- Saura-Calixto, F.; Serrano, J.; Goñi, I. Intake and Bioaccessibility of Total Polyphenols in a Whole Diet. Food Chem. 2007, 101, 492–501. [Google Scholar] [CrossRef]
- Ketnawa, S.; Reginio, F.C.; Thuengtung, S.; Ogawa, Y. Changes in Bioactive Compounds and Antioxidant Activity of Plant-Based Foods by Gastrointestinal Digestion: A Review. Crit. Rev. Food Sci. Nutr. 2021, 62, 4684–4705. [Google Scholar] [CrossRef]
- Xiao, Z.; Liu, W.; Mu, Y.; Zhang, H.; Wang, X.; Zhao, C.; Chen, J.; Liu, P. Pharmacological Effects of Salvianolic Acid B Against Oxidative Damage. Front. Pharmacol. 2020, 11, 572373. [Google Scholar] [CrossRef]
- Wen, L.; Zhao, Y.; Jiang, Y.; Yu, L.; Zeng, X.; Yang, J.; Tian, M.; Liu, H.; Yang, B. Identification of a Flavonoid C -Glycoside as Potent Antioxidant. Free Radic. Biol. Med. 2017, 110, 92–101. [Google Scholar] [CrossRef]
- Bacchetti, T.; Morresi, C.; Bellachioma, L.; Ferretti, G. Antioxidant and Pro-Oxidant Properties of Carthamus Tinctorius, Hydroxy Safflor Yellow A, and Safflor Yellow A. Antioxidants 2020, 9, 119. [Google Scholar] [CrossRef]
- Xia, W.; Lin, Y.; Gong, E.; Li, T.; Lian, F.; Zheng, B.; Liu, R. Wild Pink Bayberry Fruit: The Effect of in Vitro Gastrointestinal Digestion on Phytochemical Profiles, and Antioxidant and Antiproliferative Activities. Food Funct. 2021, 12, 2126–2136. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).