Dietary EVOO Polyphenols and Gut Microbiota Interaction: Are There Any Sex/Gender Influences?
Abstract
:1. Introduction
2. EVOO Composition and Characteristics
2.1. EVOO Polyphenols
2.2. EVOO Polyphenols and Health
2.3. Biological Properties and Mechanisms of Action of Main EVOO Polyphenols in Preclinical Models
2.3.1. Hydroxytyrosol
2.3.2. Oleuropein
2.3.3. Tyrosol
2.3.4. Oleochantal
3. Gut and Sexual Dimorphism
4. Bioavailability of Polyphenols and the Two-Way Interaction with Gut Microbiota
4.1. Gut Microbiota Metabolizes EVOO Dietary Polyphenols
4.2. EVOO Dietary Polyphenols Modify Gut Microbiota
5. EVOO Beneficial Effects on Gut Microbiota: Possible Differences between Sexes?
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- EFSA Panel on Dietetic Products. Panel Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage. EFSA J. 2011, 9, 2033. [Google Scholar] [CrossRef]
- Finicelli, M.; Squillaro, T.; Galderisi, U.; Peluso, G. Polyphenols, the Healthy Brand of Olive Oil: Insights and Perspectives. Nutrients 2021, 13, 3831. [Google Scholar] [CrossRef] [PubMed]
- Martin-Pelaez, S.; Castaner, O.; Sola, R.; Motilva, M.J.; Castell, M.; Perez-Cano, F.J.; Fito, M. Influence of Phenol-Enriched Olive Oils on Human Intestinal Immune Function. Nutrients 2016, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Cao, Z.; Li, W.; Li, H.; Lu, C.; Yang, X.; Liu, Y. Gut microbiota as an “invisible organ” that modulates the function of drugs. Biomed. Pharmacother. 2020, 121, 109653. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Millman, J.F.; Okamoto, S.; Teruya, T.; Uema, T.; Ikematsu, S.; Shimabukuro, M.; Masuzaki, H. Extra-virgin olive oil and the gut-brain axis: Influence on gut microbiota, mucosal immunity, and cardiometabolic and cognitive health. Nutr. Rev. 2021, 79, 1362–1374. [Google Scholar] [CrossRef]
- Biskup, E.; Marra, A.M.; Ambrosino, I.; Barbagelata, E.; Basili, S.; de Graaf, J.; Gonzalvez-Gasch, A.; Kaaja, R.; Karlafti, E.; Lotan, D.; et al. Awareness of sex and gender dimensions among physicians: The European federation of internal medicine assessment of gender differences in Europe (EFIM-IMAGINE) survey. Intern. Emerg. Med. 2022, 17, 1395–1404. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Bairey Merz, N.; Barnes, P.J.; Brinton, R.D.; Carrero, J.J.; DeMeo, D.L.; De Vries, G.J.; Epperson, C.N.; Govindan, R.; Klein, S.L.; et al. Sex and gender: Modifiers of health, disease, and medicine. Lancet 2020, 396, 565–582. [Google Scholar] [CrossRef]
- Morand, C.; De Roos, B.; Garcia-Conesa, M.T.; Gibney, E.R.; Landberg, R.; Manach, C.; Milenkovic, D.; Rodriguez-Mateos, A.; Van de Wiele, T.; Tomas-Barberan, F. Why interindividual variation in response to consumption of plant food bioactives matters for future personalised nutrition. Proc. Nutr. Soc. 2020, 79, 225–235. [Google Scholar] [CrossRef]
- Foshati, S.; Ghanizadeh, A.; Akhlaghi, M. The effect of extra virgin olive oil on anthropometric indices, lipid profile, and markers of oxidative stress and inflammation in patients with depression, a double-blind randomised controlled trial. Int. J. Clin. Pract. 2021, 75, e14254. [Google Scholar] [CrossRef] [PubMed]
- Longhi, R.; Santos, A.; Lopez-Yerena, A.; Rodrigues, A.P.S.; Oliveira, C.; Silveira, E.A. The Effectiveness of Extra Virgin Olive Oil and the Traditional Brazilian Diet in Reducing the Inflammatory Profile of Individuals with Severe Obesity: A Randomized Clinical Trial. Nutrients 2021, 13, 4139. [Google Scholar] [CrossRef] [PubMed]
- Podadera-Herreros, A.; Alcala-Diaz, J.F.; Gutierrez-Mariscal, F.M.; Jimenez-Torres, J.; Cruz-Ares, S.; Arenas-de Larriva, A.P.; Cardelo, M.P.; Torres-Pena, J.D.; Luque, R.M.; Ordovas, J.M.; et al. Long-term consumption of a mediterranean diet or a low-fat diet on kidney function in coronary heart disease patients: The CORDIOPREV randomized controlled trial. Clin. Nutr. 2022, 41, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Lopez, I.; Arancibia-Riveros, C.; Casas, R.; Tresserra-Rimbau, A.; Razquin, C.; Martinez-Gonzalez, M.A.; Hu, F.B.; Ros, E.; Fito, M.; Estruch, R.; et al. Changes in plasma total saturated fatty acids and palmitic acid are related to pro-inflammatory molecule IL-6 concentrations after nutritional intervention for one year. Biomed. Pharmacother. 2022, 150, 113028. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, M.A.; Ros, E.; Estruch, R. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 379, 1388–1389. [Google Scholar]
- Jimenez-Lopez, C.; Carpena, M.; Lourenco-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef]
- Ben Salem, R.; Rigane, G.; Arslan, D. Phenolic substances isolated from Olea europaea L.: A Review. J. Appl. Biochem. 2015, 108, 189–213. [Google Scholar]
- Hachicha Hbaieb, R.; Kotti, F.; Garcia-Rodriguez, R.; Gargouri, M.; Sanz, C.; Perez, A.G. Monitoring endogenous enzymes during olive fruit ripening and storage: Correlation with virgin olive oil phenolic profiles. Food Chem. 2015, 174, 240–247. [Google Scholar] [CrossRef]
- Kalua, C.M.; Bedgood, D.R.; Bishop, A.G., Jr.; Prenzler, P.D. Changes in volatile and phenolic compounds with malaxation time and temperature during virgin olive oil production. J. Agric. Food Chem. 2006, 54, 7641–7651. [Google Scholar] [CrossRef]
- Jukic Spika, M.; Perica, S.; Zanetic, M.; Skevin, D. Virgin Olive Oil Phenols, Fatty Acid Composition and Sensory Profile: Can Cultivar Overpower Environmental and Ripening Effect? Antioxidants 2021, 10, 689. [Google Scholar] [CrossRef]
- Criado-Navarro, I.; Lopez-Bascon, M.A.; Priego-Capote, F. Evaluating the Variability in the Phenolic Concentration of Extra Virgin Olive Oil According to the Commission Regulation (EU) 432/2012 Health Claim. J. Agric. Food Chem. 2020, 68, 9070–9080. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Vari, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef] [PubMed]
- Cianciosi, D.; Forbes-Hernandez, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martinez Florez, S.; Agudo Toyos, P.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. Biological Relevance of Extra Virgin Olive Oil Polyphenols Metabolites. Antioxidants 2018, 7, 170. [Google Scholar] [CrossRef]
- Martin-Pelaez, S.; Covas, M.I.; Fito, M.; Kusar, A.; Pravst, I. Health effects of olive oil polyphenols: Recent advances and possibilities for the use of health claims. Mol. Nutr. Food Res. 2013, 57, 760–771. [Google Scholar] [CrossRef] [PubMed]
- Hernaez, A.; Remaley, A.T.; Farras, M.; Fernandez-Castillejo, S.; Subirana, I.; Schroder, H.; Fernandez-Mampel, M.; Munoz-Aguayo, D.; Sampson, M.; Sola, R.; et al. Olive Oil Polyphenols Decrease LDL Concentrations and LDL Atherogenicity in Men in a Randomized Controlled Trial. J. Nutr. 2015, 145, 1692–1697. [Google Scholar] [CrossRef]
- Souza, P.A.L.; Marcadenti, A.; Portal, V.L. Effects of Olive Oil Phenolic Compounds on Inflammation in the Prevention and Treatment of Coronary Artery Disease. Nutrients 2017, 9, 1087. [Google Scholar] [CrossRef]
- Carrasco-Pancorbo, A.; Gomez-Caravaca, A.M.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Fernandez-Gutierrez, A. A simple and rapid electrophoretic method to characterize simple phenols, lignans, complex phenols, phenolic acids, and flavonoids in extra-virgin olive oil. J. Sep. Sci. 2006, 29, 2221–2233. [Google Scholar] [CrossRef]
- Servili, M.; Selvaggini, R.; Esposto, S.; Taticchi, A.; Montedoro, G.; Morozzi, G. Health and sensory properties of virgin olive oil hydrophilic phenols: Agronomic and technological aspects of production that affect their occurrence in the oil. J. Chromatogr. A 2004, 1054, 113–127. [Google Scholar] [CrossRef]
- Esposito Salsano, J.; Digiacomo, M.; Cuffaro, D.; Bertini, S.; Macchia, M. Content Variations in Oleocanthalic Acid and Other Phenolic Compounds in Extra-Virgin Olive Oil during Storage. Foods 2022, 11, 1354. [Google Scholar] [CrossRef]
- Reboredo-Rodriguez, P.; Varela-Lopez, A.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Zhang, J.; Manna, P.P.; Bompadre, S.; Quiles, J.L.; et al. Phenolic Compounds Isolated from Olive Oil as Nutraceutical Tools for the Prevention and Management of Cancer and Cardiovascular Diseases. Int. J. Mol. Sci. 2018, 19, 2305. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Merino, J.; Sun, Q.; Fitó, M.; Salas-Salvadó, J. Dietary Polyphenols, Mediterranean Diet, Prediabetes, and Type 2 Diabetes: A Narrative Review of the Evidence. Oxid. Med. Cell. Longev. 2017, 2017, 6723931. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, C.; Vari, R.; Scazzocchio, B.; De Sanctis, P.; Giovannini, C.; D’Archivio, M.; Masella, R. Anti-inflammatory Activity of Extra Virgin Olive Oil Polyphenols: Which Role in the Prevention and Treatment of Immune-Mediated Inflammatory Diseases? Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef]
- Visioli, F.; De La Lastra, C.A.; Andres-Lacueva, C.; Aviram, M.; Calhau, C.; Cassano, A.; D’Archivio, M.; Faria, A.; Fave, G.; Fogliano, V.; et al. Polyphenols and human health: A prospectus. Crit. Rev. Food Sci. Nutr. 2011, 51, 524–546. [Google Scholar] [CrossRef]
- Estruch, R.; Martinez-Gonzalez, M.A.; Corella, D.; Salas-Salvado, J.; Fito, M.; Chiva-Blanch, G.; Fiol, M.; Gomez-Gracia, E.; Aros, F.; Lapetra, J.; et al. Effect of a high-fat Mediterranean diet on bodyweight and waist circumference: A prespecified secondary outcomes analysis of the PREDIMED randomised controlled trial. Lancet Diabetes Endocrinol. 2019, 7, e6–e17. [Google Scholar] [CrossRef]
- Cordero, J.G.; Garcia-Escudero, R.; Avila, J.; Gargini, R.; Garcia-Escudero, V. Benefit of Oleuropein Aglycone for Alzheimer’s Disease by Promoting Autophagy. Oxid. Med. Cell Longev. 2018, 2018, 5010741. [Google Scholar] [CrossRef]
- Hornedo-Ortega, R.; Cerezo, A.B.; de Pablos, R.M.; Krisa, S.; Richard, T.; Garcia-Parrilla, M.C.; Troncoso, A.M. Phenolic Compounds Characteristic of the Mediterranean Diet in Mitigating Microglia-Mediated Neuroinflammation. Front. Cell Neurosci. 2018, 12, 373. [Google Scholar] [CrossRef]
- Masella, R.; Di Benedetto, R.; Vari, R.; Filesi, C.; Giovannini, C. Novel mechanisms of natural antioxidant compounds in biological systems: Involvement of glutathione and glutathione-related enzymes. J. Nutr. Biochem. 2005, 16, 577–586. [Google Scholar]
- Serreli, G.; Deiana, M. Extra Virgin Olive Oil Polyphenols: Modulation of Cellular Pathways Related to Oxidant Species and Inflammation in Aging. Cells 2020, 9, 478. [Google Scholar] [CrossRef]
- Singh, A.; Holvoet, S.; Mercenier, A. Dietary polyphenols in the prevention and treatment of allergic diseases. Clin. Exp. Allergy 2011, 41, 1346–1359. [Google Scholar] [CrossRef] [PubMed]
- Gambino, C.M.; Accardi, G.; Aiello, A.; Candore, G.; Dara-Guccione, G.; Mirisola, M.; Procopio, A.; Taormina, G.; Caruso, C. Effect of Extra Virgin Olive Oil and Table Olives on the ImmuneInflammatory Responses: Potential Clinical Applications. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Casas, R.; Estruch, R.; Sacanella, E. The Protective Effects of Extra Virgin Olive Oil on Immune-mediated Inflammatory Responses. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Del Corno, M.; Varano, B.; Scazzocchio, B.; Filesi, C.; Masella, R.; Gessani, S. Protocatechuic acid inhibits human dendritic cell functional activation: Role of PPARgamma up-modulation. Immunobiology 2014, 219, 416–424. [Google Scholar] [CrossRef]
- Tripoli, E.; Giammanco, M.; Tabacchi, G.; Di Majo, D.; Giammanco, S.; La Guardia, M. The phenolic compounds of olive oil: Structure, biological activity and beneficial effects on human health. Nutr. Res. Rev. 2005, 18, 98–112. [Google Scholar] [CrossRef]
- Santangelo, C.; Filesi, C.; Vari, R.; Scazzocchio, B.; Filardi, T.; Fogliano, V.; D’Archivio, M.; Giovannini, C.; Lenzi, A.; Morano, S.; et al. Consumption of extra-virgin olive oil rich in phenolic compounds improves metabolic control in patients with type 2 diabetes mellitus: A possible involvement of reduced levels of circulating visfatin. J. Endocrinol. Invest. 2016, 39, 1295–1301. [Google Scholar] [CrossRef]
- Jemai, H.; El Feki, A.; Sayadi, S. Antidiabetic and antioxidant effects of hydroxytyrosol and oleuropein from olive leaves in alloxan-diabetic rats. J. Agric. Food Chem. 2009, 57, 8798–8804. [Google Scholar] [CrossRef]
- Hao, J.; Shen, W.; Yu, G.; Jia, H.; Li, X.; Feng, Z.; Wang, Y.; Weber, P.; Wertz, K.; Sharman, E.; et al. Hydroxytyrosol promotes mitochondrial biogenesis and mitochondrial function in 3T3-L1 adipocytes. J. Nutr. Biochem. 2010, 21, 634–644. [Google Scholar] [CrossRef]
- Terzuoli, E.; Giachetti, A.; Ziche, M.; Donnini, S. Hydroxytyrosol, a product from olive oil, reduces colon cancer growth by enhancing epidermal growth factor receptor degradation. Mol. Nutr. Food Res. 2016, 60, 519–529. [Google Scholar] [CrossRef]
- Rigacci, S.; Stefani, M. Nutraceutical Properties of Olive Oil Polyphenols. An Itinerary from Cultured Cells through Animal Models to Humans. Int. J. Mol. Sci. 2016, 17, 843. [Google Scholar] [CrossRef]
- Lemonakis, N.; Poudyal, H.; Halabalaki, M.; Brown, L.; Tsarbopoulos, A.; Skaltsounis, A.L.; Gikas, E. The LC-MS-based metabolomics of hydroxytyrosol administration in rats reveals amelioration of the metabolic syndrome. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1041–1042, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Dagla, I.; Benaki, D.; Baira, E.; Lemonakis, N.; Poudyal, H.; Brown, L.; Tsarbopoulos, A.; Skaltsounis, A.L.; Mikros, E.; Gikas, E. Alteration in the liver metabolome of rats with metabolic syndrome after treatment with Hydroxytyrosol. A Mass Spectrometry And Nuclear Magnetic Resonance-based metabolomics study. Talanta 2018, 178, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Rosillo, M.A.; Sanchez-Hidalgo, M.; Gonzalez-Benjumea, A.; Fernandez-Bolanos, J.G.; Lubberts, E.; Alarcon-de-la-Lastra, C. Preventive effects of dietary hydroxytyrosol acetate, an extra virgin olive oil polyphenol in murine collagen-induced arthritis. Mol. Nutr. Food Res. 2015, 59, 2537–2546. [Google Scholar] [CrossRef]
- Ahamad, J.; Toufeeq, I.; Khan, M.A.; Ameen, M.S.M.; Anwer, E.T.; Uthirapathy, S.; Mir, S.R.; Ahmad, J. Oleuropein: A natural antioxidant molecule in the treatment of metabolic syndrome. Phytother. Res. 2019, 33, 3112–3128. [Google Scholar] [CrossRef] [PubMed]
- Bulotta, S.; Celano, M.; Lepore, S.M.; Montalcini, T.; Pujia, A.; Russo, D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: Focus on protection against cardiovascular and metabolic diseases. J. Transl. Med. 2014, 12, 219. [Google Scholar] [CrossRef]
- Castejon, M.L.; Rosillo, M.A.; Montoya, T.; Gonzalez-Benjumea, A.; Fernandez-Bolanos, J.G.; Alarcon-de-la-Lastra, C. Oleuropein down-regulated IL-1beta-induced inflammation and oxidative stress in human synovial fibroblast cell line SW982. Food Funct. 2017, 8, 1890–1898. [Google Scholar] [CrossRef]
- Fki, I.; Sayadi, S.; Mahmoudi, A.; Daoued, I.; Marrekchi, R.; Ghorbel, H. Comparative Study on Beneficial Effects of Hydroxytyrosol- and Oleuropein-Rich Olive Leaf Extracts on High-Fat Diet-Induced Lipid Metabolism Disturbance and Liver Injury in Rats. Biomed. Res. Int. 2020, 2020, 1315202. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.; Li, Q. Protective Effects of Oleuropein Against Cerebral Ischemia/Reperfusion by Inhibiting Neuronal Apoptosis. Med. Sci. Monit. 2018, 24, 6587–6598. [Google Scholar] [CrossRef]
- Nediani, C.; Ruzzolini, J.; Romani, A.; Calorini, L. Oleuropein, a Bioactive Compound from Olea europaea L., as a Potential Preventive and Therapeutic Agent in Non-Communicable Diseases. Antioxidants 2019, 8, 578. [Google Scholar] [CrossRef]
- Ryu, S.J.; Choi, H.S.; Yoon, K.Y.; Lee, O.H.; Kim, K.J.; Lee, B.Y. Oleuropein suppresses LPS-induced inflammatory responses in RAW 264.7 cell and zebrafish. J. Agric. Food Chem. 2015, 63, 2098–2105. [Google Scholar] [CrossRef]
- Masella, R.; Vari, R.; D’Archivio, M.; Di Benedetto, R.; Matarrese, P.; Malorni, W.; Scazzocchio, B.; Giovannini, C. Extra virgin olive oil biophenols inhibit cell-mediated oxidation of LDL by increasing the mRNA transcription of glutathione-related enzymes. J. Nutr. 2004, 134, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Cumaoglu, A.; Ari, N.; Kartal, M.; Karasu, C. Polyphenolic extracts from Olea europea L. protect against cytokine-induced beta-cell damage through maintenance of redox homeostasis. Rejuvenation Res. 2011, 14, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Nadeem, M.; Gilani, S.A.; Khan, S.; Sajid, M.W.; Amir, R.M. Antitumor Perspectives of Oleuropein and Its Metabolite Hydroxytyrosol: Recent Updates. J. Food Sci. 2018, 83, 1781–1791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moran, J.M.; Leal-Hernandez, O.; Roncero-Martin, R.; Pedrera-Zamorano, J.D. Antitumor Perspectives of Oleuropein. J. Food Sci. 2019, 84, 384. [Google Scholar] [CrossRef]
- Hamdi, H.K.; Castellon, R. Oleuropein, a non-toxic olive iridoid, is an anti-tumor agent and cytoskeleton disruptor. Biochem. Biophys. Res. Commun. 2005, 334, 769–778. [Google Scholar] [CrossRef]
- Cardeno, A.; Sanchez-Hidalgo, M.; Cortes-Delgado, A.; Alarcon de la Lastra, C. Mechanisms involved in the antiproliferative and proapoptotic effects of unsaponifiable fraction of extra virgin olive oil on HT-29 cancer cells. Nutr. Cancer 2013, 65, 908–918. [Google Scholar] [CrossRef]
- Corona, G.; Deiana, M.; Incani, A.; Vauzour, D.; Dessi, M.A.; Spencer, J.P. Inhibition of p38/CREB phosphorylation and COX-2 expression by olive oil polyphenols underlies their anti-proliferative effects. Biochem. Biophys. Res. Commun. 2007, 362, 606–611. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J.; Huang, B.; Chen, A.; Li, X. Oleuropein inhibits the proliferation and invasion of glioma cells via suppression of the AKT signaling pathway. Oncol. Rep. 2016, 36, 2009–2016. [Google Scholar] [CrossRef]
- Vivancos, M.; Moreno, J.J. Effect of resveratrol, tyrosol and beta-sitosterol on oxidised low-density lipoprotein-stimulated oxidative stress, arachidonic acid release and prostaglandin E2 synthesis by RAW 264.7 macrophages. Br. J. Nutr. 2008, 99, 1199–1207. [Google Scholar] [CrossRef]
- Di Benedetto, R.; Vari, R.; Scazzocchio, B.; Filesi, C.; Santangelo, C.; Giovannini, C.; Matarrese, P.; D’Archivio, M.; Masella, R. Tyrosol, the major extra virgin olive oil compound, restored intracellular antioxidant defences in spite of its weak antioxidative effectiveness. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 535–545. [Google Scholar] [CrossRef]
- Covas, M.I.; de la Torre, K.; Farre-Albaladejo, M.; Kaikkonen, J.; Fito, M.; Lopez-Sabater, C.; Pujadas-Bastardes, M.A.; Joglar, J.; Weinbrenner, T.; Lamuela-Raventos, R.M.; et al. Postprandial LDL phenolic content and LDL oxidation are modulated by olive oil phenolic compounds in humans. Free Radic. Biol. Med. 2006, 40, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Fragopoulou, E.; Nomikos, T.; Karantonis, H.C.; Apostolakis, C.; Pliakis, E.; Samiotaki, M.; Panayotou, G.; Antonopoulou, S. Biological activity of acetylated phenolic compounds. J. Agric. Food Chem. 2007, 55, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Rho, S.; Kim, J.; Kim, M.Y.; Lee, D.H.; Kim, S.Y.; Choi, H.; Kim, H. Neuroprotective effect of tyrosol on transient focal cerebral ischemia in rats. Neurosci. Lett. 2007, 414, 218–221. [Google Scholar] [CrossRef]
- Parkinson, L.; Keast, R. Oleocanthal, a phenolic derived from virgin olive oil: A review of the beneficial effects on inflammatory disease. Int. J. Mol. Sci. 2014, 15, 12323–12334. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.L.; Chin, K.Y. The Biological Activities of Oleocanthal from a Molecular Perspective. Nutrients 2018, 10, 570. [Google Scholar] [CrossRef]
- Lozano-Castellon, J.; Lopez-Yerena, A.; Rinaldi de Alvarenga, J.F.; Romero Del Castillo-Alba, J.; Vallverdu-Queralt, A.; Escribano-Ferrer, E.; Lamuela-Raventos, R.M. Health-promoting properties of oleocanthal and oleacein: Two secoiridoids from extra-virgin olive oil. Crit. Rev. Food Sci. Nutr. 2020, 60, 2532–2548. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Christensen, L.; Roager, H.M.; Astrup, A.; Hjorth, M.F. Microbial enterotypes in personalized nutrition and obesity management. Am. J. Clin. Nutr. 2018, 108, 645–651. [Google Scholar] [CrossRef]
- Kim, Y.S.; Unno, T.; Kim, B.Y.; Park, M.S. Sex Differences in Gut Microbiota. World J. Men’s Health 2020, 38, 48–60. [Google Scholar] [CrossRef]
- Valeri, F.; Endres, K. How biological sex of the host shapes its gut microbiota. Front. Neuroendocrinol. 2021, 61, 100912. [Google Scholar] [CrossRef] [PubMed]
- Neuman, H.; Debelius, J.W.; Knight, R.; Koren, O. Microbial endocrinology: The interplay between the microbiota and the endocrine system. FEMS Microbiol. Rev. 2015, 39, 509–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, A.; Su, J.; Liu, R.; Zhao, S.; Li, W.; Xu, X.; Li, D.; Shi, J.; Gu, B.; Zhang, J.; et al. Sexual dimorphism in glucose metabolism is shaped by androgen-driven gut microbiome. Nat. Commun. 2021, 12, 7080. [Google Scholar] [CrossRef]
- Williams, C.L.; Garcia-Reyero, N.; Martyniuk, C.J.; Tubbs, C.W.; Bisesi, J.H., Jr. Regulation of endocrine systems by the microbiome: Perspectives from comparative animal models. Gen. Comp. Endocrinol. 2020, 292, 113437. [Google Scholar] [CrossRef] [PubMed]
- Rizzetto, L.; Fava, F.; Tuohy, K.M.; Selmi, C. Connecting the immune system, systemic chronic inflammation and the gut microbiome: The role of sex. J. Autoimmun. 2018, 92, 12–34. [Google Scholar] [CrossRef]
- Cox, L.M.; Abou-El-Hassan, H.; Maghzi, A.H.; Vincentini, J.; Weiner, H.L. The sex-specific interaction of the microbiome in neurodegenerative diseases. Brain Res. 2019, 1724, 146385. [Google Scholar] [CrossRef]
- Shobeiri, P.; Kalantari, A.; Teixeira, A.L.; Rezaei, N. Shedding light on biological sex differences and microbiota-gut-brain axis: A comprehensive review of its roles in neuropsychiatric disorders. Biol. Sex Differ. 2022, 13, 12. [Google Scholar] [CrossRef]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Santos-Marcos, J.A.; Haro, C.; Vega-Rojas, A.; Alcala-Diaz, J.F.; Molina-Abril, H.; Leon-Acuna, A.; Lopez-Moreno, J.; Landa, B.B.; Tena-Sempere, M.; Perez-Martinez, P.; et al. Sex Differences in the Gut Microbiota as Potential Determinants of Gender Predisposition to Disease. Mol. Nutr. Food Res. 2019, 63, e1800870. [Google Scholar] [CrossRef]
- Vemuri, R.; Sylvia, K.E.; Klein, S.L.; Forster, S.C.; Plebanski, M.; Eri, R.; Flanagan, K.L. The microgenderome revealed: Sex differences in bidirectional interactions between the microbiota, hormones, immunity and disease susceptibility. Semin. Immunopathol. 2019, 41, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.; Saunier, K.; Hanisch, C.; Norin, E.; Alm, L.; Midtvedt, T.; Cresci, A.; Silvi, S.; Orpianesi, C.; Verdenelli, M.C.; et al. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: A cross-sectional study. Appl. Environ. Microbiol. 2006, 72, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Dominianni, C.; Sinha, R.; Goedert, J.J.; Pei, Z.; Yang, L.; Hayes, R.B.; Ahn, J. Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS ONE 2015, 10, e0124599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haro, C.; Rangel-Zuniga, O.A.; Alcala-Diaz, J.F.; Gomez-Delgado, F.; Perez-Martinez, P.; Delgado-Lista, J.; Quintana-Navarro, G.M.; Landa, B.B.; Navas-Cortes, J.A.; Tena-Sempere, M.; et al. Intestinal Microbiota Is Influenced by Gender and Body Mass Index. PLoS ONE 2016, 11, e0154090. [Google Scholar] [CrossRef]
- Takagi, T.; Naito, Y.; Inoue, R.; Kashiwagi, S.; Uchiyama, K.; Mizushima, K.; Tsuchiya, S.; Dohi, O.; Yoshida, N.; Kamada, K.; et al. Differences in gut microbiota associated with age, sex, and stool consistency in healthy Japanese subjects. J. Gastroenterol. 2019, 54, 53–63. [Google Scholar] [CrossRef]
- Mayneris-Perxachs, J.; Arnoriaga-Rodriguez, M.; Luque-Cordoba, D.; Priego-Capote, F.; Perez-Brocal, V.; Moya, A.; Burokas, A.; Maldonado, R.; Fernandez-Real, J.M. Gut microbiota steroid sexual dimorphism and its impact on gonadal steroids: Influences of obesity and menopausal status. Microbiome 2020, 8, 136. [Google Scholar] [CrossRef]
- Wang, S.; Song, F.; Gu, H.; Shu, Z.; Wei, X.; Zhang, K.; Zhou, Y.; Jiang, L.; Wang, Z.; Li, J.; et al. Assess the diversity of gut microbiota among healthy adults for forensic application. Microb. Cell Fact. 2022, 21, 46. [Google Scholar] [CrossRef]
- Correa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef]
- Kuhnau, J. The flavonoids. A class of semi-essential food components: Their role in human nutrition. World Rev. Nutr. Diet 1976, 24, 117–191. [Google Scholar]
- Chen, L.; Cao, H.; Xiao, J. Polyphenols: Absorption, bioavailability, and metabolomics. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 45–67. [Google Scholar] [CrossRef]
- Cardona, F.; Andres-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuno, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef]
- Felgines, C.; Talavera, S.; Texier, O.; Gil-Izquierdo, A.; Lamaison, J.L.; Remesy, C. Blackberry anthocyanins are mainly recovered from urine as methylated and glucuronidated conjugates in humans. J. Agric. Food Chem. 2005, 53, 7721–7727. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Clifford, M.N. Role of the small intestine, colon and microbiota in determining the metabolic fate of polyphenols. Biochem. Pharmacol. 2017, 139, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Porrini, M.; Riso, P. Factors influencing the bioavailability of antioxidants in foods: A critical appraisal. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Costa, L.G.; Lean, M.E.J.; Crozier, A. Polyphenols and health: What compounds are involved? Nutr. Metab. Cardiovasc. Dis. 2010, 20, 1–6. [Google Scholar] [CrossRef]
- Flandroy, L.; Poutahidis, T.; Berg, G.; Clarke, G.; Dao, M.C.; Decaestecker, E.; Furman, E.; Haahtela, T.; Massart, S.; Plovier, H.; et al. The impact of human activities and lifestyles on the interlinked microbiota and health of humans and of ecosystems. Sci. Total Environ. 2018, 627, 1018–1038. [Google Scholar] [CrossRef]
- Gentile, C.L.; Weir, T.L. The gut microbiota at the intersection of diet and human health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef]
- Danneskiold-Samsoe, N.B.; Dias de Freitas Queiroz Barros, H.; Santos, R.; Bicas, J.L.; Cazarin, C.B.B.; Madsen, L.; Kristiansen, K. Pastore GM, Brix S, Marostica Junior MR: Interplay between food and gut microbiota in health and disease. Food Res. Int. 2019, 115, 23–31. [Google Scholar] [CrossRef]
- Eid, H.M.; Wright, M.L.; Anil Kumar, N.V.; Qawasmeh, A.; Hassan, S.T.S.; Mocan, A.; Nabavi, S.M.; Rastrelli, L.; Atanasov, A.G.; Haddad, P.S. Significance of Microbiota in Obesity and Metabolic Diseases and the Modulatory Potential by Medicinal Plant and Food Ingredients. Front. Pharmacol. 2017, 8, 387. [Google Scholar] [CrossRef]
- Scazzocchio, B.; Minghetti, L.; D’Archivio, M. Interaction between Gut Microbiota and Curcumin: A New Key of Understanding for the Health Effects of Curcumin. Nutrients 2020, 12, 2499. [Google Scholar] [CrossRef] [PubMed]
- Anhe, F.F.; Nachbar, R.T.; Varin, T.V.; Trottier, J.; Dudonne, S.; Le Barz, M.; Feutry, P.; Pilon, G.; Barbier, O.; Desjardins, Y.; et al. Treatment with camu camu (Myrciaria dubia) prevents obesity by altering the gut microbiota and increasing energy expenditure in diet-induced obese mice. Gut 2019, 68, 453–464. [Google Scholar] [CrossRef]
- Van Hul, M.; Cani, P.D. Targeting Carbohydrates and Polyphenols for a Healthy Microbiome and Healthy Weight. Curr. Nutr. Rep. 2019, 8, 307–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molinari, R.; Merendino, N.; Costantini, L. Polyphenols as modulators of pre-established gut microbiota dysbiosis: State-of-the-art. Biofactors 2022, 48, 255–273. [Google Scholar] [CrossRef]
- Alves-Santos, A.M.; Sugizaki, C.S.A.; Lima, G.C.; Naves, M.M.V. Prebiotic effect of dietary polyphenols: A systematic review. J. Funct. Foods 2020, 74, 104169. [Google Scholar] [CrossRef]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Krga, I.; Milenkovic, D. Anthocyanins: From Sources and Bioavailability to Cardiovascular-Health Benefits and Molecular Mechanisms of Action. J. Agric. Food Chem. 2019, 67, 1771–1783. [Google Scholar] [CrossRef]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef]
- Peterson, C.T.; Vaughn, A.R.; Sharma, V.; Chopra, D.; Mills, P.J.; Peterson, S.N.; Sivamani, R.K. Effects of Turmeric and Curcumin Dietary Supplementation on Human Gut Microbiota: A Double-Blind, Randomized, Placebo-Controlled Pilot Study. J. Evid. Based Integr. Med. 2018, 23, 2515690X18790725. [Google Scholar] [CrossRef]
- Peron, G.; Sut, S.; Dal Ben, S.; Voinovich, D.; Dall’Acqua, S. Untargeted UPLC-MS metabolomics reveals multiple changes of urine composition in healthy adult volunteers after consumption of curcuma longa L. extract. Food Res. Int. 2020, 127, 108730. [Google Scholar] [CrossRef]
- Farras, M.; Martinez-Gili, L.; Portune, K.; Arranz, S.; Frost, G.; Tondo, M.; Blanco-Vaca, F. Modulation of the Gut Microbiota by Olive Oil Phenolic Compounds: Implications for Lipid Metabolism, Immune System, and Obesity. Nutrients 2020, 12, 2200. [Google Scholar] [CrossRef] [PubMed]
- Luisi, M.L.E.; Lucarini, L.; Biffi, B.; Rafanelli, E.; Pietramellara, G.; Durante, M.; Vidali, S.; Provensi, G.; Madiai, S.; Gheri, C.F.; et al. Effect of Mediterranean Diet Enriched in High Quality Extra Virgin Olive Oil on Oxidative Stress, Inflammation and Gut Microbiota in Obese and Normal Weight Adult Subjects. Front. Pharmacol. 2019, 10, 1366. [Google Scholar] [CrossRef] [PubMed]
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duenas, M.; Munoz-Gonzalez, I.; Cueva, C.; Jimenez-Giron, A.; Sanchez-Patan, F.; Santos-Buelga, C.; Moreno-Arribas, M.V.; Bartolome, B. A survey of modulation of gut microbiota by dietary polyphenols. Biomed. Res. Int. 2015, 2015, 850902. [Google Scholar] [CrossRef]
- Etxeberria, U.; Fernandez-Quintela, A.; Milagro, F.I.; Aguirre, L.; Martinez, J.A.; Portillo, M.P. Impact of polyphenols and polyphenol-rich dietary sources on gut microbiota composition. J. Agric. Food Chem. 2013, 61, 9517–9533. [Google Scholar] [CrossRef]
- Moco, S.; Martin, F.P.; Rezzi, S. Metabolomics view on gut microbiome modulation by polyphenol-rich foods. J. Proteome Res. 2012, 11, 4781–4790. [Google Scholar] [CrossRef]
- Cortes-Martin, A.; Garcia-Villalba, R.; Gonzalez-Sarrias, A.; Romo-Vaquero, M.; Loria-Kohen, V.; Ramirez-de-Molina, A.; Tomas-Barberan, F.A.; Selma, M.V.; Espin, J.C. The gut microbiota urolithin metabotypes revisited: The human metabolism of ellagic acid is mainly determined by aging. Food Funct. 2018, 9, 4100–4106. [Google Scholar] [CrossRef]
- Gonzalez-Sarrias, A.; Garcia-Villalba, R.; Romo-Vaquero, M.; Alasalvar, C.; Orem, A.; Zafrilla, P.; Tomas-Barberan, F.A.; Selma, M.V.; Espin, J.C. Clustering according to urolithin metabotype explains the interindividual variability in the improvement of cardiovascular risk biomarkers in overweight-obese individuals consuming pomegranate: A randomized clinical trial. Mol. Nutr. Food Res. 2017, 61, 1600830. [Google Scholar] [CrossRef]
- Vissers, M.N.; Zock, P.L.; Roodenburg, A.J.; Leenen, R.; Katan, M.B. Olive oil phenols are absorbed in humans. J. Nutr. 2002, 132, 409–417. [Google Scholar] [CrossRef]
- Visioli, F.; Caruso, D.; Plasmati, E.; Patelli, R.; Mulinacci, N.; Romani, A.; Galli, G.; Galli, C. Hydroxytyrosol, as a component of olive mill waste water, is dose- dependently absorbed and increases the antioxidant capacity of rat plasma. Free Radic. Res. 2001, 34, 301–305. [Google Scholar] [CrossRef]
- Rubio, L.; Macia, A.; Castell-Auvi, A.; Pinent, M.; Blay, M.T.; Ardevol, A.; Romero, M.P.; Motilva, M.J. Effect of the co-occurring olive oil and thyme extracts on the phenolic bioaccessibility and bioavailability assessed by in vitro digestion and cell models. Food Chem. 2014, 149, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Miro-Casas, E.; Covas, M.I.; Fito, M.; Farre-Albadalejo, M.; Marrugat, J.; de la Torre, R. Tyrosol and hydroxytyrosol are absorbed from moderate and sustained doses of virgin olive oil in humans. Eur. J. Clin. Nutr. 2003, 57, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Tzounis, X.; Assunta Dessi, M.; Deiana, M.; Debnam, E.S.; Visioli, F.; Spencer, J.P. The fate of olive oil polyphenols in the gastrointestinal tract: Implications of gastric and colonic microflora-dependent biotransformation. Free Radic. Res. 2006, 40, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.; Paiva-Martins, F.; Corona, G.; Debnam, E.S.; Jose Oruna-Concha, M.; Vauzour, D.; Gordon, M.H.; Spencer, J.P. Absorption and metabolism of olive oil secoiridoids in the small intestine. Br. J. Nutr. 2011, 105, 1607–1618. [Google Scholar] [CrossRef]
- Visioli, F.; Galli, C.; Bornet, F.; Mattei, A.; Patelli, R.; Galli, G.; Caruso, D. Olive oil phenolics are dose-dependently absorbed in humans. FEBS Lett. 2000, 468, 159–160. [Google Scholar] [CrossRef]
- Rodriguez-Morato, J.; Boronat, A.; Kotronoulas, A.; Pujadas, M.; Pastor, A.; Olesti, E.; Perez-Mana, C.; Khymenets, O.; Fito, M.; Farre, M.; et al. Metabolic disposition and biological significance of simple phenols of dietary origin: Hydroxytyrosol and tyrosol. Drug Metab. Rev. 2016, 48, 218–236. [Google Scholar] [CrossRef]
- Saarela, M.; Lahteenmaki, L.; Crittenden, R.; Salminen, S.; Mattila-Sandholm, T. Gut bacteria and health foods--the European perspective. Int. J. Food Microbiol. 2002, 78, 99–117. [Google Scholar] [CrossRef]
- Santos, M.M.; Piccirillo, C.; Castro, P.M.; Kalogerakis, N.; Pintado, M.E. Bioconversion of oleuropein to hydroxytyrosol by lactic acid bacteria. World J. Microbiol. Biotechnol. 2012, 28, 2435–2440. [Google Scholar] [CrossRef]
- Avila, M.; Jaquet, M.; Moine, D.; Requena, T.; Pelaez, C.; Arigoni, F.; Jankovic, I. Physiological and biochemical characterization of the two alpha-L-rhamnosidases of Lactobacillus plantarum NCC245. Microbiology 2009, 155, 2739–2749. [Google Scholar] [CrossRef]
- Marsilio, V.; Lanza, B. Characterisation of an oleuropein degrading strain of Lactobacillus plantarum. Combined effects of compounds present in olive fermenting brines (phenols, glucose and NaCl) on bacterial activity. J. Sci. Food Agric. 1998, 76, 520–524. [Google Scholar] [CrossRef]
- Landete, J.M.; Curiel, J.A.; Rodriguez, H.; de las Rivas, B.; Munoz, R. Study of the inhibitory activity of phenolic compounds found in olive products and their degradation by Lactobacillus plantarum strains. Food Chem. 2008, 107, 320–326. [Google Scholar] [CrossRef]
- Mosele, J.I.; Martin-Pelaez, S.; Macia, A.; Farras, M.; Valls, R.M.; Catalan, U.; Motilva, M.J. Faecal microbial metabolism of olive oil phenolic compounds: In vitro and in vivo approaches. Mol. Nutr. Food Res. 2014, 58, 1809–1819. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Villalba, R.; Carrasco-Pancorbo, A.; Nevedomskaya, E.; Mayboroda, O.A.; Deelder, A.M.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Exploratory analysis of human urine by LC-ESI-TOF MS after high intake of olive oil: Understanding the metabolism of polyphenols. Anal. Bioanal. Chem. 2010, 398, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Senizza, B.; Giuberti, G.; Montesano, D.; Trevisan, M.; Lucini, L. Metabolomic Study to Evaluate the Transformations of Extra-Virgin Olive Oil’s Antioxidant Phytochemicals During In Vitro Gastrointestinal Digestion. Antioxidants 2020, 9, 302. [Google Scholar] [CrossRef]
- De Bock, M.; Thorstensen, E.B.; Derraik, J.G.; Henderson, H.V.; Hofman, P.L.; Cutfield, W.S. Human absorption and metabolism of oleuropein and hydroxytyrosol ingested as olive (Olea europaea L.) leaf extract. Mol. Nutr. Food Res. 2013, 57, 2079–2085. [Google Scholar] [CrossRef]
- Cicerale, S.; Lucas, L.J.; Keast, R.S. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr. Opin. Biotechnol. 2012, 23, 129–135. [Google Scholar] [CrossRef]
- Incani, A.; Serra, G.; Atzeri, A.; Melis, M.P.; Serreli, G.; Bandino, G.; Sedda, P.; Campus, M.; Tuberoso, C.I.; Deiana, M. Extra virgin olive oil phenolic extracts counteract the pro-oxidant effect of dietary oxidized lipids in human intestinal cells. Food Chem. Toxicol. 2016, 90, 171–180. [Google Scholar] [CrossRef]
- Teixeira, L.L.; Costa, G.R.; Dorr, F.A.; Ong, T.P.; Pinto, E.; Lajolo, F.M.; Hassimotto, N.M.A. Potential antiproliferative activity of polyphenol metabolites against human breast cancer cells and their urine excretion pattern in healthy subjects following acute intake of a polyphenol-rich juice of grumixama (Eugenia brasiliensis Lam.). Food Funct. 2017, 8, 2266–2274. [Google Scholar] [CrossRef]
- Mosele, J.I.; Macia, A.; Motilva, M.J. Metabolic and Microbial Modulation of the Large Intestine Ecosystem by Non-Absorbed Diet Phenolic Compounds: A Review. Molecules 2015, 20, 17429–17468. [Google Scholar] [CrossRef]
- Selma, M.V.; Espin, J.C.; Tomas-Barberan, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Moreno-Indias, I.; Sanchez-Alcoholado, L.; Perez-Martinez, P.; Andres-Lacueva, C.; Cardona, F.; Tinahones, F.; Queipo-Ortuno, M.I. Red wine polyphenols modulate fecal microbiota and reduce markers of the metabolic syndrome in obese patients. Food Funct. 2016, 7, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- Romero, C.; Medina, E.; Vargas, J.; Brenes, M.; De Castro, A. In vitro activity of olive oil polyphenols against Helicobacter pylori. J. Agric. Food Chem. 2007, 55, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Luisa Callegari, M.; Senizza, A.; Giuberti, G.; Ruzzolini, J.; Romani, A.; Urciuoli, S.; Nediani, C.; Lucini, L. Oleuropein from olive leaf extracts and extra-virgin olive oil provides distinctive phenolic profiles and modulation of microbiota in the large intestine. Food Chem. 2022, 380, 132187. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.; Liu, M.; Ahmad, Z. Understanding the link between antimicrobial properties of dietary olive phenolics and bacterial ATP synthase. Int. J. Biol. Macromol. 2017, 101, 153–164. [Google Scholar] [CrossRef]
- Medina, E.; Brenes, M.; Romero, C.; Garcia, A.; de Castro, A. Main antimicrobial compounds in table olives. J. Agric. Food Chem. 2007, 55, 9817–9823. [Google Scholar] [CrossRef]
- Thielmann, J.; Kohnen, S.; Hauser, C. Antimicrobial activity of Olea europaea Linne extracts and their applicability as natural food preservative agents. Int. J. Food Microbiol. 2017, 251, 48–66. [Google Scholar] [CrossRef] [PubMed]
- Pallara, G.; Buccioni, A.; Pastorelli, R.; Minieri, S.; Mele, M.; Rapaccini, S.; Messini, A.; Pauselli, M.; Servili, M.; Giovannetti, L.; et al. Effect of stoned olive pomace on rumen microbial communities and polyunsaturated fatty acid biohydrogenation: An in vitro study. BMC Vet. Res. 2014, 10, 271. [Google Scholar] [CrossRef]
- Martinez, N.; Prieto, I.; Hidalgo, M.; Segarra, A.B.; Martinez-Rodriguez, A.M.; Cobo, A.; Ramirez, M.; Galvez, A.; Martinez-Canamero, M. Refined versus Extra Virgin Olive Oil High-Fat Diet Impact on Intestinal Microbiota of Mice and Its Relation to Different Physiological Variables. Microorganisms 2019, 7, 61. [Google Scholar] [CrossRef]
- Prieto, I.; Hidalgo, M.; Segarra, A.B.; Martinez-Rodriguez, A.M.; Cobo, A.; Ramirez, M.; Abriouel, H.; Galvez, A.; Martinez-Canamero, M. Influence of a diet enriched with virgin olive oil or butter on mouse gut microbiota and its correlation to physiological and biochemical parameters related to metabolic syndrome. PLoS ONE 2018, 13, e0190368. [Google Scholar] [CrossRef]
- Hidalgo, M.; Prieto, I.; Abriouel, H.; Villarejo, A.B.; Ramirez-Sanchez, M.; Cobo, A.; Benomar, N.; Galvez, A.; Martinez-Canamero, M. Changes in Gut Microbiota Linked to a Reduction in Systolic Blood Pressure in Spontaneously Hypertensive Rats Fed an Extra Virgin Olive Oil-Enriched Diet. Plant Foods Hum. Nutr. 2018, 73, 1–6. [Google Scholar] [CrossRef]
- Martin-Pelaez, S.; Mosele, J.I.; Pizarro, N.; Farras, M.; de la Torre, R.; Subirana, I.; Perez-Cano, F.J.; Castaner, O.; Sola, R.; Fernandez-Castillejo, S.; et al. Effect of virgin olive oil and thyme phenolic compounds on blood lipid profile: Implications of human gut microbiota. Eur. J. Nutr. 2017, 56, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Conterno, L.; Martinelli, F.; Tamburini, M.; Fava, F.; Mancini, A.; Sordo, M.; Pindo, M.; Martens, S.; Masuero, D.; Vrhovsek, U.; et al. Measuring the impact of olive pomace enriched biscuits on the gut microbiota and its metabolic activity in mildly hypercholesterolaemic subjects. Eur. J. Nutr. 2019, 58, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Kinross, J.M.; Darzi, A.W.; Nicholson, J.K. Gut microbiome-host interactions in health and disease. Genome Med. 2011, 3, 14. [Google Scholar] [CrossRef] [Green Version]
- Mauvais-Jarvis, F.; Berthold, H.K.; Campesi, I.; Carrero, J.J.; Dakal, S.; Franconi, F.; Gouni-Berthold, I.; Heiman, M.L.; Kautzky-Willer, A.; Klein, S.L.; et al. Sex- and Gender-Based Pharmacological Response to Drugs. Pharmacol. Rev. 2021, 73, 730–762. [Google Scholar] [CrossRef] [PubMed]
- Campesi, I.; Romani, A.; Franconi, F. The Sex-Gender Effects in the Road to Tailored Botanicals. Nutrients 2019, 11, 1637. [Google Scholar] [CrossRef]
- Iglesias-Aguirre, C.E.; Cortes-Martin, A.; Avila-Galvez, M.A.; Gimenez-Bastida, J.A.; Selma, M.V.; Gonzalez-Sarrias, A.; Espin, J.C. Main drivers of (poly)phenol effects on human health: Metabolite production and/or gut microbiota-associated metabotypes? Food Funct. 2021, 12, 10324–10355. [Google Scholar] [CrossRef]
- Olalla, J.; Garcia de Lomas, J.M.; Chueca, N.; Perez-Stachowski, X.; De Salazar, A.; Del Arco, A.; Plaza-Diaz, J.; De la Torre, J.; Prada, J.L.; Garcia-Alegria, J.; et al. Effect of daily consumption of extra virgin olive oil on the lipid profile and microbiota of HIV-infected patients over 50 years of age. Medicine 2019, 98, e17528. [Google Scholar] [CrossRef]
- Vezza, T.; Rodriguez-Nogales, A.; Algieri, F.; Garrido-Mesa, J.; Romero, M.; Sanchez, M.; Toral, M.; Martin-Garcia, B.; Gomez-Caravaca, A.M.; Arraez-Roman, D.; et al. The metabolic and vascular protective effects of olive (Olea europaea L.) leaf extract in diet-induced obesity in mice are related to the amelioration of gut microbiota dysbiosis and to its immunomodulatory properties. Pharmacol. Res. 2019, 150, 104487. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, S.; Zhong, R.; Wan, F.; Chen, L.; Liu, L.; Yi, B.; Zhang, H. Olive Fruit Extracts Supplement Improve Antioxidant Capacity via Altering Colonic Microbiota Composition in Mice. Front. Nutr. 2021, 8, 645099. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, N.; Ma, Y.; Wen, D. Hydroxytyrosol Improves Obesity and Insulin Resistance by Modulating Gut Microbiota in High-Fat Diet-Induced Obese Mice. Front. Microbiol. 2019, 10, 390. [Google Scholar] [CrossRef]
- Wang, N.; Ma, Y.; Liu, Z.; Liu, L.; Yang, K.; Wei, Y.; Liu, Y.; Chen, X.; Sun, X.; Wen, D. Hydroxytyrosol prevents PM2.5-induced adiposity and insulin resistance by restraining oxidative stress related NF-kappaB pathway and modulation of gut microbiota in a murine model. Free Radic. Biol. Med. 2019, 141, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, T.; Li, J.; Yuan, Y.; Wu, M.; Chen, F.; Deng, Z.Y.; Luo, T. Tyrosol Ameliorates the Symptoms of Obesity, Promotes Adipose Thermogenesis, and Modulates the Composition of Gut Microbiota in HFD Fed Mice. Mol. Nutr. Food Res. 2022, 66, e2101015. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, C.; Sanchez-Quesada, C.; Algarra, I.; Gaforio, J.J. The High-Fat Diet Based on Extra-Virgin Olive Oil Causes Dysbiosis Linked to Colorectal Cancer Prevention. Nutrients 2020, 12, 1705. [Google Scholar] [CrossRef] [PubMed]
- Millman, J.; Okamoto, S.; Kimura, A.; Uema, T.; Higa, M.; Yonamine, M.; Namba, T.; Ogata, E.; Yamazaki, S.; Shimabukuro, M.; et al. Metabolically and immunologically beneficial impact of extra virgin olive and flaxseed oils on composition of gut microbiota in mice. Eur. J. Nutr. 2020, 59, 2411–2425. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Wang, Y.; Fang, J.; Geng, R.; Li, M.; Zhao, Y.; Kang, S.G.; Huang, K.; Tong, T. Oleuropein Ameliorates Advanced Stage of Type 2 Diabetes in db/db Mice by Regulating Gut Microbiota. Nutrients 2021, 13, 2131. [Google Scholar] [CrossRef]
| Reference | Experimental Model | Healthy-Related Outcomes | Taxa Modifications in  GM GM | Taxa Modifications in  GM GM |
|---|---|---|---|---|
| Olalla et al., 2019 [168] |   32 HIV patients; 32 HIV patients;50 g/day EVOO for 12 weeks | EVOO consumption was associated with a decrease in total cholesterol and an increase in the alpha-diversity of the GM in males |  Actinobacteria, Eggerthella lenta; Actinobacteria, Eggerthella lenta;Verrucomicrobia, Akkermansia muciniphila; Firmicutes, Clostridia, Ruminococcuss, Ruminococcus gnavus, Lachnospiraceae; Bacteroidetes, Parabacteroides diastonis |  Bacteroidetes, Prevotella copri, Prevotella Bacteroidetes, Prevotella copri, Prevotellastercorea; Actinobacteria, Bifidobacterium, Bifidobacterium bifidum; Firmicutes, Erysipelotrichaceae, Eubacterium |
| Conterno et al., 2019 [163] |   62 healthy hypercholesterolemic subjects; 90 g of olive pomace-enriched biscuits (containing 7.1 mg/100 g HT) for 8 weeks 62 healthy hypercholesterolemic subjects; 90 g of olive pomace-enriched biscuits (containing 7.1 mg/100 g HT) for 8 weeks | Olive pomace-enriched biscuits reduced oxidized LDL cholesterol |  Bacteroidetes, Bacteroides, Rikenellaceae, Barnesiellaceae; Bacteroidetes, Bacteroides, Rikenellaceae, Barnesiellaceae;Verrucomicrobia, Akkermansia; Actinobacteria, Bifidobacterium; Proteobacteria, Enterobacteriaceae |  Firmicutes, Eubacterium; Firmicutes, Eubacterium;Bacteroidetes, Prevotella |
| Prieto et al., 2018 [160] | 26  Swiss Webster ICR (CD-1) mice; HFD enriched with butter or with EVOO (527 mg/kg polyphenols) for 12 weeks Swiss Webster ICR (CD-1) mice; HFD enriched with butter or with EVOO (527 mg/kg polyphenols) for 12 weeks | EVOO decreased plasmatic insulin level, blood pressure, and body weight |  Proteobacteria, Sutterellaceae, Marispillum Proteobacteria, Sutterellaceae, MarispillumBacteroidetes, Mucilagini bacter dageonensis | |
 Proteobacteria, Desulfovibrio Proteobacteria, Desulfovibrio | ||||
| Martinez et al., 2019 [159] | 35  Swiss Webster ICR (CD-1) mice; HFD enriched with butter or OO or EVOO (527 mg/kg polyphenols) for 12 weeks Swiss Webster ICR (CD-1) mice; HFD enriched with butter or OO or EVOO (527 mg/kg polyphenols) for 12 weeks | EVOO decreased plasmatic levels of insulin, glucose, and triglycerides |  Proteobacteria, Proteobacteria,Sutterellaceae Firmicutes, Erysipelotrichaceae | |
 Proteobacteria, Desulfovibrionaceae, Desulfovibrio, Helicobacteraceae; Tenericutes, Spiroplasmataceae Proteobacteria, Desulfovibrionaceae, Desulfovibrio, Helicobacteraceae; Tenericutes, Spiroplasmataceae | ||||
| Vezza et al., 2019 [169] | 36  C57BL/6J mice; C57BL/6J mice;HFD diet or HFD with 1, 10 or 25 mg/kg/day OLE for 5 weeks | OLE reduced basal glycaemia, inflammatory status, and insulin resistance, and improved plasma lipid profile |  Actinobacteria, Actinobacteria; Bacteroidetes, Bacteroidia, Actinobacteria, Actinobacteria; Bacteroidetes, Bacteroidia,Cytophaga; Verrumicrobia, Akkermansia | |
| Wang et al., 2021 [170] | 24  ICR mice; ICR mice;100 mg/kg b/w Chinese olive fruit extracts (containing 10 wt.% HT) for 4 weeks | Olive fruit extract treatment improved the antioxidant capacity in mice, and reduced proinflammatory cytokine level |  Firmicutes, Colidextribacter;Firmicutes/Bacteroidetes ratio Firmicutes, Colidextribacter;Firmicutes/Bacteroidetes ratio | |
 Bacteroidetes, Alloprevotella Bacteroidetes, Alloprevotella | ||||
| Liu et al., 2019 [171] | 28  C57BL/6J mice; C57BL/6J mice;HFD diet or HFD with 50 mg/kg/day HT for 8 weeks | HT improves obesity and insulin resistance, reducing chronic inflammation |  Firmicutes, Lactobacillus johnsonii Firmicutes, Lactobacillus johnsonii | |
 Firmicutes, Ruminococcaceae, Christensenellaceae, Ruminiclostridium; Firmicutes, Ruminococcaceae, Christensenellaceae, Ruminiclostridium;Proteobacteria,Desulfovibrio; Deferribacteres; Bacteroidetes, Rikenella | ||||
| Wang et al., 2019 [172] | 15  C57BL/6J mice; C57BL/6J mice;PM2.5-exposed or PM2.5-exposed treated with 50 mg/kg/day HT for 4 weeks | HT prevented visceral adipogenesis, oxidative stress, hepatic inflammation, and insulin resistance |  Verrucomicrobia, Akkermansia; Bacteroidetes, Porphyromonadaceae, Parabacteroides, Prevotellaceae UCG-001 Verrucomicrobia, Akkermansia; Bacteroidetes, Porphyromonadaceae, Parabacteroides, Prevotellaceae UCG-001 | |
 Actinobacteria, Bifidobacterium; Actinobacteria, Bifidobacterium;Firmicutes, Ruminococcaceae, Mycoplasmataceae | ||||
| Li et al., 2022 [173] | 30  C57BL/6J mice; C57BL/6J mice;HFD or HFD with 0.2% (w/w) Tyr for 16 weeks | Tyr decreased plasma triacylglycerol, total cholesterol, and fasting glucose, promoting adipose thermogenesis |  Verrucomicrobia; Verrucomicrobia;Firmicutes, Lachnospiraceae Bacterium 28_4, Colidextrybacter, Clostridia, Oscillibacter | |
 Firmicutes, Lactobacillus, Lachnospiraceae, Firmicutes, Lactobacillus, Lachnospiraceae,Bacterium DW59 | ||||
| Rodriguez-Garcia et al., [174] | 24  CD1 mice; CD1 mice;coconut-HFD diet, sunflower HFD diet, or EVOO-HFD (333.4 g/kg) for 16 weeks | EVOO diet produced a GM anti-inflammatory environment, associated with protection against CRC development |  FirmicutesLactococcus; FirmicutesLactococcus;VerrucomicrobiaAkkermansia; Firmicutes/Bacteroidetes ratio | |
 Actinobacteria, Bifidobacterium; ProteobacteriaNeisseria, Actinobacteria, Bifidobacterium; ProteobacteriaNeisseria,Pseudomonas spp.; Bacteroidetes Prevotella; Firmicutes Staphylococcus spp., Enterococcus gallinarum | ||||
| Millman et al., 2020 [175] | 20  C57BL/6J mice; C57BL/6J mice;HFD, HFD-EVOO, or HFD-flaxseed oil for 10 weeks | EVOO enhanced gut immunity, and improved metabolic health in mice |  Firmicutes, Lachnospiraceae, Allobaculum; Firmicutes, Lachnospiraceae, Allobaculum;Deferribacteres, Mucispirillum, Coriobacteriaceae | |
 Firmicutes, Clostridiales spp.; Firmicutes, Clostridiales spp.;Bacteroidetes, S24-7 spp. | ||||
| Zheng et al., 2021 [176] | 15  diabetic db/db mice; treatment with OLE (200 mg/kg) for 15 weeks diabetic db/db mice; treatment with OLE (200 mg/kg) for 15 weeks | OLE ameliorated the advanced stage of T2D, decreasing fasting glucose, and improving glucose tolerance |  Verrucomicrobia, Akkermansia; Deferribacteres Verrucomicrobia, Akkermansia; Deferribacteres | |
 Bacteroidetes, Prevotella, Bacteroidetes, Prevotella,Odoribacter, Parabacteroides; Firmicutes, Ruminococcus |
 , increase;
, increase; 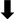 , decrease; Bold, microbiota phylum; GM, gut microbiota; EVOO, extra virgin olive oil; OO, olive oil; Tyr, tyrosol; HT, hydroxytyrosol; OLE, oleuropein; HFD, high-fat diet; PM2.5, particular matter ≤2.5 μm; T2D, type 2 diabetes.
, decrease; Bold, microbiota phylum; GM, gut microbiota; EVOO, extra virgin olive oil; OO, olive oil; Tyr, tyrosol; HT, hydroxytyrosol; OLE, oleuropein; HFD, high-fat diet; PM2.5, particular matter ≤2.5 μm; T2D, type 2 diabetes.Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Archivio, M.; Santangelo, C.; Silenzi, A.; Scazzocchio, B.; Varì, R.; Masella, R. Dietary EVOO Polyphenols and Gut Microbiota Interaction: Are There Any Sex/Gender Influences? Antioxidants 2022, 11, 1744. https://doi.org/10.3390/antiox11091744
D’Archivio M, Santangelo C, Silenzi A, Scazzocchio B, Varì R, Masella R. Dietary EVOO Polyphenols and Gut Microbiota Interaction: Are There Any Sex/Gender Influences? Antioxidants. 2022; 11(9):1744. https://doi.org/10.3390/antiox11091744
Chicago/Turabian StyleD’Archivio, Massimo, Carmela Santangelo, Annalisa Silenzi, Beatrice Scazzocchio, Rosaria Varì, and Roberta Masella. 2022. "Dietary EVOO Polyphenols and Gut Microbiota Interaction: Are There Any Sex/Gender Influences?" Antioxidants 11, no. 9: 1744. https://doi.org/10.3390/antiox11091744
APA StyleD’Archivio, M., Santangelo, C., Silenzi, A., Scazzocchio, B., Varì, R., & Masella, R. (2022). Dietary EVOO Polyphenols and Gut Microbiota Interaction: Are There Any Sex/Gender Influences? Antioxidants, 11(9), 1744. https://doi.org/10.3390/antiox11091744






