Bile Acids Induce Alterations in Mitochondrial Function in Skeletal Muscle Fibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture of Isolated Muscle Fibers
2.2. Western Blot
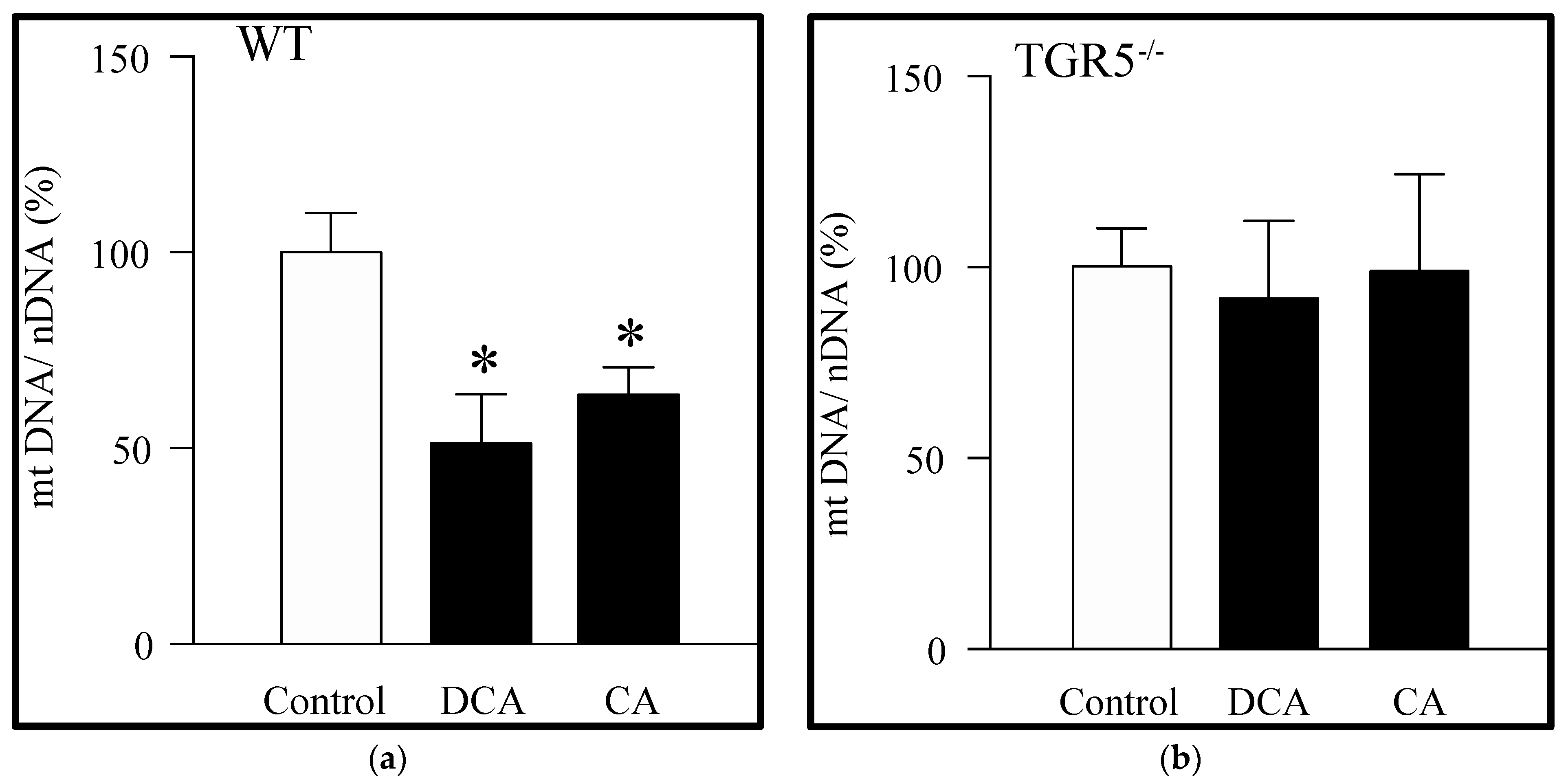
2.3. Mitochondrial DNA Quantification
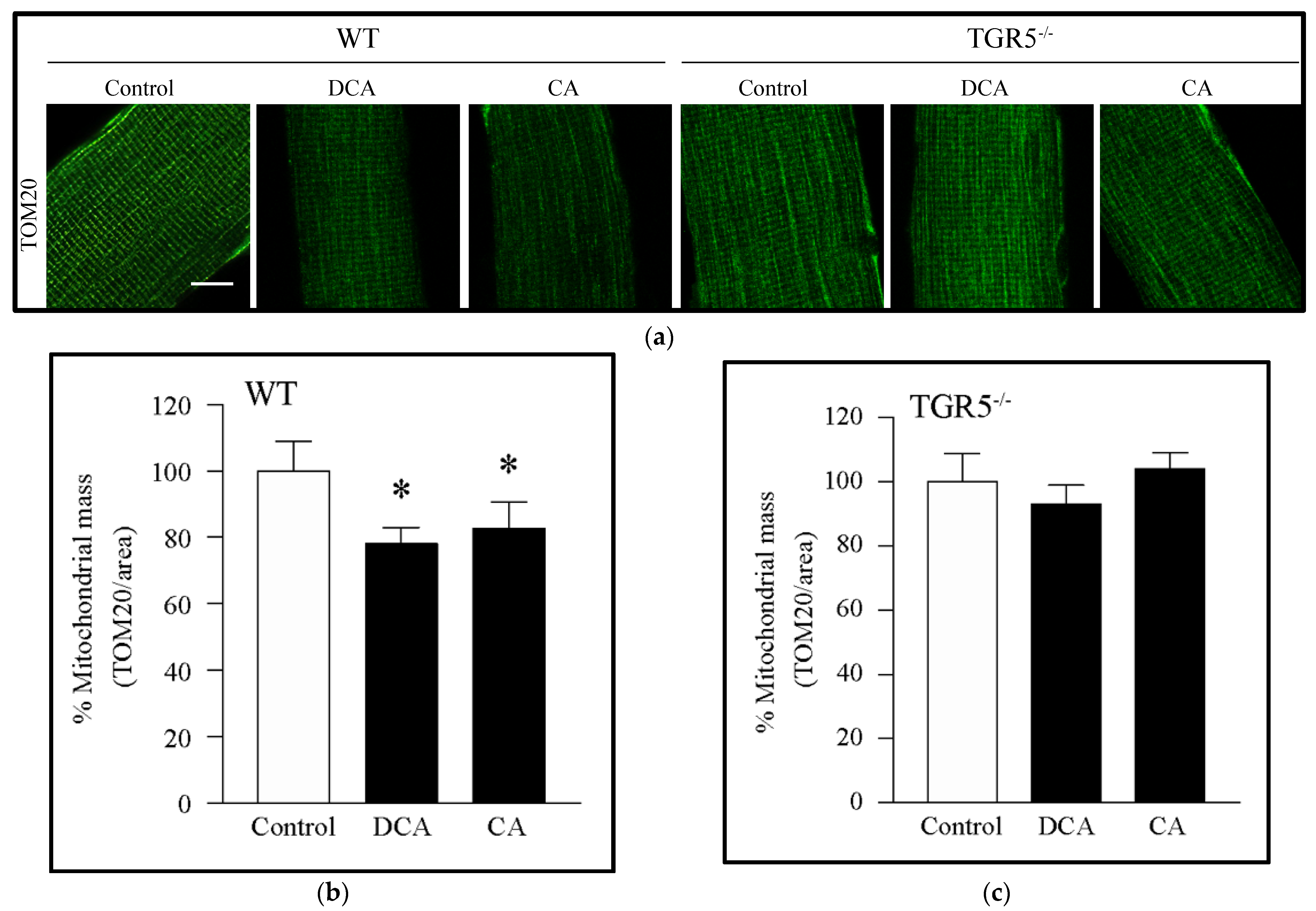
2.4. Mitochondrial Mass and Morphology
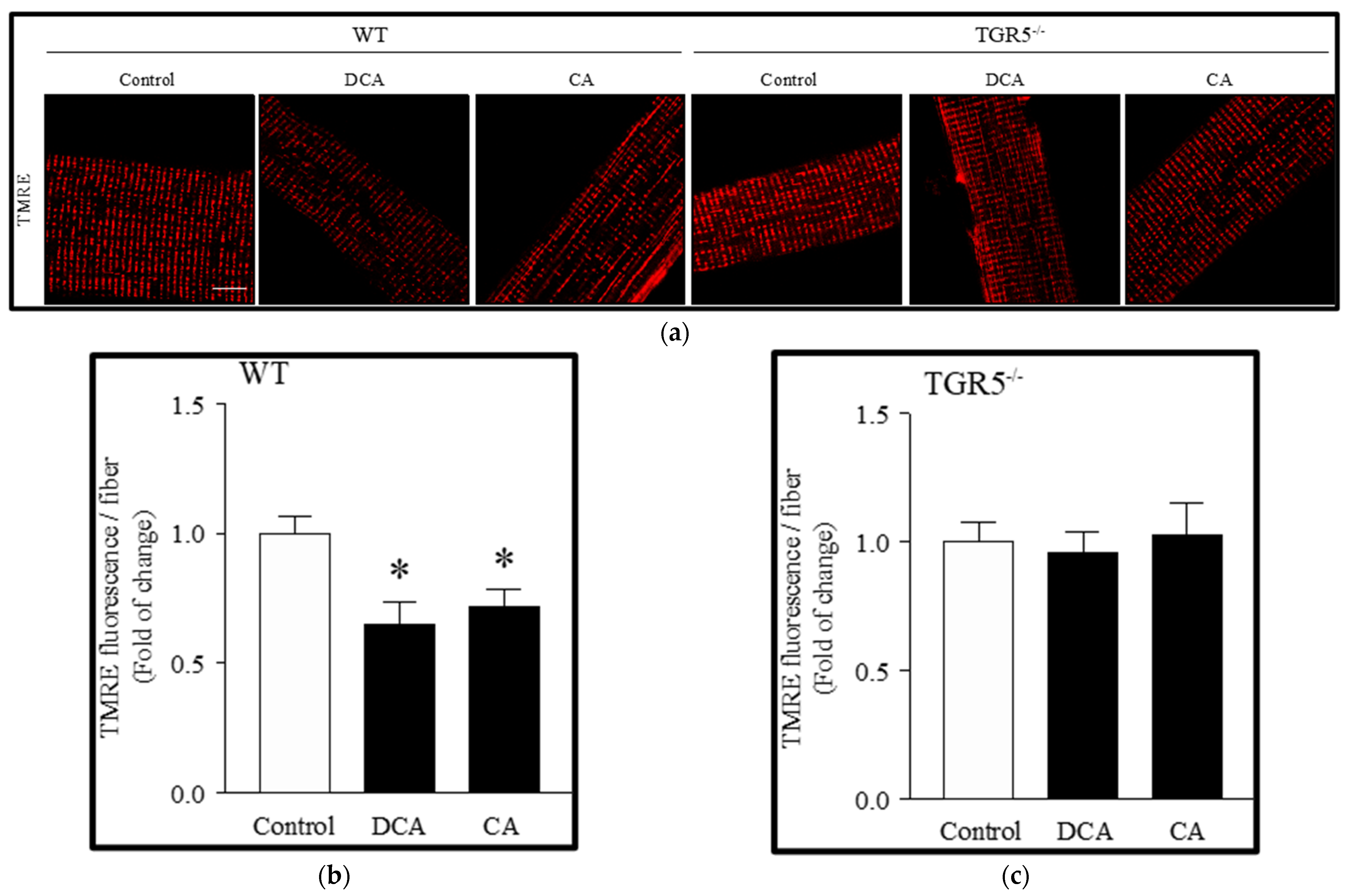
2.5. Mitochondrial Membrane Potential
2.6. Mitochondrial Reactive Oxygen Species
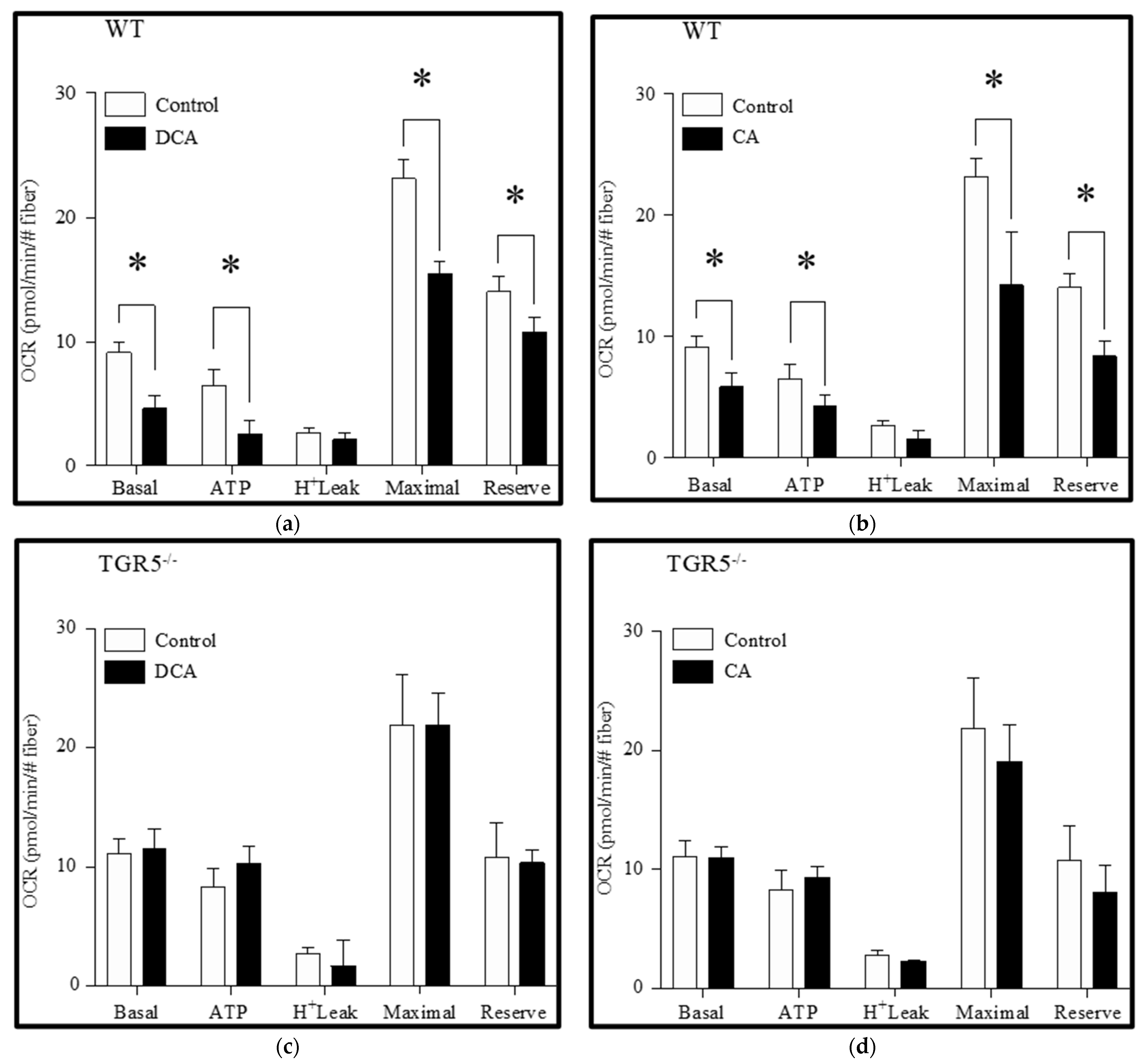
2.7. Analysis of Cellular Respiration and Mitochondrial Energetics
2.8. Statistical Analysis
3. Results
3.1. Decreased Mitochondrial Mass Induced by Deoxycholic and Cholic Acids Is Dependent on TGR5 Expression
3.2. Deoxycholic and Cholic Acids Decreased Mitochondrial Potential and Bioenergetics through a TGR5-Dependent Mechanism
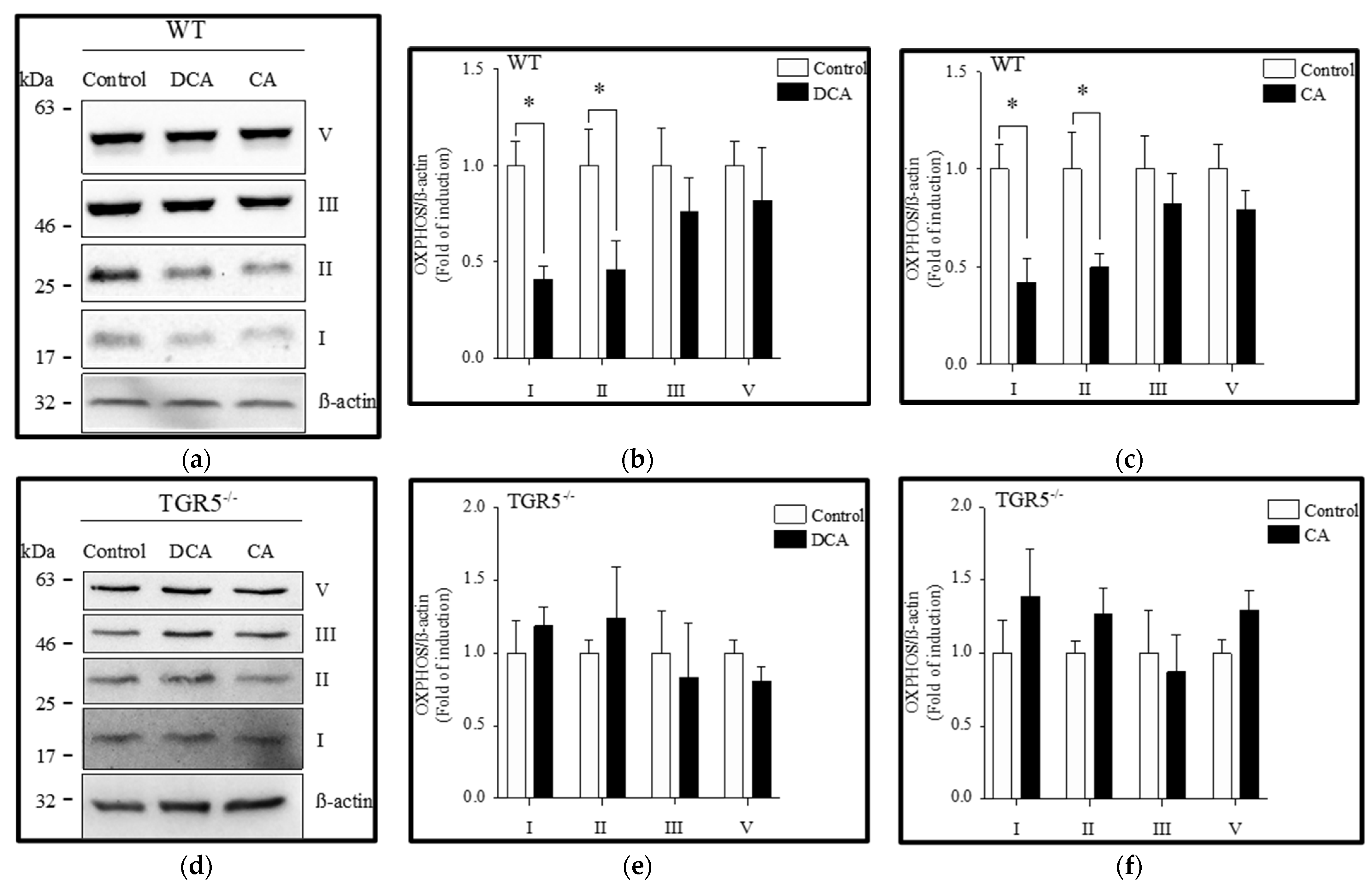
3.3. Diminution of Mitochondrial OXPHOS Complexes Induced by Deoxycholic and Cholic Acids Is Attenuated by TGR5 Deficiency
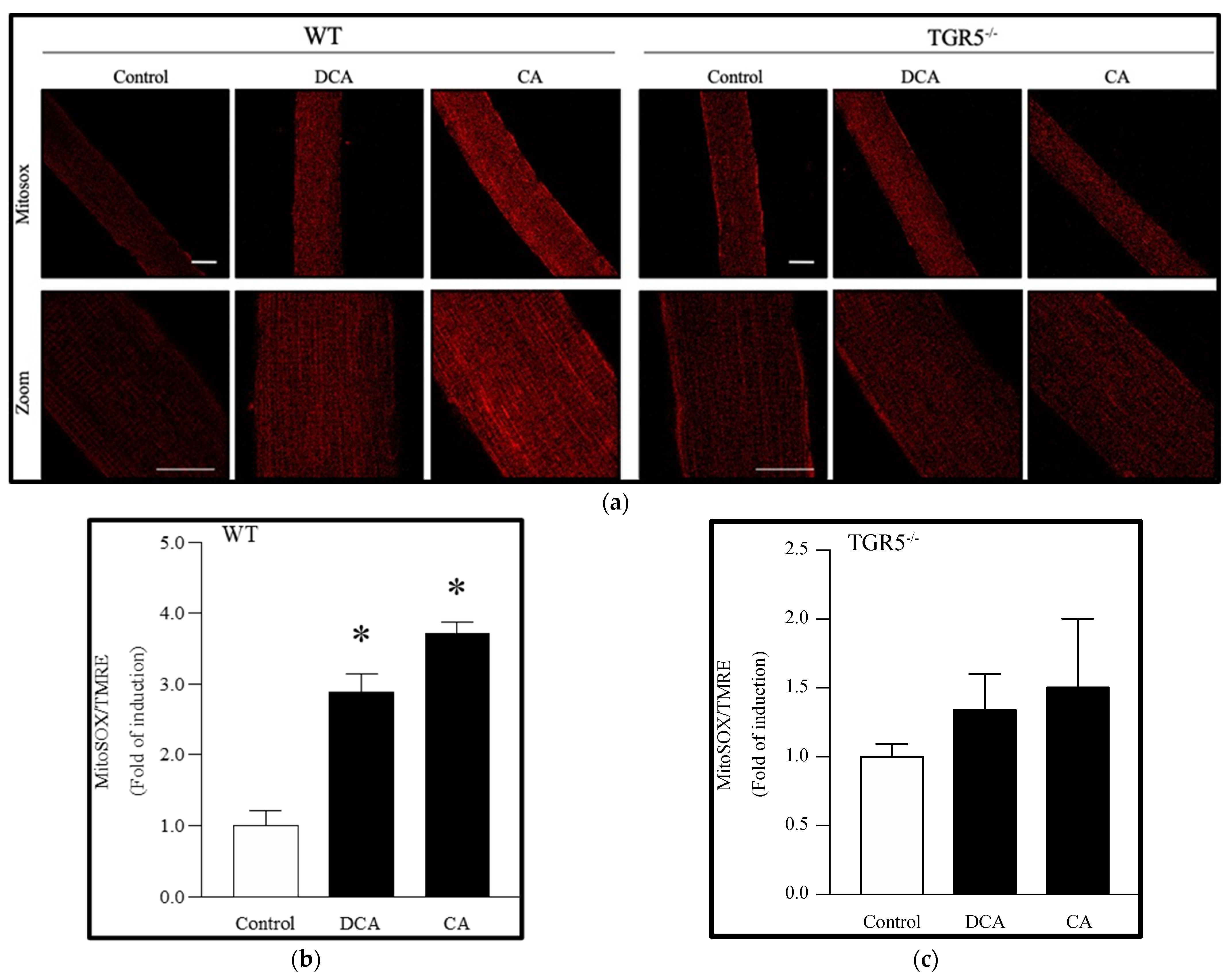
3.4. Deoxycholic and Cholic Acids Increased Mitochondrial ROS through a TGR5-Dependent Mechanism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Fernández, J.; Fernández-Montero, A.; Córdova-Martínez, A.; Pastor, D.; Martínez-Rodríguez, A.; Roche, E. Sarcopenia: Molecular Pathways and Potential Targets for Intervention. Int. J. Mol. Sci. 2020, 21, 8844. [Google Scholar] [CrossRef] [PubMed]
- CCalvani, R.; Joseph, A.-M.; Adhihetty, P.J.; Miccheli, A.; Bossola, M.; Leeuwenburgh, C.; Bernabei, R.; Marzetti, E. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol. Chem. 2013, 394, 393–414. [Google Scholar] [CrossRef] [PubMed]
- Attaix, D.; Pichard, C.; Baracos, V.E. Muscle wasting: Is mitochondrial dysfunction a key target? Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 213–214. [Google Scholar] [CrossRef]
- Abrigo, J.; Simon, F.; Cabrera, D.; Vilos, C.; Cabello-Verrugio, C. Mitochondrial Dysfunction in Skeletal Muscle Pathologies. Curr. Protein Pept. Sci. 2019, 20, 536–546. [Google Scholar] [CrossRef]
- Dasarathy, S.; Merli, M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J. Hepatol. 2016, 65, 1232–1244. [Google Scholar] [CrossRef]
- Abrigo, J.; Campos, F.; Gonzalez, F.; Aguirre, F.; Gonzalez, A.; Huerta-Salgado, C.; Conejeros, S.; Simon, F.; Arrese, M.; Cabrera, D.; et al. Sarcopenia Induced by Chronic Liver Disease in Mice Requires the Expression of the Bile Acids Membrane Receptor TGR5. Int. J. Mol. Sci. 2020, 21, 7922. [Google Scholar] [CrossRef]
- Aguirre, F.; Abrigo, J.; Gonzalez, F.; Gonzalez, A.; Simon, F.; Cabello-Verrugio, C. Protective Effect of Angiotensin 1–7 on Sarcopenia Induced by Chronic Liver Disease in Mice. Int. J. Mol. Sci. 2020, 21, 3891. [Google Scholar] [CrossRef]
- Abrigo, J.; Marín, T.; Aguirre, F.; Tacchi, F.; Vilos, C.; Simon, F.; Arrese, M.; Cabrera, D.; Cabello-Verrugio, C. N-Acetyl Cysteine Attenuates the Sarcopenia and Muscle Apoptosis Induced by Chronic Liver Disease. Curr. Mol. Med. 2019, 20, 60–71. [Google Scholar] [CrossRef]
- Abrigo, J.; Gonzalez, F.; Aguirre, F.; Tacchi, F.; González, A.; Meza, M.P.; Simon, F.; Cabrera, D.; Arrese, M.; Karpen, S.; et al. Cholic acid and deoxycholic acid induce skeletal muscle atrophy through a mechanism dependent on TGR5 receptor. J. Cell. Physiol. 2021, 236, 260–272. [Google Scholar] [CrossRef]
- Rolo, A.P.; Oliveira, P.J.; Moreno, A.J.M.; Palmeira, C.M. Bile acids affect liver mitochondrial bioenergetics: Possible relevance for cholestasis therapy. Toxicol. Sci. 2000, 57, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Penman, S.L.; Sharma, P.; Aerts, H.; Park, B.K.; Weaver, R.J.; Chadwick, A.E. Differential toxic effects of bile acid mixtures in isolated mitochondria and physiologically relevant HepaRG cells. Toxicol. In Vitro 2019, 61, 104595. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Coxito, P.M.; Sardão, V.A.; Palmeira, C.M.; Oliveira, P.J. Bile Acids Are Toxic for Isolated Cardiac Mitochondria: A Possible Cause for Hepatic-Derived Cardiomyopathies? Cardiovasc. Toxicol. 2005, 5, 63–73. [Google Scholar] [CrossRef]
- Palmeira, C.M.; Rolo, A. Mitochondrially-mediated toxicity of bile acids. Toxicology 2004, 203, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Houten, S.M.; Mataki, C.; Christoffolete, M.A.; Kim, B.W.; Sato, H.; Messaddeq, N.; Harney, J.W.; Ezaki, O.; Kodama, T.; et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 2006, 439, 484–489. [Google Scholar] [CrossRef]
- Morales, M.G.; Abrigo, J.; Acuna, M.J.; Santos, R.A.; Bader, M.; Brandan, E.; Simon, F.; Olguin, H.; Cabrera, D.; Cabello-Verrugio, C. Angiotensin-(1–7) attenuates disuse skeletal muscle atrophy in mice via its receptor, Mas. Dis. Models Mech. 2016, 9, 441–449. [Google Scholar] [CrossRef]
- Cabrera, D.; Cabello-Verrugio, C.; Solís, N.; Martín, D.S.; Cofré, C.; Pizarro, M.; Arab, J.P.; Abrigo, J.; Campos, F.; Irigoyen, B.; et al. Somatotropic Axis Dysfunction in Non-Alcoholic Fatty Liver Disease: Beneficial Hepatic and Systemic Effects of Hormone Supplementation. Int. J. Mol. Sci. 2018, 19, 1339. [Google Scholar] [CrossRef]
- Gonzalez-Ibanez, A.M.; Ruiz, L.M.; Jensen, E.; Echeverria, C.A.; Romero, V.; Stiles, L.; Shirihai, O.S.; Elorza, A.A. Erythroid Differentiation and Heme Biosynthesis Are Dependent on a Shift in the Balance of Mitochondrial Fusion and Fission Dynamics. Front. Cell. Dev. Biol. 2020, 8, 592035. [Google Scholar] [CrossRef]
- McLean, J.B.; Moylan, J.S.; Andrade, F.H. Mitochondria dysfunction in lung cancer-induced muscle wasting in C2C12 myotubes. Front. Physiol. 2014, 5, 503. [Google Scholar] [CrossRef]
- Jensen, E.L.; Gonzalez-Ibanez, A.M.; Mendoza, P.; Ruiz, L.M.; Riedel, C.A.; Simon, F.; Schuringa, J.J.; Elorza, A.A. Copper deficiency-induced anemia is caused by a mitochondrial metabolic reprograming in erythropoietic cells. Metallomics 2019, 11, 282–290. [Google Scholar] [CrossRef]
- Barbieri, E.; Battistelli, M.; Casadei, L.; Vallorani, L.; Piccoli, G.; Guescini, M.; Gioacchini, A.M.; Polidori, E.; Zeppa, S.; Ceccaroli, P.; et al. Morphofunctional and Biochemical Approaches for Studying Mitochondrial Changes during Myoblasts Differentiation. J. Aging Res. 2011, 2011, 845379. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, R.; Nair, K.S. Skeletal muscle mitochondrial dysfunction & diabetes. Indian J. Med. Res. 2007, 125, 399–410. [Google Scholar] [PubMed]
- Vazeille, E.; Codran, A.; Claustre, A.; Averous, J.; Listrat, A.; Bechet, D.; Taillandier, D.; Dardevet, D.; Attaix, D.; Combaret, L. The ubiquitin-proteasome and the mitochondria-associated apoptotic pathways are sequentially downregulated during recovery after immobilization-induced muscle atrophy. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1181–E1190. [Google Scholar] [CrossRef] [PubMed]
- Dumas, J.-F.; Simard, G.; Flamment, M.; Ducluzeau, P.-H.; Ritz, P. Is skeletal muscle mitochondrial dysfunction a cause or an indirect consequence of insulin resistance in humans? Diabetes Metab. 2009, 35, 159–167. [Google Scholar] [CrossRef]
- Hiona, A.; Sanz, A.; Kujoth, G.C.; Pamplona, R.; Seo, A.Y.; Hofer, T.; Someya, S.; Miyakawa, T.; Nakayama, C.; Samhan-Arias, A.K.; et al. Mitochondrial DNA Mutations Induce Mitochondrial Dysfunction, Apoptosis and Sarcopenia in Skeletal Muscle of Mitochondrial DNA Mutator Mice. PLoS ONE 2010, 5, e11468. [Google Scholar] [CrossRef]
- Romanello, V.; Sandri, M. Mitochondrial Biogenesis and Fragmentation as Regulators of Muscle Protein Degradation. Curr. Hypertens. Rep. 2010, 12, 433–439. [Google Scholar] [CrossRef]
- Powers, S.K.; Wiggs, M.P.; Duarte, J.A.; Zergeroglu, A.M.; Demirel, H.A. Mitochondrial signaling contributes to disuse muscle atrophy. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E31–E39. [Google Scholar] [CrossRef]
- Vincent, A.E.; Turnbull, D.M.; Eisner, V.; Hajnóczky, G.; Picard, M. Mitochondrial Nanotunnels. Trends Cell Biol. 2017, 27, 787–799. [Google Scholar] [CrossRef]
- Lavorato, M.; Iyer, V.R.; Dewight, W.; Cupo, R.R.; Debattisti, V.; Gomez, L.; De la Fuente, S.; Zhao, Y.T.; Valdivia, H.H.; Hajnóczky, G.; et al. Increased mitochondrial nanotunneling activity, induced by calcium imbalance, affects intermitochondrial matrix exchanges. Proc. Natl. Acad. Sci. USA 2017, 114, E849–E858. [Google Scholar] [CrossRef]
- Vincent, A.E.; White, K.; Davey, T.; Philips, J.; Ogden, R.T.; Lawless, C.; Warren, C.; Hall, M.G.; Ng, Y.S.; Falkous, G.; et al. Quantitative 3D Mapping of the Human Skeletal Muscle Mitochondrial Network. Cell Rep. 2019, 26, e1004. [Google Scholar] [CrossRef] [Green Version]
- Abrigo, J.; Elorza, A.A.; Riedel, C.A.; Vilos, C.; Simon, F.; Cabrera, D.; Estrada, L.; Cabello-Verrugio, C. Role of Oxidative Stress as Key Regulator of Muscle Wasting during Cachexia. Oxidative Med. Cell. Longev. 2018, 2018, 2063179. [Google Scholar] [CrossRef]
- Hood, D.A.; Memme, J.M.; Oliveira, A.N.; Triolo, M. Maintenance of Skeletal Muscle Mitochondria in Health, Exercise, and Aging. Annu. Rev. Physiol. 2019, 81, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Rolo, A.P.; Palmeira, C.M.; Holy, J.M.; Wallace, K.B. Role of Mitochondrial Dysfunction in Combined Bile Acid-Induced Cytotoxicity: The Switch Between Apoptosis and Necrosis. Toxicol. Sci. 2004, 79, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Rolo, A.P.; Palmeira, C.M.; Wallace, K.B. Mitochondrially mediated synergistic cell killing by bile acids. Biochim. Biophys. Acta 2003, 1637, 127–132. [Google Scholar] [CrossRef]
- Bradley, C.A. Adipose tissue: Bile acid-TGR5 axis promotes beiging. Nat. Rev. Endocrinol. 2018, 14, 130. [Google Scholar] [CrossRef]
- Gores, G.J.; Miyoshi, H.; Botla, R.; Aguilar, H.I.; Bronk, S.F. Induction of the mitochondrial permeability transition as a mechanism of liver injury during cholestasis: A potential role for mitochondrial proteases. Biochim. Biophys. Acta 1998, 1366, 167–175. [Google Scholar] [CrossRef]
- Botla, R.; Spivey, J.R.; Aguilar, H.; Bronk, S.F.; Gores, G.J. Ursodeoxycholate (UDCA) inhibits the mitochondrial membrane permeability transition induced by glycochenodeoxycholate: A mechanism of UDCA cytoprotection. J. Pharmacol. Exp. Ther. 1995, 272, 930–938. [Google Scholar]
- Zoratti, M.; Szabo, I. The mitochondrial permeability transition. Biochim. Biophys. Acta 1995, 1241, 139–176. [Google Scholar] [CrossRef]
- Pastorino, J.G.; Snyder, J.W.; Serroni, A.; Hoek, J.B.; Farber, J.L. Cyclosporin and carnitine prevent the anoxic death of cultured hepatocytes by inhibiting the mitochondrial permeability transition. J. Biol. Chem. 1993, 268, 13791–13798. [Google Scholar] [CrossRef]
- Lemasters, J.J.; Nieminen, A.L.; Qian, T.; Trost, L.C.; Elmore, S.P.; Nishimura, Y.; Crowe, R.A.; Cascio, W.E.; Bradham, C.A.; Brenner, D.A.; et al. The mitochondrial permeability transition in cell death: A common mechanism in necrosis, apoptosis and autophagy. Biochim. Biophys. Acta 1998, 1366, 177–196. [Google Scholar] [CrossRef]
- Campos, F.; Abrigo, J.; Aguirre, F.; Garces, B.; Arrese, M.; Karpen, S.; Cabrera, D.; Andia, M.E.; Simon, F.; Cabello-Verrugio, C. Sarcopenia in a mice model of chronic liver disease: Role of the ubiquitin-proteasome system and oxidative stress. Pflug. Arch. 2018, 470, 1503–1519. [Google Scholar] [CrossRef]
- Al Khamici, H.; Sanchez, V.C.; Yan, H.; Cataisson, C.; Michalowski, A.M.; Yang, H.H.; Li, L.; Lee, M.P.; Huang, J.; Yuspa, S.H. The oxidoreductase CLIC4 is required to maintain mitochondrial function and resistance to exogenous oxidants in breast cancer cells. J. Biol. Chem. 2022, 298, 102275. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Salas, E.; Suh, K.S.; Speransky, V.V.; Bowers, W.L.; Levy, J.M.; Adams, T.; Pathak, K.R.; Edwards, L.E.; Hayes, D.D.; Cheng, C.; et al. mtCLIC/CLIC4, an organellular chloride channel protein, is increased by DNA damage and participates in the apoptotic response to p53. Mol. Cell. Biol. 2002, 22, 3610–3620. [Google Scholar] [CrossRef]
- Andreyev, A.Y.; Kushnareva, Y.E.; Starkov, A.A. Mitochondrial metabolism of reactive oxygen species. Biochemistry 2005, 70, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Elorza, A.A.; Soffia, J.P. mtDNA Heteroplasmy at the Core of Aging-Associated Heart Failure. An Integrative View of OXPHOS and Mitochondrial Life Cycle in Cardiac Mitochondrial Physiology. Front. Cell Dev. Biol. 2021, 9, 625020. [Google Scholar] [CrossRef] [PubMed]
- Favaro, G.; Romanello, V.; Varanita, T.; Andrea Desbats, M.; Morbidoni, V.; Tezze, C.; Albiero, M.; Canato, M.; Gherardi, G.; De Stefani, D.; et al. DRP1-mediated mitochondrial shape controls calcium homeostasis and muscle mass. Nat. Commun. 2019, 10, 2576. [Google Scholar] [CrossRef] [PubMed]
- Sudevan, S.; Takiura, M.; Kubota, Y.; Higashitani, N.; Cooke, M.; Ellwood, R.A.; Etheridge, T.; Szewczyk, N.J.; Higashitani, A. Mitochondrial dysfunction causes Ca(2+) overload and ECM degradation-mediated muscle damage in C. elegans. FASEB J. 2019, 33, 9540–9550. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.L.; Cornwell, E.W.; Jackman, R.W.; Kandarian, S.C. NF-kappaB but not FoxO sites in the MuRF1 promoter are required for transcriptional activation in disuse muscle atrophy. Am. J. Physiol. Cell Physiol. 2014, 306, C762–C767. [Google Scholar] [CrossRef] [PubMed]
- Velazquez-Villegas, L.A.; Perino, A.; Lemos, V.; Zietak, M.; Nomura, M.; Pols, T.W.H.; Schoonjans, K. TGR5 signalling promotes mitochondrial fission and beige remodelling of white adipose tissue. Nat. Commun. 2018, 9, 245. [Google Scholar] [CrossRef]
- Wang, X.X.; Edelstein, M.H.; Gafter, U.; Qiu, L.; Luo, Y.; Dobrinskikh, E.; Lucia, S.; Adorini, L.; D’Agati, V.D.; Levi, J.; et al. G Protein-Coupled Bile Acid Receptor TGR5 Activation Inhibits Kidney Disease in Obesity and Diabetes. J. Am. Soc. Nephrol. 2016, 27, 1362–1378. [Google Scholar] [CrossRef]
- Shabalina, I.G.; Petrovic, N.; de Jong, J.M.; Kalinovich, A.V.; Cannon, B.; Nedergaard, J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep. 2013, 5, 1196–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrauwen, P. Skeletal muscle uncoupling protein 3 (UCP3): Mitochondrial uncoupling protein in search of a function. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Pohl, E.E.; Rupprecht, A.; Macher, G.; Hilse, K.E. Important Trends in UCP3 Investigation. Front. Physiol. 2019, 10, 470. [Google Scholar] [CrossRef] [PubMed]
- Schrauwen, P.; Hesselink, M. UCP2 and UCP3 in muscle controlling body metabolism. J. Exp. Biol. 2002, 205, 2275–2285. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abrigo, J.; Olguín, H.; Gutierrez, D.; Tacchi, F.; Arrese, M.; Cabrera, D.; Valero-Breton, M.; Elorza, A.A.; Simon, F.; Cabello-Verrugio, C. Bile Acids Induce Alterations in Mitochondrial Function in Skeletal Muscle Fibers. Antioxidants 2022, 11, 1706. https://doi.org/10.3390/antiox11091706
Abrigo J, Olguín H, Gutierrez D, Tacchi F, Arrese M, Cabrera D, Valero-Breton M, Elorza AA, Simon F, Cabello-Verrugio C. Bile Acids Induce Alterations in Mitochondrial Function in Skeletal Muscle Fibers. Antioxidants. 2022; 11(9):1706. https://doi.org/10.3390/antiox11091706
Chicago/Turabian StyleAbrigo, Johanna, Hugo Olguín, Danae Gutierrez, Franco Tacchi, Marco Arrese, Daniel Cabrera, Mayalen Valero-Breton, Alvaro A. Elorza, Felipe Simon, and Claudio Cabello-Verrugio. 2022. "Bile Acids Induce Alterations in Mitochondrial Function in Skeletal Muscle Fibers" Antioxidants 11, no. 9: 1706. https://doi.org/10.3390/antiox11091706
APA StyleAbrigo, J., Olguín, H., Gutierrez, D., Tacchi, F., Arrese, M., Cabrera, D., Valero-Breton, M., Elorza, A. A., Simon, F., & Cabello-Verrugio, C. (2022). Bile Acids Induce Alterations in Mitochondrial Function in Skeletal Muscle Fibers. Antioxidants, 11(9), 1706. https://doi.org/10.3390/antiox11091706





