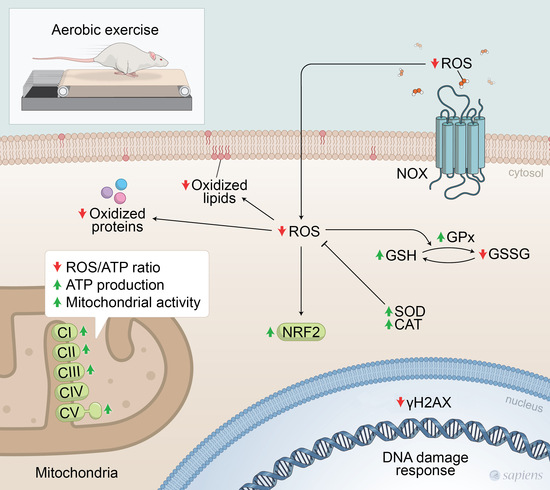
Exercise Improves Redox Homeostasis and Mitochondrial Function in White Adipose Tissue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Model
2.2. Chronic Exercise Model
2.3. Lactate Measurements
2.4. Total Body Mass and Adiposity
2.5. Histology–Adipocyte Diameter and Number per Area
2.6. NADPH Oxidase Activity
2.7. Mitochondria Isolation and Measurement of Mitochondrial Function
2.7.1. Mitochondrial Oxygen Consumption
2.7.2. Mitochondrial ATP Production
2.7.3. Mitochondrial ROS Production
2.7.4. Mitochondrial Swelling and Transmembrane Potential
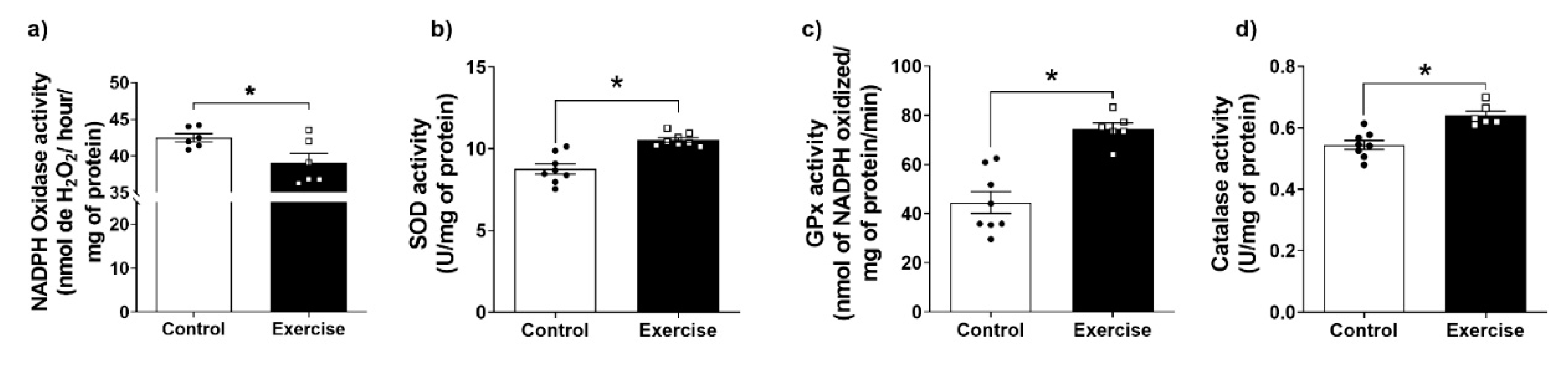
2.8. Antioxidant Enzymes Activities
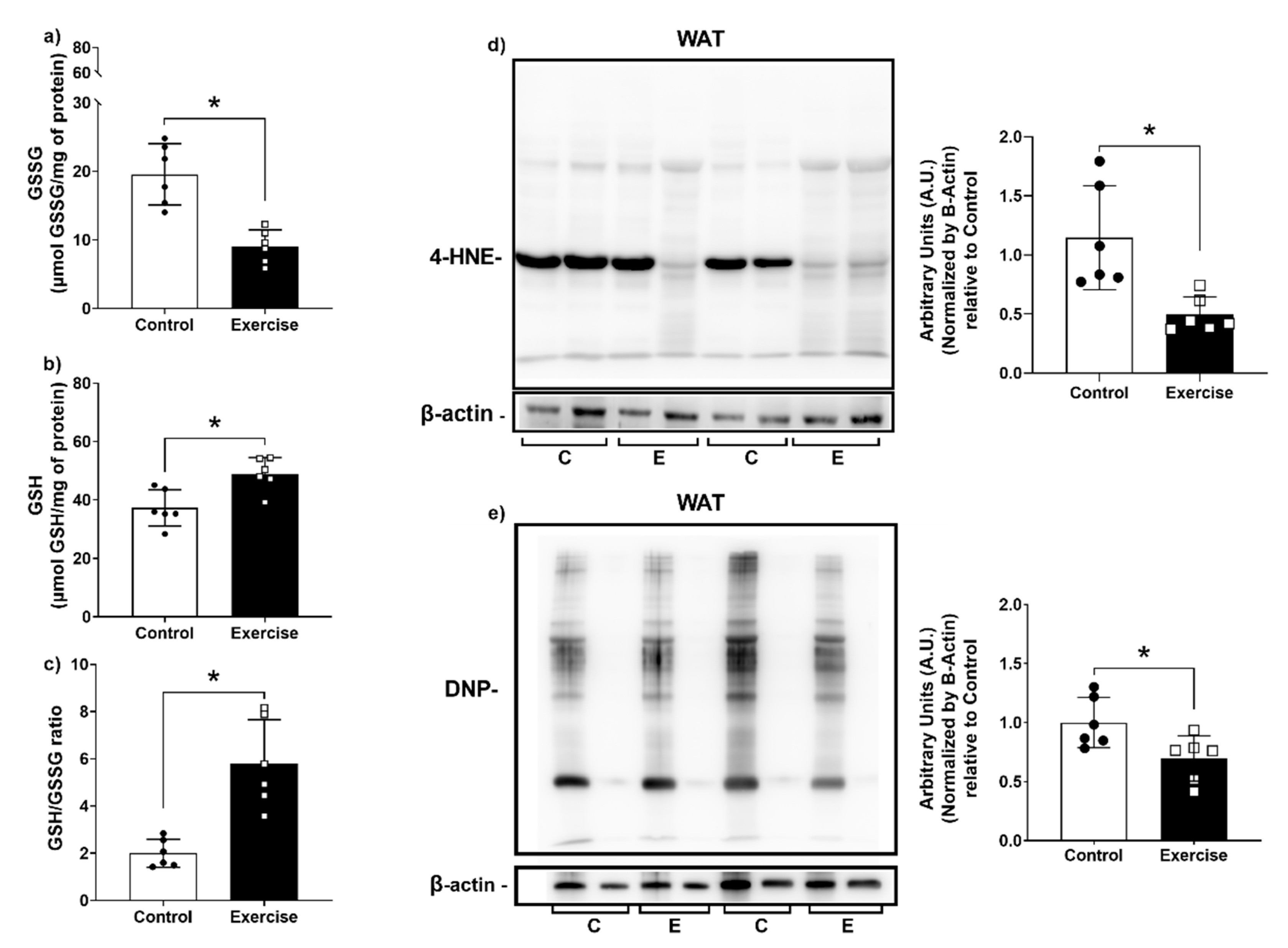
2.9. Biomarkers of Oxidative Damage
2.9.1. Quantification of GSH and GSSG Levels
2.9.2. Carbonylated Proteins by 2D OxyBlot
2.10. Protein Expression Analysis–Western Blotting
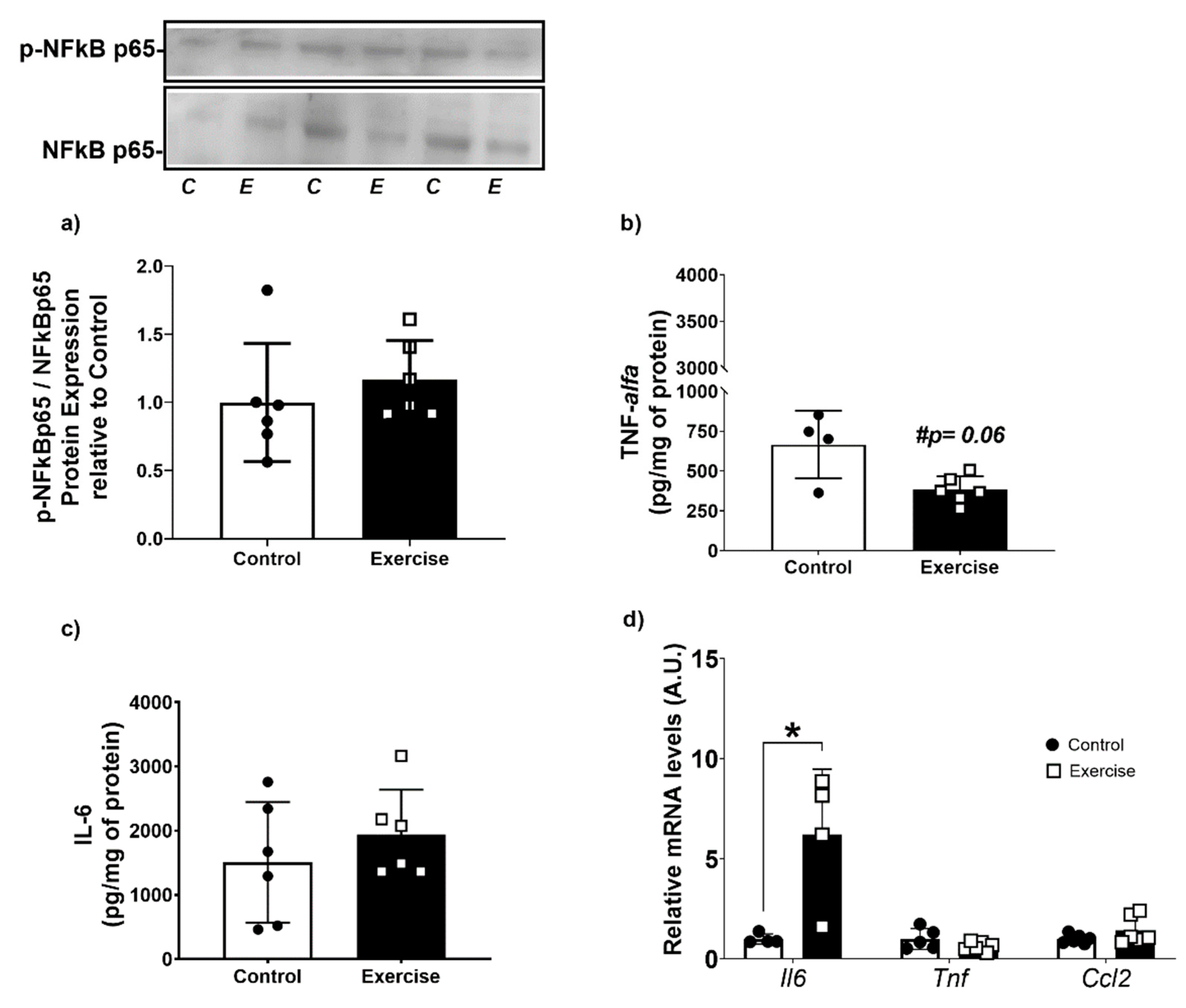
2.11. Cytokine Profile
2.12. Gene Expression by Real-Time qPCR
2.13. Statistical Analysis
3. Results
3.1. Aerobic Exercise Performance
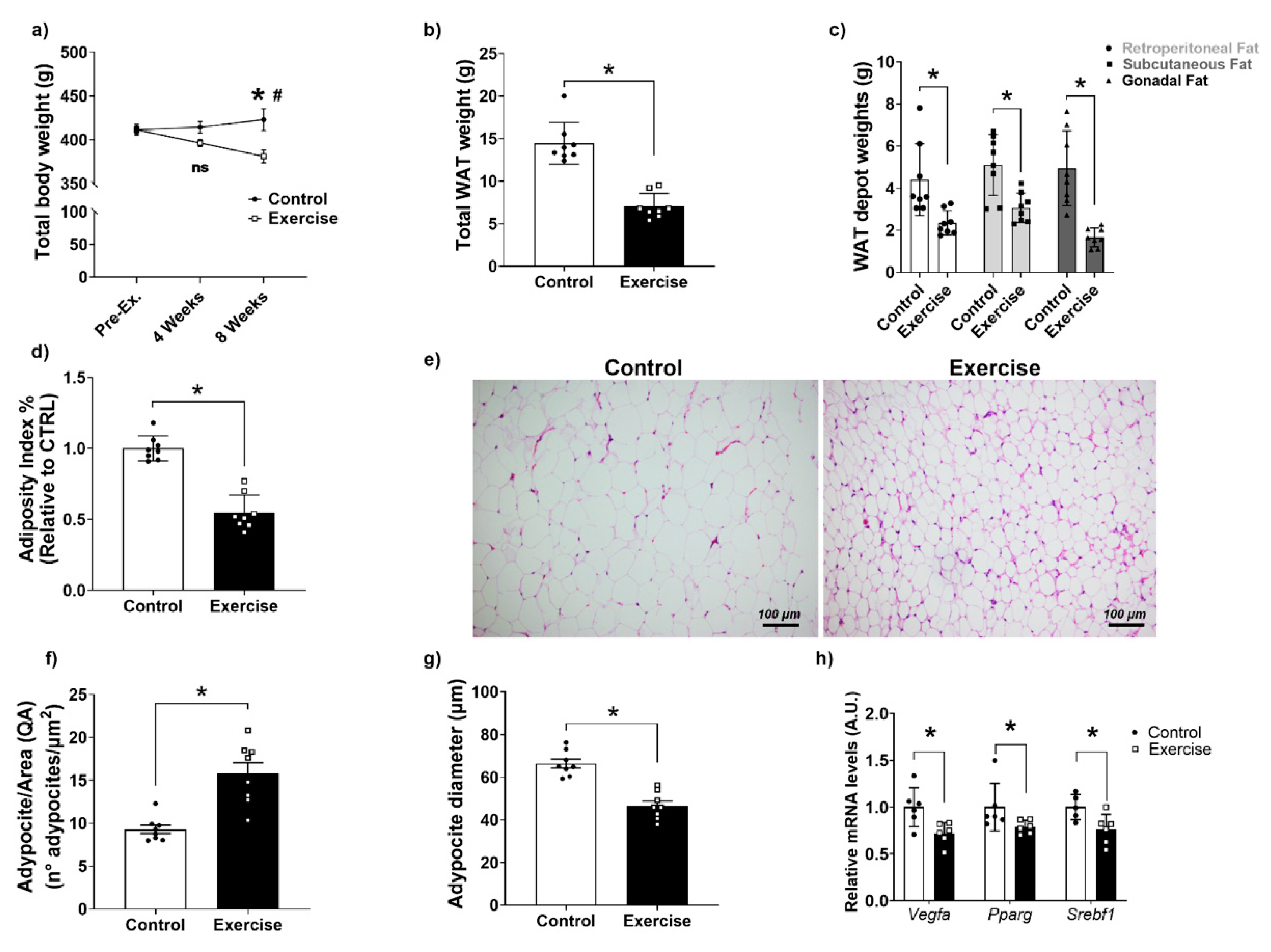
3.2. Effects of Chronic Aerobic Exercise on Body Weight, Adiposity, and WAT
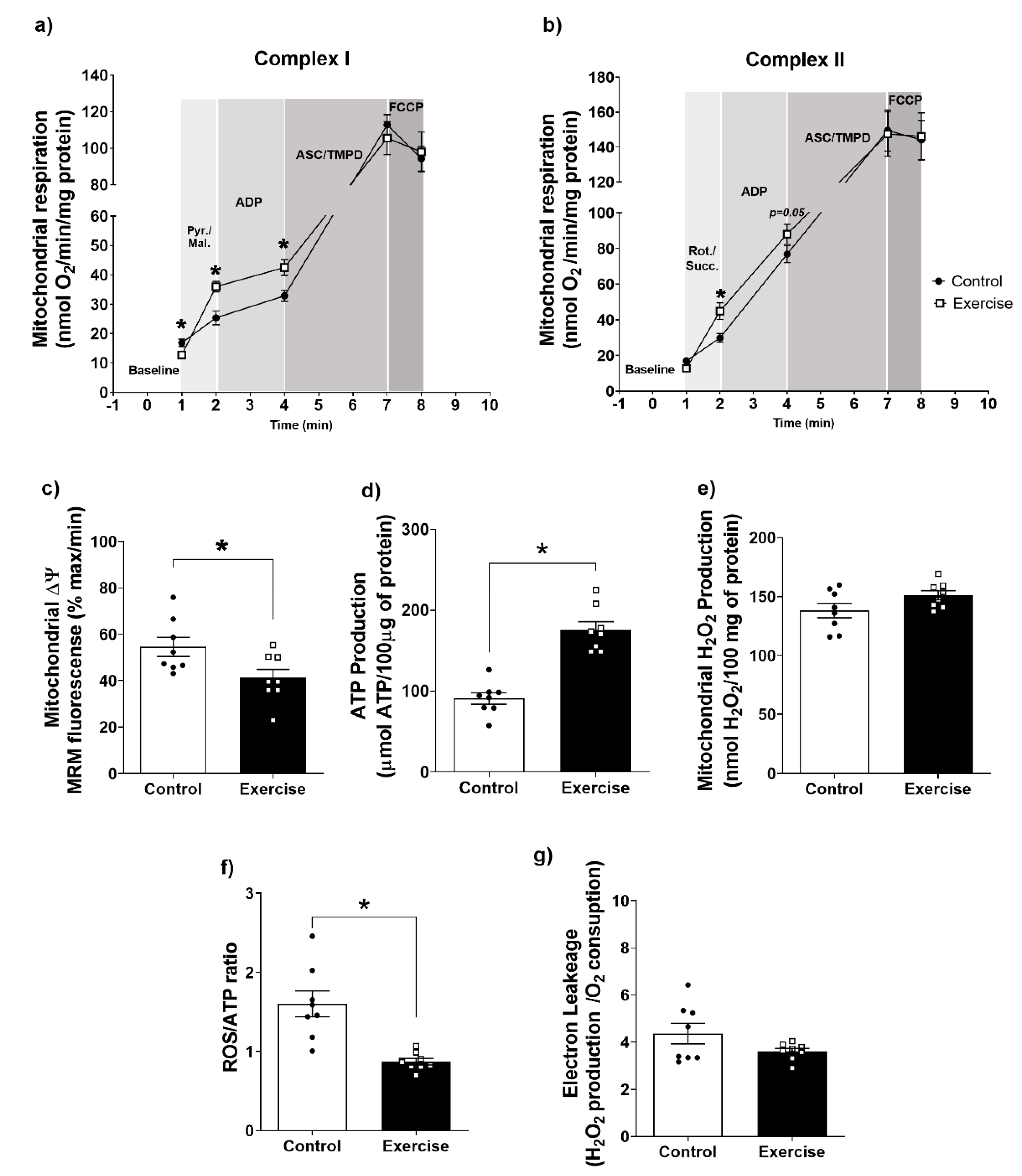
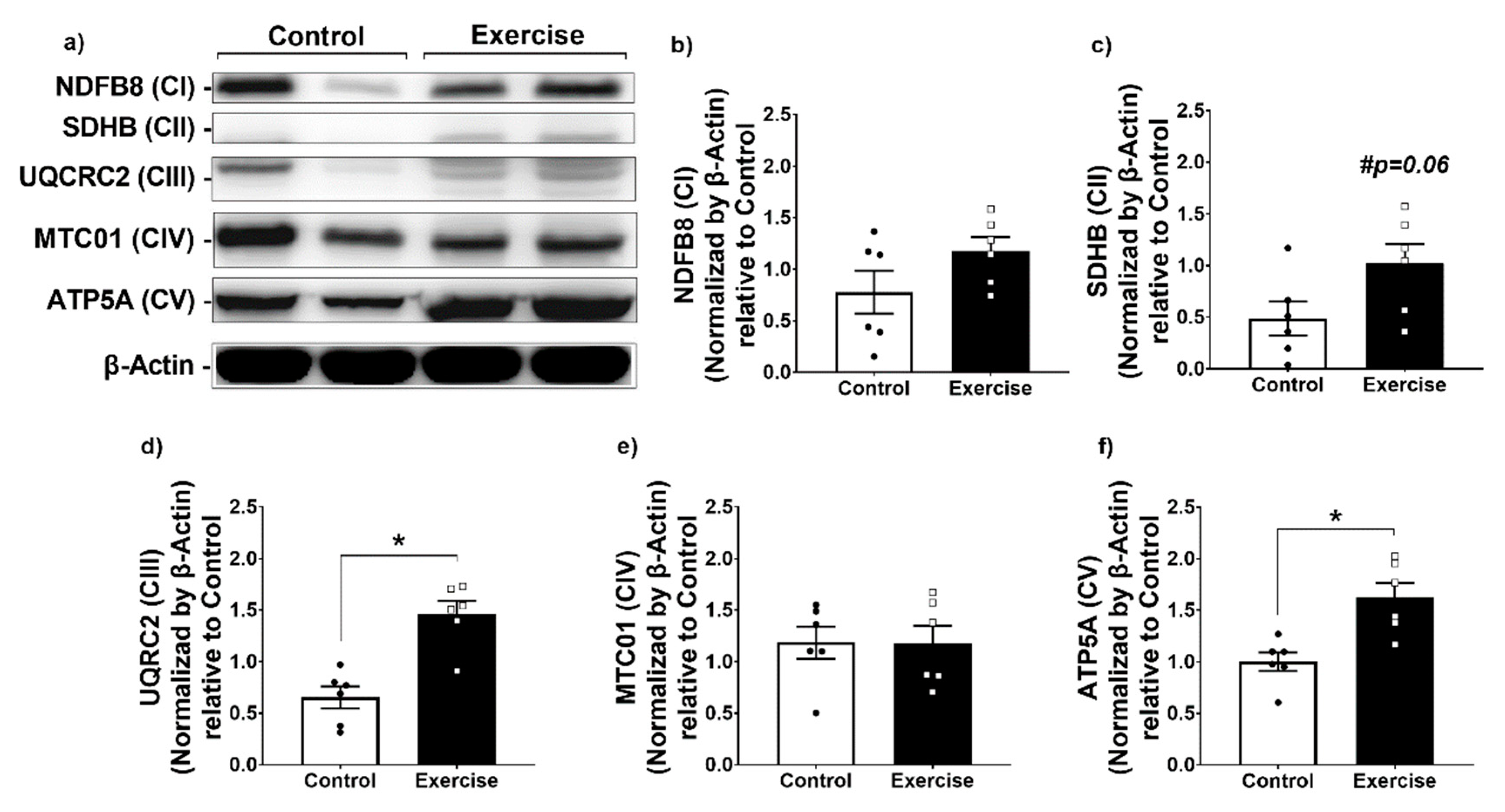
3.3. Effect of Chronic Aerobic Exercise on Mitochondrial Function in WAT
3.4. Effect of Chronic Aerobic Exercise Exposure on Redox Homeostasis and Oxidative Biomarkers
3.5. Chronic Aerobic Exercise Exposure Does Not Change Inflammatory Markers, but Increases IL-6 Gene Expression on WAT-r
3.6. Chronic Aerobic Exercise Exposure Decreases DNA Damage in WAT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Norton, K.; Norton, L.; Sadgrove, D. Position statement on physical activity and exercise intensity terminology. J. Sci. Med. Sport 2010, 13, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Taylor, A.W. Exercise and Hormesis. In The Science of Hormesis in Health and Longevity; Elsevier: Amsterdam, The Netherlands, 2018; pp. 63–73. [Google Scholar] [CrossRef]
- Ji, L.L.; Kang, C.; Zhang, Y. Exercise-induced hormesis and skeletal muscle health. Free Radic. Biol. Med. 2016, 98, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; A Baldwin, L. Defining hormesis. Hum. Exp. Toxicol. 2002, 21, 91–97. [Google Scholar] [CrossRef]
- Webb, R.; Hughes, M.G.; Thomas, A.W.; Morris, K. The Ability of Exercise-Associated Oxidative Stress to Trigger Redox-Sensitive Signalling Responses. Antioxidants 2017, 6, 63. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.A.; Ruderman, N.B. AMPK and the biochemistry of exercise: Implications for human health and disease. Biochem. J. 2009, 418, 261–275. [Google Scholar] [CrossRef]
- Aronson, D.; A Violan, M.; Dufresne, S.D.; Zangen, D.; A Fielding, R.; Goodyear, L.J. Exercise stimulates the mitogen-activated protein kinase pathway in human skeletal muscle. J. Clin. Investig. 1997, 99, 1251–1257. [Google Scholar] [CrossRef]
- Ji, L.L.; Gomezcabrera, M.; Steinhafel, N.; Vina, J. Acute exercise activates nuclear factor (NF)-ΚB signaling pathway in rat skeletal muscle. FASEB J. 2004, 18, 1499–1506. [Google Scholar] [CrossRef]
- Muthusamy, V.R.; Kannan, S.; Sadhaasivam, K.; Gounder, S.S.; Davidson, C.J.; Boeheme, C.; Hoidal, J.R.; Wang, L.; Rajasekaran, N.S. Acute exercise stress activates Nrf2/ARE signaling and promotes antioxidant mechanisms in the myocardium. Free Radic. Biol. Med. 2012, 52, 366–376. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Abreu, C.; Cardozo, L.; Stockler-Pinto, M.; Esgalhado, M.; Barboza, J.; Frauches, R.; Mafra, D. Does resistance exercise performed during dialysis modulate Nrf2 and NF-κB in patients with chronic kidney disease? Life Sci. 2017, 188, 192–197. [Google Scholar] [CrossRef]
- Magbanua, M.J.M.; Richman, E.L.; Sosa, E.V.; Jones, L.W.; Simko, J.; Shinohara, K.; Haqq, C.M.; Carroll, P.R.; Chan, J.M. Physical activity and prostate gene expression in men with low-risk prostate cancer. Cancer Causes Control 2014, 25, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Bian, Y.; Sun, Y.; Li, L.; Wang, L.; Zhao, C.; Shen, Y.; Song, Q.; Qu, Y.; Niu, S.; et al. Effects of moderate exercise over different phases on age-related physiological dysfunction in testes of SAMP8 mice. Exp. Gerontol. 2013, 48, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Fathi, R.; Nasiri, K.; Akbari, A.; Ahmadi-KaniGolzar, F.; Farajtabar, Z. Exercise protects against ethanol-induced damage in rat heart and liver through the inhibition of apoptosis and activation of Nrf2/Keap-1/HO-1 pathway. Life Sci. 2020, 256, 117958. [Google Scholar] [CrossRef] [PubMed]
- Done, A.J.; Traustadóttir, T. Nrf2 mediates redox adaptations to exercise. Redox Biol. 2016, 10, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Abramov, A.Y. The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol. Med. 2015, 88, 179–188. [Google Scholar] [CrossRef]
- Gureev, A.P.; Shaforostova, E.A.; Popov, V.N. Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction between the Nrf2 and PGC-1α Signaling Pathways. Front. Genet. 2019, 10, 435. [Google Scholar] [CrossRef]
- Holmström, K.M.; Baird, L.; Zhang, Y.; Hargreaves, I.; Chalasani, A.; Land, J.M.; Stanyer, L.; Yamamoto, M.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 Impacts Cellular Bioenergetics by Controlling Substrate Availability for Mitochondrial Respiration. Biol. Open 2013, 2, 761–770. [Google Scholar] [CrossRef]
- Kasai, S.; Shimizu, S.; Tatara, Y.; Mimura, J.; Itoh, K. Regulation of Nrf2 by Mitochondrial Reactive Oxygen Species in Physiology and Pathology. Biomolecules 2020, 10, 320. [Google Scholar] [CrossRef]
- Cordeiro, L.M.D.S.; Mario, G.; Moreira, C.C.L.; Rodrigues, A.H.; Wanner, S.P.; Soares, D.D.; Botion, L.M.; Ferreira, A.V.M. Aerobic training induces differential expression of genes involved in lipid metabolism in skeletal muscle and white adipose tissues. J. Cell. Biochem. 2019, 120, 18883–18893. [Google Scholar] [CrossRef]
- Mendham, A.E.; Larsen, S.; George, C.; Adams, K.; Hauksson, J.; Olsson, T.; Smidt, M.C.F.-D.; Nankam, P.A.N.; Hakim, O.; Goff, L.; et al. Exercise training results in depot-specific adaptations to adipose tissue mitochondrial function. Sci. Rep. 2020, 10, 3785. [Google Scholar] [CrossRef]
- Sakurai, T.; Izawa, T.; Kizaki, T.; Ogasawara, J.-E.; Shirato, K.; Imaizumi, K.; Takahashi, K.; Ishida, H.; Ohno, H. Exercise training decreases expression of inflammation-related adipokines through reduction of oxidative stress in rat white adipose tissue. Biochem. Biophys. Res. Commun. 2009, 379, 605–609. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Toppo, S.; Vanin, S.; Bosello, V.; Tosatto, S.C. TOPPO, Evolutionary and structural insights into the multifaceted glutathione peroxidase (Gpx) superfamily. Antioxid. Redox Signal. 2008, 10, 1501–1514. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.M.; Basak, A. Human catalase: Looking for complete identity. Protein Cell 2010, 1, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Sollanek, K.J.; Wiggs, M.P.; Demirel, H.; Smuder, A.J. Exercise-induced improvements in myocardial antioxidant capacity: The antioxidant players and cardioprotection. Free Radic. Res. 2013, 48, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Kwon, I.; Song, W.; Cosio-Lima, L.M.; Lee, Y. Endurance Exercise Mediates Neuroprotection Against MPTP-mediated Parkinson’s Disease via Enhanced Neurogenesis, Antioxidant Capacity, and Autophagy. Neuroscience 2018, 379, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Townsend, L.K.; Weber, A.J.; Barbeau, P.-A.; Holloway, G.P.; Wright, D.C. Reactive oxygen species-dependent regulation of pyruvate dehydrogenase kinase-4 in white adipose tissue. Am. J. Physiol. Physiol. 2020, 318, C137–C149. [Google Scholar] [CrossRef]
- Matta, L.; Fonseca, T.S.; Faria, C.C.; Lima-Junior, N.C.; De Oliveira, D.F.; Maciel, L.; Boa, L.F.; Pierucci, A.P.T.R.; Ferreira, A.C.F.; Nascimento, J.H.M.; et al. The Effect of Acute Aerobic Exercise on Redox Homeostasis and Mitochondrial Function of Rat White Adipose Tissue. Oxidative Med. Cell. Longev. 2021, 2021, 4593496. [Google Scholar] [CrossRef]
- Fortunato, R.S.; Ignácio, D.L.; Padron, S.; Peçanha, R.; Marassi, M.P.; Rosenthal, D.; Werneck-De-Castro, J.P.S.; Carvalho, D.P.; Werneck-De-Castro, J.P. The effect of acute exercise session on thyroid hormone economy in rats. J. Endocrinol. 2008, 198, 347–353. [Google Scholar] [CrossRef] [Green Version]
- Faude, O.; Kindermann, W.; Meyer, T. Lactate threshold concepts: How valid are they? Sports Med. 2009, 39, 469–490. [Google Scholar] [CrossRef]
- Cabrera, M.E.; Saidel, G.M.; Kalhan, S.C. Lactate metabolism during exercise: Analysis by an integrative systems model. Am. J. Physiol. Integr. Comp. Physiol. 1999, 277, R1522–R1536. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.; Agha-Alinejad, H.; Shamsi, M.M.; Jafari, M.; Voltarelli, F.A.; Naderi, A.; Earnest, C. Evaluation of efforts in untrained Wistar rats following exercise on forced running wheel at maximal lactate steady state. J. Exerc. Nutr. Biochem. 2017, 21, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Leopoldo, A.S.; Lima-Leopoldo, A.; Nascimento, A.; Luvizotto, R.; Sugizaki, M.; Campos, D.; Da Silva, D.; Padovani, C.; Cicogna, A.C. Classification of different degrees of adiposity in sedentary rats. Braz. J. Med Biol. Res. 2016, 49, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Zingaretti, M.C.; Cancello, R.; Ceresi, E.; Ferrara, P. Morphologic techniques for the study of brown adipose tissue and white adipose tissue. Methods Mol. Biol. 2003, 155, 21–51. [Google Scholar] [CrossRef]
- Lemonnier, D. Effect of age, sex, and site on the cellularity of the adipose tissue in mice and rats rendered obese by a high-fat diet. J. Clin. Investig. 1972, 51, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, R.S.; Braga, W.M.; Ortenzi, V.H.; Rodrigues, D.C.; Andrade, B.M.; Miranda-Alves, L.; Rondinelli, E.; Dupuy, C.; Ferreira, A.C.F.; Carvalho, D. Sexual Dimorphism of Thyroid Reactive Oxygen Species Production Due to Higher NADPH Oxidase 4 Expression in Female Thyroid Glands. Thyroid 2013, 23, 111–119. [Google Scholar] [CrossRef]
- Caldeira, D.d.A.F.; De Oliveira, D.F.; Cavalcanti-De-Albuquerque, J.P.; Nascimento, J.H.M.; Zin, W.A.; Maciel, L. Isolation of Mitochondria from Fresh Mice Lung Tissue. Front. Physiol. 2021, 12, 572870. [Google Scholar] [CrossRef]
- Maciel, L.; De Oliveira, D.F.; Monnerat, G.; De Carvalho, A.C.C.; Nascimento, J.H.M. Exogenous 10 kDa-Heat Shock Protein Preserves Mitochondrial Function After Hypoxia/Reoxygenation. Front. Pharmacol. 2020, 11, 545. [Google Scholar] [CrossRef]
- De Meis, L.; Ketzer, L.A.; Da Costa, R.M.; De Andrade, I.R.; Benchimol, M. Fusion of the Endoplasmic Reticulum and Mitochondrial Outer Membrane in Rats Brown Adipose Tissue: Activation of Thermogenesis by Ca2+. PLoS ONE 2010, 5, e9439. [Google Scholar] [CrossRef] [Green Version]
- Gedik, N.; Maciel, L.; Schulte, C.; Skyschally, A.; Heusch, G.; Kleinbongard, P. Cardiomyocyte mitochondria as targets of humoral factors released by remote ischemic preconditioning. Arch. Med. Sci. 2017, 2, 448–458. [Google Scholar] [CrossRef]
- Crapo, J.D.; McCord, J.M.; Fridovich, I. Preparation and assay of superioxide dismutases. Methods Enzymol. 1978, 53, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Flohé, L.; Günzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Arab, H.H.; Al-Shorbagy, M.Y.; Saad, M.A. Activation of autophagy and suppression of apoptosis by dapagliflozin attenuates experimental inflammatory bowel disease in rats: Targeting AMPK/mTOR, HMGB1/RAGE and Nrf2/HO-1 pathways. Chem. Interactions 2021, 335, 109368. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Wehr, N.B. Protein carbonylation: Avoiding pitfalls in the 2,4-dinitrophenylhydrazine assay. Redox Rep. 2009, 14, 159–166. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Schreck, R.; Rieberl, P.; Baeuerle, P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-xB transcription factor and HIV-1. EMBO J. 1991, 10, 2247–2258. [Google Scholar] [CrossRef]
- Barzilai, A.; Yamamoto, K.-I. DNA damage responses to oxidative stress. DNA Repair 2004, 3, 1109–1115. [Google Scholar] [CrossRef]
- Shimizu, I.; Yoshida, Y.; Suda, M.; Minamino, T. Cell Metabolism Review DNA Damage Response and Metabolic Disease. Cell Metab. 2014, 20, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Ruegsegger, G.N.; Booth, F.W. Health Benefits of Exercise. Cold Spring Harb. Perspect. Med. 2017, 8, a029694. [Google Scholar] [CrossRef] [PubMed]
- McKie, G.L.; Wright, D.C. Biochemical adaptations in white adipose tissue following aerobic exercise: From mitochondrial biogenesis to browning. Biochem. J. 2020, 477, 1061–1081. [Google Scholar] [CrossRef]
- Palou, M.; Sanchez, J.; Priego, T.; Rodriguez, A.M.; Picó, C.; Palou, A. Regional differences in the expression of genes involved in lipid metabolism in adipose tissue in response to short- and medium-term fasting and refeeding. J. Nutr. Biochem. 2010, 21, 23–33. [Google Scholar] [CrossRef]
- Palou, M.; Priego, T.; Sánchez, J.; Rodríguez, A.M.; Palou, A.; Picó, C. Gene Expression Patterns in Visceral and Subcutaneous Adipose Depots in Rats are Linked to Their Morphologic Features. Cell. Physiol. Biochem. 2009, 24, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Giles, E.D.; Steig, A.J.; Jackman, M.R.; Higgins, J.A.; Johnson, G.C.; Lindstrom, R.C.; MacLean, P.S. Exercise Decreases Lipogenic Gene Expression in Adipose Tissue and Alters Adipocyte Cellularity during Weight Regain after Weight Loss. Front. Physiol. 2016, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Tsiloulis, T.; Watt, M.J. Exercise and the Regulation of Adipose Tissue Metabolism. Prog. Mol. Biol. Transl. Sci. 2015, 135, 175–201. [Google Scholar] [CrossRef]
- De Melo, D.G.; Anaruma, C.P.; Rodrigues, K.C.D.C.; Pereira, R.M.; De Campos, T.D.P.; Canciglieri, R.S.; Ramos, C.O.; Cintra, D.E.; Ropelle, E.R.; Da Silva, A.S.R.; et al. Strength training alters the tissue fatty acids profile and slightly improves the thermogenic pathway in the adipose tissue of obese mice. Sci. Rep. 2022, 12, 6913. [Google Scholar] [CrossRef]
- Roberts, F.L.; Markby, G.R. New Insights into Molecular Mechanisms Mediating Adaptation to Exercise; A Review Focusing on Mitochondrial Biogenesis, Mitochondrial Function, Mitophagy and Autophagy. Cells 2021, 10, 2639. [Google Scholar] [CrossRef]
- Trevellin, E.; Scorzeto, M.; Olivieri, M.; Granzotto, M.; Valerio, A.; Tedesco, L.; Fabris, R.; Serra, R.; Quarta, M.; Reggiani, C.; et al. Exercise Training Induces Mitochondrial Biogenesis and Glucose Uptake in Subcutaneous Adipose Tissue Through eNOS-Dependent Mechanisms. Diabetes 2014, 63, 2800–2811. [Google Scholar] [CrossRef]
- Farhat, F.; Dupas, J.; Amérand, A.; Goanvec, C.; Feray, A.; Simon, B.; Guegueniat, N.; Moisan, C. Effect of exercise training on oxidative stress and mitochondrial function in rat heart and gastrocnemius muscle. Redox Rep. 2014, 20, 60–68. [Google Scholar] [CrossRef]
- No, M.-H.; Heo, J.-W.; Yoo, S.-Z.; Kim, C.-J.; Park, D.-H.; Kang, J.-H.; Seo, D.-Y.; Han, J.; Kwak, H.-B. Effects of aging and exercise training on mitochondrial function and apoptosis in the rat heart. Pflug. Arch. Eur. J. Physiol. 2020, 472, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Memme, J.M.; Erlich, A.T.; Phukan, G.; Hood, D.A. Exercise and mitochondrial health. J. Physiol. 2019, 599, 803–817. [Google Scholar] [CrossRef] [PubMed]
- Solmaz, S.R.; Hunte, C. Structure of Complex III with Bound Cytochrome c in Reduced State and Definition of a Minimal Core Interface for Electron Transfer. J. Biol. Chem. 2008, 283, 17542–17549. [Google Scholar] [CrossRef] [PubMed]
- Wende, A.R.; Young, M.E.; Chatham, J.; Zhang, J.; Rajasekaran, N.S.; Darley-Usmar, V.M. Redox biology and the interface between bioenergetics, autophagy and circadian control of metabolism. Free Radic. Biol. Med. 2016, 100, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhuang, J.; Hwang, P.M.; Heart, N. p53: Exercise capacity and metabolism. Curr. Opin. Oncol. 2014, 24, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Thyfault, J.P.; Bergouignan, A. Exercise and metabolic health: Beyond skeletal muscle. Diabetologia 2020, 63, 1464–1474. [Google Scholar] [CrossRef]
- Bartelt, A.; Heeren, J. Adipose tissue browning and metabolic health. Nat. Rev. Endocrinol. 2013, 10, 24–36. [Google Scholar] [CrossRef]
- Daiber, A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim. Biophys. Acta (BBA)-Bioenerg. 2010, 1797, 897–906. [Google Scholar] [CrossRef] [Green Version]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; DELLA-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Graham, K.A.; Kulawiec, M.; Owens, K.M.; Li, X.; Desouki, M.M.; Chandra, D.; Singh, K.K. NADPH oxidase 4 is an oncoprotein localized to mitochondria. Cancer Biol. Ther. 2010, 10, 223–231. [Google Scholar] [CrossRef]
- Lassègue, B.; Griendling, K.K. NADPH Oxidases: Functions and Pathologies in the Vasculature. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 653–661. [Google Scholar] [CrossRef]
- Chen, K.; Kirber, M.; Xiao, H.; Yang, Y.; Keaney, J. Regulation of ROS signal transduction by NADPH oxidase 4 localization. J. Cell Biol. 2008, 181, 1129–1139. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Den Hartigh, L.J.; Omer, M.; Goodspeed, L.; Wang, S.; Wietecha, T.; O’Brien, K.D.; Han, C.Y. Adipocyte-Specific Deficiency of NADPH Oxidase 4 Delays the Onset of Insulin Resistance and Attenuates Adipose Tissue Inflammation in Obesity. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 466–475. [Google Scholar] [CrossRef]
- Zhang, S.X.L.; Khalyfa, A.; Wang, Y.; Carreras, A.; Hakim, F.; A Neel, B.; Brady, M.J.; Qiao, Z.; Hirotsu, C.; Gozal, D. Sleep fragmentation promotes NADPH oxidase 2-mediated adipose tissue inflammation leading to insulin resistance in mice. Int. J. Obes. 2013, 38, 619–624. [Google Scholar] [CrossRef]
- DeVallance, E.; Li, Y.; Jurczak, M.J.; Cifuentes-Pagano, E.; Pagano, P.J. The Role of NADPH Oxidases in the Etiology of Obesity and Metabolic Syndrome: Contribution of Individual Isoforms and Cell Biology. Antioxid. Redox Signal. 2019, 31, 687–709. [Google Scholar] [CrossRef]
- Radak, Z.; Ishihara, K.; Tekus, E.; Varga, C.; Posa, A.; Balogh, L.; Boldogh, I.; Koltai, E. Exercise, oxidants, and antioxidants change the shape of the bell-shaped hormesis curve. Redox Biol. 2017, 12, 285–290. [Google Scholar] [CrossRef] [Green Version]
- Calabrese, E.J.; Kozumbo, W.J. The hormetic dose-response mechanism: Nrf2 activation. Pharmacol. Res. 2021, 167, 105526. [Google Scholar] [CrossRef]
- Louzada, R.A.N.; Bouviere, J.; Matta, L.P.; De Castro, J.P.S.W.; Dupuy, C.; De Carvalho, D.P.; Fortunato, R.S. Redox Signaling in Widespread Health Benefits of Exercise. Antioxidants Redox Signal. 2020, 33, 745–760. [Google Scholar] [CrossRef]
- Zoladz, J.A.; Koziel, A.; Broniarek, I.; Woyda-Ploszczyca, A.M.; Ogrodna, K.; Majerczak, J.; Celichowski, J.; Szkutnik, Z.; Jarmuszkiewicz, W. Effect of temperature on fatty acid metabolism in skeletal muscle mitochondria of untrained and endurance-trained rats. PLoS ONE 2017, 12, e0189456. [Google Scholar] [CrossRef]
- Juturu, V.; Sahin, K.; Pala, R.; Tuzcu, M.; Ozdemir, O.; Orhan, C.; Sahin, N. Curcumin prevents muscle damage by regulating NF-kB and Nrf2 pathways and improves performance: An in vivo model. J. Inflamm. Res. 2016, 9, 147–154. [Google Scholar] [CrossRef]
- Shanmugam, G.; Challa, A.K.; Devarajan, A.; Athmanathan, B.; Litovsky, S.H.; Krishnamurthy, P.; Davidson, C.J.; Rajasekaran, N.S. Exercise Mediated Nrf2 Signaling Protects the Myocardium from Isoproterenol-Induced Pathological Remodeling. Front. Cardiovasc. Med. 2019, 6, 68. [Google Scholar] [CrossRef]
- Cai, M.; Wang, H.; Li, J.-J.; Zhang, Y.-L.; Xin, L.; Li, F.; Lou, S.-J. The signaling mechanisms of hippocampal endoplasmic reticulum stress affecting neuronal plasticity-related protein levels in high fat diet-induced obese rats and the regulation of aerobic exercise. Brain Behav. Immun. 2016, 57, 347–359. [Google Scholar] [CrossRef]
- Egan, B.; Zierath, J.R. Exercise Metabolism and the Molecular Regulation of Skeletal Muscle Adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef]
- Rai, S.R.; Bhattacharyya, C.; Sarkar, A.; Chakraborty, S.; Sircar, E.; Dutta, S.; Sengupta, R. Glutathione: Role in Oxidative/Nitrosative Stress, Antioxidant Defense, and Treatments. ChemistrySelect 2021, 6, 4566–4590. [Google Scholar] [CrossRef]
- Dalleau, S.; Baradat, M.; Guéraud, F.; Huc, L. Cell death and diseases related to oxidative stress:4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef]
- Breitzig, M.; Bhimineni, C.; Lockey, R.; Kolliputi, N. 4-Hydroxy-2-nonenal: A critical target in oxidative stress? Am. J. Physiol. Cell Physiol. 2016, 311, C537–C543. [Google Scholar] [CrossRef] [Green Version]
- Morgan, M.J.; Liu, Z.-G. Crosstalk of reactive oxygen species and NF-kappaκB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Braune, J.; Weyer, U.; Hobusch, C.; Mauer, J.; Brüning, J.C.; Bechmann, I.; Gericke, M. IL-6 Regulates M2 Polarization and Local Proliferation of Adipose Tissue Macrophages in Obesity. J. Immunol. 2017, 198, 2927–2934. [Google Scholar] [CrossRef]
- Wedell-Neergaard, A.-S.; Lehrskov, L.L.; Christensen, R.H.; Legaard, G.E.; Dorph, E.; Larsen, M.K.; Launbo, N.; Fagerlind, S.R.; Seide, S.K.; Nymand, S.; et al. Exercise-Induced Changes in Visceral Adipose Tissue Mass Are Regulated by IL-6 Signaling: A Randomized Controlled Trial. Cell Metab. 2019, 29, 844–855.e3. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Petersen, A.M.W.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef]
- Vergoni, B.; Cornejo, P.-J.; Gilleron, J.; Djedaini, M.; Ceppo, F.; Jacquel, A.; Bouget, G.; Ginet, C.; Gonzalez, T.; Maillet, J.; et al. DNA Damage and the Activation of the p53 Pathway Mediate Alterations in Metabolic and Secretory Functions of Adipocytes. Diabetes 2016, 65, 3062–3074. [Google Scholar] [CrossRef]
- Rahmanian, N.; Shokrzadeh, M.; Eskandani, M. Recent advances in γH2AX biomarker-based genotoxicity assays: A marker of DNA damage and repair. DNA Repair 2021, 108, 103243. [Google Scholar] [CrossRef]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matta, L.; de Faria, C.C.; De Oliveira, D.F.; Andrade, I.S.; Lima-Junior, N.C.; Gregório, B.M.; Takiya, C.M.; Ferreira, A.C.F.; Nascimento, J.H.M.; de Carvalho, D.P.; et al. Exercise Improves Redox Homeostasis and Mitochondrial Function in White Adipose Tissue. Antioxidants 2022, 11, 1689. https://doi.org/10.3390/antiox11091689
Matta L, de Faria CC, De Oliveira DF, Andrade IS, Lima-Junior NC, Gregório BM, Takiya CM, Ferreira ACF, Nascimento JHM, de Carvalho DP, et al. Exercise Improves Redox Homeostasis and Mitochondrial Function in White Adipose Tissue. Antioxidants. 2022; 11(9):1689. https://doi.org/10.3390/antiox11091689
Chicago/Turabian StyleMatta, Leonardo, Caroline Coelho de Faria, Dahienne F. De Oliveira, Iris Soares Andrade, Niedson Correia Lima-Junior, Bianca Martins Gregório, Cristina Maeda Takiya, Andrea Claudia Freitas Ferreira, José Hamilton M. Nascimento, Denise Pires de Carvalho, and et al. 2022. "Exercise Improves Redox Homeostasis and Mitochondrial Function in White Adipose Tissue" Antioxidants 11, no. 9: 1689. https://doi.org/10.3390/antiox11091689
APA StyleMatta, L., de Faria, C. C., De Oliveira, D. F., Andrade, I. S., Lima-Junior, N. C., Gregório, B. M., Takiya, C. M., Ferreira, A. C. F., Nascimento, J. H. M., de Carvalho, D. P., Bartelt, A., Maciel, L., & Fortunato, R. S. (2022). Exercise Improves Redox Homeostasis and Mitochondrial Function in White Adipose Tissue. Antioxidants, 11(9), 1689. https://doi.org/10.3390/antiox11091689