Effects of the Partial Substitution of Corn with Wheat or Barley on the Growth Performance, Blood Antioxidant Capacity, Intestinal Health and Fecal Microbial Composition of Growing Pigs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Treatment and Experimental Design
2.2. Direction Indicators
2.2.1. Growth Performance
2.2.2. Blood Antioxidant Capacity
2.2.3. Morphology of the Small Intestine
2.2.4. Volatile Fatty Acid Analysis
2.2.5. Nutrient Digestibility
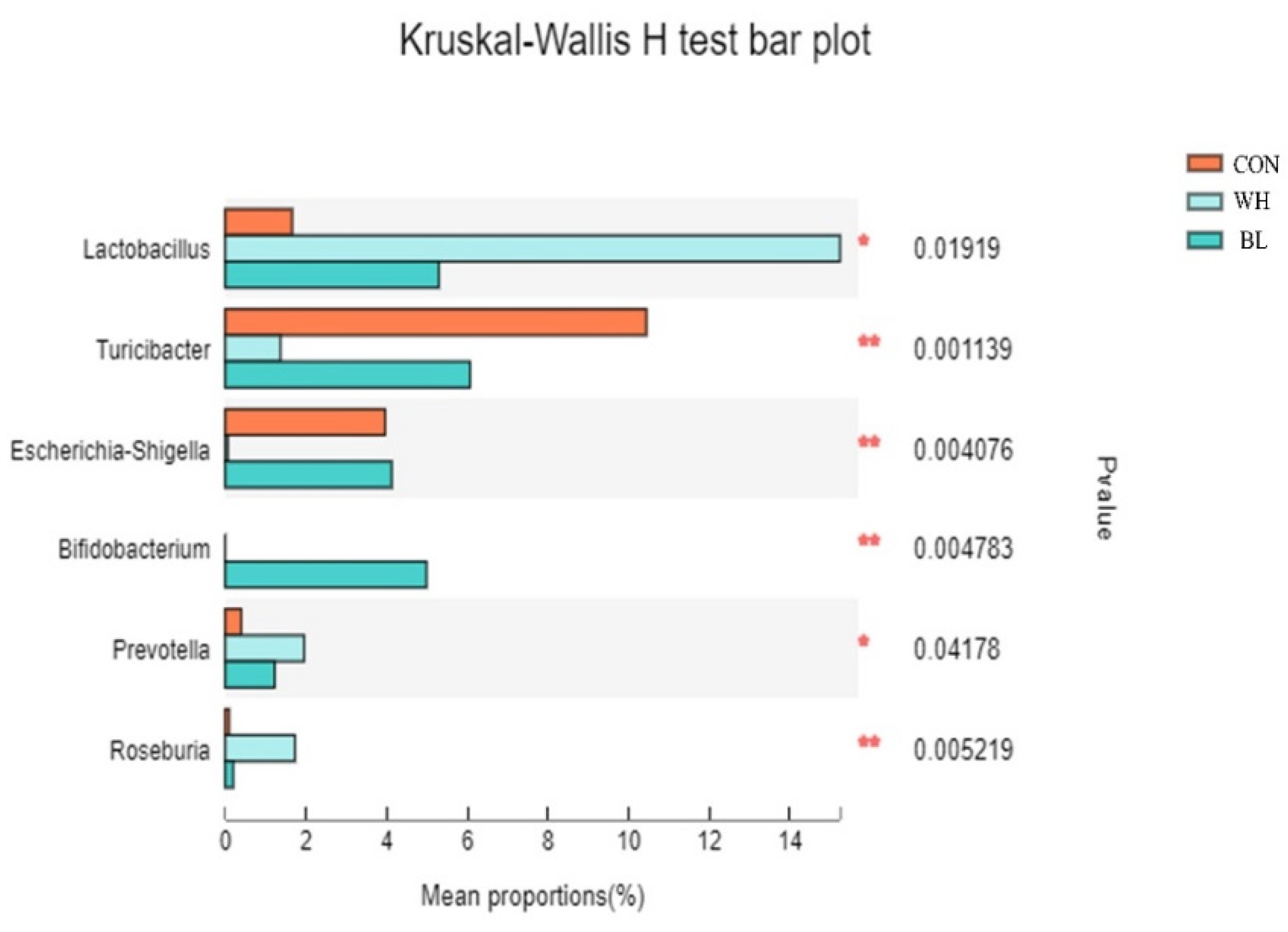
2.2.6. Microbiota Analysis by 16S RNA
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Barrera, M.; Cervantes, M.; Sauer, W.C.; Araiza, A.B.; Torrentera, N.; Cervantes, M. Ileal amino acid digestibility and performance of growing pigs fed wheat-based diets supplemented with xylanase. J. Anim. Sci. 2004, 82, 1997–2003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cromwell, G.L. Impacts of genetically modified, low-phytate corn soybean meal and transgenic pigs possessing salivary phytase on phosphorus excretion. New Dev. Regarding Pig’s Need Vitam. E A C 2002, 5, 59. [Google Scholar]
- NRC National Research Council. Nutrient Requirements of Swine, 11th ed.; National Academic Press: Washington, DC, USA, 2012. [Google Scholar]
- Yin, Y.; Wang, F.; Yang, M. Lycium barbarum polysaccharides as antibiotic substitutes improve growth Performance, serum immunity, antioxidant status, and intestinal health for weaned piglets. Front. Microbiol. 2022, 12, 819993. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zhang, C.; Yang, Y.; Mu, C.; Su, Y.; Yu, K. Long-term effects of early antibiotic intervention on blood parameters, apparent nutrient digestibility, and fecal microbial fermentation profile in pigs with different dietary protein levels. BioMed Cent. 2017, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Steven, L.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Nasir, Z.; Wang, L.F.; Young, M.G.; Swift, M.L.; Zijlstra, R.T. The effect of feeding barley on diet nutrient digestibility and growth performance of starter pigs. Anim. Feed. Sci. Tech. 2015, 210, 287–294. [Google Scholar] [CrossRef]
- Bruneau, C.D.; Chavez, E.R. Dietary preferences for cereals of nursing and weaned piglets. Livest. Prod. Sci. 1995, 41, 225–231. [Google Scholar] [CrossRef]
- Mavromichalis, I.; Hancock, J.D.; Senne, B.W.; Gugle, T.L.; Kennedy, G.A.; Hines, R.H.; Wyatt, C.L. Enzyme supplementation and particle size of wheat in diets for nursery and finishing pigs. J. Anim. Sci. 2000, 78, 3086–3095. [Google Scholar] [CrossRef] [Green Version]
- Lyberg, K.; Andersson, H.K.; Sands, J.S.; Lindberg, J.E. Influence of phytase and xylanase supplementation of a wheat-based diet on digestibility and performance in growing pigs. Acta. Agric. Scand. 2008, 58, 146–151. [Google Scholar] [CrossRef]
- Pedersen, C.; Boersma, M.G.; Stein, H.H. Digestibility of energy and phosphorus in ten samples of distillers dried grains with solubles fed to growing pigs. J. Anim. Sci. 2007, 85, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Lm, A.; Jrp, B.; Djh, B. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals—ScienceDirect. Anim. Feed. Sci. Technol. 2003, 108, 95–117. [Google Scholar]
- Willamil, J.; Badiola, I.; Devillard, E.; Geraert, P.A.; Torrallardona, D. Wheat-barley-rye- or corn-fed growing pigs respond differently to dietary supplementation with a carbohydrase complex. J. Anim. Sci. 2012, 90, 824–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Högberg, A.; Lindberg, J.E. The effect of level and type of cereal non-starch polysaccharides on the performance, nutrient utilization and gut environment of pigs around weaning. Anim. Feed. Sci. Technol. 2006, 127, 200–219. [Google Scholar] [CrossRef]
- Haenen, D.; Zhang, J.; Souza da Silva, C.; Bosch, G.; van der Meer, I.M.; van Arkel, J.; van den Borne, J.J.; Pérez Gutiérrez, O.; Smidt, H.; Kemp, B.; et al. A diet high in resistant starch modulates microbiota composition, SCFA concentrations, and gene expression in pig intestine. J. Nutr. 2013, 143, 274–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diebold, G.; Mosenthin, R.; Piepho, H.P.; Sauer, W.C. Effect of supplementation of xylanase and phospholipase to a wheat-based diet for weanling pigs on nutrient digestibility and concentrations of microbial metabolites in ileal digesta and feces. J. Anim. Sci. 2004, 82, 2647–2656. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.L.; Mcevoy, J.; Schulze, H.; Hennig, U.; Souffrant, W.B.; Mccracken, K.J. Apparent digestibility (ileal and overall) of nutrients and endogenous nitrogen losses in growing pigs fed wheat (Var. Soissons) or its by-products without or with xylanase supplementation. Livest. Prod. Sci. 2000, 62, 119–132. [Google Scholar] [CrossRef]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [Green Version]
- Louis, P.; Scott, K.P.; Duncan, S.H.; Flint, H.J. Understanding the effects of diet on bacterial metabolism in the large intestine. J. Appl. Microbiol. 2007, 102, 1197–1208. [Google Scholar] [CrossRef]
- Hamaker, B.R.; Tuncil, Y.E. A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J. Mol. Biol. 2014, 426, 3838–3850. [Google Scholar] [CrossRef]
- Drew, M.D.; Kessel, A.; Estrada, A.E.; Ekpe, E.D.; Zijlstra, R.T. Effect of dietary cereal on intestinal bacterial populations in weaned pigs. Can. J. Anim. Sci. 2002, 82, 607–609. [Google Scholar] [CrossRef]
- Canani, R.B.; Costanzo, M.D.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell. Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devillard, E.; McIntosh, F.M.; Duncan, S.H.; Wallace, R.J. Metabolism of linoleic acid by human gut bacteria: Different routes for biosynthesis of conjugated linoleic acid. J. Bacteriol. 2007, 189, 2566–2570. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Santana, C.; Castellote, C.; Castell, M.; Moltó-Puigmartí, C.; Rivero, M.; Pérez-Cano, F.J.; Franch, A. Enhancement of antibody synthesis in rats by feeding cis-9, trans-11 conjugated linoleic acid during early life. J. Nutr. Biochem. 2011, 22, 495–501. [Google Scholar] [CrossRef]
- Huang, Y.S.; Lin, Z.D.; Rong, H.; Hao, M.L.; Zhu, D.S.; Li, S.K.; Wen, X.B. Effects of conjugated linoleic acid on growth, body composition, antioxidant status, lipid metabolism and immunity parameters of juvenile Chu’s croaker, Nibea coibor. Aquacul. Res. 2018, 49, 546–556. [Google Scholar] [CrossRef]
- Mitsuoka, T. Bifidobacteria and their role in human health. J. Ind. Microbiol. 1990, 6, 263–267. [Google Scholar] [CrossRef]
- Moya-Pérez, A.; Perez-Villalba, A.; Benítez-Páez, A.; Campillo, I.; Sanz, Y. Bifidobacterium CECT 7765 modulates early stress-induced immune, neuroendocrine and behavioral alterations in mice. Brain. Behav. Immun. 2017, 65, 43–56. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, Q.; Chang, J.; Yin, Q.; Song, A.; Li, Z.; Wang, E.; Lu, F. Effects of Lactobacillus casei and Enterococcus faecalis on growth performance, immune function and gut microbiota of suckling piglets. Arch. Anim. Nutr. 2017, 71, 120–133. [Google Scholar] [CrossRef]
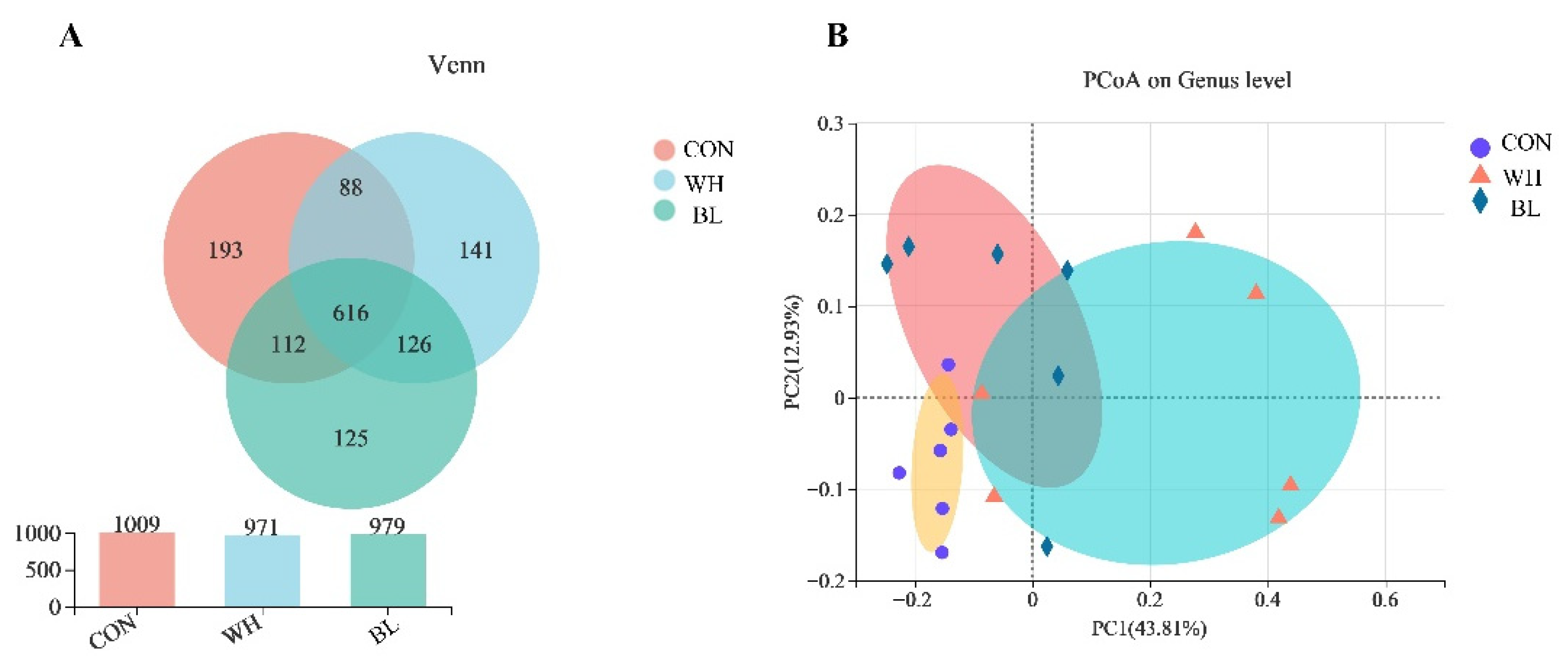
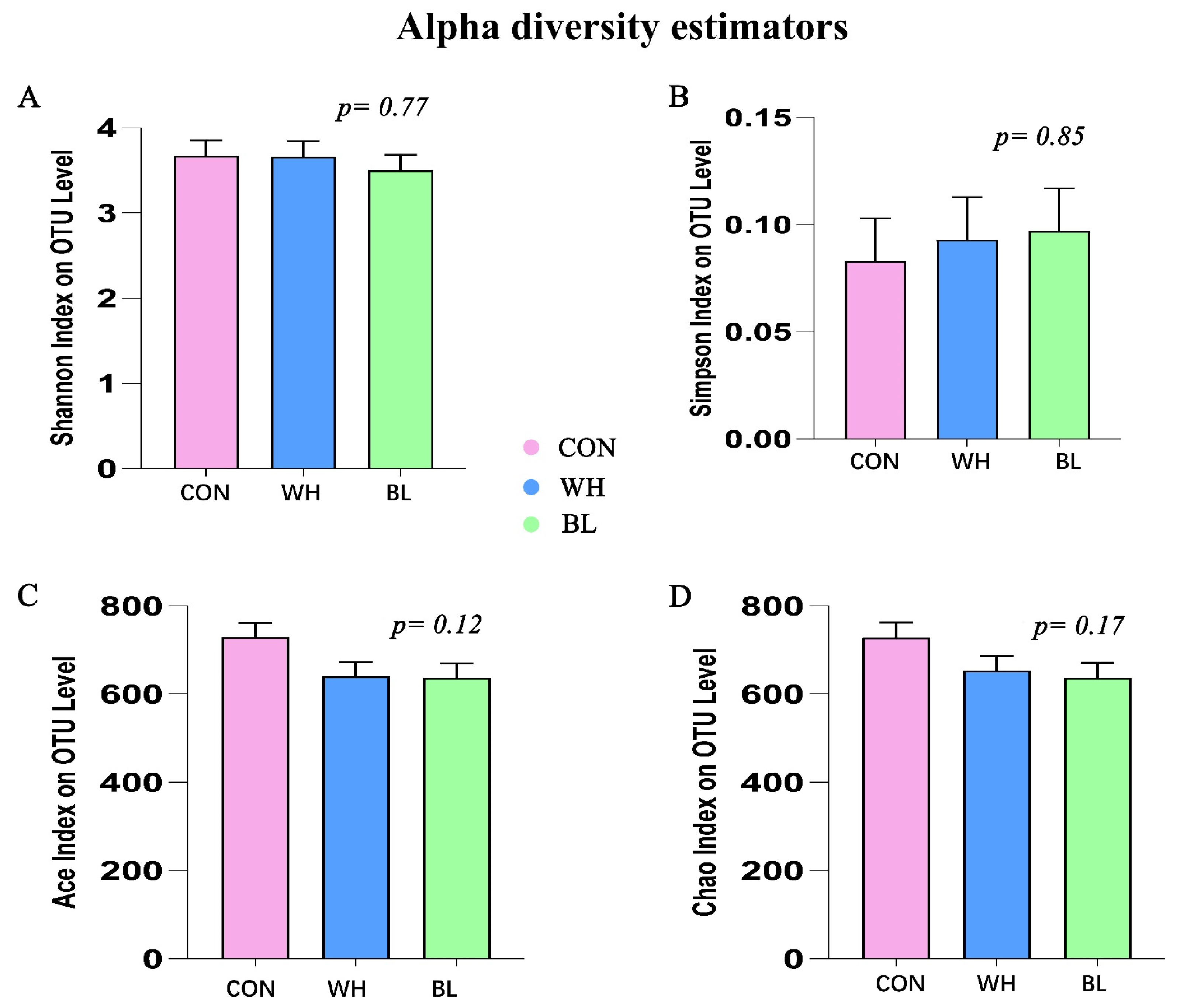
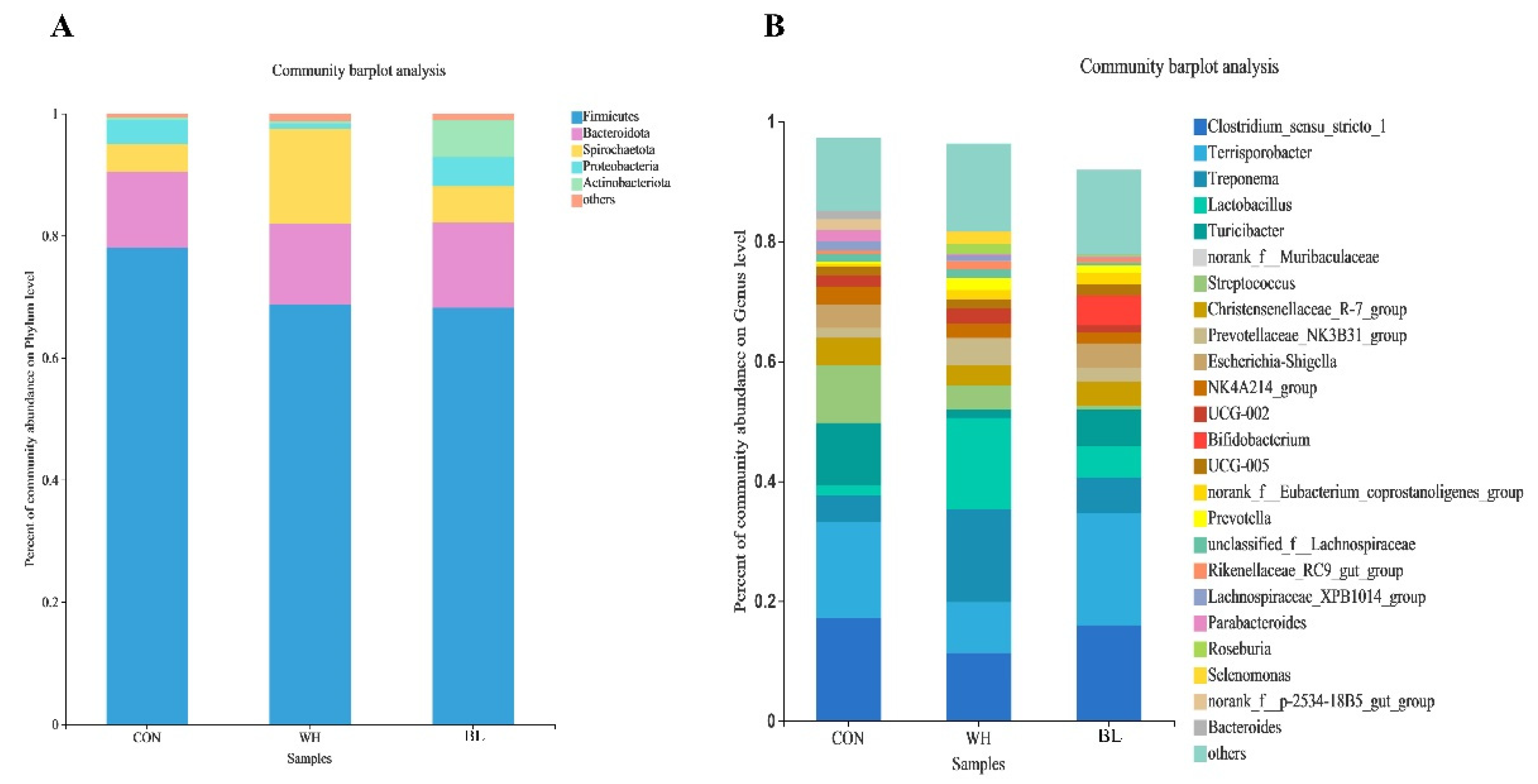
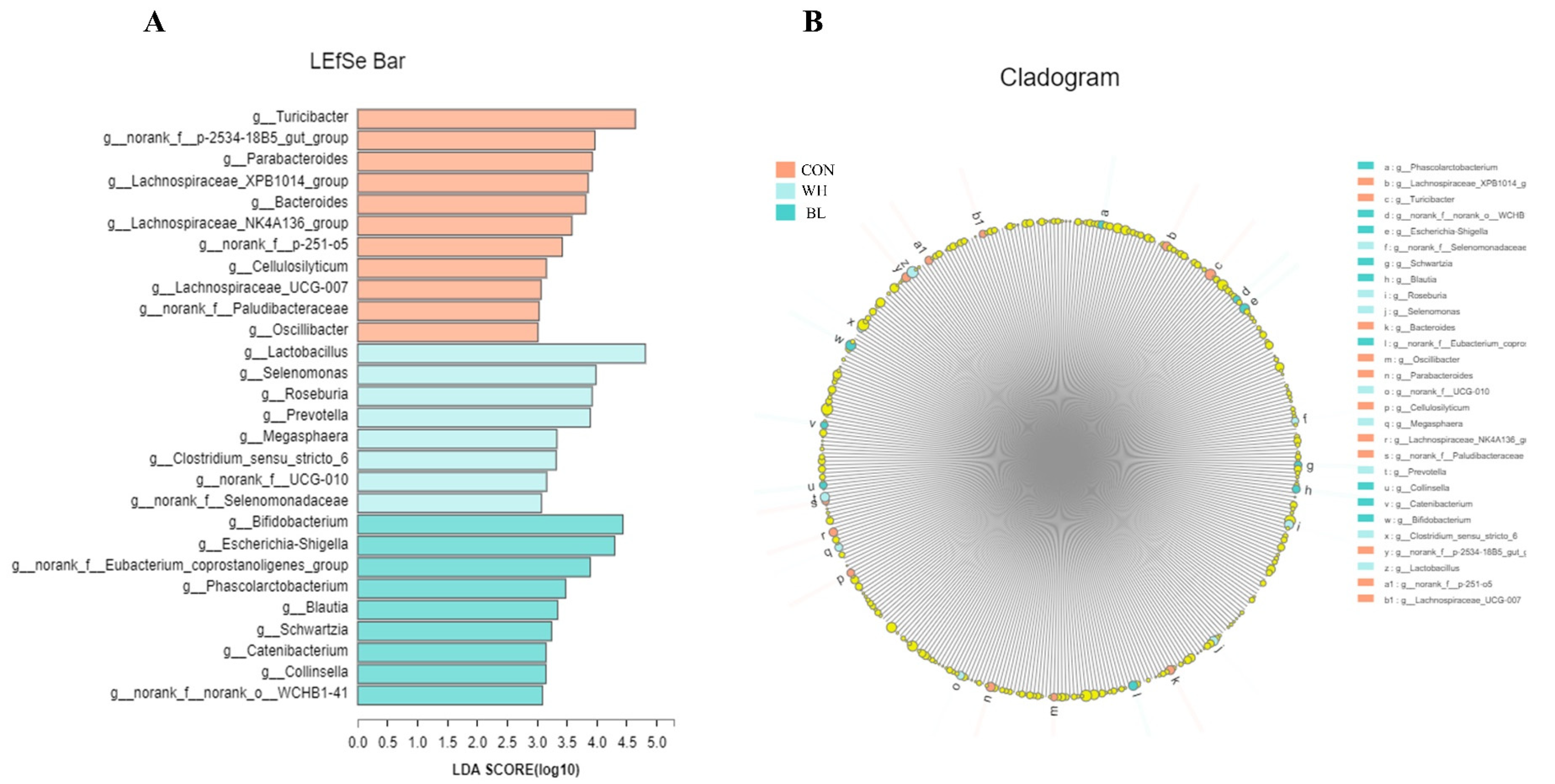
| Items | Treatments | ||
|---|---|---|---|
| CON | WH | BL | |
| Ingredients | |||
| Corn | 66.00 | 35.00 | 33.00 |
| Wheat | 0 | 38.00 | 0 |
| Barley | 0 | 0 | 37.25 |
| Soybean meal | 20.35 | 14.00 | 16.00 |
| Wheat bran | 8.00 | 7.45 | 6.39 |
| Soybean oil | 0.3 | 0.20 | 2 |
| L-lysine-HCl | 0.25 | 0.34 | 0.32 |
| DL-methionine | 0 | 0 | 0.06 |
| L-tryptophan | 0 | 0 | 0 |
| L-threonine | 0.1 | 0.06 | 0.09 |
| L-valine | 0 | 0 | 0.02 |
| Dicalcium phosphate | 1.70 | 1.70 | 1.70 |
| Limestone | 0.35 | 0.30 | 0.20 |
| Choline chloride | 0.35 | 0.35 | 0.35 |
| Salt | 0.35 | 0.35 | 0.37 |
| Cr2O3 | 0.25 | 0.25 | 0.25 |
| Vitamin-mineral premixa x | 2.00 | 2.00 | 2.00 |
| Total | 100.00 | 100.00 | 100.00 |
| Calculated composition | |||
| Digestible energy (MJ/kg) | 14.13 | 14.13 | 14.13 |
| Digestible lysine | 0.86 | 0.85 | 0.85 |
| Digestible methionine + cysteine | 0.48 | 0.48 | 0.48 |
| Digestible threonine | 0.53 | 0.52 | 0.52 |
| Digestible tryptophan | 0.15 | 0.15 | 0.15 |
| Digestible L-valine | 0.56 | 0.55 | 0.55 |
| Analyzed composition | |||
| Crude protein | 15.10 | 15.15 | 15.10 |
| Calcium | 0.56 | 0.56 | 0.56 |
| Available phosphorus | 0.43 | 0.42 | 0.43 |
| Items | Treatments | SEM 1 | p-Value | ||
|---|---|---|---|---|---|
| CON | WH | BL | |||
| Initial weight (kg) | 51.06 | 50.84 | 50.93 | 0.29 | 0.88 |
| Final weight (kg) | 78.05 | 77.83 | 77.26 | 0.31 | 0.22 |
| ADFI (g) | 2687.01 | 2679.17 | 2656.37 | 22.38 | 0.62 |
| ADG (g) | 964.29 | 963.57 | 940.48 | 7.97 | 0.10 |
| F/G | 2.79 | 2.78 | 2.82 | 0.04 | 0.69 |
| Items | Treatments | SEM 1 | p-Value | ||
|---|---|---|---|---|---|
| CON | WH | BL | |||
| SOD (IU/mL) | 124.44 | 124.78 | 125.77 | 3.61 | 0.96 |
| MDA (nmol/mL) | 1.69 | 1.73 | 1.66 | 0.08 | 0.81 |
| GSH-Px (IU/mL) | 688.52 | 678.46 | 680.18 | 11.42 | 0.72 |
| T-AOC (IU/mL) | 2.57 | 2.54 | 2.52 | 0.08 | 0.89 |
| Items | Treatments | SEM 1 | p-Value | ||
|---|---|---|---|---|---|
| CON | WH | BL | |||
| Dry matter | 81.17 | 80.58 | 80.36 | 0.27 | 0.13 |
| Organic matter | 83.29 | 82.72 | 82.54 | 0.29 | 0.19 |
| Crude protein | 75.19 | 76.31 | 76.22 | 0.57 | 0.33 |
| Gross energy | 81.28 | 80.39 | 80.46 | 0.35 | 0.17 |
| Items | Treatments | SEM 1 | p-Value | ||
|---|---|---|---|---|---|
| CON | WH | BL | |||
| Duodenum | |||||
| Villus height | 472.13 b | 535.06 a | 478.34 b | 14.73 | 0.02 |
| Crypt depth | 314.53 | 303.27 | 321.01 | 7.76 | 0.31 |
| Villus height/Crypt depth | 1.50 b | 1.76 a | 1.49 b | 0.05 | 0.01 |
| Jejunum | |||||
| Villus height | 505.47 | 496.86 | 483.62 | 10.91 | 0.39 |
| Crypt depth | 325.15 | 340.22 | 344.41 | 20.04 | 0.78 |
| Villus height/Crypt depth | 1.58 | 1.48 | 1.42 | 0.08 | 0.44 |
| Ileum | |||||
| Villus height | 497.68 | 481.12 | 510.48 | 15.91 | 0.45 |
| Crypt depth | 308.71 | 348.05 | 332.66 | 14.95 | 0.22 |
| Villus height/Crypt depth | 1.64 | 1.39 | 1.55 | 0.11 | 0.32 |
| Items | Treatments | SEM 1 | p-Value | ||
|---|---|---|---|---|---|
| CON | WH | BL | |||
| Cecum | |||||
| Acetic acid | 4956.20 b | 5180.10 b | 6214.76 a | 274.98 | 0.02 |
| Propionic acid | 2360.80 b | 2595.71 ab | 3088.21 a | 161.17 | 0.02 |
| Isobutyric acid | 64.72 | 103.71 | 85.06 | 13.03 | 0.18 |
| Butyric acid | 987.60 b | 1458.21 a | 1659.04 a | 167.98 | 0.03 |
| Isovaleric acid | 83.77 | 109.48 | 110.47 | 14.40 | 0.40 |
| Valeric acid | 165.25 | 232.45 | 238.88 | 21.78 | 0.10 |
| Total VFA | 8618.34 b | 9679.66 b | 11,396.42 a | 560.65 | 0.02 |
| Colon | |||||
| Acetic acid | 4220.56 | 4178.09 | 4633.12 | 134.98 | 0.08 |
| Propionic acid | 2444.48 b | 2156.67 b | 2885.20 a | 168.99 | 0.03 |
| Isobutyric acid | 48.58 | 36.15 | 55.44 | 5.01 | 0.08 |
| Butyric acid | 1164.78 | 1091.45 | 1355.02 | 99.94 | 0.27 |
| Isovaleric acid | 91.87 | 80.82 | 97.83 | 4.89 | 0.10 |
| Valeric acid | 196.56 | 193.67 | 206.67 | 15.01 | 0.77 |
| Total VFA 2 | 8166.82 b | 7736.85 b | 9233.28 a | 280.89 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Li, Z.; Zhang, Y. Effects of the Partial Substitution of Corn with Wheat or Barley on the Growth Performance, Blood Antioxidant Capacity, Intestinal Health and Fecal Microbial Composition of Growing Pigs. Antioxidants 2022, 11, 1614. https://doi.org/10.3390/antiox11081614
Ma X, Li Z, Zhang Y. Effects of the Partial Substitution of Corn with Wheat or Barley on the Growth Performance, Blood Antioxidant Capacity, Intestinal Health and Fecal Microbial Composition of Growing Pigs. Antioxidants. 2022; 11(8):1614. https://doi.org/10.3390/antiox11081614
Chicago/Turabian StyleMa, Xiaokang, Zhiqing Li, and Yuebo Zhang. 2022. "Effects of the Partial Substitution of Corn with Wheat or Barley on the Growth Performance, Blood Antioxidant Capacity, Intestinal Health and Fecal Microbial Composition of Growing Pigs" Antioxidants 11, no. 8: 1614. https://doi.org/10.3390/antiox11081614
APA StyleMa, X., Li, Z., & Zhang, Y. (2022). Effects of the Partial Substitution of Corn with Wheat or Barley on the Growth Performance, Blood Antioxidant Capacity, Intestinal Health and Fecal Microbial Composition of Growing Pigs. Antioxidants, 11(8), 1614. https://doi.org/10.3390/antiox11081614







