A Natural Gas Fermentation Bacterial Meal (FeedKind®) as a Functional Alternative Ingredient for Fishmeal in Diet of Largemouth Bass, Micropterus salmoides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Growth Trial and Sample Collection
2.2. Experimental Fish, Feeding and Sampling
2.3. Chemical Analysis
2.4. Liver Function, Metabolites and Immunity Response in Plasma and Distal Intestine Tissue
2.5. Histopathological Examination of the Distal Intestine
2.6. Quantitative Real-Time PCR
2.7. Western Blot
2.8. Gut Microbiota
2.9. Statistical Analysis
3. Results
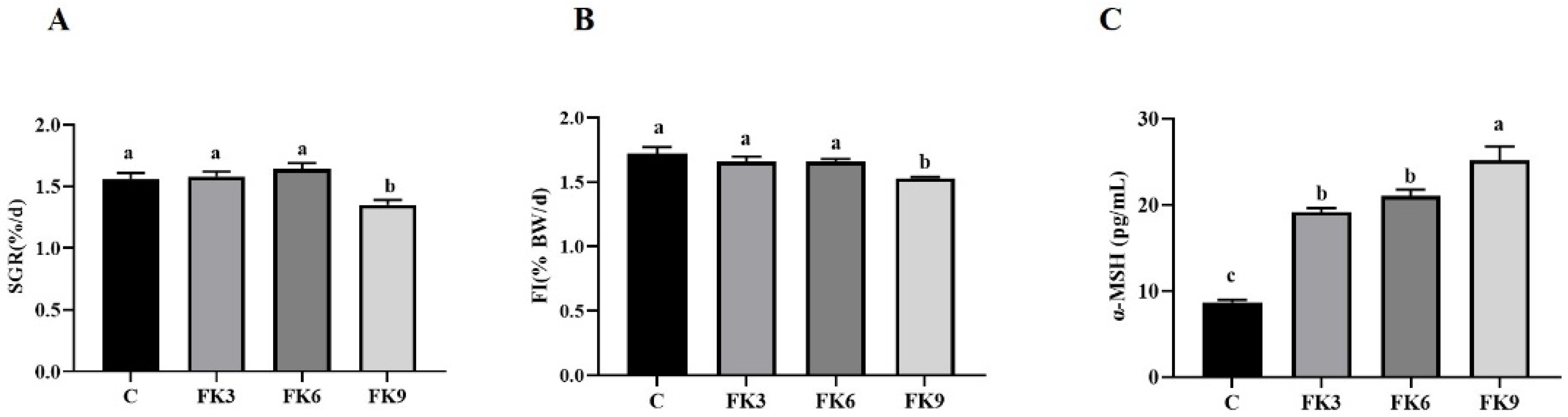
3.1. Growth Performance and Morphometric Parameters
3.2. Macro-Nutrient Compositions and Copper (Cu) Content in Whole Body, Liver Tissue and Feces
3.3. The Apparent Digestibility Coefficients (ADC) of Dry Matter, Protein, Energy and Cu in Test Diets
3.4. Liver Function and Metabolites in Plasma
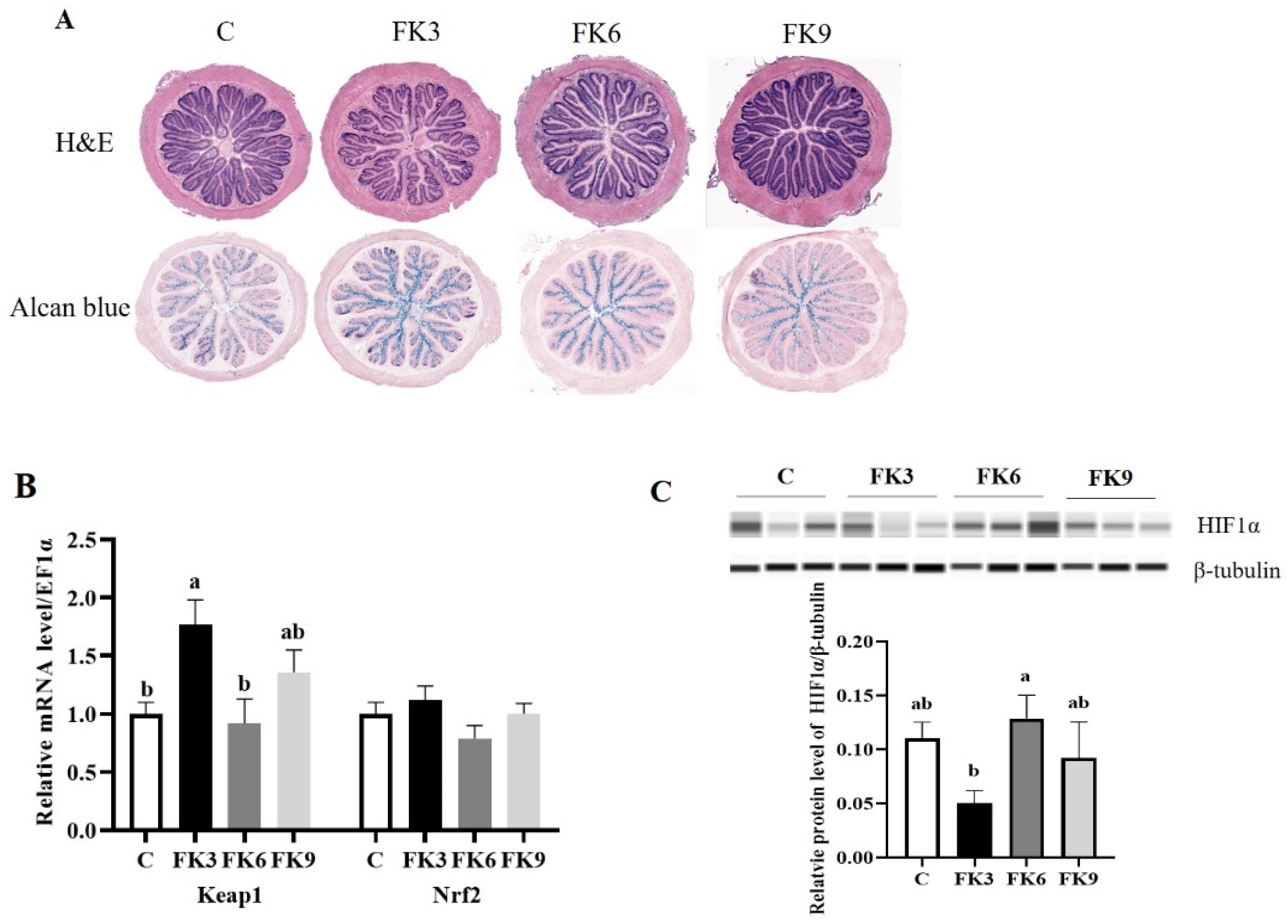
3.5. Histopathology and Systematical Antioxidant Responses
3.6. Immunity and Inflammatory Responses
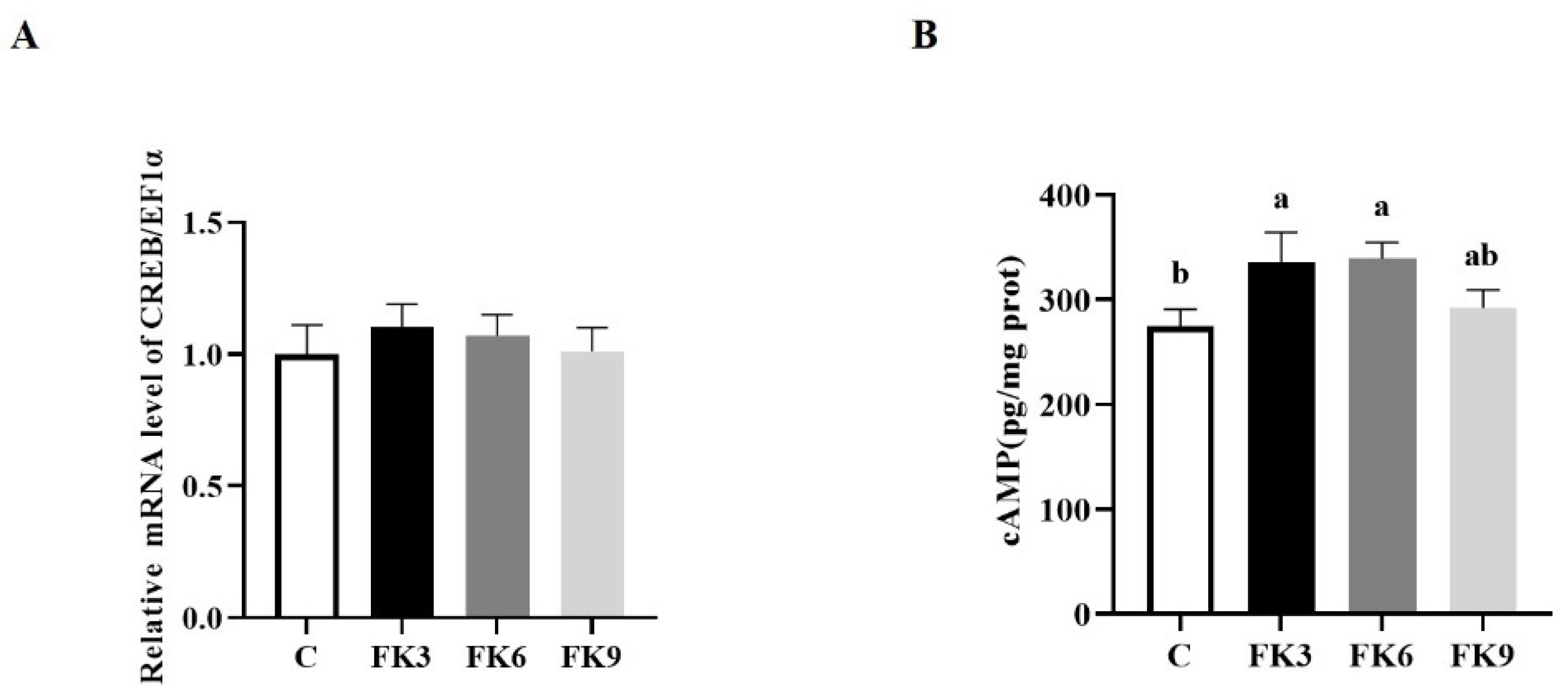
3.7. Energy Metabolism in Distal Intestine
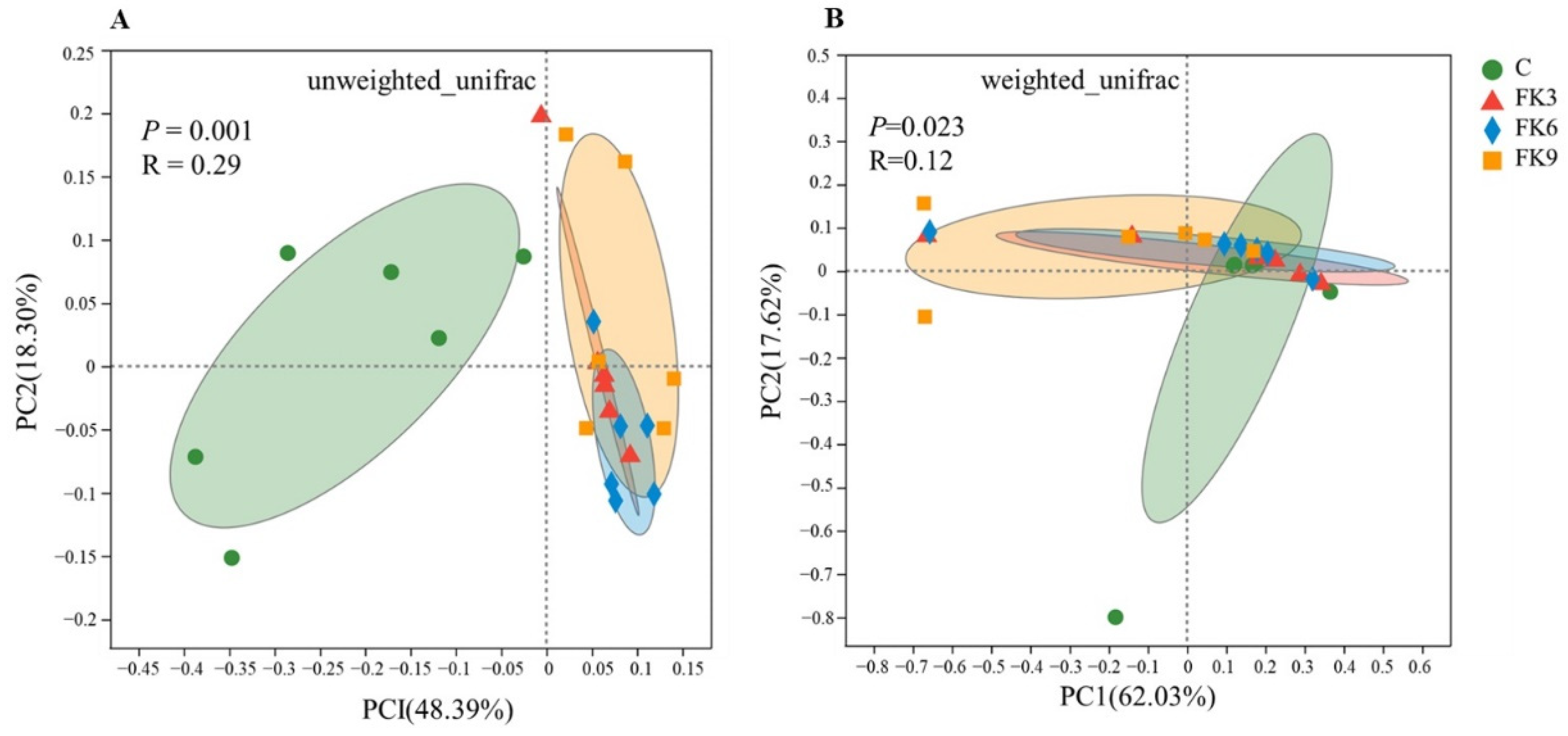
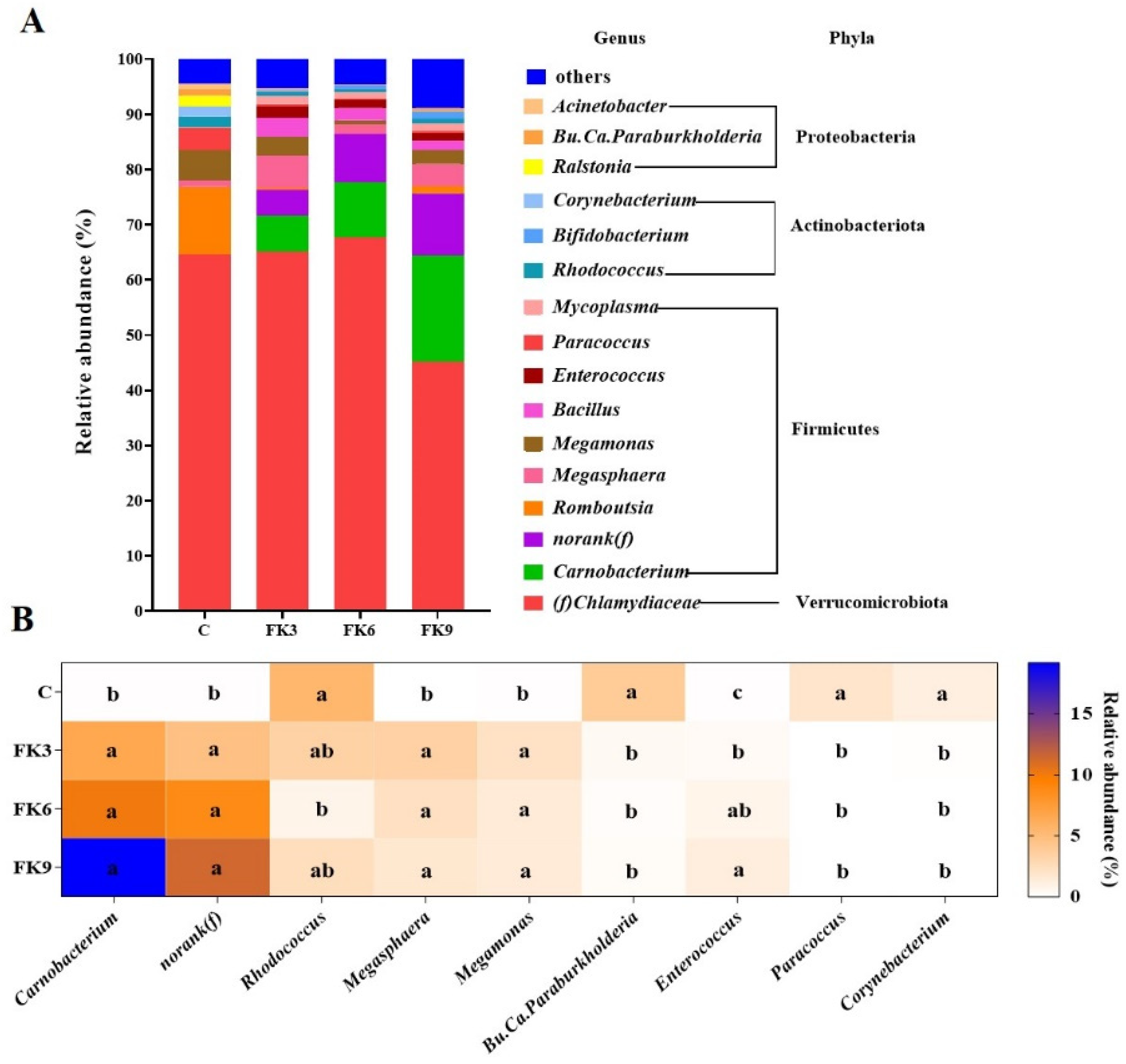
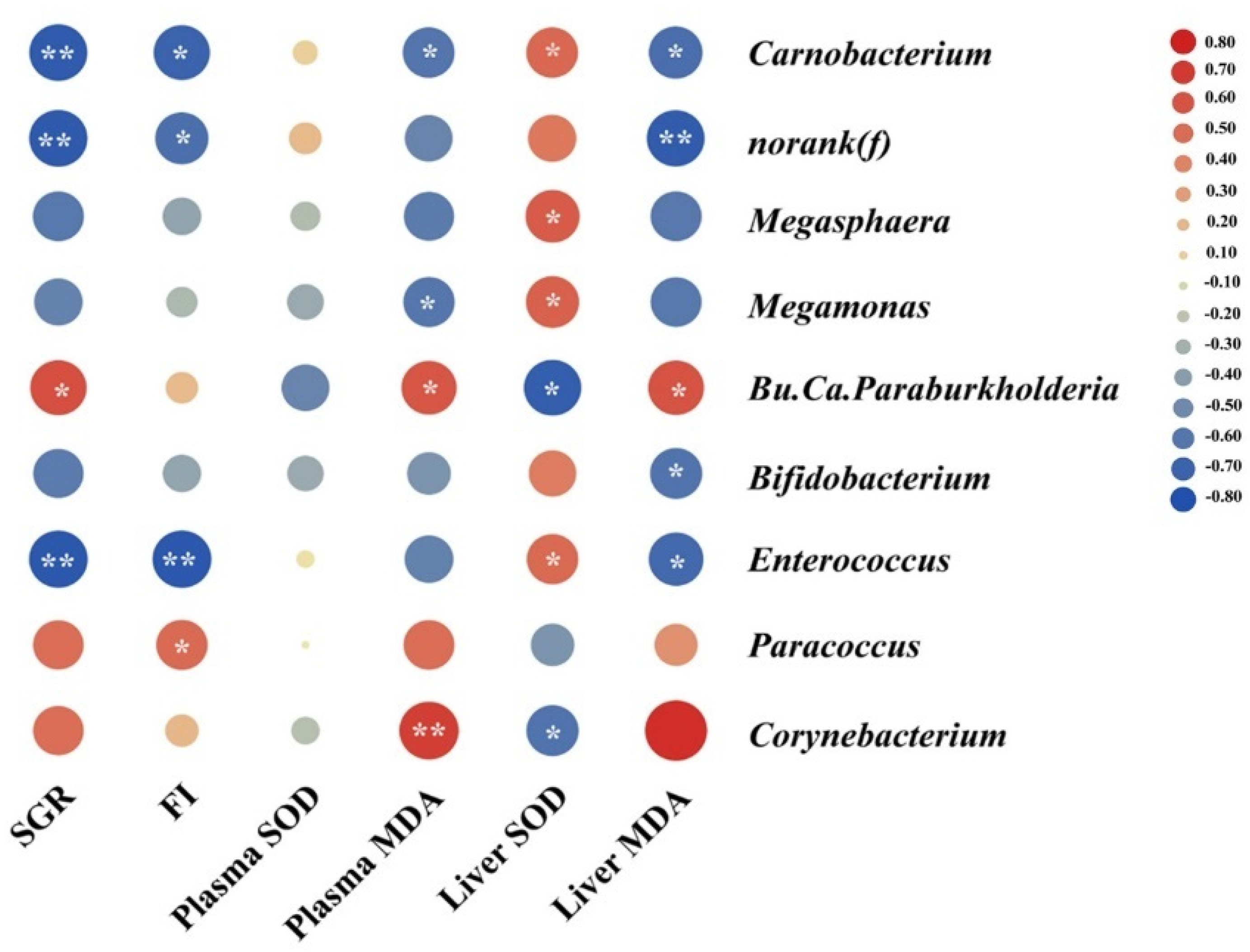
3.8. Gut Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miles, R.D.; Chapman, F.A. The benefits of fish meal in aquaculture diets. EDIS UF/IFAS Ext. 2006, 1–12. Available online: https://edis.ifas.ufl.edu/publication/fa122 (accessed on 29 June 2022). [CrossRef]
- Lu, F.; Haga, Y.; Satoh, S. Effects of replacing fish meal with rendered animal protein and plant protein sources on growth response, biological indices, and amino acid availability for rainbow trout Oncorhynchus mykiss. Fish. Sci. 2015, 81, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization of the United Nations (FAO). The State of World Fisheries and Aquaculture 2020. Sustainability in Action; FAO: Rome, Italy, 2020; p. 47. [Google Scholar] [CrossRef]
- Goytortúa-Bores, E.; Civera-Cerecedo, R.; Rocha-Meza, S.; Green-Yee, A. Partial replacement of red crab (Pleuroncodes planipes) meal for fish meal in practical diets for the white shrimp Litopenaeus vannamei. Effects on growth and in vivo digestibility. Aquaculture 2006, 256, 414–422. [Google Scholar] [CrossRef]
- Kaushik, S.J.; Seiliez, I. Protein and amino acid nutrition and metabolism in fish: Current knowledge and future needs. Aquac. Res. 2010, 41, 322–332. [Google Scholar] [CrossRef]
- Hardy, R.W.; Brezas, A. Diet formulation and manufacture. In Fish Nutrition; Academic Press: Cambridge, MA, USA, 2022; pp. 651–652. [Google Scholar]
- Barroso, F.G.; de Haro, C.; Sánchez-Muros, M.-J.; Venegas, E.; Martínez-Sánchez, A.; Pérez-Bañón, C. The potential of various insect species for use as food for fish. Aquaculture 2014, 422, 193–201. [Google Scholar] [CrossRef]
- Jones, S.W.; Karpol, A.; Friedman, S.; Maru, B.T.; Tracy, B. Recent advances in single cell protein use as a feed ingredient in aquaculture. Curr. Opin. Biotechnol. 2020, 61, 189–197. [Google Scholar] [CrossRef]
- Øverland, M.; Tauson, A.H.; Shearer, K.; Skrede, A. Evaluation of methane-utilising bacteria products as feed ingredients for monogastric animals. Arch. Anim. Nutr. 2010, 64, 171–189. [Google Scholar] [CrossRef]
- Dalton, H. The Leeuwenhoek Lecture 2000 the natural and unnatural history of methane-oxidizing bacteria. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 1207–1222. [Google Scholar] [CrossRef] [PubMed]
- Hanson, R.S.; Hanson, T. Methanotrophic bacteria. Microbiol. Rev. 1996, 60, 439–471. [Google Scholar] [CrossRef]
- Sharif, M.; Zafar, M.H.; Aqib, A.I.; Saeed, M.; Farag, M.R.; Alagawany, M.J.A. Single cell protein: Sources, mechanism of production, nutritional value and its uses in aquaculture nutrition. Aquaculture 2021, 531, 735885. [Google Scholar] [CrossRef]
- Storebakken, T.; Baeverfjord, G.; Skrede, A.; Olli, J.J.; Berge, G.M. Bacterial protein grown on natural gas in diets for Atlantic salmon, Salmo salar, in freshwater. Aquaculture 2004, 241, 413–425. [Google Scholar] [CrossRef]
- Skrede, A.; Berge, G.; Storebakken, T.; Herstad, O.; Aarstad, K.; Sundstøl, F. Digestibility of bacterial protein grown on natural gas in mink, pigs, chicken and Atlantic salmon. Anim. Feed Sci. Technol. 1998, 76, 103–116. [Google Scholar] [CrossRef]
- Øverland, M.; Petter Kjos, N.; Skrede, A. Effect of bacterial protein meal grown on natural gas on growth performance and carcass traits of pigs. Ital. J. Anim. Sci. 2004, 3, 323–336. [Google Scholar] [CrossRef]
- Matassa, S.; Boon, N.; Pikaar, I.; Verstraete, W. Microbial protein: Future sustainable food supply route with low environmental footprint. Microb. Biotechnol. 2016, 9, 568–575. [Google Scholar] [CrossRef]
- Biswas, A.; Takakuwa, F.; Yamada, S.; Matsuda, A.; Saville, R.M.; LeBlanc, A.; Silverman, J.A.; Sato, N.; Tanaka, H. Methanotroph (Methylococcus capsulatus, Bath) bacteria meal as an alternative protein source for Japanese yellowtail, Seriola quinqueradiata. Aquaculture 2020, 529, 735700. [Google Scholar] [CrossRef]
- Xu, B.; Liu, Y.; Chen, K.; Wang, L.; Sagada, G.; Tegomo, A.F.; Yang, Y.; Sun, Y.; Zheng, L.; Ullah, S.; et al. Evaluation of methanotroph (Methylococcus capsulatus, Bath) bacteria meal (FeedKind®) as an alternative protein source for juvenile black sea bream, Acanthopagrus schlegelii. Front. Mar. Sci. 2021, 8, 778301. [Google Scholar] [CrossRef]
- Zhang, Q.; Liang, H.; Longshaw, M.; Wang, J.; Ge, X.; Zhu, J.; Li, S.; Ren, M. Effects of replacing fishmeal with methanotroph (Methylococcus capsulatus, Bath) bacteria meal (FeedKind®) on growth and intestinal health status of juvenile largemouth bass (Micropterus salmoides). Fish Shellfish. Immunol. 2022, 122, 298–305. [Google Scholar] [CrossRef]
- Chama, M.K.H.; Liang, H.; Huang, D.; Ge, X.; Ren, M.; Zhang, L.; Wu, L.; Ke, J. Methanotroph (Methylococcus capsulatus, Bath) as an alternative protein source for genetically improved farmed tilapia (GIFT: Oreochromis niloticus) and its effect on antioxidants and immune response. Aquac. Rep. 2021, 21, 100872. [Google Scholar] [CrossRef]
- Chen, Y.; Chi, S.; Zhang, S.; Dong, X.; Yang, Q.; Liu, H.; Zhang, W.; Deng, J.; Tan, B.; Xie, S. Replacement of fish meal with methanotroph (Methylococcus capsulatus, Bath) bacteria meal in the diets of Pacific white shrimp (Litopenaeus vannamei). Aquaculture 2021, 541, 736801. [Google Scholar] [CrossRef]
- Jintasataporn, O.; Chumkam, S.; Triwutanon, S.; LeBlanc, A.; Sawanboonchun, J. Effects of a single cell protein (Methylococcus capsulatus, Bath) in Pacific white shrimp (Penaeus vannamei) diet on growth performance, survival rate and resistance to Vibrio parahaemolyticus, the causative agent of acute hepatopancreatic necrosis disease. Front. Mar. Sci. 2021, 8, 764042. [Google Scholar] [CrossRef]
- Romarheim, O.H.; Hetland, D.L.; Skrede, A.; Øverland, M.; Mydland, L.T.; Landsverk, T. Prevention of soya-induced enteritis in Atlantic salmon (Salmo salar) by bacteria grown on natural gas is dose dependent and related to epithelial MHC II reactivity and CD8alpha+ intraepithelial lymphocytes. Br. J. Nutr. 2013, 109, 1062–1070. [Google Scholar] [CrossRef] [Green Version]
- Romarheim, O.H.; Landsverk, T.; Mydland, L.T.; Skrede, A.; Øverland, M. Cell wall fractions from Methylococcus capsulatus prevent soybean meal-induced enteritis in Atlantic salmon (Salmo salar). Aquaculture 2013, 402–403, 13–18. [Google Scholar] [CrossRef]
- Romarheim, O.H.; Øverland, M.; Mydland, L.T.; Skrede, A.; Landsverk, T. Bacteria grown on natural gas prevent soybean meal-induced enteritis in Atlantic salmon. J. Nutr. 2011, 141, 124–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coyle, S.D.; Tidwell, J.H.; Webster, C.D. Response of largemouth bass Micropterus salmoides to dietary supplementation of lysine, methionine, and highly unsaturated fatty acids. J. World Aquac. Soc. 2000, 31, 89–95. [Google Scholar] [CrossRef]
- Bai, J.J.; Li, S.J. Genetic Breeding and Molecular Marker-Assisted Selective Breeding of Largemouth Bass; Academic Press: Cambridge, MA, USA, 2019; pp. 1–3. [Google Scholar]
- Ministry of Agriculture and Rural Affairs. China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2020; p. 25. (In Chinese)
- Huang, D.; Wu, Y.B.; Lin, Y.Y.; Chen, J.M.; Karrow, N.; Ren, X.; Wang, Y. Dietary Protein and Lipid Requirements for Juvenile Large-mouth Bass, Micropterus salmoides. J. World Aquac. Soc. 2017, 48, 782–790. [Google Scholar] [CrossRef]
- Cai, Z.N.; Qian, X.Q.; Xie, S.Q. Optimal dietary protein concentrations for largemouth bass (Micropterus salmoides) of different sizes (10–500 g), Aquacul. Int. 2020, 28, 831–840. [Google Scholar]
- Ma, S.; Liang, X.; Chen, P.; Wang, J.; Gu, X.; Qin, Y.; Blecker, C.; Xue, M. A new single-cell protein from Clostridium autoethanogenum as a functional protein for largemouth bass (Micropterus salmoides). Anim. Nutr. 2022, 10, 99–110. [Google Scholar] [CrossRef]
- Xie, X.; Wang, J.; Guan, Y.; Xing, S.; Liang, X.; Xue, M.; Wang, J.; Chang, Y.; Leclercq, E. Cottonseed protein concentrate as fishmeal alternative for largemouth bass (Micropterus salmoides) supplemented a yeast-based paraprobiotic: Effects on growth performance, gut health and microbiome. Aquaculture 2022, 551, 737898. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, L.; Chen, P.; Liang, X.; Cao, A.; Han, J.; Wu, X.; Zheng, Y.; Qin, Y.; Xue, M. Dietary bile acids enhance growth, and alleviate hepatic fibrosis induced by a high starch diet via AKT/FOXO1 and cAMP/AMPK/SREBP1 pathway in Micropterus salmoides. Front. Physiol. 2019, 10, 1430. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.F.; Chen, P.; Wu, X.L.; Xing, S.J.; Morais, S.; He, M.L.; Gu, X.; Xue, M. Effects of High starch and supplementation of an olive extraction the growth performance, hepatic antioxidant capacity and lipid metabolism of largemouth bass (Micropterus salmoides). Antioxidants 2022, 11, 577. [Google Scholar] [CrossRef]
- Wang, J.; Yun, B.; Xue, M.; Wu, X.; Zheng, Y.; Li, P. Apparent digestibility coefficients of several protein sources, and replacement of fishmeal by porcine meal in diets of Japanese seabass, Lateolabrax japonicus, are affected by dietary protein levels. Aquac. Res. 2012, 43, 117–127. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1997. [Google Scholar]
- Zhang, Y.; Chen, P.; Liang, X.F.; Han, J.; Wu, X.F.; Yang, Y.H.; Xue, M. Metabolic disorder induces fatty liver in Japanese seabass, Lateolabrax japonicas fed a full plant protein diet and regulated by cAMP-JNK/NF-kB caspase signal pathway. Fish Shellfish Immun. 2019, 90, 223–234. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Goebel, S.E. A place for DNA-DNA reassorciation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Bacteriol. 1994, 44, 846849. [Google Scholar]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.J.; Mou, M.M.; Pu, D.C.; Lin, S.M.; Chen, Y.J.; Luo, L. Effect of dietary starch level on growth, metabolism enzyme and oxidative status of juvenile largemouth bass, Micropterus salmoides. Aquaculture 2019, 498, 482–487. [Google Scholar] [CrossRef]
- Chen, P.; Zhu, Y.P.; Wu, X.F.; Gu, X.; Xue, M.; Liang, X.F. Metabolic adaptation to high starch diet in largemouth bass (Micropterus salmoides) was associated with the restoration of metabolic functions via inflammation, bile acid synthesis and energy metabolism. Br. J. Nutr. 2018, 498, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Li, X.; Song, B.; He, M.; Wu, C.; Leng, X. The potential of Clostridium autoethanogenum, a new single cell protein, in substituting fish meal in the diet of largemouth bass (Micropterus salmoides): Growth, feed utilization and intestinal histology. Aquac. Fish. 2021, in press. [CrossRef]
- Zhu, S.; Gao, W.; Wen, Z.; Chi, S.; Shi, Y.; Hu, W.; Tan, B. Partial substitution of fish meal by Clostridium autoethanogenum protein in the diets of juvenile largemouth bass (Micropterus salmoides). Aquac. Rep. 2022, 22, 100938. [Google Scholar] [CrossRef]
- Dabrowski, K.J.R.N.D. The feeding of fish larvae: Present” state of the art” and perspectives. Reprod. Nutr. Dév. 1984, 24, 807–833. [Google Scholar] [CrossRef]
- Banerjee, S.; Azad, S.; Vikineswary, S.; Selvaraj, O.; Mukherjee, T. Phototrophic bacteria as fish feed supplement. Asian-Australas. J. Anim. Sci. 2000, 13, 991–994. [Google Scholar] [CrossRef]
- Laranja, J.L.Q.; Ludevese-Pascual, G.L.; Amar, E.C.; Sorgeloos, P.; Bossier, P.; De Schryver, P. Poly-β-hydroxybutyrate (PHB) accumulating Bacillus spp. improve the survival, growth and robustness of Penaeus monodon (Fabricius, 1798) postlarvae. Vet. Microbiol. 2014, 173, 310–317. [Google Scholar] [CrossRef]
- Berge, G.M.; Baeverfjord, G.; Skrede, A.; Storebakken, T. Bacterial protein grown on natural gas as protein source in diets for Atlantic salmon, Salmo salar, in saltwater. Aquaculture 2005, 244, 233–240. [Google Scholar] [CrossRef]
- Aas, T.S.; Hatlen, B.; Grisdale-Helland, B.; Terjesen, B.F.; Bakke-McKellep, A.M.; Helland, S.J. Effects of diets containing a bacterial protein meal on growth and feed utilisation in rainbow trout (Oncorhynchus mykiss). Aquaculture 2006, 261, 357–368. [Google Scholar] [CrossRef]
- Berge, G.M.; Grisdale-Helland, B. Soy protein concentrate in diets for Atlantic halibut (Hippoglossus hippoglossus). Aquaculture 1999, 178, 139–148. [Google Scholar] [CrossRef]
- Storebakken, T.; Shearer, K.D.; Roem, A.J. Availability of protein, phosphorus and other elements in fish meal, soy-protein concentrate and phytase-treated soy-protein concentrate-based diets to Atlantic salmon, Salmo salar. Aquaculture 1998, 161, 365–379. [Google Scholar] [CrossRef]
- Liang, X.F.; Han, J.; Xue, M.; Yu, H.H.; Huang, H.Y.; Wu, X.F.; Zheng, Y.H.; Qin, Y.C.; Liang, X.F. Growth and feed intake regulation responses to anorexia, adaptation and fasting in Japanese seabss, Lateolabrax japonicas when fishmeal is totally replaced by plant protein. Aquaculture 2019, 498, 528–538. [Google Scholar] [CrossRef]
- Nagel, F.; von Danwitz, A.; Tusche, K.; Kroeckel, S.; van Bussel, C.G.; Schlachter, M.; Adem, H.; Tressel, R.-P.; Schulz, C.J. Nutritional evaluation of rapeseed protein isolate as fish meal substitute for juvenile turbot (Psetta maxima L.)—Impact on growth performance, body composition, nutrient digestibility and blood physiology. Aquaculture 2012, 356, 357–364. [Google Scholar] [CrossRef]
- Aas, T.S.; Grisdale-Helland, B.; Terjesen, B.F.; Helland, S.J. Improved growth and nutrient utilisation in Atlantic salmon (Salmo salar) fed diets containing a bacterial protein meal. Aquaculture 2006, 259, 365–376. [Google Scholar] [CrossRef]
- Storebakken, T.; Kvien, I.; Shearer, K.; Grisdale-Helland, B.; Helland, S.; Berge, G. The apparent digestibility of diets containing fish meal, soybean meal or bacterial meal fed to Atlantic salmon (Salmo salar): Evaluation of different faecal collection methods. Aquaculture 1998, 169, 195–210. [Google Scholar] [CrossRef]
- Romarheim, O. Bioprotein in diets for Atlantic halibut and Atlantic salmon, with a focus on technical feed quality and growth parameters. Master’s Thesis, Agricultural University of Norway, Oslo, Norway, 2002. (In Norwegian with English abstract). [Google Scholar]
- White, S.L.; Volkoff, H.; Devlin, R.H. Regulation of feeding behavior and food intake by appetite-regulating peptides in wild-type and growth hormone-transgenic coho salmon. Horm. Behav. 2016, 84, 18–28. [Google Scholar] [CrossRef]
- Metz, J.R.; Peters, J.J.; Flik, G. Molecular biology and physiology of the melanocortin system in fish: A review. Gen. Comp. Endocrinol. 2006, 148, 150–162. [Google Scholar] [CrossRef]
- Schjolden, J.; Schioth, H.B.; Larhammar, D.; Winberg, S.; Larson, E.T. Melanocortin peptides affect the motivation to feed in rainbow trout (Oncorhynchus mykiss). Gen. Comp. Endocrinol. 2009, 160, 134–138. [Google Scholar] [CrossRef]
- Rumsey, G.L.; Kinsella, J.E.; Shetty, K.J.; Hughes, S.G. Effect of high dietary concentrations of brewer’s dried yeast on growth performance and liver uricase in rainbow trout (Oncorhynchus mykiss). Anim. Feed Sci. Technol. 1991, 33, 177–183. [Google Scholar] [CrossRef]
- Murrell, J.C.; McDonald, I.R.; Gilbert, B. Regulation of expression of methane monooxygenases by copper ions. Trends Microbiol. 2000, 8, 221–225. [Google Scholar] [CrossRef]
- Mohanty, M.; Adhikari, S.; Mohanty, P.; Sarangi, N. Role of waterborne copper on survival, growth and feed intake of Indian major carp, Cirrhinus mrigala Hamilton. Bull. Environ. Contam. Toxicol. 2009, 82, 559–563. [Google Scholar] [CrossRef]
- Anderson, M.A.; Giusti, M.S.; Taylor, W.D. Hepatic copper concentrations and condition factors of largemouth bass (Micropterus salmoides) and common carp (Cyprinus carpio) from copper sulfate-treated and untreated reservoirs. Lake Reserv. Manag. 2001, 17, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Clearwater, S.J.; Farag, A.M.; Meyer, J.J. Bioavailability and toxicity of dietborne copper and zinc to fish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 132, 269–313. [Google Scholar] [CrossRef]
- Chen, K.; Yamamoto, F.Y.; Gatlin, D.M. Effects of inorganic and organic dietary copper supplementation on growth performance and tissue composition for juvenile red drum (Sciaenops ocellatus L.). Aquac. Nut. 2020, 26, 820–827. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Feng, L.; Jiang, W.D.; Liu, Y.; Jiang, J.; Li, S.H.; Kuang, S.Y.; Tang, L.; Zhou, X.Q. Effects of dietary copper on growth, digestive, and brush border enzyme activities and antioxidant defense of hepatopancreas and intestine for young grass carp (Ctenopharyngodon idella). Biol. Trace Elem. Res. 2013, 155, 370–380. [Google Scholar] [CrossRef]
- Ye, C.-X.; Wan, F.; Sun, Z.-Z.; Cheng, C.-H.; Ling, R.-Z.; Fan, L.-F.; Wang, A. Effect of phosphorus supplementation on cell viability, anti-oxidative capacity and comparative proteomic profiles of puffer fish (Takifugu obscurus) under low temperature stress. Aquaculture 2016, 452, 200–208. [Google Scholar] [CrossRef]
- Lei, Y.; Sun, Y.; Wang, X.; Lin, Z.; Bu, X.; Wang, N.; Du, Z.; Qin, J.; Chen, L.J.A. Effect of dietary phosphorus on growth performance, body composition, antioxidant activities and lipid metabolism of juvenile Chinese mitten crab (Eriocheir sinensis). Aquaculture 2021, 531, 735856. [Google Scholar] [CrossRef]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.C.; Chen, P.; Liang, X.F.; Yu, H.H.; Wu, X.F.; Han, J.; Luo, L.; Gu, X.; Xue, M. Plant protein diet suppressed immune function by inhibiting spiral valve intestinal mucosal barrier integrity, anti-oxidation, apoptosis, autophagy and proliferation responses in amur sturgeon (Acipenser schrenckii). Fish Shellfish Immunol. 2019, 94, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Lacher, S.E.; Levings, D.C.; Freeman, S.; Slattery, M. Identification of a functional antioxidant response element at the HIF1A locus. Redox Biol. 2018, 19, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, J.; Liu, C.; Wang, X.; Zhou, H.; Mai, K.; He, G. Slc38a9 deficiency induces apoptosis and metabolic dysregulation and leads to premature death in zebrafish. Int. J. Mol. Sci. 2022, 23, 4200. [Google Scholar] [CrossRef]
- Congleton, J.; Wagner, T. Blood-chemistry indicators of nutritional status in juvenile salmonids. J. Fish Biol. 2006, 69, 473–490. [Google Scholar] [CrossRef]
- Iwama, G.K.; Afonso, L.O.; Vijayan, M.M. Stress in fish. Ann. N. Y. Acad. Sci. 1998, 851, 304–310. [Google Scholar] [CrossRef]
- Nyblom, H.; Berggren, U.; Balldin, J.; Olsson, R. High AST/ALT ratio may indicate advanced alcoholic liver disease rather than heavy drinking. Alcohol Alcohol. 2004, 39, 336–339. [Google Scholar] [CrossRef]
- Wang, X.; Wang, G.; Qu, J.; Yuan, Z.; Pan, R.; Li, K. Calcipotriol inhibits NLRP3 signal through YAP1 activation to alleviate cholestatic liver injury and fibrosis. Front. Pharmacol. 2020, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.L.; Yu, H.H.; Liang, X.F.; Li, N.; Wang, X.; Li, F.H.; Wu, X.F.; Zheng, Y.H.; Xue, M.; Liang, X.F. Dietary butylated hydroxytoluene improves lipid metabolism, antioxidant and anti-apoptotic response of largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2018, 72, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.L.; Epand, R.F.; Rosenfeld, Y.; Peleg, A.; Barra, D.; Epand, R.M.; Shai, Y. Lipopolysaccharide, a key molecule involved in the synergism between temporins in inhibiting bacterial growth and in endotoxin neutralization. J. Biol. Chem. 2008, 283, 22907–22917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trent, M.S.; Stead, C.M.; Tran, A.X.; Hankins, J. Invited review: Diversity of endotoxin and its impact on pathogenesis. J. Endotoxin Res. 2006, 12, 205–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purcell, M.K.; Bromage, E.S.; Silva, J.; Hansen, J.D.; Badil, S.M.; Woodson, J.C.; Hershberger, P.K. Production and characterization of monoclonal antibodies to IgM of Pacific herring (Clupea pallasii). Fish Shellfish Immunol. 2012, 33, 552–558. [Google Scholar] [CrossRef]
- Mosca, A.; Leclerc, M.; Hugot, J.P. Gut microbiota diversity and human diseases: Should we reintroduce key predators in our ecosystem? Front Microbiol. 2016, 7, 455. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Jaramillo-Torres, A.; Li, Y.; Brevik, Ø.J.; Jakobsen, J.V.; Kortner, T.M.; Krogdahl, Å. Gut health and microbiota in out-of-season Atlantic Salmon (Salmo salar L.) smolts before and after seawater transfer under commercial Arctic conditions: Modulation by functional feed ingredients. Front. Mar. Sci. 2022, 9, 860081. [Google Scholar] [CrossRef]
- Wang, J.; Jaramillo-Torres, A.; Li, Y.; Kortner, T.M.; Gajardo, K.; Brevik, O.J.; Jakobsen, J.V.; Krogdahl, Å. Microbiota in intestinal digesta of Atlantic salmon (Salmo salar), observed from late freshwater stage until one year in seawater, and effects of functional ingredients: A case study from a commercial sized research site in the Arctic region. Anim. Microbiome. 2021, 3, 14. [Google Scholar] [CrossRef]
- Wang, J.; Mai, K.S.; Ai, Q.H. Conventional soybean meal as fishmeal alternative in diets of Japanese Seabass (Lateolabrax japonicus): Effects of functional additives on growth, immunity, antioxidant capacity and disease resistance. Antioxidant 2022, 11, 951. [Google Scholar] [CrossRef]
- Averina, O.V.; Poluektova, E.U.; Marsova, M.V.; Danilenko, V.N. Biomarkers and utility of the antioxidant potential of probiotic Lactobacilli and Bifidobacteria as representatives of the human gut microbiota. Biomedicines 2021, 9, 1340. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Torres, M.; Ruiz, L.; Lugli, G.A.; Ventura, M.; Margolles, A.; van Sinderen, D. Editorial: Role of Bifidobacteria in human and animal health and biotechnological applications. Front. Microbiol. 2021, 12, 785664. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Koshio, S.; Kestemont, P. Recent advances of nucleotide nutrition research in aquaculture: A review. Rev. Aquac. 2019, 12, 1028–1053. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.M.; Xu, J.; Ma, S.F.; Liang, X.F.; Wei, Z.X.; Li, D.M.; Xue, M. C1 gas protein: A potential protein substitute for advancing aquaculture sustainability. Rev. Aquac. 2022. [Google Scholar] [CrossRef]


| Ingredients | C | FK3 | FK6 | FK9 |
|---|---|---|---|---|
| Fishmeal a | 420 | 290 | 260 | 230 |
| FeedKind® b | 0 | 30 | 60 | 90 |
| Soy protein concentrated c | 150 | 250 | 250 | 250 |
| Soybean meal | 60 | 60 | 60 | 60 |
| Wheat gluten meal | 30 | 30 | 30 | 30 |
| Spay-dried blood cell powder | 50 | 50 | 50 | 50 |
| Wheat flour | 70 | 70 | 70 | 70 |
| Cassava meal | 50 | 50 | 50 | 50 |
| Microcrystalline cellulose | 54.3 | 33.7 | 31.7 | 29.6 |
| Kelp meal | 15 | 15 | 15 | 15 |
| Ca(H2PO4)2 | 5.5 | 12.3 | 13 | 13.7 |
| Lecithin oil | 20 | 20 | 20 | 20 |
| Fish oil | 20.2 | 31.2 | 32.2 | 33.2 |
| Soybean oil | 40 | 40 | 40 | 40 |
| Vitamin and mineral premix d | 14 | 14 | 14 | 14 |
| L-Lysine | 0 | 1.8 | 2.1 | 2.5 |
| DL-Methionine | 0 | 1 | 1 | 1 |
| Y2O3 | 1 | 1 | 1 | 1 |
| Total | 1000 | 1000 | 1000 | 1000 |
| Analyzed chemical compositions (g/kg, in as is basis) | ||||
| Crude protein | 502 | 500 | 498 | 500 |
| Crude lipid | 112 | 113 | 111 | 120 |
| Moisture | 34.4 | 33.8 | 42.7 | 49.7 |
| Crude ash | 110 | 103 | 97.8 | 95.6 |
| Copper (Cu, mg/kg) | 3.88 | 7.90 | 11.6 | 16.4 |
| Gross energy/(MJ/kg) | 20.6 | 20.3 | 20.8 | 19.9 |
| C | FK3 | FK6 | FK9 | |
|---|---|---|---|---|
| Essential amino acid | ||||
| Arginine | 30.3 | 31.8 | 32.1 | 32.1 |
| Histidine | 17.2 | 17.1 | 16.8 | 16.6 |
| Isoleucine | 19.8 | 20.9 | 21.1 | 20.8 |
| Leucine | 41.9 | 42.3 | 42.7 | 42.3 |
| Lysine | 36.2 | 36.1 | 36.2 | 35.9 |
| Methionine | 11.2 | 11.2 | 11.3 | 11.3 |
| Phenylalanine | 24.1 | 25.3 | 25.5 | 25.1 |
| Threonine | 21.0 | 20.5 | 20.3 | 20.5 |
| Valine | 26.4 | 27.5 | 27.7 | 27.5 |
| Non-essential amino acid | ||||
| Alanine | 29.3 | 28.6 | 28.8 | 28.8 |
| Aspartic acid | 50.8 | 52.0 | 52.4 | 51.8 |
| Cysteine | 6.0 | 5.9 | 6.2 | 5.8 |
| Glycine | 26.8 | 26.1 | 25.8 | 25.6 |
| Glutamic acid | 79.4 | 81.6 | 81.9 | 80.1 |
| Proline | 24.2 | 25.5 | 24.6 | 24.6 |
| Serine | 22.9 | 22.8 | 23.0 | 26.5 |
| Genes | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Tm (°C) | E-Values (%) | Accession Number |
|---|---|---|---|---|---|
| EF1α | TGCTGCTGGTGTTGGTGAGTT | TTCTGGCTGTAAGGGGGCTC | 60.4 | 102.8 | 119901934 |
| Keap1 | TCATTGGGGAATCACATCTTTG | TGTCCAGAAAAGTGTTGCCATC | 56.5 | 99.6 | 119899192 |
| Nrf2 | CAGACGGGGAAACAAACAATG | GGGGTAAAATACGCCACAATAAC | 55.0 | 96.8 | 119905458 |
| CREB | GGAGTCTGTATCGCTCAGCC | ACCAACGTAACTGTGGGACC | 60.0 | 97.0 | 119904409 |
| C | FK3 | FK6 | FK9 | |
|---|---|---|---|---|
| Growth performance | ||||
| SR (%) 1 | 85.00 ± 2.52 b | 94.00 ± 2.00 a | 96.00 ± 1.63 a | 96.00 ± 0.00 a |
| WGR (%) 2 | 199.1 ± 10.7 a | 202.9 ± 7.70 a | 214.2 ± 9.79 a | 158.2 ± 8.06 b |
| FCR 3 | 1.22 ± 0.07 | 1.16 ± 0.04 | 1.13 ± 0.02 | 1.22 ± 0.07 |
| PPV (%) 4 | 27.3 ± 1.4 | 28.7 ± 3.2 | 28.8 ± 4.7 | 27.0 ± 5.7 |
| PLV (%) 5 | 78.0 ± 3.7 ab | 82.0 ± 1.8 b | 93.3 ± 4.1 c | 69.3 ± 3.1 a |
| Morphometric parameters | ||||
| CF (g/cm3) 6 | 1.94 ± 0.03 a | 1.83 ± 0.03 b | 1.86 ± 0.03 ab | 1.76 ± 0.02 b |
| VSI (%) 7 | 7.03 ± 0.18 | 7.06 ± 0.23 | 7.67 ± 0.30 | 7.49 ± 0.19 |
| HSI (%) 8 | 1.49 ± 0.05 | 1.50 ± 0.08 | 1.48 ± 0.07 | 1.50 ± 0.06 |
| VAI (%) 9 | 2.72 ± 0.20 | 2.42 ± 0.14 | 2.67 ± 0.23 | 2.47 ± 0.15 |
| C | FK3 | FK6 | FK9 | |
|---|---|---|---|---|
| Whole body | ||||
| Moisture (%) | 68.4 ± 0.1 | 68.4 ± 0.3 | 68.3 ± 0.30 | 68.7 ± 0.3 |
| Ash (%) | 3.75 ± 0.03 c | 3.85 ± 0.07 bc | 3.97 ± 0.04 ab | 4.06 ± 0.03 a |
| Crude protein (%) | 16.9 ± 0.1 | 16.9 ± 0.1 | 16.7 ± 0.2 | 17.0 ± 0.0 |
| Crude lipid (%) | 9.59 ± 0.09 | 9.72 ± 0.30 | 9.77 ± 0.23 | 9.21 ± 0.18 |
| Cu (mg/kg) | 0.43 ± 0.00 | 0.50 ± 0.02 | 0.46 ± 0.04 | 0.42 ± 0.01 |
| Liver | ||||
| Crude lipid (%) | 3.69 ± 0.54 a | 2.84 ± 0.16 b | 2.99 ± 0.18 b | 2.84 ± 0.14 b |
| Cu (mg/kg) | 13.6 ± 1.20 | 14.2 ± 1.17 | 12.4 ± 0.88 | 9.55 ± 2.23 |
| Feces | ||||
| Cu (mg/kg) | 12.2 ± 1.41 c | 16.6 ± 0.43 b | 17.2 ± 1.26 b | 25.0 ± 0.59 a |
| C | FK3 | FK6 | FK9 | |
|---|---|---|---|---|
| ADCd 1 | 75.9 ± 0.3 b | 78.0 ± 0.3 a | 77.8 ± 0.3 a | 77.8 ± 0.3 a |
| ADCp 2 | 91.2 ± 0.1 b | 92.2 ± 0.1 a | 92.0 ± 0.2 a | 91.9 ± 0.1 a |
| ADCe 2 | 82.3 ± 0.2 b | 83.7 ± 0.2 a | 83.6 ± 0.2 a | 82.7 ± 0.1 b |
| ADCc 2 | 23.2 ± 9.93 b | 53.3 ± 0.60 a | 66.5 ± 2.46 a | 65.8 ± 0.58 a |
| C | FK3 | FK6 | FK9 | |
|---|---|---|---|---|
| Liver function | ||||
| ALT (U/L) | 16.4 ± 1.9 a | 8.41 ± 1.6 b | 9.14 ± 1.5 b | 12.2 ± 2.5 ab |
| AST (U/L) | 8.24 ± 0.78 | 6.47 ± 0.75 | 8.53 ± 0.63 | 8.33 ± 1.07 |
| AKP (U/L) | 84.2 ± 10.1 | 66.9 ± 8.8 | 84.0 ± 11.3 | 52.7 ± 7.7 |
| TBA (μmol/L) | 3.16 ± 0.85 ab | 3.52 ± 0.74 ab | 2.42 ± 0.31 b | 4.53 ± 0.41 a |
| Metabolites | ||||
| Glucose (mmol/L) | 4.75 ± 0.65 b | 5.51 ± 0.76 ab | 7.16 ± 0.75 a | 6.83 ± 0.78 ab |
| TP (g/L) | 23.2 ± 1.0 a | 23.7 ± 0.7 a | 20.6 ± 0.7 b | 21.5 ± 0.7 ab |
| TC (mmol/L) | 8.07 ± 1.22 | 7.67 ± 1.23 | 7.54 ± 1.14 | 7.40 ± 1.12 |
| Triglyceride (mmol/L) | 3.09 ± 0.53 | 2.48 ± 0.49 | 4.01 ± 0.64 | 2.83 ± 0.34 |
| HDL-C (mmol/L) | 2.61 ± 0.15 | 2.21 ± 0.18 | 2.21 ± 0.09 | 2.30 ± 0.19 |
| HDL-C/TC | 0.37 ± 0.06 | 0.34 ± 0.05 | 0.35 ± 0.06 | 0.35 ± 0.04 |
| LDL-C (mmol/L) | 0.45 ± 0.03 | 0.37 ± 0.05 | 0.38 ± 0.04 | 0.32 ± 0.03 |
| LDL-C/TC | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.05 ± 0.01 |
| NEFA (mEq/L) | 0.40 ± 0.04 | 0.31 ± 0.05 | 0.27 ± 0.04 | 0.28 ± 0.05 |
| C | FK3 | FK6 | FK9 | |
|---|---|---|---|---|
| Plasma | ||||
| MDA (nmol/mL) | 29.56 ± 3.10 a | 21.39 ± 2.59 b | 18.23 ± 0.99 b | 21.77 ± 1.66 b |
| SOD (U/mL) | 8.14 ± 2.44 | 9.27 ± 1.55 | 10.73 ± 1.46 | 11.84 ± 1.95 |
| SOD/MDA | 0.30 ± 0.11 c | 0.43 ± 0.06 b | 0.58 ± 0.07 a | 0.54 ± 0.07 a |
| Liver | ||||
| MDA (nmol/mg prot) | 14.8 ± 2.87 a | 5.76 ± 0.58 b | 2.78 ± 0.48 c | 2.90 ± 0.62 c |
| SOD (U/mg prot) | 13.6 ± 1.04 b | 18.6 ± 1.20 ab | 17.7 ± 2.24 ab | 22.8 ± 2.91 a |
| SOD/MDA | 6.42 ± 1.44 c | 17.3 ± 2.79 b | 39.6 ± 8.75 a | 39.3 ± 7.49 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, B.; He, X.; Ge, C.; Xue, M.; Wang, J.; Longshaw, M.; Wang, J.; Liang, X. A Natural Gas Fermentation Bacterial Meal (FeedKind®) as a Functional Alternative Ingredient for Fishmeal in Diet of Largemouth Bass, Micropterus salmoides. Antioxidants 2022, 11, 1479. https://doi.org/10.3390/antiox11081479
Guo B, He X, Ge C, Xue M, Wang J, Longshaw M, Wang J, Liang X. A Natural Gas Fermentation Bacterial Meal (FeedKind®) as a Functional Alternative Ingredient for Fishmeal in Diet of Largemouth Bass, Micropterus salmoides. Antioxidants. 2022; 11(8):1479. https://doi.org/10.3390/antiox11081479
Chicago/Turabian StyleGuo, Boyuan, Xia He, Chunyu Ge, Min Xue, Jia Wang, Matt Longshaw, Jie Wang, and Xiaofang Liang. 2022. "A Natural Gas Fermentation Bacterial Meal (FeedKind®) as a Functional Alternative Ingredient for Fishmeal in Diet of Largemouth Bass, Micropterus salmoides" Antioxidants 11, no. 8: 1479. https://doi.org/10.3390/antiox11081479
APA StyleGuo, B., He, X., Ge, C., Xue, M., Wang, J., Longshaw, M., Wang, J., & Liang, X. (2022). A Natural Gas Fermentation Bacterial Meal (FeedKind®) as a Functional Alternative Ingredient for Fishmeal in Diet of Largemouth Bass, Micropterus salmoides. Antioxidants, 11(8), 1479. https://doi.org/10.3390/antiox11081479









