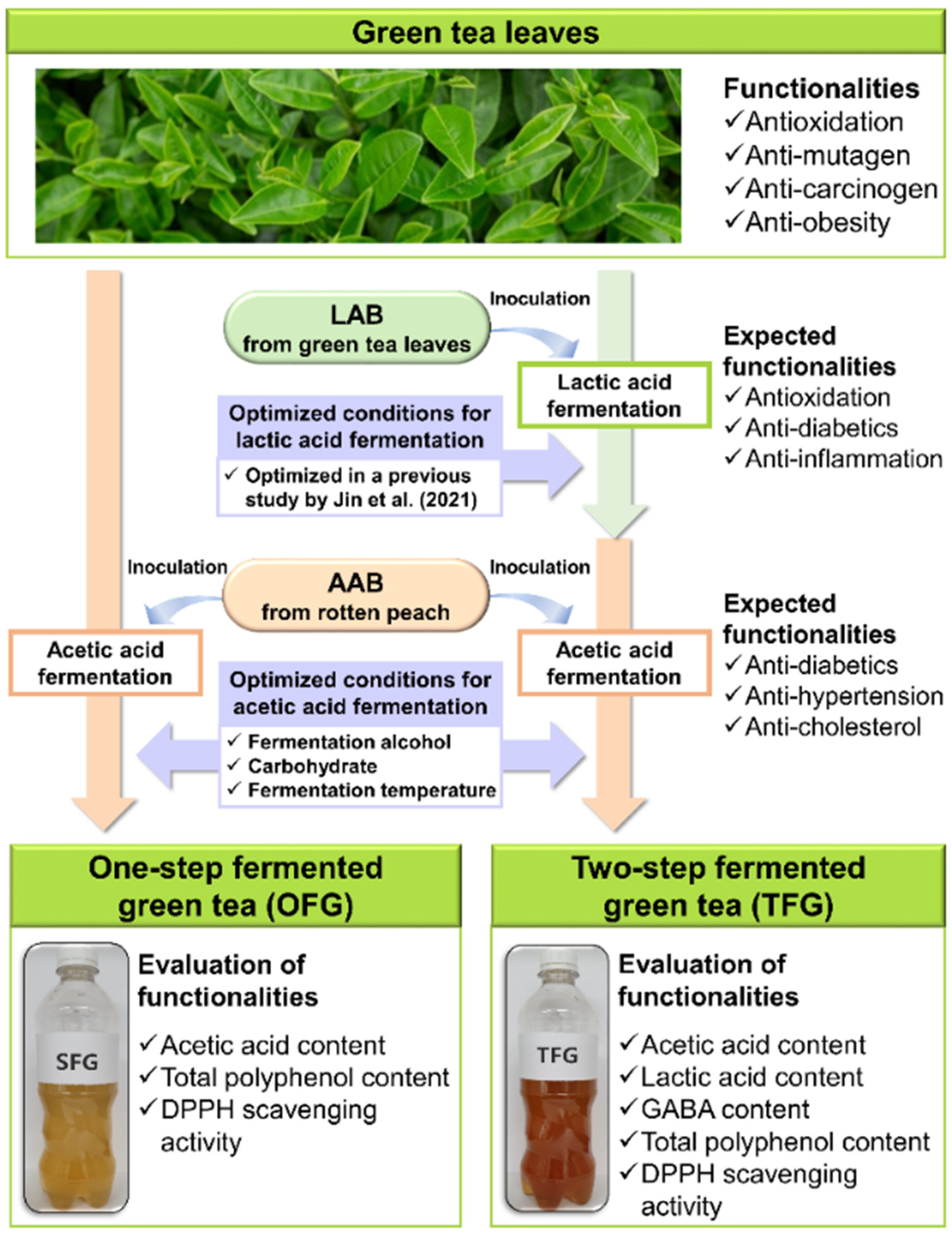
Evaluation of Bioactive Compounds and Antioxidative Activity of Fermented Green Tea Produced via One- and Two-Step Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Acetobacter Strains Used
2.1.1. Isolation of Acetobacter Strains from Over-Ripened Fruits
2.1.2. Identification of the Selected Acetobacter Strain
2.1.3. Reference Strain Used
2.2. Acetobacter Strain Selection for Acetic Acid Fermentation of OFG and TFG
2.3. Bacterial Suspension Preparation for Acetic Acid Fermentation of Green Tea
2.4. Green Tea Preparation
2.5. Lactic Acid-Fermented Green Tea Preparation for Two-Step Fermentation
2.6. Optimization of Key Factors for Acetic Acid Fermentation of Green Tea with A. pasteurianus PCH 325
2.6.1. Optimization of Fermentation Alcohol Concentration
2.6.2. Optimization of Carbohydrate Type and Concentration
2.6.3. Optimization of Fermentation Temperature
2.7. Determination of Physicochemical and Microbial Characteristics
2.8. Analysis of Contents of Organic Acids and GABA
2.9. Analysis of Contents of Fermentation Alcohol and Carbohydrates
2.10. Analysis of Antioxidative Activity
2.11. Statistical Analysis
3. Results
3.1. Acetobacter Strain Selection for Acetic Acid Fermentation of Both OFG and TFG
3.2. Optimization of Key Factors for Fermentation of OFG
3.3. Optimization of Key Factors for Fermentation of TFG
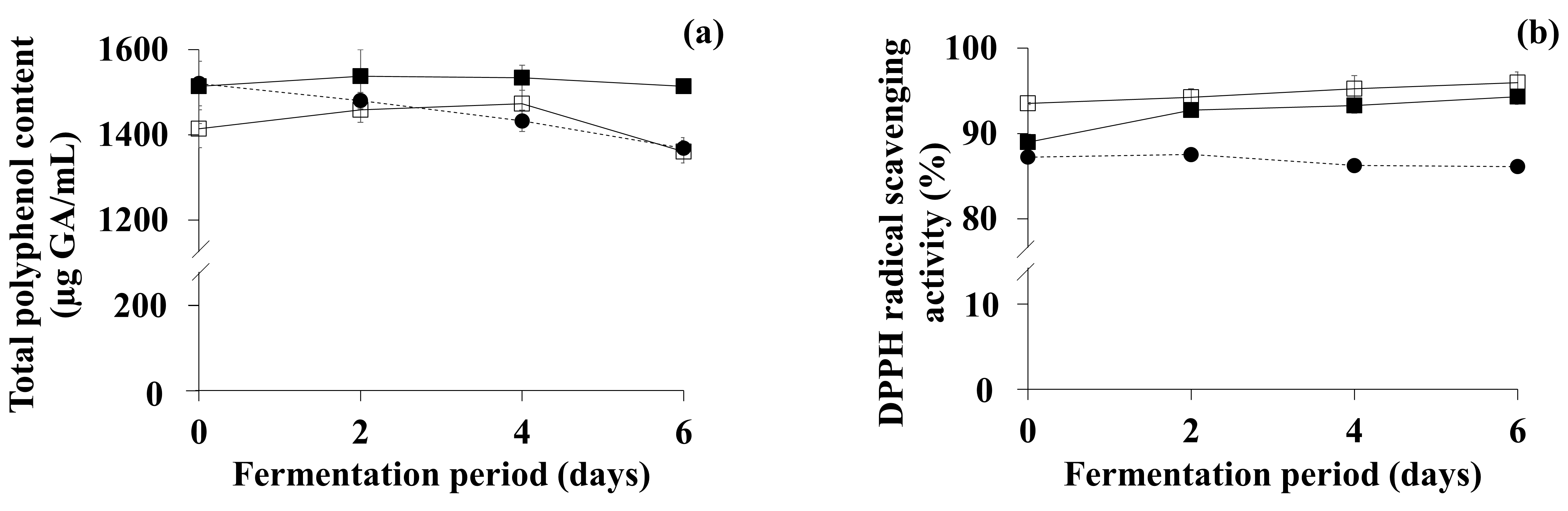
3.4. Comparison of Bioactive Compounds and DPPH Scavenging Activity of OFG and TFG
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harbowy, M.E.; Balentine, D.A.; Davies, A.P.; Cai, Y. Tea chemistry. Crit. Rev. Plant Sci. 1997, 16, 415–480. [Google Scholar] [CrossRef]
- Lee, J.-E.; Lee, B.-J.; Chung, J.-O.; Shin, H.-J.; Lee, S.-J.; Lee, C.-H.; Hong, Y.S. 1H NMR-based metabolomic characterization during green tea (Camellia sinensis) fermentation. Food Res. Int. 2011, 44, 597–604. [Google Scholar] [CrossRef]
- Fujiki, H.; Suganuma, M.; Imai, K.; Nakachi, K. Green tea: Cancer preventive beverage and/or drug. Cancer Lett. 2002, 188, 9–13. [Google Scholar] [CrossRef]
- Sharma, V.K.; Bhattacharya, A.; Kumar, A.; Sharma, H.K. Health benefits of tea consumption. Trop. J. Pharm. Res. 2007, 6, 785–792. [Google Scholar] [CrossRef]
- Suzuki, Y.; Miyoshi, N.; Isemura, M. Health-promoting effects of green tea. Proc. Jpn. Acad. Ser. B 2012, 88, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Pervin, M.; Goto, S.; Isemura, M.; Nakamura, Y. Beneficial effects of tea and the green tea catechin epigallocatechin-3-gallate on obesity. Molecules 2016, 21, 1305. [Google Scholar] [CrossRef] [PubMed]
- Kochman, J.; Jakubczyk, K.; Antoniewicz, J.; Mruk, H.; Janda, K. Health benefits and chemical composition of matcha green tea: A review. Molecules 2020, 26, 85. [Google Scholar] [CrossRef]
- Caplice, E.; Fitzgerald, G.F. Food fermentations: Role of microorganisms in food production and preservation. Int. J. Food Microbiol. 1999, 50, 131–149. [Google Scholar] [CrossRef]
- Slaa, J.; Gnode, M.; Else, H. Yeast and fermentation: The optimal temperature. J. Org. Chem. 2009, 134, a–c. [Google Scholar]
- Settanni, L.; Moschetti, G. New trends in technology and identity of traditional dairy and fermented meat production processes: Preservation of typicality and hygiene. Trends Food Sci. Technol. 2014, 37, 51–58. [Google Scholar] [CrossRef]
- Terefe, N.S.; Augustin, M.A. Fermentation for tailoring the technological and health related functionality of food products. Crit. Rev. Food Sci. Nutr. 2020, 60, 2887–2913. [Google Scholar] [CrossRef] [PubMed]
- Lacey, A.M.L.; Pérez-Santín, E.; López-Caballero, M.E.; Montero, P. Biotransformation and resulting biological properties of green tea polyphenols produced by probiotic bacteria. LWT Food Sci. Technol. 2014, 58, 633–638. [Google Scholar] [CrossRef]
- Wang, L.-C.; Pan, T.-M.; Tsai, T.-Y. Lactic acid bacteria-fermented product of green tea and Houttuynia cordata leaves exerts anti-adipogenic and anti-obesity effects. J. Food Drug Anal. 2018, 26, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.H.; Hong, J.H.; Lee, J.-H.; Yoon, H.; Pawluk, A.M.; Yun, S.J.; Mah, J.-H. Lactic acid fermented green tea with Levilactobacillus brevis capable of producing γ-aminobutyric acid. Fermentation 2021, 7, 110. [Google Scholar] [CrossRef]
- Gullo, M.; Caggia, C.; De Vero, L.; Giudici, P. Characterization of acetic acid bacteria in “traditional balsamic vinegar”. Int. J. Food Microbiol. 2006, 106, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Sengun, I.Y.; Karabiyikli, S. Importance of acetic acid bacteria in food industry. Food Control 2011, 22, 647–656. [Google Scholar] [CrossRef]
- Chang, J.M.; Fang, T.J. Survival of Escherichia coli O157: H7 and Salmonella enterica serovars Typhimurium in iceberg lettuce and the antimicrobial effect of rice vinegar against E. coli O157: H7. Food Microbiol. 2007, 24, 745–751. [Google Scholar] [CrossRef]
- Liu, L.; Yang, X. Hypolipidemic and antioxidant effects of freeze-dried powder of Shanxi mature vinegar in hyperlipidaemic mice. Food Sci. 2015, 36, 141–151. [Google Scholar]
- Salbe, A.D.; Johnston, C.S.; Buyukbese, M.A.; Tsitouras, P.D.; Harman, S.M. Vinegar lacks antiglycemic action on enteral carbohydrate absorption in human subjects. Nutr. Res. 2009, 29, 846–849. [Google Scholar] [CrossRef]
- Lee, M.-K.; Choi, S.-R.; Lee, J.; Choi, Y.-H.; Lee, J.-H.; Park, K.-U.; Kwon, S.-H.; Seo, K.-I. Quality characteristics and anti-diabetic effect of yacon vinegar. J. Korean Soc. Food Sci. Nutr. 2012, 41, 79–86. [Google Scholar] [CrossRef][Green Version]
- Kondo, S.; Tayama, K.; Tsukamoto, Y.; Ikeda, K.; Yamori, Y. Antihypertensive effects of acetic acid and vinegar on spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2001, 65, 2690–2694. [Google Scholar] [CrossRef] [PubMed]
- Fushimi, T.; Suruga, K.; Oshima, Y.; Fukiharu, M.; Tsukamoto, Y.; Goda, T. Dietary acetic acid reduces serum cholesterol and triacylglycerols in rats fed a cholesterol-rich diet. Br. J. Nutr. 2006, 95, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Kishi, M.; Fushimi, T.; Ugajin, S.; Kaga, T. Vinegar intake reduces body weight, body fat mass, and serum triglyceride levels in obese Japanese subjects. Biosci. Biotechnol. Biochem. 2009, 73, 1837–1843. [Google Scholar] [CrossRef]
- Seki, T.; Morimura, S.; Shigematsu, T.; Maeda, H.; Kida, K. Antitumor activity of rice-shochu post-distillation slurry and vinegar produced from the post-distillation slurry via oral administration in a mouse model. Biofactors 2004, 22, 103–105. [Google Scholar] [CrossRef]
- Ho, C.W.; Lazim, A.M.; Fazry, S.; Zaki, U.K.H.H.; Lim, S.J. Varieties, production, composition and health benefits of vinegars: A review. Food Chem. 2017, 221, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Wang, Y.; Zhou, R.; Yin, A.; Zhou, T. Optimization of cultural conditions for vinegar of Litchi (Litchi chinensis Sonn.) in liquid state fermentation. J. Food Nutr. Res. 2015, 3, 641–647. [Google Scholar]
- Liu, G.; Zhu, F.; Zhao, B.; Zhao, Y.; Wang, J.; Sun, M.; He, F. Optimization of acetic acid fermentation process of apple cider vinegar. Asian Agric. Res. 2020, 12, 41–49. [Google Scholar]
- Ghosh, S.; Chakraborty, R.; Chatterjee, G.; Raychaudhuri, U. Study on fermentation conditions of palm juice vinegar by response surface methodology and development of a kinetic model. Braz. J. Chem. Eng. 2012, 29, 461–472. [Google Scholar] [CrossRef]
- Liu, Y.; Xing, R.; Yang, H.; Liu, S.; Qin, Y.; Li, K.; Yu, H.; Li, P. Chitin extraction from shrimp (Litopenaeus vannamei) shells by successive two-step fermentation with Lactobacillus rhamnoides and Bacillus amyloliquefaciens. Int. J. Biol. Macromol. 2020, 148, 424–433. [Google Scholar] [CrossRef]
- Luan, C.; Zhang, M.; Devahastin, S.; Liu, Y. Effect of two-step fermentation with lactic acid bacteria and Saccharomyces cerevisiae on key chemical properties, molecular structure and flavor characteristics of horseradish sauce. LWT Food Sci. Technol. 2021, 147, 111637. [Google Scholar] [CrossRef]
- Horiuchi, J.I.; Kanno, T.; Kobayashi, M. Effective onion vinegar production by a two-step fermentation system. J. Biosci. Bioeng. 2000, 90, 289–293. [Google Scholar] [CrossRef]
- Lisdiyanti, P.; Kawasaki, H.; Seki, T.; Yamada, Y.; Uchimura, T.; Komagata, K. Identification of Acetobacter strains isolated from Indonesian sources, and proposals of Acetobacter syzygii sp. nov., Acetobacter cibinongensis sp. nov., and Acetobacter orientalis sp. nov. J. Gen. Appl. Microbiol. 2001, 47, 119–131. [Google Scholar] [CrossRef][Green Version]
- ISO 7218:2007; Microbiology of Food and Animal Feeding Stuffs—General Requirements and Guidance for Microbiological Examinations. ISO: Geneva, Switzerland, 2007.
- Leifson, E. The flagellation and taxonomy of species of Acetobacter. Anton. Leeuw. 1954, 20, 102–110. [Google Scholar] [CrossRef] [PubMed]
- NCBI. BLAST. Available online: http://www.ncbi.nlm.nih.gov/BLAST/ (accessed on 20 July 2022).
- Romero, L.E.; Cantero, D. Evaluation of ethanol evaporation losses in acetic acid fermentations. Bioprocess Eng. 1998, 18, 289–291. [Google Scholar] [CrossRef]
- Wu, X.; Yao, H.; Liu, Q.; Zheng, Z.; Cao, L.; Mu, D.; Wang, H.; Jiang, S.; Li, X. Producing acetic acid of Acetobacter pasteurianus by fermentation characteristics and metabolic flux analysis. Appl. Biochem. Biotechnol. 2018, 186, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Baranyi, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef]
- Zwietering, M.H.; De Wit, J.C.; Cuppers, H.G.A.M.; Van’t Riet, K. Modeling of bacterial growth with shifts in temperature. Appl. Environ. Microbiol. 1994, 60, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Mellefont, L.A.; Ross, T. The effect of abrupt shifts in temperature on the lag phase duration of Escherichia coli and Klebsiella oxytoca. Int. J. Food Microbiol. 2003, 83, 295–305. [Google Scholar] [CrossRef]
- Østergaard, N.B.; Christiansen, L.E.; Dalgaard, P. Stochastic modelling of Listeria monocytogenes single cell growth in cottage cheese with mesophilic lactic acid bacteria from aroma producing cultures. Int. J. Food Microbiol. 2015, 204, 55–65. [Google Scholar] [CrossRef]
- Singh, V.; Haque, S.; Niwas, R.; Srivastava, A.; Pasupuleti, M.; Tripathi, C.K.M. Strategies for fermentation medium optimization: An in-depth review. Front. Microbiol. 2017, 7, 2087. [Google Scholar] [CrossRef]
- Frey, D.D.; Wang, H. Adaptive one-factor-at-a-time experimentation and expected value of improvement. Technometrics 2006, 48, 418–431. [Google Scholar] [CrossRef]
- Crowell, E.A.; Ough, C.S. A modified procedure for alcohol determination by dichromate oxidation. Am. J. Enol. Vitic. 1979, 30, 61–63. [Google Scholar]
- Clarke, G.; Ting, K.N.; Wiart, C.; Fry, J. Radical scavenging, ferric reducing activity potential and total phenolics content indicates redundancy in use of all three assays to screen for antioxidant activity of extracts of plants from the Malaysian rainforest. Antioxidants 2013, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the antioxidant activity of plant extracts: Analysis of sensitivity and hierarchization based on the method used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Diez, L.; Guadalupe, Z.; Ayestarán, B.; Ruiz-Larrea, F. Effect of yeast mannoproteins and grape polysaccharides on the growth of wine lactic acid and acetic acid bacteria. J. Agric. Food Chem. 2010, 58, 7731–7739. [Google Scholar] [CrossRef]
- Raspor, P.; Goranovič, D. Biotechnological applications of acetic acid bacteria. Crit. Rev. Biotechnol. 2008, 28, 101–124. [Google Scholar] [CrossRef]
- Du Preez, J.C.; De Jong, F.; Botes, P.J.; Lategan, P.M. Fermentation alcohol from grain sorghum starch. Biomass 1985, 8, 101–117. [Google Scholar] [CrossRef]
- Saeki, A.; Taniguchi, M.; Matsushita, K.; Toyama, H.; Theeragool, G.; Lotong, N.; Adachi, O. Microbiological aspects of acetate oxidation by acetic acid bacteria, unfavorable phenomena in vinegar fermentation. Biosci. Biotechnol. Biochem. 1997, 61, 317–323. [Google Scholar] [CrossRef]
- Kim, J.-N.; Choo, J.-S.; Wee, Y.-J.; Yun, J.-S.; Ryu, H.-W. Culture medium optimization for acetic acid production by a persimmon vinegar-derived bacterium. Appl. Biochem. Biotechnol. 2005, 123, 861–869. [Google Scholar] [CrossRef]
- De Ory, I.; Romero, L.E.; Cantero, D. Modelling the kinetics of growth of Acetobacter aceti in discontinuous culture: Influence of the temperature of operation. Appl. Microbiol. Biotechnol. 1998, 49, 189–193. [Google Scholar] [CrossRef]
- Krisch, J.; Szajáni, B. Effects of immobilization on biomass production and acetic acid fermentation of Acetobacter aceti as a function of temperature and pH. Biotechnol. Lett. 1996, 18, 393–396. [Google Scholar] [CrossRef]
- Scalzo, R.L. Organic acids influence on DPPH scavenging by ascorbic acid. Food Chem. 2008, 107, 40–43. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, L.; Yu, G. The dominant glutamic acid metabolic flux to produce γ-amino butyric acid over proline in Nicotiana tabacum leaves under water stress relates to its significant role in antioxidant activity. J. Integr. Plant Biol. 2011, 53, 608–618. [Google Scholar] [CrossRef]
- Groussard, C.; Morel, I.; Chevanne, M.; Monnier, M.; Cillard, J.; Delamarche, A. Free radical scavenging and antioxidant effects of lactate ion: An in vitro study. J. Appl. Physiol. 2000, 89, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, M.Y.; Ji, G.E.; Lee, Y.S.; Hwang, K.T. Production of γ-aminobutyric acid in black raspberry juice during fermentation by Lactobacillus brevis GABA100. Int. J. Food Microbiol. 2009, 130, 12–16. [Google Scholar] [CrossRef]
- Park, S.-B.; Han, B.-K.; Oh, Y.J.; Lee, S.J.; Cha, S.-K.; Park, Y.-S.; Choi, H.-J. Bioconversion of green tea extract using lactic acid bacteria. Food Eng. Prog. 2012, 16, 1226–4768. [Google Scholar]
- Zubaidah, E.; Afgani, C.A.; Kalsum, U.; Srianta, I.; Blanc, P.J. Comparison of in vivo antidiabetes activity of snake fruit Kombucha, black tea Kombucha and metformin. Biocatal. Agric. Biotechnol. 2019, 17, 465–469. [Google Scholar] [CrossRef]
- Lopes, D.R.; Santos, L.O.; Prentice-Hernández, C. Antioxidant and antibacterial activity of a beverage obtained by fermentation of yerba-maté (Ilex paraguariensis) with symbiotic kombucha culture. J. Food Process Preserv. 2021, 45, e15101. [Google Scholar] [CrossRef]
- Sreeramulu, G.; Zhu, Y.; Knol, W. Kombucha fermentation and its antimicrobial activity. J. Agric. Food Chem. 2000, 48, 2589–2594. [Google Scholar] [CrossRef]
- Soumahoro, S.; Ouattara, H.G.; Droux, M.; Nasser, W.; Niamke, S.L.; Reverchon, S. Acetic acid bacteria (AAB) involved in cocoa fermentation from Ivory Coast: Species diversity and performance in acetic acid production. J. Food Sci. Technol. 2020, 57, 1904–1916. [Google Scholar] [CrossRef]
- Juneja, V.K.; Bari, M.L.; Inatsu, Y.; Kawamoto, S.; Friedman, M. Control of Clostridium perfringens spores by green tea leaf extracts during cooling of cooked ground beef, chicken, and pork. J. Food Prot. 2007, 70, 1429–1433. [Google Scholar] [CrossRef] [PubMed]
- Sirk, T.W.; Brown, E.F.; Sum, A.K.; Friedman, M. Molecular dynamics study on the biophysical interactions of seven green tea catechins with lipid bilayers of cell membranes. J. Agric. Food Chem. 2008, 56, 7750–7758. [Google Scholar] [CrossRef] [PubMed]
- Awad, H.M.; Diaz, R.; Malek, R.A.; Othman, N.Z.; Aziz, R.A.; El Enshasy, H.A. Efficient production process for food grade acetic acid by Acetobacter aceti in shake flask and in bioreactor cultures. E-J. Chem. 2012, 9, 2275–2286. [Google Scholar] [CrossRef]
- Islam, T.; Diba, F.; Miah, R.; Siddiqa, A.; Azmuda, N.; Nahar, S.; Adnan, N.; Dey, S.K.; Talukder, A.A. Optimization of acetic acid production rate by thermotolerant Acetobacter spp. Adv. Appl. Microbiol. 2017, 7, 749. [Google Scholar] [CrossRef][Green Version]
- Mangunwardoyo, W.; Hidayati, M.D. Misjiyah Influence of sucrose and Acetobacter aceti adition to acetic acid content on coconut water vinegar. IOP Conf. Ser. Earth Environ. Sci. 2020, 458, 012015. [Google Scholar]
- Sharafi, S.M.; Rasooli, I.; Beheshti-Maal, K. Isolation, characterization and optimization of indigenous acetic acid bacteria and evaluation of their preservation methods. Iran. J. Microbiol. 2010, 2, 38. [Google Scholar] [PubMed]
- Rani, K.Y.; Rao, V.R. Control of fermenters—A review. Bioprocess Eng. 1999, 21, 77–88. [Google Scholar] [CrossRef]
- Lee, A.H.; Neilson, A.P.; O’Keefe, S.F.; Ogejo, J.A.; Huang, H.; Ponder, M.; Chu, H.S.; Jin, Q.; Pilot, G.; Stewart, A.C. A laboratory-scale model cocoa fermentation using dried, unfermented beans and artificial pulp can simulate the microbial and chemical changes of on-farm cocoa fermentation. Eur. Food Res. Technol. 2019, 245, 511–519. [Google Scholar] [CrossRef]
- Teng, J.; Gong, Z.; Deng, Y.; Chen, L.; Li, Q.; Shao, Y.; Lin, L.; Xiao, W. Purification, characterization and enzymatic synthesis of theaflavins of polyphenol oxidase isozymes from tea leaf (Camellia sinensis). LWT Food Sci. Technol. 2017, 84, 263–270. [Google Scholar] [CrossRef]
- Kong, C.T.; Ho, C.W.; Ling, J.W.A.; Lazim, A.; Fazry, S.; Lim, S.J. Chemical changes and optimisation of acetous fermentation time and mother of vinegar concentration in the production of vinegar-like fermented papaya beverage. Sains Malays. 2018, 47, 2017–2026. [Google Scholar]
- Hearps, A.C.; Tyssen, D.; Srbinovski, D.; Bayigga, L.; Diaz, D.J.D.; Aldunate, M.; Cone, R.A.; Gugasyan, R.; Anderson, D.J.; Tachedjian, G. Vaginal lactic acid elicits an anti-inflammatory response from human cervicovaginal epithelial cells and inhibits production of pro-inflammatory mediators associated with HIV acquisition. Mucosal Immunol. 2017, 10, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- Adeghate, E.; Ponery, A.S. GABA in the endocrine pancreas: Cellular localization and function in normal and diabetic rats. Tissue Cell 2002, 34, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bown, A.W.; Shelp, B.J. The metabolism and functions of γ-aminobutyric acid. Plant Physiol. 1997, 115, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Axtell, R.; Mitra, A.; Miranda, M.; Lock, C.; Tsien, R.W.; Steinman, L. Inhibitory role for GABA in autoimmune inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 2580–2585. [Google Scholar] [CrossRef]
- Harrison, N.L. Mechanisms of sleep induction by GABA. J. Clin. Psychiatry 2007, 68, 6–12. [Google Scholar]
- Raj, K.C.; Ingram, L.O.; Maupin-Furlow, J.A. Pyruvate decarboxylase: A key enzyme for the oxidative metabolism of lactic acid by Acetobacter pasteurianus. Arch. Microbiol. 2001, 176, 443–451. [Google Scholar]



| Bacterial Strains 1 | pH | Growth Potential (OD600) | Green Tea Catechin Resistance | Acetic Acid Production (mg/L) | |
|---|---|---|---|---|---|
| Specific Growth Rate (h−1) | Relative Lag Time | ||||
| Acetobacter Strains Isolated From Rotten Fruits | |||||
| A. pasteurianus PCH 325 | 3.89 ± 0.00 2 | 1.92 ± 0.00 | 0.06 ± 0.00 | 1.11 ± 0.00 | 282.98 ± 25.01 |
| Other candidate strains | 3.91 ± 0.16 (3.61–4.33) 3 | 1.83 ± 0.44 (0.95–2.28) | 0.07 ± 0.03 (0.01–0.12) | 4.22 ± 2.36 (1.00–10.02) | 0.57 ± 0.44 (0.26–0.88) |
| Reference Strain of Acetobacter spp. | |||||
| A. aceti KCTC 12290 | 4.45 ± 0.03 | 0.92 ± 0.05 | 0.01 ± 0.00 | - 4 | 0.85 ± 0.25 |
| Optimization Stage (Key Factors) 1 | One-Step Fermentation | Two-Step Fermentation | ||
|---|---|---|---|---|
| Acetic Acid (mg/L) | Acetic Acid (mg/L) | GABA (mg/L) | Lactic Acid (mg/L) | |
| Control | 282.98 ± 25.01 2,A,a (100.00%) 3 | 5250.00 ± 1578.00 A,b (100.00%) | 24.77 ± 4.83 A | 243.44 ± 58.15 A |
| First stage (fermentation alcohol) | 3818.18 ± 239.56 B,a (1349.28%) | 6011.83 ± 352.42 AB,b (114.51%) | 28.10 ± 1.91 AB | 212.63 ± 80.00 A |
| Second stage (carbohydrate) | 5362.54 ± 370.70 C,a (1895.02%) | 7400.28 ± 199.85 BC,b (140.96%) | 25.99 ± 4.27 AB | 30.33 ± 3.30 B |
| Final stage (fermentation temperature) | 5997.80 ± 513.06 C,a (2119.51%) | 8349.49 ± 418.04 C,b (159.04%) | 31.49 ± 1.17 B | 88.32 ± 9.76 B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Hong, J.H.; Kim, D.; Jin, Y.H.; Pawluk, A.M.; Mah, J.-H. Evaluation of Bioactive Compounds and Antioxidative Activity of Fermented Green Tea Produced via One- and Two-Step Fermentation. Antioxidants 2022, 11, 1425. https://doi.org/10.3390/antiox11081425
Xu H, Hong JH, Kim D, Jin YH, Pawluk AM, Mah J-H. Evaluation of Bioactive Compounds and Antioxidative Activity of Fermented Green Tea Produced via One- and Two-Step Fermentation. Antioxidants. 2022; 11(8):1425. https://doi.org/10.3390/antiox11081425
Chicago/Turabian StyleXu, Huiling, Jong Hyoung Hong, Dabin Kim, Young Hun Jin, Alixander Mattay Pawluk, and Jae-Hyung Mah. 2022. "Evaluation of Bioactive Compounds and Antioxidative Activity of Fermented Green Tea Produced via One- and Two-Step Fermentation" Antioxidants 11, no. 8: 1425. https://doi.org/10.3390/antiox11081425
APA StyleXu, H., Hong, J. H., Kim, D., Jin, Y. H., Pawluk, A. M., & Mah, J.-H. (2022). Evaluation of Bioactive Compounds and Antioxidative Activity of Fermented Green Tea Produced via One- and Two-Step Fermentation. Antioxidants, 11(8), 1425. https://doi.org/10.3390/antiox11081425







