Stability and Antioxidant Activity of Hydro-Glyceric Extracts Obtained from Different Grape Seed Varieties Incorporated in Cosmetic Creams
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals
2.3. Extraction Process
2.4. Characterization of the Extract
2.4.1. Total Phenolic Content
2.4.2. Proanthocyanidin Assay Kit
2.4.3. Identification and Quantification of Polyphenols by High-Performance Liquid Chromatography (HPLC)
2.5. Antioxidant Activity
2.5.1. DiPhenyl-2-PicrylHydrazyl Free Radical Scavenging Activity (DPPH)
2.5.2. Ferric Reducing Antioxidant Power Assay (FRAP)
2.5.3. CUPRIC Ion Reducing Antioxidant Capacity Assay (CUPRAC)
2.6. Incorporation of the Extracts in Cosmetic Creams and Stability Tests
2.7. Stability Tests
2.7.1. Centrifuge Test
2.7.2. Heat-Shock Cycles
2.7.3. Viscosity and pH Tests
2.8. Total Phenolic Content and Antioxidant Activity of the Creams
2.9. Statistical Analysis
3. Results and Discussion
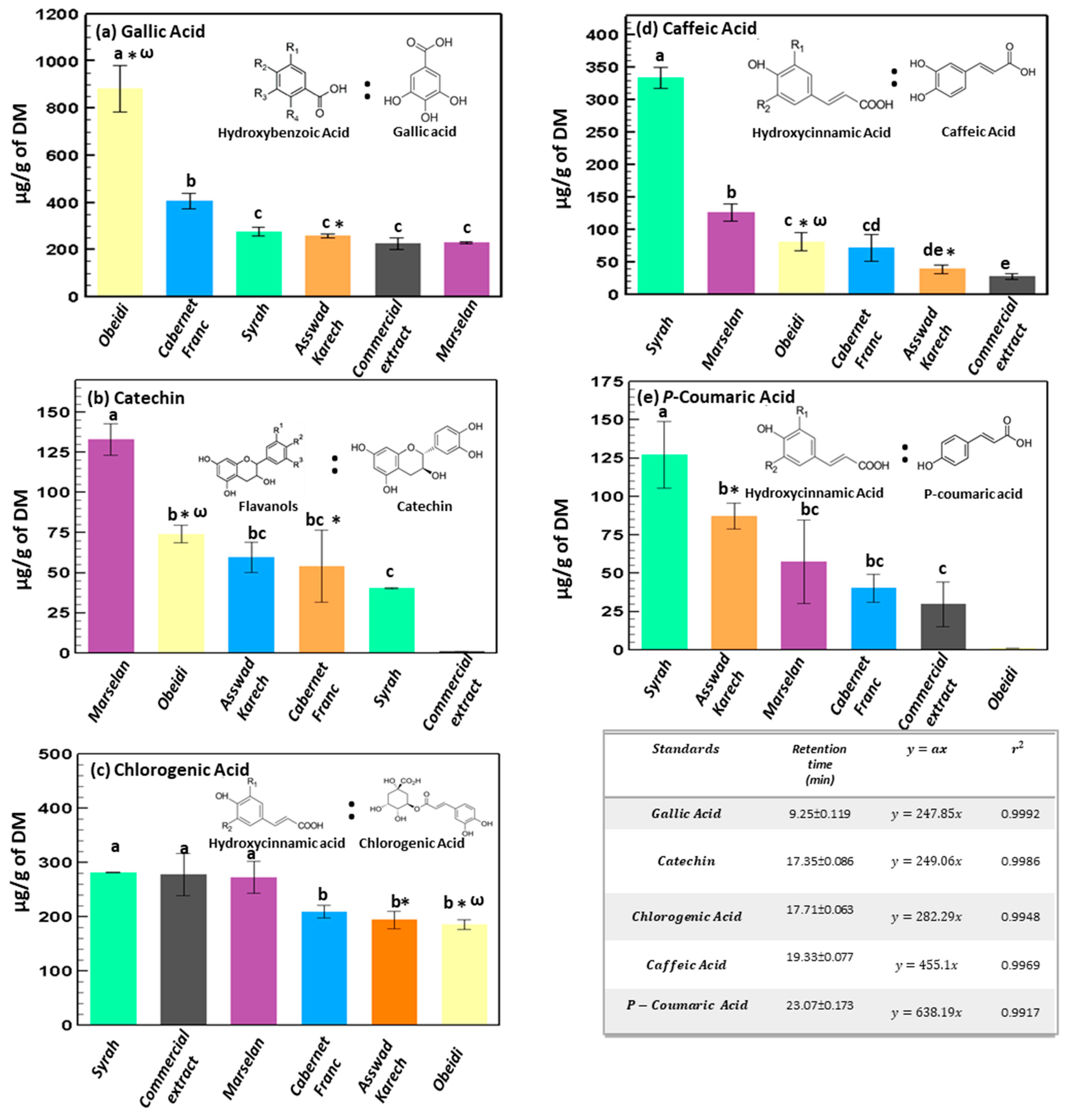
3.1. Extraction and Quantification of Phenolic Content
3.1.1. Extraction
3.1.2. Total Phenolic Content and ProAnthoCyanidin Content of the Hydro-Glyceric Extracts
3.1.3. Identification and Quantification by HPLC
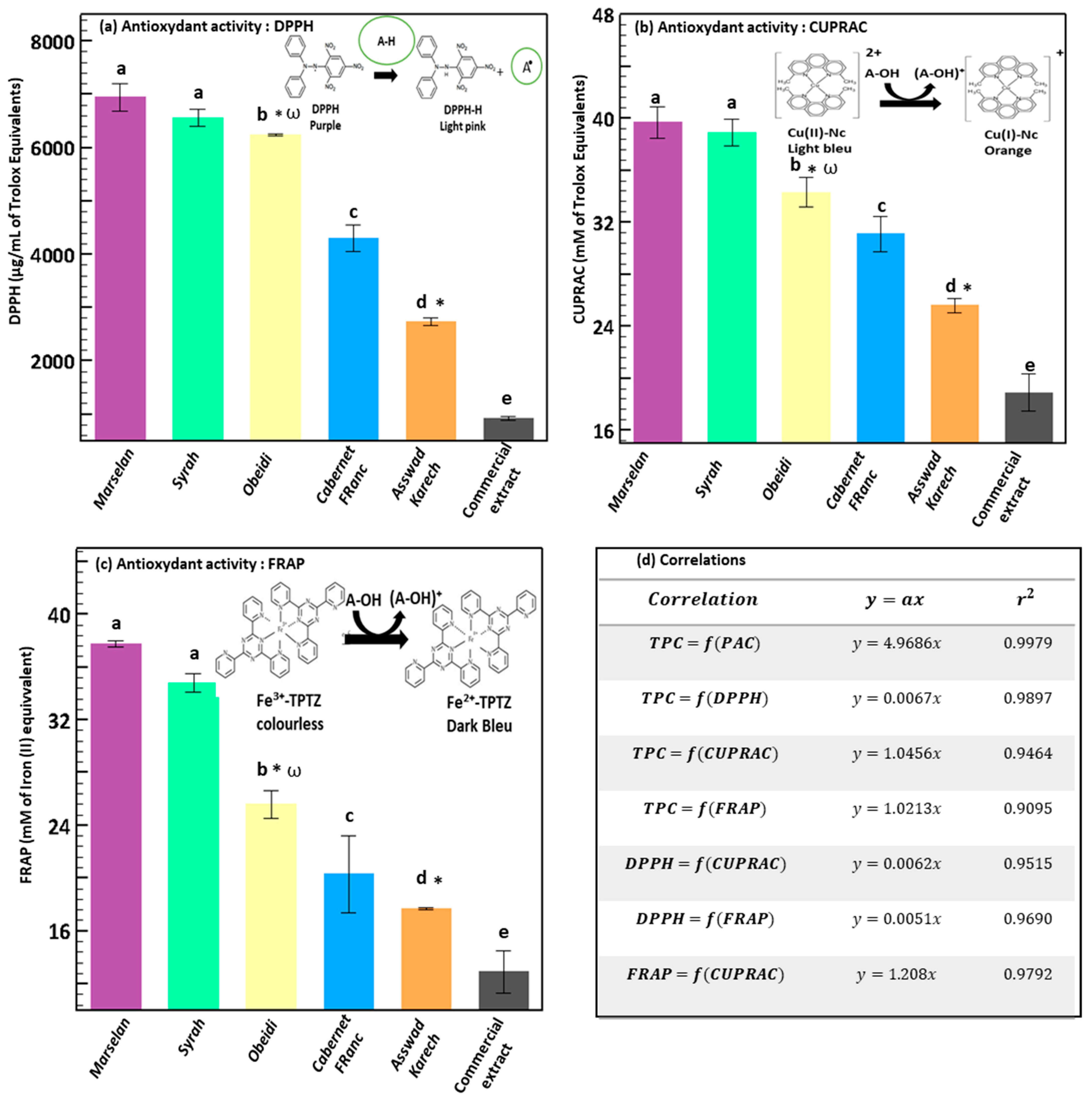
3.2. Antioxidant Activity of the Extract by DPPH, FRAP, and CUPRAC Assays
3.3. Stability Tests of Creams Containing Hydro-Glyceric Extract
3.3.1. Centrifuge Test and Heat-Cool Cycles
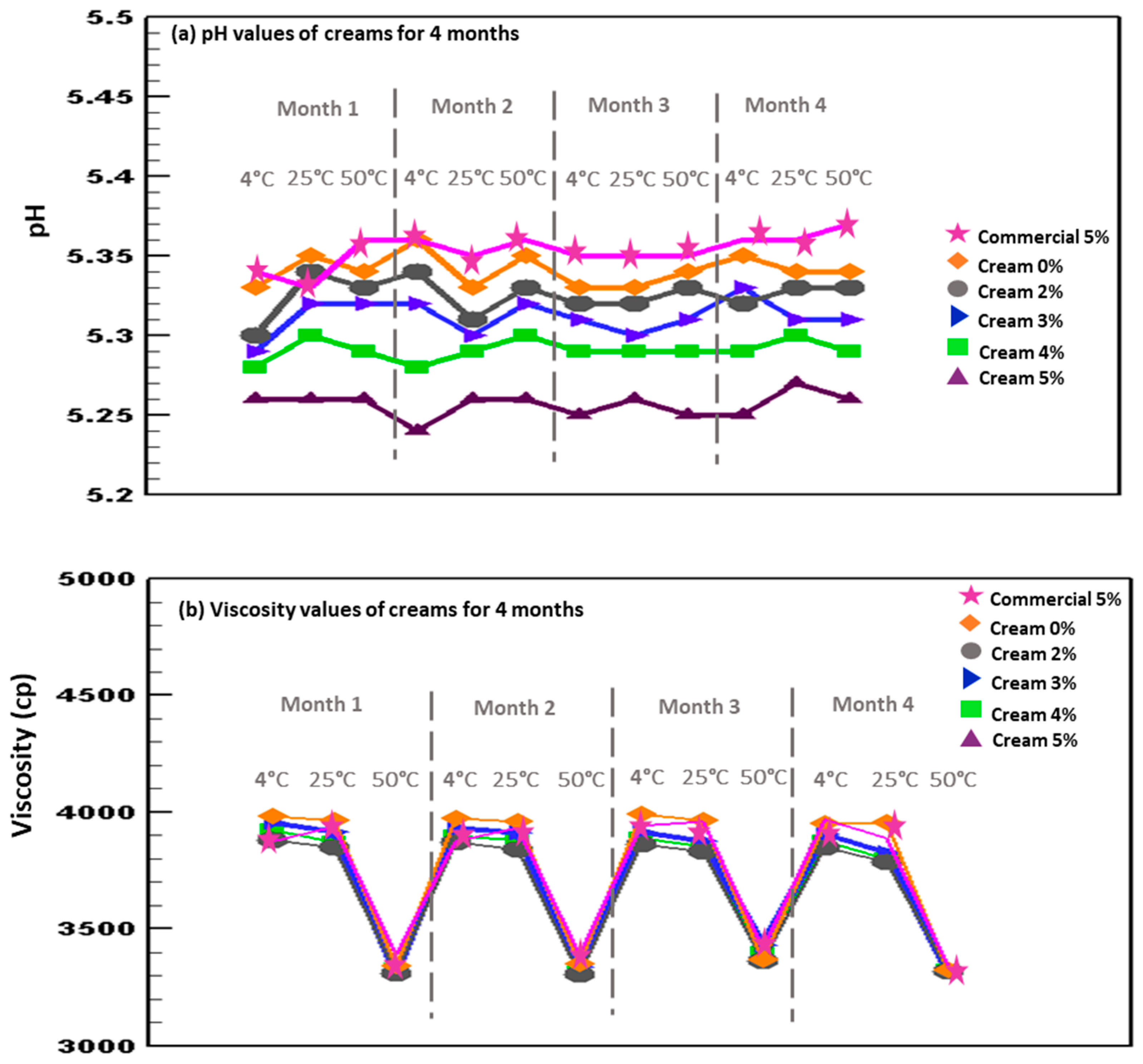
3.3.2. Measurements of pH and Viscosity
3.4. Total Phenolic Content and Antioxidant Activity of the Incorporated Extract
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yilmaz, Y.; Toledo, R.T. Major Flavonoids in Grape Seeds and Skins: Antioxidant Capacity of Catechin, Epicatechin, and Gallic Acid. J. Agric. Food Chem. 2004, 52, 255–260. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M. Functional Components of Grape Pomace: Their Composition, Biological Properties and Potential Applications. Int. J. Food Sci. Technol. 2013, 48, 221–237. [Google Scholar] [CrossRef]
- Ruggieri, L.; Cadena, E.; Martínez-Blanco, J.; Gasol, C.M.; Rieradevall, J.; Gabarrell, X.; Gea, T.; Sort, X.; Sánchez, A. Recovery of Organic Wastes in the Spanish Wine Industry. Technical, Economic and Environmental Analyses of the Composting Process. J. Clean. Prod. 2009, 17, 830–838. [Google Scholar] [CrossRef] [Green Version]
- Barbanera, M.; Cardarelli, A.; Carota, E.; Castellini, M.; Giannoni, T.; Ubertini, S. Valorization of Winery and Distillery By-Products by Hydrothermal Carbonization. Sci. Rep. 2021, 11, 23973. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, M.A.; Moral, R.; Paredes, C.; Pérez-Espinosa, A.; Moreno-Caselles, J.; Pérez-Murcia, M.D. Agrochemical Characterisation of the Solid By-Products and Residues from the Winery and Distillery Industry. Waste Manag. 2008, 28, 372–380. [Google Scholar] [CrossRef]
- Ibn Ferjani, A.; Jellali, S.; Akrout, H.; Limousy, L.; Hamdi, H.; Thevenin, N.; Jeguirim, M. Nutrient Retention and Release from Raw Exhausted Grape Marc Biochars and an Amended Agricultural Soil: Static and Dynamic Investigation. Environ. Technol. Innov. 2020, 19, 100885. [Google Scholar] [CrossRef]
- Mora-Garrido, A.B.; Cejudo-Bastante, M.J.; Heredia, F.J.; Escudero-Gilete, M.L. Revalorization of Residues from the Industrial Exhaustion of Grape By-Products. LWT 2022, 156, 113057. [Google Scholar] [CrossRef]
- Biasi, R.; Brunori, E.; Ferrara, C.; Salvati, L. Assessing Impacts of Climate Change on Phenology and Quality Traits of Vitis Vinifera L.: The Contribution of Local Knowledge. Plants 2019, 8, 121. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.A.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Dinis, L.-T.; Correia, C.; Moriondo, M.; Leolini, L.; Dibari, C.; Costafreda-Aumedes, S.; et al. A Review of the Potential Climate Change Impacts and Adaptation Options for European Viticulture. Appl. Sci. 2020, 10, 3092. [Google Scholar] [CrossRef]
- Lan, Y.; Liu, M.; Zhang, X.; Li, S.; Shi, Y.; Duan, C. Regional Variation of Chemical Characteristics in Young Marselan (Vitis Vinifera L.) Red Wines from Five Regions of China. Foods 2022, 11, 787. [Google Scholar] [CrossRef]
- Li, M.; Guo, Z.; Jia, N.; Yuan, J.; Han, B.; Yin, Y.; Sun, Y.; Liu, C.; Zhao, S. Evaluation of Eight Rootstocks on the Growth and Berry Quality of ‘Marselan’ Grapevines. Sci. Hortic. 2019, 248, 58–61. [Google Scholar] [CrossRef]
- Antonic, B.; Dordevic Jancikova, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yu, J.; Pohorly, J.E.; Kakuda, Y. Polyphenolics in Grape Seeds-Biochemistry and Functionality. J. Med. Food 2003, 6, 291–299. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The Effects of Polyphenols and Other Bioactives on Human Health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [Green Version]
- Maroun, R.G.; Rajha, H.N.; Vorobiev, E.; Louka, N. 7-Emerging Technologies for the Recovery of Valuable Compounds From Grape Processing By-Products. In Handbook of Grape Processing By-Products; Galanakis, C.M., Ed.; Academic Press: London, UK, 2017; pp. 155–181. [Google Scholar]
- Abi-Khattar, A.-M.; Rajha, H.N.; Abdel-Massih, R.M.; Maroun, R.G.; Louka, N.; Debs, E. Intensification of Polyphenol Extraction from Olive Leaves Using Ired-Irrad®, an Environmentally-Friendly Innovative Technology. Antioxidants 2019, 8, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheaib, D.; El Darra, N.; Rajha, H.N.; El-Ghazzawi, I.; Mouneimne, Y.; Jammoul, A.; Maroun, R.G.; Louka, N. Study of the Selectivity and Bioactivity of Polyphenols Using Infrared Assisted Extraction from Apricot Pomace Compared to Conventional Methods. Antioxidants 2018, 7, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajha, H.N.; Boussetta, N.; Louka, N.; Maroun, R.G.; Vorobiev, E. Electrical, Mechanical, and Chemical Effects of High-Voltage Electrical Discharges on the Polyphenol Extraction from Vine Shoots. Innov. Food Sci. Emerg. Technol. 2015, 31, 60–66. [Google Scholar] [CrossRef]
- Rajha, H.N.; Chacar, S.; Afif, C.; Vorobiev, E.; Louka, N.; Maroun, R.G. β-Cyclodextrin-Assisted Extraction of Polyphenols from Vine Shoot Cultivars. J. Agric. Food Chem. 2015, 63, 3387–3393. [Google Scholar] [CrossRef]
- Pagano, I.; Campone, L.; Celano, R.; Piccinelli, A.L.; Rastrelli, L. Green Non-Conventional Techniques for the Extraction of Polyphenols from Agricultural Food by-Products: A Review. J. Chromatogr. A 2021, 1651, 462295. [Google Scholar] [CrossRef]
- Hoss, I.; Rajha, H.N.; El Khoury, R.; Youssef, S.; Manca, M.L.; Manconi, M.; Louka, N.; Maroun, R.G. Valorization of Wine-Making By-Products’ Extracts in Cosmetics. Cosmetics 2021, 8, 109. [Google Scholar] [CrossRef]
- Baroi, A.M.; Popitiu, M.; Fierascu, I.; Sărdărescu, I.-D.; Fierascu, R.C. Grapevine Wastes: A Rich Source of Antioxidants and Other Biologically Active Compounds. Antioxidants 2022, 11, 393. [Google Scholar] [CrossRef] [PubMed]
- Kanlayavattanakul, M.; Chongnativisit, W.; Chaikul, P.; Lourith, N. Phenolic-Rich Pomegranate Peel Extract: In Vitro, Cellular, and In Vivo Activities for Skin Hyperpigmentation Treatment. Planta Med. 2020, 86, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Rasul, A.; Akhtar, N. Formulation and in Vivo Evaluation for Anti-Aging Effects of an Emulsion Containing Basil Extract Using Non- Invasive Biophysical Techniques. Daru 2011, 19, 344–350. [Google Scholar] [PubMed]
- Nichols, J.; Katiyar, S. Skin Photoprotection by Natural Polyphenols: Anti-Inflammatory, Antioxidant and DNA Repair Mechanisms. Arch. Dermatol. Res. 2009, 302, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef]
- Laguerre, M.; Lecomte, J.; Villeneuve, P. Evaluation of the Ability of Antioxidants to Counteract Lipid Oxidation: Existing Methods, New Trends and Challenges. Prog. Lipid Res. 2007, 46, 244–282. [Google Scholar] [CrossRef]
- Kusumawati, I.; Indrayanto, G. Chapter 15-Natural Antioxidants in Cosmetics. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 40, pp. 485–505. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Oxidants and Antioxidants Part A; Academic Press: London, UK, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Tuberoso, C.I.G.; Boban, M.; Bifulco, E.; Budimir, D.; Pirisi, F.M. Antioxidant Capacity and Vasodilatory Properties of Mediterranean Food: The Case of Cannonau Wine, Myrtle Berries Liqueur and Strawberry-Tree Honey. Food Chem. 2013, 140, 686–691. [Google Scholar] [CrossRef]
- Gyamfi, M.A.; Yonamine, M.; Aniya, Y. Free-Radical Scavenging Action of Medicinal Herbs from Ghana: Thonningia Sanguinea on Experimentally Induced Liver Injuries. Gen. Pharmacol. 1999, 32, 661–667. [Google Scholar] [CrossRef]
- Kallithraka, S.; Mohdaly, A.A.-A.; Makris, D.P.; Kefalas, P. Determination of Major Anthocyanin Pigments in Hellenic Native Grape Varieties (Vitis Vinifera Sp.): Association with Antiradical Activity. J. Food Compos. Anal. 2005, 18, 375–386. [Google Scholar] [CrossRef]
- Rodrigues Ueoka, A.; Pedriali Moraes, C.A. Development and Stability Evaluation of Liquid Crystal-Based Formulations Containing Glycolic Plant Extracts and Nano-Actives. Cosmetics 2018, 5, 25. [Google Scholar] [CrossRef] [Green Version]
- Whangsomnuek, N.; Mungmai, L.; Mengamphan, K.; Amornlerdpison, D. Efficiency of Skin Whitening Cream Containing Etlingera Elatior Flower and Leaf Extracts in Volunteers. Cosmetics 2019, 6, 39. [Google Scholar] [CrossRef] [Green Version]
- Stojiljković, D.; Tadić, V.; Stanković, M.; Roganović, S.; Arsić, I. Standardized Extract of Wild Apple Fruit in Alkyl-Polyglucoside-Based Cosmetic Cream-Estimation of Stability, Safety, Antioxidant Activity and Efficiency. Int. J. Cosmet. Sci. 2018, 40, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Becker, L.C.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Glycerin as Used in Cosmetics. Int. J. Toxicol. 2019, 38, 6S–22S. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.M.; Santos, L. A Potential Valorization Strategy of Wine Industry By-Products and Their Application in Cosmetics—Case Study: Grape Pomace and Grapeseed. Molecules 2022, 27, 969. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Yang, L.; Yue, X.; Li, Y.; He, R.; Deng, S.; Yang, X.; Fang, Y. Anthocyanin Profiles and Color Properties of Red Wines Made from Vitis Davidii and Vitis Vinifera Grapes. Food Sci. Hum. Wellness 2021, 10, 335–344. [Google Scholar] [CrossRef]
- Sandhu, A.K.; Gu, L. Antioxidant Capacity, Phenolic Content, and Profiling of Phenolic Compounds in the Seeds, Skin, and Pulp of Vitis Rotundifolia (Muscadine Grapes) As Determined by HPLC-DAD-ESI-MS(n). J. Agric. Food Chem. 2010, 58, 4681–4692. [Google Scholar] [CrossRef]
- Vidal-Casanella, O.; Moreno-Merchan, J.; Granados, M.; Nuñez, O.; Saurina, J.; Sentellas, S. Total Polyphenol Content in Food Samples and Nutraceuticals: Antioxidant Indices versus High Performance Liquid Chromatography. Antioxidants 2022, 11, 324. [Google Scholar] [CrossRef]
- Kosuru, R.Y.; Roy, A.; Das, S.K.; Bera, S. Gallic Acid and Gallates in Human Health and Disease: Do Mitochondria Hold the Key to Success? Mol. Nutr. Food Res. 2018, 62, 1700699. [Google Scholar] [CrossRef]
- Li, C.; Zhang, L.; Liu, C.; He, X.; Chen, M.; Chen, J. Lipophilic Grape Seed Proanthocyanidin Exerts Anti-Cervical Cancer Effects in HeLa Cells and a HeLa-Derived Xenograft Zebrafish Model. Antioxidants 2022, 11, 422. [Google Scholar] [CrossRef]
- Mandic, A.I.; Đilas, S.M.; Ćetković, G.S.; Čanadanović-Brunet, J.M.; Tumbas, V.T. Polyphenolic Composition and Antioxidant Activities of Grape Seed Extract. Int. J. Food Prop. 2008, 11, 713–726. [Google Scholar] [CrossRef]
- Isemura, M. Catechin in Human Health and Disease. Molecules 2019, 24, 528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butkeviciute, A.; Ramanauskiene, K.; Janulis, V. Formulation of Gels and Emulgels with Malus Domestica Borkh: Apple Extracts and Their Biopharmaceutical Evaluation In Vitro. Antioxidants 2022, 11, 373. [Google Scholar] [CrossRef]
- Zhao, Z.; Moghadasian, M.H. Bioavailability of Hydroxycinnamates: A Brief Review of in Vivo and in Vitro Studies. Phytochem. Rev. 2010, 9, 133–145. [Google Scholar] [CrossRef]
- Stanciu, G.; Lupsor, S.; Popescu, A.; Oancea, I. Polyphenols Isolation and Determination in Grape Seeds by HPLC/DAD; ProQuest: Ann Arbor, MI, USA, 2022. [Google Scholar]
- Xia, E.-Q.; Deng, G.-F.; Guo, Y.-J.; Li, H.-B. Biological Activities of Polyphenols from Grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as Potential Antioxidant Therapeutic Agents: Mechanism and Actions. Mutat. Res. 2005, 579, 200–213. [Google Scholar] [CrossRef]
- Zhou, K.; Raffoul, J.J. Potential Anticancer Properties of Grape Antioxidants. J. Oncol. 2012, 2012, e803294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, D.; Lameirão, F.; Delerue-Matos, C.; Rodrigues, F.; Costa, P. Characterization and Stability of a Formulation Containing Antioxidants-Enriched Castanea Sativa Shells Extract. Cosmetics 2021, 8, 49. [Google Scholar] [CrossRef]
- Ali Ishaq, M. Formulation and In Vitro Evaluation of Cream Containing Vitis Vinifera Fruit Extract. Master’s Thesis, Islamia University of Bahawalpur, Bahawalpur, Pakistan, 2020. [Google Scholar]
- Rafique, M.; Shah, N. Anti-Ageing Potential of a Cream (W/O Emulsion) Containing Grape Seed Extract (GSE): Formulation and in Vivo Evaluation of Effectiveness Using Non-Invasive Biophysical Technique. J. Clin. Exp. Dermatol. Res. 2019, 10, 2155-9554. [Google Scholar] [CrossRef] [Green Version]
- Rafique, M.; Nisar, S.; Shah, H.; Hussain, I.; Javed, I.; Nisar, N.; Riaz, R. Development of Grape Seed Extract Based Formulations by Using Non- Invasive Biophysical Technique and Its Impact on Skin Aging. Pak. J. Pharm. Sci. 2021, 34, 1621–1628. [Google Scholar]
- Ross, C.F.; Hoye, C.; Fernandez-Plotka, V.C. Influence of Heating on the Polyphenolic Content and Antioxidant Activity of Grape Seed Flour. J. Food Sci 2011, 76, C884–C890. [Google Scholar] [CrossRef]
- Vural, N.; Algan Cavuldak, Ö.; Anlı, R.E. Multi Response Optimisation of Polyphenol Extraction Conditions from Grape Seeds by Using Ultrasound Assisted Extraction (UAE). Sep. Sci. Technol. 2018, 53, 1540–1551. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salem, Y.; Rajha, H.N.; Franjieh, D.; Hoss, I.; Manca, M.L.; Manconi, M.; Castangia, I.; Perra, M.; Maroun, R.G.; Louka, N. Stability and Antioxidant Activity of Hydro-Glyceric Extracts Obtained from Different Grape Seed Varieties Incorporated in Cosmetic Creams. Antioxidants 2022, 11, 1348. https://doi.org/10.3390/antiox11071348
Salem Y, Rajha HN, Franjieh D, Hoss I, Manca ML, Manconi M, Castangia I, Perra M, Maroun RG, Louka N. Stability and Antioxidant Activity of Hydro-Glyceric Extracts Obtained from Different Grape Seed Varieties Incorporated in Cosmetic Creams. Antioxidants. 2022; 11(7):1348. https://doi.org/10.3390/antiox11071348
Chicago/Turabian StyleSalem, Yara, Hiba N. Rajha, Diana Franjieh, Israa Hoss, Maria Letizia Manca, Maria Manconi, Ines Castangia, Matteo Perra, Richard G. Maroun, and Nicolas Louka. 2022. "Stability and Antioxidant Activity of Hydro-Glyceric Extracts Obtained from Different Grape Seed Varieties Incorporated in Cosmetic Creams" Antioxidants 11, no. 7: 1348. https://doi.org/10.3390/antiox11071348
APA StyleSalem, Y., Rajha, H. N., Franjieh, D., Hoss, I., Manca, M. L., Manconi, M., Castangia, I., Perra, M., Maroun, R. G., & Louka, N. (2022). Stability and Antioxidant Activity of Hydro-Glyceric Extracts Obtained from Different Grape Seed Varieties Incorporated in Cosmetic Creams. Antioxidants, 11(7), 1348. https://doi.org/10.3390/antiox11071348














