Effects of 2-Year Nutritional and Lifestyle Intervention on Oxidative and Inflammatory Statuses in Individuals of 55 Years of Age and over at High Cardiovascular Risk
Abstract
:1. Introduction
2. Methods
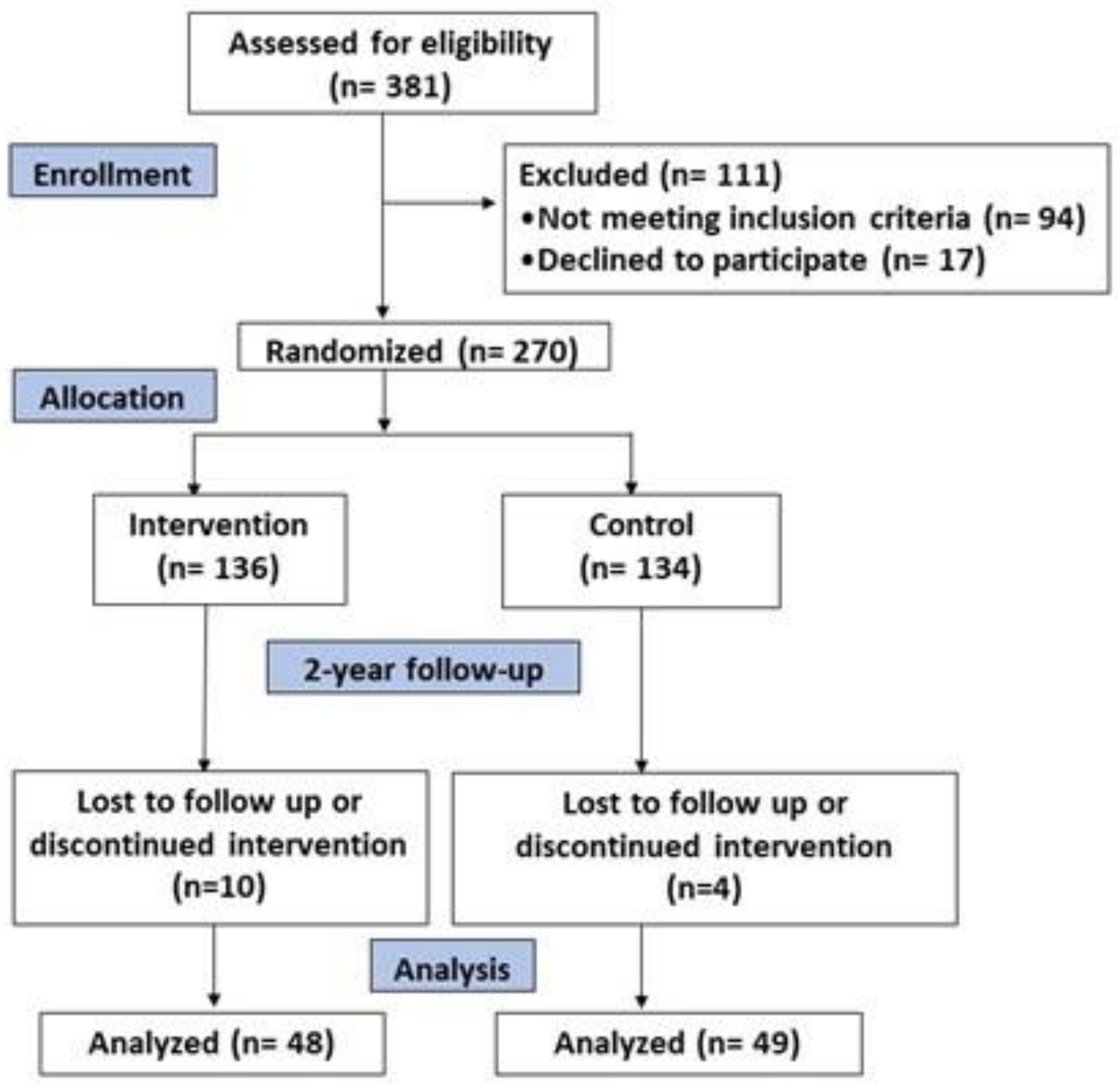
2.1. Design and Participants
2.2. Anthropometrics, Drug Intake, Mediterranean Diet and Physical Activity Characterization
2.3. Blood Collection and Analysis
2.4. Blood Samples Processing
2.5. Serum Biochemical Analysis
2.6. Enzymatic Determinations
2.7. Malondialdehyde Assay
2.8. Polyphenols Assay
2.9. 8-oxodG and 8-oxoGuo Analysis
2.10. Stimulated PBMCs and Neutrophils ROS Production
2.11. Immunoassay Kits
2.12. Statistics
3. Results
3.1. Anthropometric and Haematological Parameters
3.2. Oxidative Stress Biomarkers
3.3. Cytokine Levels
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Knowler, W.C.; Folwer, S.E.; Hamman, R.F.; Christophi, C.A.; Hoffman, H.J.; Brenneman, A.T.; Brown-Friday, J.O.; Goldberg, R.; Venditti, E.; Nathan, D.M. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009, 374, 1677. [Google Scholar] [PubMed] [Green Version]
- Espeland, M.; Pi-Sunyer, X.; Blackburn, G.; Brancati, F.L.; Bray, G.A.; Bright, R.; Clark, J.M.; Curtis, J.M.; Foreyt, J.P.; Graves, K.; et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: One-year results of the look AHEAD trial. Diabetes Care 2007, 30, 1374–1383. [Google Scholar]
- Zomer, E.; Gurusamy, K.; Leach, R.; Trimmer, C.; Lobstein, T.; Morris, S.; James, W.P.T.; Finer, N. Interventions that cause weight loss and the impact on cardiovascular risk factors: A systematic review and meta-analysis. Obes. Rev. 2016, 17, 1001–1011. [Google Scholar] [CrossRef]
- Minich, D.M.; Bland, J.S. Dietary management of the metabolic syndrome beyond macronutrients. Nutr. Rev. 2008, 66, 429–444. [Google Scholar] [CrossRef]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Godos, J.; Zappalà, G.; Bernardini, S.; Giambini, I.; Bes-Rastrollo, M.; Martinez-Gonzalez, M. Adherence to the Mediterranean diet is inversely associated with metabolic syndrome occurrence: A meta-analysis of observational studies. Int. J. Food Sci. Nutr. 2017, 68, 138–148. [Google Scholar] [CrossRef]
- Gami, A.S.; Witt, B.J.; Howard, D.E.; Erwin, P.J.; Gami, L.A.; Somers, V.K.; Montori, V.M. Metabolic Syndrome and Risk of Incident Cardiovascular Events and Death: A Systematic Review and Meta-Analysis of Longitudinal Studies. J. Am. Coll. Cardiol. 2007, 49, 403–414. [Google Scholar] [CrossRef] [Green Version]
- Maurizi, G.; Della Guardia, L.; Maurizi, A.; Poloni, A. Adipocytes properties and crosstalk with immune system in obesity-related inflammation. J. Cell. Physiol. 2018, 233, 88–97. [Google Scholar] [CrossRef]
- Lustig, R.H.; Collier, D.; Kassotis, C.; Roepke, T.A.; Kim, M.J.; Blanc, E.; Barouki, R.; Bansal, A.; Cave, M.C.; Chatterjee, S.; et al. Obesity I: Overview and molecular and biochemical mechanisms. Biochem. Pharmacol. 2022, 199, 115012. [Google Scholar] [CrossRef] [PubMed]
- Prieur, X.; Roszer, T.; Ricote, M. Lipotoxicity in macrophages: Evidence from diseases associated with the metabolic syndrome. Biochim. Biophys. Acta 2010, 1801, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [Green Version]
- Michalek, R.D.; Gerriets, V.A.; Jacobs, S.R.; Macintyre, A.N.; MacIver, N.J.; Mason, E.F.; Sullivan, S.A.; Nichols, A.G.; Rathmell, J.C. Cutting Edge: Distinct Glycolytic and Lipid Oxidative Metabolic Programs Are Essential for Effector and Regulatory CD4+ T Cell Subsets. J. Immunol. 2011, 186, 3299–3303. [Google Scholar] [CrossRef] [Green Version]
- Fox, C.J.; Hammerman, P.S.; Thompson, C.B. Fuel feeds function: Energy metabolism and the Tcell response. Nat. Rev. Immunol. 2005, 5, 844–852. [Google Scholar] [CrossRef]
- Raval, F.M.; Nikolajczyk, B.S. The Bidirectional relationship between metabolism and immune responses. Discoveries 2013, 1, e6. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Legrand, R.; Asakawa, A.; Amitani, H.; Francois, M.; Tennoune, N.; Coëffier, M.; Claeyssens, S.; Rego, J.-C.D.; Déchelotte, P.; et al. Anti-ghrelin immunoglobulins modulate ghrelin stability and its orexigenic effect in obese mice and humans. Nat. Commun. 2013, 4, 2685. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Lin, H. Inflammation initiates a vicious cycle between obesity and nonalcoholic fatty liver disease. Immun. Inflamm. Dis. 2021, 9, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Durrer Schutz, D.; Busetto, L.; Dicker, D.; Farpour-Lambert, N.; Pryke, R.; Toplak, H.; Widmer, D.; Yumuk, V.; Schutz, Y. European Practical and Patient-Centred Guidelines for Adult Obesity Management in Primary Care. Obes. Facts 2019, 12, 40–66. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Díaz-López, A.; Ruiz-Canela, M.; Basora, J.; Fitó, M.; Corella, D.; Serra-Majem, L.; Wärnberg, J.; Romaguera, D.; Estruch, R.; et al. Effect of a Lifestyle Intervention Program with Energy-Restricted Mediterranean Diet and Exercise on Weight Loss and Cardiovascular Risk Factors: One-Year Results of the PREDIMED-Plus Trial. Diabetes Care 2019, 42, 777–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julibert, A.; Bibiloni, M.D.M.; Gallardo-Alfaro, L.; Abbate, M.; Martínez-González, M.; Salas-Salvadó, J.; Corella, D.; Fitó, M.; Martínez, J.A.; Alonso-Gómez, Á.M.; et al. Metabolic Syndrome Features and Excess Weight Were Inversely Associated with Nut Consumption after 1-Year Follow-Up in the PREDIMED-Plus Study. J. Nutr. 2020, 150, 3161–3170. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Ma, Y.; Mo, D.; Zhang, S.; Xiang, M.; Zhang, Z. Methodology of an exercise intervention program using social incentives and gamification for obese children. BMC Pub. Health 2019, 19, 1–10. [Google Scholar] [CrossRef]
- IDF. The International Diabetes Federation Consensus Worldwide Definition of the Metabolic Syndrome; IDF Communications: Brussels, Belgium, 2006. [Google Scholar]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Molina, L.; Sarmiento, M.; Peñafiel, J.; Donaire, D.; Garcia-Aymerich, J.; Gomez, M.; Ble, M.; Ruiz, S.; Frances, A.; Schröder, H.; et al. Validation of the Regicor Short Physical Activity Questionnaire for the Adult Population. PLoS ONE 2017, 12, e0168148. [Google Scholar] [CrossRef]
- Elosua, R.; Garcia, M.; Aguilar, A.; Molina, L.; Covas, M.I.; Marrugat, J. Validation of the Minnesota Leisure Time Physical Activity Questionnaire in Spanish Women. Investigators of the MARATDON Group. Med. Sci. Sports Exerc. 2000, 32, 1431–1437. [Google Scholar] [CrossRef]
- Elosua, R.; Marrugat, J.; Molina, L.; Pons, S.; Pujol, E. Validation of the Minnesota Leisure Time Physical Activity Questionnaire in Spanish men. The MARATHOM Investigators. Am. J. Epidemiol. 1994, 139, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; López-Fontana, C.; Varo, J.J.; Sánchez-Villegas, A.; Martinez, J.A. Validation of the Spanish version of the physical activity questionnaire used in the Nurses’ Health Study and the Health Professionals’ Follow-up Study. Public Health Nutr. 2005, 8, 920–927. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Leon, A.S.; Jacobs, D.R.; Montoye, H.J.; Sallis, J.F.; Paffenbarger, R.S. Compendium of Physical Activities: Classification of energy costs of human physical activities. Med. Sci. Sports Exerc. 1993, 25, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Álvarez, I.; Martinez-Gonzalez, M.A.; Sánchez-Tainta, A.; Corella, D.; Díaz-López, A.; Fito, M.; Vioque, J.; Romaguera, D.; Martínez, J.A.; Wärnberg, J.; et al. Adherence to an energy-restricted Mediterranean diet score and prevalence of cardiovascular risk factors in the PREDIMED-plus: A cross-sectional study. Rev. Española Cardiol. 2019, 72, 925–934. [Google Scholar] [CrossRef]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bøyum, A. Separation of White Blood Cells. Nature 1964, 204, 793–794. [Google Scholar] [CrossRef]
- Busquets-Cortés, C.; Capó, X.; Bibiloni, M.D.M.; Martorell, M.; Ferrer, M.D.; Argelich, E.; Bouzas, C.; Carreres, S.; Tur, J.A.; Pons, A.; et al. Peripheral blood mononuclear cells antioxidant adaptations to regular physical activity in elderly people. Nutrients 2018, 10, 1555. [Google Scholar] [CrossRef] [Green Version]
- Capo, X.; Martorell, M.; Sureda, A.; Tur, J.A.; Pons, A. Effects of docosahexaenoic supplementation and in vitro vitamin C on the oxidative and inflammatory neutrophil response to activation. Oxidative Med. Cell. Longev. 2015, 2015, 187849. [Google Scholar] [CrossRef] [Green Version]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Capó, X.; Bouzas, C.; Mateos, D.; Pons, A.; Tur, J.A.; Sureda, A. Metabolic syndrome is associated with oxidative stress and proinflammatory state. Antioxidants 2020, 9, 236. [Google Scholar] [CrossRef] [Green Version]
- Busquets-Cortés, C.; Capó, X.; Argelich, E.; Ferrer, M.D.; Mateos, D.; Bouzas, C.; Abbate, M.; Tur, J.A.; Sureda, A.; Pons, A. Effects of micromolar steady-state hydrogen peroxide exposure on inflammatory and redox gene expression in immune cells from humans with metabolic syndrome. Nutrients 2018, 10, 1920. [Google Scholar] [CrossRef] [Green Version]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Flohé, L.; Otting, F. Superoxide dismutase assays. Methods Enzymol. 1984, 105, 93–104. [Google Scholar] [PubMed]
- Capeillère-Blandin, C. Oxidation of guaiacol by myeloperoxidase: A two-electron-oxidized guaiacol transient species as a mediator of NADPH oxidation. Biochem. J. 1998, 336, 395–404. [Google Scholar] [CrossRef] [Green Version]
- Medina-Remón, A.; Tresserra-Rimbau, A.; Pons, A.; Tur, J.A.; Martorell, M.; Ros, E.; Buil-Cosiales, P.; Sacanella, E.; Covas, M.I.; Corella, D.; et al. Effects of total dietary polyphenols on plasma nitric oxide and blood pressure in a high cardiovascular risk cohort. The PREDIMED randomized trial. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Kjær, L.K.; Cejvanovic, V.; Henriksen, T.; Petersen, K.M.; Hansen, T.; Pedersen, O.; Christensen, C.K.; Torp-Pedersen, C.; Gerds, T.A.; Brandslund, I.; et al. Cardiovascular and All-Cause Mortality Risk Associated with Urinary Excretion of 8-oxoGuo, a Biomarker for RNA Oxidation, in Patients With Type 2 Diabetes: A Prospective Cohort Study. Diabetes Care 2017, 40, 1771–1778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henriksen, T.; Hillestrøm, P.R.; Poulsen, H.E.; Weimann, A. Automated method for the direct analysis of 8-oxo-guanosine and 8-oxo-2’-deoxyguanosine in human urine using ultraperformance liquid chromatography and tandem mass spectrometry. Free Radic. Biol. Med. 2009, 47, 629–635. [Google Scholar] [CrossRef]
- Rasmussen, S.T.; Andersen, J.T.; Nielsen, T.K.; Cejvanovic, V.; Petersen, K.M.; Henriksen, T.; Weimann, A.; Lykkesfeldt, J.; Poulsen, H.E. Simvastatin and oxidative stress in humans: A randomized, double-blinded, placebo-controlled clinical trial. Redox Biol. 2016, 9, 32–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulsen, H.E.; Weimann, A.; Henriksen, T.; Kjær, L.K.; Larsen, E.L.; Carlsson, E.R.; Christensen, C.K.; Brandslund, I.; Fenger, M. Oxidatively generated modifications to nucleic acids in vivo: Measurement in urine and plasma. Free Radic. Biol. Med. 2019, 145, 336–341. [Google Scholar] [CrossRef]
- Agnoli, C.; Sieri, S.; Ricceri, F.; Giraudo, M.T.; Masala, G.; Assedi, M.; Panico, S.; Mattiello, A.; Tumino, R.; Giurdanella, M.C.; et al. Adherence to a Mediterranean diet and long-term changes in weight and waist circumference in the EPIC-Italy cohort. Nutr. Diabetes 2018, 8, 22. [Google Scholar]
- Esposito, K.; Kastorini, C.M.; Panagiotakos, D.B.; Giugliano, D. Mediterranean diet and weight loss: Meta-analysis of randomized controlled trials. Metab. Syndr. Relat. Disord. 2011, 9, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Buckland, G.; Bach, A.; Serra-Majem, L. Obesity and the Mediterranean diet: A systematic review of observational and intervention studies. Obes. Rev. 2008, 9, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Cano-Ibáñez, N.; Bueno-Cavanillas, A.; Martínez-González, M.Á.; Salas-Salvadó, J.; Corella, D.; Freixer, G.; Romaguera, D.; Vioque, J.; Alonso-Gómez, Á.M.; Wärnberg, J.; et al. Effect of changes in adherence to Mediterranean diet on nutrient density after 1-year of follow-up: Results from the PREDIMED-Plus Study. Eur. J. Nutr. 2020, 59, 2395–2409. [Google Scholar] [CrossRef] [PubMed]
- Nani, A.; Murtaza, B.; Sayed Khan, A.; Khan, N.A.; Hichami, A. Antioxidant and anti-inflammatory potential of polyphenols contained in mediterranean diet in obesity: Molecular mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef] [PubMed]
- Sheikhansari, G.; Soltani-Zangbar, M.S.; Pourmoghadam, Z.; Kamrani, A.; Azizi, R.; Aghebati-Maleki, L.; Danaii, S.; Koushaeian, L.; Hojat-Farsangi, M.; Yousefi, M. Oxidative stress, inflammatory settings, and microRNA regulation in the recurrent implantation failure patients with metabolic syndrome. Am. J. Reprod. Immunol. 2019, 82, e13170. [Google Scholar] [CrossRef] [PubMed]
- Sladoje, D.P.; Kisić, B.; Mirić, D. The Monitoring of Protein Markers of Inflammation and Serum Lipid Concentration in Obese Subjects with Metabolic Syndrome. J. Med. Biochem. 2017, 36, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Kurajoh, M.; Fukumoto, S.; Murase, T.; Nakamura, T.; Ishihara, T.; Go, H.; Yamamoto, K.; Nakatani, S.; Tsuda, A.; Morioka, T.; et al. Insulin Resistance Associated with Plasma Xanthine Oxidoreductase Activity Independent of Visceral Adiposity and Adiponectin Level: MedCity21 Health Examination Registry. Int. J. Endocrinol. 2019, 2019, 1762161. [Google Scholar] [CrossRef]
- Vorbach, C.; Harrison, R.; Capecchi, M.R. Xanthine oxidoreductase is central to the evolution and function of the innate immune system. Trends Immunol. 2003, 24, 512–517. [Google Scholar] [CrossRef]
- Richette, P.; Poitou, C.; Manivet, P.; Denis, J.; Bouillot, J.L.; Clément, K.; Oppert, J.M.; Bardin, T. Weight Loss, Xanthine Oxidase, and Serum Urate Levels: A Prospective Longitudinal Study of Obese Patients. Arthritis Care Res. 2016, 68, 1036–1042. [Google Scholar] [CrossRef]
- Sureda, A.; del Bibiloni, M.; Martorell, M.; Buil-Cosiales, P.; Marti, A.; Pons, A.; Tur, J.A.; Martinez-Gonzalez, M.Á. Mediterranean diets supplemented with virgin olive oil and nuts enhance plasmatic antioxidant capabilities and decrease xanthine oxidase activity in people with metabolic syndrome: The PREDIMED study. Mol. Nutr. Food Res. 2016, 60, 2654–2664. [Google Scholar] [CrossRef]
- Mathew, A.V.; Li, L.; Byun, J.; Guo, Y.; Michailidis, G.; Jaiswal, M.; Chen, Y.E.; Pop-Busui, R.; Pennathur, S. Therapeutic Lifestyle Changes Improve HDL Function by Inhibiting Myeloperoxidase-Mediated Oxidation in Patients with Metabolic Syndrome. Diabetes Care 2018, 41, 2431–2437. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.J.; Yen, C.H.; Huang, Y.C.; Lee, B.J.; Hsia, S.; Lin, P.T. Relationships between Inflammation, Adiponectin, and Oxidative Stress in Metabolic Syndrome. PLoS ONE 2012, 7, 8–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.-S.; Kor, C.-T.; Chen, T.-Y.; Liu, K.-H.; Shih, K.-L.; Su, W.-W.; Wu, H.-M. Relationships between Serum Uric Acid, Malondialdehyde Levels, and Carotid Intima-Media Thickness in the Patients with Metabolic Syndrome. Oxidative Med. Cell. Longev. 2019, 2019, 6859757. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Kim, H.; Barnes, R.C.; Talcott, S.T.; Mertens-Talcott, S.U. Obesity-Associated Diseases Biomarkers Are Differently Modulated in Lean and Obese Individuals and Inversely Correlated to Plasma Polyphenolic Metabolites After 6 Weeks of Mango (Mangifera indica L.) Consumption. Mol. Nutr. Food Res. 2018, 62, e1800129. [Google Scholar] [CrossRef]
- Novotny, J.A.; Chen, T.Y.; Terekhov, A.I.; Gebauer, S.K.; Baer, D.J.; Ho, L.; Pasinetti, G.M.; Ferruzzi, M.G. The effect of obesity and repeated exposure on pharmacokinetic response to grape polyphenols in humans. Mol. Nutr. Food Res. 2017, 61, 1700043. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; De Bittencourt, P.I.H. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016, 473, 4527–4550. [Google Scholar] [CrossRef]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Bouzas, C.; Capó, X.; Mateos, D.; Ugarriza, L.; Tur, J.A.; Sureda, A. Peripheral Blood Mononuclear Cells Oxidative Stress and Plasma Inflammatory Biomarkers in Adults with Normal Weight, Overweight and Obesity. Antioxidants 2021, 10, 813. [Google Scholar] [CrossRef]
- Versleijen, M.; Roelofs, H.; Preijers, F.; Roos, D.; Wanten, G. Parenteral lipids modulate leukocyte phenotypes in whole blood, depending on their fatty acid composition. Clin. Nutr. 2005, 24, 822–829. [Google Scholar] [CrossRef]
- Jaudszus, A.; Gruen, M.; Watzl, B.; Ness, C.; Roth, A.; Lochner, A.; Barz, D.; Gabriel, H.; Rothe, M.; Jahreis, G. Evaluation of suppressive and pro-resolving effects of EPA and DHA in human primary monocytes and T-helper cells. J. Lipid Res. 2013, 54, 923–935. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, L.R.; Olsen, R.H.; Anholm, C.; Astrup, A.; Eugen-Olsen, J.; Fenger, M.; Simonsen, L.; Walzem, R.L.; Haugaard, S.B.; Prescott, E. Effects of 1 year of exercise training versus combined exercise training and weight loss on body composition, low-grade inflammation and lipids in overweight patients with coronary artery disease: A randomized trial. Cardiovasc. Diabetol. 2019, 18, 127. [Google Scholar] [CrossRef]
- Petelin, A.; Bizjak, M.; Černelič-Bizjak, M.; Jurdana, M.; Jakus, T.; Jenko-Pražnikar, Z. Low-grade inflammation in overweight and obese adults is affected by weight loss program. J. Endocrinol. Investig. 2014, 37, 745–755. [Google Scholar] [CrossRef]
- Porter Starr, K.N.; Orenduff, M.; McDonald, S.R.; Mulder, H.; Sloane, R.; Pieper, C.F.; Bales, C.W. Influence of Weight Reduction and Enhanced Protein Intake on Biomarkers of Inflammation in Older Adults with Obesity. J. Nutr. Gerontol. Geriatr. 2019, 38, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Moschen, A.R.; Molnar, C.; Enrich, B.; Geiger, S.; Ebenbichler, C.F.; Tilg, H. Adipose and liver expression of interleukin (IL)-1 family members in morbid obesity and effects of weight loss. Mol. Med. 2011, 17, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.I.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.P.; Sheu, W.H.H.; Lee, I.T.; Lee, W.J.; Wang, J.S.; Liang, K.W.; Lee, W.L.; Lin, S.Y. Weight loss reduces serum monocyte chemoattractant protein-1 concentrations in association with improvements in renal injury in obese men with metabolic syndrome. Clin. Chem. Lab. Med. 2015, 53, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.H.; Park, H.S.; Kim, K.S.; Choi, W.H.; Ahn, C.W.; Kim, B.T.; Kim, S.M.; Lee, S.Y.; Ahn, S.M.; Kim, Y.K.; et al. Effect of weight loss on some serum cytokines in human obesity: Increase in IL-10 after weight loss. J. Nutr. Biochem. 2008, 19, 371–375. [Google Scholar] [CrossRef]
- Carpi, S.; Scoditti, E.; Massaro, M.; Polini, B.; Manera, C.; Digiacomo, M.; Salsano, J.E.; Poli, G.; Tuccinardi, T.; Doccini, S.; et al. The extra-virgin olive oil polyphenols oleocanthal and oleacein counteract inflammation-related gene and miRNA expression in adipocytes by attenuating NF-κB activation. Nutrients 2019, 11, 2855. [Google Scholar] [CrossRef] [Green Version]
- Larsen, E.L.; Weimann, A.; Poulsen, H.E. Interventions targeted at oxidatively generated modifications of nucleic acids focused on urine and plasma markers. Free Radic. Biol. Med. 2019, 145, 256–283. [Google Scholar] [CrossRef]
- Cejvanovic, V.; Asferg, C.; Kjær, L.K.; Andersen, U.B.; Linneberg, A.; Frystyk, J.; Henriksen, T.; Flyvbjerg, A.; Christiansen, M.; Weimann, A.; et al. Markers of oxidative stress in obese men with and without hypertension. Scand. J. Clin. Lab. Investig. 2016, 76, 620–625. [Google Scholar] [CrossRef]
- Carlsson, E.R.; Fenger, M.; Henriksen, T.; Kjaer, L.K.; Worm, D.; Hansen, D.L.; Madsbad, S.; Poulsen, H.E. Reduction of oxidative stress on DNA and RNA in obese patients after Roux-en-Y gastric bypass surgery-An observational cohort study of changes in urinary markers. PLoS ONE 2020, 15, e0243918. [Google Scholar] [CrossRef]
- Mitjavila, M.T.; Fandos, M.; Salas-Salvadó, J.; Covas, M.-I.; Borrego, S.; Estruch, R.; Lamuela-Raventos, R.M.; Corella, D.; Martinez-Gonzalez, M.A.; Sánchez, J.M.; et al. The Mediterranean diet improves the systemic lipid and DNA oxidative damage in metabolic syndrome individuals. A randomized, controlled, trial. Clin. Nutr. 2013, 32, 172–178. [Google Scholar] [CrossRef]

| Baseline | 2-Year | ANCOVA | |||
|---|---|---|---|---|---|
| Control (n = 49) | Intervention (n = 48) | Control (n = 49) | Intervention (n = 48) | NIxT | |
| Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | ||
| Weight (kg) | 88.0 ± 1.19 | 87.0 ± 1.17 | 87.5 ± 1.32 | 83.9 ± 1.20 | 0.439 |
| Height (cm) | 162.7 ± 0.78 | 162.8 ± 0.82 | 162.7 ± 0.83 | 162.4 ± 0.87 | 0.497 |
| BMI (kg/m2) | 33.2 ± 0.33 | 32.7 ± 0.30 | 33.0 ± 0.40 | 31.7 ± 0.32 | 0.132 |
| WHtR | 0.689 ± 0.005 | 0.679 ± 0.005 | 0.679 ± 0.006 | 0.657 ± 0.006 | 0.033 |
| Abdominal obesity (cm) | 111.9 ± 0.89 | 110.4 ± 0.86 | 110.6 ± 1.07 | 106.7 ± 0.92 | 0.104 |
| Systolic blood pressure (mmHg) | 143.2 ± 1.54 | 141.2 ± 1.47 | 138.9 ± 1.65 | 136.1 ± 1.73 | 0.039 |
| Diastolic blood pressure (mmHg) | 82.4 ± 0.78 | 82.7 ± 0.76 | 76.4 ± 0.87 * | 75.9 ± 0.86 * | <0.001 |
| Glucose (mg/dL) | 117.5 ± 3.04 | 118.5 ± 3.25 | 115.5 ± 3.22 | 114.9 ± 2.96 | 0.823 |
| Triglycerides (mg/dL) | 154.6 ± 6.10 | 148.2 ± 6.57 | 152.1 ± 6.57 | 132.6 ± 5.49 | 0.376 |
| HDL-cholesterol (mg/dL) | 45.0 ± 0.94 | 43.5 ± 0.87 | 45.6 ± 1.17 | 46.0 ± 0.98 | 0.672 |
| Mediterranean Diet adherence (score) | 7.31 ± 0.23 | 7.68 ± 0.20 | 10.86 ± 0.29 * | 12.66 ± 0.23 * | <0.001 |
| Total physical activity (MET·min/week) | 3250 ± 272 | 2763 ± 222 | 3026 ± 254 | 3040 ± 298 | 0.424 |
| N (%) | N (%) | N (%) | N (%) | p-value | |
| Antidiabetic drug intake | 19 (39.1) | 16 (33.6) | 20 (41.7) | 17 (34.7) | 0.496 |
| Antihypertensive drug intake | 37 (75.9) | 39 (81.7) | 38 (78.7) | 42 (87.1) | 0.133 |
| Baseline | 2-Year | ANCOVA | |||
|---|---|---|---|---|---|
| Control (n = 49) | Intervention (n = 48) | Control (n = 49) | Intervention (n = 48) | NIxT | |
| Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | ||
| Hematocrit (%) | 43.0 ± 0.4 | 42.8 ± 0.3 | 43.0 ± 0.4 | 43.0 ± 0.3 | 0.668 |
| Erythrocytes (106/mm3) | 4.77 ± 0.04 | 4.78 ± 0.04 | 4.75 ± 0.05 | 4.73 ± 0.04 | 0.776 |
| Leukocytes (103/mm3) | 7.32 ± 0.13 | 7.45 ± 0.20 | 7.41 ± 0.18 | 7.35 ± 0.19 | 0.390 |
| Neutrophils (103/mm3) | 4.56 ± 0.41 | 4.17 ± 0.16 | 4.12 ± 0.13 | 4.77 ± 0.67 | 0.752 |
| Lymphocytes (103/mm3) | 2.44 ± 0.06 | 2.39 ± 0.07 | 2.39 ± 0.08 | 2.30 ± 0.07 | 0.924 |
| Monocytes (103/mm3) | 0.61 ± 0.01 | 0.65 ± 0.02 | 0.62 ± 0.02 | 0.618 ± 0.02 | 0.174 |
| Eosinophils (103/mm3) | 0.26 ± 0.04 | 0.231 ± 0.02 | 0.228 ± 0.02 | 0.223 ± 0.01 | 0.855 |
| Basophils (103/mm3) | 0.055 ± 0.007 | 0.056 ± 0.007 | 0.053 ± 0.003 | 0.060 ± 0.005 | 0.948 |
| Baseline | 2-Year | ANCOVA | |||
|---|---|---|---|---|---|
| Control (n = 49) | Intervention (n = 48) | Control (n = 49) | Intervention (n = 48) | NIxT | |
| Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | ||
| Plasma enzymes | |||||
| CAT (kat/L sang) | 48.0 ± 3.76 | 53.3 ± 4.16 | 98.15 ± 6.05 * | 84.1 ± 6.93 * | <0.001 |
| SOD (pkat/L sang) | 162.9 ± 13.7 | 155.2 ± 14.0 | 188.4 ± 12.5 | 170.1 ± 6.29 | 0.174 |
| MPO (μkat/mL sang) | 57.5 ± 4.23 | 63.5 ± 4.85 | 29.1 ± 2.21 * | 27.2 ± 1.55 * | <0.001 |
| XOD (ng/mL) | 0.430 ± 0.046 | 0.449 ± 0.046 | 0.424 ± 0.036 | 0.286 ± 0.029 | 0.402 |
| Oxidative Damage | |||||
| MDA plasma (nM) | 1.190 ± 0.085 | 1.025 ± 0.105 | 0.485 ± 0.035 * | 0.467 ± 0.035 * | <0.001 |
| MDA urine/creatinine (mM/mM) | 94.4 ± 10.8 | 96.1 ± 13.3 | 100.5 ± 7.99 | 111.0 ± 12.5 | 0.652 |
| Polyphenols plasma (mg/mL) | 0.058 ± 0.003 | 0.057 ± 0.002 | 0.102 ± 0.009 * | 0.087 ± 0.009 * | <0.001 |
| Polyphenols urine/creatinine (g/L/mM) | 11.2 ± 0.58 | 11.4 ± 0.72 | 13.5 ± 1.1 | 13.7 ± 0.922 | 0.941 |
| 8oxoGuo urine/creatinine (nM/mM) | 2.04 ± 0.085 | 2.01 ± 0.073 | 1.91 ± 0.076 | 1.76 ± 0.055 | 0.566 |
| 8oxodG urine/creatinine (nM/mM) | 1.49 ± 0.092 | 1.40 ± 0.073 | 1.26 ± 0.045 | 1.17 ± 0.051 | 0.247 |
| ROS production | |||||
| PBMCs Zym (RLU/min·103 cells) | 3424 ± 261 | 3617 ± 282 | 2892 ± 153 | 2881 ± 146 | 0.373 |
| PBMCs LPS (RLU/min·103 cells) | 1327 ± 104 | 1312 ± 100 | 1240 ± 74 | 1210 ± 68 | 0.986 |
| Neutrophils Zym (RLU/min·103 cells) | 11219 ± 661 | 11341 ± 714 | 9750 ± 674 | 9248 ± 416 | 0.182 |
| Neutrophils LPS (RLU/min·103 cells) | 3339 ± 198 | 3490 ± 251 | 2877 ± 249 | 2633 ± 180 * | 0.022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Bouzas, C.; García, S.; Mateos, D.; Gómez, C.; Gámez, J.M.; Poulsen, H.E.; Tur, J.A.; Sureda, A. Effects of 2-Year Nutritional and Lifestyle Intervention on Oxidative and Inflammatory Statuses in Individuals of 55 Years of Age and over at High Cardiovascular Risk. Antioxidants 2022, 11, 1326. https://doi.org/10.3390/antiox11071326
Monserrat-Mesquida M, Quetglas-Llabrés M, Bouzas C, García S, Mateos D, Gómez C, Gámez JM, Poulsen HE, Tur JA, Sureda A. Effects of 2-Year Nutritional and Lifestyle Intervention on Oxidative and Inflammatory Statuses in Individuals of 55 Years of Age and over at High Cardiovascular Risk. Antioxidants. 2022; 11(7):1326. https://doi.org/10.3390/antiox11071326
Chicago/Turabian StyleMonserrat-Mesquida, Margalida, Magdalena Quetglas-Llabrés, Cristina Bouzas, Silvia García, David Mateos, Cristina Gómez, José M. Gámez, Henrik E. Poulsen, Josep A. Tur, and Antoni Sureda. 2022. "Effects of 2-Year Nutritional and Lifestyle Intervention on Oxidative and Inflammatory Statuses in Individuals of 55 Years of Age and over at High Cardiovascular Risk" Antioxidants 11, no. 7: 1326. https://doi.org/10.3390/antiox11071326
APA StyleMonserrat-Mesquida, M., Quetglas-Llabrés, M., Bouzas, C., García, S., Mateos, D., Gómez, C., Gámez, J. M., Poulsen, H. E., Tur, J. A., & Sureda, A. (2022). Effects of 2-Year Nutritional and Lifestyle Intervention on Oxidative and Inflammatory Statuses in Individuals of 55 Years of Age and over at High Cardiovascular Risk. Antioxidants, 11(7), 1326. https://doi.org/10.3390/antiox11071326










