Antioxidative Role of Hygrophila erecta (Brum. F.) Hochr. on UV-Induced Photoaging of Dermal Fibroblasts and Melanoma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Extraction of MEH
2.2. Liquid Chromatography—Mass Spectrometry (LC–MS) Analysis
2.3. Free Radical Scavenging Activity
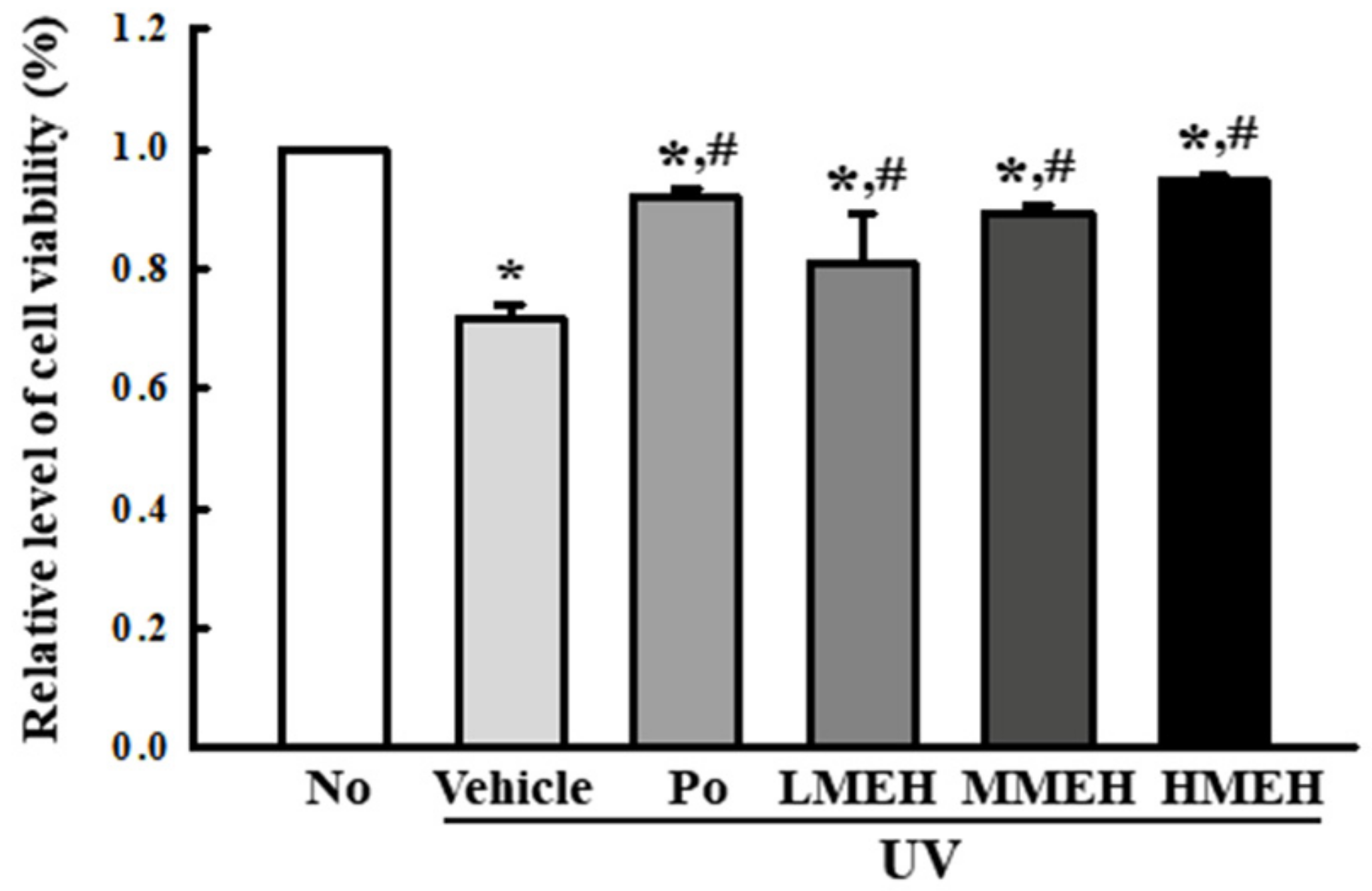
2.4. Cell Viability Analysis
2.5. Apoptotic Cells Analysis
2.6. Determination of Intracellular ROS Levels
2.7. Nitric Oxide (NO) Concentration
2.8. Superoxide Dismutase (SOD) Activity Analysis
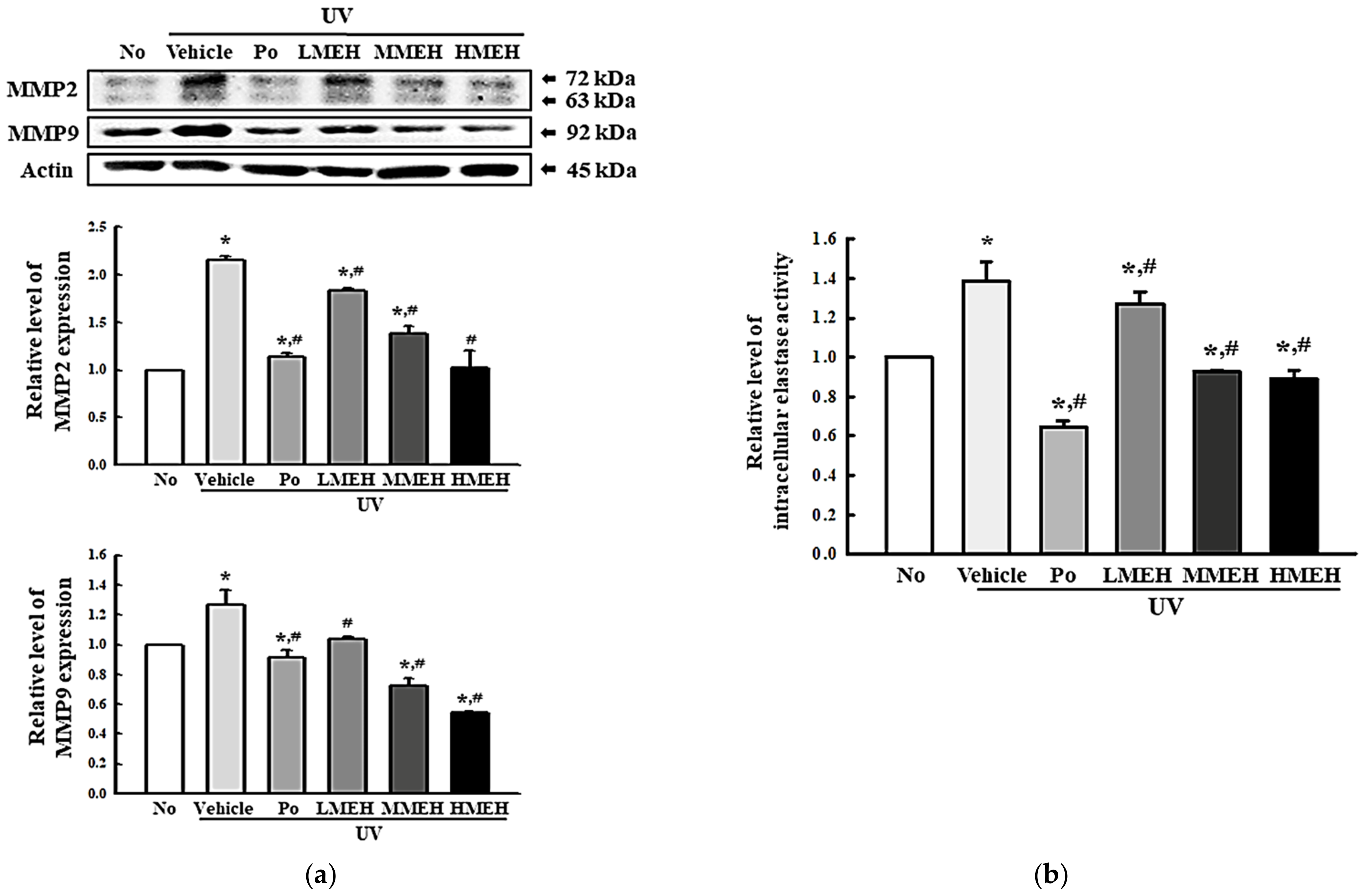
2.9. Intracellular Elastase Activity Assay
2.10. Melanin Content Analysis
2.11. Intracellular Tyrosinase Activity Assay
2.12. Western Blot Analysis
2.13. Quantitative Real Time-PCR (RT-qPCR) Analysis
2.14. Statistical Significance Analysis
3. Results
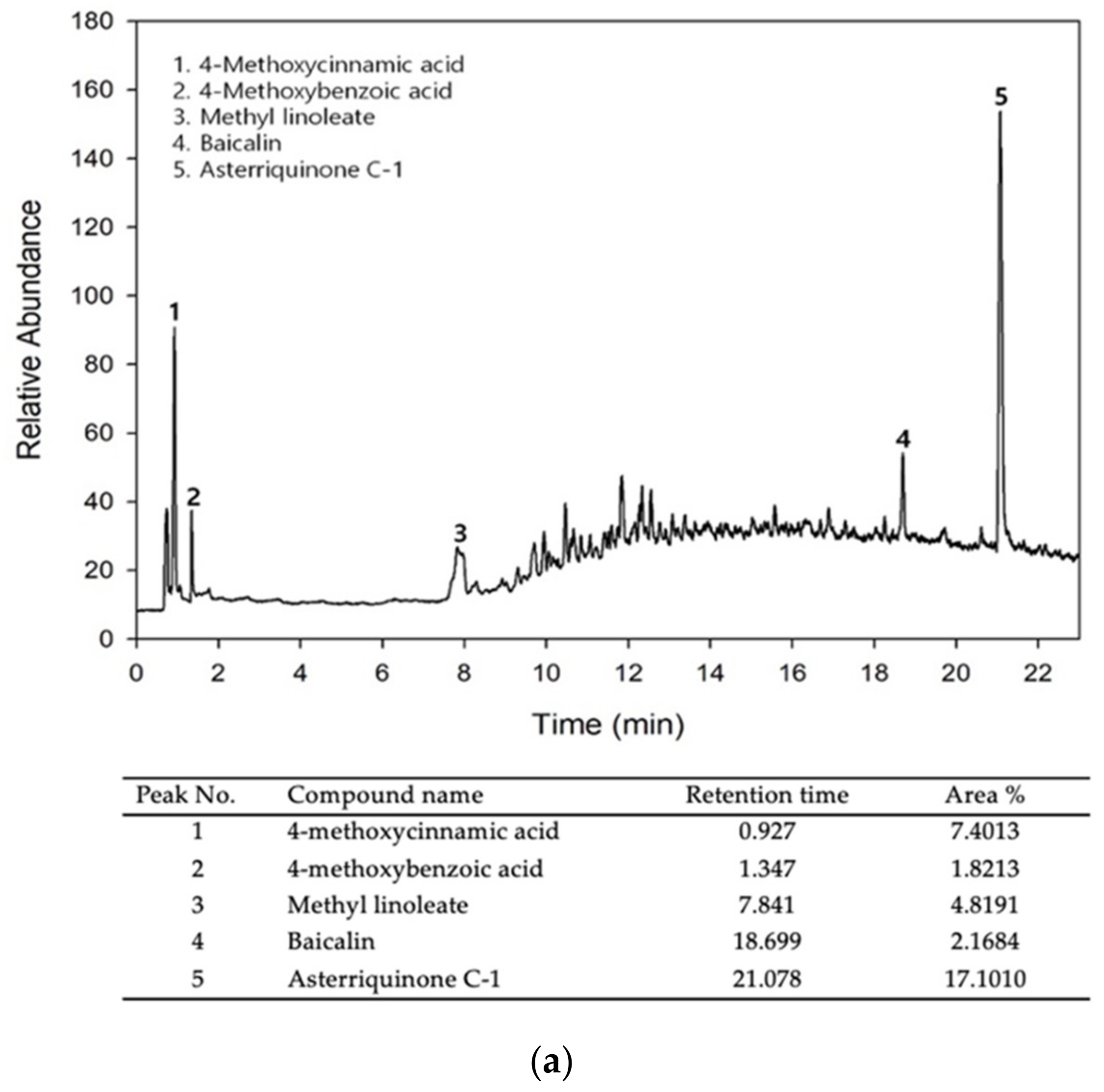
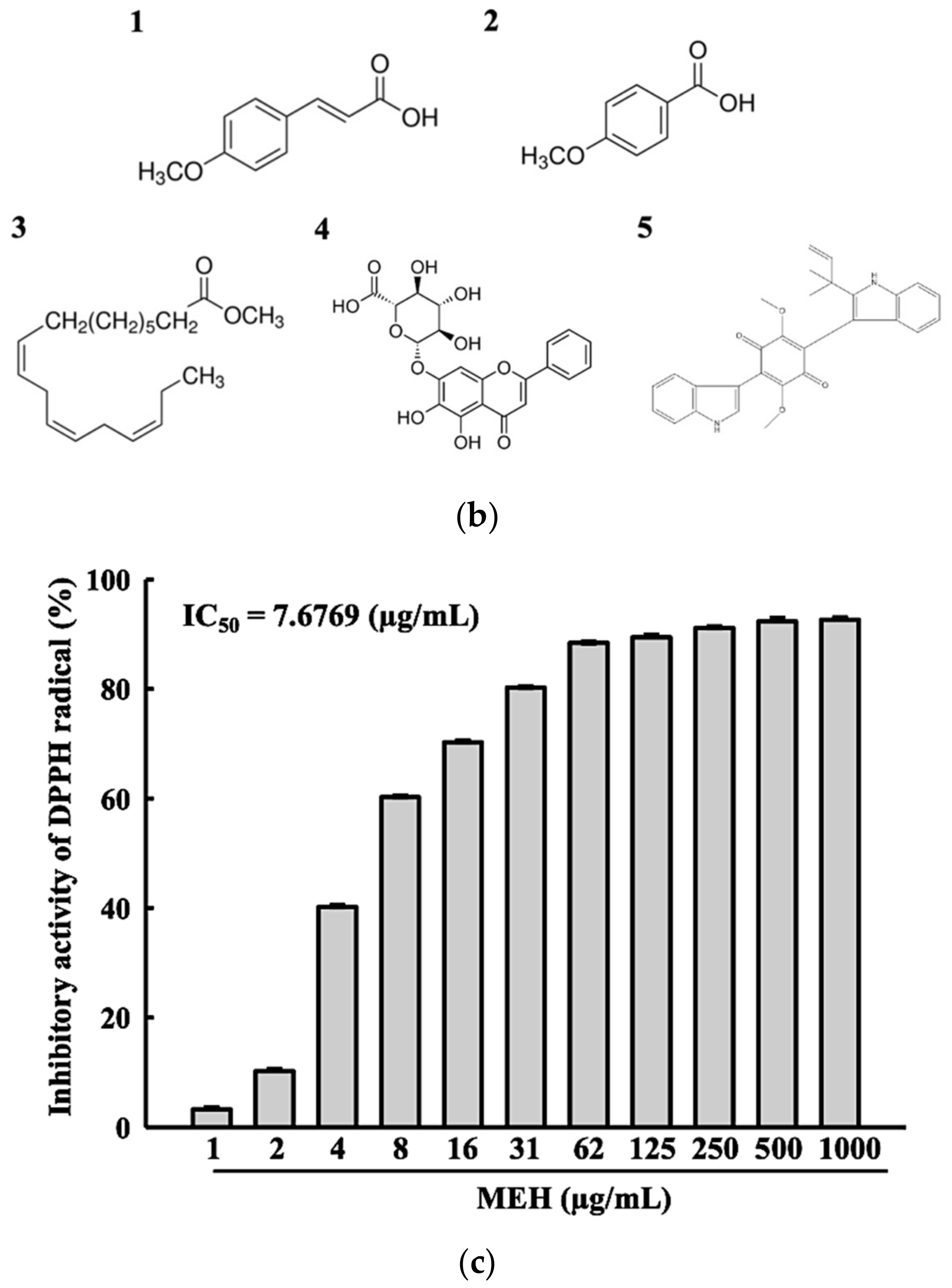
3.1. Bioactive Components and Free Radical Scavenging Activity of MEH
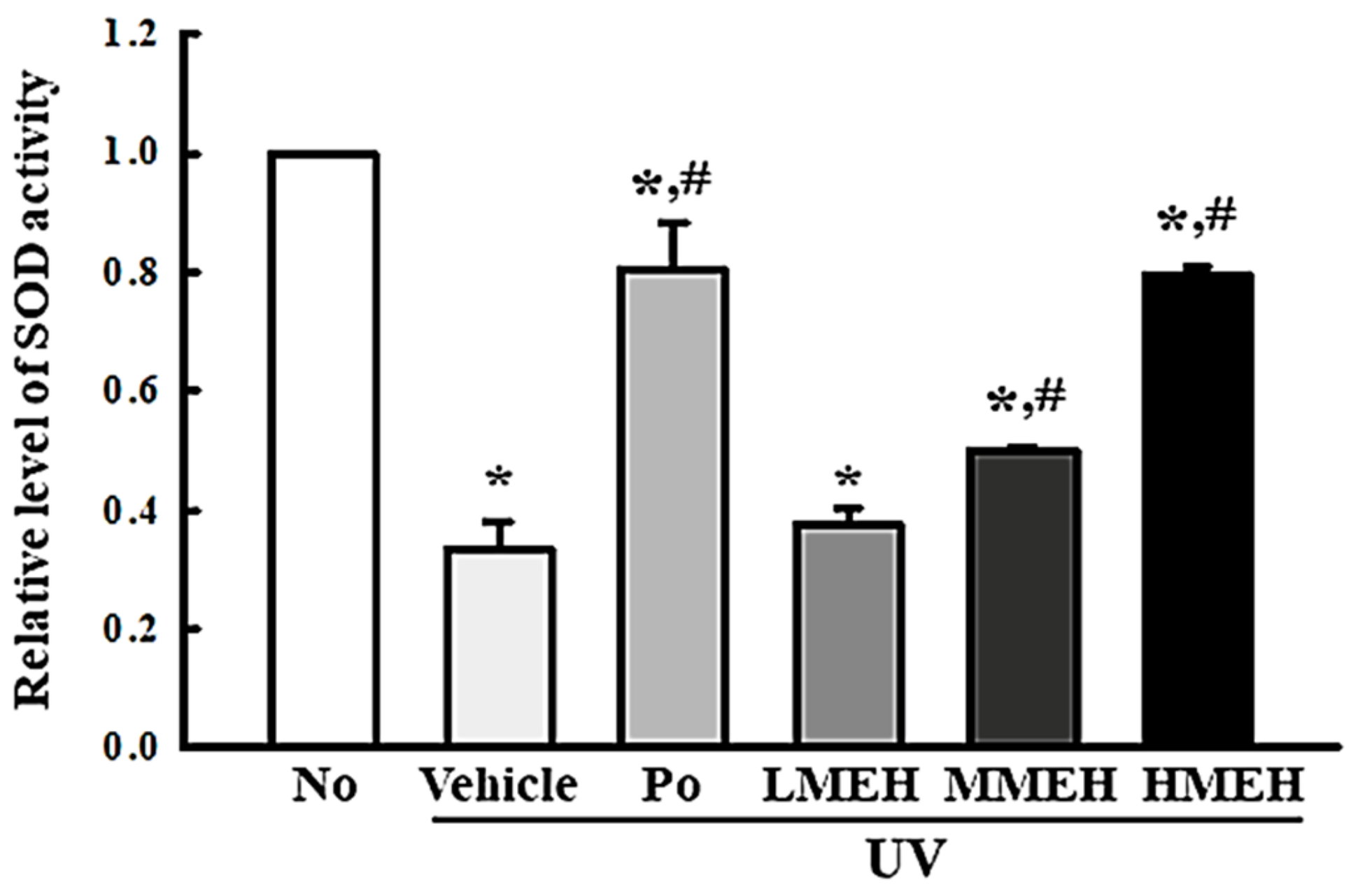
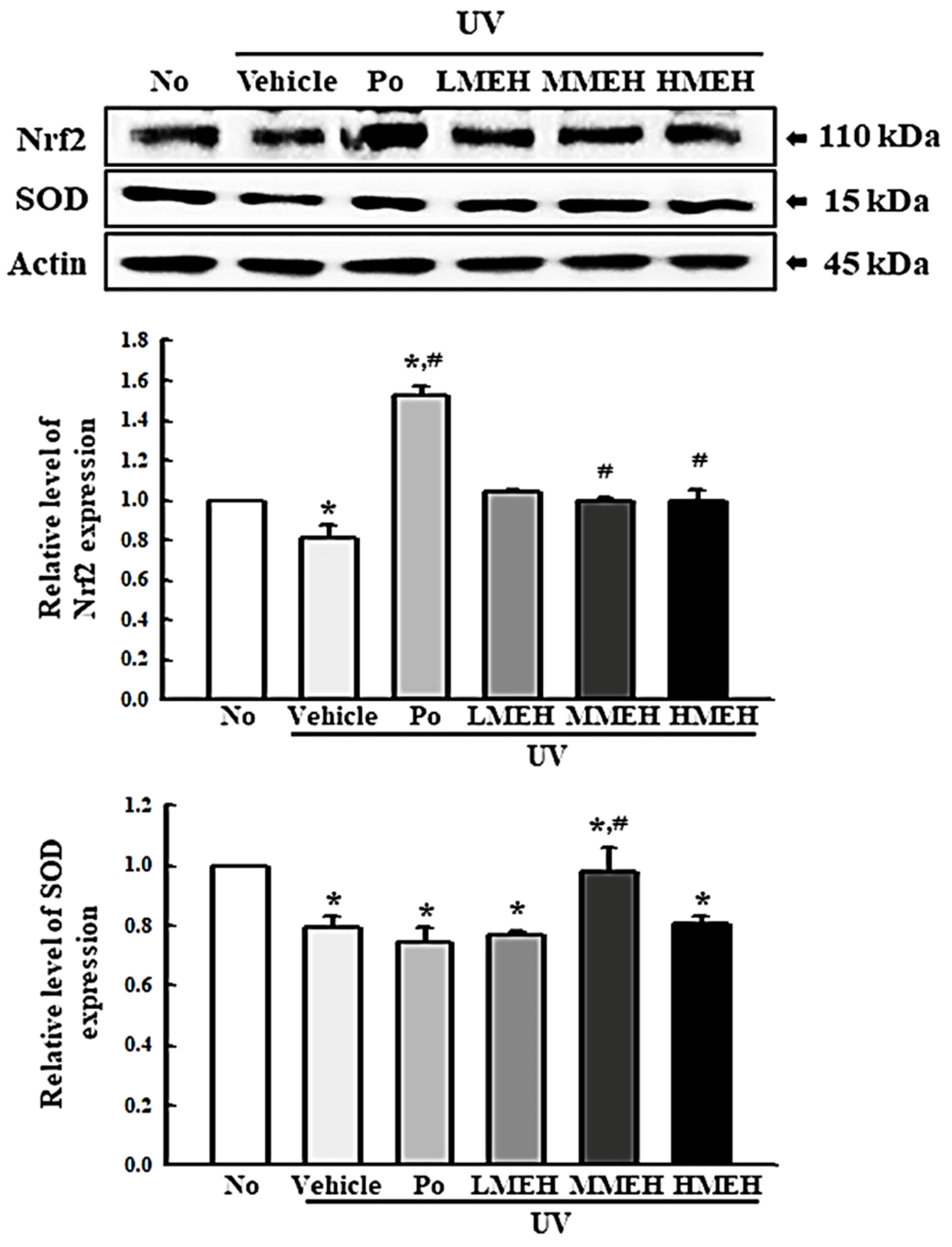
3.2. Improvement of the Antioxidant Defense System of UV-Irradiated NHDF Cells by MEH
3.3. Recovery Effect of MEH on Apoptosis of UV-Irradiated NHDF Cells
3.4. Effect of MEH on the Regulation of ECM Structural Proteins in UV-Irradiated NHDF Cells
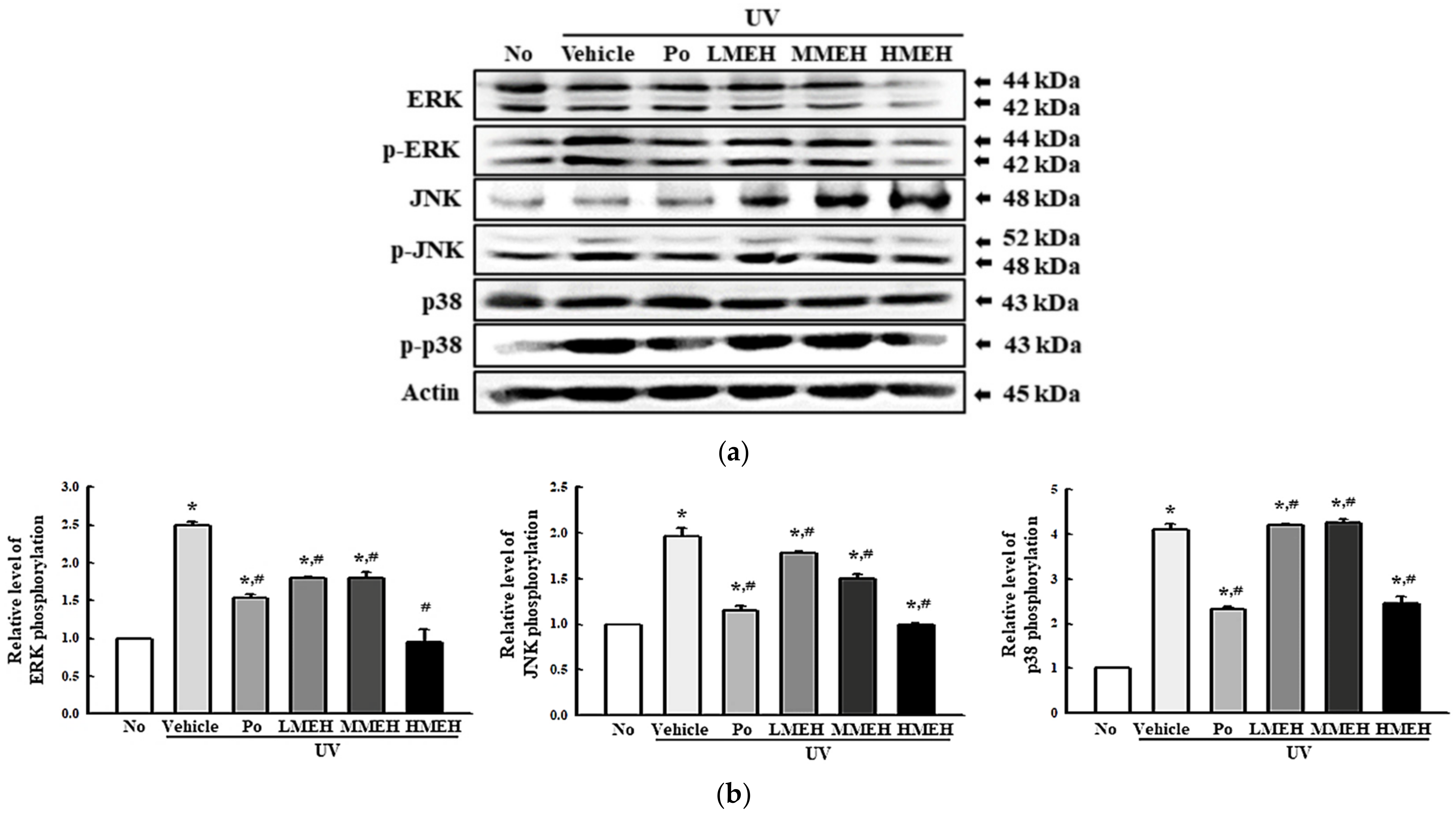
3.5. Role of MAPK Signaling Pathway during the Inhibition of UV-Induced Overexpression of MMPs in NHDF Cells
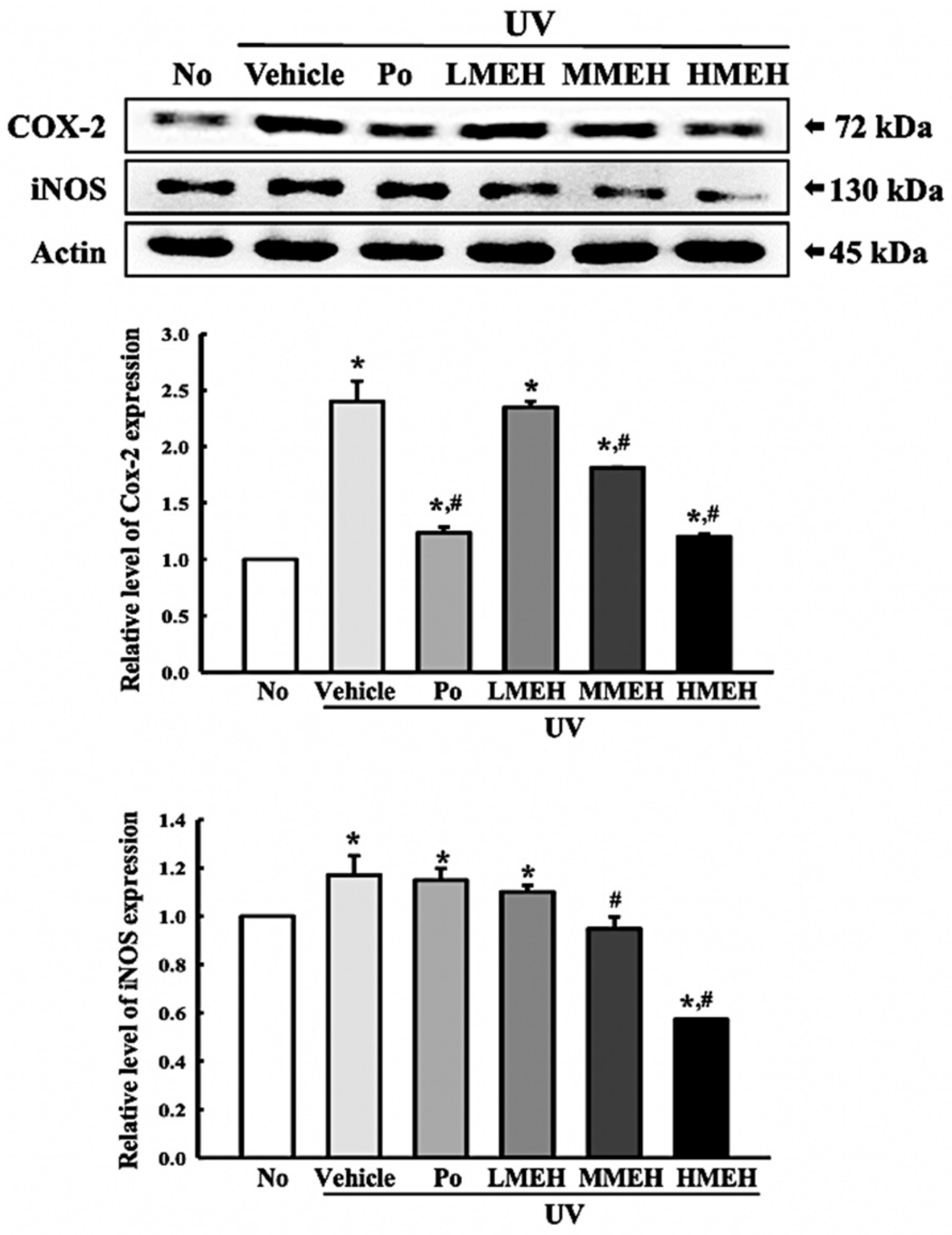
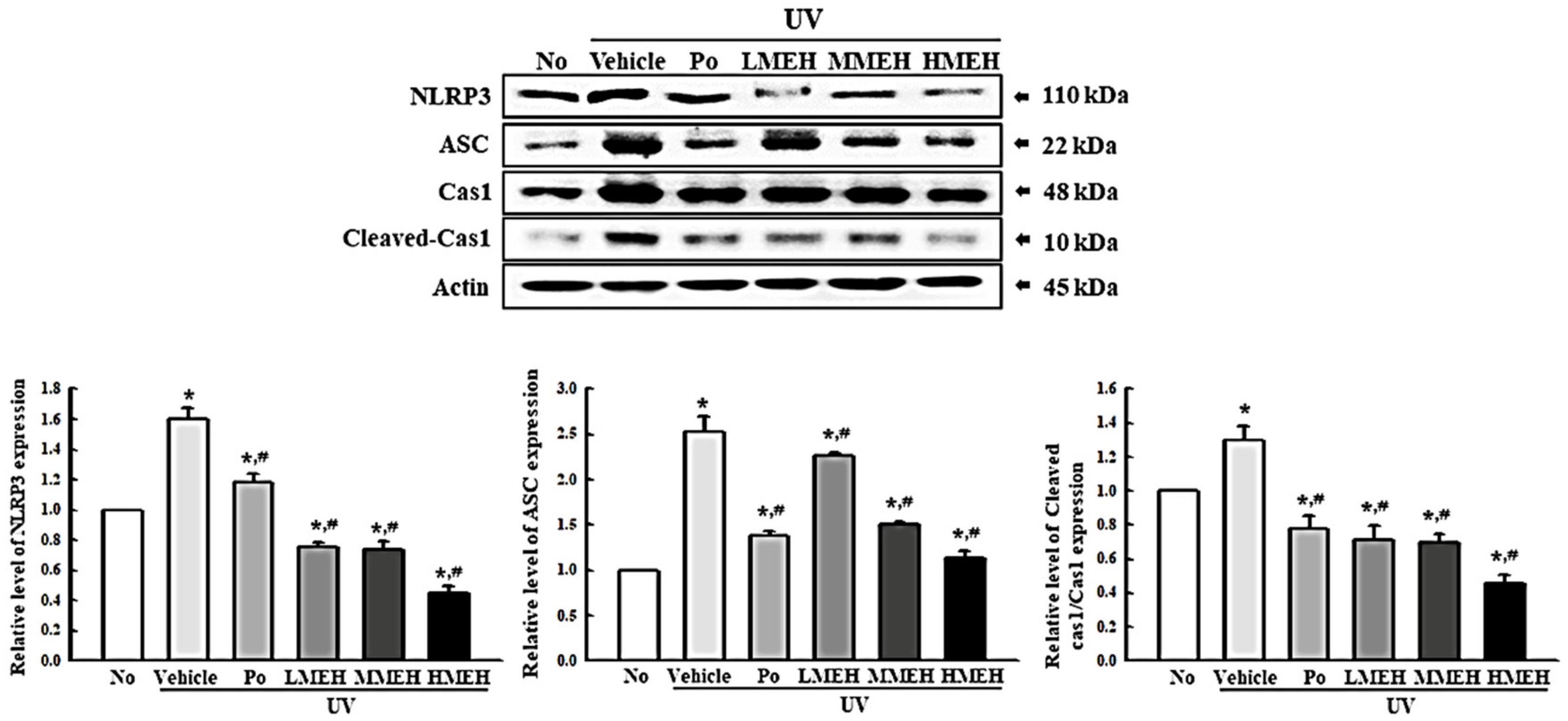
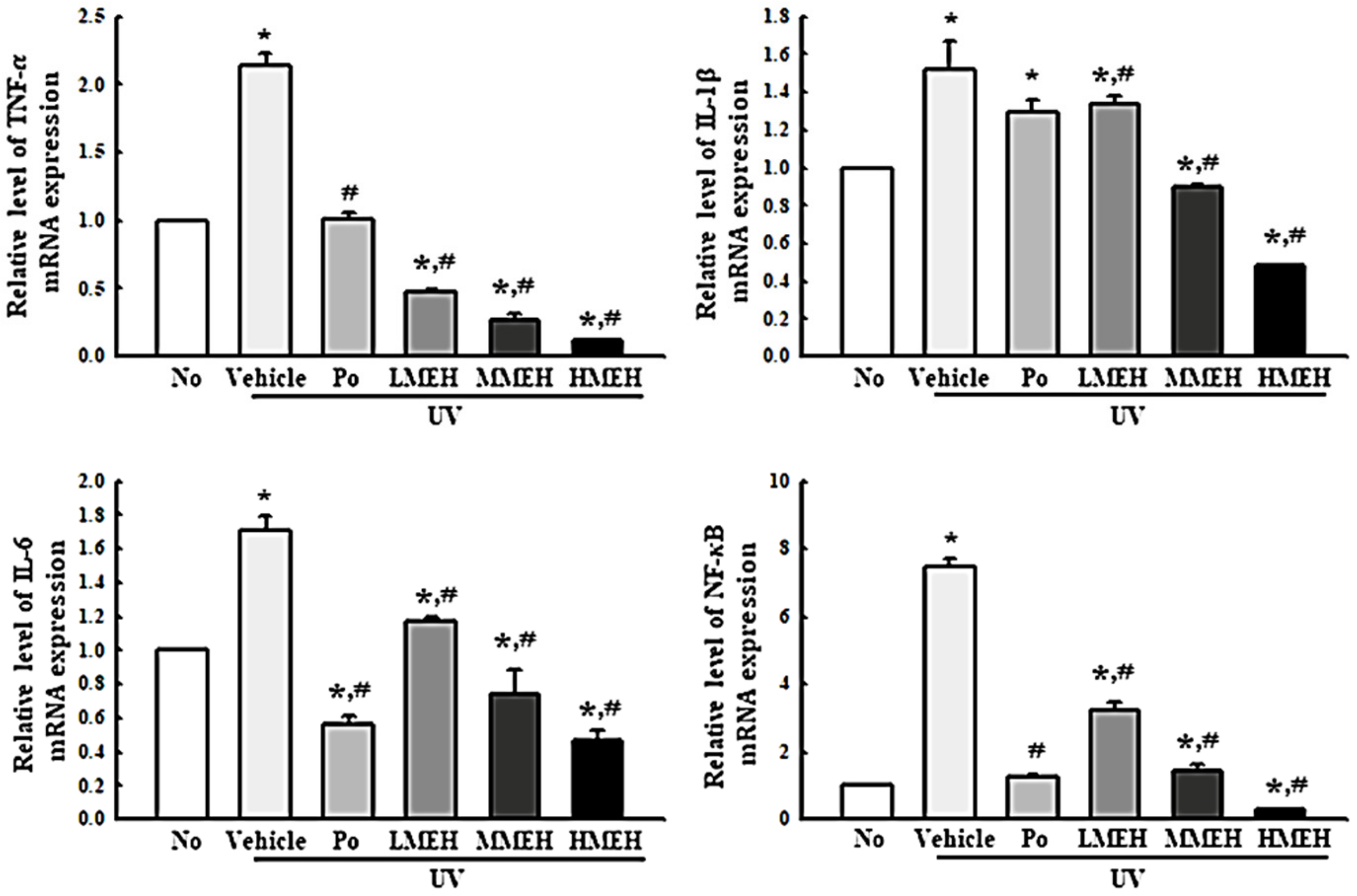
3.6. Effect of MEH on Inflammatory Response in UV-Irradiated NHDF Cells
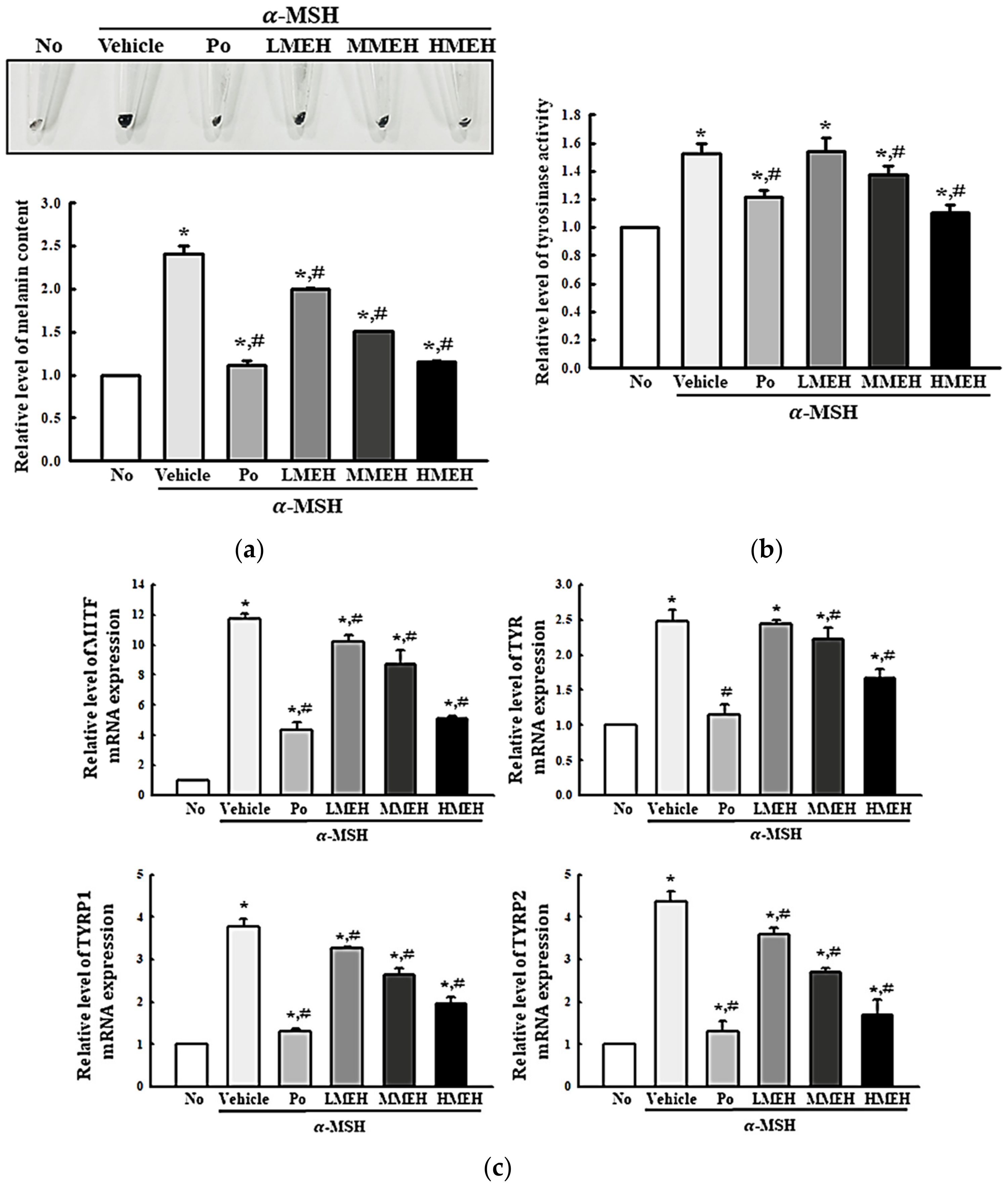
3.7. Effect of MEH on the Melanin Contents in α-Melanocyte-Stimulating Hormone (MSH)-Stimulated B16F1 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, B.C.; Chang, C.J. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat. Chem. Biol. 2011, 7, 504–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, A.; Thatai, P.; Sapra, B. Need of UV protection and evaluation of efficacy of sunscreens. J. Cosmet. Sci. 2014, 65, 315–345. [Google Scholar] [PubMed]
- Corsini, E.; Sangha, N.; Feldman, S.R. Epidermal stratification reduces the effects of UV-B (but not UV-A) on keratinocyte cytokine production and cytotoxicity. Photodermatol. Photoimmunol. Photomed. 1997, 13, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.Y.; Liu, S.X. Pathogenesis of photoaging in human dermal fibroblasts. Int. J. Dermatol. Venereol. 2020, 3, 37–42. [Google Scholar] [CrossRef]
- Dong, K.K.; Damaghi, N.; Picart, S.D.; Markova, N.G.; Obayashi, K.; Okano, Y.; Masaki, H.; Grether-Beck, S.; Krutmann, J.; Smiles, K.A.; et al. UV-induced DNA damage initiates release of MMP-1 in human skin. Exp. Dermatol. 2008, 17, 1037–1044. [Google Scholar] [CrossRef]
- Wiegand, C.; Raschke, C.; Elsner, P. Skin Aging: A Brief Summary of Characteristic Changes. In Textbook of Aging Skin; Farage, M., Miller, K., Maibach, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Poon, F.; Kang, S.; Chien, A.L. Mechanisms and treatments of photoaging. Photodermatol. Photoimmunol. Photomed. 2015, 31, 65–74. [Google Scholar] [CrossRef]
- Weiss, J.S.; Ellis, C.N.; Headington, J.T.; Tincoff, T.; Hamilton, T.A.; Voorhees, J.J. Topical tretinoin improves photoaged skin. A double-blind vehicle controlled study. JAMA 1988, 259, 527–532. [Google Scholar] [CrossRef]
- Sefton, J.; Kligman, A.M.; Kopper, S.C.; Lue, J.C.; Gibson, J.R. Photodamage pilot study: A double-blind, vehicle-controlled study to assess the efficacy and safety of tazarotene 0.1% gel. J. Am. Acad. Dermatol. 2000, 43, 656–663. [Google Scholar] [CrossRef]
- Robert, S.; Stern, M.D. Treatment of photoaging. N. Engl. J. Med. 2004, 350, 1526–1534. [Google Scholar] [CrossRef]
- David, M.; Hodak, E.; Lowe, N.J. Adverse effects of retinoids. Med. Toxicol. Advers. Drug Exp. 1988, 3, 273–288. [Google Scholar] [CrossRef]
- Hong, Y.H.; Kim, J.H.; Cho, J.Y. Photoaging protective effects of Ranunculus bulumei methanol extract. Evid. Based Complementary Altern. Med. 2020, 2020, 11. [Google Scholar] [CrossRef] [Green Version]
- Son, D.J.; Jung, J.C.; Choi, Y.M.; Ryu, H.Y.; Lee, S.; Davis, B.A. Wheat extract oil (WEO) attenuates UVB-induced photoaging via collagen synthesis in human keratinocytes and hairless mice. Nutrients 2020, 12, 300. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.I.; Lee, J.H.; Kim, J.M.; Jung, T.D.; Cho, B.Y.; Choi, S.H.; Lee, D.W.; Kim, J.; Kim, J.Y.; Lee, O.H. Ulmus macrocarpa Hance extracts attenuated H2O2 and UVB-induced skin photoaging by activating antioxidant enzymes and inhibiting MAPK Pathways. Int. J. Mol. Sci. 2017, 18, 1200. [Google Scholar] [CrossRef]
- Hassan, S.M.; Al Aqil, A.A.; Attimarad, M. Determination of crude saponin and total flavonoids content in guar meal. Adv. Med. Plant Res. 2013, 1, 24–28. [Google Scholar]
- Lee, S.J.; Kim, J.E.; Choi, Y.J.; Gong, J.E.; Park, S.H.; Douangdeuane, B.; Souliya, O.; Park, J.M.; Lee, H.S.; Kim, B.H.; et al. Therapeutic effects of Dipterocarpus tuberculatus with high antioxidative activity against UV-induced photoaging of NHDF cells and nude Mice. Antioxidants 2021, 10, 791. [Google Scholar] [CrossRef]
- Cannell, R.J.P.; Kellan, S.J.; Owsianka, A.M.; Walker, J.M. Results of a large scale screen of microalgae for the production of protease inhibitors. Planta Med. 1988, 54, 10–14. [Google Scholar] [CrossRef]
- Hosoi, J.; Abe, E.; Suda, T.; Kuroki, T. Regulation of melanin synthesis of B16 mouse melanoma cells by 1 alpha, 25-dihydroxyvitamin D3 and retinoic acid. Cancer Res. 1985, 45, 1474–1478. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- McCabe, M.C.; Hill, R.C.; Calderone, K.; Cui, Y.; Yan, Y.; Quan, T.; Fisher, G.J.; Hansen, K.C. Alterations in extracellular matrix composition during aging and photoaging of the skin. Matrix Biol. Plus 2020, 8, 100041. [Google Scholar] [CrossRef]
- Kwon, K.R.; Alam, M.B.; Park, J.H.; Kim, T.H.; Lee, S.H. Attenuation of UVB-induced photoaging by polyphenolic-rich Spatholobus suberectus stem extract via modulation of MAPK/AP-1/MMPs signaling in human keratinocytes. Nutrients 2019, 11, 1341. [Google Scholar] [CrossRef] [Green Version]
- Suschek, C.V.; Schnorr, O.; Kolb-Bachofen, V. The role of iNOS in chronic inflammatory processes in vivo: Is it damage-promoting, protective, or active at all? Curr. Mol. Med. 2004, 13, 763–775. [Google Scholar] [CrossRef]
- Maranduca, M.A.; Branisteanu, D.; Serban, D.N.; Branisteanu, D.C.; Stoleriu, G.; Manolache, N.; Serban, I.L. Synthesis and physiological implications of melanic pigments. Oncol. Lett. 2019, 17, 4183–4187. [Google Scholar] [CrossRef] [Green Version]
- Petruk, G.; Del Giudice, R.; Rigano, M.M.; Monti, D.M. Antioxidants from plants protect against skin photoaging. Oxid. Med. Cell. Longev. 2018, 2018, 1454936. [Google Scholar] [CrossRef] [Green Version]
- Henry, M.; Roussel, J.; Andary, C. Verbascoside production in callus and suspension cultures of Hygrophila erecta. Phytochemistry 1987, 26, 1961–1963. [Google Scholar] [CrossRef]
- Borg, J.; Toazara, J.; Hietter, H.; Henry, M.; Schmitt, G.; Luu, B. Neurotrophic effect of naturally occurring long-chain fatty alcohols on cultured CNS neurons. FEBS Lett. 1987, 213, 406–410. [Google Scholar] [CrossRef] [Green Version]
- Borg, J.; Kesslak, P.J.; Cotman, C.W. Peripheral administration of a long-chain fatty alcohol promotes septal cholinergic neurons survival after fimbria-fornix transection. Brain Res. 1990, 518, 295–298. [Google Scholar] [CrossRef]
- Moosbrugger, I.; Bischoff, P.; Beck, J.P.; Luu, B.; Borg, J. Studies on the immunological effects of fatty alcohols—I. Effects of n-hexacosanol on murine macrophages in culture. Int. J. Immunopharmacol. 1992, 14, 293–302. [Google Scholar] [CrossRef]
- Mithi, F.M.; Ahsan, M.; Hasan, C.M.; Azam, Z. Isolation and characterization of secondary metabolites and evaluation of biological activity of Hygrophila erecta (Burm.f.) Hochr. Br. J. Med. Health Res. 2020, 7, 10. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, Q.X.; Wang, Q.; Song, K.K.; Qiu, L. Inhibitory effects of cinnamic acid and its derivatives on the diphenolase activity of mushroom (Agaricus bisporus) tyrosinase. Food Chem. 2005, 92, 707–712. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Hsu, W.H.; Yibchok-anun, S. Mechanisms of p-methoxycinnamic acid-induced increase in insulin secretion. Horm. Metab. Res. 2011, 43, 766–773. [Google Scholar] [CrossRef]
- Whelan, J. The health implications of changing linoleic acid intakes. Prostaglandins Leukot. Essent. Fat. Acids 2008, 79, 165–167. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Banu, J.; Rahman, M.; Causey, J.; Fernandes, G. Biological effects of conjugated linoleic acids in health and disease. J. Nutr. Biochem. 2006, 17, 789–810. [Google Scholar] [CrossRef]
- Pinto, M.; Araújo, S.G.; Morais, M.I.; Sá, N.P.; Lima, C.M.; Rosa, C.A.; Siqueira, E.P.; Johann, S.; Lima, L. Antifungal and antioxidant activity of fatty acid methyl esters from vegetable oils. An. Acad. Bras. Cienc. 2017, 89, 1671–1681. [Google Scholar] [CrossRef] [Green Version]
- Georgousaki, K.; Tsafantakis, N.; Gumeni, S.; Lambrinidis, G.; González-Menéndez, V.; Tormo, J.R.; Genilloud, O.; Trougakos, I.P.; Fokialakis, N. Biological evaluation and in silico study of benzoic acid derivatives from Bjerkandera adusta targeting proteostasis network modules. Molecules 2020, 25, 666. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Li, X.M.; Gloer, J.B.; Wang, B.G. Isolation, characterization, and antioxidant activity of bromophenols of the marine red alga Rhodomela confervoides. J. Agric. Food Chem. 2011, 59, 9916–9921. [Google Scholar] [CrossRef]
- Seo, G.W.; Cho, J.Y.; Kuk, J.H.; Wee, J.H.; Moon, J.H.; Kim, S.H.; Park, K.H. Identification of antioxidative substances in Allium fistulosum L. by GC-MS. Korean J. Food Sci. Technol. 2003, 35, 988–993. [Google Scholar]
- Moghaddam, E.; Teoh, B.T.; Sam, S.S.; Lani, R.; Hassandarvish, P.; Chik, Z.; Yueh, A.; Abubakar, S.; Zandi, K. Baicalin, a metabolite of baicalein with antiviral activity against dengue virus. Sci. Rep. 2014, 4, 5452. [Google Scholar] [CrossRef] [Green Version]
- Shirinzadeh, H.; Eren, B.; Gurer-Orhan, H.; Suzen, S.; Ozden, S. Novel indole-based analogs of melatonin: Synthesis and in vitro antioxidant activity studies. Molecules 2010, 15, 2187–2202. [Google Scholar] [CrossRef] [Green Version]
- Estevão, M.S.; Carvalho, L.C.; Ribeiro, D.; Couto, D.; Freitas, M.; Gomes, A.; Ferreira, L.M.; Fernandes, E.; Marques, M.M. Antioxidant activity of unexplored indole derivatives: Synthesis and screening. Eur. J. Med. Chem. 2010, 45, 4869–4878. [Google Scholar] [CrossRef]
- Lim, J.Y.; Kim, O.K.; Lee, J.; Lee, M.J.; Kang, N.; Hwang, J.K. Protective effect of the standardized green tea seed extract on UVB-induced skin photoaging in hairless mice. Nutr. Res. Pract. 2014, 8, 398–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, P.; Alam, M.B.; Lee, S.H. Protection of UVB-induced photoaging by fuzhuan-brick tea aqueous extract via MAPKs/Nrf2-mediated down-regulation of MMP-1. Nutrients 2019, 11, 60. [Google Scholar] [CrossRef] [Green Version]
- Jeon, J.; Sung, J.; Lee, H.; Kim, Y.; Jeong, H.S.; Lee, J. Protective activity of caffeic acid and sinapic acid against UVB-induced photoaging in human fibroblasts. J. Food Biochem. 2019, 43, e12701. [Google Scholar] [CrossRef]
- Laura, M.; Gloria, M.; Federica, L.; Mario, D.; Stefano, P.; Enrico, S.; Marina, M. Effects of Vitis vinifera L. leaves extract on UV radiation damage in human keratinocytes (HaCaT). J. Photochem. Photobiol. B Biol. 2020, 204, 111810. [Google Scholar] [CrossRef]
- Imokawa, G.; Ishida, K. Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging I: Reduced skin elasticity, highly associated with enhanced dermal elastase activity, triggers wrinkling and sagging. Int. J. Mol. Sci. 2015, 16, 7753–7775. [Google Scholar] [CrossRef] [Green Version]
- Hašová, M.; Crhák, T.; Safránková, B.; Dvořáková, J.; Muthný, T.; Velebný, V.; Kubala, L. Hyaluronan minimizes effects of UV irradiation on human keratinocytes. Arch. Dermatol. 2011, 303, 277–284. [Google Scholar] [CrossRef]
- Hruza, L.L.; Pentland, A.P. Mechanisms of UV-induced inflammation. J. Investig. Dermatol. 1993, 100, 35S–41S. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.W.; Karin, M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J. Clin. Investig. 2007, 117, 1175–1183. [Google Scholar] [CrossRef]
- Smyth, M.J.; Dunn, G.P.; Schreiber, R.D. Cancer immunosurveillance and immunoediting: The roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv. Immunol. 2006, 90, 1–50. [Google Scholar] [CrossRef] [PubMed]
- Tayed, M.A.; Marletta, M.A. Macrophage oxidation of L-arginine to nitric oxide, nitrite, and nitrate. J. Biol. Chem. 1989, 264, 19654–19658. [Google Scholar] [CrossRef]
- Simmons, D.L.; Botting, R.M.; Hla, T. Cyclooxygenase isozymes: The biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 2004, 56, 387–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.W.; Cheng, Y.C.; Hung, Y.C.; Lee, C.H.; Fang, J.Y.; Li, W.T.; Wu, Y.R.; Pan, T.L. Red raspberry extract protects the skin against UVB-induced damage with antioxidative and anti-inflammatory properties. Oxid. Med. Cell. Longev. 2019, 2019, 9529676. [Google Scholar] [CrossRef] [Green Version]
- Ha, S.J.; Lee, J.H.; Kim, H.J.; Song, K.M.; Lee, N.H.; Kim, Y.E.; Lee, H.K.; Kim, Y.H.; Jung, S.K. Preventive effect of Rhus javanica extract on UVB-induced skin inflammation and photoaging. J. Funct. Foods 2016, 27, 589–599. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Yamaguchi, Y.; Sakaki, S.; Takenaka, H. Pleurochrysis carterae hot-Water extract inhibits melanogenesis in murine melanoma cells. Cosmetics 2019, 6, 60. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.S.; Choi, M.J.; Park, S.Y.; Park, S.K. Inhibitory effects of Hoelen extract on melanogenesis in B16/F1 melanoma cells. Phytother. Res. 2010, 24, 1359–1364. [Google Scholar] [CrossRef]
- Choi, E.K.; Guo, H.; Choi, J.K.; Jang, S.K.; Shin, K.; Cha, Y.S.; Choi, Y.; Seo, D.W.; Lee, Y.B.; Joo, S.S.; et al. Extraction conditions of white rose petals for the inhibition of enzymes related to skin aging. Lab. Anim. Res. 2015, 31, 148–152. [Google Scholar] [CrossRef] [Green Version]
- Seong, N.W.; Oh, W.J.; Kim, I.S.; Kim, S.J.; Seo, J.E.; Park, C.E.; Kim, D.Y.; Ko, J.W.; Kim, J.C. Efficacy and local irritation evaluation of Eriobotrya japonica leaf ethanol extract. Lab. Anim. Res. 2019, 35, 4. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.J.; Kim, J.E.; Choi, Y.J.; Jin, Y.J.; Roh, Y.J.; Seol, A.Y.; Song, H.J.; Park, S.H.; Uddin, M.S.; Lee, S.W.; et al. Antioxidative Role of Hygrophila erecta (Brum. F.) Hochr. on UV-Induced Photoaging of Dermal Fibroblasts and Melanoma Cells. Antioxidants 2022, 11, 1317. https://doi.org/10.3390/antiox11071317
Lee SJ, Kim JE, Choi YJ, Jin YJ, Roh YJ, Seol AY, Song HJ, Park SH, Uddin MS, Lee SW, et al. Antioxidative Role of Hygrophila erecta (Brum. F.) Hochr. on UV-Induced Photoaging of Dermal Fibroblasts and Melanoma Cells. Antioxidants. 2022; 11(7):1317. https://doi.org/10.3390/antiox11071317
Chicago/Turabian StyleLee, Su Jin, Ji Eun Kim, Yun Ju Choi, You Jeong Jin, Yu Jeong Roh, A Yun Seol, Hee Jin Song, So Hae Park, Md. Salah Uddin, Sang Woo Lee, and et al. 2022. "Antioxidative Role of Hygrophila erecta (Brum. F.) Hochr. on UV-Induced Photoaging of Dermal Fibroblasts and Melanoma Cells" Antioxidants 11, no. 7: 1317. https://doi.org/10.3390/antiox11071317
APA StyleLee, S. J., Kim, J. E., Choi, Y. J., Jin, Y. J., Roh, Y. J., Seol, A. Y., Song, H. J., Park, S. H., Uddin, M. S., Lee, S. W., & Hwang, D. Y. (2022). Antioxidative Role of Hygrophila erecta (Brum. F.) Hochr. on UV-Induced Photoaging of Dermal Fibroblasts and Melanoma Cells. Antioxidants, 11(7), 1317. https://doi.org/10.3390/antiox11071317







