Content of Phenolic Acids as a Marker of Polish Honey Varieties and Relationship with Selected Honey-Quality-Influencing Variables
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Identification of the Varieties of Honey
2.2.2. Determination of Water Content
2.2.3. Determination of Electrical Conductivity
2.2.4. Determination of Color Intensity
2.2.5. Determination of Color on the Pfund Scale
2.2.6. Determination of Total Phenolic Content (TPC)
2.2.7. Determination of Radical Scavenging Activity by DPPH Assay
2.2.8. Determination of FRAP
2.2.9. Preparation of Samples for HPLC Analysis–Isolation of Phenolic Compounds
2.2.10. HPLC Analysis
2.2.11. Statistical Analysis
3. Results
3.1. Varieties of Bee Honey
3.2. Selected Quality Parameters
3.3. Profile of Phenolic Acids and the Variety of Honey
3.4. Correlations
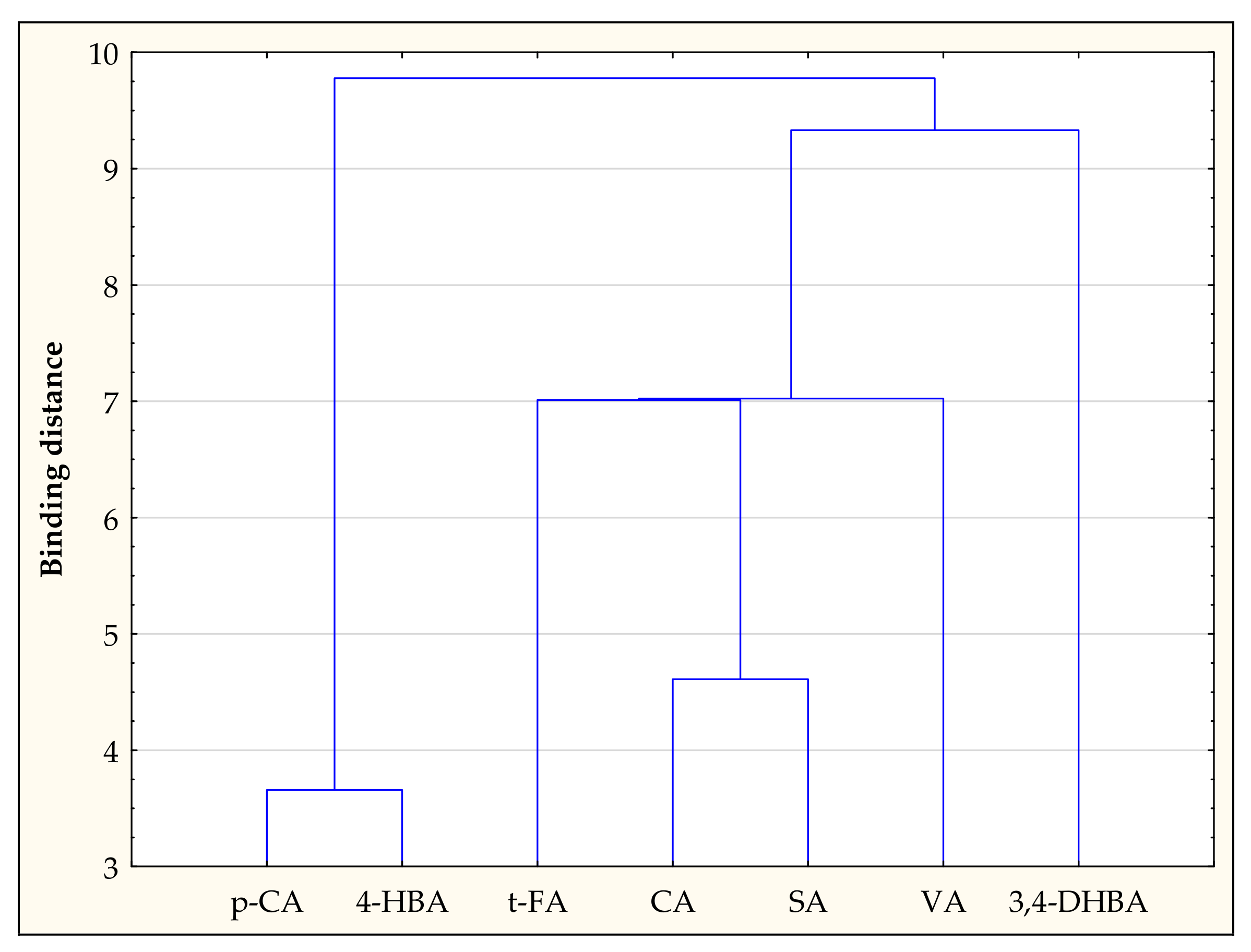
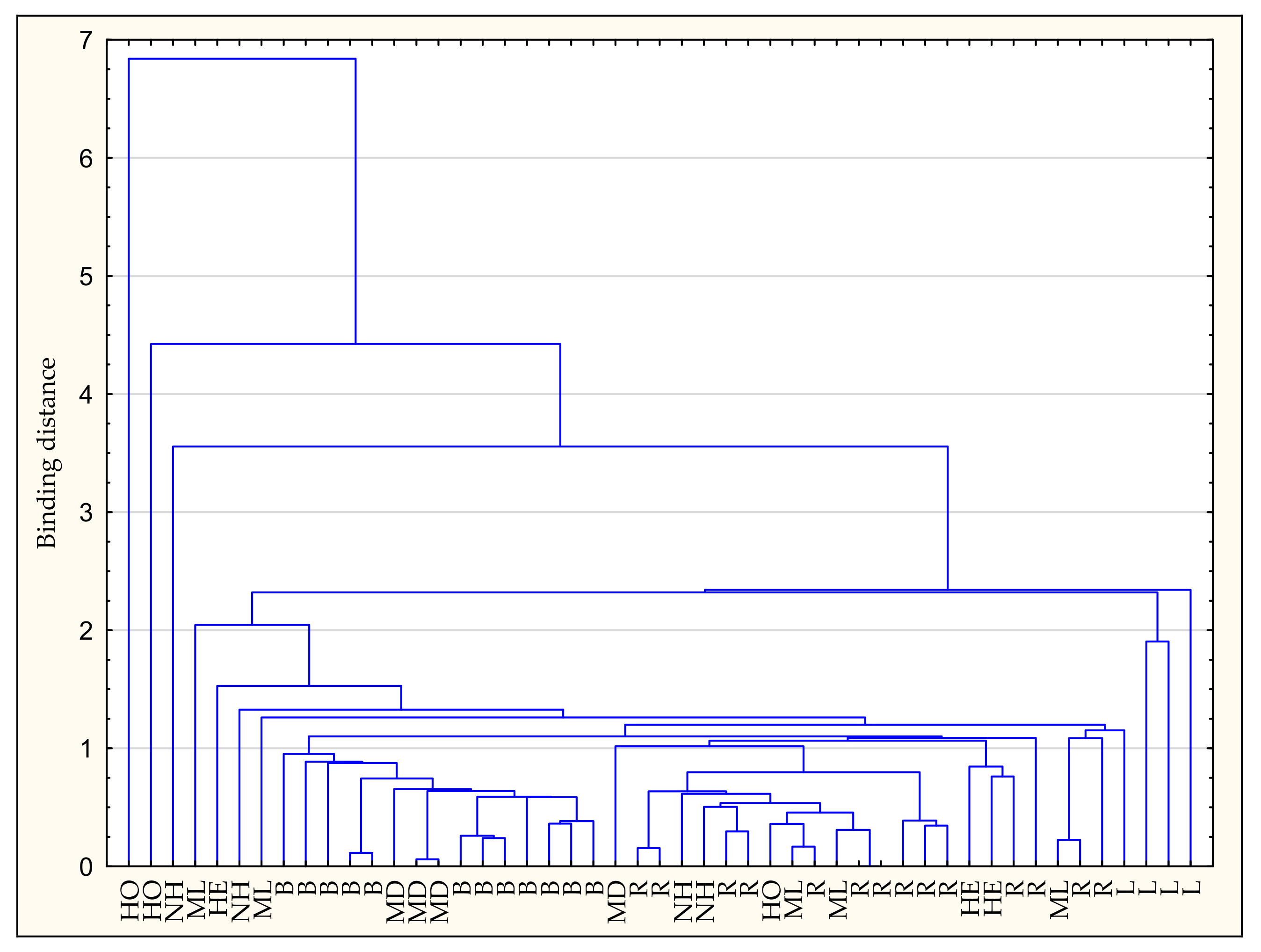
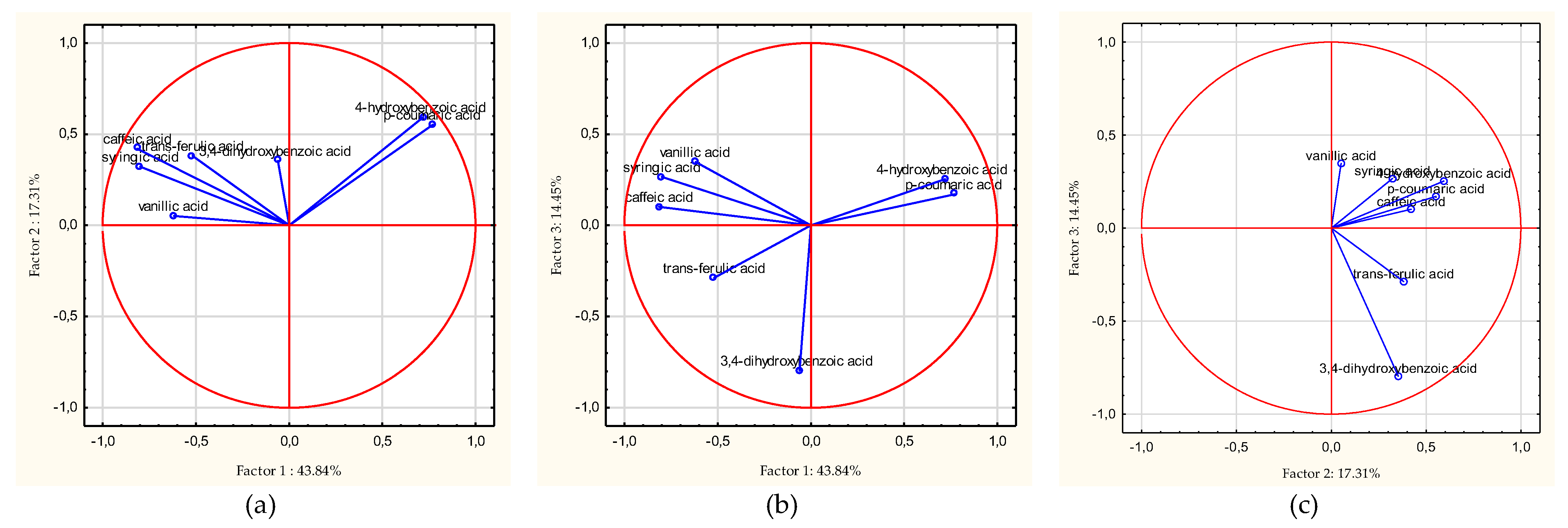
3.5. Chemometric Analyzes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Puścion-Jakubik, A.; Socha, K.; Borawska, M.H. Comparative study of labelled bee honey from Poland and the result of the melissopalynological analysis. J. Apic. Res. 2020, 59, 928–938. [Google Scholar] [CrossRef]
- Major, N.; Marković, K.; Krpan, M.; Sarić, G.; Hruškar, M.; Vahčić, N. Rapid honey characterization and botanical classification by an electronic tongue. Talanta 2011, 85, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Simova, S.; Atanassov, A.; Shishiniova, M.; Bankova, V. A rapid differentiation between oak honeydew honey and nectar and other honeydew honeys by NMR spectroscopy. Food Chem. 2012, 134, 1706–1710. [Google Scholar] [CrossRef]
- Lenhardt, L.; Bro, R.; Zeković, I.; Dramićanin, T.; Dramićanin, M.D. Fluorescence spectroscopy coupled with PARAFAC and PLS DA for characterization and classification of honey. Food Chem. 2015, 175, 284–291. [Google Scholar] [CrossRef]
- Zhou, J.; Yao, L.; Li, Y.; Chen, L.; Wu, L.; Zhao, J. Floral classification of honey using liquid chromatography-diode array detection-tandem mass spectrometry and chemometric analysis. Food Chem. 2014, 145, 941–949. [Google Scholar] [CrossRef]
- Dżugan, M.; Tomczyk, M.; Sowa, P.; Grabek-Lejko, D. Antioxidant Activity as Biomarker of Honey Variety. Molecules 2018, 23, 2069. [Google Scholar] [CrossRef] [Green Version]
- Al-Waili, N.; Salom, K.; Al-Ghamdi, A.A. Honey for wound healing, ulcers, and burns; data supporting its use in clinical practice. Sci. World J. 2011, 11, 766–787. [Google Scholar] [CrossRef]
- Braithwaite, I.; Hunt, A.; Riley, J.; Fingleton, J.; Kocks, J.; Corin, A.; Helm, C.; Sheahan, D.; Tofield, C.; Montgomery, B.; et al. Randomised controlled trial of topical kanuka honey for the treatment of rosacea. BMJ Open 2015, 5, e007651. [Google Scholar] [CrossRef]
- Oduwole, O.; Udoh, E.E.; Oyo-Ita, A.; Meremikwu, M.M. Honey for acute cough in children. Cochrane Database Syst. Rev. 2018, 4, CD007094. [Google Scholar] [CrossRef]
- Saha, A.; Chattopadhyay, S.; Azam, M.; Sur, P.K. The role of honey in healing of bedsores in cancer patients. South Asian J. Cancer 2012, 1, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Martinello, M.; Mutinelli, F. Antioxidant Activity in Bee Products: A Review. Antioxidants 2021, 10, 71. [Google Scholar] [CrossRef]
- Vacek, J.; Ulrichová, J.; Klejdus, B.; Šimánek, V. Analytical methods and strategies in the study of plant polyphenolics in clinical samples. Anal. Methods 2010, 6, 604–613. [Google Scholar] [CrossRef]
- Olas, B. Honey and Its Phenolic Compounds as an Effective Natural Medicine for Cardiovascular Diseases in Humans? Nutrients 2020, 12, 283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regulation of the Minister of Agriculture and Rural Development dated 14 January 2009. On Methods of Analysis Related to the Performance Evaluation of Honey [In Polish, Rozporządzenie Ministra Rolnictwa I Rozwoju Wsi Z Dnia 14 Stycznia 2009 R. W Sprawie Metod Analiz Związanych Z Dokonywaniem Oceny Miodu]. 2009; Dz.U. 2009 nr 17 poz. 94. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20090170094 (accessed on 2 March 2022).
- Beretta, G.; Granata, P.; Ferrero, M.; Orioli, M.; Facino, R.M. Standarization of antioxidant properties of honey by a combination of spectrophotometric/fluorimetric assays and chemometrics. Anal. Chim. Acta 2005, 533, 185–191. [Google Scholar] [CrossRef]
- Ferreira, I.C.; Aires, E.; Barreira, J.C.; Estevinho, L.M. Antioxidant activity of Portuguese honey samples: Different contributions of the entire honey and phenolic extract. Food Chem. 2009, 114, 1438–1443. [Google Scholar] [CrossRef]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Maamri, S.; Djireb, F.; Stocker, P. Phenolic extracts from various Algerian plants as strong inhibitors of porcine liver carboxylesterase. J. Enzym. Inhib. Med. Chem. 2006, 21, 719–726. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Starowicz, M.; Ostaszyk, A.; Zieliński, H. The Relationship between the Browning Index, Total Phenolics, Color, and Antioxidant Activity of Polish-Originated Honey Samples. Foods 2021, 10, 967. [Google Scholar] [CrossRef]
- Ecem Bayram, N.; Canli, D.; Gercek, Y.C.; Bayram, S.; Çelik, S.; Güzel, F.; Morgil, H.; Oz, G.C. Macronutrient ans micronutrient levels and phenolic compound characteristics of monofloral honey samples. J. Food Nutr. Res. 2020, 59, 311–322. [Google Scholar]
- Dimitrova, B.; Gevrenova, R.; Anklam, E. Analysis of phenolic acids in honeys of different floral origin by solid-phase extraction and high-performance liquid chromatography. Phytochem. Anal. 2007, 18, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Jasicka-Misiak, I.; Poliwoda, A.; Dereń, M.; Kafarski, P. Phenolic compounds and abscisic acid as potential markers for the floral origin of two Polish unifloral honeys. Food Chem. 2012, 131, 1149–1156. [Google Scholar] [CrossRef]
- Pasini, F.; Gardini, S.; Marcazzan, G.L.; Caboni, M.F. Buckwheat honeys: Screening of composition and properties. Food Chem. 2013, 141, 2802–2811. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Hu, Y.; Zhou, J.; Chen, L.; Lu, X. Systematic review of the characteristic markers in honey of various botanical, geographic, and entomological origins. ASC Food Sci. Technol. 2022, 2, 206–220. [Google Scholar] [CrossRef]
- Cabras, P.; Angioni, A.; Tuberoso, C.; Floris, I.; Reniero, F.; Guillou, C.; Ghelli, S. Homogentisic acid: A phenolic acid as a marker of strawberry-tree (Arbutus unedo) honey. J. Agric. Food Chem. 1999, 47, 4064–4067. [Google Scholar] [CrossRef]
- Jerković, I.; Kuś, P.M.; Tuberoso, C.I.; Šarolić, M. Phytochemical and physical-chemical analysis of Polish willow (Salix spp.) honey: Identification of the marker compounds. Food Chem. 2014, 145, 8–14. [Google Scholar] [CrossRef]
- Jasicka-Misiak, I.; Makowicz, E.; Stanek, N. Polish Yellow Sweet Clover (Melilotus officinalis L.) Honey, Chromatographic Fingerprints, and Chemical Markers. Molecules 2017, 22, 138. [Google Scholar] [CrossRef] [Green Version]
- Cheng, N.; Wang, Y.; Cao, W. The Protective Effect of Whole Honey and Phenolic Extract on Oxidative DNA Damage in Mice Lymphocytes Using Comet Assay. Plant Foods Hum. Nutr. 2017, 72, 388–395. [Google Scholar] [CrossRef]
- Liu, Y.M.; Shen, J.D.; Xu, L.P.; Li, H.B.; Li, Y.C.; Yi, L.T. Ferulic acid inhibits neuro-inflammation in mice exposed to chronic unpredictable mild stress. Int. Immunopharmacol. 2017, 45, 128–134. [Google Scholar] [CrossRef]
- Wang, T.; Gong, X.; Jiang, R.; Li, H.; Du, W.; Kuang, G. Ferulic acid inhibits proliferation and promotes apoptosis via blockage of PI3K/Akt pathway in osteosarcoma cell. Am. J. Transl. Res. 2016, 8, 968–980. [Google Scholar] [PubMed]
- Ou, C.; Shi, N.; Yang, Q.; Zhang, Y.; Wu, Z.; Wang, B.; Compans, R.W.; He, C. Protocatechuic acid, a novel active substance against avian influenza virus H9N2 infection. PLoS ONE 2014, 9, e111004. [Google Scholar] [CrossRef] [Green Version]
- Amalan, V.; Vijayakumar, N.; Indumathi, D.; Ramakrishnan, A. Antidiabetic and antihyperlipidemic activity of p-coumaric acid in diabetic rats, role of pancreatic GLUT 2: In vivo approach. Biomed. Pharm. 2016, 84, 230–236. [Google Scholar] [CrossRef]
- Wilczyńska, A. Phenolic content and antioxidant activity of different types of Polish honey—A short report. Pol. J. Food Nutr. Sci. 2010, 60, 309–313. [Google Scholar]
- Pentoś, K.; Łuczycka, D.; Oszmiański, J.; Lachowicz, S.; Pasternak, G. Polish honey as a source of antioxidants—A comparison with Manuka honey. J. Apic. Res. 2020, 59, 939–945. [Google Scholar] [CrossRef]

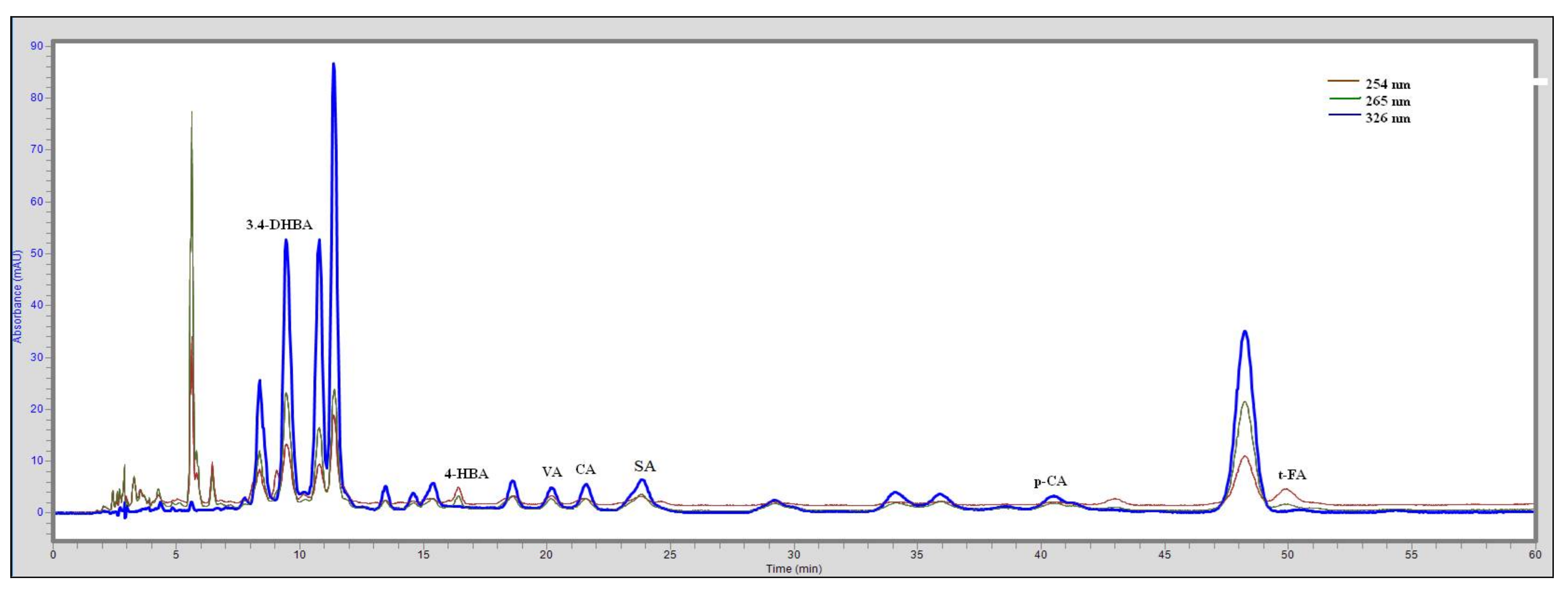
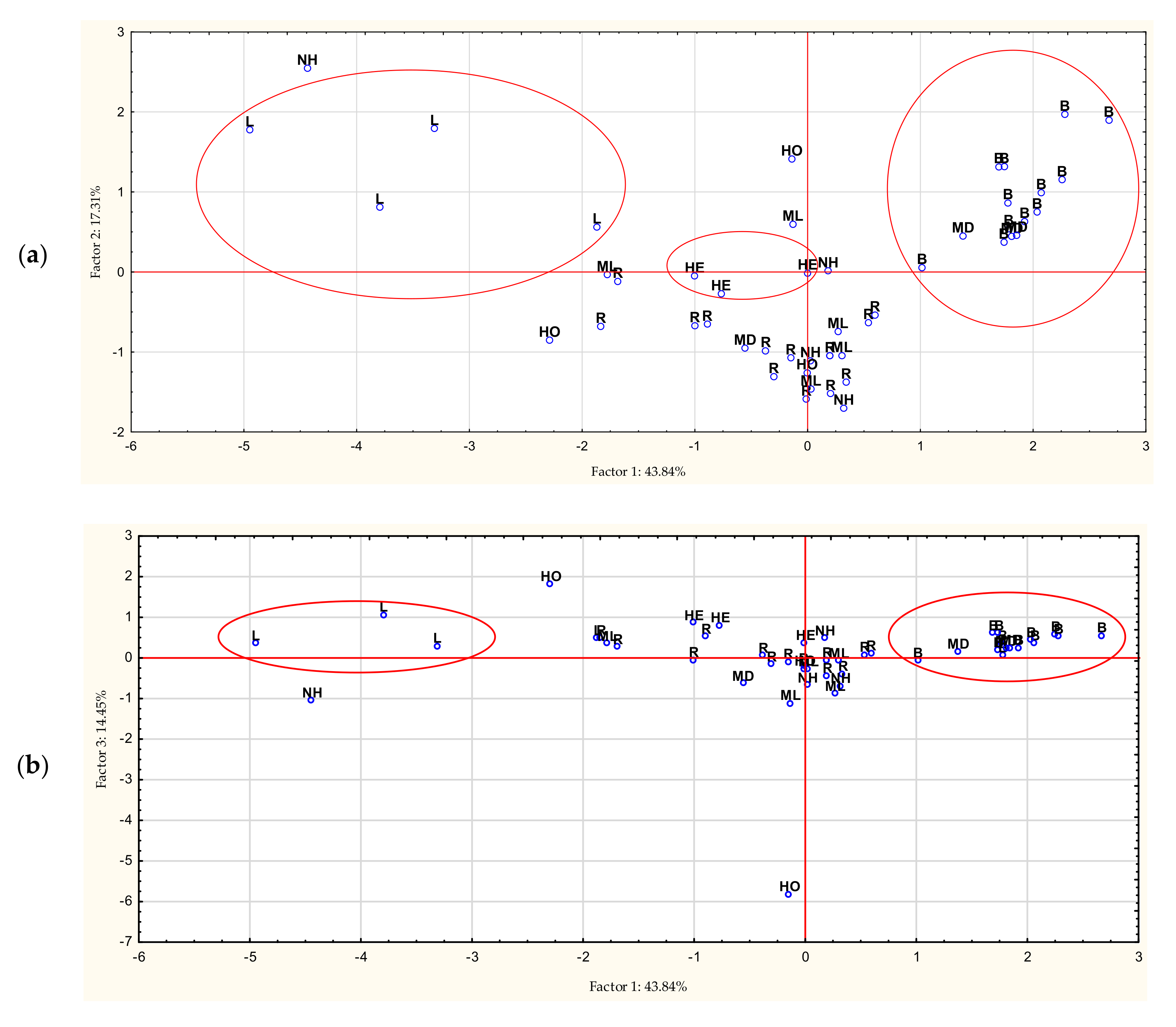
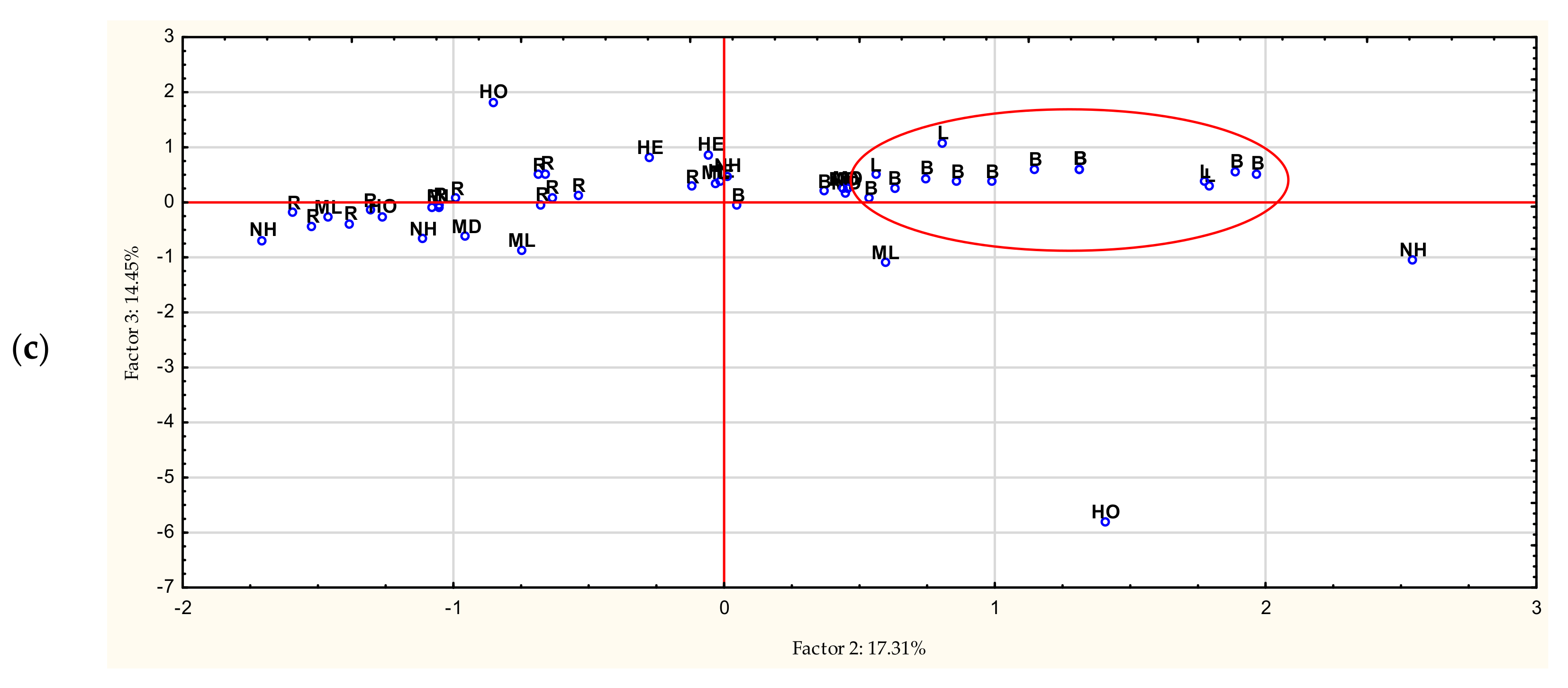
| Compounds | RT | LOD (mg/100 g) | LOQ (mg/100 g) |
|---|---|---|---|
| 3,4–DHBA | 9.183 | 0.099 | 0.300 |
| 4–HBA | 16.570 | 0.092 | 0.278 |
| VA | 20.284 | 0.089 | 0.271 |
| CA | 21.756 | 0.106 | 0.322 |
| SA | 23.886 | 0.147 | 0.445 |
| p–CA | 40.572 | 0.138 | 0.418 |
| t–FA | 50.040 | 0.084 | 0.255 |
| Variety–Declarations of Beekeepers | The Number of Attempts Correctly Classified | The Number of Attempts Is Classified Incorrectly |
|---|---|---|
| buckwheat (n = 15) | 12 | 3 |
| dandelion (n = 4) | 0 | 4 |
| heather (n = 3) | 3 | 0 |
| honeydew (n = 3) | 3 | 0 |
| linden (n = 9) | 4 | 5 |
| multi-flower light (n = 3) | 3 | 0 |
| nectar–honeydew (n = 4) | 3 | 1 |
| rape (n = 8) | 8 | 0 |
| Variety (Sign) | Colour Scale (mm Pfund) | Colour Intensity (mAU) | TPC (mg GAE/100 g) | Water Content (%) | Electrical Conductivity (mS × cm−1) | DPPH (% Free Radical Scavenging) | FRAP (Equivalent of µmol Fe2+/mL) | |
|---|---|---|---|---|---|---|---|---|
| Buckwheat (B) | Av. ± SD Min-Max Med Q1-Q3 | 166.4 ± 29.4 | 1816.0 ± 688.0 | 182.60 ± 61.08 | 18.9 ± 0.5 | 0.400 ± 0.043 | 42.0 ± 4.5 | 0.402 ± 0.010 |
| 125.8–218.5 | 711.0–2634.7 | 44.95–241.87 | 18.1–19.9 | 0.326–0.507 | 34.9–52.7 | 0.379–0.417 | ||
| 159.8 | 2109.2 | 196.59 | 18.9 | 0.391 | 41.1 | 0.403 | ||
| 147.9–189.0 | 1229.0–2291.8 | 142.29–236.63 | 18.4–19.3 | 0.380–0.416 | 39.8–44.0 | 0.398–0.409 | ||
| Heather (He) | 125.2 ± 14.8 | 575.8 ± 179.5 | 91.78 ± 4.25 | 19.2 ± 0.7 | 0.552 ± 0.027 | 46.4 ± 3.7 | 0.141 ± 0.002 | |
| 111.1–140.7 | 468.0–783.0 | 87.72–96.20 | 18.6–19.9 | 0.534–0.583 | 42.3–49.5 | 0.139–0.143 | ||
| 124.0 | 476.3 | 91.42 | 19.0 | 0.538 | 47.5 | 0.140 | ||
| 111.1–140.7 | 468.0–783.0 | 87.72–96-20 | 18.6–19.9 | 0.5334–0.583 | 42.3–49 | 0.139–0.143 | ||
| Honeydew (Ho) | 109.9 ± 95.9 | 587.1 ± 327.0 | 86.0 ± 55.3 | 16.3 ± 0.6 | 1.728 ± 1.072 | 58.6 ± 4.0 | 0.323 ± 0.017 | |
| 49.8–220.5 | 215.3–830.0 | 42.8–148.3 | 15.7–16.8 | 1.041–2.963 | 55.9–63.2 | 0.312–0.343 | ||
| 59.5 | 716.0 | 67.07 | 16.4 | 1.181 | 56.7 * B | 0.315 | ||
| 49.8–220.5 | 215.3–830.0 | 42.78–148.30 | 15.7–16.8 | 1.041–1.922 | 55.9–63.2 | 0.312–0.343 | ||
| Linden (L) | 43.5 ± 19.6 | 84.0 ± 44.0 | 29.23 ± 10.60 | 16.7 ± 0.7 | 0.502 ± 0.104 | 58.6 ± 1.4 | 0.083 ± 0.012 | |
| 20.0–64.2 | 49.0–148.3 | 18.24–43.69 | 15.7–17.1 | 0.396–0.597 | 56.6–59.7 | 0.071–0.099 | ||
| 44.9 ** B | 69.3 *** B | 27.50 ** B | 16.9 | 0.508 | 59.0 ** B | 0.081 | ||
| 27.8–59.2 | 57.5–110.5 | 22.44–36.03 | 16.2–17.1 | 0.413–0.592 | 57.5–59.7 | 0.075–0.091 | ||
| Multifloral dark (Md) | 124.4 ± 25.6 | 1424.7 ± 803.1 | 187.6 ± 194.3 | 19.2 ± 0.8 | 0.416 ± 0.026 | 56.7 ± 2.8 | 0.218 ± 0.015 | |
| 91.9–154.1 | 280.0–2160.0 | 55.60–467.83 | 18.1–20.0 | 0.380–0.437 | 53.2–59.3 | 0.198–0.232 | ||
| 125.8 | 1629.3 | 113.50 | 19.3 | 0.423 | 57.2 * B | 0.221 | ||
| 107.3–141.5 | 953.7–1895.7 | 56.37–318.85 | 18.7–19.7 | 0.397–0.435 | 54.4–59.0 | 0.206–0.230 | ||
| Multifloral light (Ml) | 33.3 ± 24.3 | 155.6 ± 90.9 | 32.04 ± 3.80 | 18.6 ± 0.7 | 0.431 ± 0.109 | 45.3 ± 6.5 | 0.052 ± 0.032 | |
| 1.0–64.7 | 64.0–272.0 | 28.86–38.26 | 18.0–19.3 | 0.308–0.584 | 37.4–52.8 | 0.014–0.090 | ||
| 37.4 *** B | 128.0 * B | 30.85 ** B | 18.1 | 0.452 | 46.8 | 0.062 ** B | ||
| 18.9–44.2 | 85.0–229.0 | 29.44–32.79 | 18.1–19.3 | 0.344–0.466 | 39.8–49.6 | 0.023–0.070 | ||
| Nectar–honeydew (Nh) | 115.2 ± 55.2 | 322.6 ± 231.7 | 57.08 ± 11.54 | 17.8 ± 1.7 | 0.641 ± 0.031 | 57.4 ± 4.2 | 0.205 ± 0.010 | |
| 75.4–192.6 | 93.7–623.0 | 47.20–70.74 | 16.5–20.3 | 0.609–0.670 | 52.4–61.8 | 0.197–0.219 | ||
| 96.5 | 286.8 | 55.19 | 17.2 | 0.642 | 57.8 * B | 0.203 | ||
| 75.5–155.0 | 145.8–499.3 | 47.51–66.65 | 16.8–18.8 | 0.614–0.667 | 54.1–60.8 | 0.198–0.213 | ||
| Rape (R) | 81.47 ± 31.88 | 127.7 ± 48.69 | 33.18 ± 6.28 | 18.7 ± 0.8 | 0.284 ± 0.092 | 47.9 ± 5.7 | 0.030 ± 0.012 | |
| 17.5–125.6 | 62.0–231.0 | 20.40–43.94 | 17.7–20.6 | 0.169–0.449 | 37.8–58.7 | 0.012–0.058 | ||
| 84.8 ** B | 126.0 *** B | 35.10 ** B | 18.6 | 0.242 ** Ho, ** Nh | 48.0 | 0.030 *** B | ||
| 66.4–98.8 | 86.0–150.3 | 30.17–36.74 | 18.1–19.1 | 0.215–0.352 | 45.6–52.3 | 0.022–0.035 |
| Variety (Sign) | 3,4-DHBA | 4-HBA | CA | p-CA | VA | SA | t-FA | |
|---|---|---|---|---|---|---|---|---|
| Buckwheat (B) | Av. ± SD Min-Max Med Q1-Q3 | 1.403 ± 0.419 | 3.203 ± 0.736 | 0.194 ± 0.073 | 0.784 ± 0.129 | 0.151 ± 0.043 | 0.186 ± 0.127 | 0.095 ± 0.050 |
| 0.784–2.233 | 1.699–4.432 | <LOD-0.325 | 0.558–1.004 | <LOD-0.193 | <LOD-0.329 | <LOD-0.175 | ||
| 1.421 | 3.129 | 0.207 | 0.804 | 0.165 | <LOD | <LOD | ||
| 1.101–1.558 | 2.869–3.515 | 0.177–0.219 | 0.678–0.870 | <LOD-0.180 | <LOD-0.198 | <LOD-0.152 | ||
| Heather (He) | 0.539 ± 0.056 | 0.895 ± 0.172 | 0.215 ± 0.025 | 0.386 ± 0.059 | 0.162 ± 0.143 | 0.860 ± 0.159 | 0.106 ± 0.092 | |
| 0.505–0.604 | 0.736–1.078 | 0.189–0.239 | 0.340–0.452 | <LOD-0.273 | 0.705–1.023 | <LOD-0.166 | ||
| 0.509 | 0.873 | 0.216 | 0.367 | 0.211 | 0.852 | 0.152 | ||
| 0.505–0.604 | 0.736–1.078 | 0.189–0.239 | 0.340–0.452 | <LOD-0.273 | 0.705–1.023 | <LOD-0.166 | ||
| Honeydew (Ho) | 7.646 ± 12.383 | 0.287 ± 0.090 | 0.252 ± 0.024 | 0.249 ± 0.089 | 0.368 ± 0.472 | 0.166 ± 0.183 | 0.475 ± 0.493 | |
| 0.354–21.944 | 0.184–0.348 | 0.225–0.268 | 0.171–0.346 | 0.133–0.913 | <LOD-0.317 | <LOD-0.985 | ||
| 0.639 | 0.329 | 0.264 | 0.230 | 0.107 | <LOD | 0.442 | ||
| 0.354–21.944 | 0.184–0.348 | 0.225–0.268 | 0.171–0.346 | 0.133–0.913 | <LOD-0.317 | <LOD-0.985 | ||
| Linden (L) | 2.064 ± 0.278 | 0.200 ± 0.051 | 1.679 ± 0.338 | 0.212 ± 0.211 | 0.312 ± 0.080 | 1.085 ± 0.276 | 1.973 ± 2.142 | |
| 1.818–2.454 | 0.152–0.271 | 1.227–1.998 | <LOD-0.403 | 0.240–0.402 | 0.726–1.399 | <LOD-3.982 | ||
| 1.993 | 0.188 * B | 1.746 ** B | 0.221 * B | 0.304 * B | 1.107 * L | 1.954 | ||
| 1.872–2.257 | 0.165–0.235 | 1.427–1.931 | 0.151–0.392 | 0.245–0.380 | 0.910–1.259 | 0.123–3.822 | ||
| Multifloral dark (Md) | 0.680 ± 0.258 | 1.572 ± 0.964 | 0.261 ± 0.075 | 0.631 ± 0.325 | 0.108 ± 0.047 | <LOD | 0.633 ± 1.164 | |
| 0.443–1.045 | 0.185–2.235 | 0.190–0.334 | 0.144–0.804 | <LOD-0.146 | <LOD | <LOD-2.376 | ||
| 0.615 | 1.934 | 0.260 | 0.789 | 0.128 | <LOD | 0.087 | ||
| 0.517–0.842 | 0.917–2.227 | 0.197–0.325 | 0.463–0.800 | <LOD-0.136 | <LOD | <LOD-1.267 | ||
| Multifloral light (Ml) | 0.646 ± 0.551 | 0.191 ± 0.061 | 0.332 ± 0.333 | 0.357 ± 0.185 | 0.108 ± 0.070 | 0.178 ± 0.396 | 1.741 ± 2.479 | |
| 0.193–1.596 | 0.108–0.251 | <LOD-0.885 | 0.158–0.235 | <LOD-0.165 | <LOD-0.885 | 0.094–5.654 | ||
| 0.541 | 0.179 ** B | 0.225 | 0.373 | 0.118 | <LOD | 0.155 | ||
| 0.354–0.545 | 0.167–0.251 | 0.203–0.346 | 0.235–0.377 | <LOD-0.145 | <LOD | <LOD -2.782 | ||
| Nectar–honeydew (Nh) | 1.019 ± 0.849 | 0.235 ± 0.068 | 0.512 ± 0.667 | 0.299 ± 0.280 | 0.116 ± 0.033 | 0.410 ± 0.541 | 2.773 ± 4.607 | |
| 0.233–2.222 | 0.140–0.298 | <LOD-1.493 | 0.154–0.717 | <LOD-0.189 | <LOD-1.141 | 0.090–9.656 | ||
| 0.810 | 0.251 * B | 0.278 | 0.178 | 0.107 | 0.249 | 0.673 * B | ||
| 0.488–1.550 | 0.193–0.277 | 0.134–0.891 | 0.174–0.457 | <LOD-0.152 | <LOD-0.819 | 0.193–5.353 | ||
| Rape (R) | 0.455 ± 0.379 | 0.256 ± 0.139 | 0.334 ± 0.245 | 0.262 ± 0.145 | 0.102 ± 0.068 | 0.267 ± 0.313 | 0.124 ± 0.060 | |
| <LOD-1.482 | 0.109–0.484 | 0.177–0.870 | 0.155–0.581 | <LOD-0.246 | <LOD-0.847 | <LOD-0.237 | ||
| 0.350 ** L, ** B | 0.174 *** B | 0.227 | 0.242 *** B | 0.114 | 0.165 | 0.128 | ||
| 0.265–0.385 | 0.153–0.411 | 0.199–0.237 | 0.168–0.325 | <LOD-0.127 | <LOD-0.419 | 0.096–0.155 |
| Parameter 1 | Parameter 2 | r | p |
|---|---|---|---|
| Color in Pfund scale | Colour intensity | 0.82 | 0.001 |
| Color in Pfund scale | TPC | 0.77 | 0.001 |
| Color in Pfund scale | Diastase number | 0.51 | 0.001 |
| Color in Pfund scale | 3,4-DHBA | 0.75 | 0.001 |
| Color in Pfund scale | SA | −0.33 | 0.021 |
| Color in Pfund scale | p-CA | 0.51 | 0.001 |
| Color in Pfund scale | t-FA | −0.57 | 0.001 |
| Color in Pfund scale | CA | −0.45 | 0.001 |
| Colour intensity | TPC | 0.90 | 0.001 |
| Colour intensity | Diastase number | 0.51 | 0.001 |
| Colour intensity | Water | 0.33 | 0.020 |
| Colour intensity | 4-HBA | 0.84 | 0.001 |
| Colour intensity | VA | −0.39 | 0.005 |
| Colour intensity | SA | −0.45 | 0.001 |
| Colour intensity | p-CA | 0.68 | 0.001 |
| Colour intensity | t-FA | −0.52 | 0.001 |
| Colour intensity | CA | −0.46 | 0.001 |
| DPPH | Water | −0.37 | 0.008 |
| DPPH | p-CA | −0.35 | 0.01 |
| DPPH | t-FA | 0.45 | 0.001 |
| TPC | Diastase number | 0.58 | 0.001 |
| TPC | 3,4-DHBA | 0.33 | 0.020 |
| TPC | 4-HBA | 0.79 | 0.001 |
| TPC | VA | −0.30 | 0.038 |
| TPC | p-CA | 0.60 | 0.001 |
| TPC | t-FA | −0.57 | 0.001 |
| TPC | CA | −0.31 | 0.001 |
| Diastase number | 4-HBA | 0.56 | 0.001 |
| Diastase number | p-CA | 0.55 | 0.001 |
| Water | Electrical conductivity | −0.37 | 0.009 |
| Water | 4-HBA | 0.31 | 0.026 |
| Water | VA | −0.37 | 0.009 |
| Water | p-CA | 0.32 | 0.023 |
| Water | CA | −0.36 | 0.011 |
| Electrical conductivity | 3,4-DHBA | 0.40 | 0.005 |
| Electrical conductivity | VA | 0.29 | 0.040 |
| Electrical conductivity | CA | 0.42 | 0.002 |
| FRAP | Colour in Pfund scale | 0.68 | 0.001 |
| FRAP | Colour intensity | 0.82 | 0.001 |
| FRAP | TPC | 0.82 | 0.001 |
| FRAP | Diastase number | 0.50 | 0.001 |
| FRAP | Electrical conductivity | 0.38 | 0.008 |
| FRAP | 3,4-DHBA | 0.53 | 0.001 |
| FRAP | 4-HBA | 0.73 | 0.001 |
| FRAP | p-CA | 0.58 | 0.001 |
| FRAP | t-FA | −0.38 | 0.006 |
| 3,4-DHBA | SA | 0.30 | 0.034 |
| 3,4-DHBA | CA | 0.37 | 0.009 |
| 4-HBA | SA | −0.29 | 0.040 |
| 4-HBA | p-CA | 0.82 | 0.001 |
| 4-HBA | t-FA | −0.46 | 0.001 |
| 4-HBA | CA | −0.36 | 0.011 |
| VA | SA | 0.60 | 0.000 |
| VA | p-CA | −0.32 | 0.024 |
| VA | CA | 0.60 | 0.001 |
| SA | p-CA | −0.31 | 0.028 |
| SA | CA | 0.51 | 0.001 |
| p-CA | t-FA | −0.30 | 0.038 |
| p-CA | CA | −0.29 | 0.040 |
| t-FA | CA | 0.47 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puścion-Jakubik, A.; Karpińska, E.; Moskwa, J.; Socha, K. Content of Phenolic Acids as a Marker of Polish Honey Varieties and Relationship with Selected Honey-Quality-Influencing Variables. Antioxidants 2022, 11, 1312. https://doi.org/10.3390/antiox11071312
Puścion-Jakubik A, Karpińska E, Moskwa J, Socha K. Content of Phenolic Acids as a Marker of Polish Honey Varieties and Relationship with Selected Honey-Quality-Influencing Variables. Antioxidants. 2022; 11(7):1312. https://doi.org/10.3390/antiox11071312
Chicago/Turabian StylePuścion-Jakubik, Anna, Elżbieta Karpińska, Justyna Moskwa, and Katarzyna Socha. 2022. "Content of Phenolic Acids as a Marker of Polish Honey Varieties and Relationship with Selected Honey-Quality-Influencing Variables" Antioxidants 11, no. 7: 1312. https://doi.org/10.3390/antiox11071312
APA StylePuścion-Jakubik, A., Karpińska, E., Moskwa, J., & Socha, K. (2022). Content of Phenolic Acids as a Marker of Polish Honey Varieties and Relationship with Selected Honey-Quality-Influencing Variables. Antioxidants, 11(7), 1312. https://doi.org/10.3390/antiox11071312








